Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis Ticks Collected in Eastern Canada
Abstract
:1. Introduction
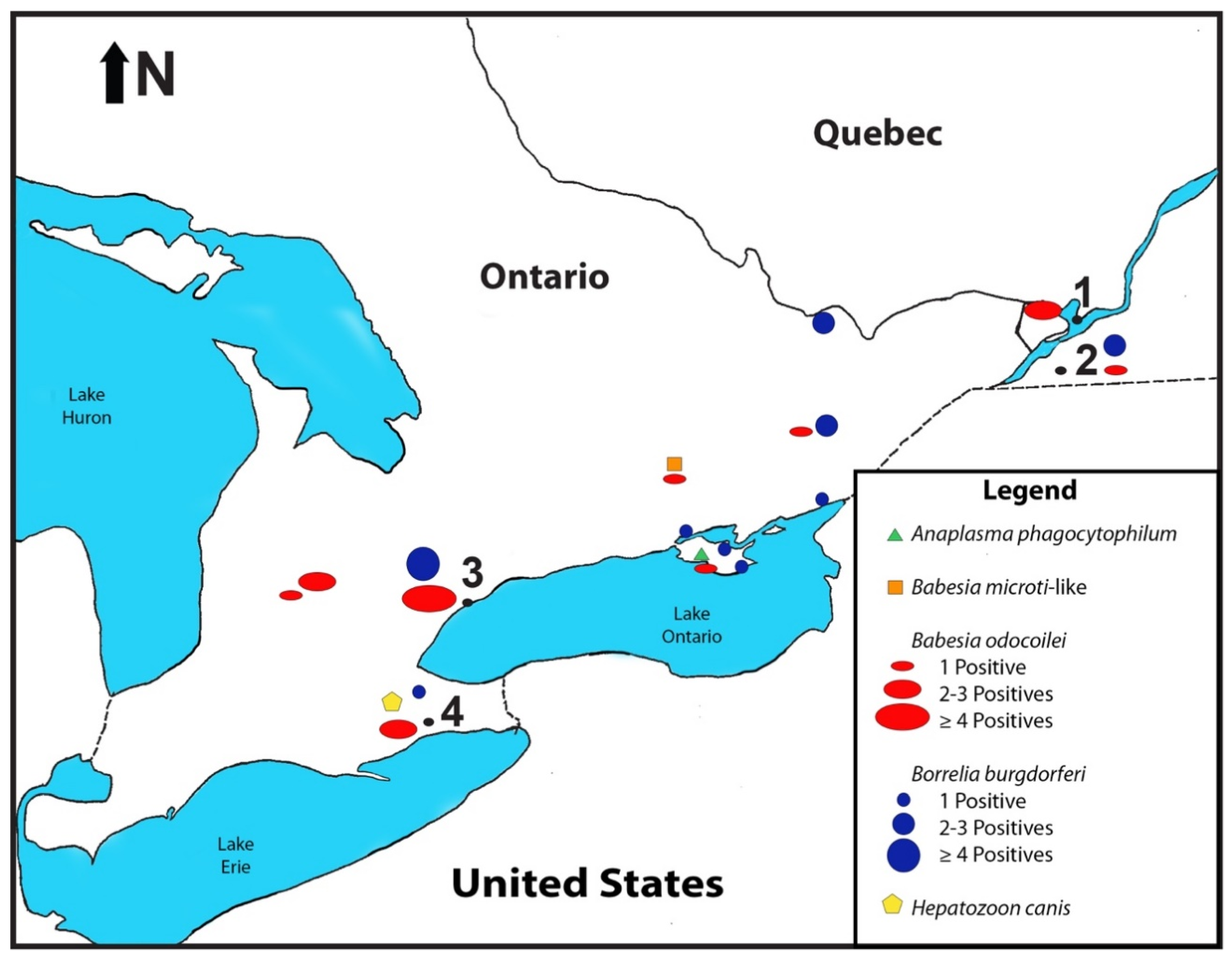
2. Results
2.1. Tick Collection
2.2. Pathogen Detection
3. Discussion
3.1. Pathogen-Positive I. scapularis
3.2. Distribution of B. odocoilei
3.3. Comparative Relationship between Babesia Spp.
3.4. Source of B. odocoilei-Infected Ticks
3.5. Pathways of B. odocoilei
3.6. Phylogeny of B. microti-Like Piroplasm
3.7. Ixodes scapularis Parasitize Mammalian Hosts
3.8. Novel Discovery of H. canis in Canada
3.9. American Robins Are Competent Reservoirs of B. burgdorferi Sensu Lato
3.10. Human Babesiosis as a Tick-Borne Zoonosis
3.11. Endemic Area Not Required to Contract Tick-Borne Diseases
3.12. Diversity of Pathogens in I. scapularis
4. Materials and Methods
4.1. Tick Collection
4.2. Pathogen Detection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magnarelli, L.A.; Dumler, J.S.; Anderson, J.F.; Johnson, R.C.; Fikrig, E. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J. Clin. Microbiol. 1995, 33, 3054–3057. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.D.; Reed, K.D.; Hofkes, J.M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J. Clin. Microbiol. 1996, 34, 724–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stricker, R.B. Lyme disease: A potential polymicrobial infection. ASM News 2003, 69, 265. [Google Scholar]
- Benach, J.L.; Coleman, J.L.; Habicht, G.S.; MacDonald, A.; Grunwaldt, E.; Giron, J.A. Serological evidence for simultaneous occurrences of Lyme disease and babesiosis. J. Infect. Dis. 1985, 152, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Mintz, E.D.; Gadbaw, J.J.; Magnarelli, L.A. Babesia microti, human babesiosis, and Borrelia burgdorferi in Connecticut. J. Clin. Microbiol. 1991, 29, 2779–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial nature of tick-borne diseases. mBio 2019, 10, e02055-19. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, W.A.; Sonenshine, D.E.; Noden, B.H. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: London, UK, 2019; pp. 603–672. ISBN 978-0-12-814043-7. [Google Scholar]
- Scott, J.D.; Pascoe, E.L.; Sajid, M.S.; Foley, J.E. Detection of Babesia odocoilei in Ixodes scapularis ticks collected from songbirds in Ontario and Quebec, Canada. Pathogens 2020, 9, 781. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Bierman, B.C.; Durden, L.A. Far-reaching dispersal of Borrelia burgdorferi sensu lato-infected blacklegged ticks by migratory songbirds in Canada. Healthcare 2018, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Beati, L.; Mazerolle, D.F.; Geddes, G.; Durden, L.A. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J. Parasitol. 2005, 91, 780–790. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, Influenza A and enteropathogens. Clin. Med. Res. 2002, 1, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.D.; Clark, K.L.; Coble, N.M.; Ballantyne, T.R. Presence of Babesia odocoilei and Borrelia burgdorferi sensu stricto in a tick and dual parasitism of Amblyomma inornatum and Ixodes scapularis on a bird in Canada. Healthcare 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.D.; Clark, K.L.; Coble, N.M.; Ballantyne, T.R. Detection and transstadial passage of Babesia species and Borrelia burgdorferi sensu lato in ticks collected from avian and mammalian hosts in Canada. Healthcare 2019, 7, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Kessing, F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Scott, J.D.; Sajid, M.S.; Pascoe, E.L.; Foley, J.E. Detection of Babesia odocoilei in humans with babesiosis symptoms. Diagnostics 2021, 11, 947. [Google Scholar] [CrossRef]
- Emerson, H.R.; Wright, W.T. The isolation of a Babesia in white-tailed deer. Bull. Wildl. Dis. Assoc. 1968, 4, 142–143. [Google Scholar] [CrossRef] [Green Version]
- Emerson, H.R.; Wright, W.T. Correction. J. Wildl. Dis. 1970, 6, 519. [Google Scholar] [CrossRef]
- Holman, P.J.; Madeley, J.; Craig, T.M.; Allsopp, B.A.; Allsopp, M.T.; Petrini, K.R.; Waghela, S.D.; Wagner, G.G. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J. Wildl. Dis. 2000, 36, 518–530. [Google Scholar] [CrossRef]
- Eshoo, M.W.; Carolan, H.E.; Massire, C.; Chou, D.M.; Crowder, C.D.; Rounds, M.A.; Phillipson, C.A.; Schutzer, S.E.; Ecker, D.J. Survey of Ixodes pacificus ticks in California reveals a diversity of microorganisms and a novel and widespread Anaplasmataceae species. PLoS ONE 2015, 10, e0135828. [Google Scholar] [CrossRef] [PubMed]
- Waldrup, K.A.; Kocan, A.A.; Barker, R.W.; Wagner, G.G. Transmission of Babesia odocoilei in white-tailed deer (Odocoileus virginianus) by Ixodes scapularis (Acari: Ixodidae). J. Wildl. Dis. 1990, 26, 390–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, S.A.; Roy, P.L.; Hickling, G.J.; Walker, E.D.; Foster, E.S. Zoonotic pathogens in Ixodes scapularis, Michigan. Emerg. Infect. Dis. 2007, 13, 1131–1133. [Google Scholar] [CrossRef]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Grindle, N.; Civitello, D.; Clay, K.; Fuqua, C. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 2008, 45, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Livengood, J.; Hutchinson, M.L.; Thirumalapura, N.; Tewari, D. Detection of Babesia, Borrelia, Anaplasma, and Rickettsia spp. in adult black-legged ticks (Ixodes scapularis) from Pennsylvania, United States, with Luminex Multiplex Bead Assay. Vector Borne Zoonotic Dis. 2020, 20, 406–411. [Google Scholar] [CrossRef]
- Scott, J.D.; Pascoe, E.L.; Sajid, M.S.; Foley, J.E. Detection of Babesia odocoilei in Ixodes scapularis ticks collected in southern Ontario, Canada. Pathogens 2021, 10, 327. [Google Scholar] [CrossRef]
- Waldrup, K.A.; Kocan, A.A.; Qureshi, T.; Davis, D.S.; Baggett, D.; Wagner, G.G. Serological prevalence and isolation of Babesia odocoilei among white-tailed deer (Odocoileus virginianus) in Texas and Oklahoma. J. Wildl. Dis. 1989, 25, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Perry, B.D.; Nichols, D.K.; Cullom, E.S. Babesia odocoilei Emerson and Wright, 1970 in white-tailed deer, Odocoileus virginianus (Zimmermann), in Virginia. J. Wildl. Dis. 1985, 21, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shock, B.C.; Moncayo, A.; Cohen, S.; Mitchell, E.A.; Williamson, P.C.; Lopez, G.; Garrison, L.E.; Yabsley, M.J. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis. 2014, 5, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Schoelkopf, L.; Hutchinson, C.E.; Bendele, K.G.; Goff, W.L.; Willette, M.; Rasmussen, J.M.; Holman, P.J. New ruminant hosts and wider geographic range identified for Babesia odocoilei (Emerson and Wright 1970). J. Wildl. Dis. 2005, 41, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.P., Jr.; Elias, S.P.; Borelli, T.J.; Missaghi, B.; York, B.J.; Kessler, R.A.; Lubelczyk, C.B.; Lacombe, E.H.; Hayes, C.M.; Coulter, M.S.; et al. Human babesiosis, Maine, USA, 1995–2011. Emerg. Infect. Dis. 2014, 20, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Pattullo, K.M.; Wobeser, G.; Lockerbie, B.P.; Burgess, H.J. Babesia odocoilei infection in a Saskatchewan elk (Cervus elaphus canadensis) herd. J. Vet. Diagn. Investig. 2013, 25, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.I. Lyme and Other Co-infections: Parasitic, Viral, and Fungal Infectivity. In Why Can’t I Get Better? St. Martin’s Press: New York, NY, USA, 2013; pp. 134–162. ISSN 978-1-250-019-0. [Google Scholar]
- Kinderlehrer, D.A. Babesia. In Recovery from Lyme disease: The Integrative Medicine Guide to Diagnosing and Treating Tick-Bore Illness; Skyhorse Publishing: New York, NY, USA, 2021; pp. 93–101. ISBN 978-1-5107-6205-3. [Google Scholar]
- Scott, J.D.; Pascoe, E.L.; Sajid, M.S.; Foley, J.E. Monitoring of nesting songbirds detects established population of blacklegged ticks and associated Lyme disease endemic area in Canada. Healthcare 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baneth, G.; Mathew, J.S.; Shkap, V.; Macintire, D.K.; Barta, J.R.; Ewing, S.A. Canine hepatozoonosis: Two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol. 2003, 19, 27–31. [Google Scholar] [CrossRef]
- Macintire, D.K.; Vincent-Johnson, N.; Dillon, A.R.; Blagburn, B.; Lindsay, D.; Whitley, E.M.; Banfield, C. Hepatozoonosis in dogs: 22 cases (1989–1994). J. Am. Vet. Med. Assoc. 1997, 210, 916–922. [Google Scholar] [PubMed]
- Kaufman, R.W. Integument and ecdysis. In Biology of Ticks, 2nd ed.; Sonenshine, E.E., Roe, R.M., Eds.; Oxford University Press: Oxford, UK, 2013; Volume 1, pp. 99–196. ISBN 9780199744053. [Google Scholar]
- Richter, D.; Spielman, A.; Momar, N.; Matuschka, F.-R. Competence of American Robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 2000, 6, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babes, V. Sur l’hémoglobinurie bactérienne du boeuf. C. R. Acad. Sci. 1888, 107, 692–694. [Google Scholar]
- Smith, T.; Kilborne, F.L. Investigation into the Nature, Causation, and Prevention of Southern Cattle Fever, 1st ed.; United States Government Printing Office: Washington, DC, USA, 1983; pp. 85–116.
- Škrabalo, Z.; Deanović, Z. Piroplasmosis in man: Report on a case. Doc. Med. Geogr. Trop. 1957, 9, 11–16. [Google Scholar]
- Schetters, T. Mechanisms involved in the persistence of Babesia canis infection in dogs. Pathogens 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.; Zintl, A.; Hildebrandt, A.; Hunfeld, K.-P.; Weiss, L. Zoonotic babesiosis: Overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010, 1, 3–10. [Google Scholar] [CrossRef]
- Scholtens, R.G.; Braff, E.H.; Healy, R.R.; Gleason, N. A case of babesiosis in man in the United States. Am. J. Trop. Med. Hyg. 1968, 17, 810–813. [Google Scholar] [CrossRef]
- Western, K.A.; Benson, G.D.; Gleason, N.N.; Healy, G.R.; Schultz, M.G. Babesiosis in a Massachusetts resident. N. Engl. J. Med. 1970, 283, 854–856. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Caccio, S.; Gherlinzoni, F.; Aspöck, H.; Siemenda, S.B.; Piccaluga, P.; Martinelli, G.; Edelhofer, R.; Hollenstain, U.; Poletti, G.; et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg. Infect. Dis. 2003, 9, 942–948. [Google Scholar] [CrossRef]
- Shih, C.M.; Liu, L.P.; Chung, W.C.; Ong, S.J.; Wang, C.C. Human babesiosis in Taiwan: Asymptomatic infection with a Babesia microti-like organism in Taiwanese woman. J. Clin. Microbiol. 1997, 35, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjemtrup, A.M.; Conrad, P.A. Human babesiosis: An emerging tick-borne disease. Int. J. Parasitol. 2000, 30, 1323–1337. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Persing, D.H.; Precigout, E.A.; Goff, W.L.; Mathiesen, D.A.; Taylor, P.W.; Eberhard, M.L.; Gorenflot, A.F. A fatal case of babesiosis in Missouri: Identification of another piroplasm that infects humans. Ann. Intern. Med. 1996, 124, 643–650. [Google Scholar] [CrossRef]
- Ord, R.L.; Lobo, C.A. Human babesiosis: Pathogens, prevalence, diagnosis, and treatment. Cur. Clin Microbiol. Rep. 2015, 2, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Cho, S.-H.; Joo, H.-N.; Tsuji, M.; Cho, S.-R.; Park, I.-J.; Chung, G.-T.; Ju, J.-W.; Cheun, H.-I.; Lee, H.-W.; et al. First case of human babesiosis in Korea: Detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine Babesia. J. Clin. Microbiol. 2007, 45, 2084–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef] [Green Version]
- Tonnetti, L.; Young, C.; Kessler, D.A.; Williamson, P.C.; Reik, R.; Proctor, M.C.; Brès, V.; Deisting, B.; Bakkour, S.; Schneider, W. Transcription-mediated amplification blood donation screening for Babesia. Transfusion 2020, 60, 317–325. [Google Scholar] [CrossRef]
- Brennan, M.B.; Herwaldt, B.L.; Kazmierczak, J.J.; Weiss, J.W.; Klein, C.L.; Leith, C.P.; He, R.; Oberley, M.J.; Tonnetti., L.; Wilkins, P.P.; et al. Transmission of Babesia microti parasites by solid organ transplantation. Emerg. Infect. Dis. 2016, 22, 1869–1876. [Google Scholar] [CrossRef] [Green Version]
- New, D.L.; Quinn, J.B.; Qureshi, M.Z.; Sigler, S.J. Vertically transmitted babesiosis. J. Pediatr. 1997, 131, 163–164. [Google Scholar] [CrossRef]
- Fox, L.M.; Winger, S.; Ahmed, A.; Arnold, A.; Chou, J.; Rhein, L.; Levy, O. Neonatal babesiosis: Case report and review of the literature. Pediatr. Infect. Dis. J. 2006, 25, 169–173. [Google Scholar] [CrossRef]
- Cornett, J.K.; Malhotra, A.; Hart, D. Vertical transmission of babesiosis from a pregnant, splenectomized mother to her neonate. Infect. Dis. Clin. Pract. 2012, 20, 408–410. [Google Scholar] [CrossRef]
- Iyer, S.; Goodman, K. Congenital babesiosis from maternal exposure: A case report. J. Emerg. Med. 2019, 56, E39–E41. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, P.E.; Lattner, B.P.; Duray, P.H.; Barbour, A.G.; Johnson, P.C. Culture positive seronegative transplacental Lyme borreliosis infect morality. Arthritis Rheum. 1987, 30, 50. [Google Scholar]
- MacDonald, A.B. Human fetal borreliosis, toxemia of pregnancy, and fetal death. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 1986, 263, 189. [Google Scholar] [CrossRef]
- Trevisan, G.; Stinco, G.; Cinco, M. Neonatal skin lesions due to spirochetal infection; A case of congenital Lyme borreliosis? Int. J. Dermatol. 1997, 36, 677–680. [Google Scholar] [PubMed]
- Horowitz, R.I. Lyme disease and pregnancy: Implication of chronic infection, PCR testing, and prenatal treatment. In Proceeding of the 16th International Scientific Conference on Lyme Disease & Other Tick-Borne Disorders, Hartford, CT, USA, 7–8 June 2003. [Google Scholar]
- Gardner, T. Lyme disease. In Infectious Diseases of the Fetus and Newborn Infant, 5th ed.; Remington, J.S., Klein, J.O., Eds.; W.B. Sanders Company: Philadelphia, PA, USA, 2001; Chapter 11; pp. 519–641. ISBN 0-7216-7976-5. [Google Scholar]
- Eisen, R.J.; Eisen, L.; Beard, C.B. Country-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penzhorn, B.L.; Oosthuizen, M.C. Babesia species of domestic cats: Molecular characterization has opened Pandora’s box. Front. Vet. Sci. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E.; Clifford, C.M. The genus Ixodes in the United States: A scanning electron microscope study and key to the adults. J. Med. Entomol. 1978, 15, 1–38. [Google Scholar] [CrossRef]
- Keirans, J.E.; Hutcheson, H.J.; Durden, L.A.; Klompen, J.S.H. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 1996, 33, 297–318. [Google Scholar] [CrossRef]
- Durden, L.A.; Keirans, J.E. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical Veterinary Importance, Monographs; Thomas Say Publications in Entomology, Entomological Society of America: Lanham, MD, USA, 1996; p. 95. ISBN 0-938522-57. [Google Scholar]
- Foley, J.; Tinoco-Gracia, L.; Rodriguez-Lomelf, M.; Estrada-Guzmán, J.; Fierro, M.; Mattar-Lopez, E.; Peterson, A.; Pascoe, E.; Gonzalez, Y.; Hori-Oshima, S.; et al. Unbiased assessment of abundance of Rhipicephalus sanguineus sensu lato ticks, canine exposure to spotted fever group Rickettsia, risk factor in Mexicali, México. Am. J. Trop. Med. Hyg. 2019, 101, 22–32. [Google Scholar] [CrossRef]
- Barbour, A.G.; Bunikis, J.; Tranvinsky, B.; Hoen, A.B.; Diuk-Wasser, M.A.; Fish, D.; Tsao, J.I. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009, 8, 1120–1131. [Google Scholar] [CrossRef] [Green Version]
- Drazenovich, N.; Foley, J.; Brown, R.N. Use of real-time quantitative PCR targeting the msp2 protein gene to identify cryptic Anaplasma phagocytophilum infections in wildlife and domestic animals. Vector Borne Zoonotic Dis. 2006, 6, 83–90. [Google Scholar] [CrossRef]
- Black, W.C., 4th; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodidae) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [Green Version]
- Beati, L.; Keirans, J.E. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12 ribosomal DNA gene sequences and morphological characters. J. Parasitol. 2001, 87, 32–48. [Google Scholar] [CrossRef]


| Source | No. of Hosts | No. Ticks Collected | Ixodes scapularis | Ticks Tested | Pathogens | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L | N | M | F | Bbsl | Bab | Aph | Hcn | ||||
| Songbirds | |||||||||||
| Lincoln’s Sparrow, Melospiza lincolnii | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Indigo Bunting, Passerina cyanea | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| House Wren, Troglodytes aedon | 4 | 5 | 0 | 5 | 0 | 0 | 5 | 1 | 0 | 0 | 1 |
| Common Yellowthroat, Geothlypis trichas | 5 | 5 | 3 | 2 | 0 | 0 | 5 | 0 | 3 | 0 | 0 |
| Veery, Catharus fuscescens | 3 | 3 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Baltimore Oriole, Icterus galbula | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 0 |
| American Robin, Turdus migratorius | 1 | 3 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| Song Sparrow, Melospiza melodia | 2 | 4 | 0 | 3 | 0 | 0 | 3 | 1 | 1 | 0 | 0 |
| Black-capped Chickadee Poecile atricapillus | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eastern Towhee, Pipilo erythrophthalmus | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Ovenbird, Seiurus aurocapilla | 1 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Northern Waterthrush, Parkesia noveboracensis | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Mammals | |||||||||||
| Dog, Canis familiaris | 32 | 39 | 0 | 0 | 4 | 35 | 39 | 5 | 3 | 0 | 0 |
| Cat, Felis catus | 9 | 11 | 0 | 0 | 2 | 9 | 11 | 1 | 3 | 1 | 0 |
| North American porcupine, Erethizon dorsatum | 1 | 5 | 0 | 0 | 0 | 5 | 5 | 3 | 1 | 0 | 0 |
| Human, Homo sapiens | 4 | 4 | 0 | 0 | 0 | 4 | 4 | 2 | 0 | 0 | 0 |
| Flagging | |||||||||||
| Vegetation | 0 | 25 | 0 | 0 | 8 | 17 | 25 | 14 | 5 | 0 | 0 |
| Total | 69 | 113 | 7 | 20 | 14 | 70 | 111 | 29 | 18 | 1 | 1 |
| Geographic Location. | Source | Life Stage | Pathogen | GenBank Accession Number |
|---|---|---|---|---|
| Cayuga, ON | Common Yellowthroat | N | B. odocoilei | MZ841618 |
| Belwood, ON | Cat, domestic | F | B. odocoilei | MZ841619 |
| Montée Biggar, QC | Song Sparrow | N | B. odocoilei | OK041475 |
| Ste-Anne-de-Belleville, QC | Common Yellowthroat | L | B. odocoilei | MZ841620 |
| Toronto, ON | Vegetation | M | B. odocoilei | MZ841621 |
| Fergus, ON | Dog, domestic | F | B. odocoilei | MZ841622 |
| Westport, ON | Porcupine | F | B. odocoilei | MZ841623 |
| Bloomfield, ON | Dog, domestic | F | B. odocoilei | MZ841624 |
| Tweed, ON | Cat, domestic | F | B. microti-like | MZ841625 |
| Tweed, ON | Dog, domestic | F | B. odocoilei | OK041476 |
| Cayuga, ON | House Wren | N | H. canis | MZ841626 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, J.D.; Pesapane, R.R. Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis Ticks Collected in Eastern Canada. Pathogens 2021, 10, 1265. https://doi.org/10.3390/pathogens10101265
Scott JD, Pesapane RR. Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis Ticks Collected in Eastern Canada. Pathogens. 2021; 10(10):1265. https://doi.org/10.3390/pathogens10101265
Chicago/Turabian StyleScott, John D., and Risa R. Pesapane. 2021. "Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis Ticks Collected in Eastern Canada" Pathogens 10, no. 10: 1265. https://doi.org/10.3390/pathogens10101265
APA StyleScott, J. D., & Pesapane, R. R. (2021). Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis Ticks Collected in Eastern Canada. Pathogens, 10(10), 1265. https://doi.org/10.3390/pathogens10101265






