A Broad-Based Mosquito Yeast Interfering RNA Pesticide Targeting Rbfox1 Represses Notch Signaling and Kills Both Larvae and Adult Mosquitoes
Abstract
:1. Introduction
2. Results and Discussion
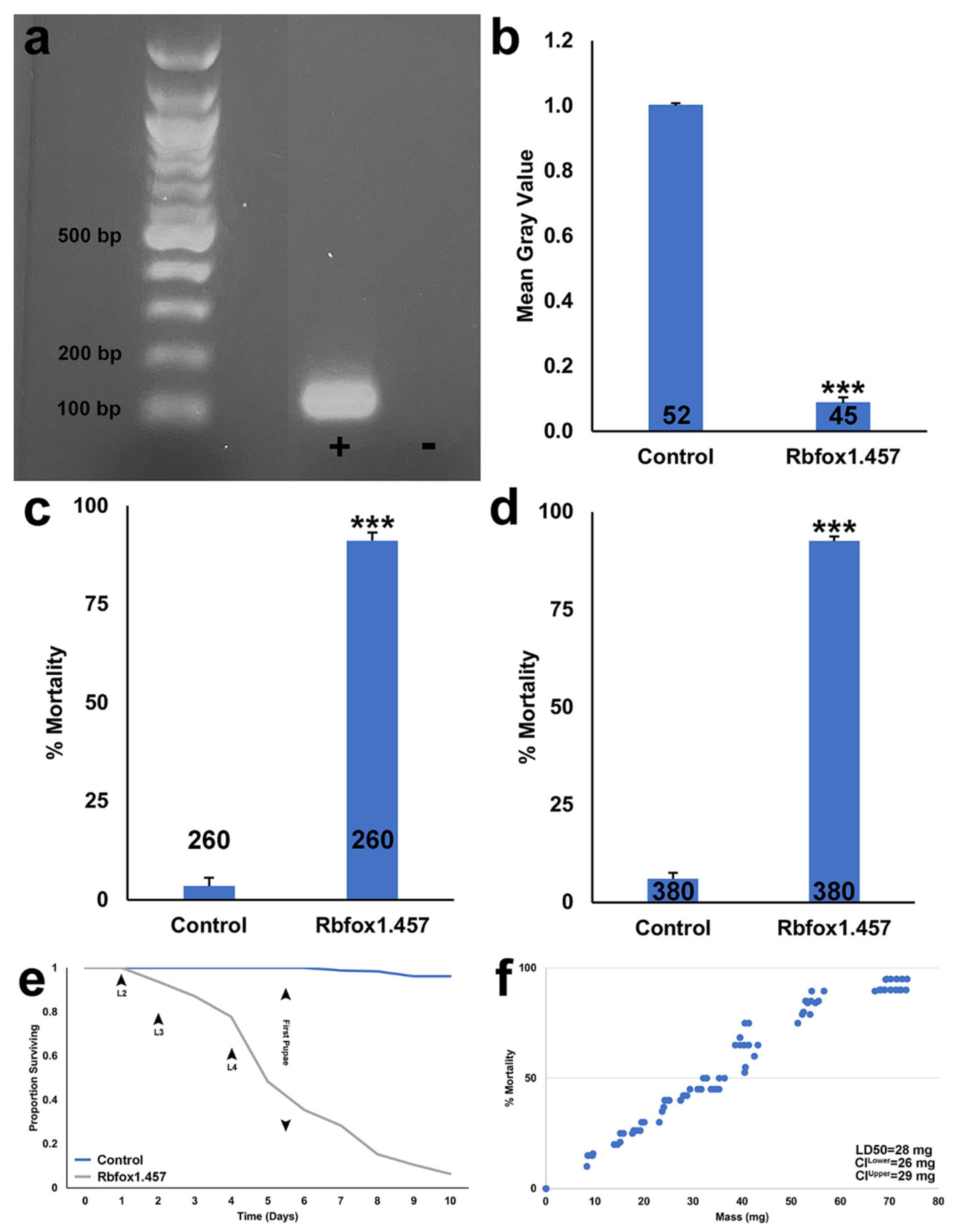
2.1. Silencing Rbfox1 Results in A. aegypti Larval Mortality
2.2. Sugar Baited Delivery of Rbfox1.457 siRNA
2.3. Development of an RNAi-Based Yeast Pesticide That Can Be Deployed as an ATSB
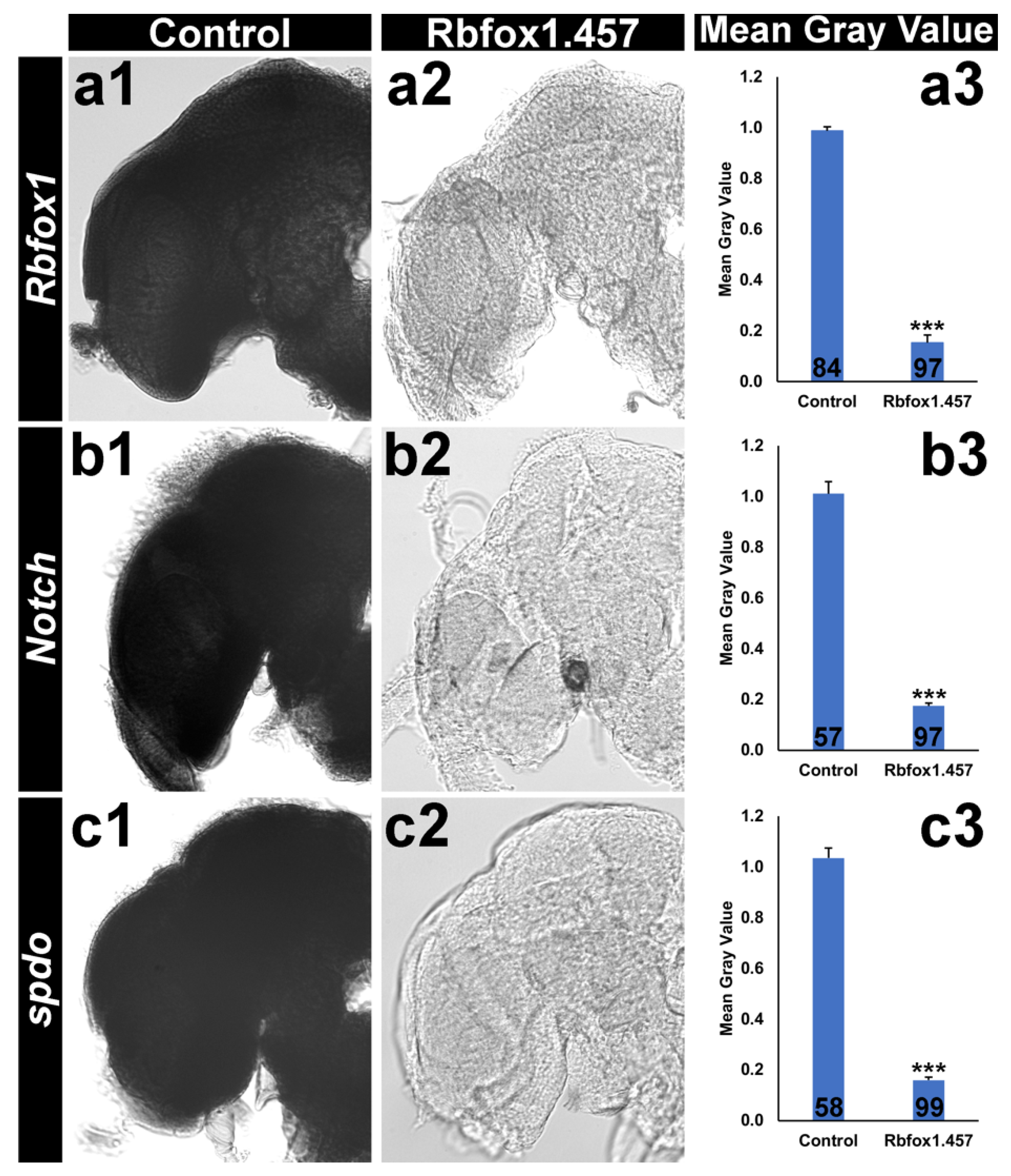
2.4. Silencing Rbfox1 Represses Notch Signaling
2.5. Rbfox1.457 Yeast Pesticides Function as Broad-Range Mosquito Insecticides but Are Not Found to Be Toxic to Select Non-Target Arthropods
3. Materials and Methods
3.1. Mosquito Rearing
3.2. Identification of siRNA #457
3.3. Adult siRNA-ATSB Trials
3.4. Generation of Yeast Interfering RNA Larvicide Strains and Yeast Culturing
3.5. Larvicide Trials
3.5.1. Laboratory Assays
3.5.2. Semi-Field Trials
3.5.3. Yeast ATSB Simulated Field Trials
3.6. Whole Mount In Situ Hybridization
3.7. Evaluation of Non-target Species
3.7.1. O. fasciatus
3.7.2. H. convergens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Airs, P.M.; Bartholomay, L.C. RNA interference for mosquito and mosquito-borne disease control. Insects 2017, 8, 4. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Pesticides. Available online: https://www.epa.gov/pesticides (accessed on 1 March 2019).
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Wiltshire, R.M.; Duman-Scheel, M. Advances in oral RNAi for disease vector mosquito research and control. Curr. Opin. Insect Sci. 2020, 40, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hapairai, L.K.; Mysore, K.; Chen, Y.; Harper, E.I.; Scheel, M.P.; Lesnik, A.M.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Lure-and-kill yeast interfering RNA larvicides targeting neural genes in the human disease vector mosquito Aedes aegypti. Sci. Rep. 2017, 7, 13223. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Harper, E.I.; Chen, Y.; Eggleson, K.K.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Wei, N.; et al. Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality. Malar. J. 2017, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Hapairai, L.K.; Mysore, K.; Sun, L.; Li, P.; Wang, C.W.; Scheel, N.D.; Lesnik, A.; Scheel, M.P.; Igiede, J.; Wei, N.; et al. Characterization of an adulticidal and larvicidal interfering RNA pesticide that targets a conserved sequence in mosquito G protein-coupled dopamine 1 receptor genes. Insect Biochem. Mol. Biol. 2020, 120, 103359. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Li, P.; Wang, C.W.; Scheel, N.D.; Lesnik, A.; Igiede, J.; Scheel, M.P.; Wei, N.; et al. Characterization of a dual-action adulticidal and larvicidal interfering RNA pesticide targeting the Shaker gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2020, 14, e0008479. [Google Scholar] [CrossRef]
- Mysore, K.; Li, P.; Wang, C.W.; Hapairai, L.K.; Scheel, N.D.; Realey, J.S.; Sun, L.; Roethele, J.B.; Severson, D.W.; Wei, N.; et al. Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, e0007422. [Google Scholar] [CrossRef]
- Mysore, K.; Li, P.; Wang, C.W.; Hapairai, L.K.; Scheel, N.D.; Realey, J.S.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Characterization of a broad-based mosquito yeast interfering RNA larvicide with a conserved target site in mosquito semaphorin-1a genes. Parasit. Vectors 2019, 12, 256. [Google Scholar] [CrossRef]
- Conboy, J.G. Developmental regulation of RNA processing by Rbfox proteins. Wiley Interdiscip. Rev. RNA 2017, 8, e1398. [Google Scholar] [CrossRef]
- Shukla, J.P.; Deshpande, G.; Shashidhara, L.S. Ataxin 2-binding protein 1 is a context-specific positive regulator of Notch signaling during neurogenesis in Drosophila melanogaster. Development 2017, 144, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Nikonova, E.; Kao, S.Y.; Ravichandran, K.; Wittner, A.; Spletter, M.L. Conserved functions of RNA-binding proteins in muscle. Int. J. Biochem. Cell Biol. 2019, 110, 29–49. [Google Scholar] [CrossRef]
- Shibata, H.; Huynh, D.P.; Pulst, S.M. A novel protein with RNA-binding motifs interacts with ataxin-2. Hum. Mol. Genet. 2000, 9, 1303–1313. [Google Scholar] [CrossRef]
- Gazzara, M.R.; Mallory, M.J.; Roytenberg, R.; Lindberg, J.P.; Jha, A.; Lynch, K.W.; Barash, Y. Ancient antagonism between CELF and RBFOX families tunes mRNA splicing outcomes. Genome Res. 2017, 27, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, M.M.; Shcherbata, H.R. Stress-dependent miR-980 regulation of Rbfox1/A2bp1 promotes ribonucleoprotein granule formation and cell survival. Nat. Commun. 2018, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Tastan, O.Y.; Maines, J.Z.; Li, Y.; McKearin, D.M.; Buszczak, M. Drosophila ataxin 2-binding protein 1 marks an intermediate step in the molecular differentiation of female germline cysts. Development 2010, 137, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Rosario, A.; Bhargava, V.; Hillebrand, J.; Kollipara, R.K.; Ramaswami, M.; Buszczak, M. Repression of Pumilio protein expression by Rbfox1 promotes germ cell differentiation. Dev. Cell 2016, 36, 562–571. [Google Scholar] [CrossRef]
- Bajpai, R.; Sambrani, N.; Stadelmayer, B.; Shashidhara, L.S. Identification of a novel target of D/V signaling in Drosophila wing disc: Wg-independent function of the organizer. Gene Expr. Patterns 2004, 5, 113–121. [Google Scholar] [CrossRef]
- Usha, N.; Shashidhara, L.S. Interaction between Ataxin-2 Binding Protein 1 and Cubitus-interruptus during wing development in Drosophila. Dev. Biol. 2010, 341, 389–399. [Google Scholar] [CrossRef]
- Guven-Ozkan, T.; Busto, G.U.; Schutte, S.S.; Cervantes-Sandoval, I.; O’Dowd, D.K.; Davis, R.L. MiR-980 is a memory suppressor microRNA that regulates the autism-susceptibility gene A2bp1. Cell Rep. 2016, 14, 1698–1709. [Google Scholar] [CrossRef]
- Nazario-Toole, A.E.; Robalino, J.; Okrah, K.; Corrada-Bravo, H.; Mount, S.M.; Wu, L.P. The splicing factor RNA-binding Fox Protein 1 mediates the cellular immune response in Drosophila melanogaster. J. Immunol. 2018, 201, 1154–1164. [Google Scholar] [CrossRef]
- Koizumi, K.; Higashida, H.; Yoo, S.; Islam, M.S.; Ivanov, A.I.; Guo, V.; Pozzi, P.; Yu, S.H.; Rovescalli, A.C.; Tang, D.; et al. RNA interference screen to identify genes required for Drosophila embryonic nervous system development. Proc. Natl. Acad. Sci. USA 2007, 104, 5626–5631. [Google Scholar] [CrossRef] [PubMed]
- Tunstall, N.E.; Herr, A.; de Bruyne, M.; Warr, C.G. A screen for genes expressed in the olfactory organs of Drosophila melanogaster identifies genes involved in olfactory behaviour. PLoS ONE 2012, 7, e35641. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Calderon, G.I.; Emrich, S.J.; MacCallum, R.M.; Maslen, G.; Dialynas, E.; Topalis, P.; Ho, N.; Gesing, S.; VectorBase, C.; Madey, G.; et al. VectorBase: An updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015, 43, D707–D713. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef]
- Sousa-Paula, L.C.; Otranto, D.; Dantas-Torres, F. Lutzomyia longipalpis (Sand Fly). Trends Parasitol. 2020, 36, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.D. Attractive toxic sugar bait (ATSB) for control of mosquitoes and its impact on non-target organisms: A review. Int. J. Environ. Res. Public Health 2017, 14, 398. [Google Scholar] [CrossRef]
- Faraji, A.; Unlu, I. The eye of the tiger, the thrill of the gight: Effective larval and adult control measures against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), in North America. J. Med. Entomol. 2016, 53, 1029–1047. [Google Scholar] [CrossRef]
- Duman-Scheel, M. Saccharomyces cerevisiae (baker’s yeast) as an interfering RNA expression and delivery system. Curr. Drug Targets 2019, 20, 942–952. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Li, P.; Roethele, J.B.; Sun, L.; Igiede, J.; Misenti, J.K.; Duman-Scheel, M. A functional requirement for sex-determination M/m locus region lncRNA genes in Aedes aegypti female larvae. Sci. Rep. 2021, 11, 10657. [Google Scholar] [CrossRef]
- Mysore, K.; Sun, L.; Roethele, J.B.; Li, P.; Igiede, J.; Misenti, J.K.; Duman-Scheel, M. A conserved female-specific larval requirement for MtnB function facilitates sex separation in multiple species of disease vector mosquitoes. Parasit. Vectors 2021, 14, 338. [Google Scholar] [CrossRef]
- Skeath, J.B.; Doe, C.Q. Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development 1998, 125, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.S. Larvicides and larvicidal formulations for the control of Culex pipiens fatigans. Bull. World Health Organ. 1967, 37, 311–315. [Google Scholar] [PubMed]
- World Health Organization. Larval Source Management: A Supplementary Measure for Malaria Vector Control: An Operational Manual; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Center for Disease Control. Larvicides. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/community/larvicides.html (accessed on 3 July 2021).
- Afrane, Y.A.; Mweresa, N.G.; Wanjala, C.L.; Gilbreath III, T.M.; Zhou, G.; Lee, M.C.; Githeko, A.K.; Yan, G. Evaluation of long-lasting microbial larvicide for malaria vector control in Kenya. Malar. J. 2016, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Kahindi, S.C.; Muriu, S.; Derua, Y.A.; Wang, X.; Zhou, G.; Lee, M.C.; Mwangangi, J.; Atieli, H.; Githeko, A.K.; Yan, G. Efficacy and persistence of long-lasting microbial larvicides against malaria vectors in western Kenya highlands. Parasit. Vectors 2018, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Derua, Y.A.; Kweka, E.J.; Kisinza, W.N.; Githeko, A.K.; Mosha, F.W. Bacterial larvicides used for malaria vector control in sub-Saharan Africa: Review of their effectiveness and operational feasibility. Parasit. Vectors 2019, 12, 426. [Google Scholar] [CrossRef]
- Derua, Y.A.; Kahindi, S.C.; Mosha, F.W.; Kweka, E.J.; Atieli, H.E.; Zhou, G.; Lee, M.C.; Githeko, A.K.; Yan, G. Susceptibility of Anopheles gambiae complex mosquitoes to microbial larvicides in diverse ecological settings in western Kenya. Med. Vet. Entomol. 2019, 33, 220–227. [Google Scholar] [CrossRef]
- Getachew, D.; Balkew, M.; Tekie, H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar. J. 2020, 19, 65. [Google Scholar] [CrossRef]
- Khallaayoune, K.; Qualls, W.A.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Kravchenko, V.D.; Xue, R.D.; Schlein, Y.; Beier, J.C.; Muller, G.C. Attractive toxic sugar baits: Control of mosquitoes with the low-risk active ingredient dinotefuran and potential impacts on nontarget organisms in Morocco. Environ. Entomol. 2013, 42, 1040–1045. [Google Scholar] [CrossRef]
- Qualls, W.A.; Muller, G.C.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Beier, J.C.; Smith, M.L.; Scott, J.M.; Kravchenko, V.D.; Hausmann, A.; et al. Evaluation of attractive toxic sugar bait (ATSB)-barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in sub-tropical environments in Florida. Acta Trop. 2014, 131, 104–110. [Google Scholar] [CrossRef]
- Fulcher, A.; Scott, J.M.; Qualls, W.A.; Muller, G.C.; Xue, R.D. Attractive toxic sugar baits mixed with pyriproxyfen sprayed on plants against adult and larval Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 896–899. [Google Scholar] [CrossRef]
- Revay, E.E.; Muller, G.C.; Qualls, W.A.; Kline, D.L.; Naranjo, D.P.; Arheart, K.L.; Kravchenko, V.D.; Yefremova, Z.; Hausmann, A.; Beier, J.C.; et al. Control of Aedes albopictus with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in St. Augustine, Florida. Parasitol. Res. 2014, 113, 73–79. [Google Scholar] [CrossRef]
- Junnila, A.; Revay, E.E.; Muller, G.C.; Kravchenko, V.; Qualls, W.A.; Xue, R.D.; Allen, S.A.; Beier, J.C.; Schlein, Y. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015, 152, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Seeger, K.E.; Scott, J.M.; Muller, G.C.; Qualls, W.A.; Xue, R.D. Effect of common species of Florida landscaping plants on the efficacy of attractive toxic sugar baits against Aedes albopictus. J. Am. Mosq. Control. Assoc. 2017, 33, 139–141. [Google Scholar] [CrossRef]
- Sippy, R.; Rivera, G.E.; Sanchez, V.; Heras, F.; Morejon, B.; Beltran, E.; Hikida, R.S.; Lopez-Latorre, M.A.; Aguirre, A.; Stewart-Ibarra, A.M.; et al. Ingested insecticide to control Aedes aegypti: Developing a novel dried attractive toxic sugar bait device for intra-domiciliary control. Parasit. Vectors 2020, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.C.; Junnila, A.; Schlein, Y. Effective control of adult Culex pipiens by spraying an attractive toxic sugar bait solution in the vegetation near larval habitats. J. Med. Entomol. 2010, 47, 63–66. [Google Scholar] [CrossRef]
- Muller, G.C.; Junnila, A.; Qualls, W.; Revay, E.E.; Kline, D.L.; Allan, S.; Schlein, Y.; Xue, R.D. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. Med. Vet. Entomol. 2010, 24, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Qualls, W.A.; Scott-Fiorenzano, J.; Muller, G.C.; Arheart, K.L.; Beier, J.C.; Xue, R.D. Evaluation and adaptation of attractive toxic sugar baits for Culex tarsalis and Culex quinquefasciatus control in the Coachella Valley, Southern California. J. Am. Mosq. Control. Assoc. 2016, 32, 292–299. [Google Scholar] [CrossRef]
- Beier, J.C.; Muller, G.C.; Gu, W.; Arheart, K.L.; Schlein, Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar. J. 2012, 11, 31. [Google Scholar] [CrossRef]
- Qualls, W.A.; Muller, G.C.; Traore, S.F.; Traore, M.M.; Arheart, K.L.; Doumbia, S.; Schlein, Y.; Kravchenko, V.D.; Xue, R.D.; Beier, J.C. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar. J. 2015, 14, 301. [Google Scholar] [CrossRef]
- Tenywa, F.C.; Kambagha, A.; Saddler, A.; Maia, M.F. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. Malar. J. 2017, 16, 338. [Google Scholar] [CrossRef]
- Furnival-Adams, J.E.C.; Camara, S.; Rowland, M.; Koffi, A.A.; Ahoua Alou, L.P.; Oumbouke, W.A.; N’Guessan, R. Indoor use of attractive toxic sugar bait in combination with long-lasting insecticidal net against pyrethroid-resistant Anopheles gambiae: An experimental hut trial in Mbe, central Cote d’Ivoire. Malar. J. 2020, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Diarra, R.A.; Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; et al. Testing configurations of attractive toxic sugar bait (ATSB) stations in Mali, West Africa, for improving the control of malaria parasite transmission by vector mosquitoes and minimizing their effect on non-target insects. Malar. J. 2021, 20, 184. [Google Scholar] [CrossRef]
- MonSanto. Docket ID: EPA-HQ-OPP-2013-0485. Available online: https://www.apsnet.org/members/outreach/ppb/Documents/Monsanto%20posted%20written%20comment%20Jan%202014.pdf (accessed on 5 May 2014).
- Casanova, C.; Andrighetti, M.T.; Sampaio, S.M.; Marcoris, M.L.; Colla-Jacques, F.E.; Prado, A.P. Larval breeding sites of Lutzomyia longipalpis (Diptera: Psychodidae) in visceral leishmaniasis endemic urban areas in Southeastern Brazil. PLoS Negl. Trop. Dis. 2013, 7, e2443. [Google Scholar] [CrossRef]
- Vivero, R.J.; Torres-Gutierrez, C.; Bejarano, E.E.; Pena, H.C.; Estrada, L.G.; Florez, F.; Ortega, E.; Aparicio, Y.; Muskus, C.E. Study on natural breeding sites of sand flies (Diptera: Phlebotominae) in areas of Leishmania transmission in Colombia. Parasit. Vectors 2015, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.N.; Pita-Pereira, D.; Costa, S.G.; Brazil, R.P.; Moraes, C.S.; Diaz-Albiter, H.M.; Genta, F.A. Transmission blocking sugar baits for the control of Leishmania development inside sand flies using environmentally friendly beta-glycosides and their aglycones. Parasit. Vectors 2018, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- McDermott, E.G.; Morris, E.K.; Garver, L.S. Sodium ascorbate as a potential toxicant in attractive sugar baits for control of adult mosquitoes (Diptera: Culicidae) and sand flies (Diptera: Psychodidae). J. Med. Entomol. 2019, 56, 1359–1367. [Google Scholar] [CrossRef]
- Clemons, A.; Mori, A.; Haugen, M.; Severson, D.W.; Duman-Scheel, M. Culturing and egg collection of Aedes aegypti. Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5507. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Wong, S.; Ryan, C.P.; Whyard, S. Oral delivery of double-stranded RNA in larvae of the yellow fever mosquito, Aedes aegypti: Implications for pest mosquito control. J. Insect Sci. 2013, 13, 69. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Tomchaney, M.; Mysore, K.; Sun, L.; Li, P.; Emrich, S.J.; Severson, D.W.; Duman-Scheel, M. Examination of the genetic basis for sexual dimorphism in the Aedes aegypti (dengue vector mosquito) pupal brain. Biol. Sex. Differ. 2014, 5, 10. [Google Scholar] [CrossRef]
- van Dijken, J.P.; Bauer, J.; Brambilla, L.; Duboc, P.; Francois, J.M.; Gancedo, C.; Giuseppin, M.L.; Heijnen, J.J.; Hoare, M.; Lange, H.C.; et al. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb. Technol. 2000, 26, 706–714. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Wei, N.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Duman-Scheel, M. Preparation and use of a yeast shRNA delivery system for gene silencing in mosquito larvae. Methods Mol. Biol. 2019, 1858, 213–231. [Google Scholar] [CrossRef]
- Patel, N.H. In situ hybridization to whole mount Drosophila embryos. In A Laboratory Guide to RNA: Isolation, Analysis, and Synthesis; Krieg, P.A., Ed.; Wiley-Liss: New York, NY, USA, 1996; pp. 357–370. [Google Scholar]
- Haugen, M.; Tomchaney, M.; Kast, K.; Flannery, E.; Clemons, A.; Jacowski, C.; Simanton Holland, W.; Le, C.; Severson, D.; Duman-Scheel, M. Whole-mount in situ hybridization for analysis of gene expression during Aedes aegypti development. Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5509. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2019, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Sun, L.; Tomchaney, M.; Sullivan, G.; Adams, H.; Piscoya, A.S.; Severson, D.W.; Syed, Z.; Duman-Scheel, M. siRNA-Mediated silencing of doublesex during female development of the dengue vector mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 2015, 9, e0004213. [Google Scholar] [CrossRef]
- Hunter, W.; Ellis, J.; Vanengelsdorp, D.; Hayes, J.; Westervelt, D.; Glick, E.; Williams, M.; Sela, I.; Maori, E.; Pettis, J.; et al. Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PloS Pathog. 2010, 6, e1001160. [Google Scholar] [CrossRef] [PubMed]


| Experiment 1 | % Mortality | p Value Control vs. Treatment | n |
|---|---|---|---|
| Larval soaking | |||
| Control siRNA | 0 ± 0 | 4.4−13 | 40 |
| Rbfox1 siRNA | 73 ± 2.5 | ||
| Adult microinjection | |||
| Control siRNA | 5 | 4.0−3 | 20 |
| Rbfox1 siRNA | 45 | ||
| ATSB/siRNA feeding | |||
| Control siRNA | 8 ± 5.3 | 3.5−10 | 37 |
| Rbfox1 siRNA | 77 ± 7.1 | 42 |
| Feeding Rate (%) | ||||
|---|---|---|---|---|
| Experiment 1 | Species | Control | Rbfox1.457 | n |
| siRNA ATSB | A. aegypti | 57 ± 7 | 63 ± 8.5 | 65 |
| Yeast ATSB | A. aegypti | 100 ± 0 | 100 ± 0 | 150 |
| A. gambiae | 100 ± 0 | 100 ± 0 | 75 | |
| C. quinquefasciatus | 100 ± 0 | 100 ± 0 | 225 | |
| A. albopictus | 89 ± 0.5 | 91 ± 1 | 225 | |
| Test Organism 1 | Control | Rbfox1.457 | n |
|---|---|---|---|
| D. magna adults | 100 ± 0 | 98 ± 3.5 | 40 |
| D. melanogaster larvae | 100 ± 0 | 100 ± 0 | 60 |
| D. melanogaster adults | 100 ± 0 | 99 ± 1 | 60 |
| H. convergens adults | 90 ± 0 | 90 ± 7 | 20 |
| O. fasciatus adults | 80 ± 7 | 77.5 ± 18 | 40 |
| T. castaneum adults | 100 ± 0 | 100 ± 0 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mysore, K.; Sun, L.; Hapairai, L.K.; Wang, C.-W.; Roethele, J.B.; Igiede, J.; Scheel, M.P.; Scheel, N.D.; Li, P.; Wei, N.; et al. A Broad-Based Mosquito Yeast Interfering RNA Pesticide Targeting Rbfox1 Represses Notch Signaling and Kills Both Larvae and Adult Mosquitoes. Pathogens 2021, 10, 1251. https://doi.org/10.3390/pathogens10101251
Mysore K, Sun L, Hapairai LK, Wang C-W, Roethele JB, Igiede J, Scheel MP, Scheel ND, Li P, Wei N, et al. A Broad-Based Mosquito Yeast Interfering RNA Pesticide Targeting Rbfox1 Represses Notch Signaling and Kills Both Larvae and Adult Mosquitoes. Pathogens. 2021; 10(10):1251. https://doi.org/10.3390/pathogens10101251
Chicago/Turabian StyleMysore, Keshava, Longhua Sun, Limb K. Hapairai, Chien-Wei Wang, Joseph B. Roethele, Jessica Igiede, Max P. Scheel, Nicholas D. Scheel, Ping Li, Na Wei, and et al. 2021. "A Broad-Based Mosquito Yeast Interfering RNA Pesticide Targeting Rbfox1 Represses Notch Signaling and Kills Both Larvae and Adult Mosquitoes" Pathogens 10, no. 10: 1251. https://doi.org/10.3390/pathogens10101251
APA StyleMysore, K., Sun, L., Hapairai, L. K., Wang, C.-W., Roethele, J. B., Igiede, J., Scheel, M. P., Scheel, N. D., Li, P., Wei, N., Severson, D. W., & Duman-Scheel, M. (2021). A Broad-Based Mosquito Yeast Interfering RNA Pesticide Targeting Rbfox1 Represses Notch Signaling and Kills Both Larvae and Adult Mosquitoes. Pathogens, 10(10), 1251. https://doi.org/10.3390/pathogens10101251






