Study on Mechanical Strength and Chloride Corrosion Resistance of Composite Mortars Mixed with Steel Slag, Bayer Red Mud, and Phosphogypsum
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportion and Sample Preparation
2.3. Test Methods
3. Results and Discussion
3.1. Mechanical Properties
3.2. Hydration Heat
3.3. Chloride Corrosion Resistance
3.3.1. Chloride Migration Coefficient and Electric Flux
3.3.2. Corrosion Resistance Coefficient
3.4. Microstructure
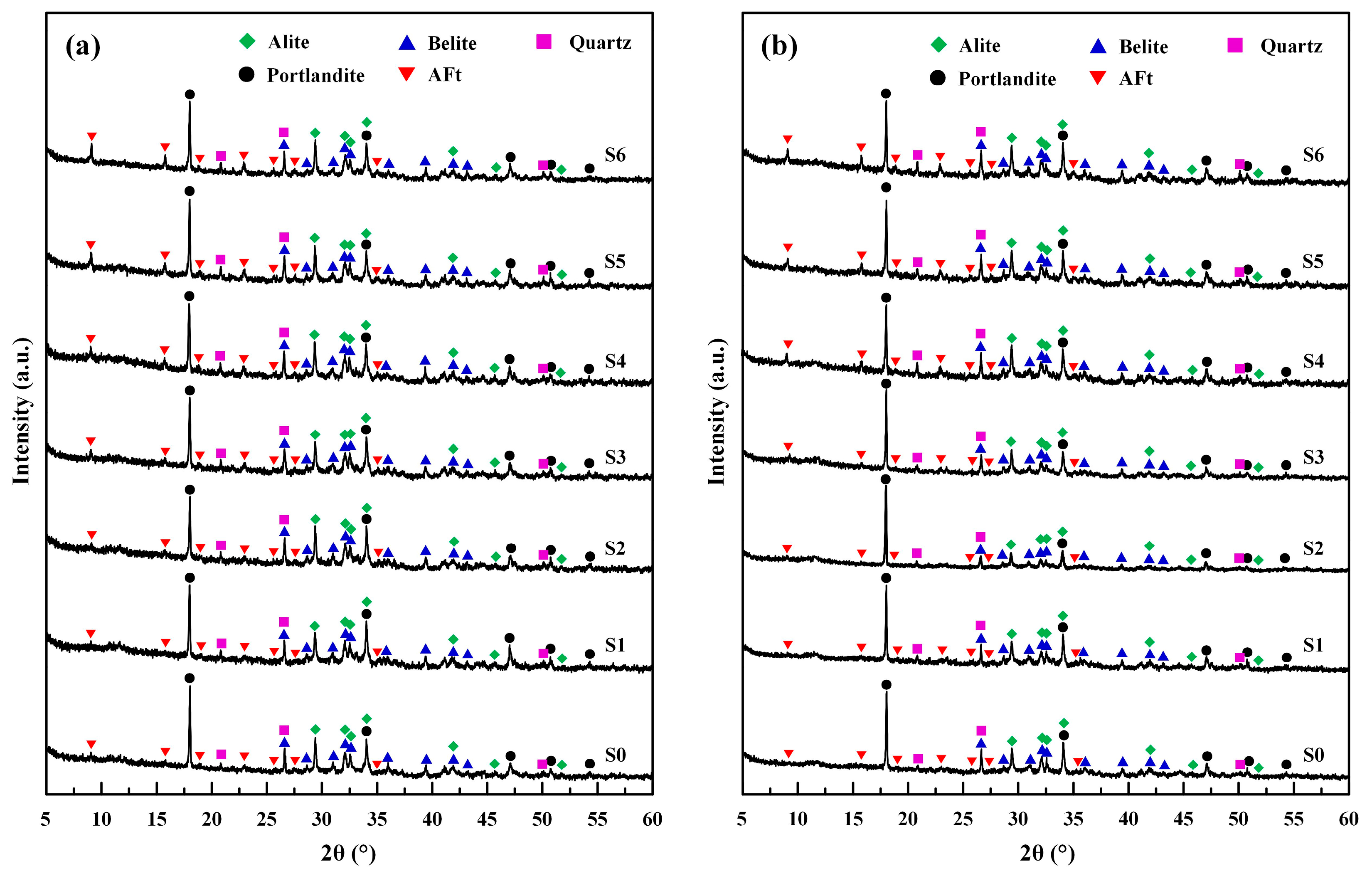
3.4.1. XRD
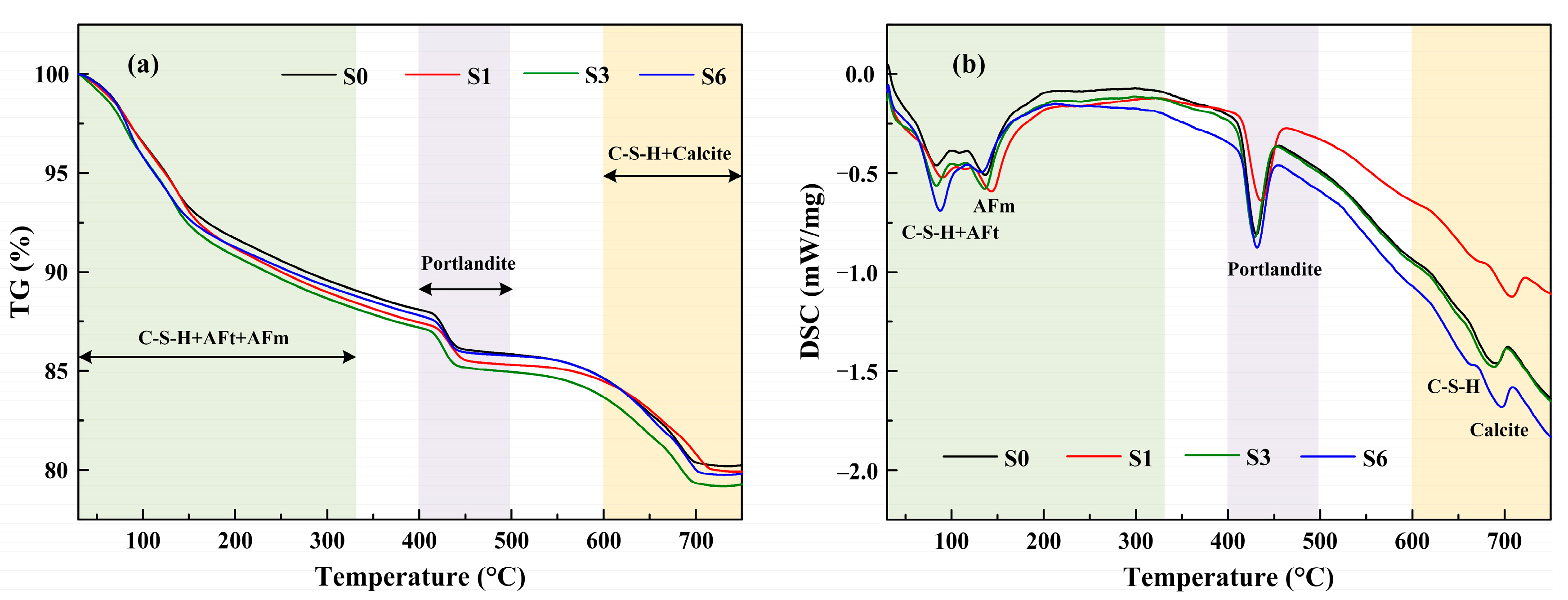
3.4.2. TG–DSC
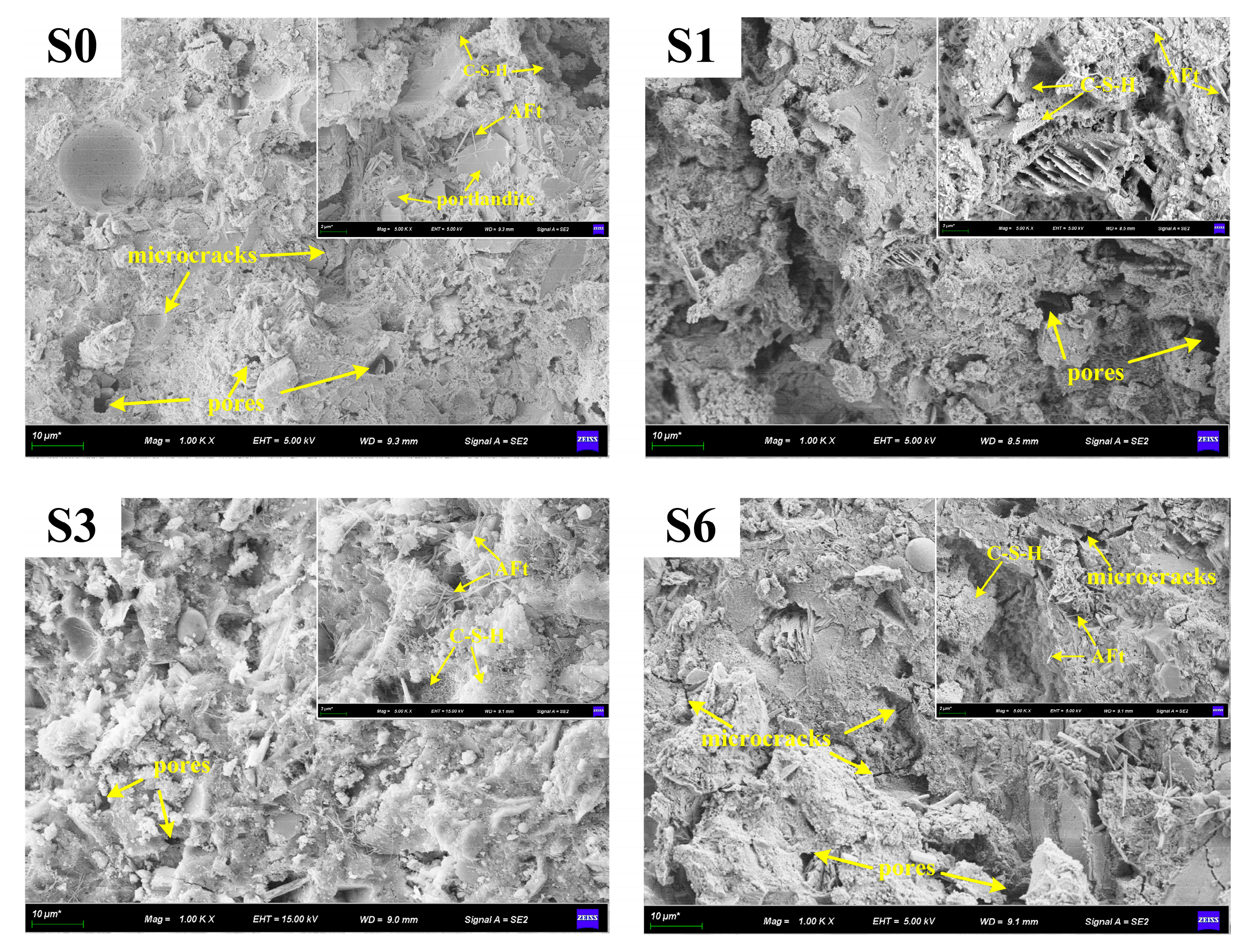
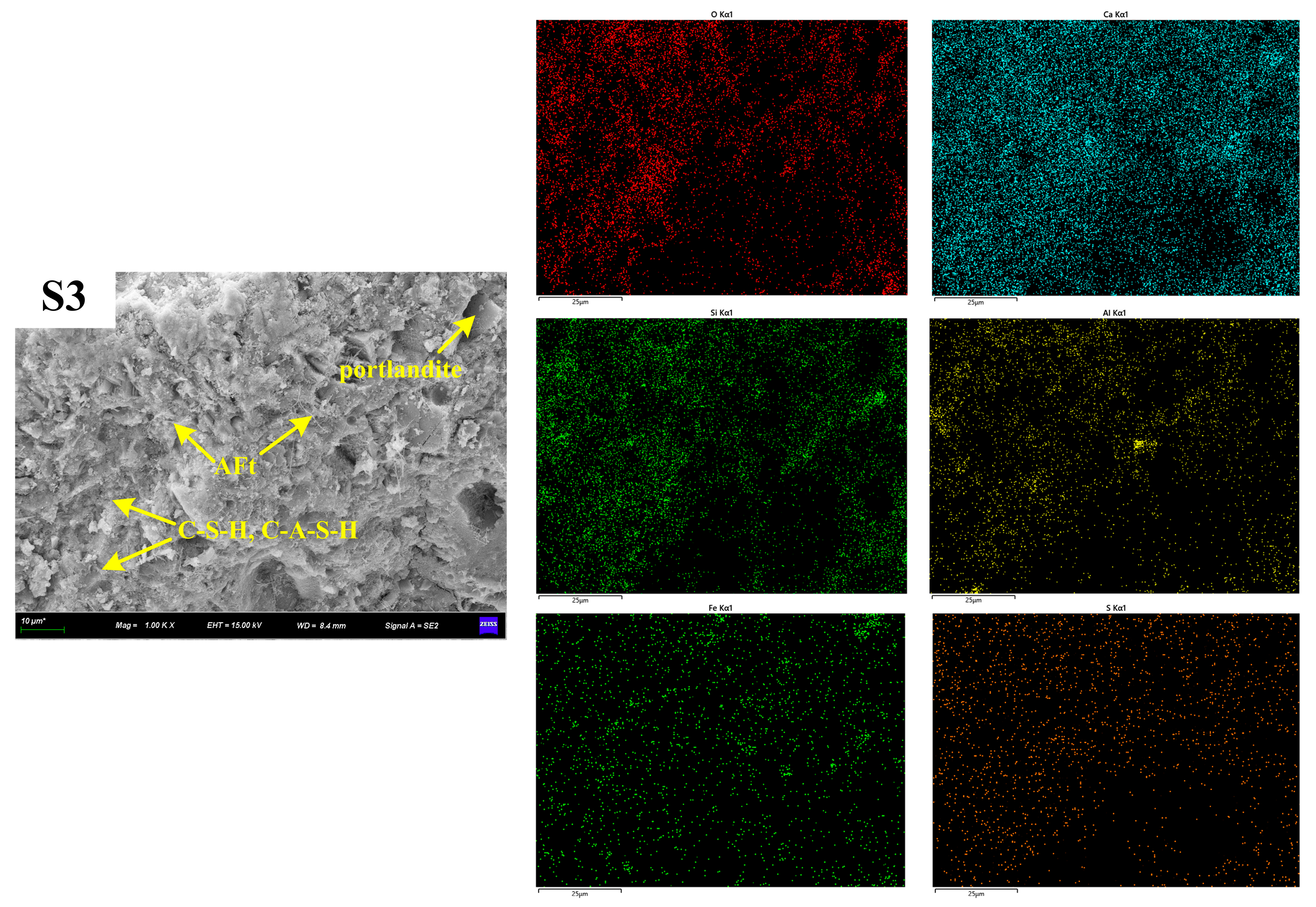
3.4.3. SEM-EDS
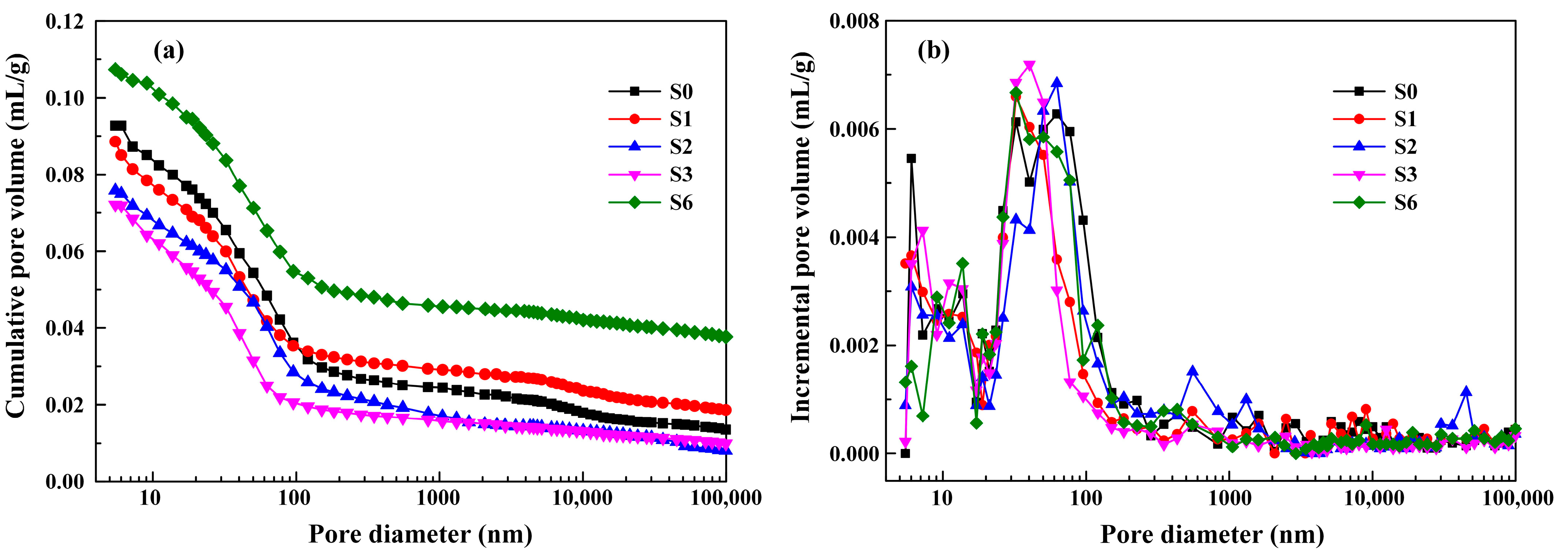
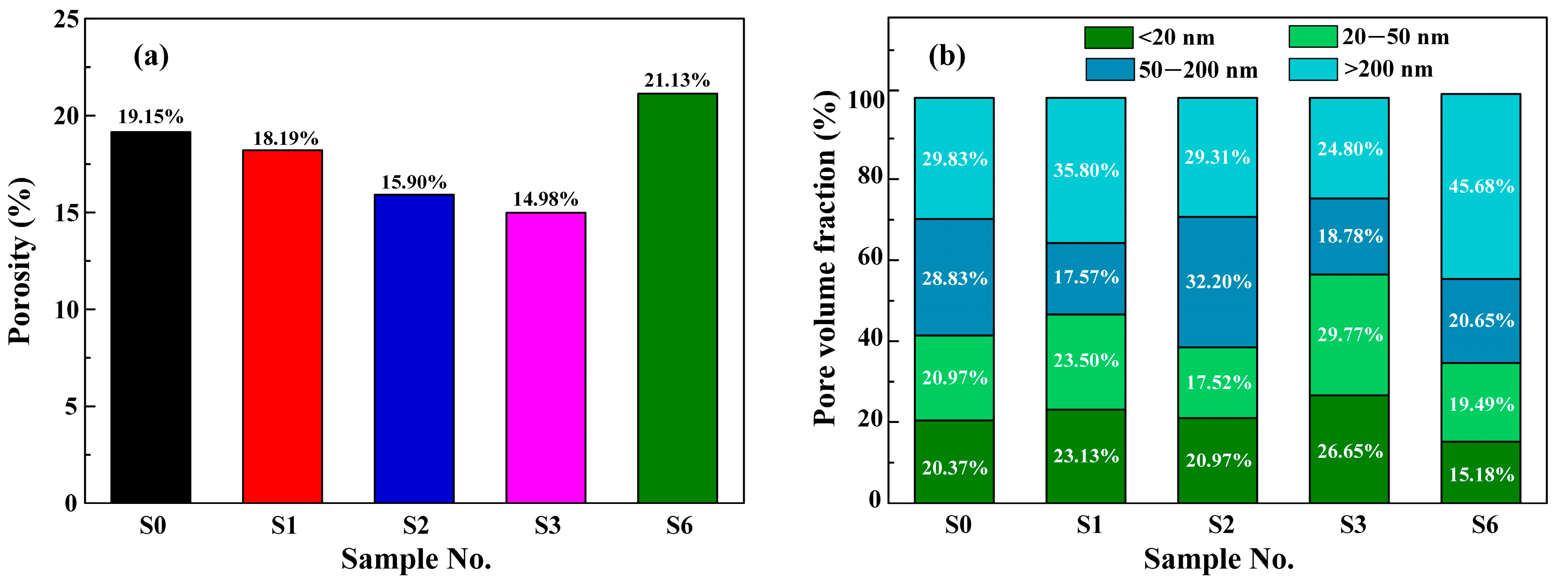
3.4.4. Pore Structure
4. Conclusions
- Using single BRM to replace BOFS, the mechanical strength and chloride corrosion resistance of the composite mortars slightly increased. Further, the 28 d compressive strength and 120 d corrosion resistance coefficient KC of the composite mortars, respectively, increased by 3.60% and 9.18% after the addition of 10 wt% BRM.
- With the increase in PG, the compressive strength, flexural strength, chloride migration coefficient DRCM, and electric flux Q of the composite mortars increased first and then gradually decreased, in contrast to the corrosion resistance coefficient KC. Further, sample S3, with 6 wt% BRM and 4 wt% PG, achieved the highest strength and chloride corrosion resistance.
- The alkali activation of BRM promoted the hydration of active minerals in BOFS, resulting in an increase in the amount of portlandite and C-S-H gel generated. Moreover, the free aluminum in BRM dissolved into C-S-H gel to induce the generation C-A-S-H gel during the hydration process. The increase in hydration products and the generation of C-A-S-H gel were the primary factors in improving the properties of the composite mortars.
- The PG additive promoted the generation of needle rod-like ettringite, and inhibited the transformation of ettringite into monosulphate. The generation of ettringite and the filling effect of PG was benefited for decreasing the porosity and amount of harmful pores, resulting in the enhancement of the mechanical strength and chloride corrosion resistance. But excess PG reduced the generation content of portlandite and C-S-H gel, which caused adverse effects on the properties of the composite mortars.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Deng, Z.; Davis, S.J.; Giron, C.; Ciaos, P. Monitoring global carbon emissions in 2021. Nat. Rev. Earth Environ. 2022, 3, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Muller, H.S.; Haist, M.; Vogel, M. Assessment of the sustainability potential of concrete and concrete structures considering their environmental impact, performance and lifetime. Constr. Build. Mater. 2014, 67, 321–337. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Ziegler-Rodriguez, K.; Laso, J.; Quispe, I.; Aldaco, R.; Kahhat, R. Production of cement in Peru: Understanding carbon–related environmental impacts and their policy implications. Resour. Conserv. Recycl. 2019, 142, 283–292. [Google Scholar] [CrossRef]
- Mohamad, N.; Muthusamy, K.; Embong, R.; Kusbiantoro, A.; Hashim, M.H. Environmental impact of cement production and Solutions: A review. Mater. Today Proc. 2022, 48, 741–746. [Google Scholar] [CrossRef]
- Pelisser, F.; Barcelos, A.; Santos, D.; Peterson, M.; Bernardin, A.M. Lightweight concrete production with low portland cement consumption. J. Clean. Prod. 2012, 23, 68–74. [Google Scholar] [CrossRef]
- Arora, A.; Almujaddidi, A.; Kianmofrad, F.; Mobasher, B.; Neithalath, N. Material design of economical ultra–high performance concrete (UHPC) and evaluation of their properties. Cem. Concr. Compos. 2019, 104, 103346. [Google Scholar] [CrossRef]
- Tekin, İ.; Dirikolu, İ.; Gökçe, H.S. A regional supplementary cementitious material for the cement industry: Pistachio shell ash. J. Clean. Prod. 2021, 285, 124810. [Google Scholar] [CrossRef]
- Li, Y.; Pei, S.; Pan, S.Y.; Chiang, P.C.; Lu, C.; Ouyang, T. Carbonation and utilization of basic oxygen furnace slag coupled with concentrated water from electrodeionization. J. CO2 Util. 2018, 25, 46–55. [Google Scholar] [CrossRef]
- Mo, L.; Yang, S.; Huang, B.; Xu, L.; Feng, S.; Deng, M. Preparation, microstructure and property of carbonated artificial steel slag aggregate used in concrete. Cem. Concr. Compos. 2020, 113, 103715. [Google Scholar] [CrossRef]
- Feng, J.; Sun, J. A comparison of the 10–year properties of converter steel slag activated by high temperature and an alkaline activator. Constr. Build. Mater. 2020, 234, 116948. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H. Utilization of steel slag powder as a combined admixture with ground granulated blast–furnace slag in cement based materials. J. Mater. Civ. Eng. 2013, 25, 1990–1993. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hou, G.; Yan, P. Preparation of sustainable and green cement–based composite binders with high–volume steel slag powder and ultrafine blast furnace slag powder. J. Clean. Prod. 2021, 289, 125133. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Santos, W.F.; Brouwers, H.J.H. Air granulated basic Oxygen furnace (BOF) slag application as a binder: Effect on strength, volumetric stability, hydration study, and environmental risk. Constr. Build. Mater. 2023, 367, 130342. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Zhuang, S. The soundness of steel slag with different free CaO and MgO contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Q.; Liu, J.; Wu, Q. Material Properties of steel slag–cement binding materials prepared by precarbonated steel slag. J. Mater. Civ. Eng. 2018, 30, 04018208. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.E.; Papadimitriou, G.D.; Tsibouki, Z. Properties and hydration of blended cements with steelmaking slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Wei, J.; Li, J. Investigation on mechanical properties, durability and micro–structural development of steel slag blended cements. J. Therm. Anal. Calorim. 2012, 110, 633–639. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; LI, L.; Wang, J.; Shao, N.; Wang, D. Microstructure and phase evolution of alkali–activated steel slag during early age. Constr. Build. Mater. 2019, 204, 158–165. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, D. Hydration and mechanical properties of cement–steel slag system incorporating different activators. Constr. Build. Mater. 2023, 363, 129981. [Google Scholar] [CrossRef]
- Qi, L.; Liu, J.; Liu, Q. Compound effect of CaCO3 and CaSO4·2H2O on the strength of steel slag–cement binding materials. Mater. Res. 2016, 19, 269–275. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, P.; Hu, C.; Xia, H.; Liang, Z. Study on the mechanical properties and hydration behavior of steel slag–red mud–electrolytic manganese residue based composite mortar. Appl. Sci. 2023, 13, 5913. [Google Scholar] [CrossRef]
- Wu, F.; Xiao, B.; Yang, F. Rheological and strength properties of steel–slag cemented paste backfill: Link to gypsum type and dosage. Minerals 2023, 13, 421. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhang, Z.; Zhang, W.; Lu, Y.; Wang, Y.; Yang, T. In–depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Build. Eng. 2022, 52, 104449. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Ma, W.; Hu, J.; Cai, Z. Investigation on the application of steel slag–fly ash–phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Gao, Y.; Liu, C.; Qi, Y. Workability and microstructural properties of red-mud-based geopolymer with different particle sizes. Adv. Cem. Res. 2021, 33, 210–223. [Google Scholar] [CrossRef]
- Li, W.; Ma, L.; Qiu, S.; Yin, X.; Dai, Q.; Du, W. Sustainable utilization of phosphogypsum in multi-solid waste recycled aggregates: Environmental impact and economic viability. Sustainability 2024, 16, 1161. [Google Scholar] [CrossRef]
- Hu, C.; Xiang, W.; Chen, P.; Yang, Y.; Zhou, L.; Jiang, J.; Li, S.; Ming, Y.; Li, Q. Impact of bayer red mud on the working and mechanical properties of composite cement mortar. J. Renew. Mater. 2023, 11, 3945–3956. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z. Investigation on phosphogypsum–steel slag–granulated blast-furnace slag–limestone cement. Constr. Build. Mater. 2010, 24, 1296–1301. [Google Scholar] [CrossRef]
- AlArab, A.; Hamad, B.; Assaad, J.J. Strength and durability of concrete containing ceramic waste powder and blast furnace slag. J. Mater. Civ. Eng. 2022, 34, 04021392. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Liang, H.; Jiang, J.; Yang, Y.; He, Z. Synergistic enhancement of mechanical property of the high replacement low–calcium ultrafine fly ash blended cement paste by multiple chemical activators. J. Build. Eng. 2020, 32, 101520. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z.; Wang, D.; Yan, P. Hydration heat evolution and kinetics of blended cement containing steel slag at different temperatures. Thermochim. Acta. 2015, 605, 43–51. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, M.; Qin, S.; Liu, T.; Tong, W.; Zhou, A.; Jivkov, A. Calcium leaching from cement hydrates exposed to sodium sulfate solutions. Constr. Build. Mater. 2022, 351, 128975. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, H.; Chen, B.; Cui, Q. Development and optimization of phosphogypsum-based geopolymer cement. Constr. Build. Mater. 2023, 369, 130577. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, G.; Liu, Y. Early hydration heat of calcium sulfoaluminate cement with influences of supplementary cementitious materials and water to binder ratio. Materials 2021, 14, 642. [Google Scholar] [CrossRef]
- Chang, H.; Fan, S.; Guo, Z.; Ma, Y.; Li, Z.; Zuo, Z.; Liu, J.; Yang, L. Chloride binding behavior of calcium silicate hydrates and Friedel’s salt of pastes. J. Build. Eng. 2023, 76, 107318. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, B.; Liang, F.; Xue, Z.; Qian, B.; Wang, J.; Zhou, L.; Zhang, J.; Liang, R.; Li, Y.; et al. Use of untreated phosphogypsum as a raw material for autoclavedaerated concrete preparation. J. Build. Eng. 2023, 64, 105607. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Yang, K.; Liu, C.; Guan, X.; Liu, S.; Jing, G. Effects of synthetic CSH-tartaric acid nanocomposites on the properties of ordinary Portland cement. Cem. Concr. Compos. 2022, 129, 104466. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, G.; Zhao, K.; Wang, M.; Wu, B.; Li, S.; Chen, Q.; Geng, J. Mechanical properties, permeability and microstructure of steam-cured fly ash mortar mixed with phosphogypsum. Constr. Build. Mater. 2023, 400, 132582. [Google Scholar] [CrossRef]
- Zhu, X.; Richardson, I.G. Morphology-structural change of C–A–S–H gel in blended cements. Cem. Concr. Res. 2023, 168, 107156. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Guo, J.; Zhang, H. Fractal characteristics of geopolymer mortar containing municipal solid waste incineration fly ash and its correlations to pore structure and strength. Fractal Fract. 2022, 6, 676. [Google Scholar] [CrossRef]
- Shen, A.; Lin, S.; Guo, Y.; He, T.; Lyu, Z. Relationship between flexural strength and pore structure of pavement concrete under fatigue loads and freeze-thaw interaction in seasonal frozen regions. Constr. Build. Mater. 2018, 174, 684–692. [Google Scholar] [CrossRef]

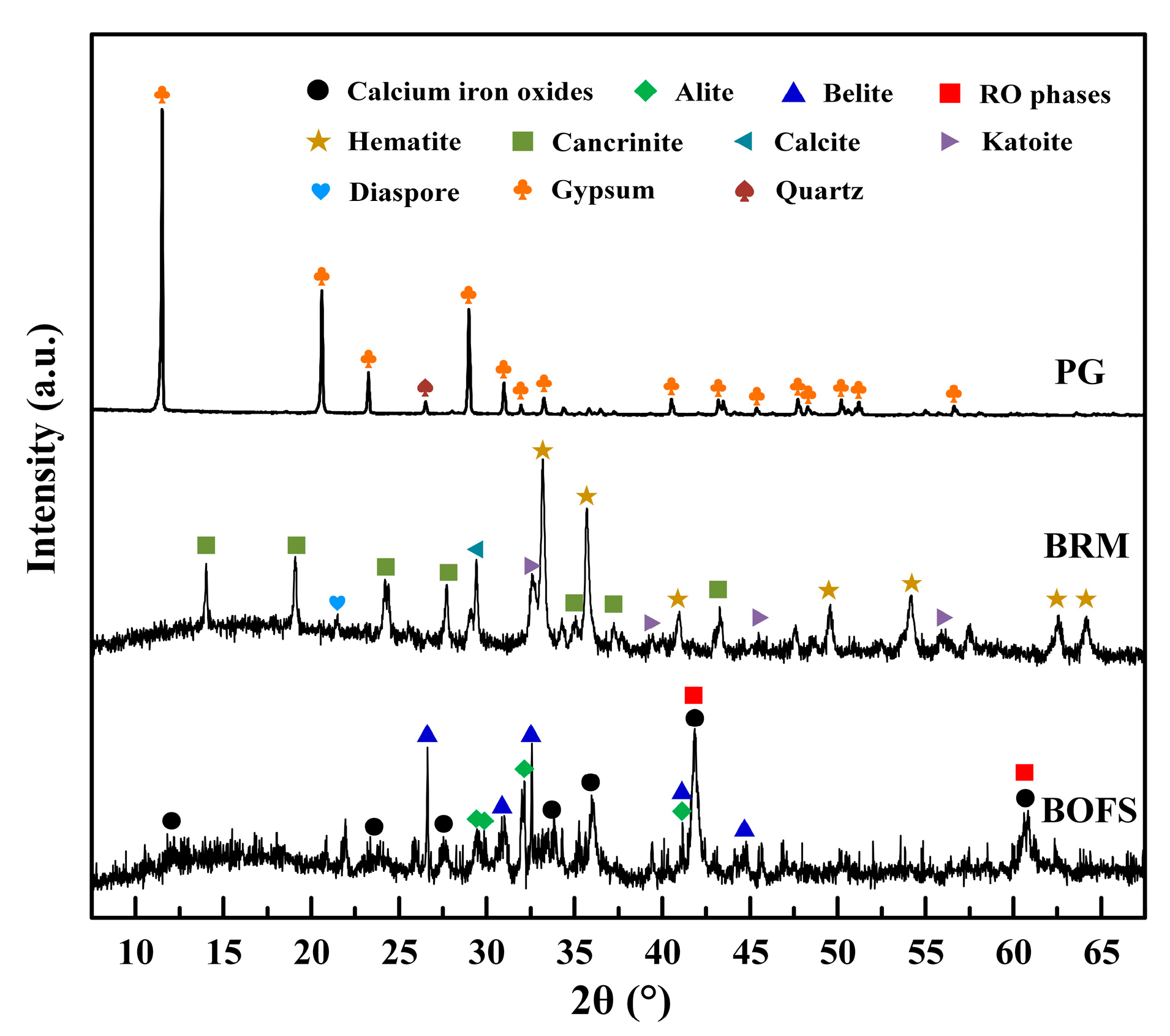
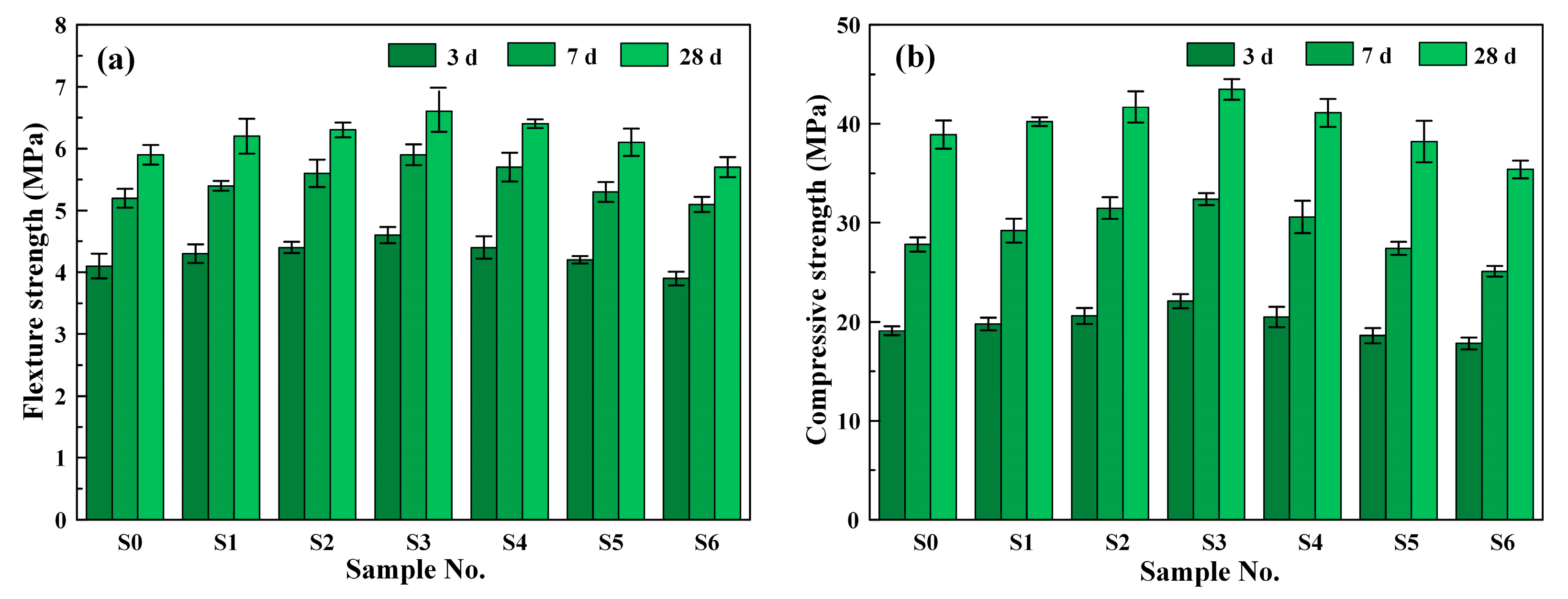
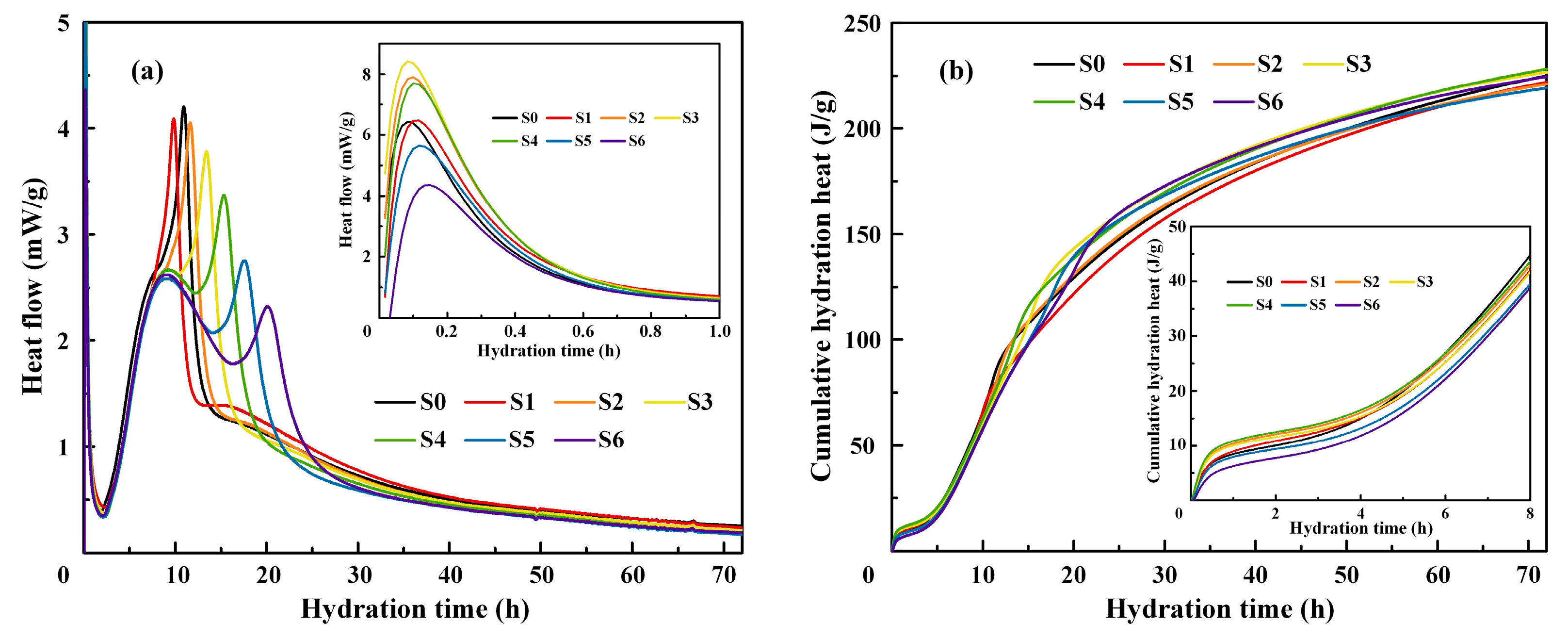

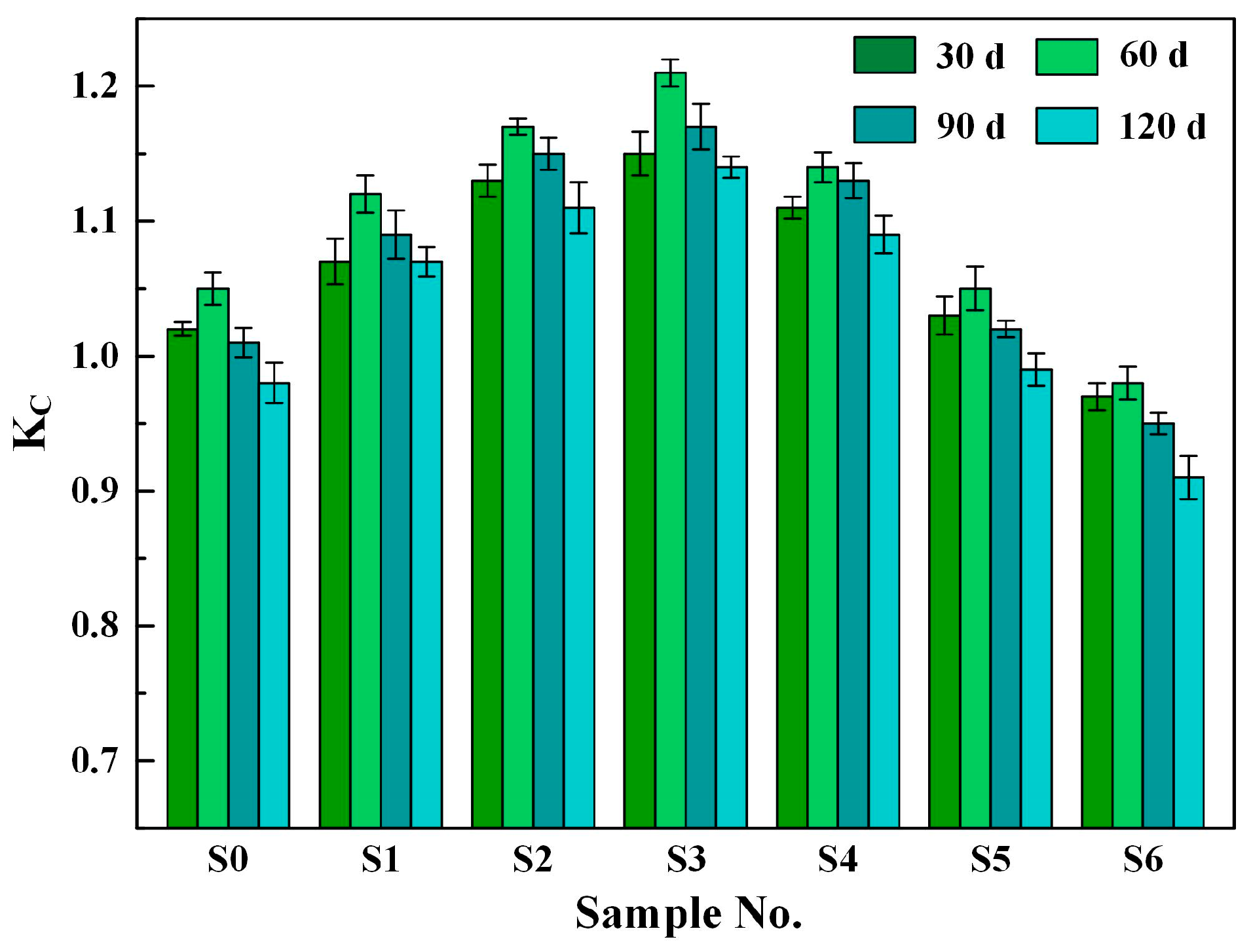
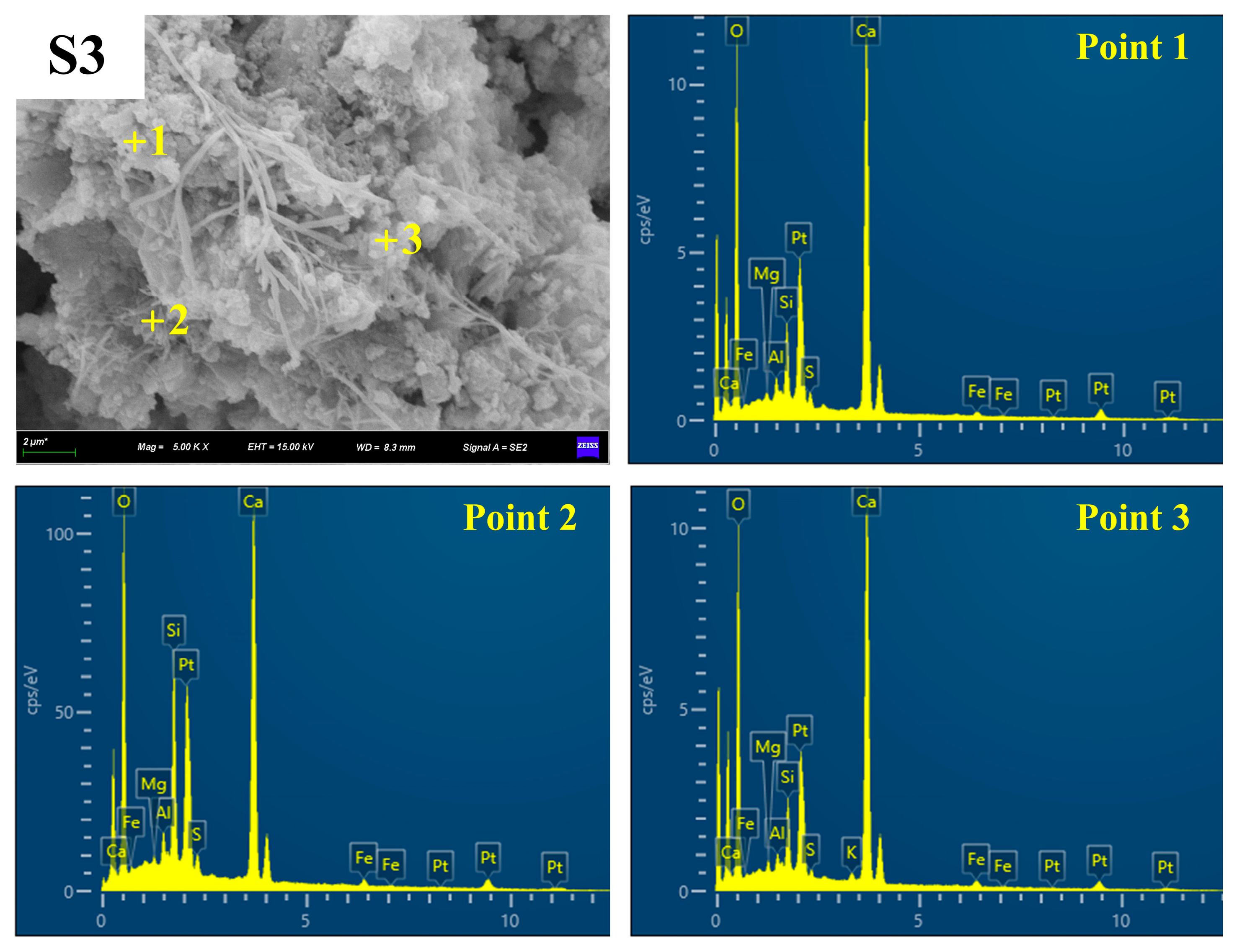
| Raw Material | SiO2 | CaO | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 24.76 | 60.25 | 5.64 | 3.71 | 0.88 | 3.22 | 0.16 | 0.70 | 0.68 |
| BOFS | 26.95 | 29.67 | 5.66 | 24.15 | 5.33 | 0.31 | 0.09 | 0.08 | 7.76 |
| BRM | 14.49 | 16.82 | 20.39 | 30.58 | 0.33 | 0.52 | 7.15 | 0.19 | 9.53 |
| PG | 4.07 | 36.98 | 0.36 | 0.27 | 0.03 | 35.17 | 0.05 | 0.02 | 23.05 |
| Sample No. | Cement | BOFS | BRM | PG | Sand | Water |
|---|---|---|---|---|---|---|
| S0 | 410.16 | 175.78 | 0 | 0 | 1757.82 | 292.97 |
| S1 | 410.16 | 117.19 | 58.59 | 0.00 | 1757.82 | 292.97 |
| S2 | 410.16 | 117.19 | 46.88 | 11.72 | 1757.82 | 292.97 |
| S3 | 410.16 | 117.19 | 35.16 | 23.44 | 1757.82 | 292.97 |
| S4 | 410.16 | 117.19 | 23.44 | 35.16 | 1757.82 | 292.97 |
| S5 | 410.16 | 117.19 | 11.72 | 46.88 | 1757.82 | 292.97 |
| S6 | 410.16 | 117.19 | 0.00 | 58.59 | 1757.82 | 292.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Wang, Q.; Xiang, W.; Zhang, T.; Li, Y.; Chen, R. Study on Mechanical Strength and Chloride Corrosion Resistance of Composite Mortars Mixed with Steel Slag, Bayer Red Mud, and Phosphogypsum. Buildings 2025, 15, 1510. https://doi.org/10.3390/buildings15091510
Hu C, Wang Q, Xiang W, Zhang T, Li Y, Chen R. Study on Mechanical Strength and Chloride Corrosion Resistance of Composite Mortars Mixed with Steel Slag, Bayer Red Mud, and Phosphogypsum. Buildings. 2025; 15(9):1510. https://doi.org/10.3390/buildings15091510
Chicago/Turabian StyleHu, Cheng, Qijie Wang, Weiheng Xiang, Tao Zhang, Yanguang Li, and Ruhua Chen. 2025. "Study on Mechanical Strength and Chloride Corrosion Resistance of Composite Mortars Mixed with Steel Slag, Bayer Red Mud, and Phosphogypsum" Buildings 15, no. 9: 1510. https://doi.org/10.3390/buildings15091510
APA StyleHu, C., Wang, Q., Xiang, W., Zhang, T., Li, Y., & Chen, R. (2025). Study on Mechanical Strength and Chloride Corrosion Resistance of Composite Mortars Mixed with Steel Slag, Bayer Red Mud, and Phosphogypsum. Buildings, 15(9), 1510. https://doi.org/10.3390/buildings15091510





