Factors Affecting Synthesized C-S-H CO2 Uptake: Initial Alkalinity and Ca/Si
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Mineralization of C-S-H
2.3. Microstructural Characterization of C-S-H
2.4. Mineral Composition Analysis
2.5. Structural Testing of C-S-H
2.6. Pore Size Distribution Testing of Synthesized C-S-H
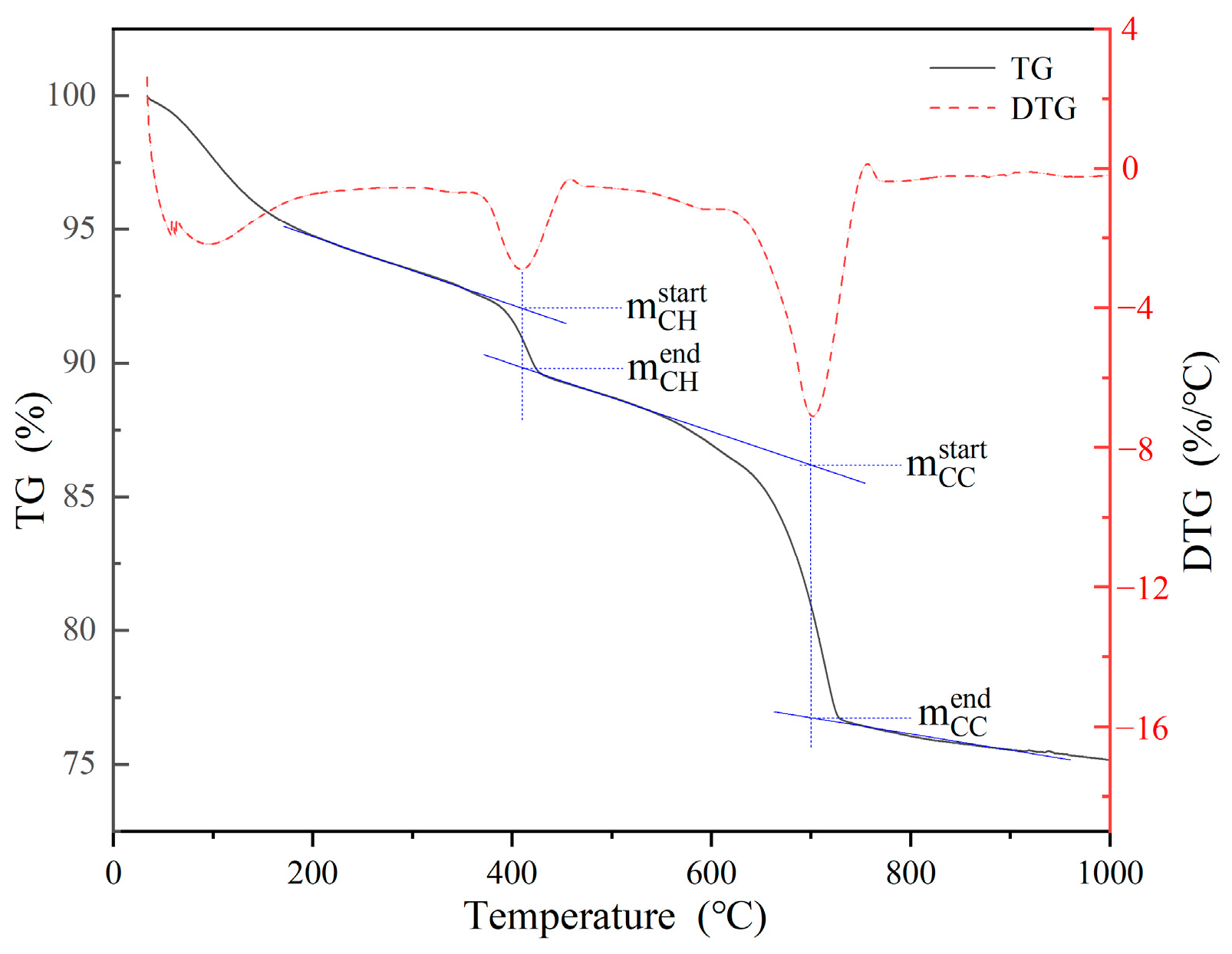
2.7. Calculation of CO2 Uptake
3. Results
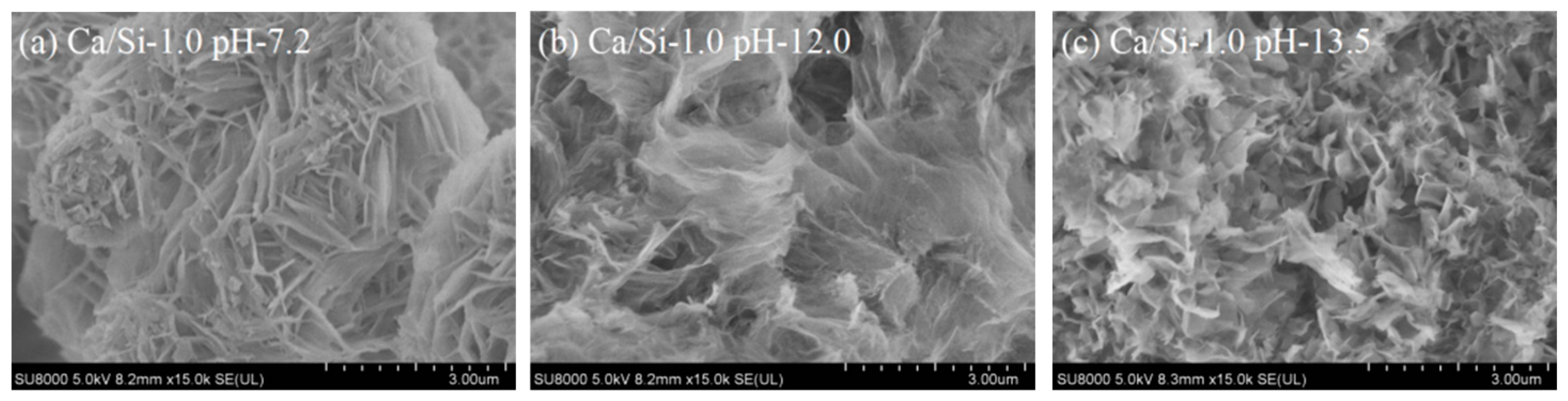
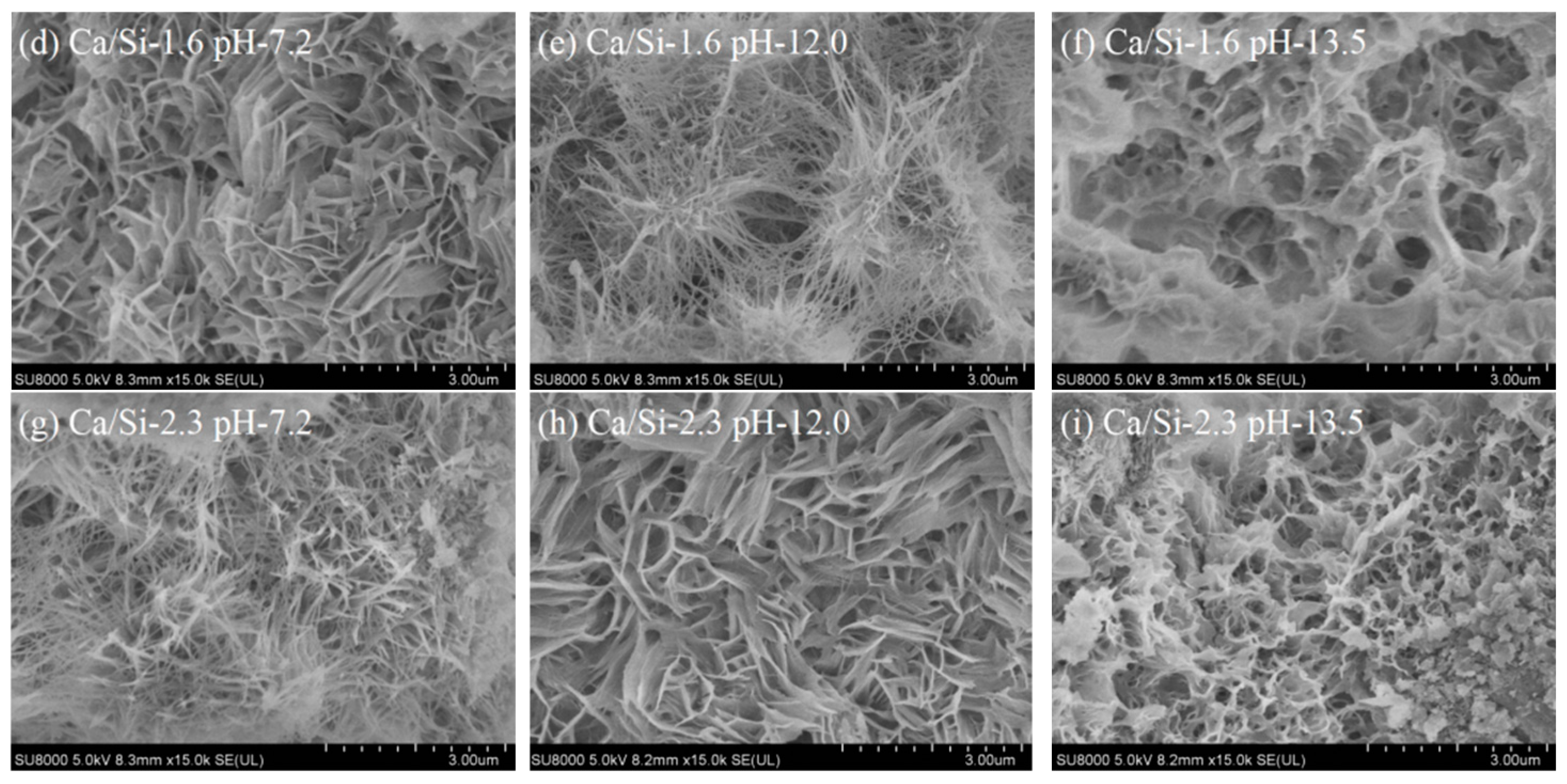
3.1. Microstructure of Synthesized C-S-H
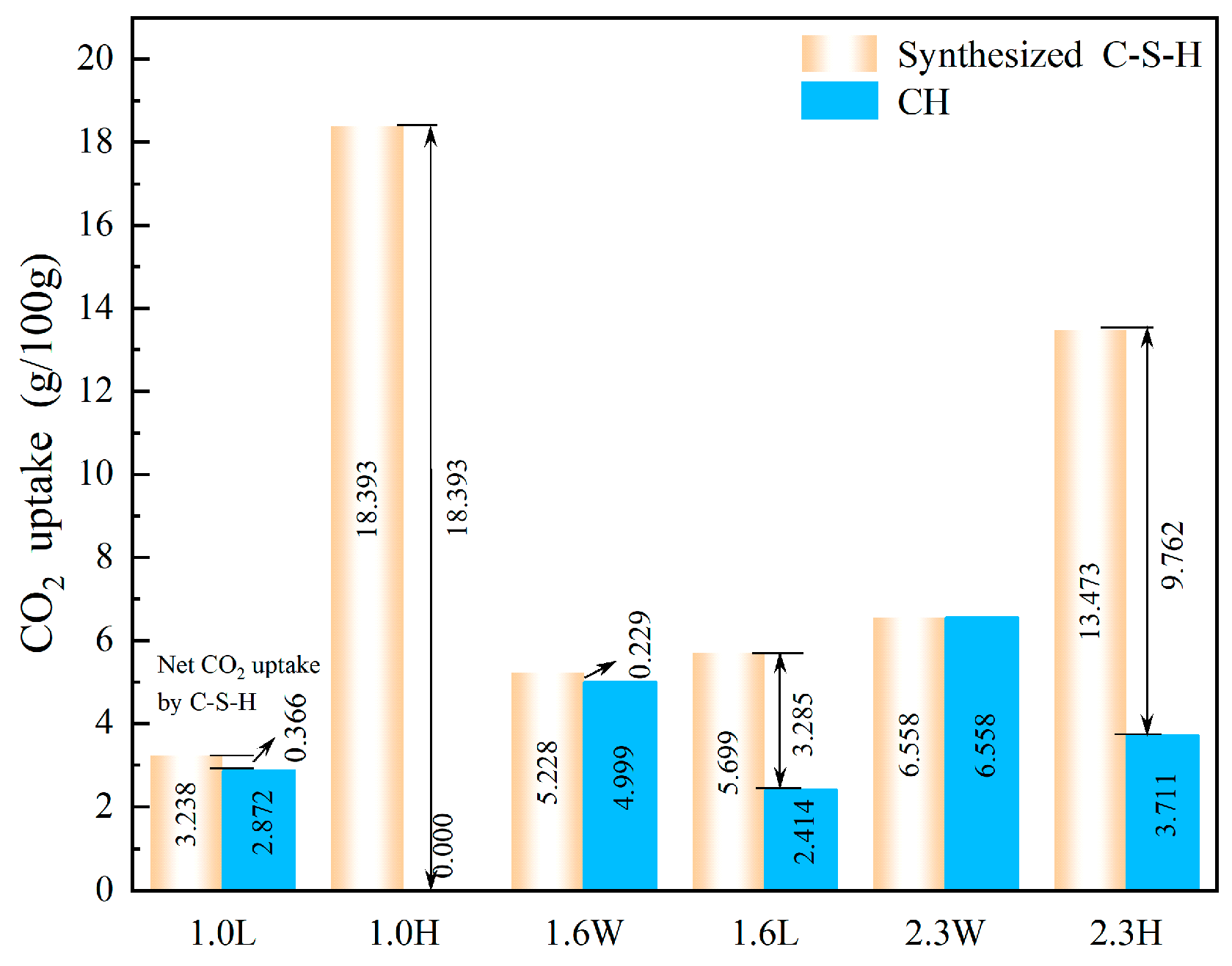
3.2. CO2 Uptake by Synthesized C-S-H
4. Discussion
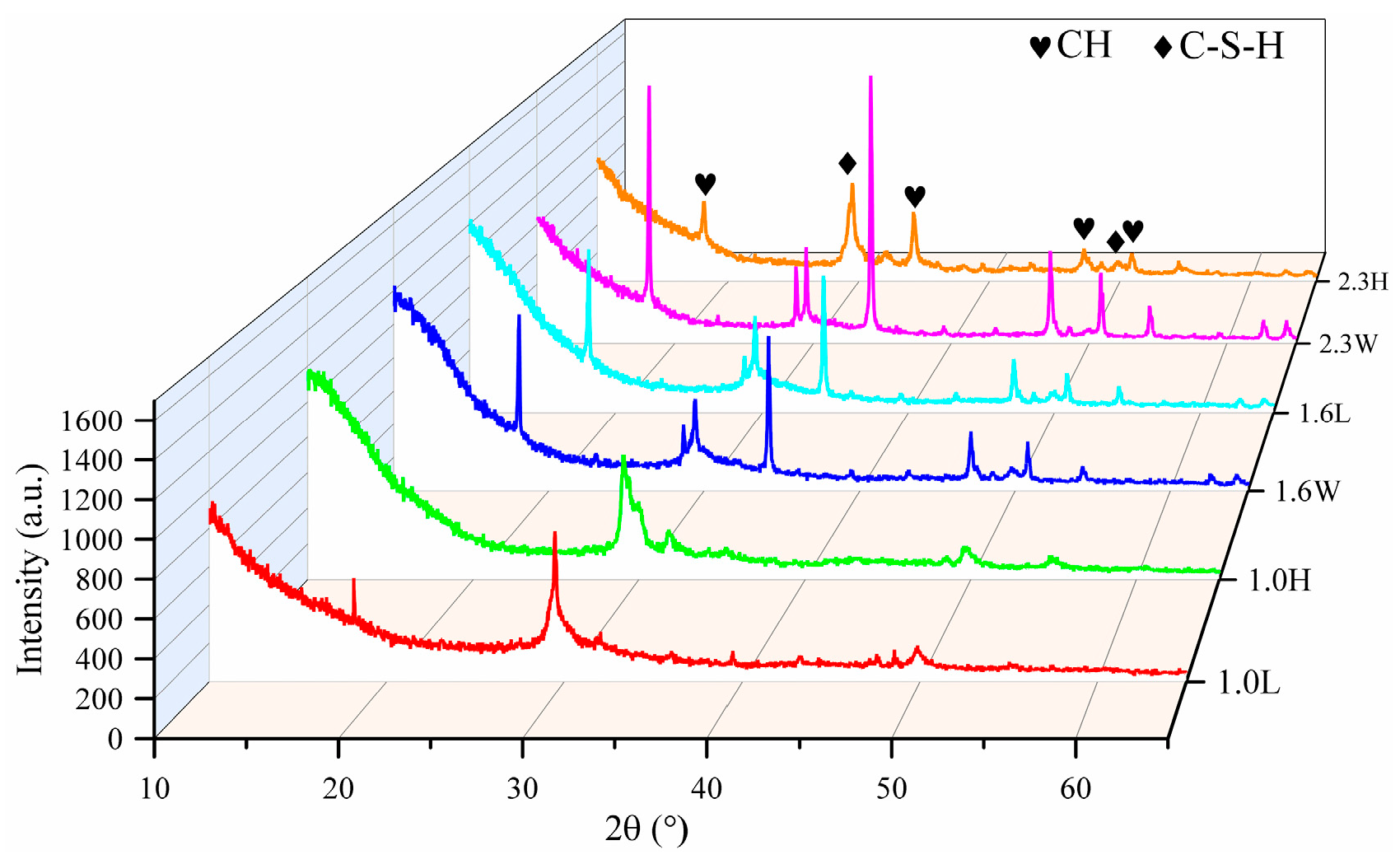
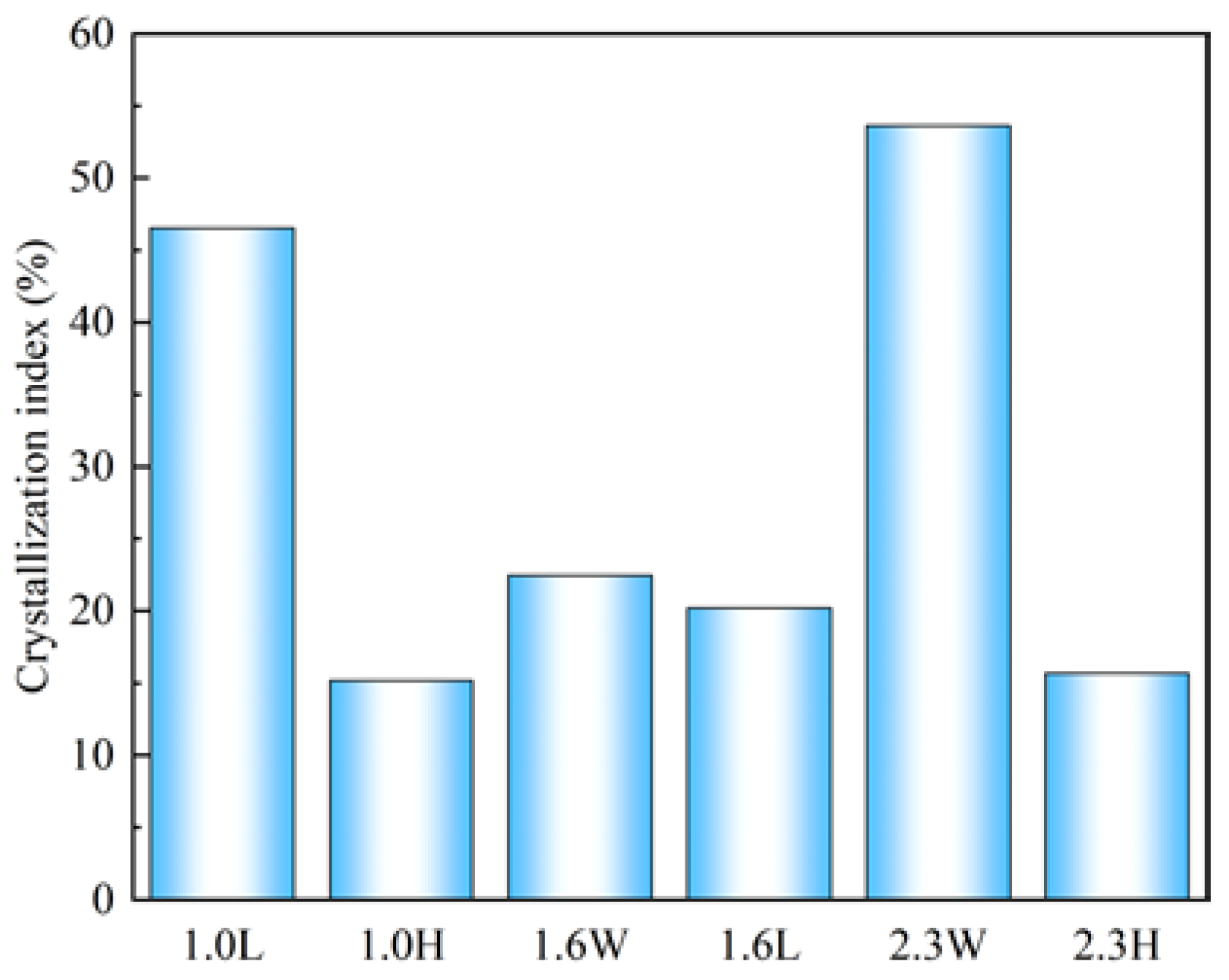
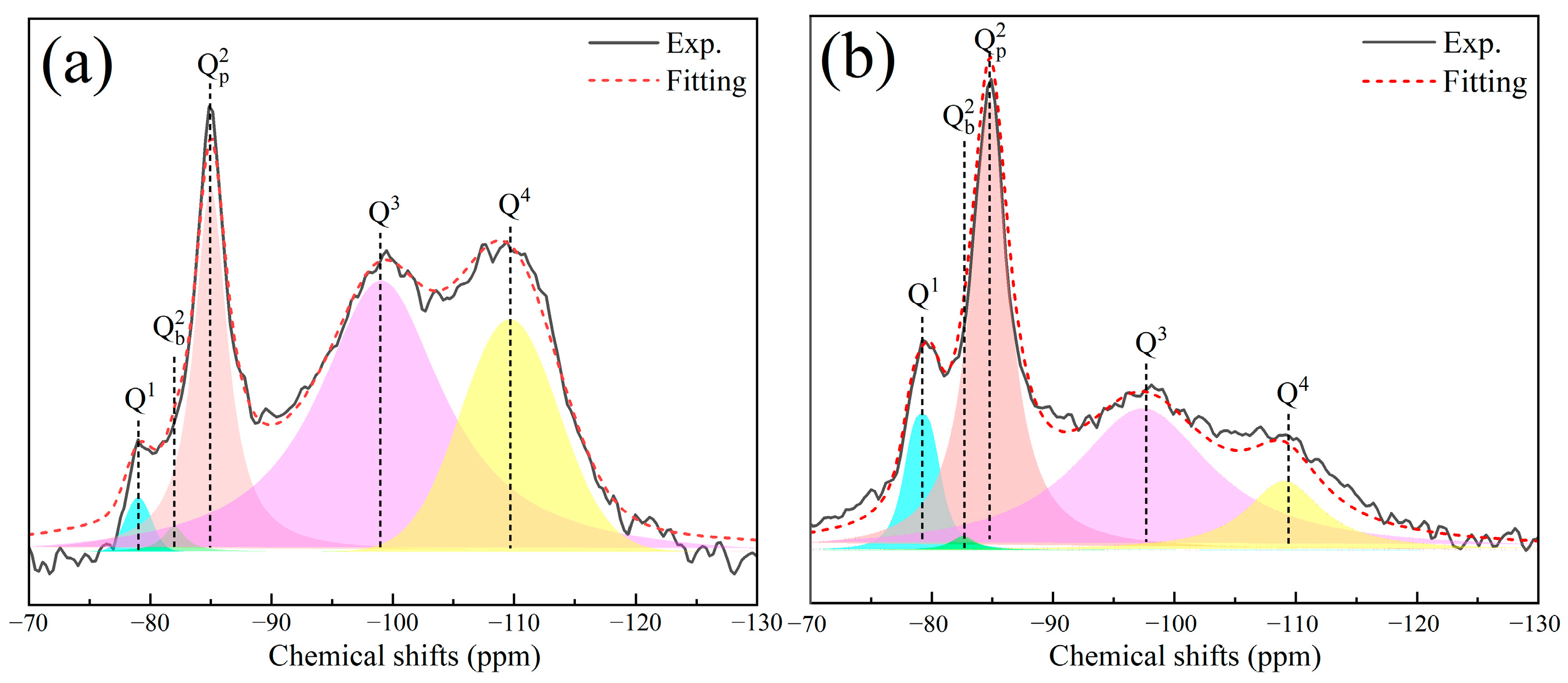
4.1. Effect of Alkalinity and Ca/Si on the Crystallinity and Polymerization Degree of C-S-H
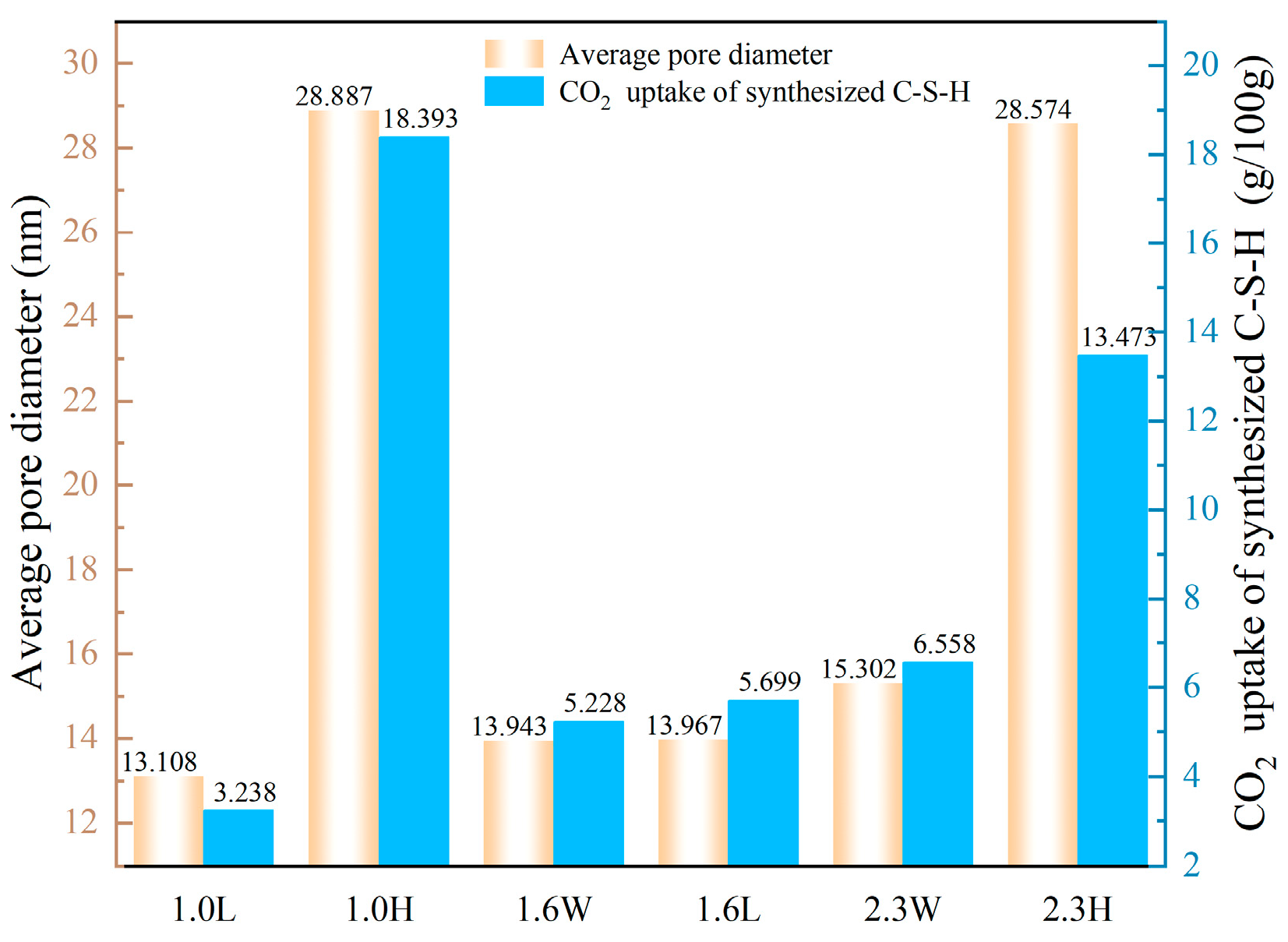
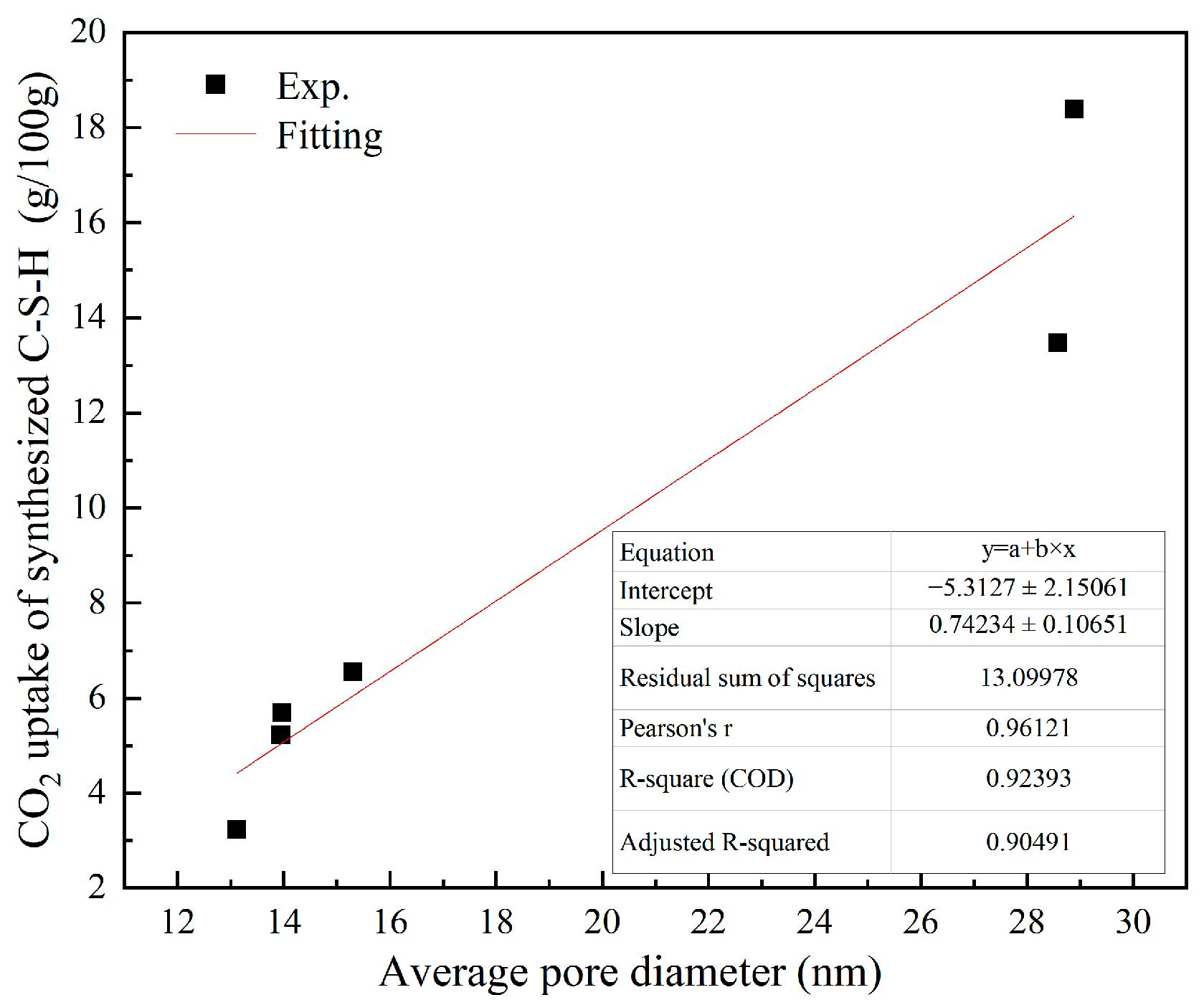
4.2. Effect of Pore Structure of Synthesized C-S-H on CO2 Uptake
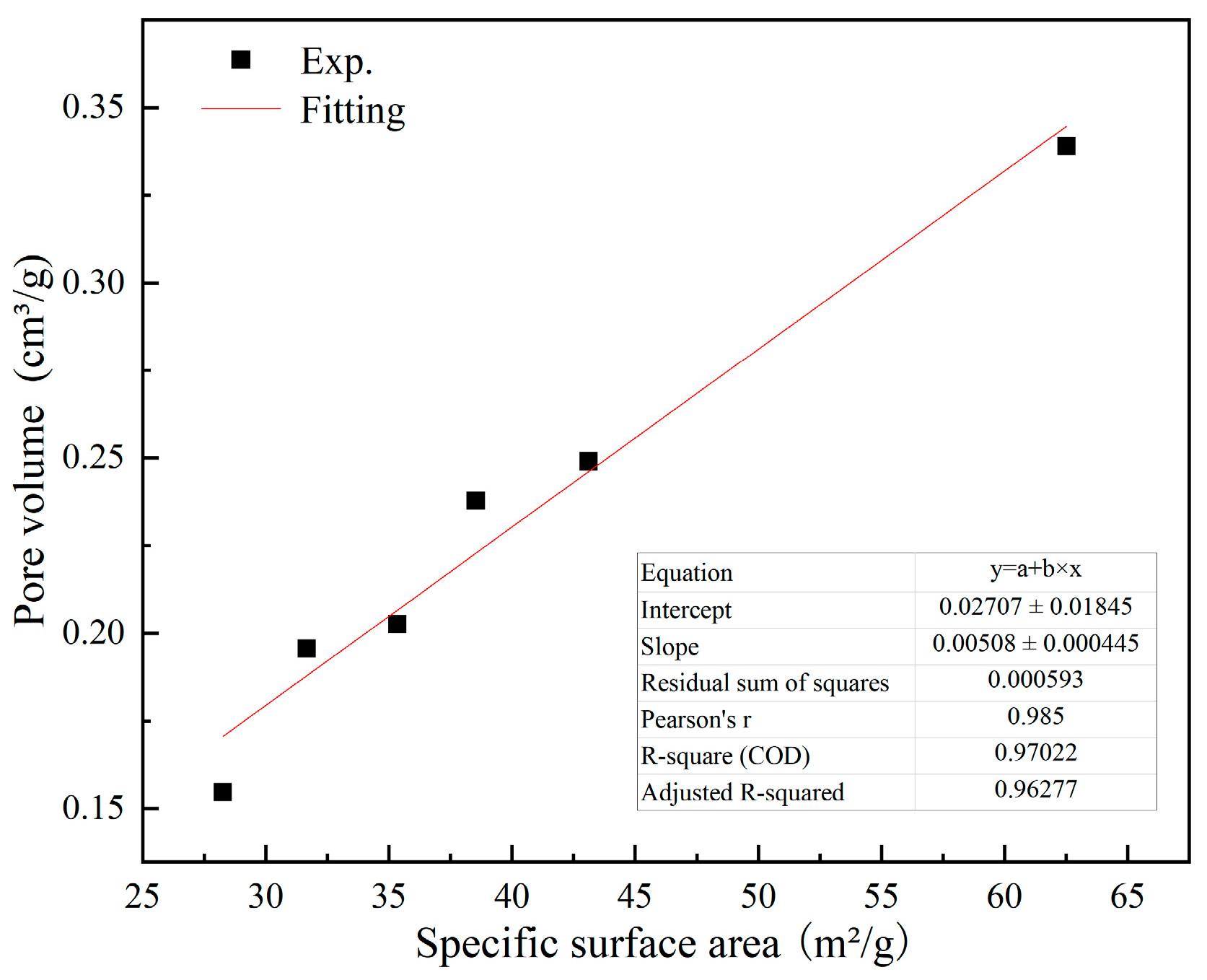
4.3. Effect of C-S-H Nanostructural Characteristics on CO2 Uptake
5. Conclusions
- C-S-H synthesized under conditions of pH 7.2 to 12.0 and Ca/Si ratios of 1.0 to 2.3 are flocculent, fibrous, and needle-like structures with high crystallinity and mainly linked by Q1. At the same pH, increasing the Ca/Si ratio leads to an increase in the proportion of Q1. The C-S-H synthesized under conditions of pH 12.0 to 13.5 and Ca/Si ratios of 1.0 to 2.3 is closer to a poor crystallinity structure with a predominant Q1 group when the Ca/Si ratio approaches 1.6 to 2.3 and the pH approaches 13.5, resembling a short-chain structure of silicon-oxygen tetrahedron and exhibiting a porous structure. This is primarily attributed to the tendency of OH− ions to protonate the silanol groups (>Si–OH), which facilitates the uptake of Ca2+ ions. This, in turn, weakens the extent of crosslinking between Si–OCa+ in Q1 and thus reduces the formation of Q2.
- Under the pH ranging from 7.2 to 13.5 and Ca/Si ratio from 1.0 to 2.3, the C-S-H synthesized after 6 h of CO2 curing shows that an increase in Ca/Si ratio results in higher CO2 uptake by the C-S-H when the pH is between 7.2 and 12.0. However, once the pH reaches 13.5, there is no positive correlation between the CO2 uptake and Ca/Si ratio. Instead, C-S-H synthesized at a Ca/Si ratio of 1.0 exhibits the highest CO2 uptake (18.393 g/100 g). This can be attributed to two factors: first, the average pore diameter of C-S-H synthesized at high pH (13.5) is larger; second, the silicon-oxygen tetrahedral chains primarily consisting of Q2 units are more likely to release Ca2+ and participate in carbonation reactions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bertos, M.F.; Simons, S.J.R. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar]
- Shtepenko, O.; Hills, C. The effect of carbon dioxide on β-dicalcium silicate and Portland cement. Chem. Eng. J. 2006, 118, 107–118. [Google Scholar]
- Monkman, S.; Shao, Y.-X. Assessing the carbonation behavior of cementitious materials. J. Mater. Civ. Eng. 2006, 18, 768–776. [Google Scholar]
- El-Hassan, H.; Shao, Y.-X. Carbon storage through concrete block carbonation curing. J. Clean Energy Technol. 2014, 2, 287–291. [Google Scholar]
- Liu, B.; Qin, J. New perspectives on utilization of CO2 sequestration technologies in cement-based materials. Constr. Build. Mater. 2021, 272, 121660. [Google Scholar]
- Wang, T.; Huang, H.; Hu, X.-T. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate. Chem. Eng. J. 2017, 323, 320–329. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiery, M. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar]
- Justnes, H.; Skocek, J. Microstructural changes of hydrated cement blended with fly ash upon carbonation. Cem. Concr. Res. 2020, 137, 106192. [Google Scholar]
- Visser, J.H.M. Influence of the carbon dioxide concentration on the resistance to carbonation of concrete. Constr. Build. Mater. 2014, 67, 8–13. [Google Scholar] [CrossRef]
- Li, L.; Liu, Q. Dimensional change of cement paste subjected to carbonation in CO2 sequestration and utilization context: A critical review on the mechanisms. J. CO2 Util. 2023, 70, 102444. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Stark, J. Recent advances in the field of cement hydration and microstructure analysis. Cem. Concr. Res. 2011, 41, 666–678. [Google Scholar] [CrossRef]
- Chang, J.; Fang, Y.-F. Effects of calcium to silicon ratios on accelerating carbonation of calcium silicate hydrate. J. Chin. Ceram. Soc. 2014, 42, 1377–1382. [Google Scholar]
- Liu, X.; Feng, P. Effects of pH on the nano/micro structure of calcium silicate hydrate (C-S-H) under sulfate attack. Cem. Concr. Res. 2021, 140, 106306. [Google Scholar] [CrossRef]
- ROSENQVIST, M.; BERTRON, A. Concrete alteration due to 55 years of exposure to river water: Chemical and mineralogical characterisation. Cem. Concr. Res. 2017, 92, 110–120. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhong, H.-X. Effect of Calcium-Silicon Ratio of Calcium Silicate Hydrate on Its Structure, Zeta Potential and Adsorption Capacity of Superplasticizer. Bull. Chin. Ceram. Soc. 2020, 39, 1798–1804. [Google Scholar]
- He, Y.; Zhao, X. Effect of C/S ratio on morphology and structure of hydrothermally synthesized calcium silicate hydrate. J Wuhan Univ Technol. 2011, 26, 770–773. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Liu, Z.-T. Decalcification Behavior of Calcium Silicate Hydrate with Different Ca/Si Ratios. J. Chin. Ceram. Soc. 2023, 51, 2924–2930. [Google Scholar]
- Matsuyama, H.; Young, J.F. Effects of pH on precipitation of quasi-crystalline calcium silicate hydrate in aqueous solution. Adv. Cem. Res. 2000, 12, 29–33. [Google Scholar] [CrossRef]
- Duque-Redondo, E.; Bonnaud, P.A. A comprehensive review of C-S-H empirical and computational models, their applications, and practical aspects. Cem. Concr. Res. 2022, 156, 106784. [Google Scholar]
- Chen, J.J.; Thomas, J.J. Solubility and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 1499–1519. [Google Scholar]
- Ntafalias, E.; Koutsoukos, P.G. Spontaneous precipitation of calcium silicate hydrate in aqueous solutions. Cryst. Res. Technol. 2010, 45, 39–47. [Google Scholar]
- Shen, X.; Feng, P. Toward the formation mechanism of synthetic calcium silicate hydrate (C-S-H)-pH and kinetic considerations. Cem. Concr. Res. 2023, 172, 107248. [Google Scholar]
- Liu, X.; Feng, P. Carbonation behavior of calcium silicate hydrate (C-S-H): Its potential for CO2 capture. Chem. Eng. J. 2022, 431, 134243. [Google Scholar]
- Steiner, S.; Lothenbach, B. Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite. Cem. Concr. Res. 2020, 135, 106116. [Google Scholar]
- Black, L.; Garbev, K. Surface carbonation of synthetic C-S-H samples: A comparison between fresh and aged C-S-H using X-ray photoelectron spectroscopy. Cem. Concr. Res. 2008, 38, 745–750. [Google Scholar]
- Li, J.; Yu, Q.J. Effects of Ca/Si ratio, aluminum and magnesium on the carbonation behavior of calcium silicate hydrate. Materials 2019, 12, 1268. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C–S–H and C–A–S–H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar]
- Wu, B.; Ye, G. Carbonation mechanism of different kind of C-S-H: Rate and products. In Proceedings of the RILEM Conference on Materials, Systems and Structures in Civil Engineering 2016-Segment on Concrete with Supplementary Cementitious Materials, Hong Kong, China, 9 September 2016. [Google Scholar]
- Lei, Y.S.; Han, T.; Wang, H.Q. Preparation and characterization of calcium silicate hydrate (C-S-H) synthesized by the hydrothermal method. Bull. Chin. Ceram. Soc. 2014, 33, 465–469. [Google Scholar]
- Zhu, Y.Y.; Zhu, G.Y.; Qi, F. Research progress on preparation and comprehensive utilization of solid waste based calcium silicate hydrates. Bull. Chin. Ceram. Soc. 2024, 43, 517–531. [Google Scholar]
- Zeng, L.; He, M.; Mao, D. Influence of hydrothermal synthesis conditions on pore structure of calcium silicate hydrates. Non-Met. Mines 2017, 40, 10–13. [Google Scholar]
- Tajuelo, R.E.; Richardson, I.G. Composition, silicate anion structure and morphology of calcium silicate hydrates (C-S-H) synthesized by silica-lime reaction and by controlled hydration of tricalcium silicate (C3S). Adv. Appl. Ceram. 2015, 114, 362–371. [Google Scholar] [CrossRef]
- Xie, J.; Yang, F.; Tan, N. Calcium sulphoaluminate cement from solid waste with nano-TiO2 addition for high-efficiency CO2 capture. Constr. Build. Mater. 2023, 367, 130267. [Google Scholar]
- Lu, B.; He, P.; Liu, J. Microstructure of Portland cement paste subjected to different CO2 concentrations and further water curing. J. CO2 Util. 2021, 53, 101714. [Google Scholar]
- Khan, R.I.; Ashraf, W.; Olek, J. Amino acids as performance-controlling additives in carbonation-activated cementitious materials. Cem. Concr. Res. 2021, 147, 106501. [Google Scholar]
- Zelić, J.; Rušić, D.; Krstulović, R. Kinetic analysis of thermal decomposition of Ca(OH)2 formed during hydration of commercial Portland cement by DSC. J. Therm. Anal. Calorim. 2002, 67, 613–622. [Google Scholar]
- Francioso, V.; Moro, C.; Martinez-Lage, I. Curing temperature: A key factor that changes the effect of TiO2 nanoparticles on mechanical properties, calcium hydroxide formation and pore structure of cement mortars. Cem. Concr. Comp. 2019, 104, 103374. [Google Scholar]
- Plank, J.; Schönlein, M. Study on the early crystallization of calcium silicate hydrate (C-S-H) in the presence of polycarboxylate superplasticizers. J. Organomet. Chem. 2018, 869, 227–232. [Google Scholar]
- Kanchanason, V.; Plank, J. Role of pH on the structure, composition and morphology of C-S-H–PCE nanocomposites and their effect on early strength development of Portland cement. Cem. Concr. Res. 2017, 102, 90–98. [Google Scholar]
- Black, L.; Breen, C. Structural features of C–S–H (I) and its carbonation in air–a Raman spectroscopic study. Part II: Carbonated phases. J. Am. Ceram. 2007, 90, 908–917. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, S.Y.; Miron, G.D. Effect of alkali hydroxide on calcium silicate hydrate (CSH). Cem. Concr. Res. 2022, 151, 106636. [Google Scholar] [CrossRef]
- Wu, J.; Liao, H.; Ma, Z. Effect of different initial CaO/SiO2 molar ratios and curing times on the preparation and formation mechanism of calcium silicate hydrate. Materials 2023, 16, 717. [Google Scholar] [CrossRef]
- La Plante, E.C.; Oey, T. Enhancing silicate dissolution kinetics in hyperalkaline environments. J. Phys. Chem. C 2019, 123, 3687–3695. [Google Scholar] [CrossRef]
- Garg, N.; Özçelik, V.O. Nanoscale ordering and depolymerization of calcium silicate hydrates in the presence of alkalis. J. Phys. Chem. C 2019, 123, 24873–24883. [Google Scholar] [CrossRef]
- Dupuis, R.; Pellenq, R. Dissociation mechanisms of dissolved alkali silicates in sodium hydroxide. J. Phys. Chem. C 2020, 124, 8288–8294. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Trinh, T.T. Mechanism of the initial stage of silicate oligomerization. J. Am. Chem. Soc. 2011, 133, 6613–6625. [Google Scholar] [CrossRef]
- Li, D.-K.; Zhao, W.-Y. Molecular dynamics study on the chemical bound, physical adsorbed and ultra-confined water molecules in the nano-pore of calcium silicate hydrate. Constr. Build. Mater. 2017, 151, 563–574. [Google Scholar] [CrossRef]



| Sample No. | Ca/Si | pH |
|---|---|---|
| 1.0W | 1.0 | 7.2 |
| 1.0L | 1.0 | 12 |
| 1.0H | 1.0 | 13.5 |
| 1.6W | 1.6 | 7.2 |
| 1.6L | 1.6 | 12 |
| 1.6H | 1.6 | 13.5 |
| 2.3W | 2.3 | 7.2 |
| 2.3L | 2.3 | 12 |
| 2.3H | 2.3 | 13.5 |
| 1.0LC | 1.0 | 12 |
| 1.0HC | 1.0 | 13.5 |
| 1.6WC | 1.6 | 7.2 |
| 1.6LC | 1.6 | 12 |
| 2.3WC | 2.3 | 7.2 |
| 2.3HC | 2.3 | 13.5 |
| Sample No. | MCL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | (%) | (ppm) | (%) | (ppm) | (%) | (ppm) | (%) | (ppm) | (%) | ||
| 1.0 H | −78.8 | 40.2 | −82.3 | 24.6 | −84.5 | 35.2 | / | / | 5.0 | ||
| 2.3 H | −78.4 | 59.0 | −81.8 | 14.2 | −84.7 | 26.7 | / | / | 3.4 | ||
| 2.3 W | −79.2 | 50.2 | −82.1 | 9.3 | −84.9 | 40.5 | / | / | 4.0 | ||
| Sample No. | MCL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | (%) | (ppm) | (%) | (ppm) | (%) | (ppm) | (%) | (ppm) | (%) | ||
| 1.0 H | −78.8 | 40.2 | −82.3 | 24.6 | −84.5 | 35.2 | / | / | 5.0 | ||
| 2.3 H | −78.4 | 59.0 | −81.8 | 14.2 | −84.7 | 26.7 | / | / | 3.4 | ||
| 1.0 HC | −79.1 | 1.5 | −82.0 | 0.8 | −85.0 | 18.9 | −99.0 | 55.2 | −109.7 | 23.6 | 135.2 |
| 2.3 HC | −79.3 | 7.8 | −82.7 | 0.8 | −84.9 | 37.4 | −97.4 | 42.6 | −108.9 | 11.4 | 25.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Zhang, K.; Xie, G.; Shi, K.; Zhu, Y. Factors Affecting Synthesized C-S-H CO2 Uptake: Initial Alkalinity and Ca/Si. Buildings 2025, 15, 1264. https://doi.org/10.3390/buildings15081264
Gong J, Zhang K, Xie G, Shi K, Zhu Y. Factors Affecting Synthesized C-S-H CO2 Uptake: Initial Alkalinity and Ca/Si. Buildings. 2025; 15(8):1264. https://doi.org/10.3390/buildings15081264
Chicago/Turabian StyleGong, Jingwei, Kai Zhang, Gangchuan Xie, Kebin Shi, and Ying Zhu. 2025. "Factors Affecting Synthesized C-S-H CO2 Uptake: Initial Alkalinity and Ca/Si" Buildings 15, no. 8: 1264. https://doi.org/10.3390/buildings15081264
APA StyleGong, J., Zhang, K., Xie, G., Shi, K., & Zhu, Y. (2025). Factors Affecting Synthesized C-S-H CO2 Uptake: Initial Alkalinity and Ca/Si. Buildings, 15(8), 1264. https://doi.org/10.3390/buildings15081264





