Comparative Study on the Foaming and Fireproof Properties of PDMS Foam Composites with Different Inorganic Fillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
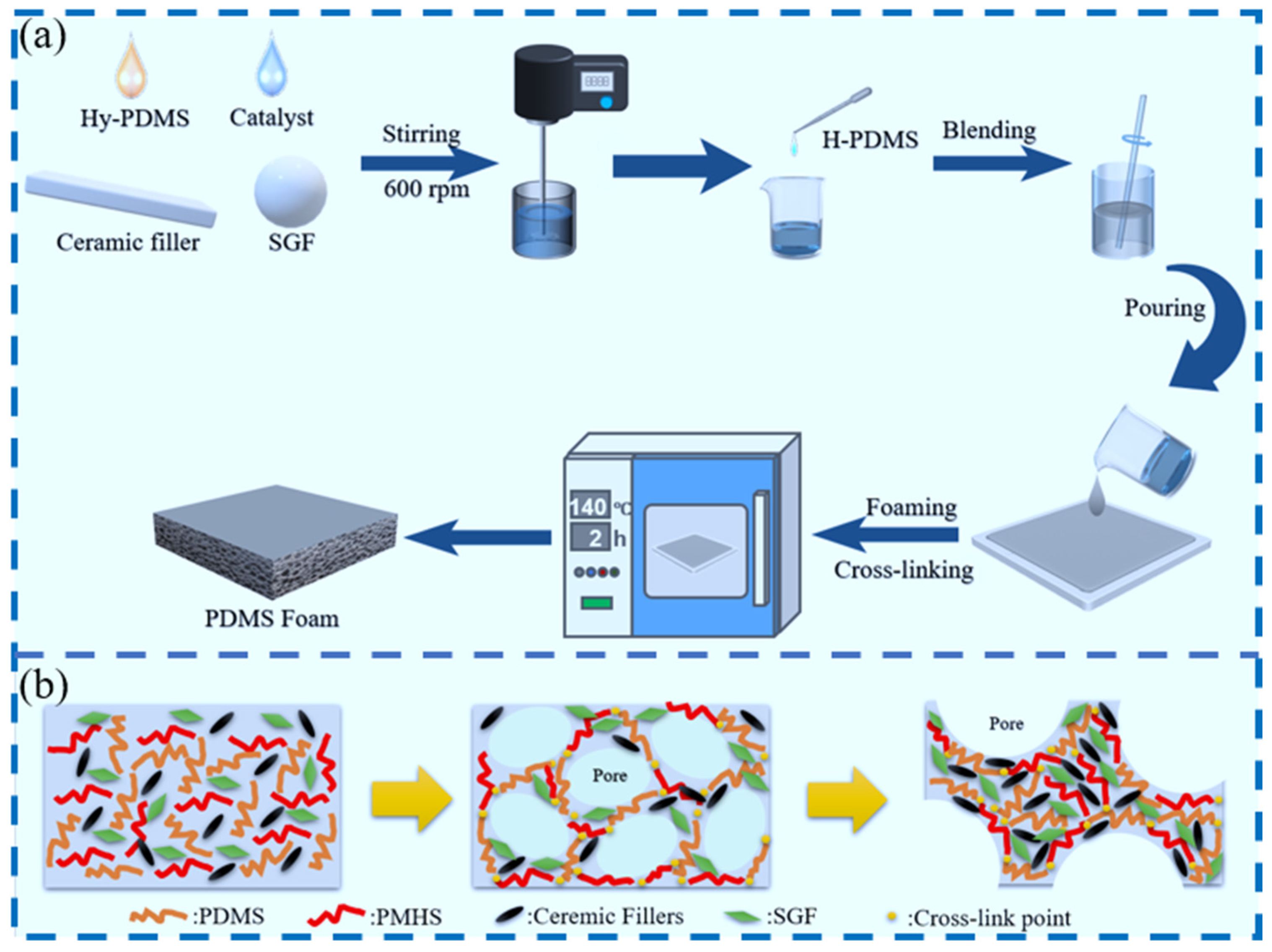
2.2. Preparation of PDMS Foam
2.3. Characterization
2.3.1. Apparent Density Test
2.3.2. Cell Diameter Test
2.3.3. Porosity Test
2.3.4. Open-Cell Content Test
2.3.5. Rotational Viscosity Test
2.3.6. Mechanical Test
2.3.7. X-Ray Diffraction (XRD)
2.3.8. X-Ray Fluorescence (XRF)
2.3.9. Thermogravimetric Analysis (TGA)
2.3.10. Scanning Electron Microscope (SEM)
2.3.11. Ceramification Test
2.3.12. Fireproof Performance Test
3. Results and Discussion
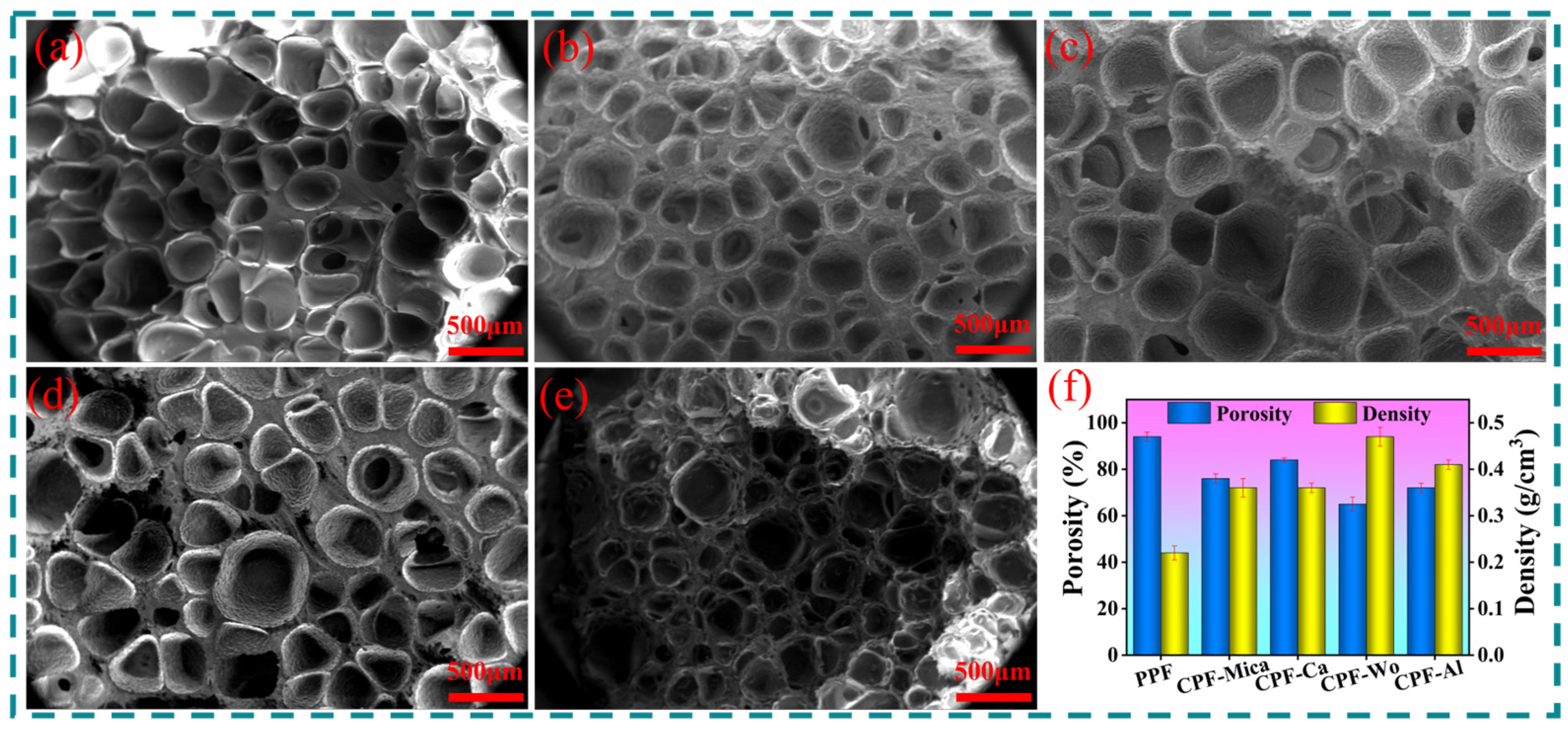
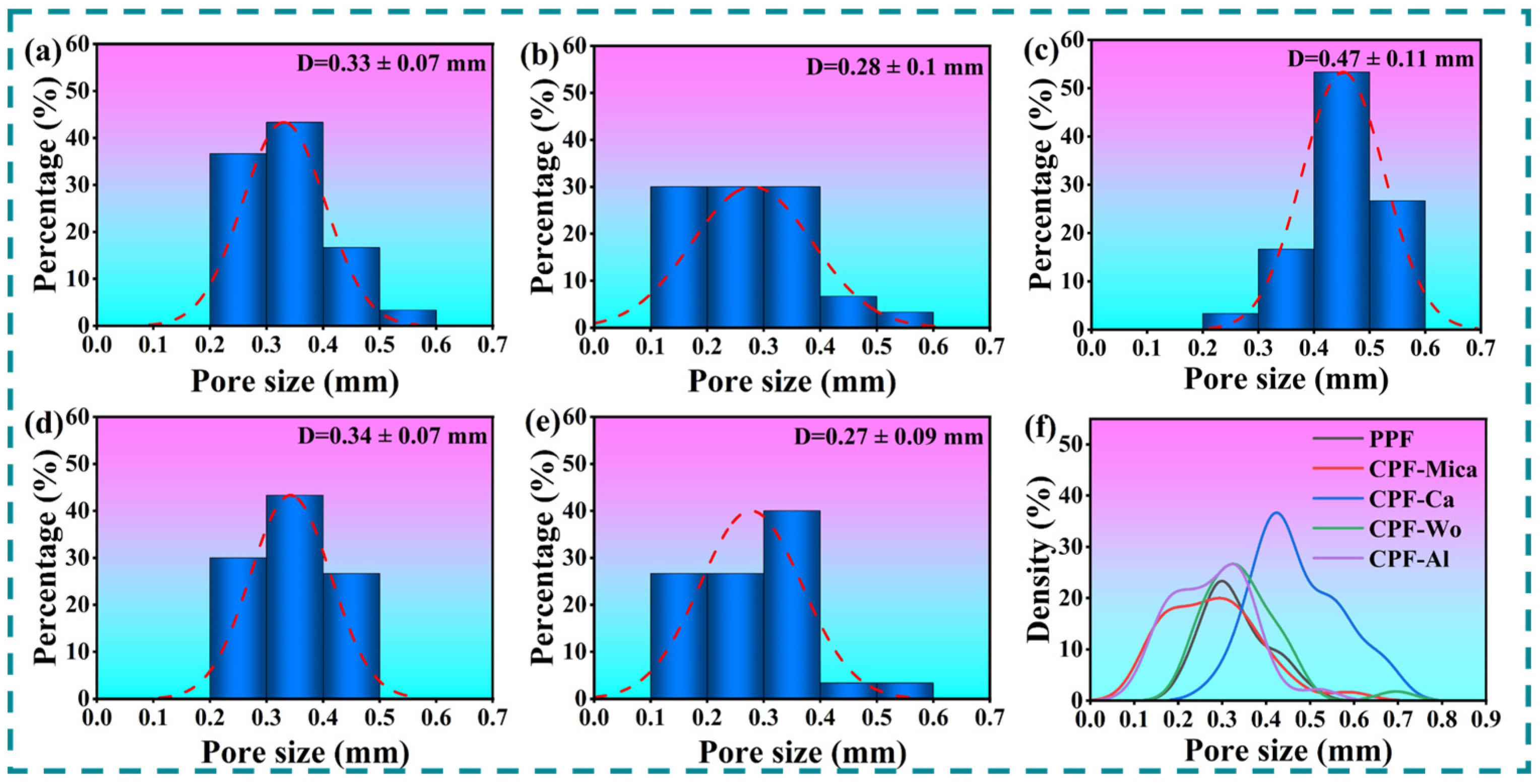
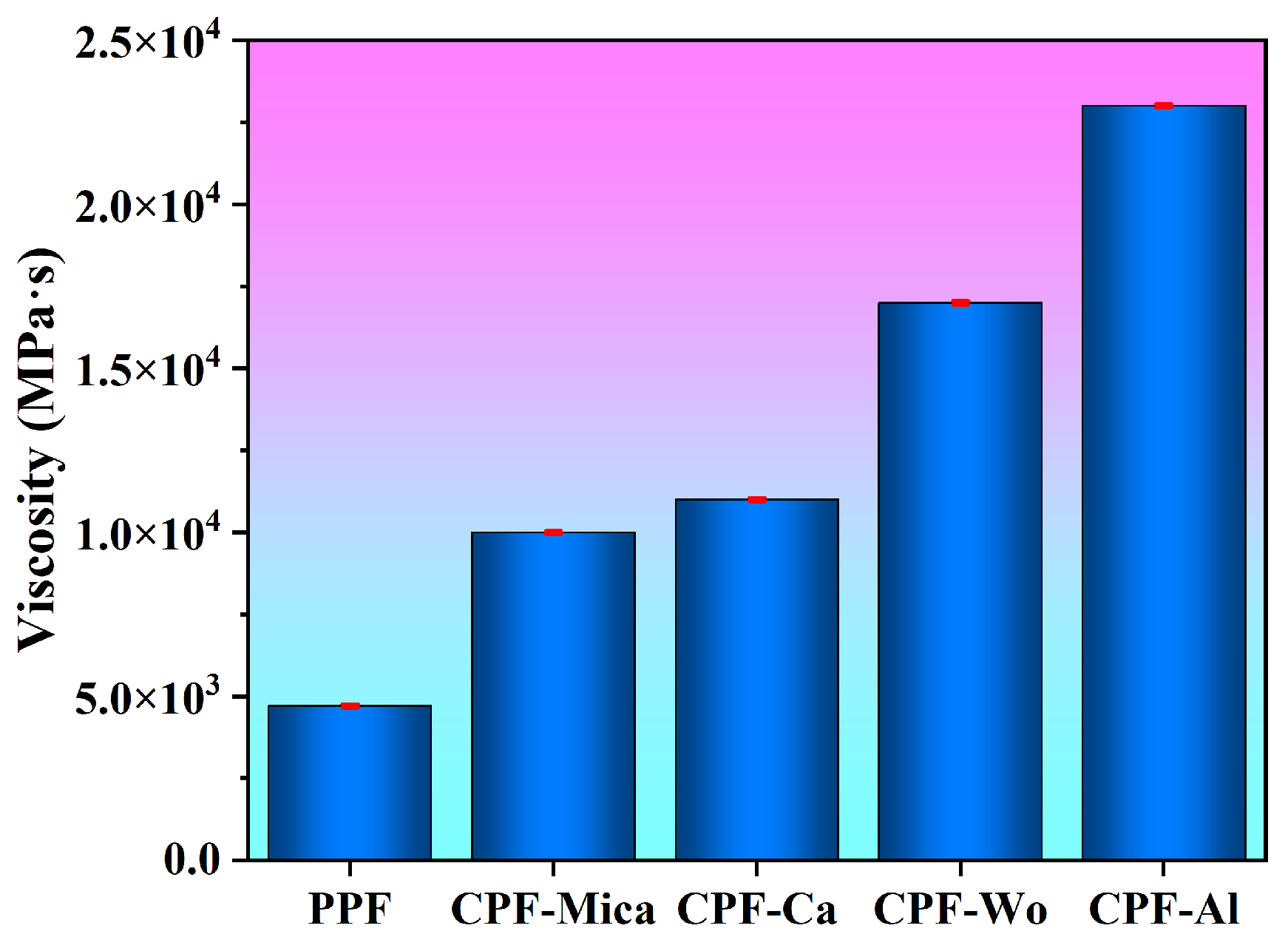
3.1. Analysis of Foaming Properties
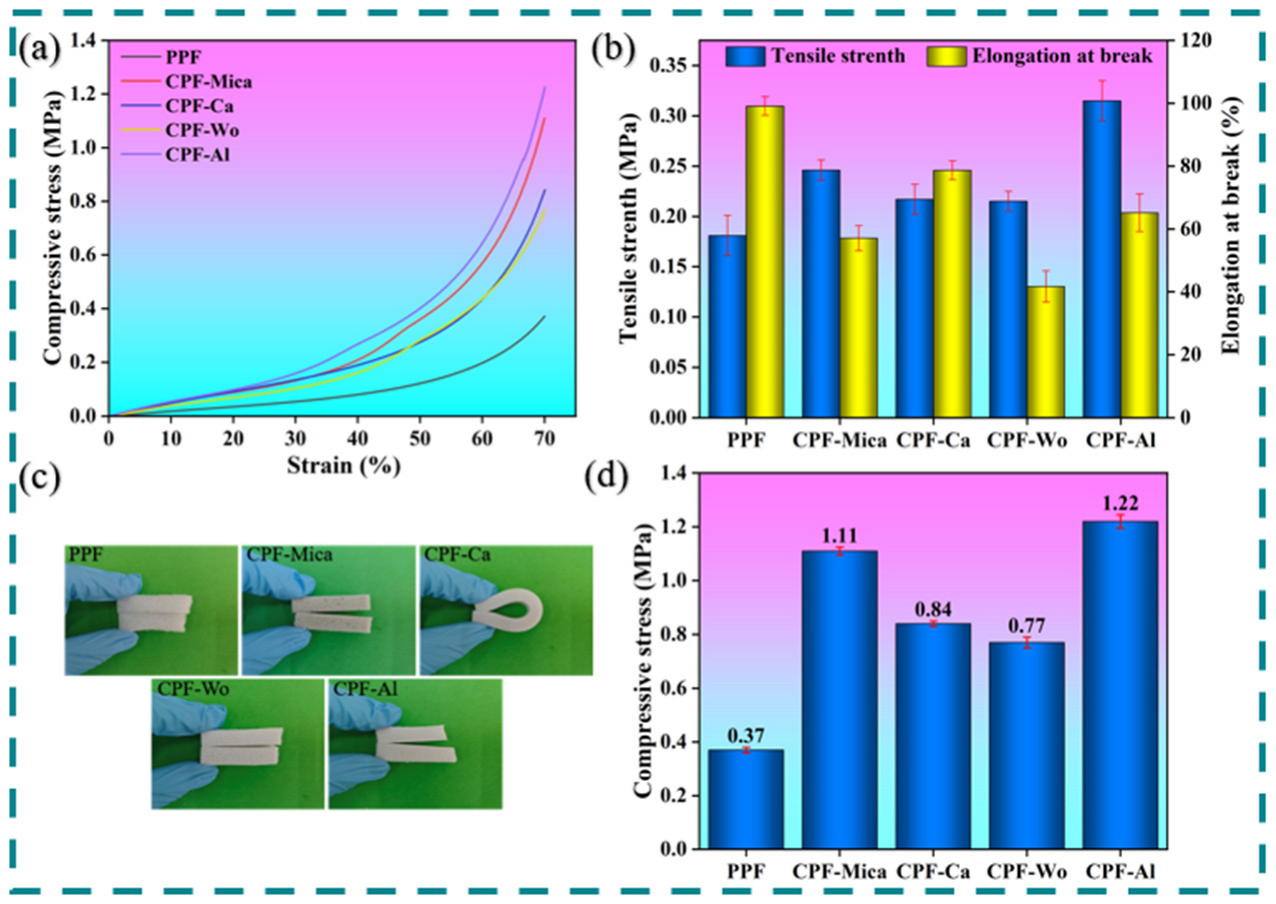
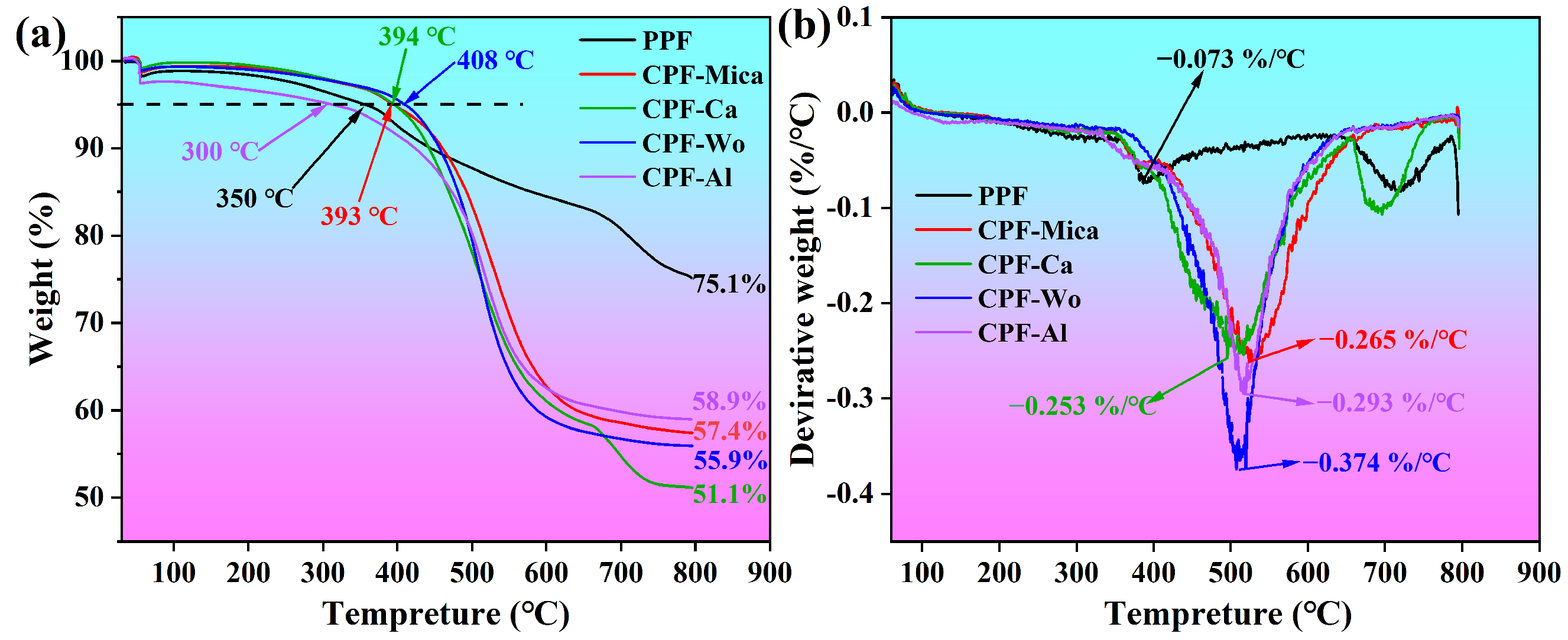
3.2. Analysis of Mechanical and Thermal Performance
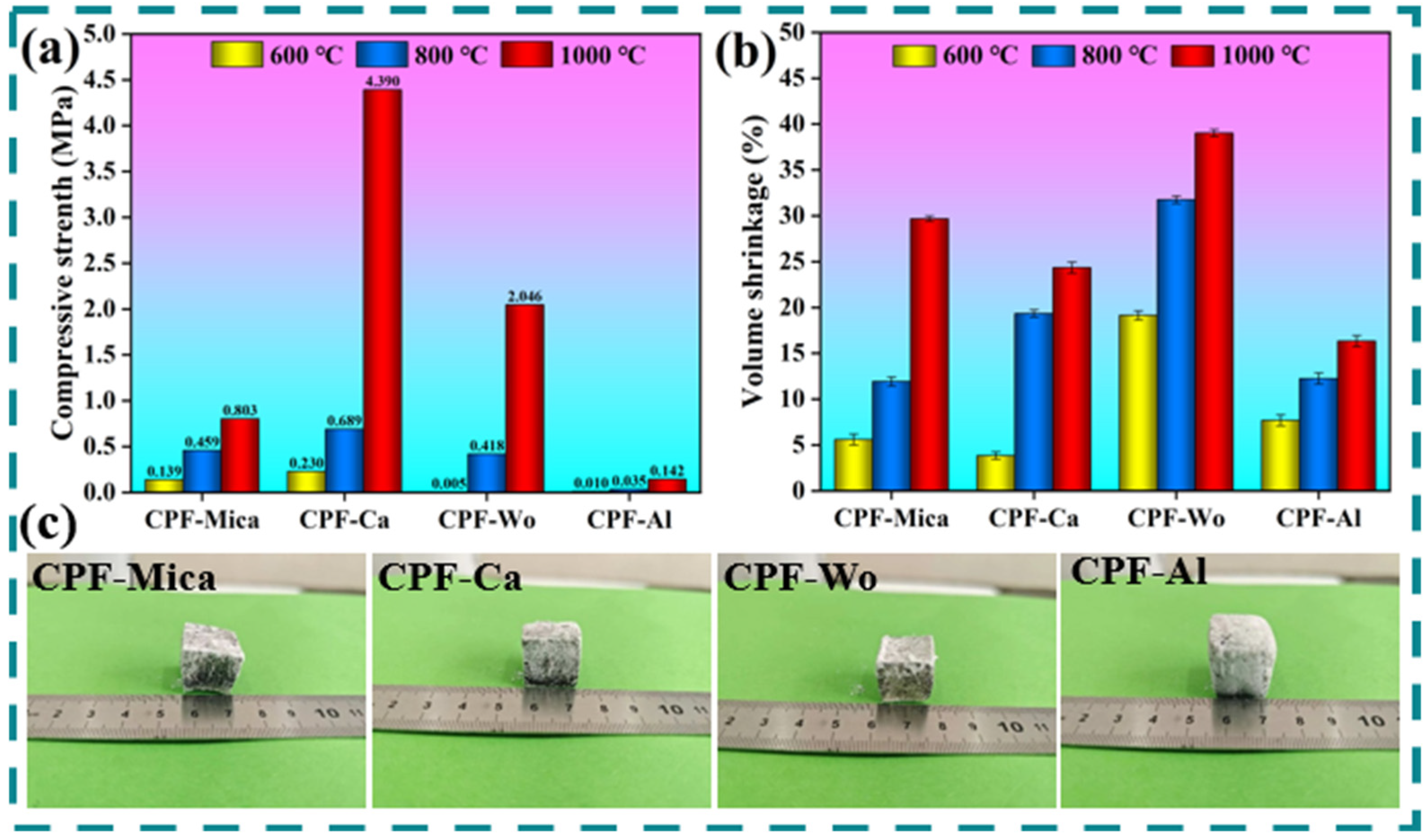
3.3. Analysis of Ceramification Performance
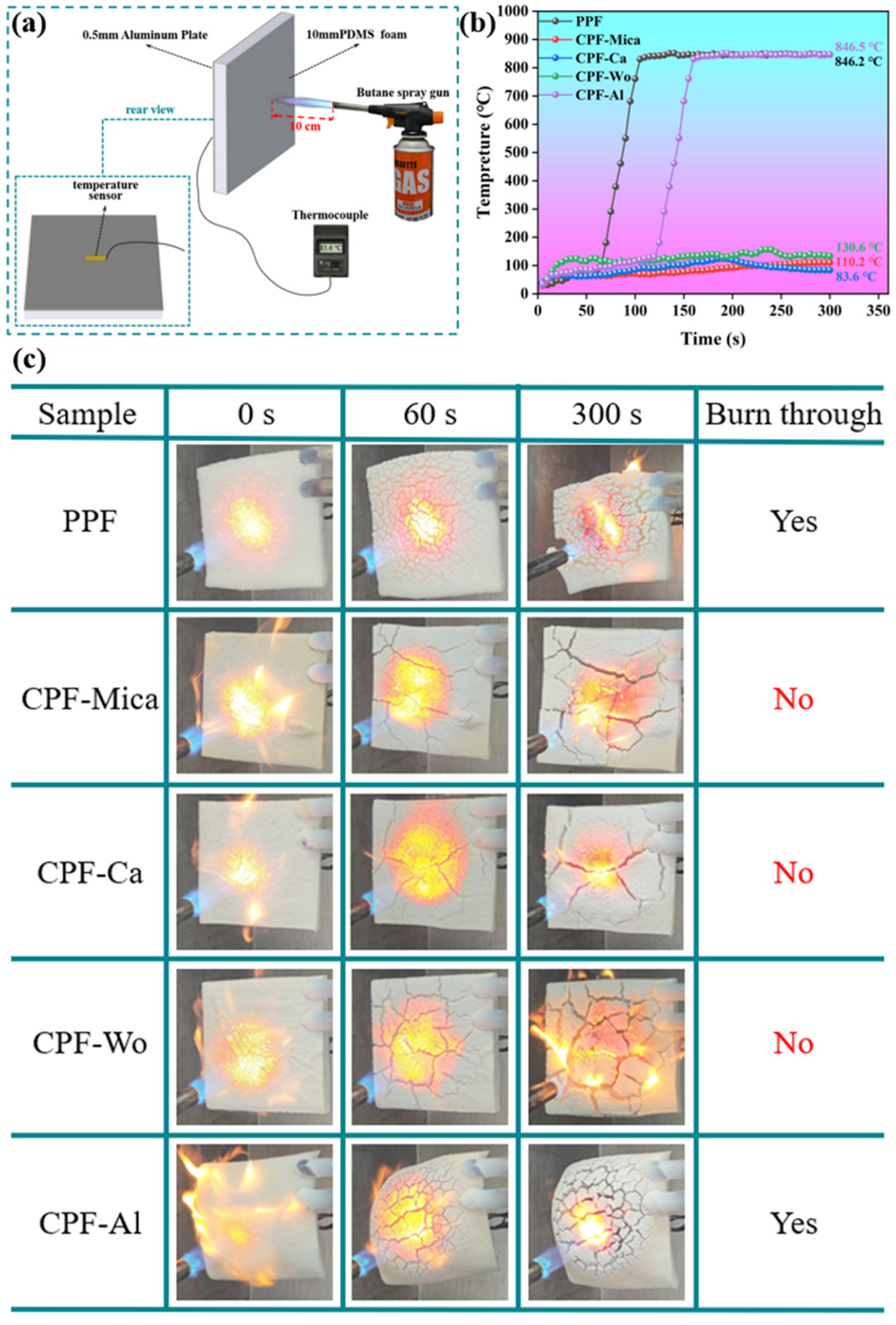
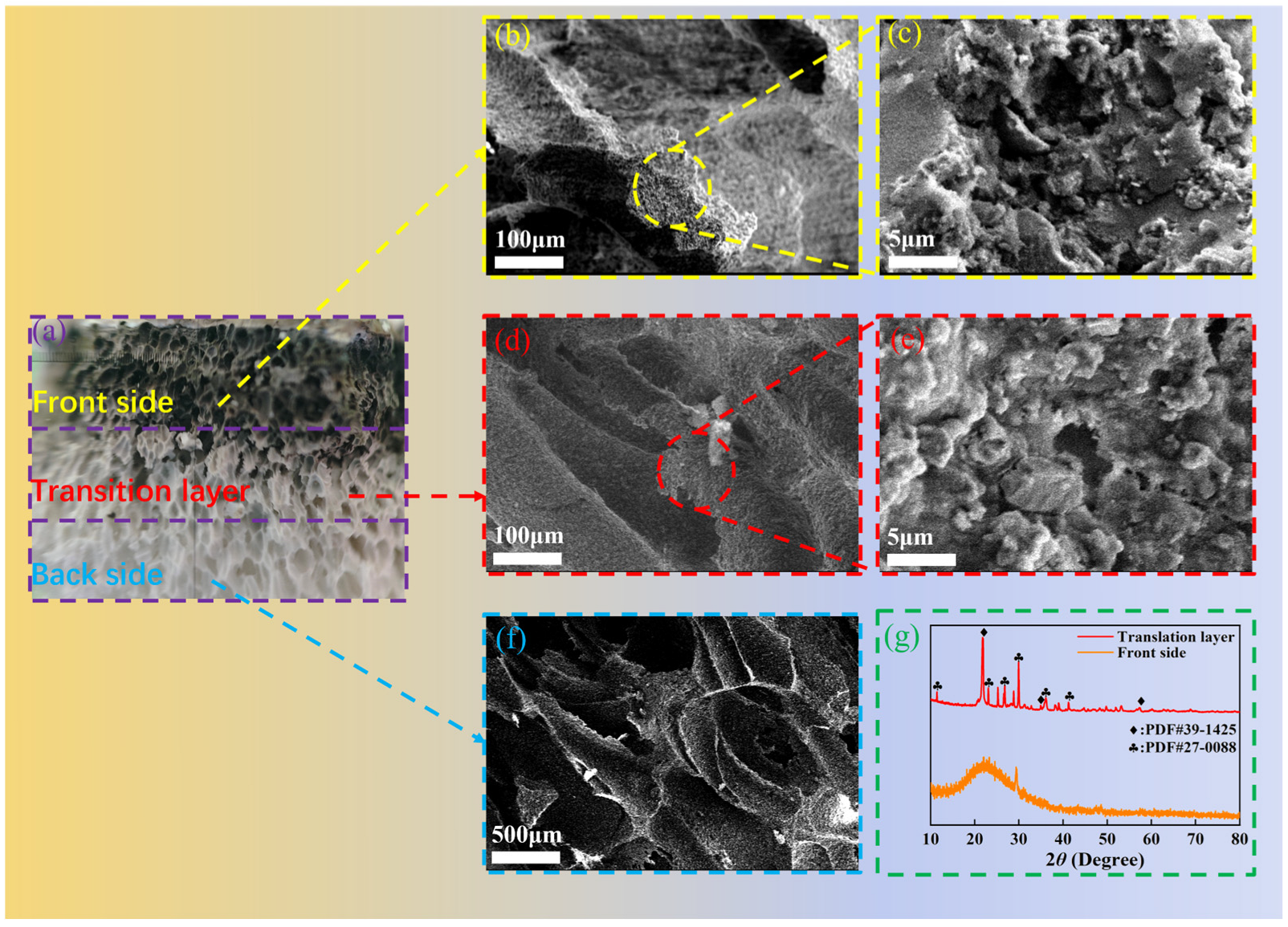
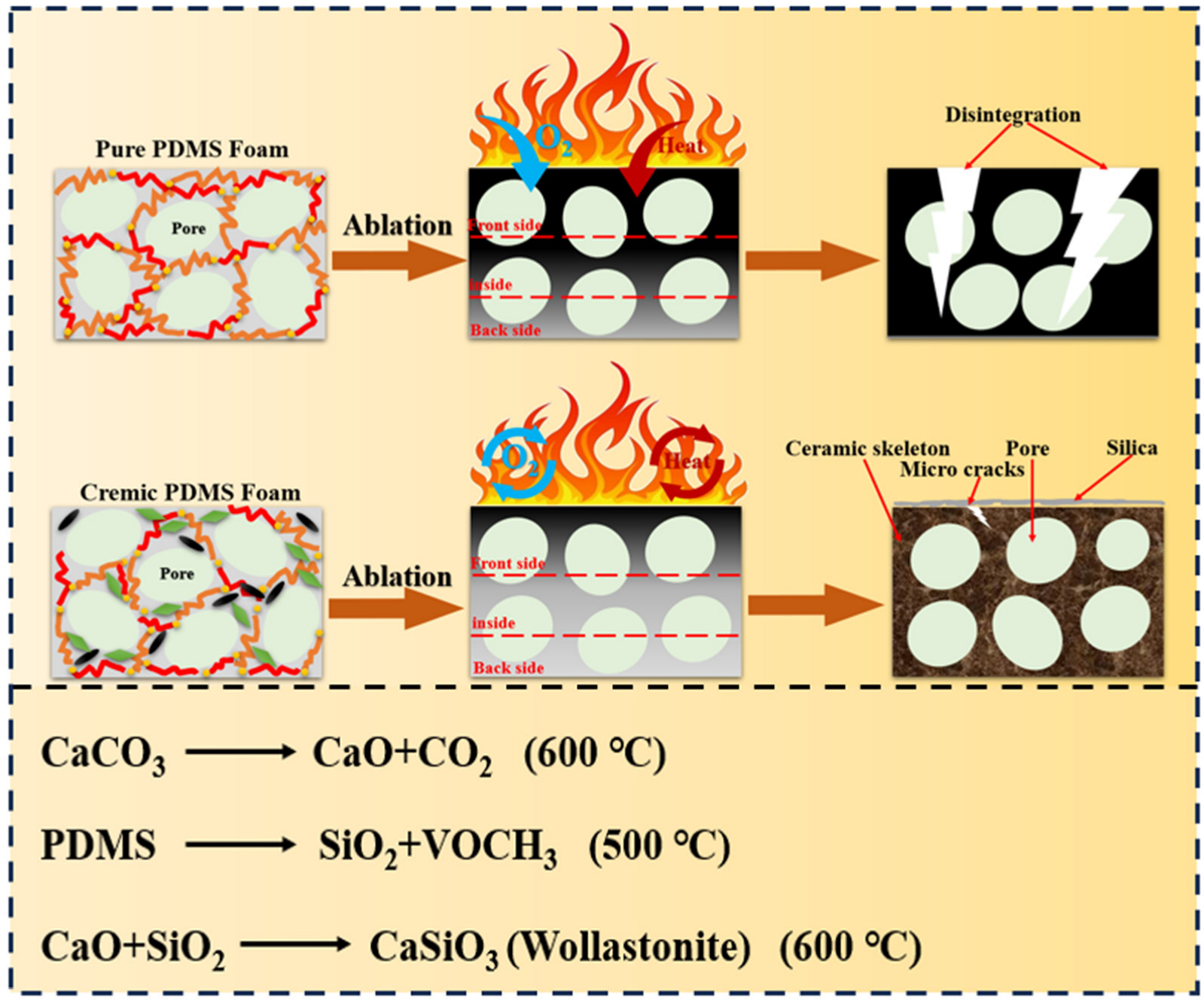
3.4. Analysis of Fireproof Performance and Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, S.; Deng, X.; Beiyuan, J.; Chen, X.; Liu, D.; Lv, D.; Duan, C.; Lin, L.; Cha, R.; Xie, W. Review of synthetic polymer-based thermal insulation materials in construction and building. J. Build. Eng. 2024, 97, 110846. [Google Scholar]
- Chen, J.; Liu, X.; Tian, Y.; Zhu, W.; Yan, C.; Shi, Y.; Kong, L.B.; Qi, H.J.; Zhou, K. 3D-Printed anisotropic polymer materials for functional applications. Adv. Mater. 2022, 34, 2102877. [Google Scholar]
- Yuen, A.C.Y.; Chen, T.B.Y.; Cordero, I.M.D.C.; Liu, H.; Li, A.; Yang, W.; Cheung, S.C.P.; Chan, Q.N.; Kook, S.; Yeoh, G.H. Developing a solid decomposition kinetics extraction framework for detailed chemistry pyrolysis and combustion modelling of building polymer composites. J. Anal. Appl. Pyrolysis 2022, 163, 105500. [Google Scholar]
- Ao, X.; Vázquez-López, A.; Mocerino, D.; González, C.; Wang, D.-Y. Flame retardancy and fire mechanical properties for natural fiber/polymer composite: A review. Compos. Part B Eng. 2024, 268, 111069. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Li, H.; Zeng, X.; Lin, J.; Wu, J.; Zhou, Y.; Chen, G.; Lai, X. Multifunctional ceramifiable silicone foam for smart fire fighting. Chem. Eng. J. 2024, 496, 154149. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Morgan, A.B. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Deng, Z.; Shi, M.; Huang, Z.; Yu, X.; Wu, Y.; Yang, X. Oxidation resistance, ablation resistance and in situ ceramization mechanism of Al-coated carbon fiber/boron phenolic resin ceramizable composites modified with Ti3SiC2. Ceram. Int. 2023, 49, 32490–32502. [Google Scholar]
- Yang, C.; Gong, M.; Yang, F.; Liang, J.; Jin, P. Molecular Dynamics Simulation of Ceramic Polyolefin for Fire Resistant Cable. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012025. [Google Scholar]
- Wang, Y.; Lai, X.; Li, H.; Liu, T.; Zeng, X. Significantly improve fire safety of silicone rubber by efficiently catalyzing ceramization on fluorophlogopite. Compos. Commun. 2021, 25, 100683. [Google Scholar] [CrossRef]
- Liu, T.; Yao, Y.; Zhao, D.; Hu, F.; Yun, C.; Jiang, G.; Shen, Y.; Wang, T. Improving thermal insulation and fire resistance of ceramifiable EVA/ceramic hybrid composites via low temperature sintering and foaming strategy. Ceram. Int. 2024, 50, 6207–6219. [Google Scholar]
- Liu, L.; Xiang, D.; Wu, L. Improved thermal conductivity of ceramic-epoxy composites by constructing vertically aligned nanoflower-like AlN network. Ceram. Int. 2022, 48, 10438–10446. [Google Scholar]
- Li, Y.-M.; Hu, S.-L.; Wang, D.-Y. Polymer-based ceramifiable composites for flame retardant applications: A review. Compos. Commun. 2020, 21, 100405. [Google Scholar]
- Ma, Z.; Zhang, J.; Maluk, C.; Yu, Y.; Seraji, S.M.; Yu, B.; Wang, H.; Song, P. A lava-inspired micro/nano-structured ceramifiable organic-inorganic hybrid fire-extinguishing coating. Matter 2022, 5, 911–932. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, M.; Zhu, J.; Liu, Z.; Geng, Y.; Xie, C.; Chen, X.; Liu, L.; Song, P. A ceramifiable organic-inorganic fire extinguishing hybrid coating capable of cyclic fire-warning. Polym. Degrad. Stab. 2025, 234, 111208. [Google Scholar] [CrossRef]
- Shen, J.; Sun, Q.; Li, L.; Zhang, J.; Sheng, J. Improved shape stability and ceramifiable properties of ceramifiable polyethylene composites by crystallization reaction. Polym. Degrad. Stab. 2022, 200, 109965. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Guo, L.; Xie, W.; Zhao, B.; Liu, M.; Yin, C.; Li, Q. Preparation and performance of ceramifiable flame-retardant silicone sealant. J. Appl. Polym. Sci. 2024, 141, e54860. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Yang, M.; Zhong, S.; Wang, Y.; Zhang, P.; Xia, L. An advanced ceramifiable organic-inorganic hybrid polysiloxane coating with superior moisture-resistant property. J. Eur. Ceram. Soc. 2025, 45, 116924. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wu, Y.Y.; Liu, S.C.; Li, Y.; Guan, Z.Q.; Cao, C.F.; Zhang, G.D.; Tuten, B.T.; Gao, J.F.; Shi, Y.Q. Pottery-Inspired Flexible Fire-Shielding Ceramifiable Silicone Foams for Exceptional Long-Term Thermal Protection. Adv. Funct. Mater. 2025, 35, 2413362. [Google Scholar] [CrossRef]
- Chai, W.; Su, X.; Xia, Y.; Gao, M.; Li, Y.; Liao, C.; Zheng, Z. An eco-friendly, bio-inspired and synergistic flame retardant system with outstanding water tolerance and ultra-low addition for silicone rubber. Constr. Build. Mater. 2023, 365, 130005. [Google Scholar] [CrossRef]
- Chai, W.; Su, X.; Xia, Y.; Gao, M.; Li, Y.; Liao, C.; Zheng, Z. Construction of a novel halogen-free and phosphorus-free and synergistic flame retardant system with low addition in simultaneously improving the flame retardancy, water resistance and mechanical properties for silicone rubber. Appl. Clay Sci. 2023, 238, 106926. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, F.; Fan, Y.; Song, Y.; He, N.; Lai, G.; Gong, Z.; Shen, J. Highly transparent acrylate epoxidized soybean oil based UV–curable silicone–modified coatings with good thermal stability and flame retardancy. Prog. Org. Coat. 2022, 165, 106769. [Google Scholar] [CrossRef]
- Li, Y.-T.; Liu, W.-J.; Shen, F.-X.; Zhang, G.-D.; Gong, L.-X.; Zhao, L.; Song, P.; Gao, J.-F.; Tang, L.-C. Processing, thermal conductivity and flame retardant properties of silicone rubber filled with different geometries of thermally conductive fillers: A comparative study. Compos. Part B Eng. 2022, 238, 109907. [Google Scholar] [CrossRef]
- Deng, J.; Kang, F.R.; Xiao, Y.; Shu, C.M.; Wang, W.F.; Laiwang, B.; Liu, Z.C. Effects of platinum compounds/superfine aluminum hydroxide/ultrafine calcium carbonate on the flame retardation and smoke suppression of silicone foams. J. Appl. Polym. Sci. 2020, 137, 47679. [Google Scholar]
- Zhao, D.; Liu, T.; Xu, Y.; Zhang, J.; Shen, Y.; Wang, T. Investigation of the thermal degradation kinetics of ceramifiable silicone rubber-based composite. J. Therm. Anal. Calorim. 2023, 148, 6487–6499. [Google Scholar] [CrossRef]
- Wang, J.; Ji, C.; Yan, Y.; Zhao, D.; Shi, L. Mechanical and ceramifiable properties of silicone rubber filled with different inorganic fillers. Polym. Degrad. Stab. 2015, 121, 149–156. [Google Scholar]
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Parys, G.; Rybiński, P.; Zarzecka-Napierała, M.; Imiela, M.; Gozdek, T.; Siciński, M.; Okraska, M. Effect of mineral filler additives on flammability, processing and use of silicone-based ceramifiable composites. Polym. Bull. 2018, 75, 1731–1751. [Google Scholar]
- Xu, Y.; Zhao, D.; Fang, X.; Jiang, G.; Shen, Y.; Wang, T. “Mica/silicate glass frit-armored skeleton” in PDMS composite foam for improving fire-proofing performance. J. Appl. Polym. Sci. 2023, 140, e53938. [Google Scholar]
- ASTM D3574-2022; Standard Test Methods for Flexible Cellular Materials—Slab, Bonded, and Molded Urethane Foams. ASTM International: West Conshohocken, PA, USA, 2022.
- GB/T 2794-2013; Test Method for Viscosity Determination of Adhesives with Rotational Viscometers (Rotational Viscometer Method). Standards Press of China: Beijing, China, 2013.
- ASTM D1056-19; Standard Specification for Flexible Cellular Materials—Sponge or Expanded Rubber. ASTM International: West Conshohocken, PA, USA, 2019.
- Ataei, M.; Shaayegan, V.; Wang, C.; Costa, F.; Han, S.; Park, C.B.; Bussmann, M. Numerical analysis of the effect of the local variation of viscosity on bubble growth and deformation in polymer foaming. J. Rheol. 2019, 63, 895–903. [Google Scholar]
- Li, L.; Jia, C.; Liu, Y.; Fang, B.; Zhu, W.; Li, X.; Schaefer, L.A.; Li, Z.; Zhang, F.; Feng, X. Nanograin–glass dual-phasic, elasto-flexible, fatigue-tolerant, and heat-insulating ceramic sponges at large scales. Mater. Today 2022, 54, 72–82. [Google Scholar] [CrossRef]

| Sample | Hy-PDMS a | Vi- PDMS b | H-PDMS c | Mica | CaCO3 | Wo | Al2O3 | SGF | Pt Catalyst |
|---|---|---|---|---|---|---|---|---|---|
| PPF | 70 | 20 | 10 | 0 | 0 | 0 | 0 | 0 | 0.8 |
| CPF-Mica | 70 | 20 | 10 | 40 | 0 | 0 | 0 | 20 | 0.8 |
| CPF-Ca | 70 | 20 | 10 | 0 | 40 | 0 | 0 | 20 | 0.8 |
| CPF-Wo | 70 | 20 | 10 | 0 | 0 | 40 | 0 | 20 | 0.8 |
| CPF-Al | 70 | 20 | 10 | 0 | 0 | 0 | 40 | 20 | 0.8 |
| Element | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | Fe2O3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Mica | 1.53 | 1.20 | 22.33 | 61.01 | 7.90 | 2.80 | 0.31 | 2.35 | 0.56 |
| Wo | / | 2.20 | 0.10 | 47.56 | 0.01 | 49.80 | / | 0.16 | 0.17 |
| SGF | 17.64 | 0.16 | 5.69 | 55.69 | 4.46 | 6.98 | 8.89 | 0.18 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Yang, M.; Hu, F.; Jiang, G.; Shen, Y. Comparative Study on the Foaming and Fireproof Properties of PDMS Foam Composites with Different Inorganic Fillers. Buildings 2025, 15, 1172. https://doi.org/10.3390/buildings15071172
He X, Yang M, Hu F, Jiang G, Shen Y. Comparative Study on the Foaming and Fireproof Properties of PDMS Foam Composites with Different Inorganic Fillers. Buildings. 2025; 15(7):1172. https://doi.org/10.3390/buildings15071172
Chicago/Turabian StyleHe, Xin, Mengmeng Yang, Fangzhou Hu, Guodong Jiang, and Yucai Shen. 2025. "Comparative Study on the Foaming and Fireproof Properties of PDMS Foam Composites with Different Inorganic Fillers" Buildings 15, no. 7: 1172. https://doi.org/10.3390/buildings15071172
APA StyleHe, X., Yang, M., Hu, F., Jiang, G., & Shen, Y. (2025). Comparative Study on the Foaming and Fireproof Properties of PDMS Foam Composites with Different Inorganic Fillers. Buildings, 15(7), 1172. https://doi.org/10.3390/buildings15071172





