Performance and Microstructure Characterization of Grouting Materials for Tailings Mined-Out Area Prepared by All-Solid Waste
Abstract
1. Introduction
2. Materials
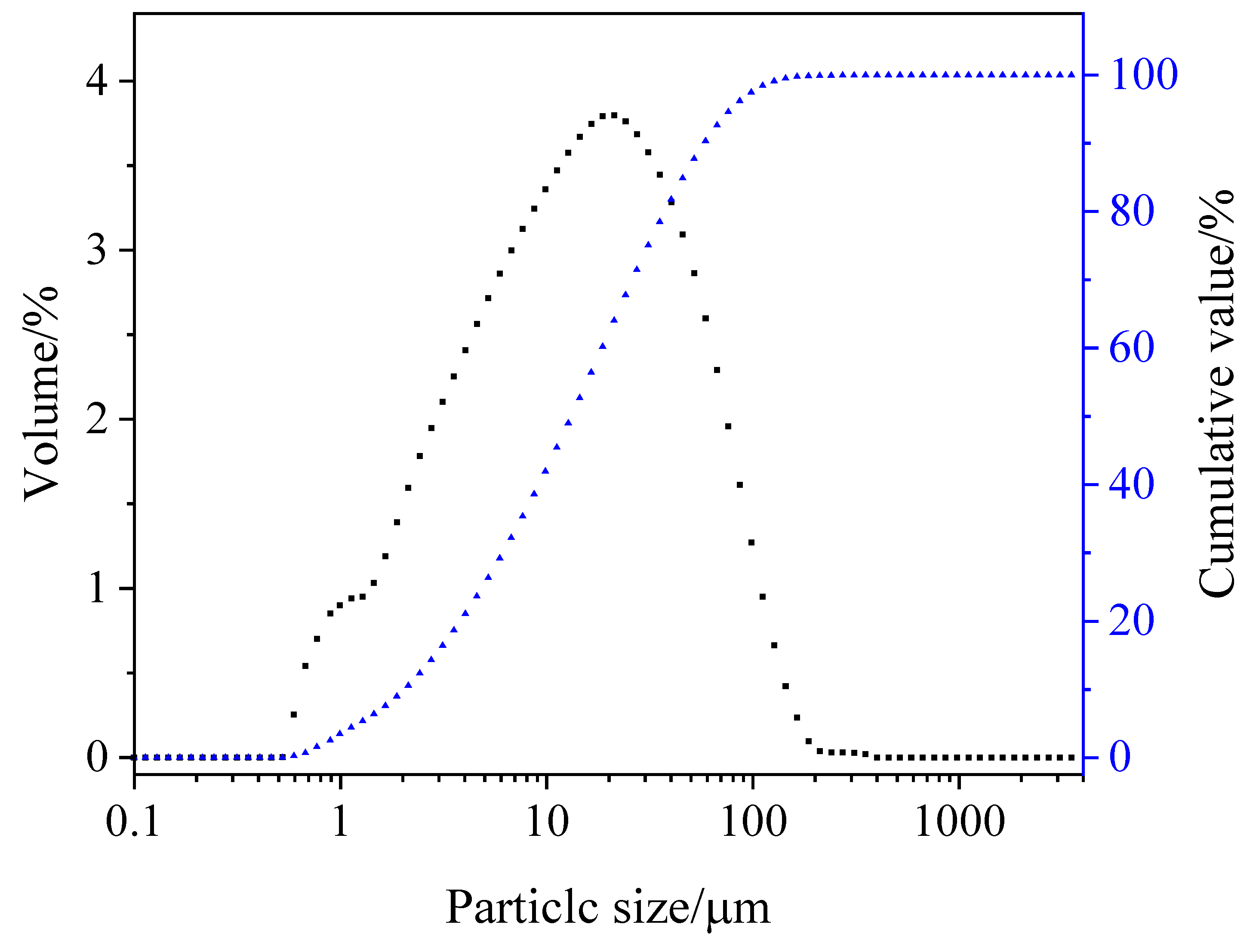
2.1. Coal Gangue
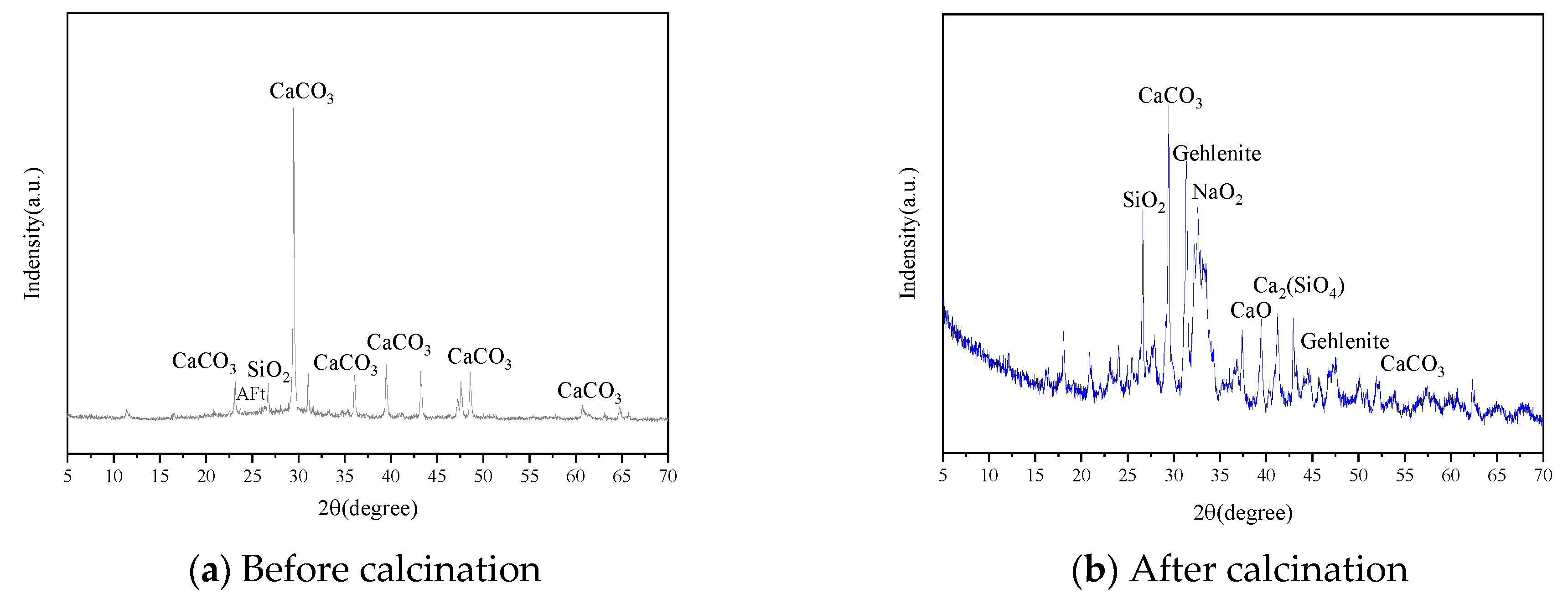
2.2. Alkali Activators
2.3. Water
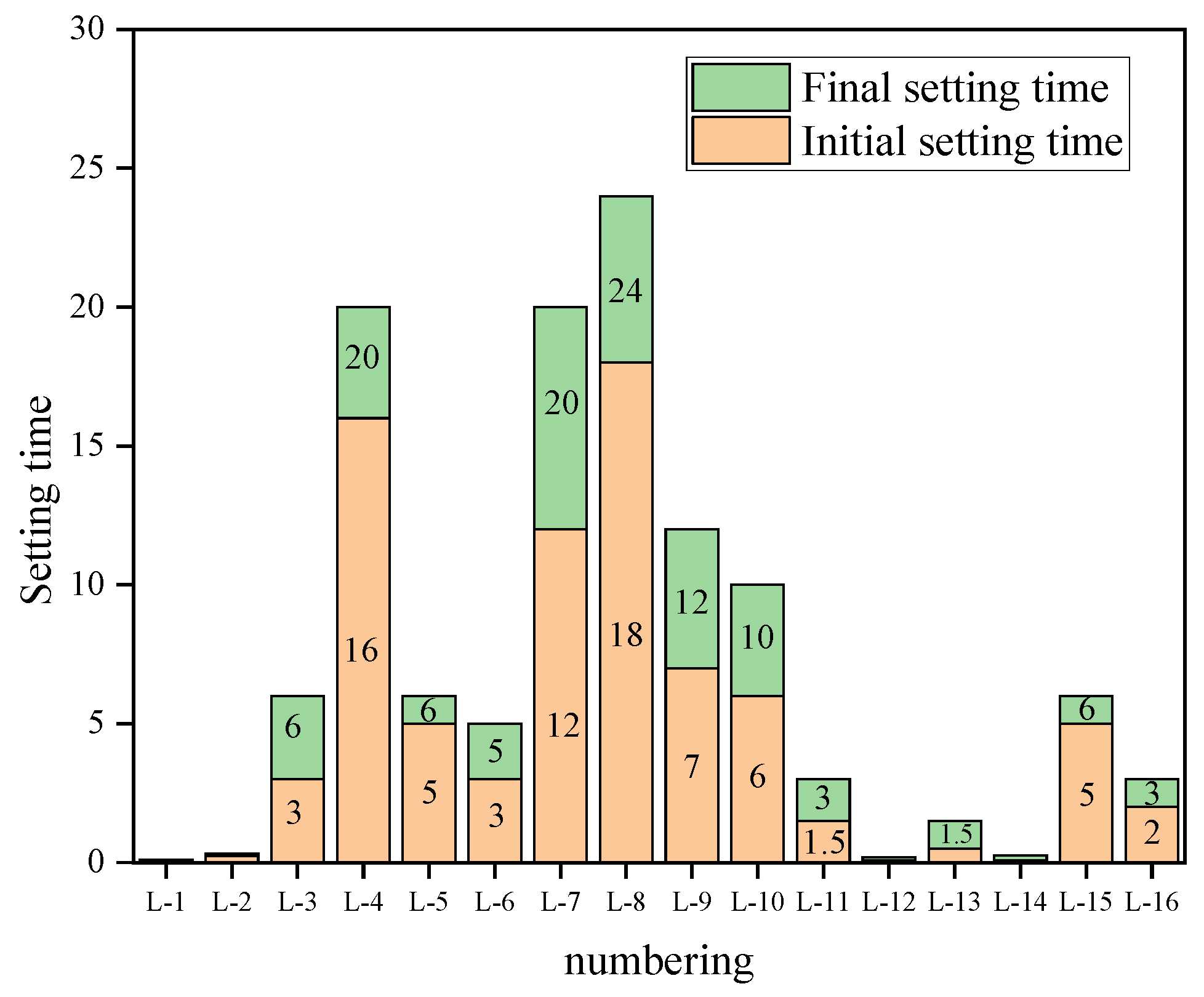
2.4. Research Methodology for Performance Testing
3. Test Scheme
3.1. Mix Design
3.2. Specimen Preparation
4. Results and Analysis
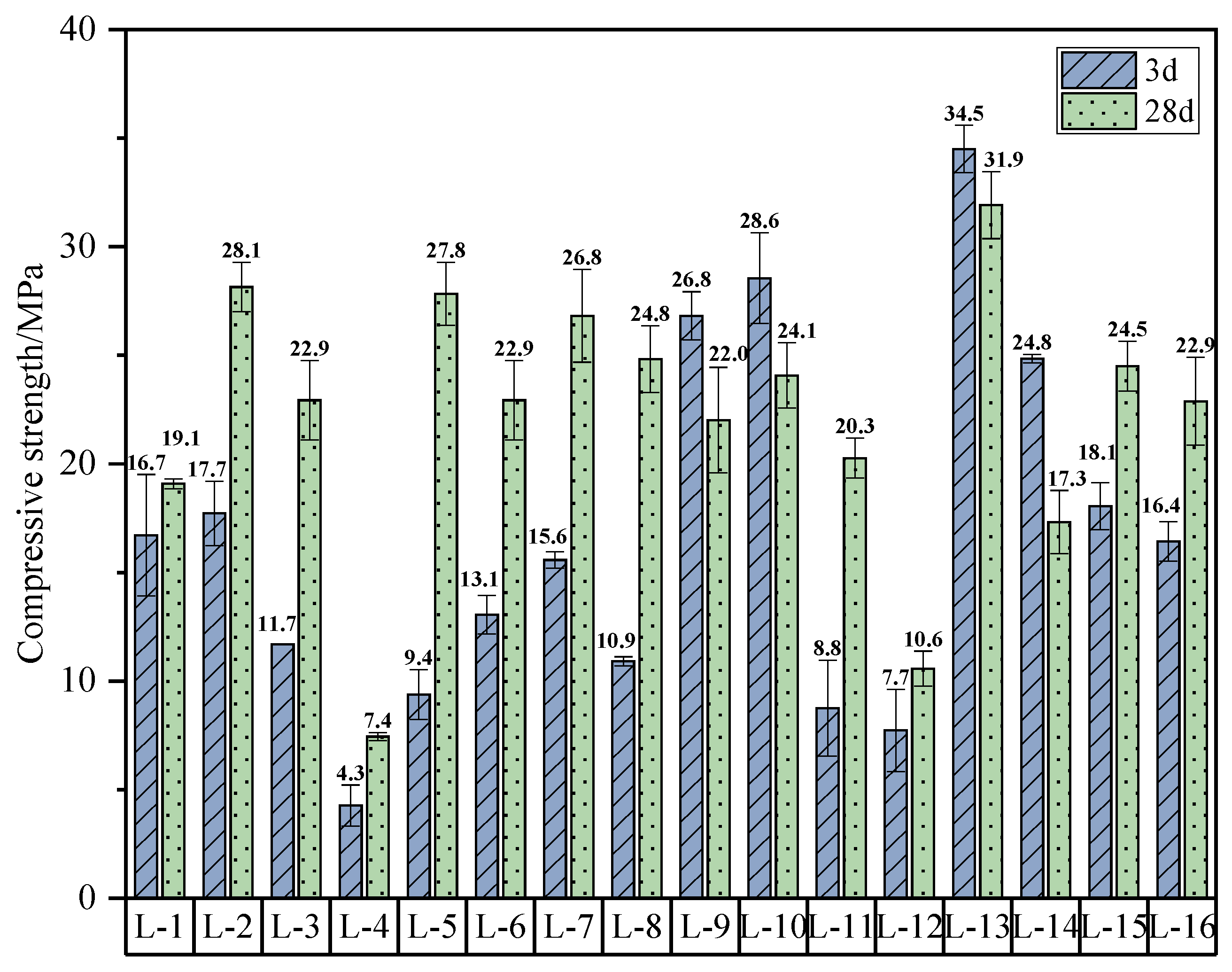
4.1. CS
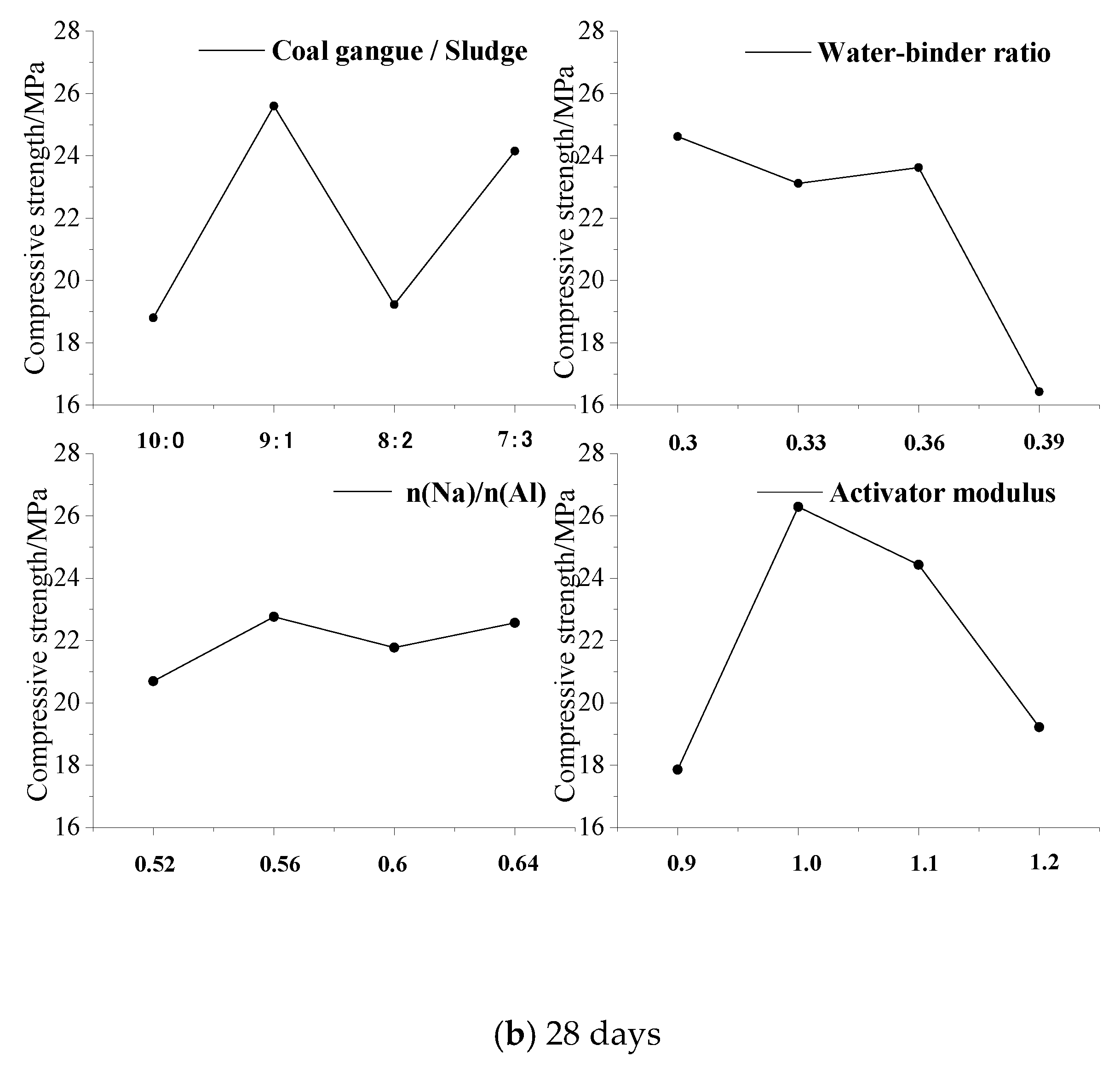
4.1.1. Range Analysis
4.1.2. Sensitivity Analysis
4.1.3. Analysis of Variance
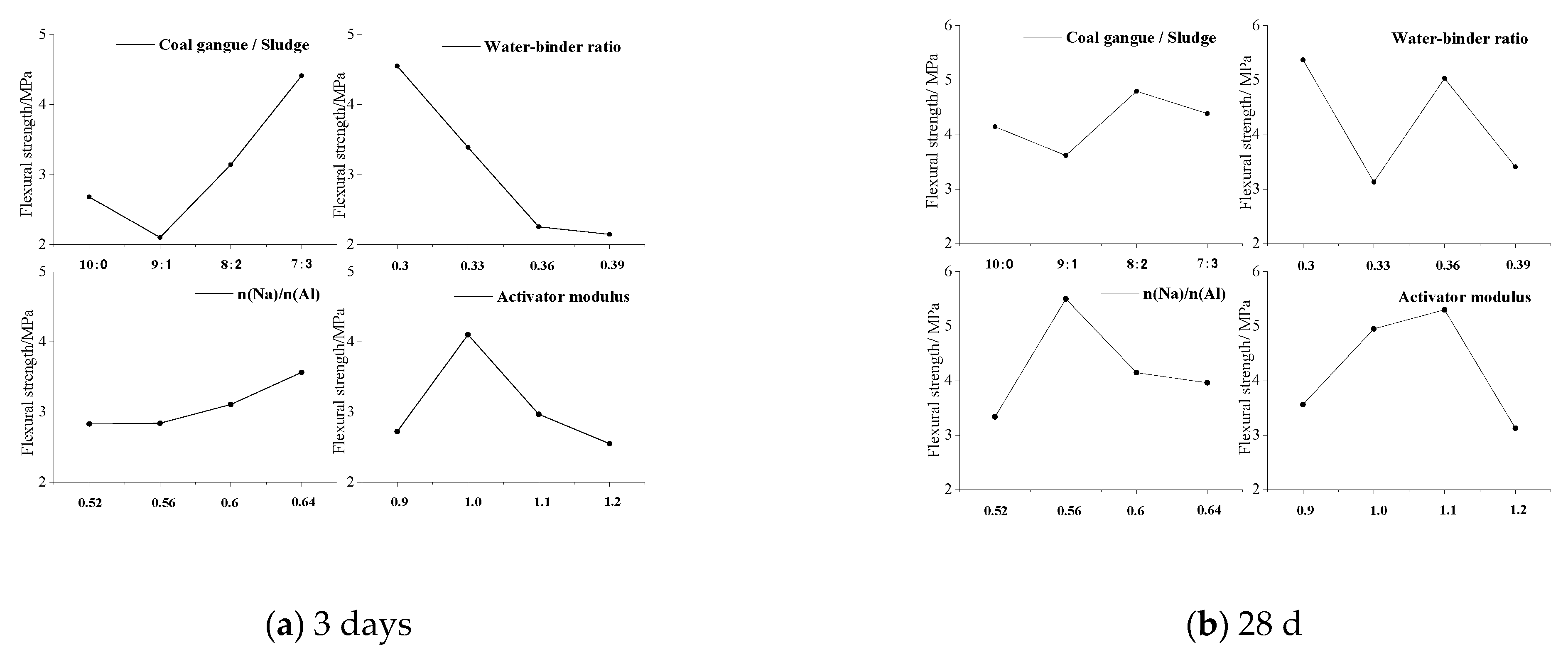
4.2. Flexural Strength
4.2.1. Range Analysis
4.2.2. Sensitivity Analysis
4.2.3. Analysis of Variance
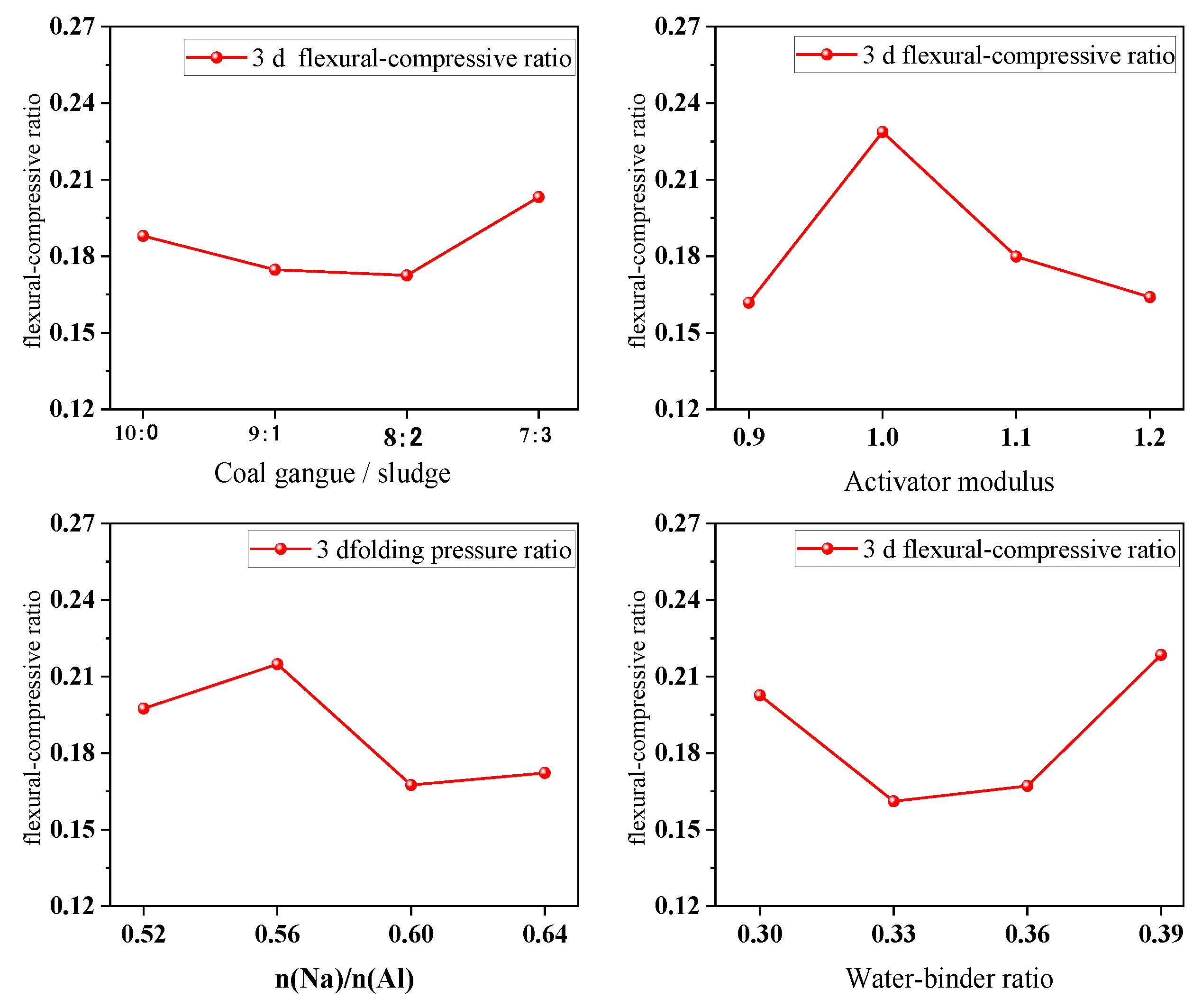
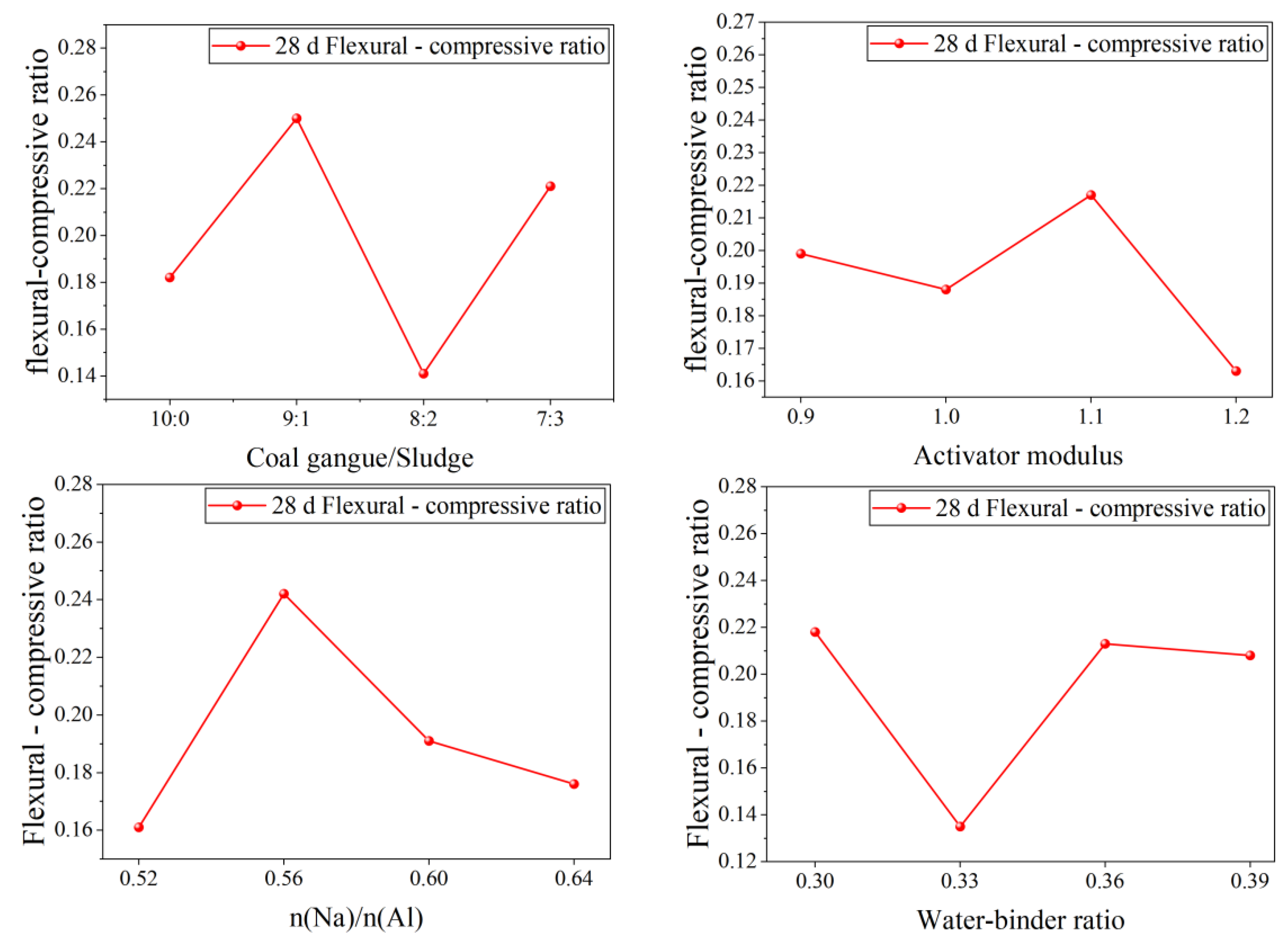
4.3. Flexural–Compressive Ratio
4.4. Microscopic Mechanism Analysis
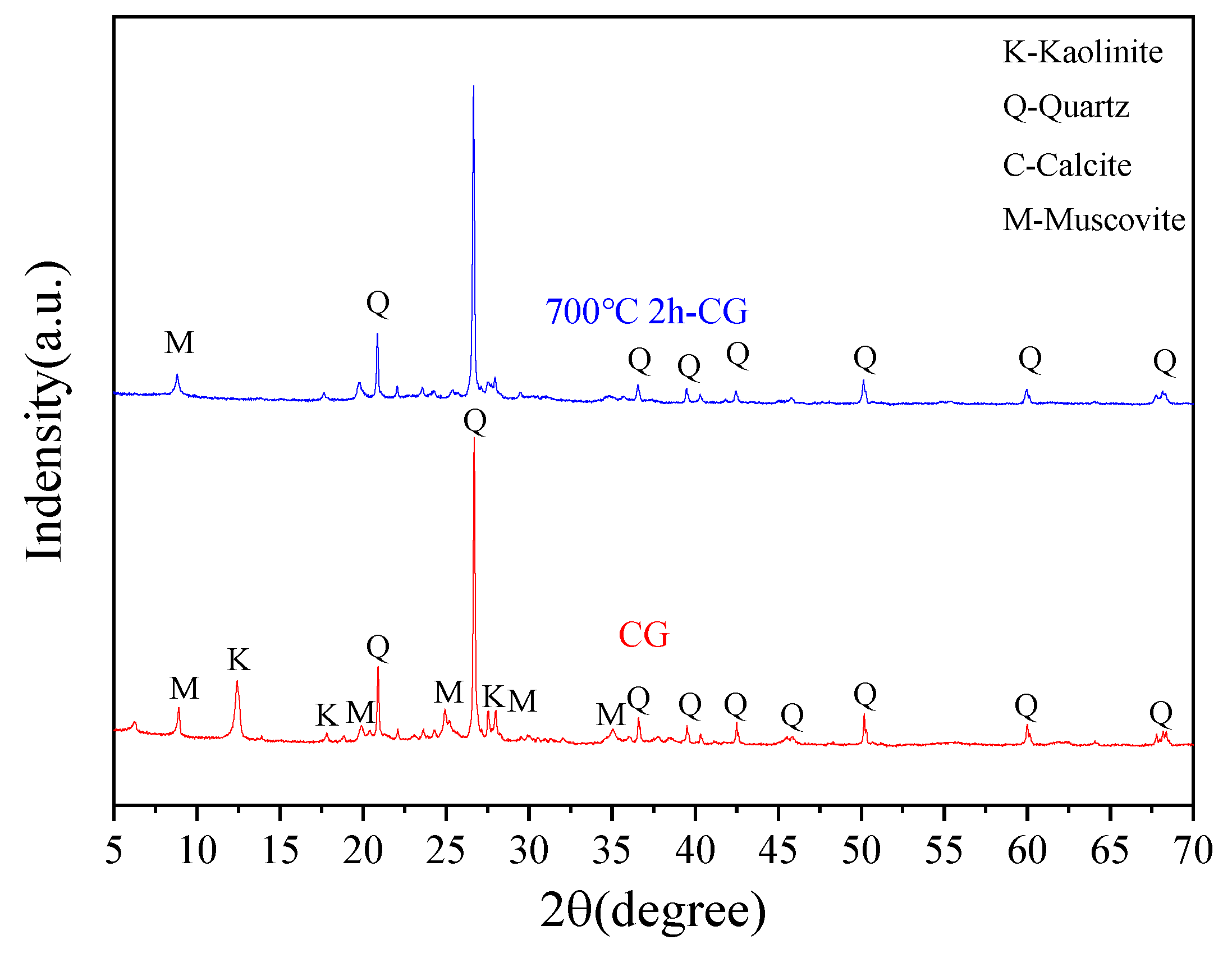
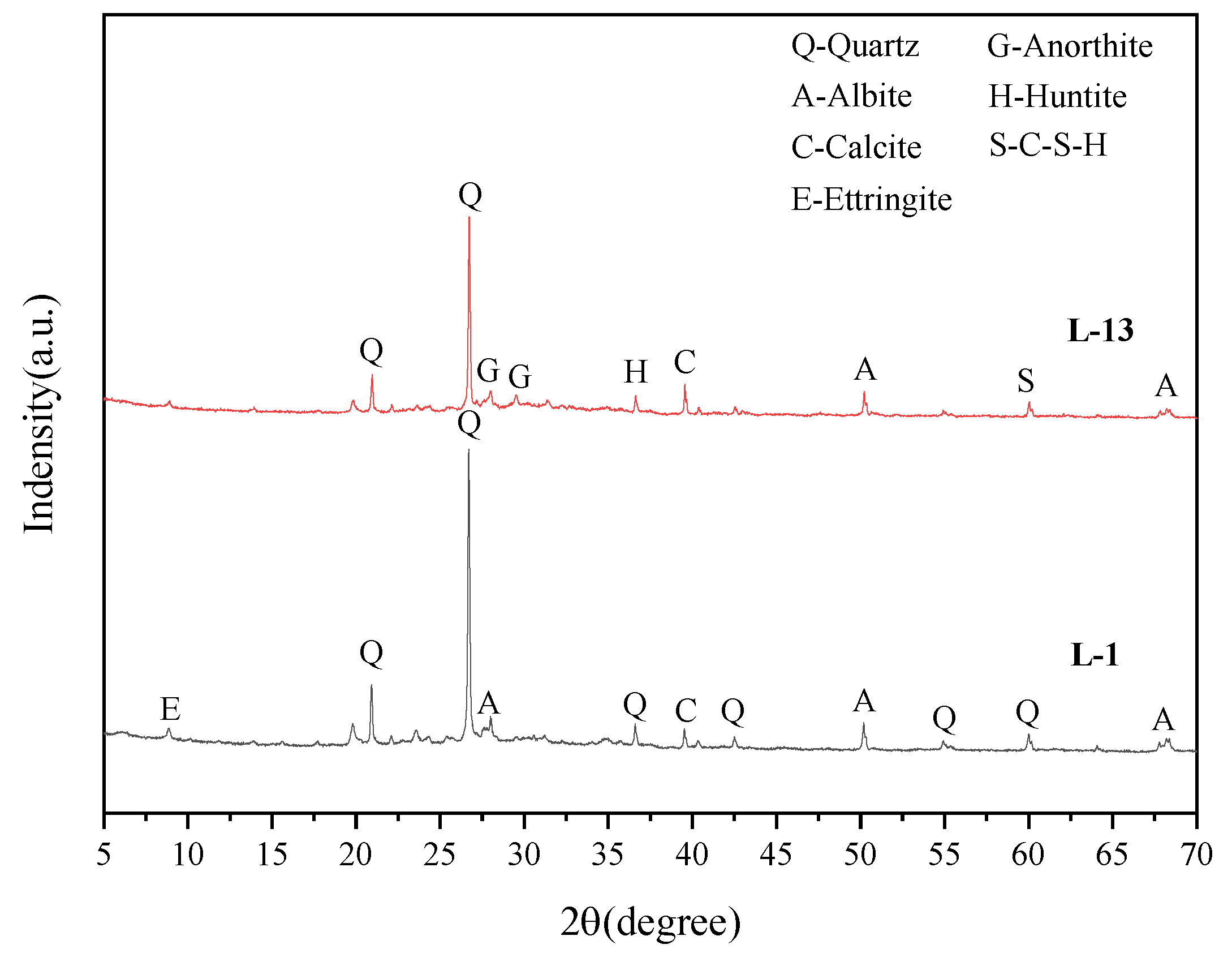
4.4.1. XRD
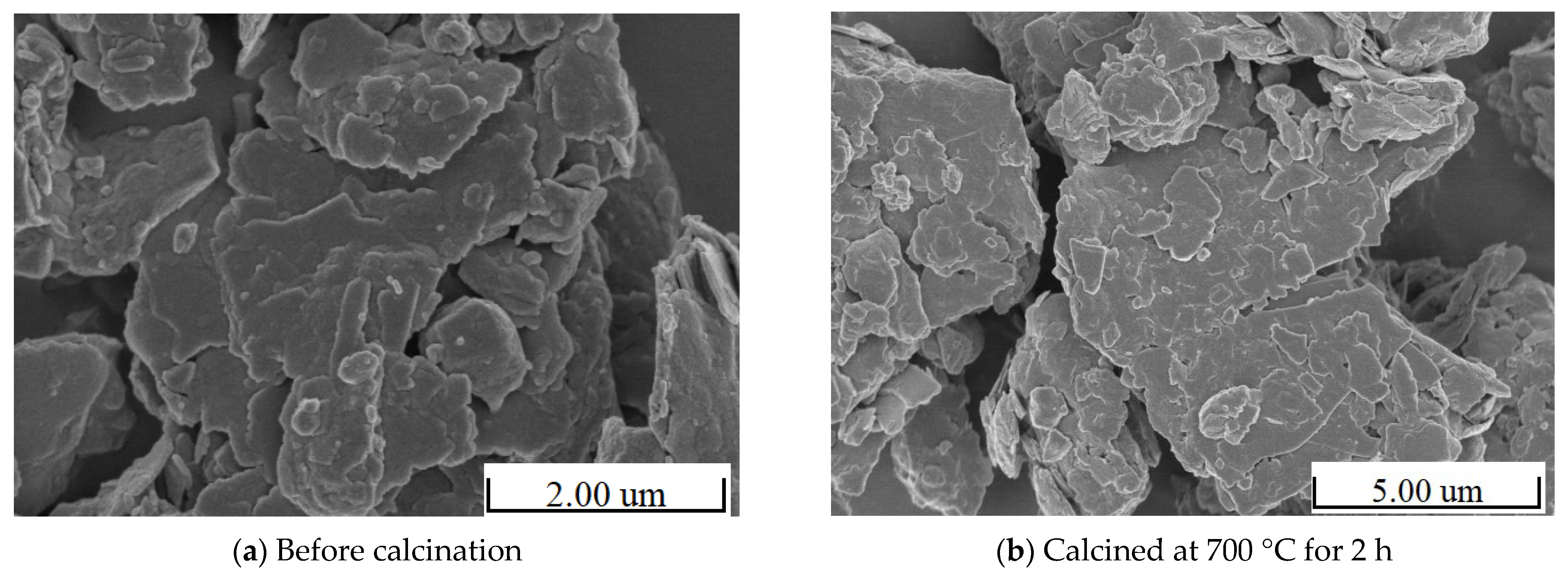
4.4.2. SEM
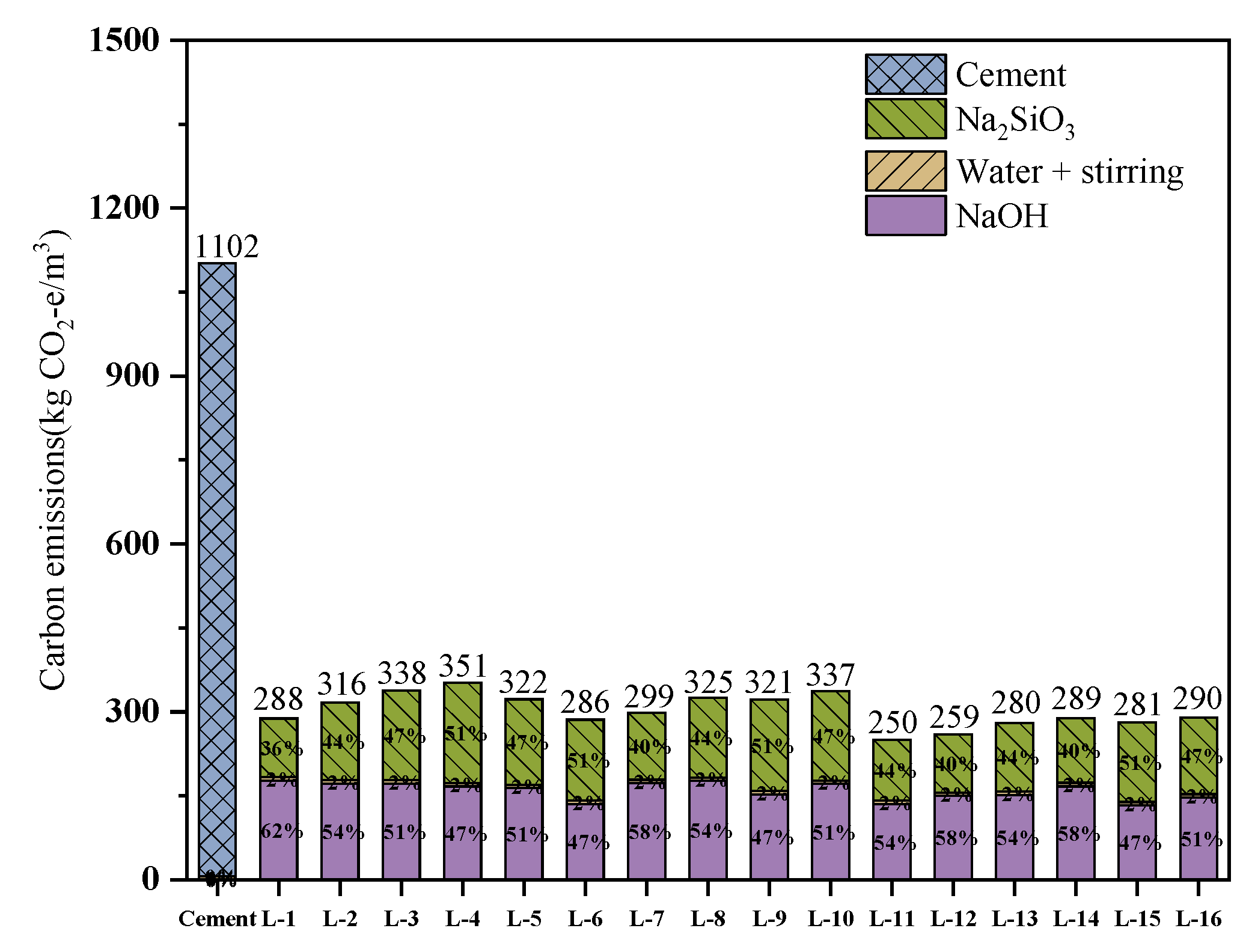
4.5. Carbon Footprint Analysis of Geopolymer
| Materials and Process | Carbon Emission Factor (kg CO2-e/kg) | Data Sources |
|---|---|---|
| Coal gangue | - | - |
| Sludge | - | - |
| NaOH | 1.43 | [25] |
| Na2SiO3 | 0.78 | [25] |
| Portland cement | 0.91 | [26] |
| Water | 0.0002 | [26] |
| Agitation | 0.003 | [27] |
5. Conclusions and Future Work
- When the coal gangue/sludge ratio is 7:3, the water–binder ratio is 0.3, the sodium-to-aluminum ratio is 0.64, and the activator modulus is 1.0, the geopolymer achieves a 3-day compressive strength (CS) of 34.5 MPa, a 3-day flexural strength (FS) of 7.85 MPa, and a final setting time of 90 min, demonstrating its suitability for use as a grouting material in engineering practice. However, the lower water–binder ratio may limit fluidity, requiring a balance between workability and early strength in practical grouting applications.
- According to the analysis of variance and range analysis, the water–binder ratio, sodium-to-aluminum ratio, activator modulus, and the amount of coal gangue replaced by sludge have an effect on the mechanical properties of CSG, among which the water–binder ratio has the most significant effect. The CS of CSG increases with the decrease in the water–binder ratio, and the crack resistance decreases first and then increases with the increase in the water–binder ratio.
- Sludge and coal gangue can be used as good complementary materials to prepare all-solid-waste geopolymer. The calcium-containing minerals in sludge supplement the deficiency of low calcium content in coal gangue and generate more calcium-containing minerals in the hydration process, which is helpful to improve the mechanical properties of geopolymer.
- The carbon emissions of alkali-activated normal-temperature-solidified geopolymer are much lower than those of cement paste, and the carbon emissions of the optimal ratio L-13 specimen are 74.6% lower than those of cement paste. It can therefore be used as a new sustainable cementitious material in green construction.
- Future research could explore the scalability of this all-solid-waste system and draw inspiration from other successful cases of alternative material utilization [11] to further enhance its field applicability in diverse harsh environments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, Y.; Shi, C.J.; Yang, N.; Yang, J. Research Progresses on Durability of Magnesium Phosphate Cement Based Materials. J. Chin. Ceram. Soc. 2014, 42, 486–493. [Google Scholar]
- Davidovits, J. Properties of geopolymer cements. In Proceedings of the First International Conference on Alkaline Cements and Concretes, Scientific Research, Kiev, Ukraine, 11–14 October 1994; pp. 131–149. [Google Scholar]
- Zhou, M.; Bai, J.T.; Guo, L.Z.; Zhang, Y.Z. Optimization of Grouting Material Proportion of Coal Gangue Geopolymer Based on Response Surface Methodology. Mater. Rep. 2023, 37, 123–131. [Google Scholar]
- Sha, F.; Liu, R.T.; Li, S.C.; Lin, C.; Li, Z.; Liu, B.; Bai, J. Application on different types of cementitious grouts for water-leakage operational tunnels. J. Cent. South Univ. (Sci. Technol.) 2016, 47, 4163–4172. [Google Scholar]
- Guo, L.Z.; Zhou, M.; Wang, L.J.; Xie, P.; Zhang, X. Preparation and Properties of Coal-Based Solid Waste Geopolymer Grouting Materials. J. Build. Mater. 2022, 25, 1092–1100. [Google Scholar]
- Feng, H.; Zhang, X.M.; Ou, X.F.; Zhang, C.; Jiang, J.; Guo, T.F.; Zhou, X.S. Experimental Study on Geopolymer Grouting Material in Rapid Grouting Reinforcement of Fractured Rock. J. South China Univ. Technol. (Nat. Sci. Ed.) 2020, 48, 43–50. [Google Scholar]
- Li, Z.F.; Liu, C.; Wang, C.; Zhang, J.; Wang, Y.; Gao, Y. Experimental Study on Grouting Material of Red Mud–blast Furnace Slag–steel Slag Ternary System. Adv. Eng. Sci. 2021, 53, 203–211. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, M.; Wang, X.; Li, C.; Jia, H. Preparation of coal gangue-slag-fly ash geopolymer grouting materials. Constr. Build. Mater. 2022, 328, 126997. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Y.; Li, J.; Ouyang, S.; Fan, B.; Liu, Y.; Hou, G. Preparation of the geopolymer grouting material by coal-based solid wastes for the aquiclude key strata and its application. Constr. Build. Mater. 2023, 408, 133539. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Das, A.K.; Qi, Y. High performance natural seawater coral sand ECC (HP-NSCS-ECC) in coastal conditions: Experimental characterization, microstructure, and sustainability. Constr. Build. Mater. 2025, 499, 143860. [Google Scholar] [CrossRef]
- Luo, X.; Xu, J.; Bai, E.; Li, W. Systematic study on the basic characteristics of alkali-activated slag-fly ash cementitious material system. Constr. Build. Mater. 2012, 29, 482–486. [Google Scholar] [CrossRef]
- Zhou, J.F.; Chen, S.K.; Das, A.K. Application of sludge as a sustainable material inbuilding materials: A comprehensive review. J. Build. Eng. 2025, 111, 113434. [Google Scholar] [CrossRef]
- Pan, Y.C.; Lv, X.B.; Tao, J.; Yu, H.J.; Yang, S.J.; Cui, J.G.; Ren, G.Q.; Xiong, J. Research progress of sludge pretreatment and resource recycling technology. Appl. Chem. Ind. 2024, 53, 900–905. [Google Scholar] [CrossRef]
- Xu, Z.F.; Zou, X.T.; Chen, J. Preparation of Thermal Activation Sludge and Coal Gangue Polymer. Integr. Ferroelectr. 2015, 160, 1–9. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, W.H.; Guo, L.X.; Wang, Z. Study on mechanical properties and pore structurecharacteristics of alkali activation sludge-cement composites. Mater. Today Commun. 2023, 35, 105469. [Google Scholar] [CrossRef]
- Albitar, M.; Ali, M.S.M.; Visintin, P.; Drechsler, M. Effect of granulated lead smelter slag on strength of fly ash-based geopolymer concrete. Constr. Build. Mater. 2015, 83, 128–135. [Google Scholar] [CrossRef]
- Sadique, M.; Al-Nageim, H.; Atherton, W.; Seton, L.; Dempster, N. Mechano-chemical activation of high-Ca fly ash by cement free blending and gypsum aided grinding. Constr. Build. Mater. 2013, 43, 480–489. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland cement. Standard Press of China: Beijing, China, 2011.
- Wang, F.; Liu, Z.; Han, L.; Xie, F. Preparation and Properties of Activated Coal Gangue Geopolymer. Bull. Chin. Ceram. Soc. 2021, 40, 914–920. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhou, M.; Li, K.; Xie, Y.G. Research Progress on Performance Degradation and Corrosion Failure of Cement-based Materials in Carbonate Environment. Mater. Rep. 2023, 37, 92–100. [Google Scholar]
- Das, A.K.; Xiao, J. Upcycling waste glass bottles as a binder within engineered cementitious composites (ECCs): Experimental investigation and environmental impact assessment. Clean. Mater. 2025, 16, 100311. [Google Scholar] [CrossRef]
- Liao, G.; Wang, D.; Wang, W.; He, Y. Microstructure, strength development mechanism, and CO2 emission analyses of alkali-activated fly ash-slag mortars. J. Clean. Prod. 2024, 442, 141116. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, J.; Wen, G.; Xi, Z.; Li, S. Orthogonal test of alkali-activated slag solidified construction spoil, fluidity, compressive strength, water resistance and carbon emission. J. Clean. Prod. 2024, 434, 140201. [Google Scholar] [CrossRef]
- Zannerni, G.M.; Fattah, K.P.; Al-Tamimi, A.K. Ambient-cured geopolymer concrete with single alkali activator. Sustain. Mater. Technol. 2020, 23, e00131. [Google Scholar] [CrossRef]








| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | Burning Vector |
|---|---|---|---|---|---|---|---|---|
| Wt% | 50.8 | 28.1 | 6.2 | 3.7 | 1.2 | 0.6 | 1.2 | 7.9 |
| Chemical Composition | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| Wt% | 41.95 | 32.64 | 13.24 | 3.7 | 3.63 | 1.74 | 1.24 | 0.52 |
| Factor | Coal Gangue/Sludge A | Water–Binder Ratio B | n (Na)/n (Al) C | Activator Modulus D | Vacant Column | |
|---|---|---|---|---|---|---|
| Level | ||||||
| 1 | 10:0 | 0.30 | 0.52 | 0.9 | 1 | |
| 2 | 9:1 | 0.33 | 0.56 | 1.0 | 1 | |
| 3 | 8:2 | 0.36 | 0.60 | 1.1 | 1 | |
| 4 | 7:3 | 0.39 | 0.64 | 1.2 | 1 | |
| Numbering | Coal Gangue/g | Sludge/g | NaOH/g | Water Glass/g | Water/g |
|---|---|---|---|---|---|
| L-1 | 1400 | - | 147 | 459.3 | 420 |
| L-2 | 1400 | - | 149.6 | 640.1 | 462 |
| L-3 | 1400 | - | 155.8 | 770.1 | 504 |
| L-4 | 1400 | - | 160.8 | 912.8 | 546 |
| L-5 | 1260 | 140 | 143.8 | 710.6 | 420 |
| L-6 | 1260 | 140 | 116.2 | 659.3 | 462 |
| L-7 | 1260 | 140 | 151.3 | 556.2 | 504 |
| L-8 | 1260 | 140 | 161.3 | 690 | 546 |
| L-9 | 1120 | 280 | 137.7 | 781.6 | 420 |
| L-10 | 1120 | 280 | 155 | 766 | 462 |
| L-11 | 1120 | 280 | 115.3 | 493.2 | 504 |
| L-12 | 1120 | 280 | 130.8 | 480.9 | 546 |
| L-13 | 980 | 420 | 140 | 599.1 | 420 |
| L-14 | 980 | 420 | 142.1 | 522.3 | 462 |
| L-15 | 980 | 420 | 116.2 | 659.2 | 504 |
| L-16 | 980 | 420 | 131.7 | 651.1 | 546 |
| Test Number | 3 d | 28 d | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| Coal Gangue/Sludge | Water–Binder Ratio | n(Na)/n(Al) | Activator Modulus | Coal Gangue/Sludge | Water–Binder Ratio | n(Na)/n(Al) | Activator Modulus | |
| K1 | 13.19 | 22.45 | 14.33 | 16.81 | 18.81 | 24.62 | 20.70 | 17.86 |
| K2 | 12.23 | 21.04 | 13.22 | 17.97 | 25.60 | 23.12 | 22.76 | 26.29 |
| K3 | 17.97 | 13.52 | 18.57 | 16.51 | 19.23 | 23.63 | 21.77 | 24.43 |
| K4 | 23.46 | 9.84 | 20.73 | 15.55 | 24.16 | 16.43 | 22.56 | 19.22 |
| Ri | 11.22 | 12.61 | 7.51 | 2.42 | 6.80 | 8.19 | 2.06 | 8.43 |
| Primary and secondary order of factors | B > A > C > D | D > B > A > C | ||||||
| The optimum level | A4 | B1 | C4 | D2 | A2 | B1 | C2 | D2 |
| Optimal combination | A4B1C4D2 | A2B1C2D2 | ||||||
| Influencing Factors | Standards of Significance | 3 d | 28 d | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Degrees of Freedom Between Groups | Within-Group Degrees of Freedom | Mean Square Between Groups | Mean Square Within Group | F | p | Significance | Degrees of Freedom Between Groups | Within-Group Degrees of Freedom | Mean Square Between Groups | Mean Square Within Group | F | p | Significance | ||
| A(Coal gangue/sludge) | F0.1(3, 28) = 2.9467 | 3 | 28 | 178.87 | 52.38 | 3.4151 | 0.039 | ** | 3 | 28 | 195.26 | 50.24 | 3.8863 | 0.025 | ** |
| B(water–binder ratio) | F0.05(3, 28) = 2.291 | 3 | 28 | 263.05 | 43.36 | 6.0671 | 0.004 | *** | 3 | 28 | 177.12 | 53.33 | 3.3212 | 0.043 | ** |
| C(Sodium-to- aluminum ratio) | F0.01(3, 28) = 4.568 | 3 | 28 | 80.68 | 62.90 | 1.2827 | 0.292 | - | 3 | 28 | 10.61 | 67.85 | 0.1564 | 0.928 | - |
| D(activator modulus) | 3 | 28 | 19.90 | 69.41 | 0.2868 | 0.828 | - | 3 | 28 | 201.67 | 50.57 | 3.9881 | 0.025 | ** | |
| Test Number | 3d | 28d | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| Coal Gangue/Sludge | Water–Binder Ratio | n(Na)/n(Al) | Activator Modulus | Coal Gangue/Sludge | Water–Binder Ratio | n(Na)/n(Al) | Activator Modulus | |
| K1 | 2.68 | 4.55 | 2.83 | 2.72 | 4.15 | 5.37 | 3.34 | 3.56 |
| K2 | 2.11 | 3.39 | 2.84 | 4.11 | 3.62 | 3.13 | 5.50 | 4.95 |
| K3 | 3.14 | 2.26 | 3.11 | 2.97 | 4.80 | 5.03 | 4.15 | 5.30 |
| K4 | 4.41 | 2.15 | 3.57 | 2.55 | 4.39 | 3.41 | 3.96 | 3.13 |
| Ri | 2.31 | 2.40 | 0.74 | 1.56 | 1.18 | 2.25 | 2.17 | 2.18 |
| Primary and secondary order of factors | B > A > D > C | B > D > C > A | ||||||
| The optimum level | A4 | B1 | C4 | D2 | A3 | B1 | C2 | D3 |
| Optimal combination | A4B1C4D2 | A3B1C2D3 | ||||||
| Influencing Factors | Standards of Significance | 3 d | 28 d | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Degrees of Freedom Between Groups | Within-Group Degrees of Freedom | Mean Square Between Groups | Mean Square Within Group | F | p | Significance | Degrees of Freedom Between Groups | Within-Group Degrees of Freedom | Mean Square Between Groups | Mean Square Within Group | F | p | Significance | ||
| A(Coal gangue/sludge) | F0.1(3, 28) = 2.9467 | 3 | 28 | 6.71 | 1.60 | 4.2006 | 0.016 | *** | 3 | 28 | 0.70 | 1.74 | 0.4037 | 0.75 | - |
| B(water–binder ratio) | F0.05(3, 28) = 2.291 | 3 | 28 | 9.44 | 1.37 | 6.8851 | 0.001 | *** | 3 | 28 | 6.13 | 1.17 | 5.2409 | 0.006 | *** |
| C(sodium-to-aluminum ratio) | F0.01(3, 28) = 4.568 | 3 | 28 | 1.38 | 2.23 | 0.6186 | 0.606 | - | 3 | 28 | 3.75 | 1.43 | 2.6326 | 0.070 | * |
| D(activator modulus) | 3 | 28 | 3.31 | 2.03 | 1.6306 | 0.203 | - | 3 | 28 | 4.79 | 1.31 | 3.6457 | 0.030 | ** | |
| Material Type | 3-day CS (MPa) | 28-day CS (MPa) | Carbon Emission (kg CO2-e/m3) | SER (MPa/(kg CO2-e/m3)) |
|---|---|---|---|---|
| L-13 geopolymer | 34.5 | 31.9 | 280 | 0.123 |
| Cement paste (Reference) | - | 30.0 * | 1102 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Chen, M.; Zhou, B.; Yao, X.; Liu, S.; Chang, Y.; Chen, S. Performance and Microstructure Characterization of Grouting Materials for Tailings Mined-Out Area Prepared by All-Solid Waste. Buildings 2025, 15, 4177. https://doi.org/10.3390/buildings15224177
Gao Y, Chen M, Zhou B, Yao X, Liu S, Chang Y, Chen S. Performance and Microstructure Characterization of Grouting Materials for Tailings Mined-Out Area Prepared by All-Solid Waste. Buildings. 2025; 15(22):4177. https://doi.org/10.3390/buildings15224177
Chicago/Turabian StyleGao, Yongwei, Mengya Chen, Borui Zhou, Xianhua Yao, Shiwen Liu, Yiqian Chang, and Shengqiang Chen. 2025. "Performance and Microstructure Characterization of Grouting Materials for Tailings Mined-Out Area Prepared by All-Solid Waste" Buildings 15, no. 22: 4177. https://doi.org/10.3390/buildings15224177
APA StyleGao, Y., Chen, M., Zhou, B., Yao, X., Liu, S., Chang, Y., & Chen, S. (2025). Performance and Microstructure Characterization of Grouting Materials for Tailings Mined-Out Area Prepared by All-Solid Waste. Buildings, 15(22), 4177. https://doi.org/10.3390/buildings15224177







