Evolution of Cementitious Binders: Overview of History, Environmental Impacts, and Emerging Low-Carbon Alternatives
Abstract
1. Introduction
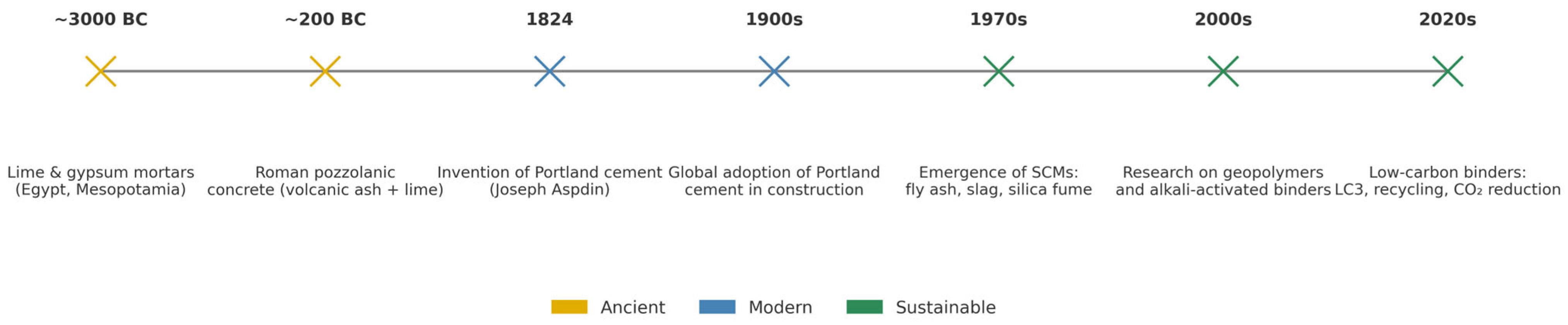
2. Historical Development and Production of Cementitious Binders
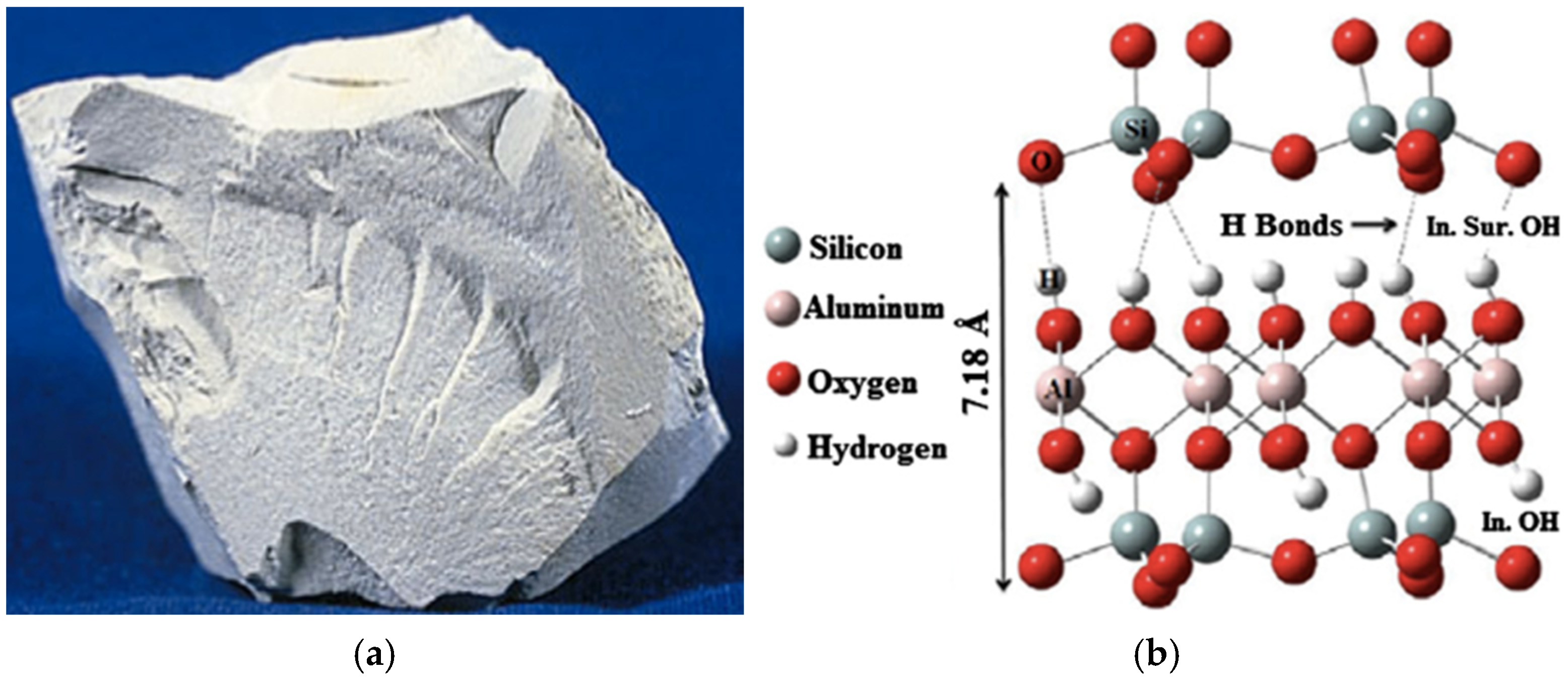
2.1. Early Binders and Neolithic Innovations
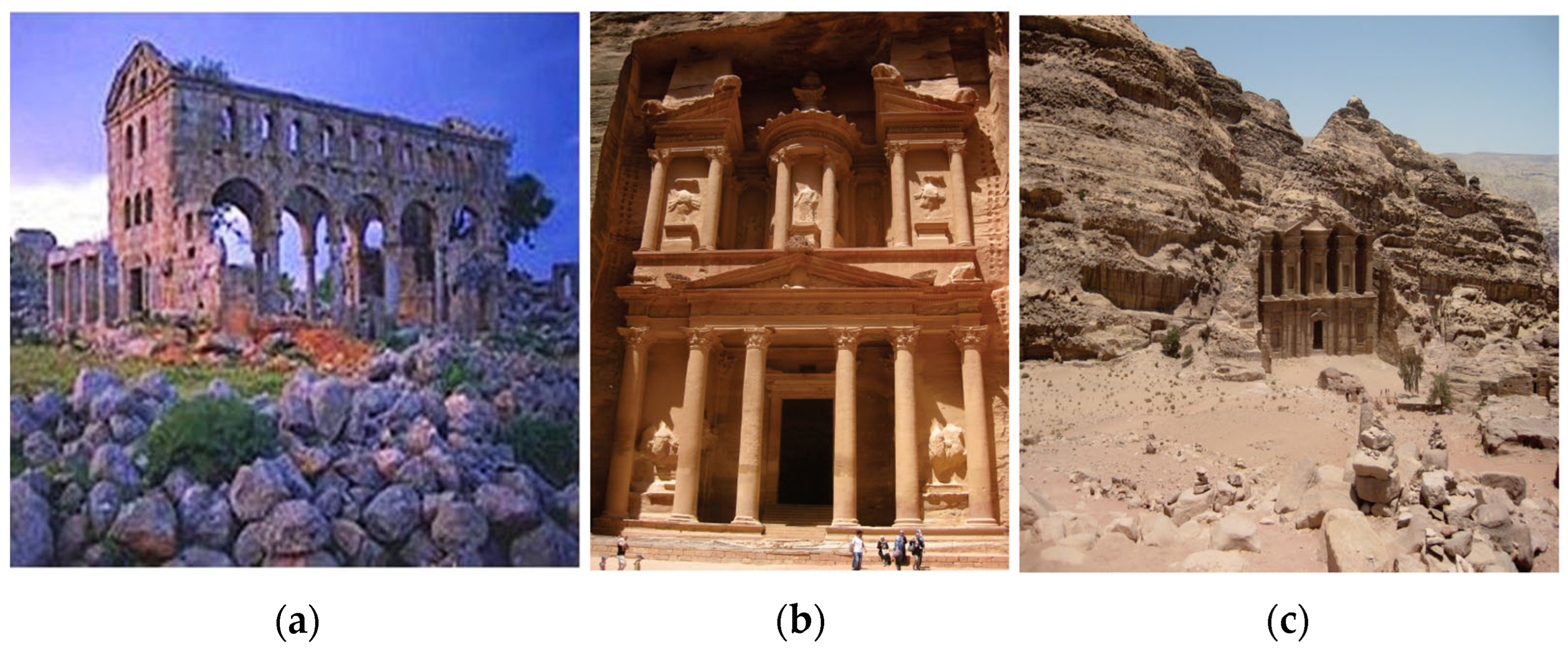
2.2. Roman Advances in Pozzolanic Binders
2.3. Industrial Innovations and the Birth of Portland Cement
2.4. Global Expansion of the Cement Production
3. Environmental Challenges of Cement Manufacturing
3.1. CO2 Emissions and Climate Impact
3.2. Energy Demand and Resource Consumption
| Emission | Value/Statement | References |
|---|---|---|
| Global cement production (2023) | ≈4.1 billion metric tons (2023) | [87] |
| Total CO2 emissions from cement production (2023) | 1.56 billion metric tons CO2 (2023)—stated as double that of 2000 | |
| CO2 intensity (per ton cement) | ~1.0 t CO2/t cement (until 2001); 0.87 t CO2/t cement (by 2010) | [72] |
| Share of global CO2 emissions (industry) | Two ranges reported: 7–8% in some studies; ≈5–7% in others → overall 5–8% | [58,59,60] |
| Energy consumption (global/industrial share) | ~2% of total global energy; ~5% of industrial energy consumption; also reported as 5% of total industrial energy | [62,69] |
| Historical/projected production trend (by 2050) | Production rose ~1.0 → ~3.6 billion t; projected up to ~5.8 billion t by 2050; another estimate cites 18 billion t by 2050 | [80] |
3.3. Sources of Emissions in Cement Manufacture
4. Supplementary Cementitious Materials (SCMs)
4.1. SCMs: Definitions, Background, and Historical Development
4.2. Research Status of the Emerging SCMs
4.3. Limitations and Risks of Using SCMs
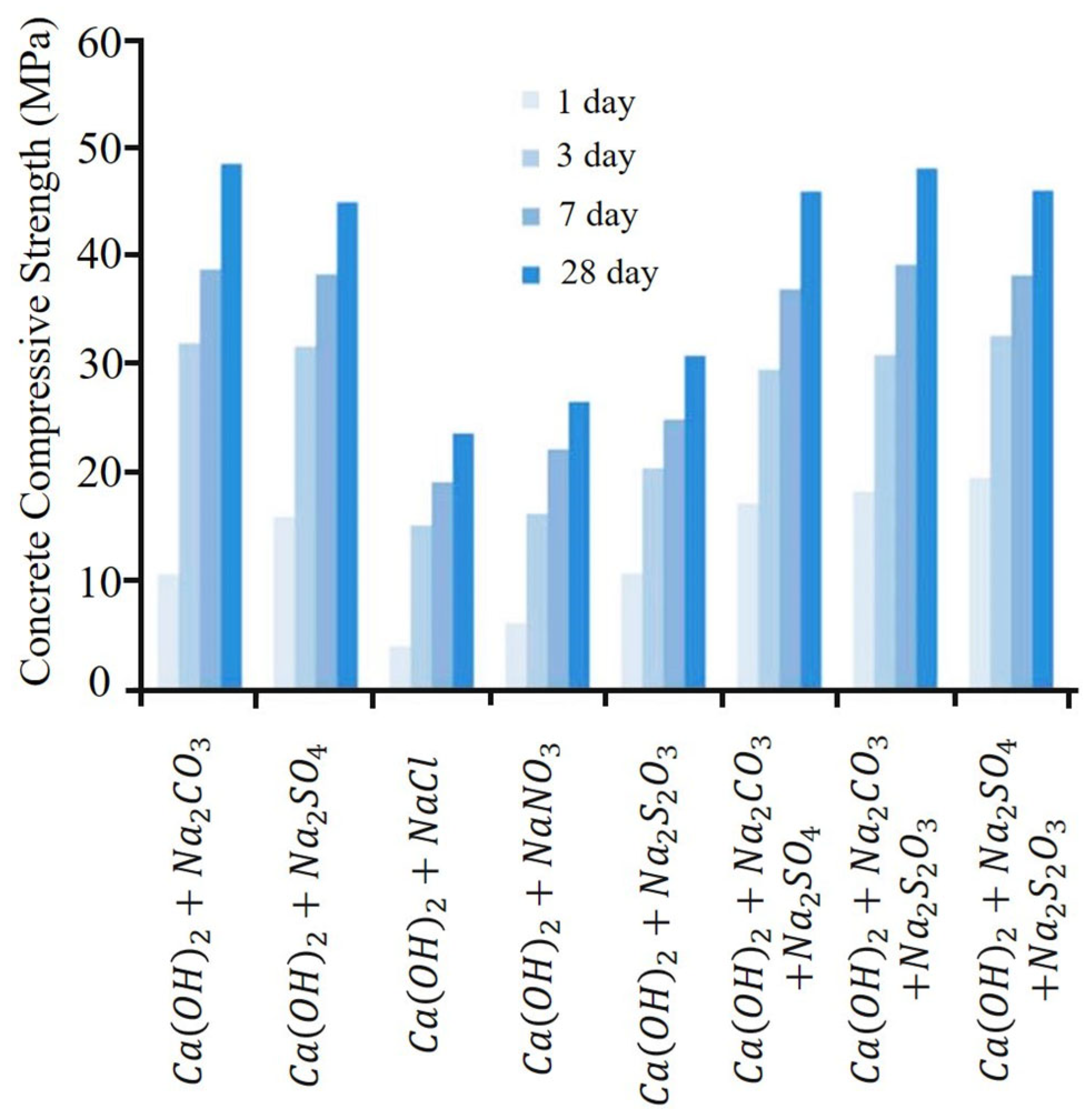
5. Alkali-Activated Binders (AABs)
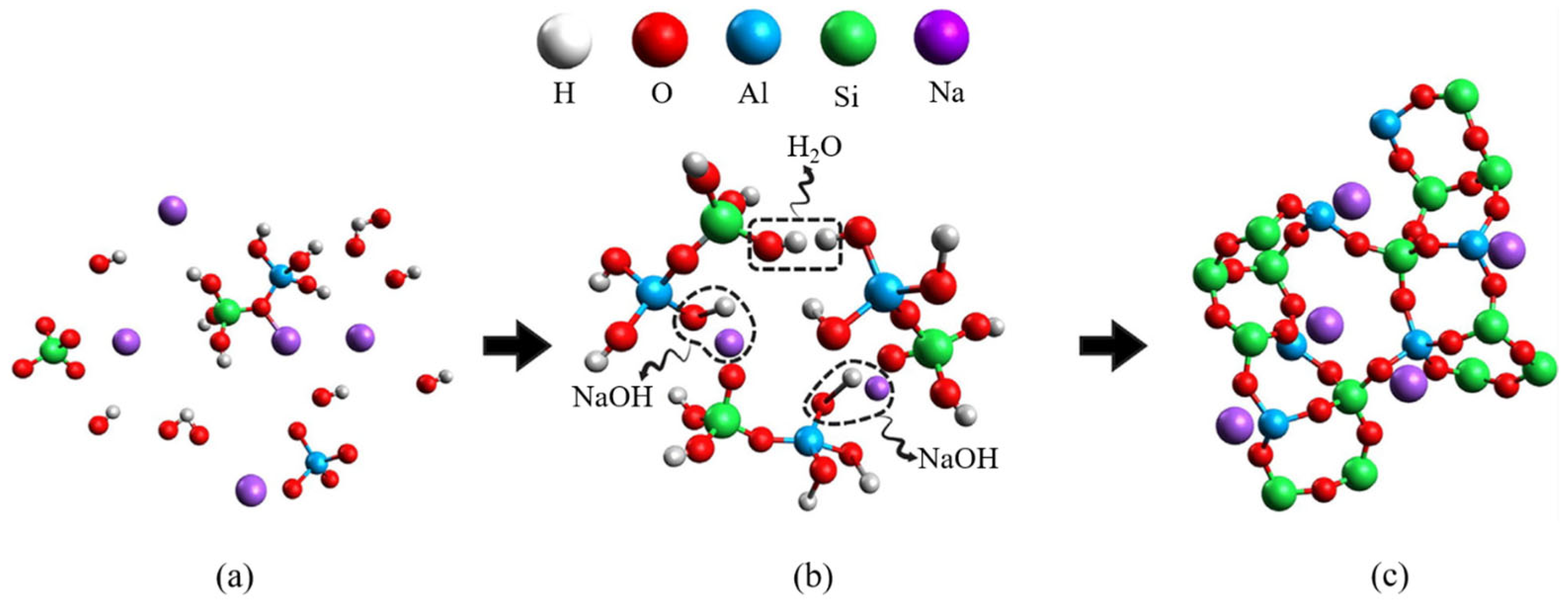
5.1. AAB Technology and Background
5.2. Properties and Advantages of AABs
| References | Year | Significance |
|---|---|---|
| Feret [146] | 1939 | Slags used for cement. |
| Purdon [105] | 1940 | Alkali–slag combinations. |
| Glukhovsky [147] | 1959 | Theoretical basis and development of alkaline cement. |
| Glukhovsky et al. [148] | 1979 | First called “alkaline cements.” |
| Davidovits [149] | 1989 | “Geopolymer term.” |
| Malinowski et al. [22] | 1991 | Ancient aqueducts characterized. |
| Forss [150] | 1983 | F-cement (slag–alkali–superplasticizer). |
| Barnes et al. [151] | 1984 | Ancient building materials characterized. |
| Krivenko [152] | 1986 | DSc thesis, R2O–RO–SiO2–H2O. |
| Malek et al. [153] | 1986 | Slag cement-low level radioactive waste forms. |
| Davidovits [154] | 1987 | Ancient and modern concretes compared. |
| Roy and Langton [155] | 1989 | Ancient concrete analogs. |
| Talling and Brandstetr [156] | 1989 | Alkali-activated slag. |
| Wu et al. [157] | 1990 | Activation of slag cement. |
| Roy and Silsbee [158] | 1991 | Alkali-activated cements: an overview. |
| Palomo and Glasser [159] | 1992 | CBC with metakaolin |
| Roy and Malek [160] | 1993 | Slag cement. |
| Glukhovsky [161] | 1994 | Ancient, modern, and future concrete. |
| Krivenko [162] | 1992 | Alkaline cements. |
| Wang and Scivener [163] | 1995 | Slag and alkali-activated microstructure. |
| Shi [164] | 1996 | Strength, pore structure, and permeability of alkali-activated slag. |
| Fernández-Jiménez et al. [165] | 1997 | Studies of alkali-activated slag cements. |
| Katz [166] | 1998 | Microstructure of alkali-activated fly ash. |
| Davidovits [167] | 1999 | Chemistry of geopolymeric systems, technology. |
| Roy [59] | 1999 | Opportunities and challenges of alkali-activated cement. |
| Palomo et al. [168] | 1998 | Alkali-activated fly ash is a cement for the future. |
| Gong and Yang [169] | 2000 | Alkali-activated red mud–slag cement. |
| Puertas et al. [170] | 2000 | Alkali-activated fly ash/slag cement. |
| Bakharev et al. [171] | 2000 | Alkali-activated slag concrete. |
| Palomo and Palacios [172] | 2003 | Immobilization of hazardous wastes. |
| Grutzeck et al. [173] | 2004 | Zeolite formation. |
| Sun et al. [174] | 2006 | Sialite technology. |
| Duxson et al. [175] | 2007 | Geopolymer technology: the current state of the art. |
| Hajimohammadi et al. [176] | 2008 | One-part geopolymer. |
| Provis and Devente [177] | 2009 | Geopolymers: structure, processing, properties, and industrial applications. |
| Haha et al. [94] | 2011 | Durability of alkali-activated slag and fly ash concrete |
| Junior et al. [178] | 2013 | Properties of alkali-activated concrete with varying mix design. |
| Provis et al. [179] | 2015 | Geopolymers: structure, properties, and applications. |
| Liu et al. [180] | 2016 | Utilization of waste materials in alkali-activated binders. |
| Provis, J. L. [181] | 2018 | Development of low-carbon alkali-activated materials. |
| Khalifa et al. [182] | 2020 | Advances in the use of metakaolin in alkali-activated binders. |
| Labianca et al. [134] | 2022 | Incorporation of sustainable materials in alkali-activated binders. |
| Chen et al. [183] | 2023 | Incorporation of sustainable materials in alkali-activated binders. |
| Manjunath et al. [184] | 2024 | Incorporation of sustainable materials in alkali-activated binders. |
| Murali et al. [185] | 2024 | Incorporation of sustainable materials in alkali-activated binders. |
| Park et al. [186] | 2024 | Incorporation of sustainable materials in alkali-activated binders. |
| Singh et al. [187] | 2025 | Incorporation of sustainable materials in alkali-activated binders. |
| Lemougna et al. [188] | 2025 | Incorporation of sustainable materials in alkali-activated binders. |
| Amin et al. [189] | 2025 | Durability study of concrete incorporating sustainable materials in alkali-activated binders. |
| Amin et al. [190] | 2025 | Durability study of concrete incorporating sustainable materials in alkali-activated binders. |
| Zhang et al. [191] | 2025 | Incorporation of sustainable materials in alkali-activated binders. |
| Xiang et al. [192] | 2025 | Incorporation of sustainable materials in alkali-activated binders. |
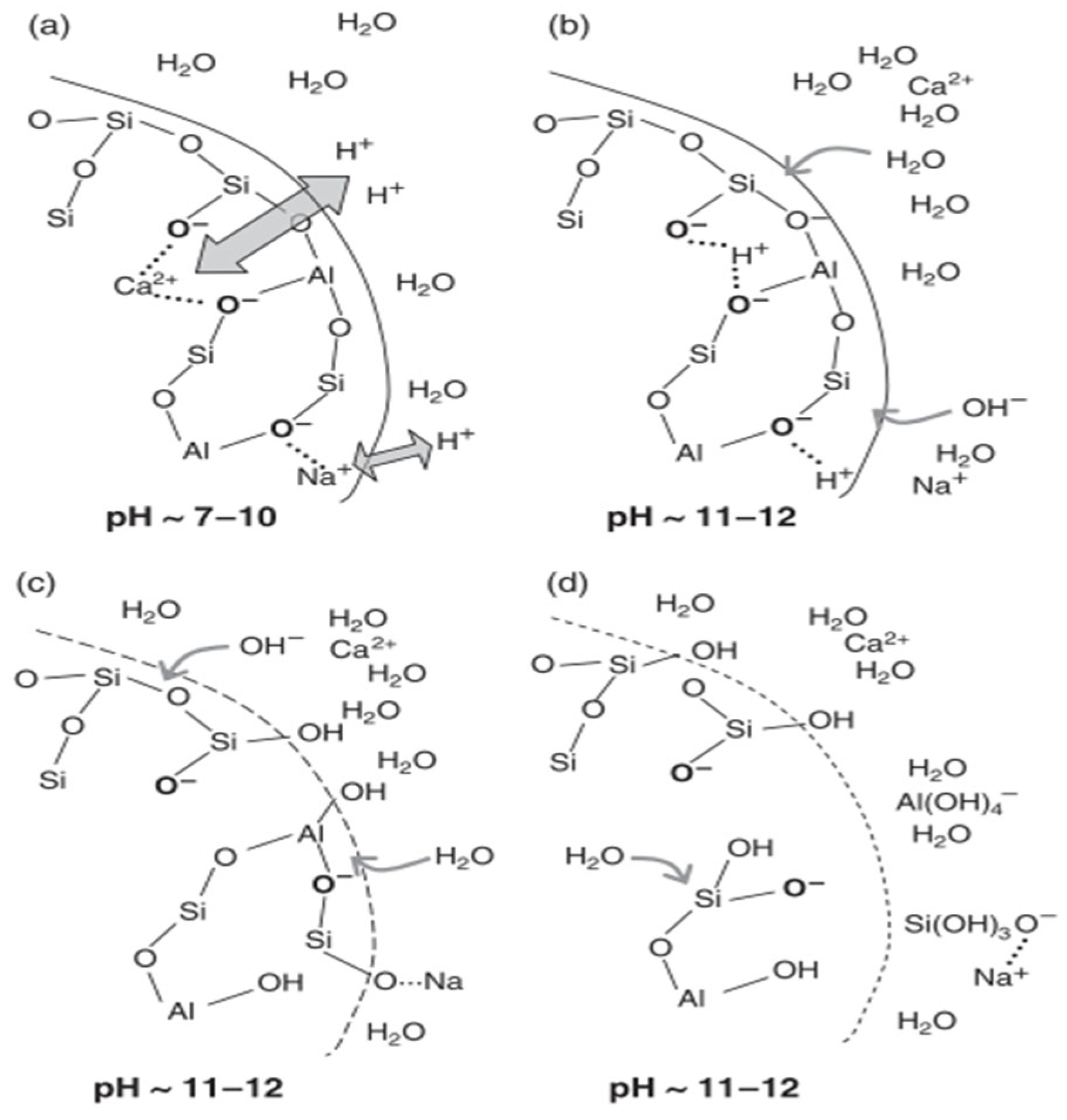
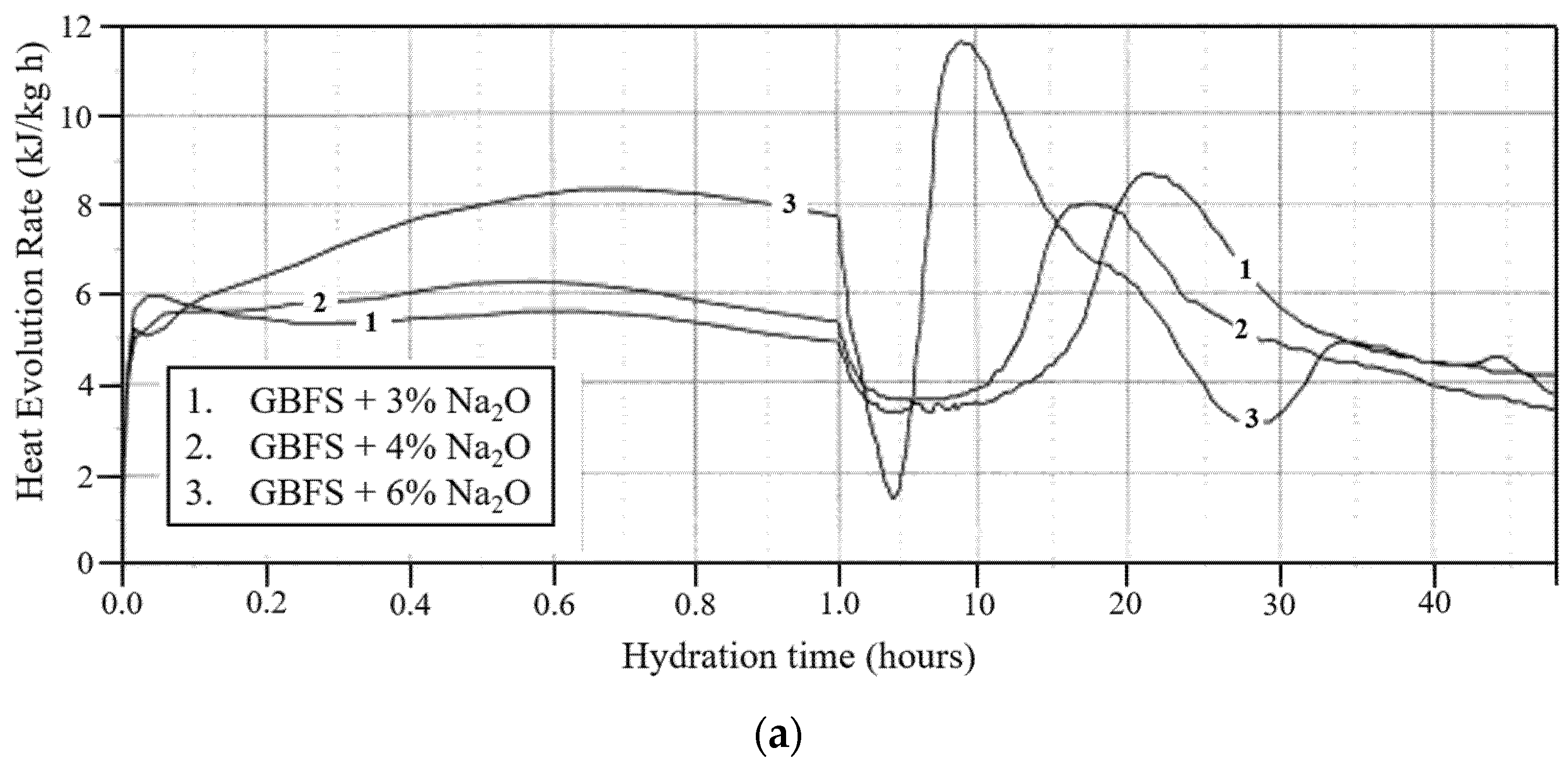
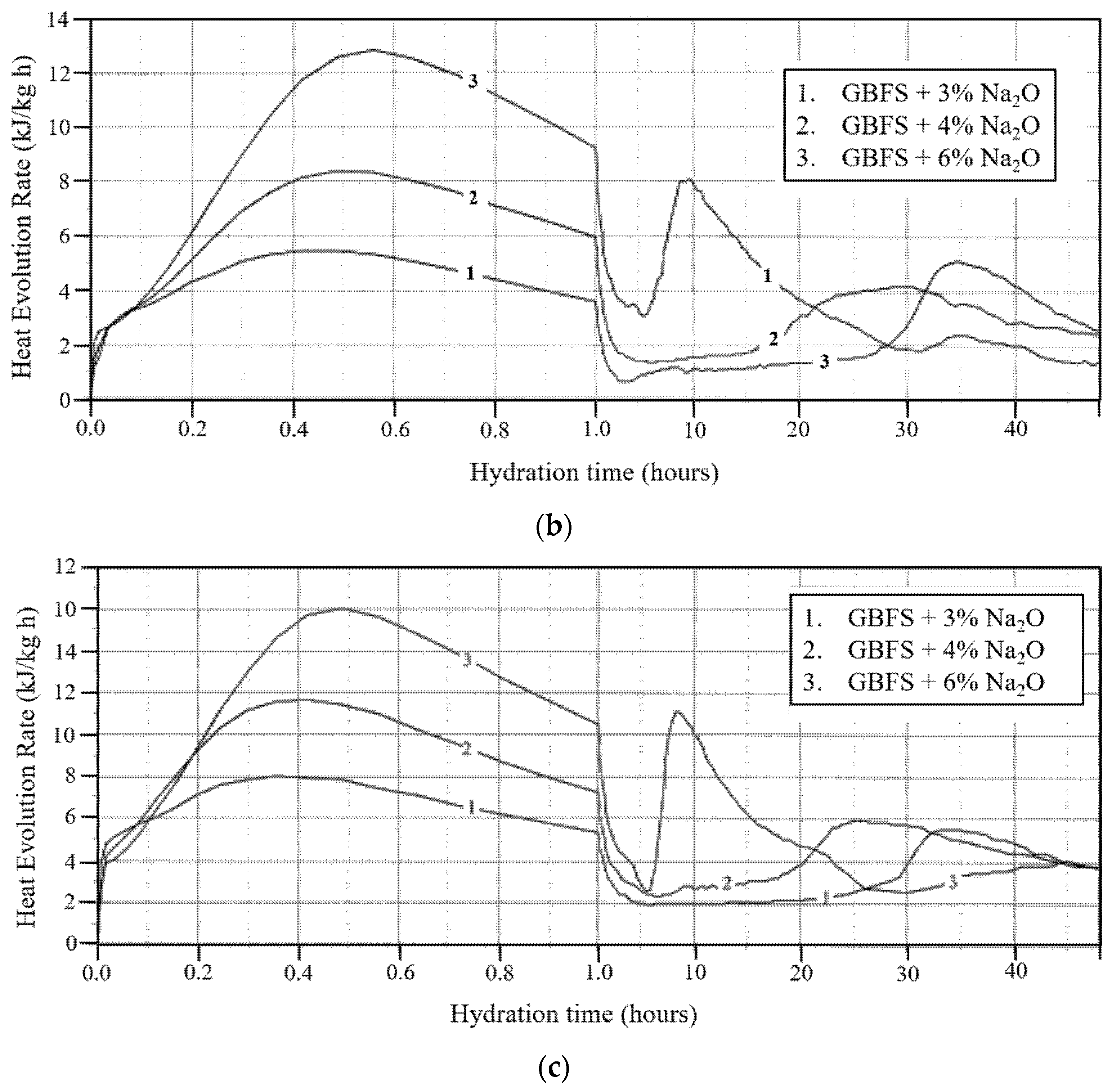
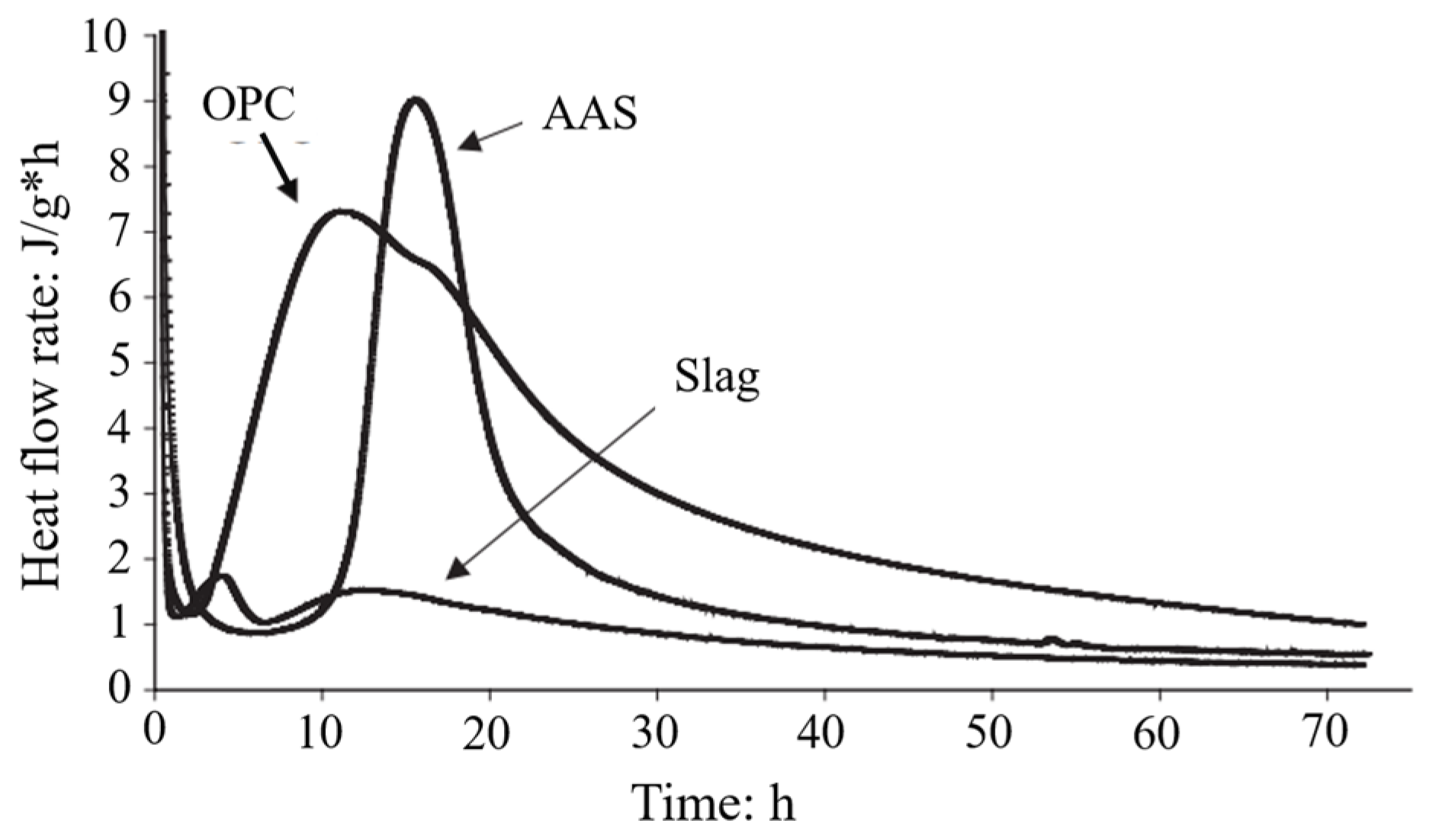
5.3. Reaction Mechanism
5.4. Challenges and Practical Barriers for AABs
- Safety and handling concerns with alkaline activators (NaOH, sodium silicate).
- High embodied carbon and cost of some activators, requiring careful LCA accounting.
- Feedstock variability and pre-treatment needs for slags, fly ash, or agricultural residues.
- Limited long-term durability evidence across diverse exposure conditions.
- Absence of standardized codes, test methods, and acceptance criteria.
- Supply-chain and economic uncertainties at an industrial scale.
6. Comparison Between AABs and Portland Cement
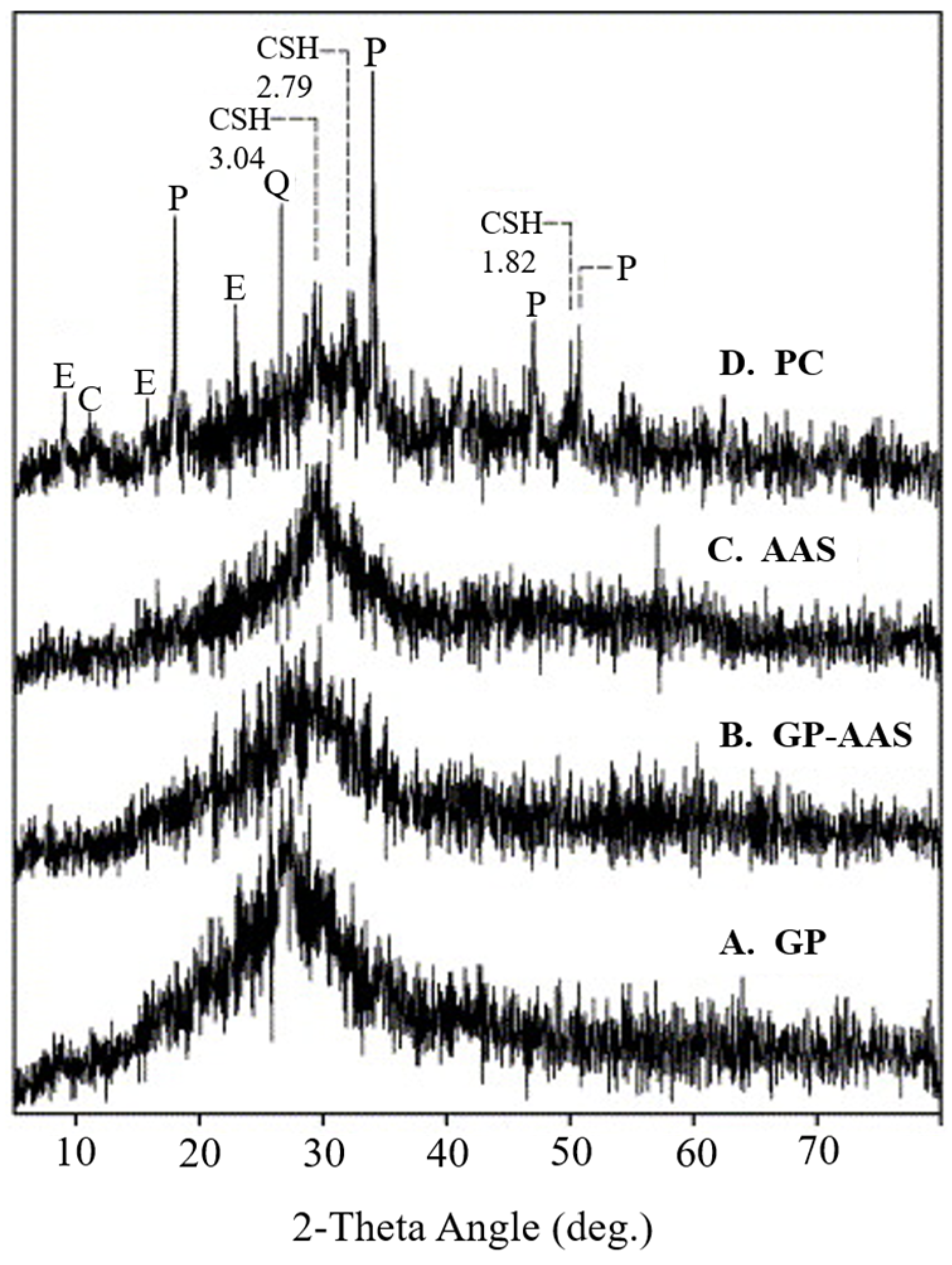
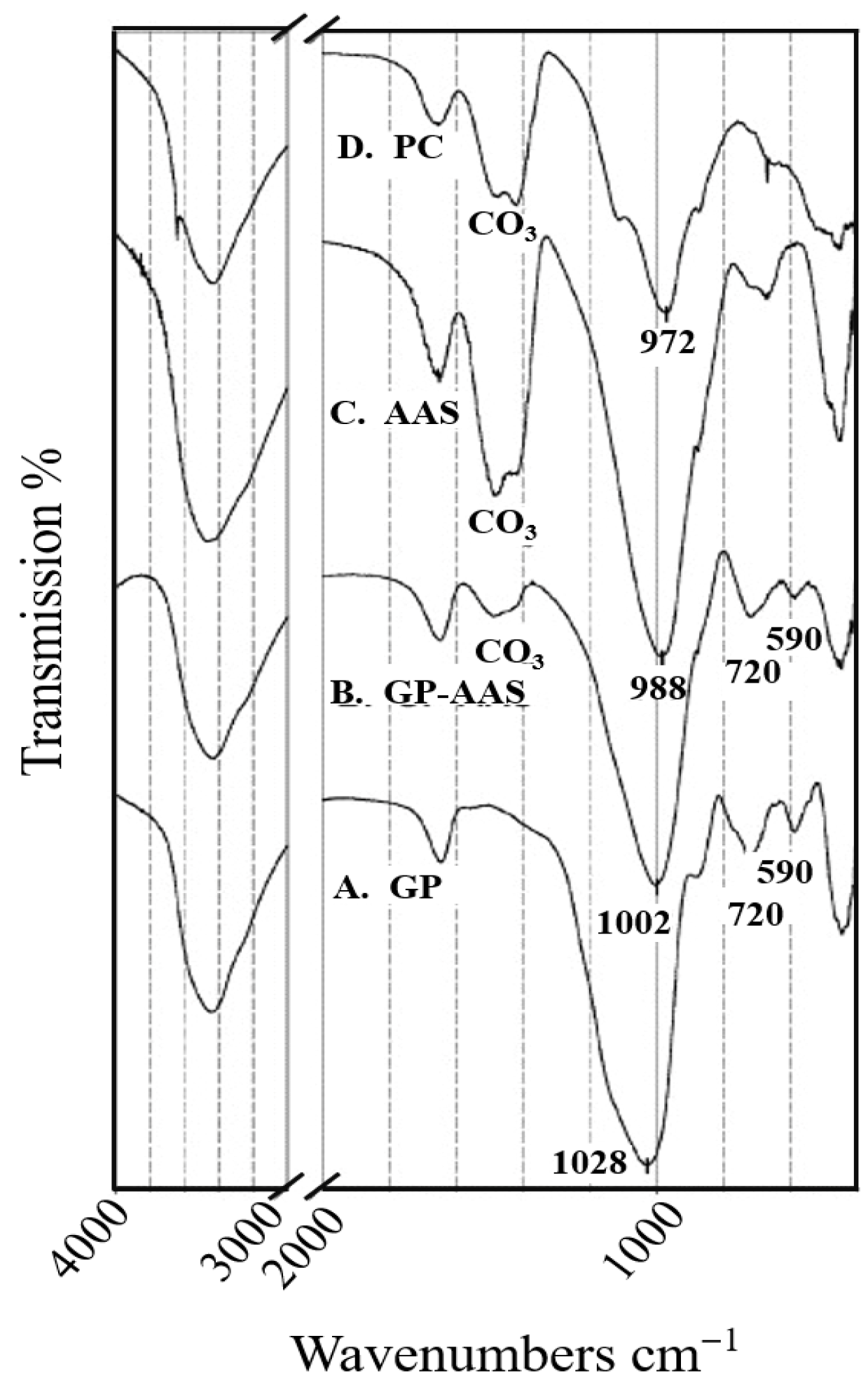
6.1. Material Characterization
6.2. Resistance to Acid Attack
6.3. Alkali-Silica Reaction (ASR)
6.4. Resistance to High Temperature
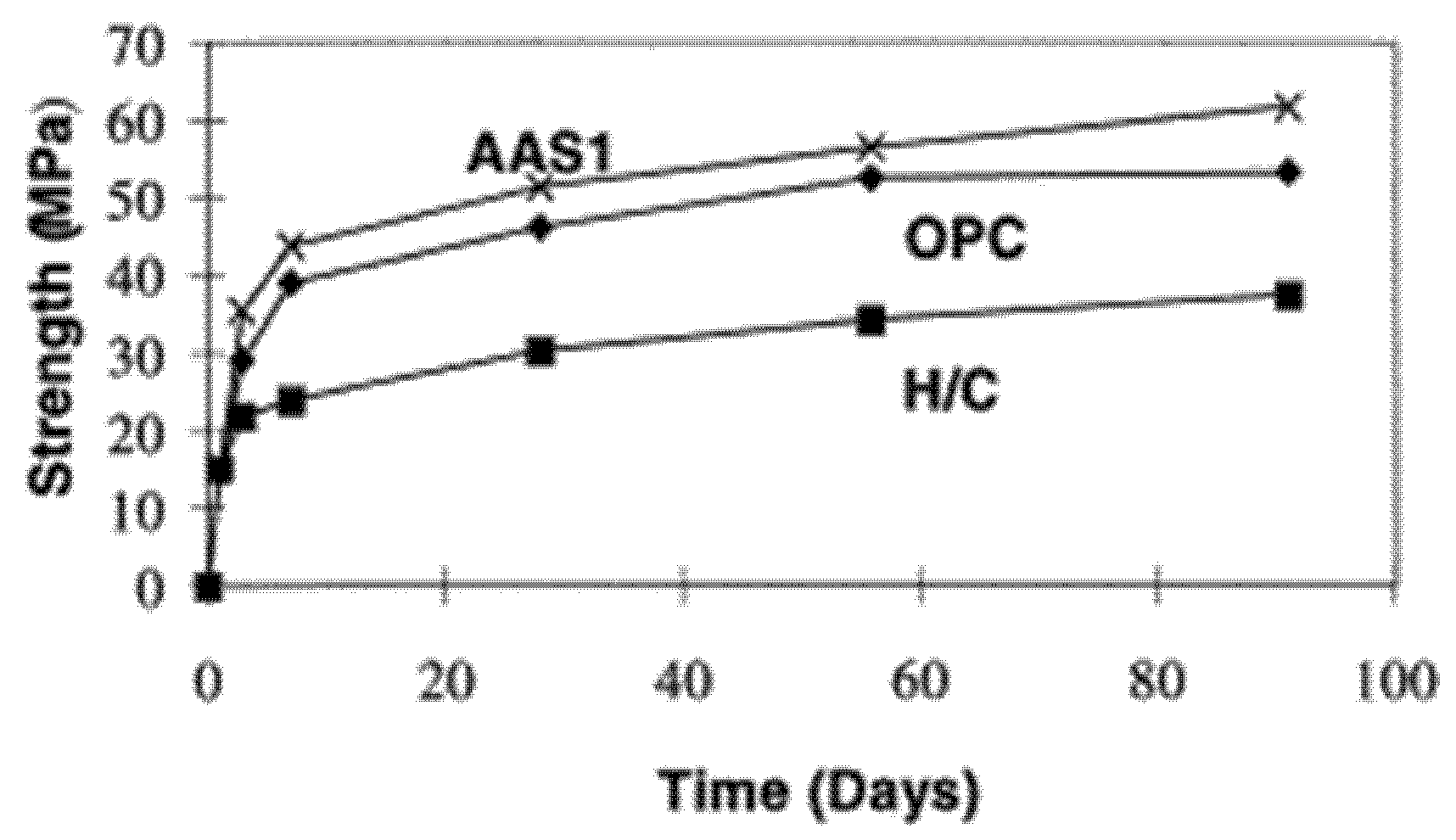
6.5. Strength Development
7. Conclusions and Future Perspectives
7.1. Conclusions
- The development and innovation of binders have evolved from lime mortars in ancient civilizations to Portland cement, driven by the availability of local resources and technological advancements. The rise of Portland cement paralleled rapid industrialization and infrastructure growth in the 19th and 20th centuries.
- Although Portland cement is dominating as the main binder in the modern era, its large-scale production is a main source of CO2 emissions and high energy consumption.
- Rapid innovation in developing different supplementary cementitious materials (SCMs) from sustainable sources provides partial solutions to reducing clinker use and lowering emissions. However, their widespread adoption in the construction industry remains limited.
- Alkali-activated binders (AABs) and geopolymer technologies represent promising alternatives to Portland cement. Although AABs outperform Portland cement in durability, acid resistance, high-temperature stability, and strength development, the complex handling of activators, the lack of standardized codes limit their applications. To increase the adoption of AABs further, usability, scalability, and regulatory hurdles need to be addressed.
7.2. Future Perspectives
- Expand the applicability and scalability of alkali-activated binders (AABs) and geopolymers as viable alternatives to traditional Portland cement locally produced from diverse industrial and agricultural by-products to meet local construction demands.
- Conduct extensive studies on the long-term durability of concrete with sustainable binders derived from industrial and agricultural wastes, including examining carbonation resistance, chloride penetration, and chemical attack to ensure their performance under aggressive environments.
- Establish regulatory frameworks and industry standards that promote the application of AABs and geopolymers, enabling broader acceptance and integration into the construction industry.
- Undertake life cycle assessments (LCA) to evaluate the effects of AABs and geopolymers on the embodied carbon, energy demand, and cost analyses of concrete to validate real-world feasibility.
- Support large-scale construction trials to bridge the gap between laboratory research and field applications, providing critical data on constructability, performance, and scalability.
- Strengthen interdisciplinary collaboration between material scientists, civil engineers, policymakers, and industry stakeholders to foster innovation in this field and adoption through proper policy management, standards and risk assessments.
- To examine the sustainability trade-offs between mechanical performance, cost and emissions, life-cycle assessment (LCA), and life-cycle cost (LCC) analysis should be performed that captures embodied energy and CO2 emissions. A combined LCA–LCC approach will enable the adoption of rational decision-making in order to balance performance, affordability, and environmental benefits.
- Digital construction methods such as 3D-printed concrete will play a significant role in adopting sustainable binders as such construction significantly uses cement. Three-dimensional-printed concrete has seen a significant rise in its adoption in the construction industry due to efficient utilization of materials, reducing formwork requirements, and enabling topology-optimized designs that reduce material waste as well as reduce binder consumption. Thus, research should focus on examining the effects of various sustainable binders in the extrusion and setting of 3D-printed concrete as well as their structural performance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacLaren, D.C.; White, M.A. Cement: Its chemistry and properties. J. Chem. Educ. 2003, 80, 623. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Mohamad, N.; Muthusamy, K.; Embong, R.; Kusbiantoro, A.; Hashim, M.H. Environmental impact of cement production and Solutions: A review. Mater. Today Proc. 2022, 48, 741–746. [Google Scholar] [CrossRef]
- Ahmed, M.; Mim, N.J.; Biswas, W.; Shaikh, F.; Zhang, X.; Patel, V.I. Effects of Partial Replacement of Cement with Fly Ash on the Mechanical Properties of Fiber-Reinforced Rubberized Concrete Containing Waste Tyre Rubber and Macro-Synthetic Fibers. Buildings 2025, 15, 2685. [Google Scholar] [CrossRef]
- Favier, A.; De Wolf, C.; Scrivener, K.; Habert, G. A Sustainable Future for the European Cement and Concrete Industry: Technology Assessment for Full Decarbonisation of the Industry by 2050; ETH Zurich: Zürich, Switzerland, 2018. [Google Scholar]
- Trout, E.A. The history of calcareous cements. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 1–29. [Google Scholar]
- Zajac, M.; Skocek, J.; Ben Haha, M.; Deja, J. CO2 mineralization methods in cement and concrete industry. Energies 2022, 15, 3597. [Google Scholar] [CrossRef]
- Ahmed, M.; Emara, M.; Patel, V.I.; Chen, W.; Zhang, X.; Hamoda, A. Experimental and numerical analysis of the flexural performance of concrete-filled steel tubular members with partial replacement of fine aggregates with sawdust. Eng. Struct. 2025, 343, 121050. [Google Scholar] [CrossRef]
- Ahmed, M.; Hamoda, A.; Yehia, S.A.; Shahin, R.I.; Abadel, A.A.; Chen, W.; Zhang, X. Flexural performance of reinforced concrete beams containing timber waste. Structures 2025, 79, 109455. [Google Scholar] [CrossRef]
- Mim, N.J.; Ahmed, M.; Zhang, X.; Shaikh, F.; Hamoda, A.; Patel, V.I.; Abadel, A.A. Investigation of Fresh, Mechanical, and Durability Properties of Rubberized Fibre-Reinforced Concrete Containing Macro-Synthetic Fibres and Tyre Waste Rubber. Buildings 2025, 15, 2778. [Google Scholar] [CrossRef]
- Hamoda, A.; Abadel, A.A.; Sennah, K.; Ahmed, M.; Zhang, X.; Emara, M. Shear Strengthening of RC Beams Incorporating Post-Tensioned Bars and Engineered Cementitious Composite Reinforced with Palm Fronds. Buildings 2024, 14, 3277. [Google Scholar] [CrossRef]
- Hamoda, A.; Abadel, A.A.; Shahin, R.I.; Ahmed, M.; Baktheer, A.; Yehia, S.A. Shear strengthening of simply supported deep beams using galvanized corrugated sheet filled with high-performance concrete. Case Stud. Constr. Mater. 2024, 21, e04085. [Google Scholar] [CrossRef]
- Hamoda, A.; Bahrami, A.; Abadel, A.A.; Ahmed, M.; Ghalla, M. Strengthening Reinforced Concrete Walls with Externally Bonded Galvanized Steel Sheets and Near-Surface Mounted Steel Bars. Buildings 2025, 15, 636. [Google Scholar] [CrossRef]
- Hamoda, A.; Shahin, R.I.; Abadel, A.A.; Sennah, K.; Ahmed, M.; Yehia, S.A. Shear strengthening of normal concrete deep beams with openings using strain-hardening cementitious composites with glass fiber mesh. Structures 2025, 71, 107994. [Google Scholar] [CrossRef]
- Huang, H.; Guo, M.; Zhang, W.; Huang, M. Seismic behavior of strengthened RC columns under combined loadings. J. Bridge Eng. 2022, 27, 05022005. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Yuan, Y.; Bai, H. Theoretical analysis on the lateral drift of precast concrete frame with replaceable artificial controllable plastic hinges. J. Build. Eng. 2022, 62, 105386. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef]
- Clifford, B.; McGee, W. Cyclopean Cannibalism. A method for recycling rubble. In Proceedings of the 38th Annual Conference of the Association for Computer Aided Design in Architecture (ACADIA), Mexico City, Mexico, 18–20 October 2018; pp. 404–413. [Google Scholar]
- Blezard, R.G. The history of calcareous cements. In Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1–32. [Google Scholar]
- Clifford, B.; McGee, W.; Muhonen, M. Recovering cannibalism in architecture with a return to cyclopean masonry. Nexus Netw. J. 2018, 20, 583–604. [Google Scholar] [CrossRef]
- Malinowski, R.; Garfinkel, Y. Prehistory of concrete. Concr. Int. 1991, 13, 62–68. [Google Scholar]
- Gençer, F.; Hamamcıoğlu-Turan, M. Hellenistic masonry techniques in southern and western Anatolia. J. Archaeol. Sci. Rep. 2022, 45, 103642. [Google Scholar] [CrossRef]
- Gan, M. Cement and Concrete; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Okonkwo, P.; Adefila, S. The kinetics of calcination of high calcium limestone. Int. J. Eng. Sci. Technol. 2012, 4, 391–400. [Google Scholar]
- Yesilata, B.; Bulut, H.; Turgut, P. Experimental study on thermal behavior of a building structure using rubberized exterior-walls. Energy Build. 2011, 43, 393–399. [Google Scholar] [CrossRef]
- Kavitha, R.; Vivekananthan, M.R.; Dhanagopal, K.; Divyabharathi, P.; Prabhakaran, M.; Ramprathap, M. An overview of water proofing system in concrete structures. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Mydin, M.A.O.; Nawi, M.N.M.; Munaaim, M.A.C. Assessment of waterproofing failures in concrete buildings and structures. Malays. Constr. Res. J. 2017, 2, 166–179. [Google Scholar]
- Shariatmadari, N.; Mohebbi, H.; Javadi, A.A. Surface stabilization of soils susceptible to wind erosion using volcanic ash–based geopolymer. J. Mater. Civ. Eng. 2021, 33, 04021345. [Google Scholar] [CrossRef]
- Gromicko, N.; Shepard, K. The History of Concrete. Six Sigma Deploy. 2003. Available online: https://www.nachi.org/history-of-concrete.htm (accessed on 14 October 2025).
- Britannica, E. Celluloid. Available online: https://www.britannica.com/technology/celluloid (accessed on 1 August 2024).
- Joshi, R.; Lohumi, S.; Joshi, R.; Kim, M.S.; Qin, J.; Baek, I.; Cho, B.-K. Raman spectral analysis for non-invasive detection of external and internal parameters of fake eggs. Sens. Actuators B Chem. 2020, 303, 127243. [Google Scholar] [CrossRef]
- Henry, M.; Therrien, T. Essential Natural Plasters: A Guide to Materials, Recipes, and Use; New Society Publishers: Gabriola, BC, Canada, 2018; Volume 7. [Google Scholar]
- Jahren, P.; Sui, T. History of Concrete: A Very old and Modern Material; World Scientific: London, UK, 2017. [Google Scholar]
- Angel, C.C. The Spatial Ordering of Nabataea: An Integrated Analysis of the Geography, Architecture, and Morphology of Nabataean Petra; University of Arkansas: Fayetteville, Arkansas, 2017. [Google Scholar]
- Zwerger, K. Wood and Wood Joints: Building Traditions of Europe, Japan and China; Birkhäuser: Basel, Switzerland, 2023. [Google Scholar]
- Sakir, S.; Raman, S.N.; Safiuddin, M.; Kaish, A.A.; Mutalib, A.A. Utilization of by-products and wastes as supplementary cementitious materials in structural mortar for sustainable construction. Sustainability 2020, 12, 3888. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Characterization of ancient, byzantine and later historic mortars by thermal and X-ray diffraction techniques. Thermochim. Acta 1995, 269, 779–795. [Google Scholar] [CrossRef]
- Andreotti, A.; Bonaduce, I.; Cantisani, E.; Degano, I.; Duman, B.; Ismaelli, T.; Salvadori, B.; Vettori, S. Ancient restoration practices in the Monumental Nymphaeum at Tripolis ad Maeandrum (Turkey): Multi-analytical approach on Roman and Byzantine bonding mortars. J. Cult. Herit. 2023, 63, 71–80. [Google Scholar] [CrossRef]
- Hughes, D.; Swann, S.; Gardner, A. Roman cement: Part one: Its origins and properties. J. Archit. Conserv. 2007, 13, 21–36. [Google Scholar] [CrossRef]
- Wang, D.; Zentar, R.; Abriak, N.E. Durability and swelling of solidified/stabilized dredged marine soils with class-F fly ash, cement, and lime. J. Mater. Civ. Eng. 2018, 30, 04018013. [Google Scholar] [CrossRef]
- Jahandari, S.; Toufigh, M.M.; Li, J.; Saberian, M. Laboratory study of the effect of degrees of saturation on lime concrete resistance due to the groundwater level increment. Geotech. Geol. Eng. 2018, 36, 413–424. [Google Scholar] [CrossRef]
- Delatte, N.J. Lessons from Roman cement and concrete. J. Prof. Issues Eng. Educ. Pract. 2001, 127, 109–115. [Google Scholar] [CrossRef]
- Thurston, A. Parker’s “Roman” Cement. Trans. Newcom. Soc. 1938, 19, 193–206. [Google Scholar] [CrossRef]
- Harrisson, A.M. Constitution and specification of Portland cement. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 501. [Google Scholar]
- Halstead, P. The early history of Portland cement. Trans. Newcom. Soc. 1961, 34, 37–54. [Google Scholar] [CrossRef]
- Hall, C. On the history of portland cement after 150 years. J. Chem. Educ. 1976, 53, 222. [Google Scholar] [CrossRef]
- The Eddystone Lighthouse. Available online: https://www.mylearning.org/stories/john-smeaton/1629 (accessed on 1 September 2025).
- Bridges. Available online: https://www.mylearning.org/stories/john-smeaton/1629 (accessed on 1 September 2025).
- Bailey, J. Science of Key Building Materials—Cementitious Substances, Iron and Steel. In Inventive Geniuses Who Changed the World: Fifty-Three Great British Scientists and Engineers and Five Centuries of Innovation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 341–361. [Google Scholar]
- Das, S.; Kizilkanat, A.B.; Chowdhury, S.; Stone, D.; Neithalath, N. Temperature-induced phase and microstructural transformations in a synthesized iron carbonate (siderite) complex. Mater. Des. 2016, 92, 189–199. [Google Scholar] [CrossRef]
- Gartmann, F. Sement i Norge 100 år (Cement in Norway 100 years); Norcem: Oslo, Norway, 1990; Available online: https://www.nb.no/items/fa897b8a0b2a0d2fe1f30b1f26a9c71c?page=0 (accessed on 14 October 2025).
- Hughes, D.; Sugden, D.; Jaglin, D.; Mucha, D. Calcination of Roman cement: A pilot study using cement-stones from Whitby. Constr. Build. Mater. 2008, 22, 1446–1455. [Google Scholar] [CrossRef]
- Hughes, D.C.; Jaglin, D.; Kozłowski, R.; Mucha, D. Roman cements—Belite cements calcined at low temperature. Cem. Concr. Res. 2009, 39, 77–89. [Google Scholar] [CrossRef]
- Klemmensen, B. Cement production in Denmark. In Green Industrial Restructuring: International Case Studies and Theoretical Interpretations; Springer: Berlin/Heidelberg, Germany, 2001; pp. 323–352. [Google Scholar]
- Bjerge, L.-M.; Brevik, P. CO2 capture in the cement industry, Norcem CO2 capture project (Norway). Energy Procedia 2014, 63, 6455–6463. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Malhotra, V. Introduction: Sustainable development and concrete technology. Concr. Int. 2002, 24, 22. [Google Scholar]
- Roy, D.M. Alkali-activated cements opportunities and challenges. Cem. Concr. Res. 1999, 29, 249–254. [Google Scholar] [CrossRef]
- Abellán-García, J.; Santofimio-Vargas, M.A.; Torres-Castellanos, N. Analysis of metakaolin as partial substitution of ordinary Portland cement in Reactive Powder Concrete. Adv. Civ. Eng. Mater. 2020, 9, 368–386. [Google Scholar] [CrossRef]
- Panjaitan, T.W.S.; Dargusch, P.; Wadley, D. Carbon management in an emissions-intensive industry in a developing economy: Cement manufacturing in Indonesia. Case Study Environ. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Emam, M.A. Factors affecting the mechanical properties of alkali activated ground granulated blast furnace slag concrete. Constr. Build. Mater. 2019, 197, 339–355. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Shi, Y.; Yang, K.; Zhou, J.; Umar, H.A.; Long, G.; Xie, Y. A comparative study on mechanical properties and environmental impact of UHPC with belite cement and portland cement. J. Clean. Prod. 2022, 380, 135003. [Google Scholar] [CrossRef]
- Rehan, R.; Nehdi, M. Carbon dioxide emissions and climate change: Policy implications for the cement industry. Environ. Sci. Policy 2005, 8, 105–114. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different additives on the properties of alkali-activated slag—A guide for Civil Engineer. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Pérez, O.F.A.; Arrieta, V.S.; Ospina, J.H.G.; Herrera, S.H.; Rojas, C.F.R.; Navarro, A.M.S. Carbon dioxide emissions from traditional and modified concrete. A review. Environ. Dev. 2024, 52, 101036. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes—A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Rodríguez Gómez, N.; Alonso Carreño, M.; Grasa Adiego, G.; Abanades García, J.C. Process for Capturing CO2 Arising from the Calcination of the CaCO3 Used in Cement Manufacture. Environ. Sci. Technol. 2008, 42, 6980–6984. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Prasad, B. Mechanical behavior of non-silicate based alkali-activated ground granulated blast furnace slag. Constr. Build. Mater. 2019, 198, 494–500. [Google Scholar] [CrossRef]
- Arunvivek, G.; Anandaraj, S.; Kumar, P.; Pratap, B.; Sembeta, R.Y. Compressive strength modelling of cenosphere and copper slag-based geopolymer concrete using deep learning model. Sci. Rep. 2025, 15, 27849. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gong, X.Z.; Cui, S.P.; Wang, Z.H.; Zheng, Y.; Chi, B.C. CO2 emissions due to cement manufacture. Mater. Sci. Forum 2011, 685, 181–187. [Google Scholar] [CrossRef]
- Carbon Dioxide Emissions from the Manufacture of Cement Worldwide from 1960 to 2023. Available online: https://www.statista.com/statistics/1299532/carbon-dioxide-emissions-worldwide-cement-manufacturing/ (accessed on 1 September 2025).
- Petit, J.-R.; Jouzel, J.; Raynaud, D.; Barkov, N.I.; Barnola, J.-M.; Basile, I.; Bender, M.; Chappellaz, J.; Davis, M.; Delaygue, G. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 1999, 399, 429–436. [Google Scholar] [CrossRef]
- Paswan, R.K.; Kumar, P.; Kumar, V.; Sembeta, R.Y. Mechanical properties of alkali activated slag binder based concrete at elevated temperatures. Discov. Sustain. 2025, 6, 744. [Google Scholar] [CrossRef]
- Sahu, A.; Kumar, P.; Pratap, B.; Gogineni, A.; Sembeta, R.Y. Thermal and mechanical performance of geopolymer concrete with recycled aggregate and copper slag as fine aggregate. Sci. Rep. 2025, 15, 28968. [Google Scholar] [CrossRef]
- Kassim, U.; Ong, B.P. Performance of concrete incorporating of clam shell as partially replacement of ordinary Portland cement (OPC). J. Adv. Res. Appl. Mech. 2019, 55, 12–21. [Google Scholar]
- Xie, J.; Wu, Z.; Zhang, X.; Hu, X.; Shi, C. Trends and developments in low-heat portland cement and concrete: A review. Constr. Build. Mater. 2023, 392, 131535. [Google Scholar] [CrossRef]
- Torres, M.; Puertas, F.; Blanco-Varela, M. Preparación de cementos alcalinos a partir de residuos vítreos. solubilidad de residuos vítreos en medios fuertemente básicos. In Proceedings of the XII Congreso Nacional de Materiales, XII Congreso Iberoamericano de Materiales, Alicante, Spain, 25–27 September 2024. [Google Scholar]
- Kumar, P.S.; Kumar, P.; Manikandan, N.; Thejasree, P.; Sunheriya, N.; Giri, J.; Chadge, R.; Sathish, T.; Kumar, A.; Ammarullah, M.I. A sustainable bioengineering approach for enhancing black cotton soil stability using waste foundry sand. Int. J. Low-Carbon Technol. 2025, 20, 1112–1120. [Google Scholar] [CrossRef]
- Juenger, M.C.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Kirkpatrick, R.J. Innovation in use and research on cementitious material. Cem. Concr. Res. 2008, 38, 128–136. [Google Scholar] [CrossRef]
- Tkachenko, N.; Tang, K.; McCarten, M.; Reece, S.; Kampmann, D.; Hickey, C.; Bayaraa, M.; Foster, P.; Layman, C.; Rossi, C. Global database of cement production assets and upstream suppliers. Sci. Data 2023, 10, 696. [Google Scholar] [CrossRef]
- De Schepper, M.; De Buysser, K.; Van Driessche, I.; De Belie, N. The regeneration of cement out of Completely Recyclable Concrete: Clinker production evaluation. Constr. Build. Mater. 2013, 38, 1001–1009. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Zhang, G.; Xiong, B. Energy revolution: From a fossil energy era to a new energy era. Nat. Gas Ind. B 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Kumar, P.; Upadhyay, R.; Sharma, K.K.; Madhuri, S.; Gogineni, A.; Vagestan, P.K. Mechanical performance of fiber reinforced concrete incorporating rice husk Ash and brick aggregate. Innov. Infrastruct. Solut. 2025, 10, 362. [Google Scholar] [CrossRef]
- Teker Ercan, E.E.; Andreas, L.; Cwirzen, A.; Habermehl-Cwirzen, K. Wood ash as sustainable alternative raw material for the production of concrete—A review. Materials 2023, 16, 2557. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A.; Real, S.; Meireles, I. Industrial production of recycled cement: Energy consumption and carbon dioxide emission estimation. Environ. Sci. Pollut. Res. 2023, 30, 8778–8789. [Google Scholar] [CrossRef]
- Yang, K.-H.; Jung, Y.-B.; Cho, M.-S.; Tae, S.-H. Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J. Clean. Prod. 2015, 103, 774–783. [Google Scholar] [CrossRef]
- Duxson, P. Geopolymer precursor design. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 37–49. [Google Scholar]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Onyenokporo, N.C. Supplementary cementitious materials as sustainable partial replacement for cement in the building industry. Int. J. Archit. Environ. Eng. 2022, 16, 74–84. [Google Scholar]
- Aprianti, E. A huge number of artificial waste material can be supplementary cementitious material (SCM) for concrete production–a review part II. J. Clean. Prod. 2017, 142, 4178–4194. [Google Scholar] [CrossRef]
- Haha, M.B.; Termkhajornkit, P.; Ouzia, A.; Uppalapati, S.; Huet, B. Low clinker systems-Towards a rational use of SCMs for optimal performance. Cem. Concr. Res. 2023, 174, 107312. [Google Scholar]
- Panesar, D.K.; Zhang, R. Performance comparison of cement replacing materials in concrete: Limestone fillers and supplementary cementing materials–A review. Constr. Build. Mater. 2020, 251, 118866. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, S.; Kumar, P.S.; Yuvaraj, M.; Purushotham, D.; Kumar, S.; Vashistha, K.K.; Kumar, A.; Gogineni, A. Thermal performance prediction for alkali-activated concrete using GGBFS, NaOH, and sodium silicate. Civ. Eng. Infrastruct. J. 2024, in press. [Google Scholar] [CrossRef]
- Thomas, M. Supplementary Cementing Materials in Concrete; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Kumar, P.; Pratap, B.; Sahu, A. Recycled aggregate with GGBS geopolymer concrete behaviour on elevated temperatures. J. Struct. Fire Eng. 2025, 16, 118–137. [Google Scholar] [CrossRef]
- Malhotra, V.; Hammings, R. Blended cements in North America—A review. Cem. Concr. Compos. 1995, 17, 23–35. [Google Scholar] [CrossRef]
- Fidjestol, P.; Dastol, M. The history of silica fume in concrete from novelty to key ingredient in high performance concrete. In Proceedings of the Congresso Brasileiro do Concreto, Salvador, Brazil, 4–9 September 2008. [Google Scholar]
- Kumar, P.; Gogineni, A.; Upadhyay, R. Mechanical performance of fiber-reinforced concrete incorporating rice husk ash and recycled aggregates. J. Build. Pathol. Rehabil. 2024, 9, 144. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K.; Geng, Y. Evaluation and mitigation of cement CO2 emissions: Projection of emission scenarios toward 2030 in China and proposal of the roadmap to a low-carbon world by 2050. Mitig. Adapt. Strateg. Glob. Change 2019, 24, 301–328. [Google Scholar] [CrossRef]
- García-Segura, T.; Yepes, V.; Alcalá, J. Life cycle greenhouse gas emissions of blended cement concrete including carbonation and durability. Int. J. Life Cycle Assess. 2014, 19, 3–12. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Bano, S.; Kumar, P. Soft computing techniques for analysing the mechanical properties of egg shell powder-based concrete: DOI registering. Adv. Civ. Archit. Eng. 2024, 15, 119–132. [Google Scholar] [CrossRef]
- Purdon, A. The action of alkalis on blast-furnace slag. J. Soc. Chem. Ind. 1940, 59, 191–202. [Google Scholar]
- Zain, M.F.M.; Islam, M.N.; Mahmud, F.; Jamil, M. Production of rice husk ash for use in concrete as a supplementary cementitious material. Constr. Build. Mater. 2011, 25, 798–805. [Google Scholar] [CrossRef]
- Thiedeitz, M.; Schmidt, W.; Härder, M.; Kränkel, T. Performance of rice husk ash as supplementary cementitious material after production in the field and in the lab. Materials 2020, 13, 4319. [Google Scholar] [CrossRef] [PubMed]
- Isberto, C.D.; Labra, K.L.; Landicho, J.M.B.; De Jesus, R. Optimized preparation of rice husk ash (RHA) as a supplementary cementitious material. GEOMATE J. 2019, 16, 56–61. [Google Scholar] [CrossRef]
- Hasan, N.M.S.; Sobuz, M.H.R.; Khan, M.M.H.; Mim, N.J.; Meraz, M.M.; Datta, S.D.; Rana, M.J.; Saha, A.; Akid, A.S.M.; Mehedi, M.T. Integration of rice husk ash as supplementary cementitious material in the production of sustainable high-strength concrete. Materials 2022, 15, 8171. [Google Scholar] [CrossRef]
- Amin, M.N.; Khan, K.; Arab, A.M.A.; Farooq, F.; Eldin, S.M.; Javed, M.F. Prediction of sustainable concrete utilizing rice husk ash (RHA) as supplementary cementitious material (SCM): Optimization and hyper-tuning. J. Mater. Res. Technol. 2023, 25, 1495–1536. [Google Scholar] [CrossRef]
- Mosaberpanah, M.A.; Umar, S.A. Utilizing rice husk ash as supplement to cementitious materials on performance of ultra high performance concrete—A review. Mater. Today Sustain. 2020, 7, 100030. [Google Scholar] [CrossRef]
- Jhatial, A.A.; Goh, W.I.; Mastoi, A.K.; Rahman, A.F.; Kamaruddin, S. Thermo-mechanical properties and sustainability analysis of newly developed eco-friendly structural foamed concrete by reusing palm oil fuel ash and eggshell powder as supplementary cementitious materials. Environ. Sci. Pollut. Res. 2021, 28, 38947–38968. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Tahir, M.M.; Mirza, J.; Khalid, N.H.A.; Asaad, M.A.; Husein, A.A.; Sarbini, N.N. Synergism between palm oil fuel ash and slag: Production of environmental-friendly alkali activated mortars with enhanced properties. Constr. Build. Mater. 2018, 170, 235–244. [Google Scholar] [CrossRef]
- Fode, T.A.; Jande, Y.A.C.; Kivevele, T. Effects of different supplementary cementitious materials on durability and mechanical properties of cement composite–Comprehensive review. Heliyon 2023, 9, e17924. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Stoica, A.E.; Vrabec, M.; Šmuc Rogan, N.; Sturm, S.; Ow-Yang, C.; Gulgun, M.A.; Bundur, Z.B.; Ciuca, I.; Vasile, B.S. End-of-life materials used as supplementary cementitious materials in the concrete industry. Materials 2020, 13, 1954. [Google Scholar] [CrossRef] [PubMed]
- Adamu, M.; Alanazi, H.; Ibrahim, Y.E.; Abdellatief, M. Mechanical, microstructural characteristics and sustainability analysis of concrete incorporating date palm ash and eggshell powder as ternary blends cementitious materials. Constr. Build. Mater. 2024, 411, 134753. [Google Scholar] [CrossRef]
- Awal, A.A.; Shehu, I.; Ismail, M. Effect of cooling regime on the residual performance of high-volume palm oil fuel ash concrete exposed to high temperatures. Constr. Build. Mater. 2015, 98, 875–883. [Google Scholar] [CrossRef]
- Thomas, B.S.; Yang, J.; Bahurudeen, A.; Abdalla, J.A.; Hawileh, R.A.; Hamada, H.M.; Nazar, S.; Jittin, V.; Ashish, D.K. Sugarcane bagasse ash as supplementary cementitious material in concrete–a review. Mater. Today Sustain. 2021, 15, 100086. [Google Scholar] [CrossRef]
- Yadav, A.L.; Sairam, V.; Muruganandam, L.; Srinivasan, K. An overview of the influences of mechanical and chemical processing on sugarcane bagasse ash characterisation as a supplementary cementitious material. J. Clean. Prod. 2020, 245, 118854. [Google Scholar] [CrossRef]
- Agwa, I.S.; Zeyad, A.M.; Tayeh, B.A.; Adesina, A.; de Azevedo, A.R.; Amin, M.; Hadzima-Nyarko, M. A comprehensive review on the use of sugarcane bagasse ash as a supplementary cementitious material to produce eco-friendly concretes. Mater. Today Proc. 2022, 65, 688–696. [Google Scholar] [CrossRef]
- Jittin, V.; Minnu, S.; Bahurudeen, A. Potential of sugarcane bagasse ash as supplementary cementitious material and comparison with currently used rice husk ash. Constr. Build. Mater. 2021, 273, 121679. [Google Scholar] [CrossRef]
- Cordeiro, G.; Paiva, O.; Toledo Filho, R.; Fairbairn, E.; Tavares, L. Long-term compressive behavior of concretes with sugarcane bagasse ash as a supplementary cementitious material. J. Test. Eval. 2018, 46, 564–573. [Google Scholar] [CrossRef]
- Arshad, S.; Sharif, M.B.; Irfan-ul-Hassan, M.; Khan, M.; Zhang, J.-L. Efficiency of supplementary cementitious materials and natural fiber on mechanical performance of concrete. Arab. J. Sci. Eng. 2020, 45, 8577–8589. [Google Scholar] [CrossRef]
- Acordi, J.; Luza, A.; Fabris, D.; Raupp-Pereira, F.; De Noni Jr, A.; Montedo, O. New waste-based supplementary cementitious materials: Mortars and concrete formulations. Constr. Build. Mater. 2020, 240, 117877. [Google Scholar] [CrossRef]
- Fantilli, A.P.; Jóźwiak-Niedźwiedzka, D. Supplementary cementitious materials in concrete, part I. Materials 2021, 14, 2291. [Google Scholar] [CrossRef] [PubMed]
- Wirth, X.; Benkeser, D.; Yeboah, N.N.; Shearer, C.; Kurtis, K.; Burns, S. Evaluation of alternative fly ashes as supplementary cementitious materials. ACI Mater. J. 2019, 116, 69–77. [Google Scholar] [CrossRef]
- Yang, Y.; Takasu, K.; Suyama, H.; Ji, X.; Xu, M.; Liu, Z. Comparative analysis of Woody biomass fly Ash and class F fly Ash as supplementary cementitious materials in mortar. Materials 2024, 17, 3723. [Google Scholar] [CrossRef]
- Thomas, B.S.; Yang, J.; Mo, K.H.; Abdalla, J.A.; Hawileh, R.A.; Ariyachandra, E. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Build. Eng. 2021, 40, 102332. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Water resistance of magnesium oxychloride cement wood board with the incorporation of supplementary cementitious materials. Constr. Build. Mater. 2020, 255, 119145. [Google Scholar] [CrossRef]
- Martínez-García, R.; Jagadesh, P.; Zaid, O.; Șerbănoiu, A.A.; Fraile-Fernández, F.J.; de Prado-Gil, J.; Qaidi, S.M.; Grădinaru, C.M. The present state of the use of waste wood ash as an eco-efficient construction material: A review. Materials 2022, 15, 5349. [Google Scholar] [CrossRef]
- Ahmed, A.; Kamau, J. A review of the use of corncob ash as a supplementary cementitious material. Eur. J. Eng. Technol. Res. 2017, 2, 1–6. [Google Scholar]
- Shakouri, M.; Exstrom, C.L.; Ramanathan, S.; Suraneni, P. Hydration, strength, and durability of cementitious materials incorporating untreated corn cob ash. Constr. Build. Mater. 2020, 243, 118171. [Google Scholar] [CrossRef]
- Bheel, N.; Ali, M.O.A.; Liu, Y.; Tafsirojjaman, T.; Awoyera, P.; Sor, N.H.; Bendezu Romero, L.M. Utilization of corn cob ash as fine aggregate and ground granulated blast furnace slag as cementitious material in concrete. Buildings 2021, 11, 422. [Google Scholar] [CrossRef]
- Labianca, C.; Ferrara, C.; Zhang, Y.; Zhu, X.; De Feo, G.; Hsu, S.-C.; You, S.; Huang, L.; Tsang, D.C. Alkali-activated binders—A sustainable alternative to OPC for stabilization and solidification of fly ash from municipal solid waste incineration. J. Clean. Prod. 2022, 380, 134963. [Google Scholar] [CrossRef]
- Wei, M.; Chen, L.; Lei, N.; Li, H.; Huang, L. Mechanical properties and microstructures of thermally activated ultrafine recycled fine powder cementitious materials. Constr. Build. Mater. 2025, 475, 141195. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, Z.; Su, Z.; Cao, W.; Shi, D.; Hao, X.; Zhang, S.; Wang, Z.; Xu, X.; Zhu, J. A diffusion-controlled kinetic model for binder burnout in a green part fabricated by binder jetting based on the thermal decomposition kinetics of TEG-DMA. Addit. Manuf. 2025, 105, 104793. [Google Scholar] [CrossRef]
- Higuchi, A.M.D.; dos Santos Marques, M.G.; Ribas, L.F.; de Vasconcelos, R.P. Use of glass powder residue as an eco-efficient supplementary cementitious material. Constr. Build. Mater. 2021, 304, 124640. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, R.; Bai, Y.; Huang, B.; Ma, Y. Influence of waste glass powder as a supplementary cementitious material (SCM) on physical and mechanical properties of cement paste under high temperatures. J. Clean. Prod. 2022, 340, 130778. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Bameri, M.; Mohammadhasani, M.; Khaloo, A.; Ashour, A.; NikKhah, M. Influence of Waste Glass Powder as a Supplementary Cementitious Material (SCM) on the Mechanical Properties, Expansion, Environmental Impact, and Microstructure of Cementitious Mortar. Eng. Rep. 2025, 7, e70238. [Google Scholar] [CrossRef]
- Dai, T.; Fang, C.; Liu, T.; Zheng, S.; Lei, G.; Jiang, G. Waste glass powder as a high temperature stabilizer in blended oil well cement pastes: Hydration, microstructure and mechanical properties. Constr. Build. Mater. 2024, 439, 137359. [Google Scholar] [CrossRef]
- Hassan, A.; Alomayri, T.; Noaman, M.F.; Zhang, C. 3D printed concrete for sustainable construction: A review of mechanical properties and environmental impact. Arch. Comput. Methods Eng. 2025, 32, 2713–2743. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, Y.; Zhang, W.; Ye, Z.; Li, Z.; Liang, Y. Thermal insulation and high-temperature resistant cement-based materials with different pore structure characteristics: Performance and high-temperature testing. J. Build. Eng. 2025, 101, 111839. [Google Scholar] [CrossRef]
- Shi, T.; Li, K.-M.; Wang, C.-Z.; Jin, Z.; Hao, X.-K.; Sun, P.; Han, Y.-X.; Pan, C.-G.; Fu, N.; Wang, H.-B. Fracture Toughness of Recycled Carbon Fibers Reinforced Cement Mortar and its Environmental Impact Assessment. Case Stud. Constr. Mater. 2025, 22, e04866. [Google Scholar] [CrossRef]
- Paswan, R.K.; Gogineni, A.; Sharma, S.; Kumar, P. Predicting split tensile strength in Portland and geopolymer concretes using machine learning algorithms: A comparative study. J. Build. Pathol. Rehabil. 2024, 9, 129. [Google Scholar] [CrossRef]
- Feret, R. Slags for the manufacture of cement. Rev. Mater. Constr. Trav. Publ. 1939, 1–145. [Google Scholar]
- Glukhovsky, V. Gruntosilikaty (Soil Silicates); Gosstroyizdat: Kiev, Ukraine, 1959. [Google Scholar]
- Glukhovsky, V.; Krivenko, P. Alkaline and Alkaline-Alkaline Earth Hydraulic Binders and Concretes; Vyscha Shkola Publish: Kiev, Ukraine, 1979. [Google Scholar]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Forss, B. Experiences from the use of F-cement—A binder based on alkali-activated blastfurnace slag. Alkalis Concr. Dan. Concr. Assoc. Cph. Den. 1983, 101–104. [Google Scholar]
- Barnes, M.W.; Langton, C.A.; Roy, D.M. Leaching of saltstone. MRS Online Proc. Libr. (OPL) 1984, 44, 865. [Google Scholar] [CrossRef]
- Krivenko, P. Sintez Vyazhushchih so Special’nymi Svojstvami v Sisteme Me2O-MeO-Me2O3-SiO2-H2O. Ph.D. Thesis, KPI, Kyiv, Ukraine, 1986. [Google Scholar]
- Malek, R.I.; Roy, D.; Langton, C. Slag Cement-Low Level Radioactive Waste Forms at Savannah River Plant. Am. Ceram. Soc. Bull. 1986, 65. [Google Scholar]
- Davidovits, J. Ancient and modern concretes: What is the real difference? Concr. Int. 1987, 9, 23–28. [Google Scholar]
- Roy, D.M.; Langton, C. Studies of Ancient Concrete as Analogs of Cementitious Sealing Materials for a Repository in Tuff; Los Alamos National Lab.(LANL): Los Alamos, NM, USA, 1989. [Google Scholar]
- Talling, B.; Brandstetr, J. Present state and future of alkali-activated slag concretes. Spec. Publ. 1989, 114, 1519–1546. [Google Scholar]
- Wu, X.; Jiang, W.; Roy, D.M. Early activation and properties of slag cement. Cem. Concr. Res. 1990, 20, 961–974. [Google Scholar] [CrossRef]
- Roy, D.M.; Silsbee, M. Alkali activated cementitious materials: An overview. MRS Online Proc. Libr. (OPL) 1991, 245, 153. [Google Scholar] [CrossRef]
- Palomo, A.; Glasser, F.P. Chemically-bonded cementitious materials based on metakaolin. Br. ceramic. Trans. J. 1992, 91, 107–112. [Google Scholar]
- Roy, D.M.; Malek, R. Hydration of slag cement. In Progress in Cement and Concrete–Mineral Admixtures in Cement and Concrete; Ghosh, S.N., Ed.; ABI books Pvt. Ltd.: Delhi, India, 1993; Volume 4, pp. 84–117. [Google Scholar]
- Glukhovsky, V. Ancient, modern and future concretes. In Proceedings of the First International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 11–14 October 1994; pp. 1–8. [Google Scholar]
- Krivenko, P. Special Slag Alkaline Cements; Budivelnyk: Kiev, Ukraine, 1992. [Google Scholar]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Shi, C. Strength, pore structure and permeability of alkali-activated slag mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F. Alkali-activated slag cements: Kinetic studies. Cem. Concr. Res. 1997, 27, 359–368. [Google Scholar] [CrossRef]
- Katz, A. Microscopic study of alkali-activated fly ash. Cem. Concr. Res. 1998, 28, 197–208. [Google Scholar] [CrossRef]
- Davidovits, J. Chemistry of Geopolymeric systems, terminology. In 99 Geopolymer International Conference Proceedings; Davidovits, J., Davidovits, R., James, C., Eds.; Geopolymer Institute: Saint-Quentin, France, 1999. [Google Scholar]
- Palomo, A.; Grutzeck, M.; Blanco, M. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Gong, C.; Yang, N. Effect of phosphate on the hydration of alkali-activated red mud–slag cementitious material. Cem. Concr. Res. 2000, 30, 1013–1016. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Effect of admixtures on properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Palomo, A.; Palacios, M. Alkali-activated cementitious materials: Alternative matrices for the immobilisation of hazardous wastes: Part II. Stabilisation Chromium lead. Cem. Concr. Res. 2003, 33, 289–295. [Google Scholar] [CrossRef]
- Grutzeck, M.; Kwan, S.; DiCola, M. Zeolite formation in alkali-activated cementitious systems. Cem. Concr. Res. 2004, 34, 949–955. [Google Scholar] [CrossRef]
- Sun, H.H.; Li, Y.; Wang, N.; Zhao, Y.H. Sialite technology and cycle economy. Key Eng. Mater. 2007, 336, 1895–1897. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; Van Deventer, J.S. One-part geopolymer mixes from geothermal silica and sodium aluminate. Ind. Eng. Chem. Res. 2008, 47, 9396–9405. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Júnior, N.T.A.; Lima, V.M.; Torres, S.M.; Basto, P.E.; Neto, A.A.M. Experimental investigation of mix design for high-strength alkali-activated slag concrete. Constr. Build. Mater. 2021, 291, 123387. [Google Scholar]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Yang, E.-H. Alkali-activated ground granulated blast-furnace slag incorporating incinerator fly ash as a potential binder. Constr. Build. Mater. 2016, 112, 1005–1012. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J. A new supplementary cementitious material: Walnut shell ash. Constr. Build. Mater. 2023, 408, 133852. [Google Scholar] [CrossRef]
- Manjunath, B.; Ouellet-Plamondon, C.M.; Ganesh, A.; Das, B.; Bhojaraju, C. Valorization of coffee cherry waste ash as a sustainable construction material. J. Build. Eng. 2024, 97, 110796. [Google Scholar] [CrossRef]
- Murali, G.; Lee, D.; Wong, L.S.; Abdulkadir, I. Towards sustainable construction: Strength and microstructural assessment of Dillenia suffruticosa and Acacia auriculiformis leaf ash as novel supplementary cementitious materials. J. Build. Eng. 2024, 97, 110727. [Google Scholar] [CrossRef]
- Park, C.; Lee, J. Transforming biowaste into sustainable supplementary cementitious materials. J. Build. Eng. 2024, 98, 110976. [Google Scholar] [CrossRef]
- Singh, A.; Panghal, H.; Rath, D.K.; Kumar, R.; Chaudhary, S. Evaluating the potential of oil seed extract ashes from niger, cotton, and flaxseed as sustainable supplementary cementitious materials. Sustain. Energy Technol. Assess. 2025, 76, 104285. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Gouda, S.; Adediran, A.; Isteri, V.; Tanskanen, P.; Kilpimaa, K. Recycling analcime residues of lithium production from spodumene ore in eco-friendly cementitious binders. Case Stud. Constr. Mater. 2025, 22, e04556. [Google Scholar] [CrossRef]
- Amin, M.T.E.; Sarker, P.K.; Shaikh, F.U.A. Effect of lithium slag as supplementary cementitious material on sulphate attack resistance of cement mortar. J. Build. Eng. 2025, 108, 112858. [Google Scholar] [CrossRef]
- Amin, M.T.E.; Sarker, P.K.; Shaikh, F.U.A.; Hosan, A. Chloride permeability and chloride-induced corrosion of concrete containing lithium slag as a supplementary cementitious material. Constr. Build. Mater. 2025, 471, 140629. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.; Zhou, X.; Zheng, Y.; Liu, Z. Effect of alkali activator and granulated blast furnace slag on the properties of lithium slag-based high-strength lightweight aggregates. Sci. Rep. 2025, 15, 30115. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Y.; Chen, Y.; Deng, J.; Li, S.; Sun, G. Synergistic improvement in cement performance via combined use of cationic polymer-grafted nano-silica with silica fume and fly ash. J. Build. Eng. 2025, 112, 113977. [Google Scholar] [CrossRef]
- Kuehl, H. Slag Cement and Process of Making the Same. U.S. Patent No. 900,939, 13 October 1908. [Google Scholar]
- Glukhovsky, V. Soil Silicates; Gosstroyizdat: Kiev, Ukraine, 1959; Volume 154. [Google Scholar]
- Krivenko, P. Synthesis of Cementitious Materials of the Me2O-MeO-Me2O3-SiO2-H2O System with Required Properties. Ph.D. Thesis, KISI Publish Kiev USSR, Kyiv, Ukraine, 1986. [Google Scholar]
- Glukhovsky, V.; Rostovskaja, G.; Rumyna, G. High strength slag-alkaline cements. In Proceedings of the Seventh International Congress on the Chemistry of Cement, Paris, France; 1980; pp. 164–168. [Google Scholar]
- Glukhovsky, V. Slag-Alkali Concretes Produced from Fine-Grained Aggregate; Vishcha Shkolay: Kiev, Ukraine, 1981. [Google Scholar]
- Davidovits, J.; Cordi, S. Synthesis of new high temperature geo-polymers for reinforced plastics/composites. Spe Pactec 1979, 79, 151–154. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer chemistry and sustainable development. The poly (sialate) terminology: A very useful and simple model for the promotion and understanding of green-chemistry. In Proceedings of the 2005 Geopolymer Conference, Saint-Quentin, France, 29 June–1 July 2005; pp. 9–15. [Google Scholar]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J. The geopolymerisation of natural alumino-silicates. In Proceedings of the 2nd International Conference Geopolymere, Paris, France, 30 June–2 July 1999; pp. 43–63. [Google Scholar]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition–A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Caglar, B.; Çırak, Ç.; Tabak, A.; Afsin, B.; Eren, E. Covalent grafting of pyridine-2-methanol into kaolinite layers. J. Mol. Struct. 2013, 1032, 12–22. [Google Scholar] [CrossRef]
- Feng, X.; Garboczi, E.J.; Bentz, D.P.; Stutzman, P.E.; Mason, T.O. Estimation of the degree of hydration of blended cement pastes by a scanning electron microscope point-counting procedure. Cem. Concr. Res. 2004, 34, 1787–1793. [Google Scholar] [CrossRef]
- Pratap, B.; Paswan, R.K.; Kumar, P. Mechanical and micro-characterization of alkali-activated concrete under high-temperature exposure. Struct. Concr. 2025, 26, 1911–1923. [Google Scholar] [CrossRef]
- Brough, A.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Pratap, B.; Kumar, P.; Shubham, K.; Chaudhary, N. Soft computing-based investigation of mechanical properties of concrete using ready-mix concrete waste water as partial replacement of mixing portable water. Asian J. Civ. Eng. 2024, 25, 1255–1266. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali–slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- van Jaarsveld, J.; Van Deventer, J. Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Pratap, B.; Kumar, P. Effect of the elevated temperature on the mechanical properties of geopolymer concrete using fly ash and ground granulated blast slag. J. Struct. Fire Eng. 2024, 15, 409–425. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Calorimetric study of alkaline activation of calcium hydroxide–metakaolin solid mixtures. Cem. Concr. Res. 2001, 31, 25–30. [Google Scholar] [CrossRef]
- Kumar, P.; Pratap, B.; Sharma, S.; Kumar, I. Compressive strength prediction of fly ash and blast furnace slag-based geopolymer concrete using convolutional neural network. Asian J. Civ. Eng. 2024, 25, 1561–1569. [Google Scholar] [CrossRef]
- Lee, W.; Van Deventer, J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Z.; Su, X.; Feng, Y.; Xu, T.; Zheng, Z.; Liu, Y.; Li, P. Improving sulfate and chloride resistance in eco-friendly marine concrete: Alkali-activated slag system with mineral admixtures. Constr. Build. Mater. 2024, 411, 134333. [Google Scholar] [CrossRef]
- Jin, M.; Li, W.; Wang, X.; Tang, J.; Teng, L.; Ma, Y.; Zeng, H. Investigating the dual influence of freezing-thawing cycles and chloride ion penetration on GGBS-AEA concrete deterioration. J. Build. Eng. 2023, 78, 107759. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Effect of elevated temperature curing on properties of alkali-activated slag concrete. Cem. Concr. Res. 1999, 29, 1619–1625. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W. Identification of silicooxygen rings in SiO2 based on IR spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 1819–1823. [Google Scholar] [CrossRef]
- Gogineni, A.; Panday, I.K.; Kumar, P.; Paswan, R.K. Predictive modelling of concrete compressive strength incorporating GGBS and alkali using a machine-learning approach. Asian J. Civ. Eng. 2024, 25, 699–709. [Google Scholar] [CrossRef]
- Li, W.; Tang, Z.; Tam, V.W.; Zhao, X.; Wang, K. A review on durability of alkali-activated system from sustainable construction materials to infrastructures. ES Mater. Manuf. 2019, 4, 2–19. [Google Scholar] [CrossRef]
- Davidovits, J.; Comrie, D.C.; Paterson, J.H.; Ritcey, D.J. Geopolymeric concretes for environmental protection. Concr. Int. 1990, 12, 30–40. [Google Scholar]
- Palomo, A.; Blanco-Varela, M.T.; Granizo, M.; Puertas, F.; Vazquez, T.; Grutzeck, M. Chemical stability of cementitious materials based on metakaolin. Cem. Concr. Res. 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Shi, C.; Stegemann, J. Acid corrosion resistance of different cementing materials. Cem. Concr. Res. 2000, 30, 803–808. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Song, X.; Marosszeky, M.; Brungs, M.; Munn, R. Durability of fly ash based geopolymer concrete against sulphuric acid attack. In Proceedings of the International Conference on Durability of Building Materials and Components, Lyon, France, 17–20 April 2005. [Google Scholar]
- Fernando, P.-T.; João, C.-G.; Said, J. Durability and environmental performance of alkali-activated tungsten mine waste mud mortars. J. Mater. Civ. Eng. 2010, 22, 897–904. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Mejía de Gutiérrez, R.; Provis, J.L. Performance of alkali-activated slag mortars exposed to acids. J. Sustain. Cem. Based Mater. 2012, 1, 138–151. [Google Scholar] [CrossRef]
- Israel, D.; Macphee, D.E.; Lachowski, E. Acid attack on pore-reduced cements. J. Mater. Sci. 1997, 32, 4109–4116. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Stanton, T.E. Expansion of concrete through reaction between cement and aggregate. Trans. Am. Soc. Civ. Eng. 1942, 107, 54–84. [Google Scholar] [CrossRef]
- Wood, J.; Johnson, R. The appraisal and maintenance of structures with alkali-silica reaction. Struct. Eng. 1993, 2, 19–23. [Google Scholar]
- Fernández-Jiménez, A.; Puertas, F. The alkali–silica reaction in alkali-activated granulated slag mortars with reactive aggregate. Cem. Concr. Res. 2002, 32, 1019–1024. [Google Scholar] [CrossRef]
- Puertas, F. Cementos de escorias activadas alcalinamente: Situación actual y perspectivas de futuro. Mater. Construcción 1995, 45, 53–64. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to alkali–aggregate reaction. Cem. Concr. Res. 2001, 31, 331–334. [Google Scholar] [CrossRef]
- Pawlasová, S.; Skavara, F. High-temperature properties of geopolymer materials. In Proceedings of the Alkali Activated Materials-Research, Production and Utilization 3rd Conference, Prague, Czech Republic, 21–22 June 2007; p. 524. [Google Scholar]
- Kumar, P.; Pratap, B. Feature engineering for predicting compressive strength of high-strength concrete with machine learning models. Asian J. Civ. Eng. 2024, 25, 723–736. [Google Scholar] [CrossRef]
- Gogineni, A.; Panday, I.K.; Kumar, P.; Paswan, R.K. Predicting compressive strength of concrete with fly ash and admixture using XGBoost: A comparative study of machine learning algorithms. Asian J. Civ. Eng. 2024, 25, 685–698. [Google Scholar] [CrossRef]
- Bortnovsky, O.; Dvorakova, K.; Roubicek, P.; Bousek, J.; Prudkova, Z.; Baxa, P. Development, properties and production of geopolymers based on secondary raw materials. In Proceedings of the Alkali Activated Materials–Research, Production and Utilization 3rd Conference, Prague, Czech Republic, 21–22 June 2007; pp. 83–96. [Google Scholar]
- Kong, D.L.; Sanjayan, J.G.; Sagoe-Crentsil, K. Factors affecting the performance of metakaolin geopolymers exposed to elevated temperatures. J. Mater. Sci. 2008, 43, 824–831. [Google Scholar] [CrossRef]
- Krivenko, P.; Guziy, S. Fire resistant alkaline portland cements. In Proceedings of the Alkali activated materials–research, production and utilization, Prague, Czech Republic, 21–22 June 2007; pp. 333–347. [Google Scholar]
- Gruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag: Comparison with ordinary Portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- Lou, B.; Vrålstad, T. Strength development of metakaolin-based alkali-activated cement. Appl. Sci. 2023, 13, 13062. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, P.K.; Iqbal, M.A. A Comprehensive Study on Optimizing Activator Composition for Enhanced Strength and Micro-Structure in High-Strength Alkali-Activated Slag Binders. Iran. J. Sci. Technol. Trans. Civ. Eng. 2024, 48, 3173–3187. [Google Scholar] [CrossRef]
- Salami, B.A.; Ibrahim, M.; Algaifi, H.A.; Alimi, W.; Ewebajo, A.O. A review on the durability performance of alkali-activated binders subjected to chloride-bearing environment. Constr. Build. Mater. 2022, 317, 125947. [Google Scholar] [CrossRef]
- Komaragiri, M.S.; Subhani, S.M. Mechanical and durability assessment of slag based alkali-activated binders incorporating granite dust. J. Build. Pathol. Rehabil. 2025, 10, 7. [Google Scholar] [CrossRef]
- Chakravarthy, G.S.; GuhaRay, A.; Kar, A. Effect of soil burial exposure on durability of alkali-activated binder-treated jute geotextile. Innov. Infrastruct. Solut. 2021, 6, 62. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Workability and mechanical properties of alkali activated slag concrete. Cem. Concr. Res. 1999, 29, 455–458. [Google Scholar] [CrossRef]
- Bernal, S.A.; De Gutiérrez, R.M.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Bernal, S.; De Gutierrez, R.; Delvasto, S.; Rodriguez, E. Performance of an alkali-activated slag concrete reinforced with steel fibers. Constr. Build. Mater. 2010, 24, 208–214. [Google Scholar] [CrossRef]
- Li, Z.; Delsaute, B.; Lu, T.; Kostiuchenko, A.; Staquet, S.; Ye, G. A comparative study on the mechanical properties, autogenous shrinkage and cracking proneness of alkali-activated concrete and ordinary Portland cement concrete. Constr. Build. Mater. 2021, 292, 123418. [Google Scholar] [CrossRef]
- Charitha, V.; Athira, G.; Bahurudeen, A.; Shekhar, S. Carbonation of alkali activated binders and comparison with the performance of ordinary Portland cement and blended cement binders. J. Build. Eng. 2022, 53, 104513. [Google Scholar] [CrossRef]
- Nodehi, M.; Aguayo, F.; Madey, N.; Zhou, L. A comparative review of polymer, bacterial-based, and alkali-activated (also geopolymer) binders: Production, mechanical, durability, and environmental impacts (life cycle assessment (LCA)). Constr. Build. Mater. 2024, 422, 135816. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Y.; Zhang, Z.; Liu, K.; Li, Y.; Shi, L.; Sun, D. The durability of alkali-activated materials in comparison with ordinary Portland cements and concretes: A review. Engineering 2020, 6, 695–706. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.; Puertas, F. Alkali-activated slag mortars: Mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Early age strength and workability of slag pastes activated by NaOH and Na2CO3. Cem. Concr. Res. 1998, 28, 655–664. [Google Scholar] [CrossRef]
- Atiş, C.D.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rahman, M.K.; Johari, M.A.M.; Nasir, M.; Oladapo, E.A. Chloride diffusion and chloride-induced corrosion of steel embedded in natural pozzolan-based alkali activated concrete. Constr. Build. Mater. 2020, 262, 120669. [Google Scholar] [CrossRef]
- Zhang, X.; Long, K.; Liu, W.; Li, L.; Long, W.-J. Carbonation and chloride ions’ penetration of alkali-activated materials: A review. Molecules 2020, 25, 5074. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.A. A review of durability and strength characteristics of alkali-activated slag concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Kumar, P.; Gogineni, A.; Ahmed, M.; Chen, W. Evolution of Cementitious Binders: Overview of History, Environmental Impacts, and Emerging Low-Carbon Alternatives. Buildings 2025, 15, 3811. https://doi.org/10.3390/buildings15213811
Kumar A, Kumar P, Gogineni A, Ahmed M, Chen W. Evolution of Cementitious Binders: Overview of History, Environmental Impacts, and Emerging Low-Carbon Alternatives. Buildings. 2025; 15(21):3811. https://doi.org/10.3390/buildings15213811
Chicago/Turabian StyleKumar, Amit, Pramod Kumar, Abhilash Gogineni, Mizan Ahmed, and Wensu Chen. 2025. "Evolution of Cementitious Binders: Overview of History, Environmental Impacts, and Emerging Low-Carbon Alternatives" Buildings 15, no. 21: 3811. https://doi.org/10.3390/buildings15213811
APA StyleKumar, A., Kumar, P., Gogineni, A., Ahmed, M., & Chen, W. (2025). Evolution of Cementitious Binders: Overview of History, Environmental Impacts, and Emerging Low-Carbon Alternatives. Buildings, 15(21), 3811. https://doi.org/10.3390/buildings15213811