Effects of Incorporating Iron-Rich Slag on the Performance of Calcium Sulfoaluminate Cement: Strength Development, Hydration Mechanisms and Microstructure
Abstract
1. Introduction
2. Experimental Method
2.1. Raw Materials
2.2. Mechanical Grinding Treatment of IRS
2.3. Mix Proportions and Specimens Preparation
2.4. Test Method
2.4.1. Compressive Strength Test
2.4.2. Hydration Heat Test
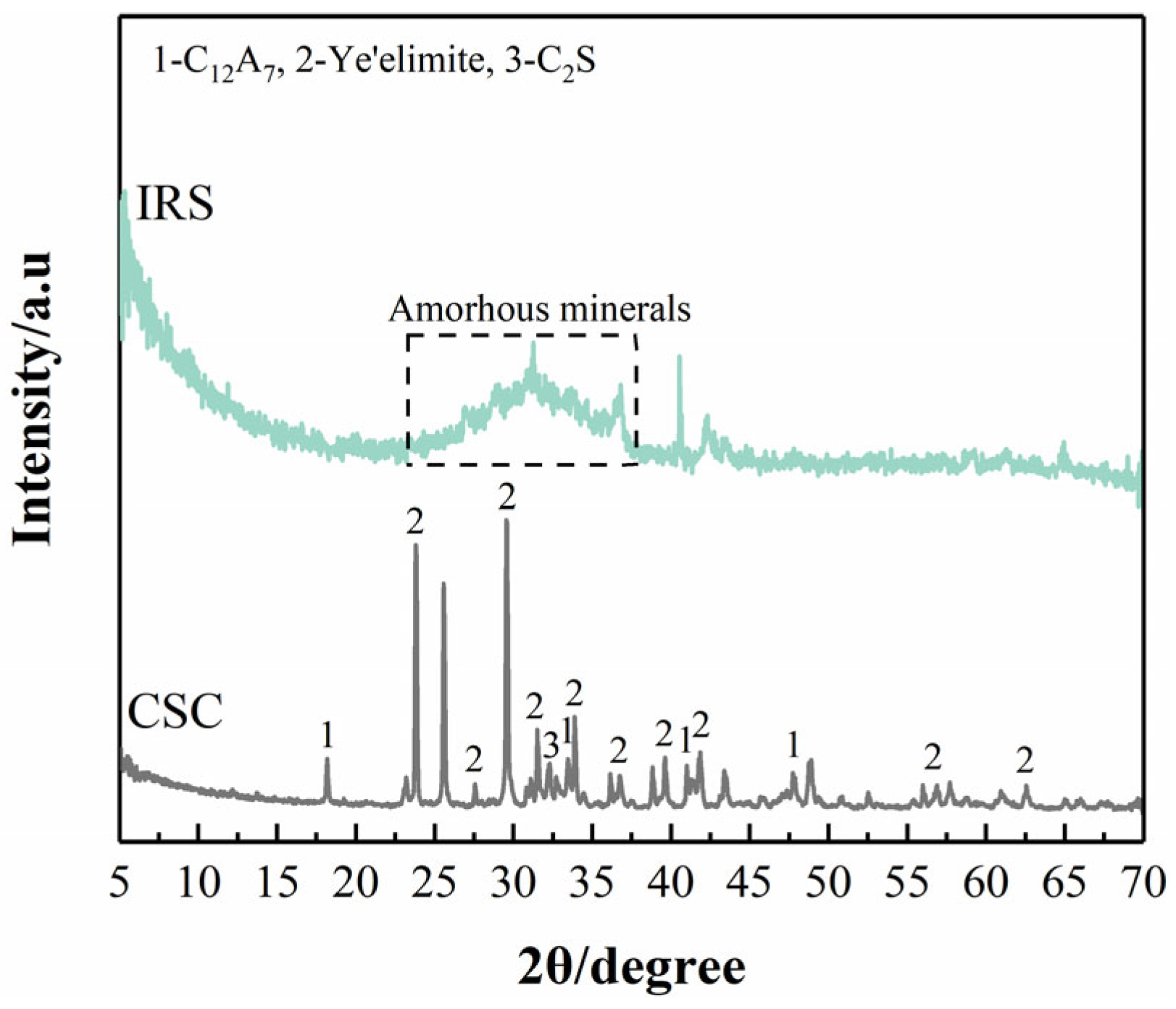
2.4.3. Solid-Phase Analyses
2.4.4. Microstructure Analyses
3. Results
3.1. Physical and Chemical Properties of IRS Treated by Mechanical Grinding
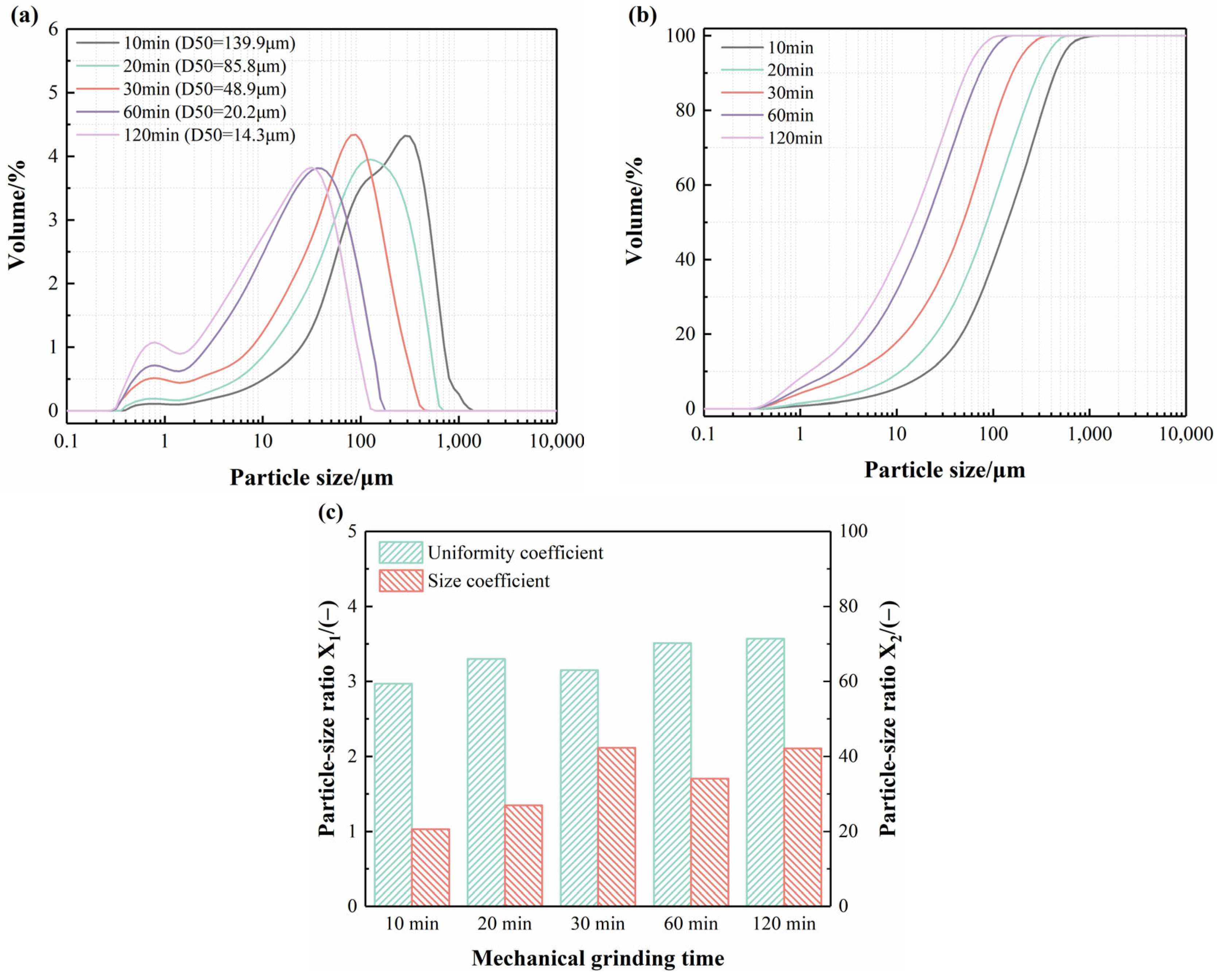
3.1.1. Particle Size Distribution of IRS
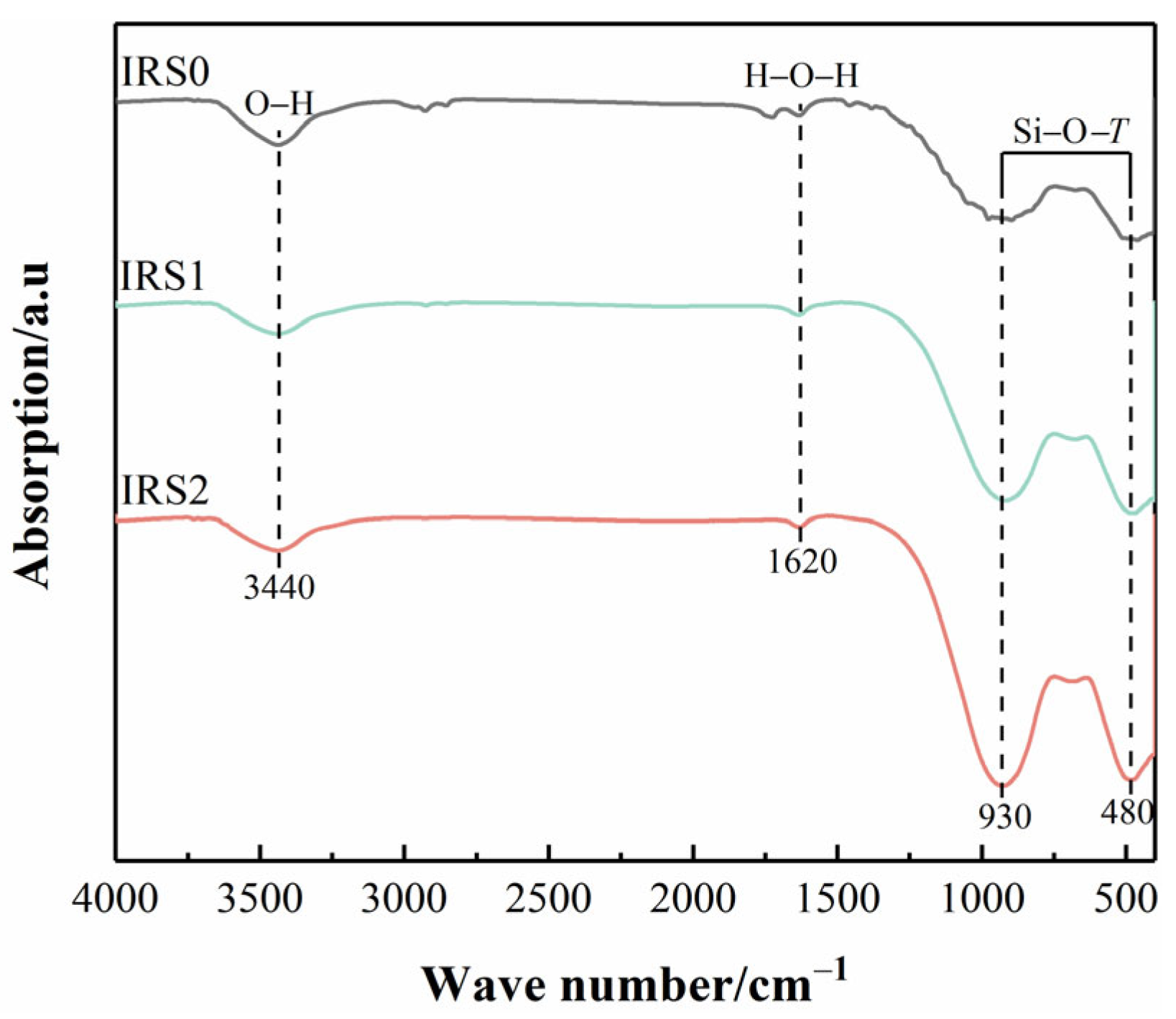
3.1.2. Chemical Bond Activity of IRS
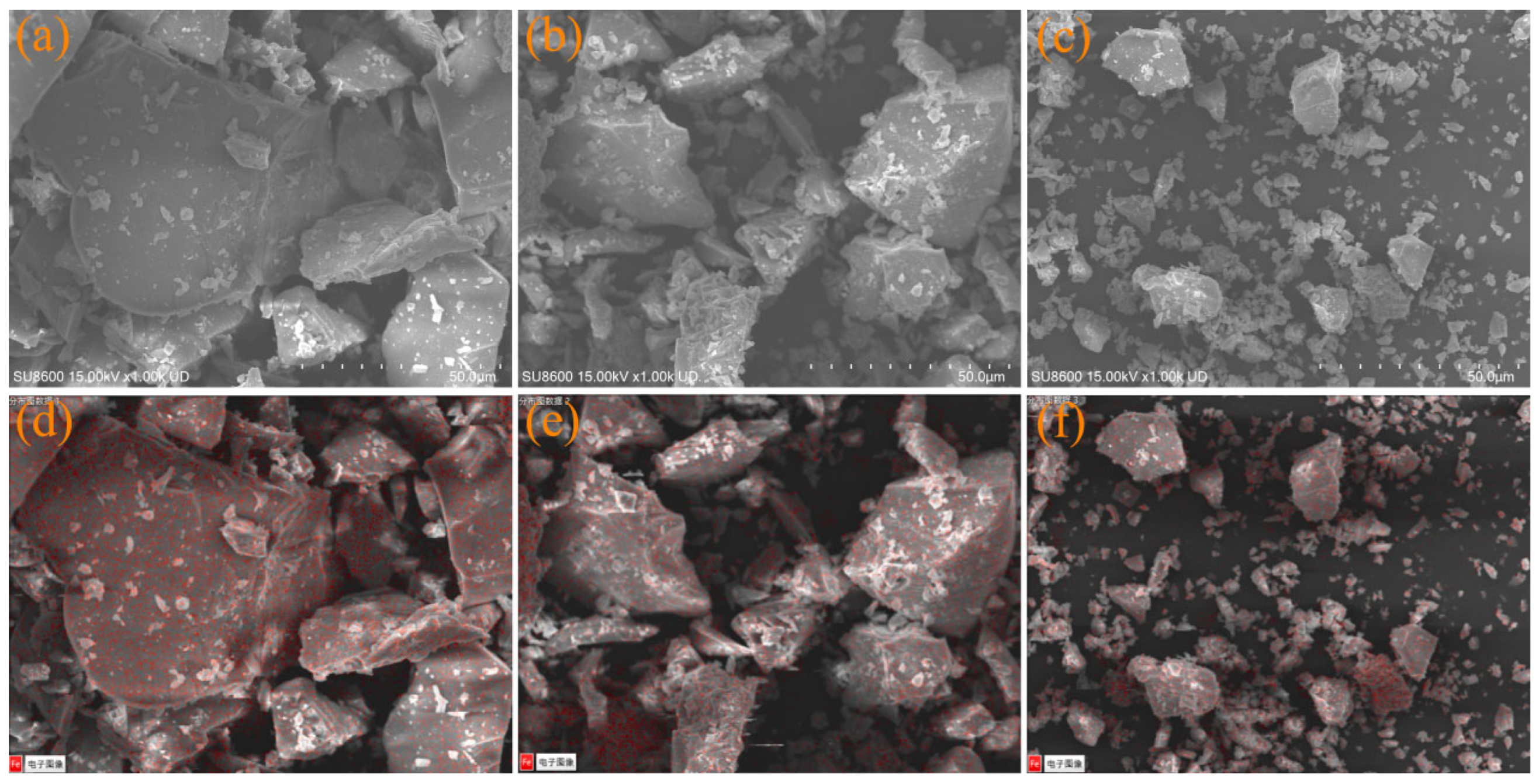
3.1.3. Morphological Characteristics of IRS
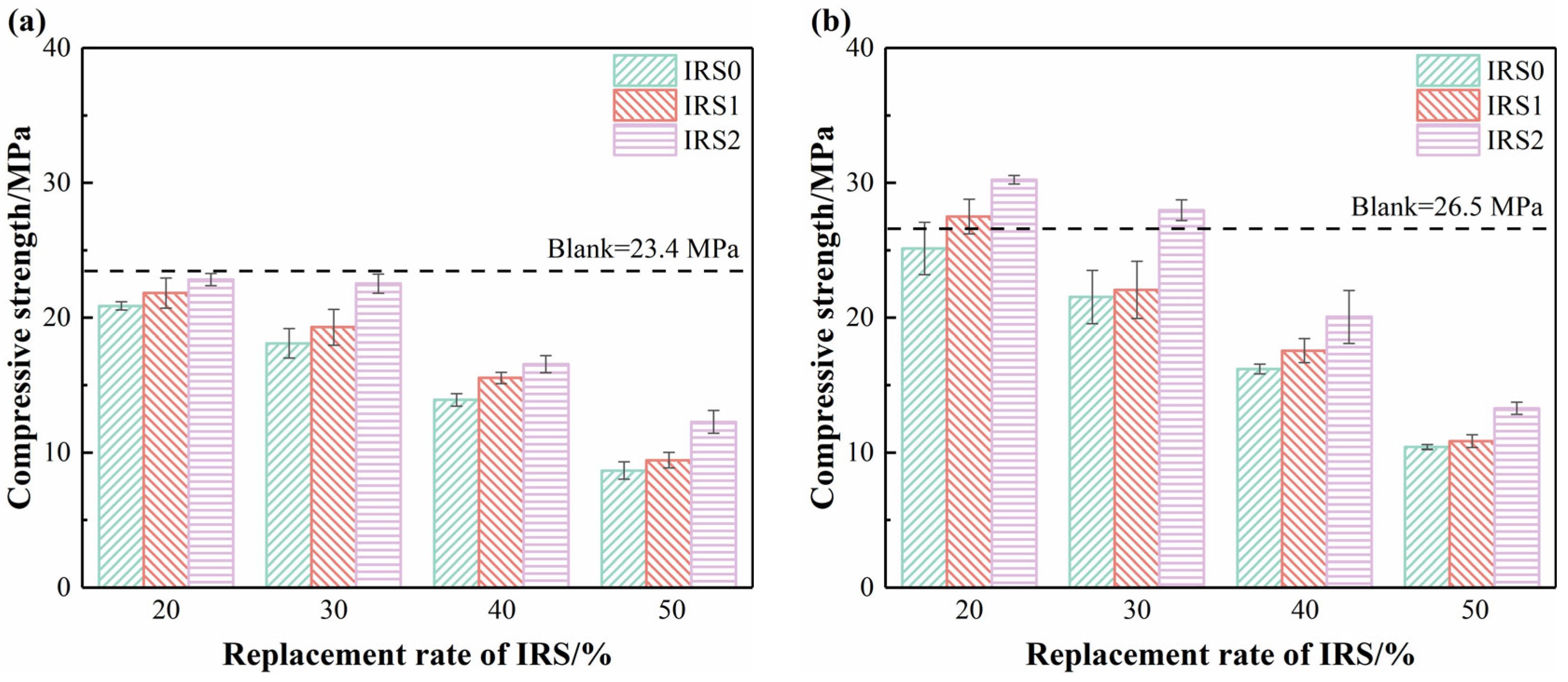
3.2. Compressive Strength
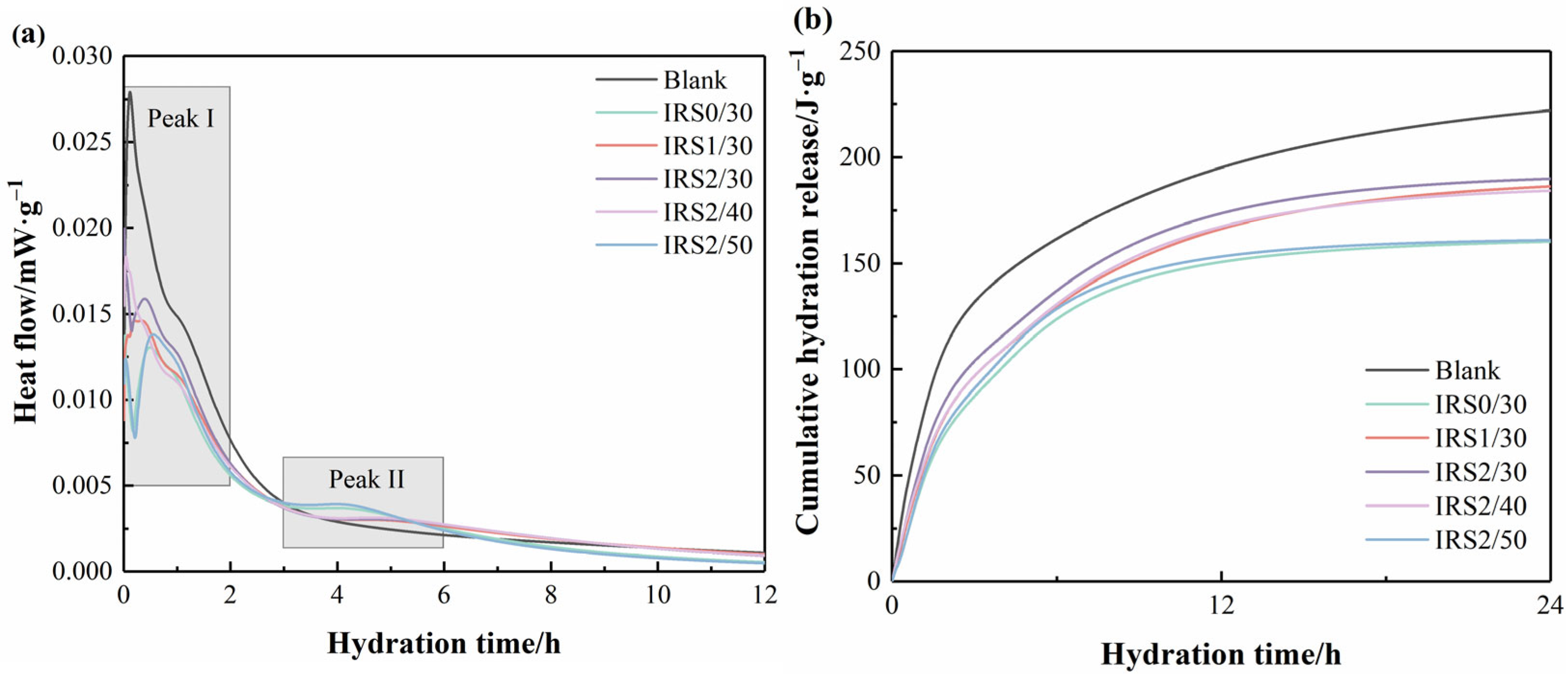
3.3. Hydration Heat
3.4. Solid Phase Analysis
3.4.1. XRD Analysis
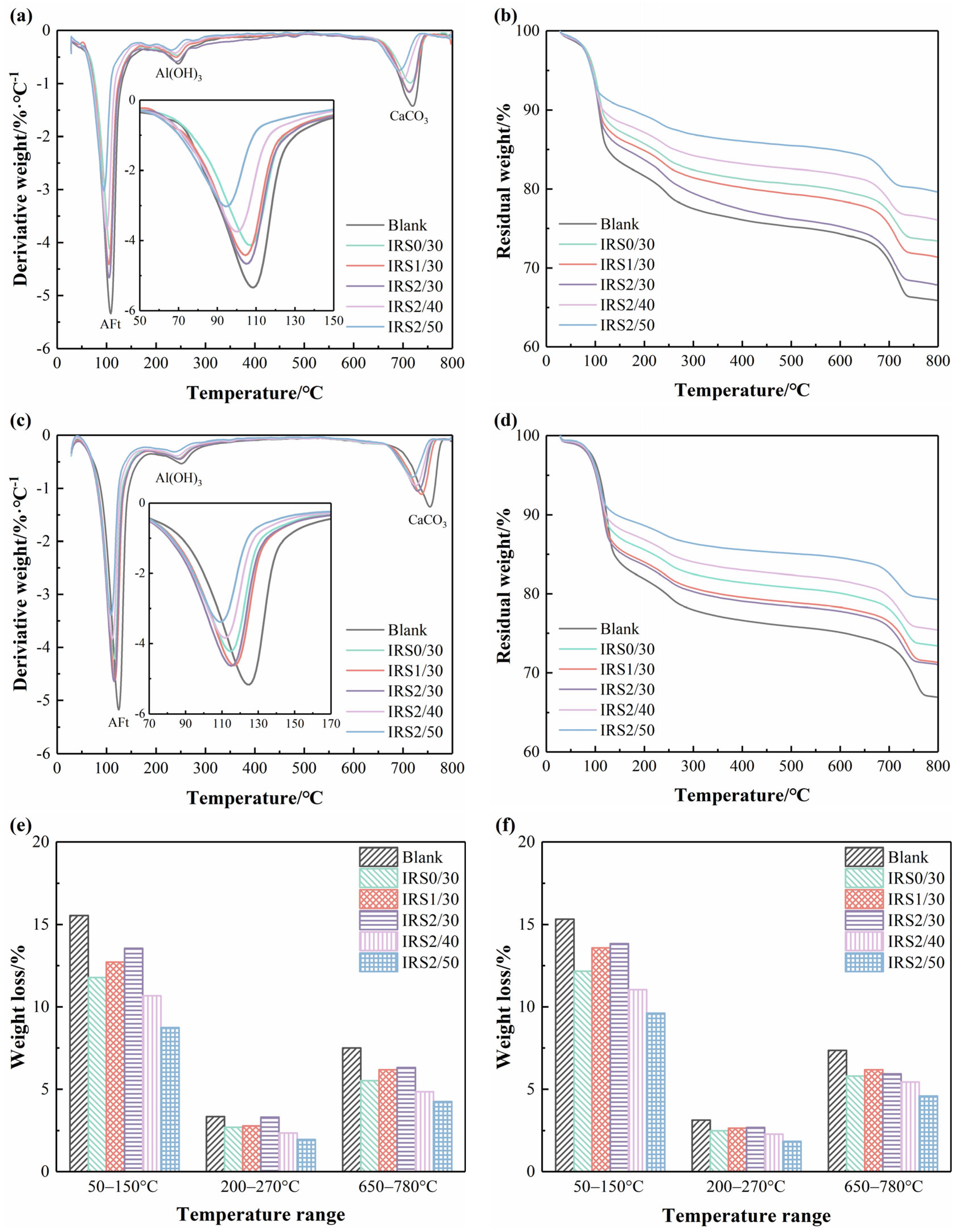
3.4.2. TG/DTG Analysis
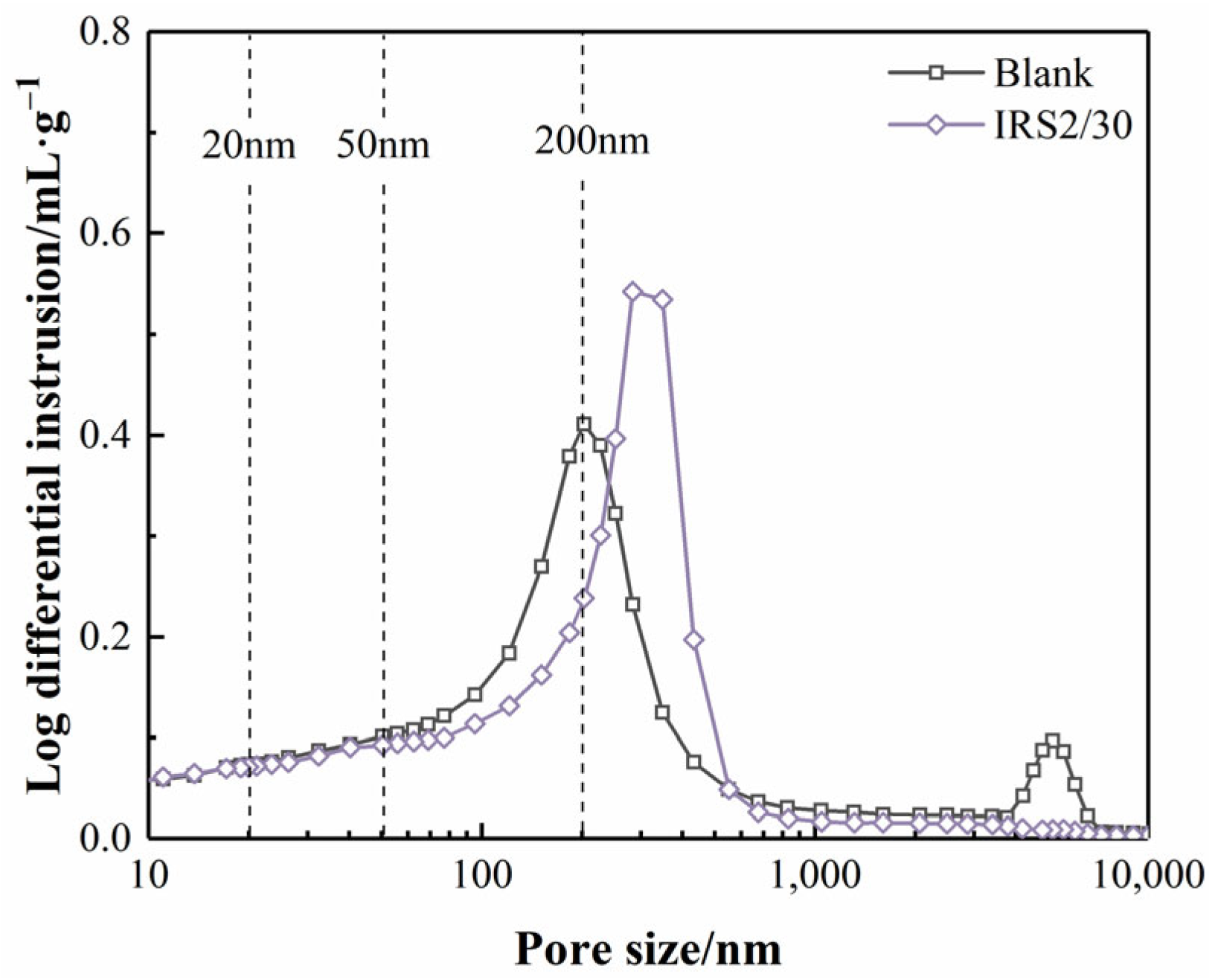
3.5. Microstructure
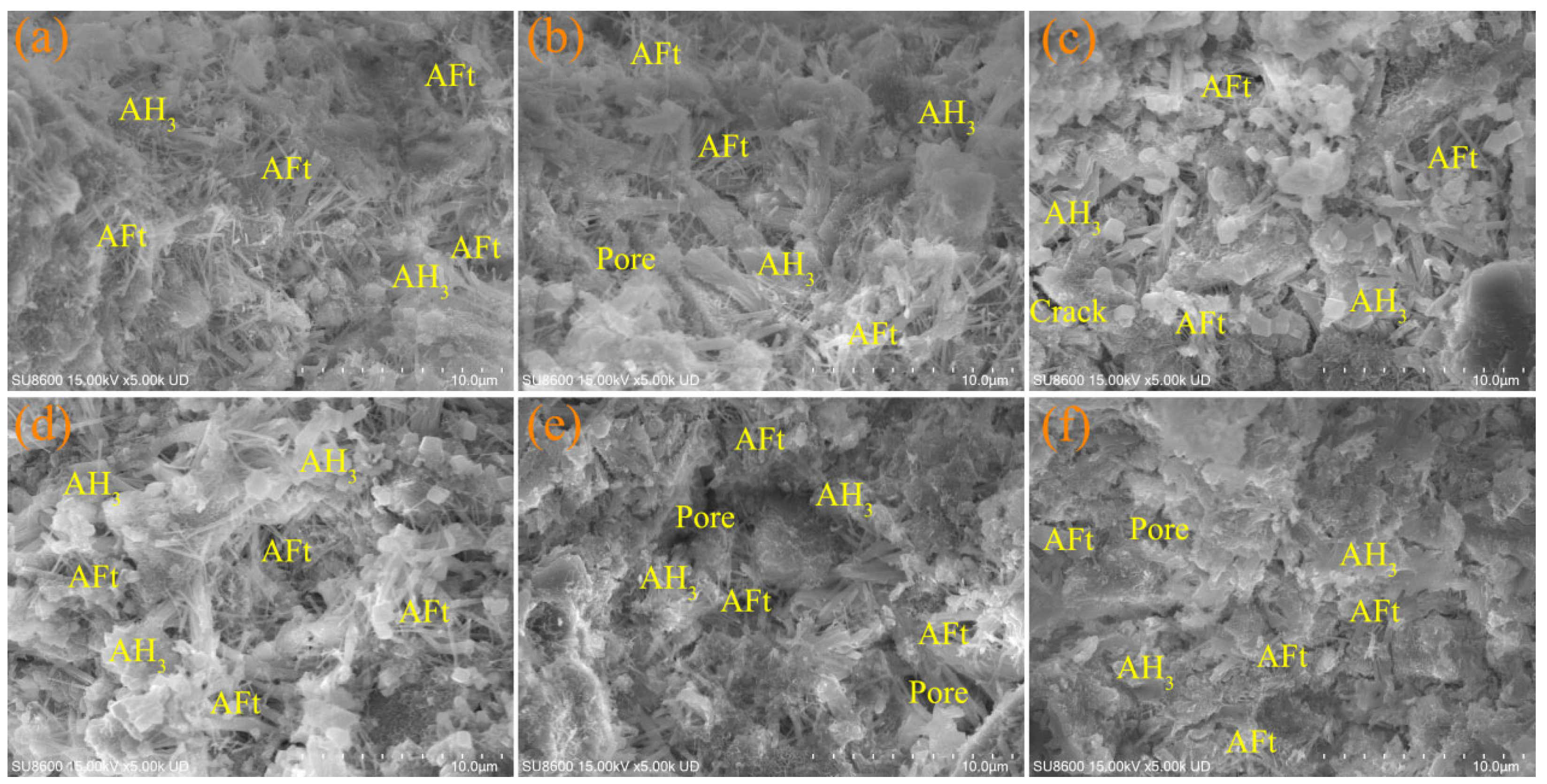
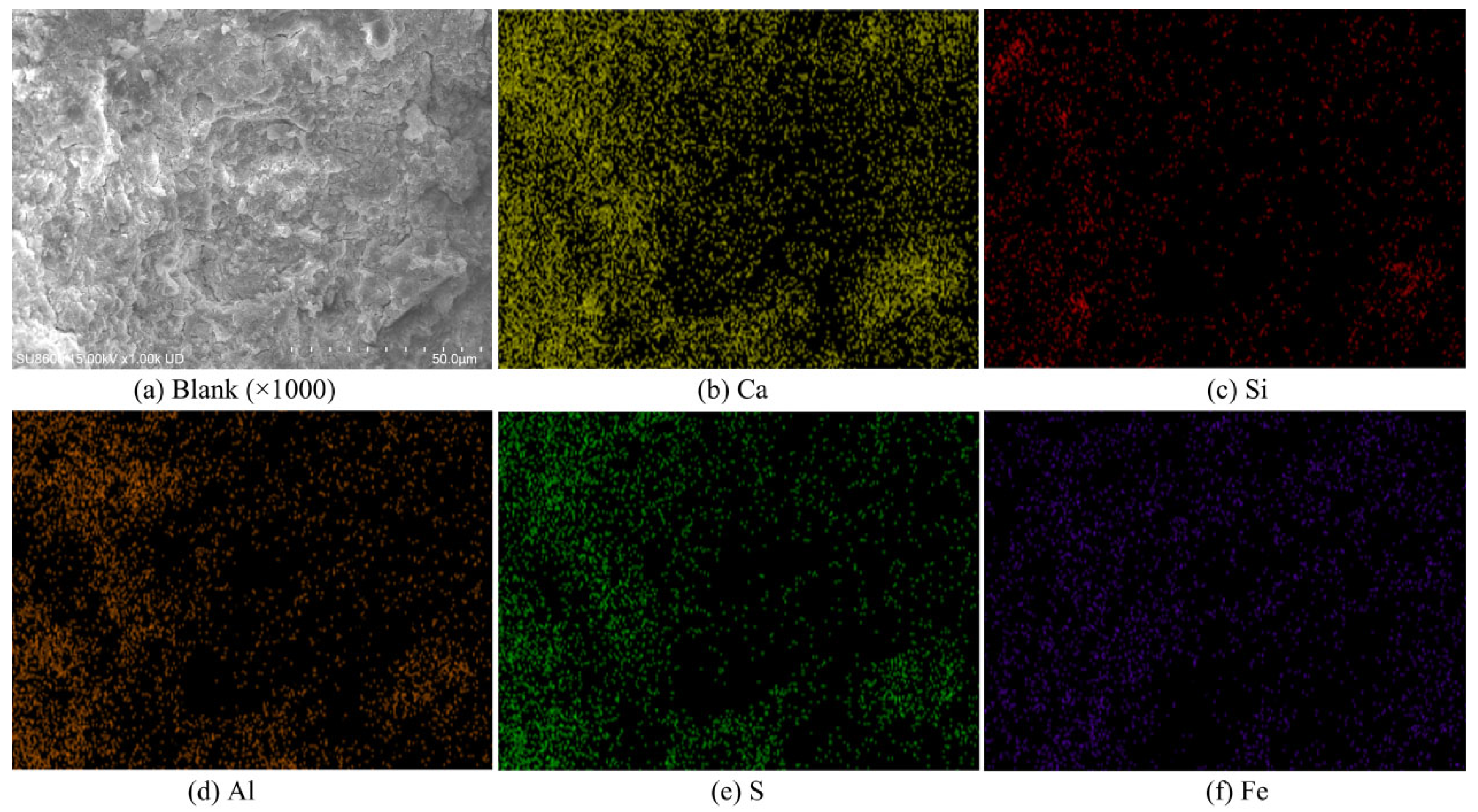
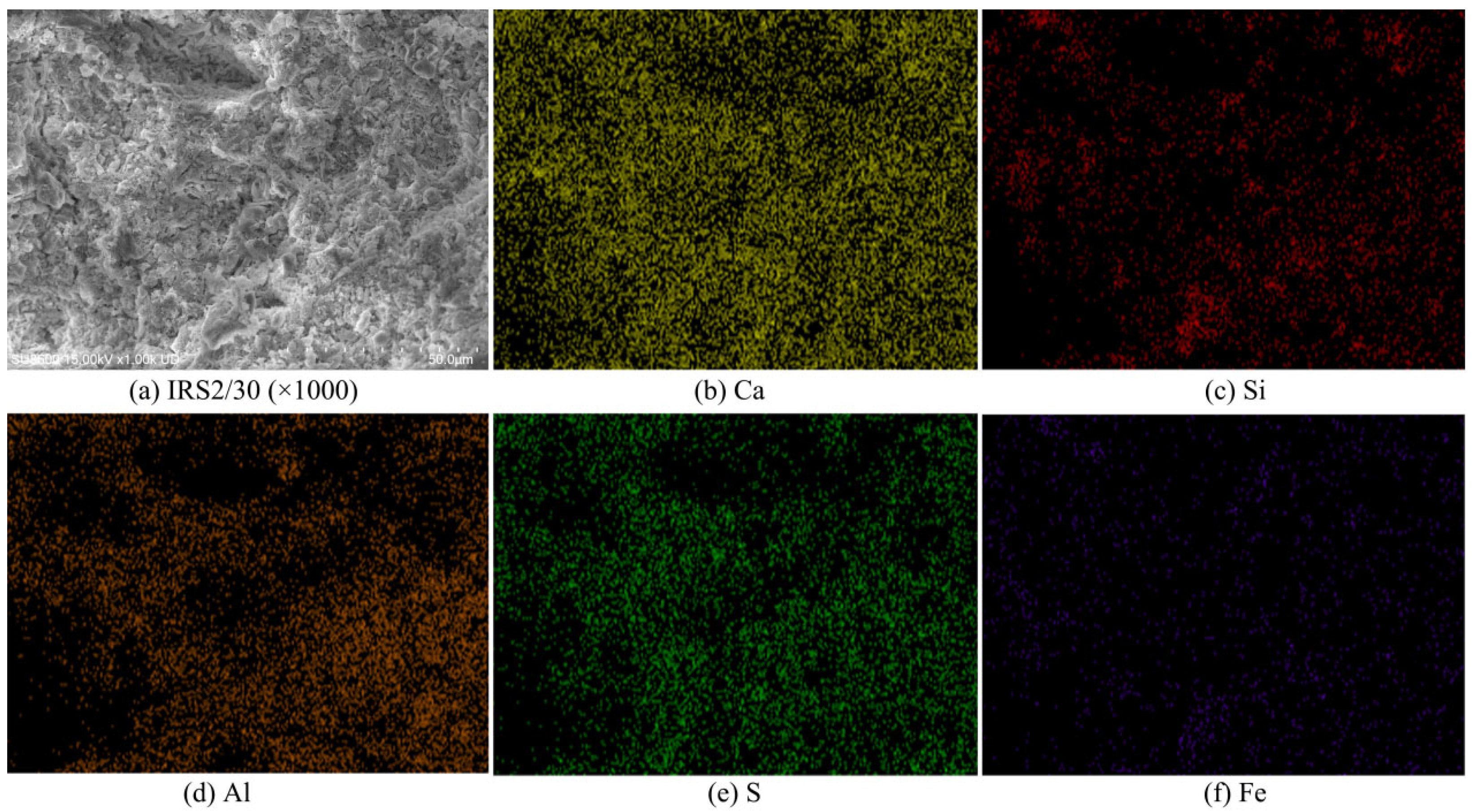
3.5.1. SEM
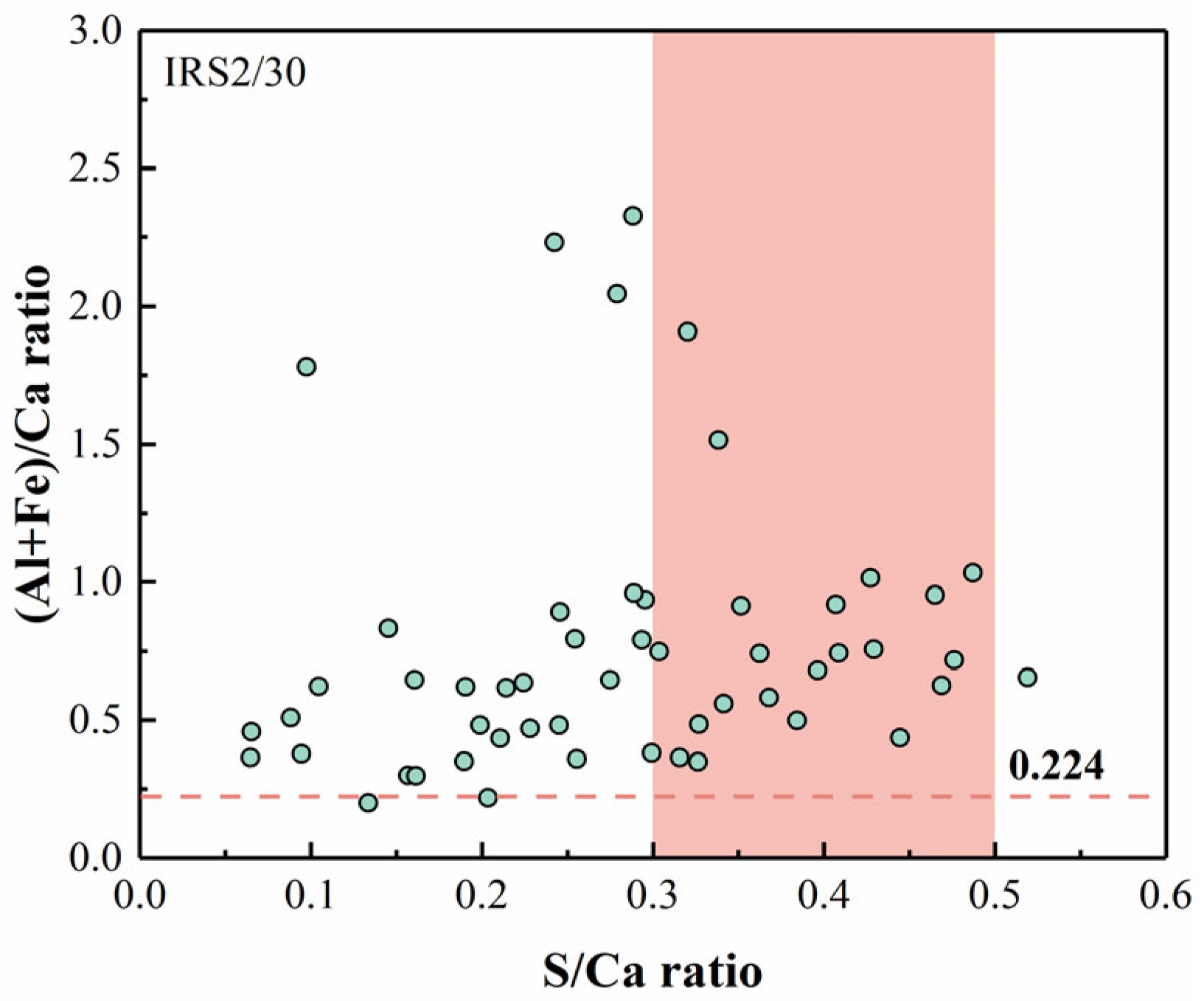
3.5.2. EDS
3.6. Discussion
4. Conclusions
- (1)
- Mechanical grinding effectively reduced the particle size of IRS. The D50 of IRS decreased to 85.8 μm, 48.9 μm, and 14.3 μm with grinding times of 20 min, 30 min, and 120 min, respectively. Although prolonged grinding did not improve the uniformity of IRS particles or optimize the size distribution, it significantly increased the contact area between IRS and other hydrates, thereby enhancing its reactivity.
- (2)
- Under the impact of the dilution effect, incorporating IRS reduces the 3 d compressive strength of CSC mortar. However, when the content of IRS2 does not exceed 30%, the 28 d compressive strength of these CSC mortars becomes comparable to that of the pure CSC mortar. Specifically, the IRS2/30 achieved a 28 d compressive strength of 28.0 MPa.
- (3)
- The incorporation of IRS reduced the hydration heat release of CSC. A decrease in IRS particle size exposed more reactive sites, which in turn accelerated the release efficiency of Fe3+. Moreover, increasing the fineness of IRS effectively promoted the hydration of C4A3Š in CSC, facilitated the formation of AFt and AH3, and enhanced the overall degree of hydration of CSC.
- (4)
- Increasing the fineness of IRS contributed to a denser microstructure in CSC-based material, especially at 28 days. EDS analyses indicated the potential substitution of Al3+ by Fe3+ in AFt, which is hypothesized to influence the strength development of CSC-based materials. However, further advanced characterization is required to confirm the extent and mechanism of this substitution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, X.; Duan, G.; Huang, Y.; Li, J.; Li, C.; Hou, P. Study on the hydration of slag-belite calcium sulfoaluminate-Portland cement composite cementitious system. Constr. Build. Mater. 2024, 433, 136713. [Google Scholar] [CrossRef]
- Chen, J.; Xie, B.; Lu, Z.; He, S.; Ma, S. Early hydration characteristics and kinetics model of ordinary portland cement-calcium sulfoaluminate cement composites. Materials 2025, 18, 2559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Z.; Xiao, M.; Cui, C.; Liu, H. Utilization of marine-dredged sediment and calcium sulfoaluminate cement for preparing non-sintered ceramsites: Properties and microstructure. J. Mar. Sci. Eng. 2025, 13, 891. [Google Scholar] [CrossRef]
- Sun, H.; Lin, T.; Zhuo, K.; Qian, J.; Chen, X.; Zhang, J.; Sun, Y. Sulfate optimization in ettringite rich cements by SO3/Al2O3 molar ratio: A comparative study of calcium sulfoaluminate and aluminate cements. Case Stud. Constr. Mater. 2024, 21, e03701. [Google Scholar] [CrossRef]
- Tchekwagep, J.J.K.; Li, Z.; Shifeng, H.; Huang, J.; Tchakouté, H.K. Transforming waste into sustainable insulation: A novel thermal insulation board utilizing treated rice husk ash, bagasse ash, and expanded vermiculite in calcium sulfoaluminate cement composite. J. Clean. Prod. 2025, 512, 145699. [Google Scholar] [CrossRef]
- Ndiaye, K.; Samson, G.; Ginestet, S.; Rouviere, Y.; Cyr, M. Foamed calcium sulfoaluminate cement with controlled porous network for daily or seasonal heat storage in ettringite. J. Build. Eng. 2024, 98, 111284. [Google Scholar] [CrossRef]
- Leone, M.; Blasi, G.; Colonna, D. Long-term flexural response of reinforced calcium sulfoaluminate/cement concrete beams. Eng. Struct. 2023, 292, 116585. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, W.; Yuan, J.; Zhang, Z.; Wu, G. Study on seawater corrosion resistance of calcium sulfoaluminate cement-based novel grouting materials. Mater. Lett. 2024, 366, 136509. [Google Scholar] [CrossRef]
- Cai, X.; Yang, D.; Zhang, D.; Cui, J.; Wang, W.; Liu, L. Development of high-early-strength low-carbon engineered cementitious composites with calcium sulfoaluminate cement incorporating high-volume fly ash. Case Stud. Constr. Mater. 2023, 18, e01959. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Pan, Z.; Li, H.; Wu, Z.; Li, L.; Xiong, Z. Use of supplementary cementitious materials in seawater–sea sand concrete: State-of-the-art review. Constr. Build. Mater. 2024, 425, 136009. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.; Kim, N.; Yoon, H.N.; Kim, S.; Bae, J.-H.; Jang, D.; Cho, A.; Lee, H.K. Characterization of hydrates in quaternary Portland cement-blast furnace slag-calcium sulfoaluminate clinker-limestone or gypsum systems: Experimental and thermodynamic modeling approaches. Cem. Concr. Compos. 2025, 162, 106126. [Google Scholar] [CrossRef]
- Alsaif, A.; Fares, G.; Alhozaimy, A. Effectiveness of ultra-high-performance concrete containing recycled tire steel fibers and high-volume scoria rock powder as natural pozzolan. Case Stud. Constr. Mater. 2024, 21, e03841. [Google Scholar] [CrossRef]
- Khamseh, B.; Shourijeh, P.T.; Binesh, S.M. Strength and deformation characteristics of cement-stabilized Fe-rich fine iron ore tailings. Constr. Build. Mater. 2025, 463, 140101. [Google Scholar] [CrossRef]
- Zeng, H.; Ramanathan, S.; Kim, H.J. Effect of copper mine tailings and copper slag on the hydration, microstructure, and mechanical property in Portland cement. Results Eng. 2025, 26, 104890. [Google Scholar] [CrossRef]
- Astoveza, J.; Trauchessec, R.; Migot-Choux, S.; Soth, R.; Pontikes, Y. Iron-rich slag addition in ternary binders of Portland cement, aluminate cement and calcium sulfate. Cem. Concr. Res. 2022, 153, 106689. [Google Scholar] [CrossRef]
- Astoveza, J.; Trauchessec, R.; Soth, R.; Pontikes, Y. Properties of calcium aluminate blended cement incorporating iron-rich slag: Evolution over a curing period of 1 year. Constr. Build. Mater. 2021, 282, 122569. [Google Scholar] [CrossRef]
- Liu, K.; He, B.; Wang, X.; Wang, L.; Jin, J.; Han, P.; Bai, X. Fabrication of iron-rich copper slag cement using low-concentration phosphoric acid as medium: Workability and mechanism. J. Build. Eng. 2025, in press. [Google Scholar] [CrossRef]
- Hallet, V.; Pedersen, M.T.; Lothenbach, B.; Winnefeld, F.; De Belie, N.; Pontikes, Y. Hydration of blended cement with high volume iron-rich slag from non-ferrous metallurgy. Cem. Concr. Res. 2022, 151, 106624. [Google Scholar] [CrossRef]
- Beersaerts, G.; Soete, J.; Giels, M.; Eykens, L.; Lucas, S.; Pontikes, Y. 3D printing of an iron-rich slag based hybrid mortar. A durable, sustainable and cost-competitive product? Cem. Concr. Compos. 2023, 144, 105304. [Google Scholar] [CrossRef]
- Lyu, K.; Qu, Y.; Liu, X.; Kou, F.; Xie, X.; Shi, J.; Shah, S.P. Mechanical activation of MSWIFA: Performance trade-offs between electric grinding and ball milling. Constr. Build. Mater. 2025, 493, 143110. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, C.; Cui, C.; Su, Y.; He, X.; Zhi, Z.; Chen, S.; Yang, C.; Guan, S. Effect of ground granulated blast-furnace slag slurries by wet-grinding on the mechanical properties and hydration process of hemihydrate phosphogypsum. Mater. Today Commun. 2024, 41, 111006. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, L.; Wang, F. Properties of (Al, Fe)-ettringite solid solution: Experiment, atomic simulation, and thermodynamics modeling. Cem. Concr. Res. 2024, 182, 107556. [Google Scholar] [CrossRef]
- Chang, J.; Zeng, T.; Li, J. Influence of iron substitution on the microstructure and properties of ettringite in calcium sulfoaluminate cement: A comprehensive study on the Al/(Fe+Al) ratios. J. Build. Eng. 2024, 86, 108855. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, B.; Zhu, W. Performance and hydration mechanisms of ultrafine iron ore tailings enhanced supersulfated cement with high phosphogypsum content. Cem. Concr. Compos. 2025, 157, 105891. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, T.; Ouyang, G.; Li, Z.; Deng, Y. Enhancement of polyferric sulphate on the carbonation resistance of phosphogypsum-based excess-sulphate slag cement: Focus on iron-doped ettringite and microstructure alteration. Constr. Build. Mater. 2025, 494, 143224. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Canonico, F.; Paul, G. CSA and slag: Towards CSA composite binders. Adv. Cem. Res. 2018, 31, 147–158. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of China: Beijing, China, 2021.
- JC/T 958-2005; Flow Table for Determine Mortar Fluidity. Standardization Administration of China: Beijing, China, 2005.
- Zhu, H.; Ma, M.; He, X.; Zheng, Z.; Su, Y.; Yang, J.; Zhao, H. Effect of wet-grinding steel slag on the properties of Portland cement: An activated method and rheology analysis. Constr. Build. Mater. 2021, 286, 122823. [Google Scholar] [CrossRef]
- Li, H.-W.; Wang, R.; Wei, M.-W.; Lei, N.-Z.; Sun, H.-X.; Fan, J.-J. Mechanical properties and hydration mechanism of high-volume ultra-fine iron ore tailings cementitious materials. Constr. Build. Mater. 2022, 353, 129100. [Google Scholar] [CrossRef]
- Kotake, N.; Kuboki, M.; Kiya, S.; Kanda, Y. Influence of dry and wet grinding conditions on fineness and shape of particle size distribution of product in a ball mill. Adv. Powder Technol. 2011, 22, 86–92. [Google Scholar] [CrossRef]
- Hu, Z.; Gu, X.; Li, Z.; Hu, Z.; Liu, J.; Wang, S.; Wang, H. Sustainable valorization of coal gasification slag through optimized grinding kinetics: Composite cement compressive strength enhancement and environmental assessment. Environ. Res. 2025, 277, 121601. [Google Scholar] [CrossRef]
- Gu, X.; Hu, Z.; Hu, Z.; Li, Z.; Liu, J.; Ge, X.; Wang, H. Coal gasification slag-based composite cement under the regulation of grinding speed grinding kinetics: Parameter optimization, hydration mechanism and properties analysis. Adv. Powder Technol. 2025, 36, 105029. [Google Scholar] [CrossRef]
- Möschner, G.; Lothenbach, B.; Winnefeld, F.; Ulrich, A.; Figi, R.; Kretzschmar, R. Solid solution between Al-ettringite and Fe-ettringite (Ca6[Al1−xFex(OH)6]2(SO4)3·26H2O). Cem. Concr. Res. 2009, 39, 482–489. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Lu, L.; Zhang, W.; Wang, S.; Wang, W.; Cheng, X. Phosphogypsum as a component of calcium sulfoaluminate cement: Hazardous elements immobilization, radioactivity and performances. J. Clean. Prod. 2020, 248, 119287. [Google Scholar] [CrossRef]
- Jacques, K.T.J.; Fengzhen, Y.; Shoude, W.; Piqi, Z.; Shifeng, H.; Xin, C. Analysis of the phases and functions of the various compounds of calcium sulfoaluminate cement after exposure to high temperature. J. Mater. Res. Technol. 2023, 25, 4154–4170. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, N.; Xu, L.; Yu, L.; Huang, L.; Wu, K. Evaluation of the efflorescence resistance of calcium sulfoaluminate cement mortar: From indoor accelerated testing to outdoor exposure. J. Mater. Res. Technol. 2023, 22, 2447–2461. [Google Scholar] [CrossRef]
- Ba, M.; Xie, J.; Ma, X.; Ma, H.; Ren, X.; Lin, R.; Shen, Y. Effects of secondary aluminum ash sintered ground powder on properties of calcium sulfoaluminate cement-based grouting materials. Constr. Build. Mater. 2025, 462, 140015. [Google Scholar] [CrossRef]
- Tanguler-Bayramtan, M.; Turk, S.; Yaman, I.O. Hydration characteristics of calcium sulfoaluminate cements synthesized using an industrial symbiosis framework. Constr. Build. Mater. 2024, 447, 138090. [Google Scholar] [CrossRef]
- Bouibes, A.; Laanaiya, M.; Lacarrière, L. Microscopic effect of iron dosage on the stability of Fe-doped ettringite. J. Am. Ceram. Soc. 2024, 107, 5127–5138. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Su, Y.; Yang, J.; Strnadel, B.; Wang, X. Efficiency of wet-grinding on the mechano-chemical activation of granulated blast furnace slag (GBFS). Constr. Build. Mater. 2019, 199, 185–193. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Wu, J.; Gao, Y.; Shao, J.; Wang, C.; Su, T.; Cao, F.; Zhang, W.; Yang, Q.; et al. Preparation of high-belite calcium sulfoaluminate cement and calcium sulfoaluminate cement from industrial solid waste: A review. Sustainability 2025, 17, 4269. [Google Scholar] [CrossRef]
- Akerele, D.D.; Aguayo, F. A comparative evaluation of polymer-modified rapid-set calcium sulfoaluminate concrete: Bridging the gap between laboratory shrinkage and the field strain performance. Buildings 2025, 15, 2759. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, F.-Y.; Tan, D.-Y.; Yang, A.-W. A deep learning informed-mesoscale cohesive numerical model for investigating the mechanical behavior of shield tunnels with crack damage. Structures 2024, 66, 106902. [Google Scholar] [CrossRef]
- Wang, F.; Zhai, W.; Zhao, S.; Man, J. A novel unsupervised PINN framework with dynamically self-adaptive strategy for solid mechanics. J. Comput. Phys. 2025, 542, 114373. [Google Scholar] [CrossRef]


| CaO | Al2O3 | SO3 | SiO2 | Fe2O3 | MgO | ZnO | K2O | MnO | |
|---|---|---|---|---|---|---|---|---|---|
| CSC/% | 56.09 | 15.02 | 16.78 | 4.51 | 4.03 | 1.13 | 0.02 | 0.32 | 0.05 |
| IRS/% | 12.09 | 6.74 | 4.09 | 20.30 | 43.08 | 1.28 | 4.52 | 1.78 | 1.69 |
| Fineness/m2·kg−1 | Setting Time/min | Compressive Strength/MPa (w/b Raito = 0.42) | Flexural Strength/MPa (w/b Raito = 0.42) | |||
|---|---|---|---|---|---|---|
| Initial | Final | 1 d | 3 d | 1 d | 3 d | |
| 40 | 10 | 15 | 37.2 | 45.1 | 6.1 | 6.5 |
| Specimens ID | CSC/g | IRS0/g | IRS1/g | IRS2/g | Sand/g | Water/g |
|---|---|---|---|---|---|---|
| Blank | 450 | / | / | / | 1350 | 225 |
| IRS0/20 | 360 | 90 | / | / | 1350 | 225 |
| IRS0/30 | 315 | 135 | / | / | 1350 | 225 |
| IRS0/40 | 270 | 180 | / | / | 1350 | 225 |
| IRS0/50 | 225 | 225 | / | / | 1350 | 225 |
| IRS1/20 | 360 | / | 90 | / | 1350 | 225 |
| IRS1/30 | 315 | / | 135 | / | 1350 | 225 |
| IRS1/40 | 270 | / | 180 | / | 1350 | 225 |
| IRS1/50 | 225 | / | 225 | / | 1350 | 225 |
| IRS2/20 | 360 | / | / | 90 | 1350 | 225 |
| IRS2/30 | 315 | / | / | 135 | 1350 | 225 |
| IRS2/40 | 270 | / | / | 180 | 1350 | 225 |
| IRS2/50 | 225 | / | / | 225 | 1350 | 225 |
| Specimens ID | Elements (%) | ||||
|---|---|---|---|---|---|
| Ca | Si | Al | S | Fe | |
| Blank | 66.15 | 3.35 | 16.40 | 13.92 | 0.00 |
| IRS2/30 | 52.24 | 5.91 | 17.37 | 15.92 | 8.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Liu, H.; Yang, X.; Peng, C.; Wei, T.; Li, H. Effects of Incorporating Iron-Rich Slag on the Performance of Calcium Sulfoaluminate Cement: Strength Development, Hydration Mechanisms and Microstructure. Buildings 2025, 15, 3654. https://doi.org/10.3390/buildings15203654
Wang R, Liu H, Yang X, Peng C, Wei T, Li H. Effects of Incorporating Iron-Rich Slag on the Performance of Calcium Sulfoaluminate Cement: Strength Development, Hydration Mechanisms and Microstructure. Buildings. 2025; 15(20):3654. https://doi.org/10.3390/buildings15203654
Chicago/Turabian StyleWang, Rong, Haixing Liu, Xiaohua Yang, Chao Peng, Taibing Wei, and Huawei Li. 2025. "Effects of Incorporating Iron-Rich Slag on the Performance of Calcium Sulfoaluminate Cement: Strength Development, Hydration Mechanisms and Microstructure" Buildings 15, no. 20: 3654. https://doi.org/10.3390/buildings15203654
APA StyleWang, R., Liu, H., Yang, X., Peng, C., Wei, T., & Li, H. (2025). Effects of Incorporating Iron-Rich Slag on the Performance of Calcium Sulfoaluminate Cement: Strength Development, Hydration Mechanisms and Microstructure. Buildings, 15(20), 3654. https://doi.org/10.3390/buildings15203654






