Abstract
The engineering applicability of alkali-activated mortar (AAM) is limited by high shrinkage and fast setting time. In this study, the shrinkage performance of AAM was regulated by adding desulfurization gypsum (DG), and the effects of DG content on its workability, corrosion resistance, and mechanical properties were systematically investigated. The test included fluidity, setting time, compressive strength, drying shrinkage, water erosion resistance, and sulfate erosion resistance and was combined with microscopic analysis to reveal its phase composition and micro-morphology. The results show that DG can significantly prolong the setting time and reduce the drying shrinkage. With a DG content of 10%, alkali-activated materials exhibited a setting time similar to that of OPC, and the 56-d drying shrinkage of the AAM was reduced by 20.2%. However, the fluidity, water erosion resistance, and sulfate resistance decreased with an increase in DG content. When the DG content was 10%, the fluidity of the AAM reached 126 mm, and its setting time was equivalent to that of OPC. The mechanical properties showed a trend of increasing first and then decreasing. The optimum was reached when the DG content was 6%. The 28-d compressive strength of AAM-6 was 63.25 MPa, and after 60 days of water erosion and sulfate corrosion its residual strength was still higher than that of OPC in the same environment. Microscopic analysis showed that DG promoted the formation of ettringite, which filled pores with age and formed a dense structure, thereby improving mechanical properties and inhibiting shrinkage. This study enhances the engineering applicability of AAM while enabling high-value utilization of industrial solid waste for sustainable construction materials.
1. Introduction
Consumption of Portland cement has increased significantly against the backdrop of global economic growth, and it has become one of the most used synthetic materials. The world consumed 4.4 billion tons of Portland cement in 2021, and cement production is expected to grow by 12 to 23% in 2050 [1]. The cement industry is one of the major CO2 pollution sources in the world, with about 94% of CO2 emissions per ton of Portland cement [2]. Large amounts of CO2 emissions can easily contribute to the greenhouse effect, leading to a decline in air quality and ecosystem damage. With the advancement of the “double carbon” strategy, the cement industry needs to implement carbon reduction development targets, and it is urgent to develop environmentally friendly cementitious materials [3].
Alkali-activated materials (AAMs) are a kind of unburned low-carbon cementitious material prepared by activating industrial solid waste (such as fly ash, slag, and steel slag) as silicon–aluminum raw materials with alkaline activators (such as water glass and NaOH) at room temperature [4]. The material has the advantage of a simple preparation process and low energy consumption compared to ordinary Portland cement (OPC). At the same time, it exhibits excellent impermeability, frost resistance, high-temperature resistance, and corrosion resistance and is considered the most promising alternative to Portland cement [5]. AAMs are characterized by their reliance on highly concentrated alkaline activators and rapid hydration kinetics. The strongly alkaline environment is the primary factor inducing rapid condensation and hardening. It efficiently activates the aluminosilicate precursors in slag and fly ash, causing their rapid dissolution and subsequent hydration reaction, thereby leading to the swift formation of C-A-S-H gel [6]. This series of reaction processes occurs at a significantly faster rate than the conventional hydration of minerals like C2S and C3S in OPC. Although this gives them excellent early strength, it also leads to two key defects: (1) the collapse and recombination of the gel during drying and the refinement of the matrix pore size can lead to significant shrinkage [7]; (2) an excessively fast setting time leads to insufficient engineering applicability. In particular, microcracks induced by drying shrinkage not only reduce the mechanical properties but also accelerate the penetration of corrosive media and severely impair the durability of the material [8]. To facilitate the engineering applications of alkali-activated mortar (AAM), it is imperative to improve their shrinkage performance.
At present, the control methods for the high-shrinkage characteristics of alkali-activated materials include adjusting the parameters of activators, using shrinkage-reducing agents or expansion agents, and improving curing conditions (internal curing or high-temperature curing). Adjusting the alkali activator parameters can reduce the drying shrinkage by optimizing the Na2O content or by using a weak alkali activator to reduce the hydration reaction rate [9]. Internal curing effects provide additional water by the addition of saturated lightweight aggregates or super absorbent, creating a micro-curing environment that compensates for the autogenous shrinkage of the material [10]. By expanding the amount of pores and bound water in the gel, high-temperature curing can significantly enhance the shrinkage characteristics of materials [11]. However, the first two methods generally reduce the mechanical strength, and high-temperature curing significantly increases the complexity of the curing process. As typical chemical shrinkage agents, ethylene glycol and polyethylene glycol can effectively alleviate the shrinkage deformation induced by capillary stress by reducing the surface tension of pore solutions of materials [12]. While its optimal content is difficult to control accurately, fiber reinforcement suppresses shrinkage through physical confinement and crack bridging mechanisms. However, this method suffers from problems such as poor fiber dispersion and excessive incorporation, leading to a decrease in matrix strength and workability [13]. By promoting the crystal growth of hydration products, the expansion agent can effectively inhibit the drying shrinkage [14]. Studies have shown that the incorporation of gypsum can significantly coarsen the pore structure and reduce the drying shrinkage [15]. As the main by-product of lime–limestone recovery of sulfur dioxide from flue gas in thermal power plants, desulfurization gypsum (DG) has successfully replaced natural gypsum in the fields of cement retarder [16], concrete admixture [17], and gypsum products [18]. However, due to the expanding nature of gypsum, the content in conventional cement-based materials usually does not exceed 5% [19]. The annual output of DG in China is more than 130 million tons, and the large stockpiles not only occupy land resources but also cause serious environmental pressure [20,21]. Recent studies have begun to explore the utilization of DG in AAMs. DG provides SO42− ions that can promote the dissolution of aluminum phases and facilitate the formation of ettringite (AFt) [22]. The resulting acicular AFt crystals can fill microcracks, thereby enhancing the density of the matrix [23]. Several studies have reported that DG can improve the early-age strength and drying shrinkage resistance of slag–fly ash-based geopolymers [24]. However, a systematic investigation into the effect of DG content on the comprehensive properties of AAMs is still lacking. Furthermore, the optimal dosage and the underlying micro-mechanisms, particularly the role of AFt in microstructural evolution, require further clarification.
This study used DG as a cementitious material component and systematically investigated the effects of DG on the workability, mechanical properties, shrinkage performance, and corrosion resistance of AAM. The phase composition and micro-morphology of DG-based alkali-activated materials were revealed by FTIR, XRD, and SEM. In contrast to previous studies, this work clarifies the optimal dosage of DG in AAM and evaluates its long-term performance in an erosive environment, thereby offering a new pathway for the high-value-added utilization of industrial solid waste in green building materials.
2. Experimental Program
2.1. Raw Materials
In this study, S95 slag powder, grade II fly ash, and DG were used as alkali-activated cementitious materials. The “S95” designation indicates a grade of slag powder with a 28-day activity index of ≥95%, reflecting its high reactivity. The slag powder, with a specific surface area of 402 m2/kg, is produced at the Rizhao Iron and Steel Plant in Shandong Province, China. The fly ash (FA) and ordinary Portland cement with a strength rating of 42.5, is from Shandong Shanshui Cement Group Co., Ltd. in Jinan, China. DG was taken from a coal-fired power plant in Qingdao (Shandong, China). The pH value of the desulfurization gypsum (DG) was measured as 8.5 in a solid-to-deionized water ratio of 1:5 (by mass) suspension. The chemical composition of the cementitious material is shown in Table 1. Figure 1 suggests the particle size distribution of the powder. The order of fineness of the four kinds of powder from fine to coarse (particle size from small to large) is cement, slag powder, DG, and fly ash. The alkali activator consists of water glass and NaOH, with water glass containing 26.98% SiO2 and 8.53% Na2O. The fine aggregate was ordinary medium sand with a fine modulus of 2.6, a mud content of 0.83%, and a bulk density of 1465 kg/m3.

Table 1.
Chemical composition of raw materials (wt.%).
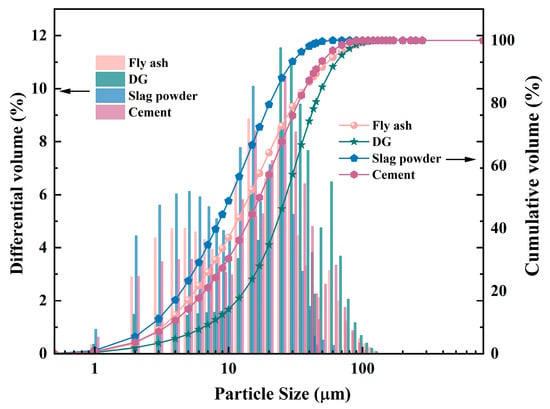
Figure 1.
Particle size distribution of raw materials.
2.2. Mix Proportion
The AAM was prepared by replacing fly ash with DG in proportions of 0%, 2%, 4%, 6%, 8%, and 10%, and the cement mortar was used as a control group. The results of the preliminary test show that an excessively high DG substitution rate (>10%) will lead to significant loss of workability and may damage the strength development. Therefore, the maximum content of DG was set to 10% in this study. Based on the previous results [25,26], the total amount of alkali-activated cementitious materials was set at 440 kg/m3, of which 70% was slag powder and 30% fly ash. To ensure that the AAM had mechanical strength, the alkali concentration (the mass ratio of Na2O to cementitious material was set to 3.5%, the modulus (the molar ratio of SiO2 to Na2O) was 1.0, and the sol ratio (the mass ratio of alkali activator solution to cementitious material) was 0.42. The quantities of water and activator (water glass and solid NaOH) were determined. The specific mix proportion is shown in Table 2.

Table 2.
Mix proportion of AAM (kg/m3).
2.3. Testing Method
2.3.1. Workability
According to the GB/T 2419-2024 standard [27], the workability of an AAM was assessed by measuring its fluidity under specified conditions. The thoroughly mixed mortar was poured into the mold in two layers. The first layer was filled to 2/3 of the height of the mold and compacted, and then the second layer of mortar was filled. The mortar should be slightly higher than the test mold after stamping. Then, the conical mold was lifted up vertically, and the platform was opened and immediately vibrated 25 times. After the vibration was complete, the bottom surface diameter of the mortar was measured in both perpendicular directions using a vernier caliper, with the mean value taken as the fluidity of the mortar.
In accordance with GB/T 1346-2024 [28], the cement paste setting time was measured using a Vicat apparatus. First, the cementitious material was weighed to 500 g, a measured amount of alkali activator solution was added, and the mixture was thoroughly stirred. A uniformly stirred slurry was injected into the test mold, and after surface leveling was applied, the Vicat instrument was used for testing. As the test rod sank to 6 ± 1 mm below the base plate, the amount of alkali-activated solution present at that point was recorded, which represented the standard water consumption. Subsequently, the prepared paste sample was immediately cured under specified conditions. The initial setting state was determined by observing the immersion of the Vicat test needle, which was immersed into the bottom surface of the sample to 4 ± 1 mm. The sample after the initial setting was removed from the glass plate, flipped 180°, and continued curing. When the needle used to test the setting time penetrated the sample to a depth of 0.5 mm, the sample was determined to have reached its final setting time.
2.3.2. Mechanical Property
According to the relevant specifications of GB/T 17671-2021 [29], a prism specimen of 40 mm × 40 mm × 160 mm was used to determine the mechanical strength of AAM. The mixture was poured into the mold and a shaker was used to compact it for 60 s to remove entrained air and achieve consolidation. After the surface was leveled, the sample was moved to a standard curing box to cure until the specified testing age was reached.
2.3.3. Shrinkage Performance and Corrosion Resistance
In accordance with standard JGJ/T 70-2009 [30], the drying shrinkage of AAM was tested on prism specimens with the same size as those used for compressive strength testing. Upon completion of sample preparation, they were placed in a standard curing chamber (temperature approximately 20 °C, relative humidity greater than 95%) for 7 days of curing, after which their initial length was measured. The samples then transferred to a test chamber with a temperature of 20 ± 2 °C and relative humidity of 60 ± 5% for further curing. The lengths of the samples were measured after 7, 14, 21, 28, and 56 days to characterize the time-varying drying shrinkage law of AAM.
As required by ASTM C267-01 specifications [31], AAM containing DG with a curing age of 28 d was immersed in a 5% magnesium sulfate solution and tap water to evaluate its corrosion resistance. The effect of DG on the corrosion resistance of AAM was deter-mined by measuring the mass and compressive strength of the samples after 30 days and 60 days of immersion.
2.3.4. Microstructure
The microstructure of DG-based AAM was characterized using alkali-activated cement paste samples. FTIR, XRD, and SEM analysis methods were used to characterize the phase composition and microstructure of DG-based alkali-activated materials. The step speed of the XRD test instrument was 0.2 s/step, and the scanning range was 5°~60°. The magnification of SEM was 1000×, 5000×, and 20,000×, and the test wavelength of FTIR was 400–4000 cm−1.
3. Results and Discussion
3.1. Workability
3.1.1. Fluidity
Figure 2a illustrates the fluidity test setup and a representative tested specimen. Figure 2b shows the effect of the DG content on the fluidity of the AAM mixture, indicating that the fluidity of the AAM mixture decreased significantly with increasing DG content. The fluidity of AAM-4 and AAM-10 was reduced by 4.8% and 13.1%, respectively, com-pared to the control group AAM-0. This phenomenon is mainly attributed to the following reasons: (1) compared with fly ash, DG has finer particles (Figure 1), and higher content will lead to a significant increase in AAM water demand [32]; (2) fly ash has a morphological effect, and its application in AAM can significantly improve its fluidity [33]. Under the condition that the amount of alkali activator solution is constant, the amount of fly ash gradually decreased as the DG content increased, resulting in a gradual decrease in the AAM fluidity. The data in Figure 2 also shows that the workability of AAM was significantly lower than that of cement mortar, and the fluidity of AAM-0 was 31% lower than that of OPC. This is because the alkali-activated material mainly relies on water glass and NaOH as activators, resulting in a highly viscous system [34]. Moreover, the hydration reaction rate of alkali-activated cementitious materials is significantly faster than that of ordinary Portland cement, resulting in significantly lower fluidity for AAM than for OPC.
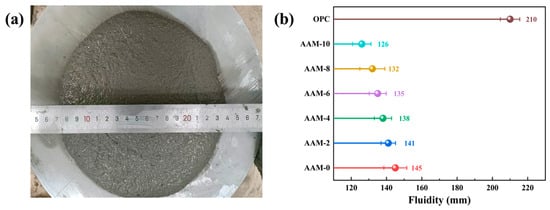
Figure 2.
Fluidity of AAM mixture: (a) fluidity test, (b) test result.
3.1.2. Setting Time
Figure 3 illustrates the effect of DG on the setting time of alkali-activated cement, showing that DG can effectively prolong the initial and final setting times of alkali-activated cement, which gradually increases with the DG content. With a DG content of 4.0%, the initial and final setting times of AAM-4 were 35.7% and 14.6% longer, respectively, than those of AAM-0. As the DG content increased to 10.0%, the setting time of AAM-10 was further prolonged, with its initial and final setting times being 121.4% and 26.8% higher than those of AAM-0, respectively. When DG is used in place of fly ash, the concentration of Ca2+ in the system can be significantly increased, changing the original Ca/Si molar ratio. This change inhibits the dissolution–condensation hydration reaction of active substances such as [SiO4]4− and [AlO4]5− [35], thereby delaying the setting time. It is worth noting that when the DG content was 2.0%, the initial setting time was further shortened, and the initial setting time of AAM-2 was 25 min less than that of AAM-0. This may be due to the fact that DG is alkaline and SO42− in DG promotes nucleation of early AFt, thus shortening the initial setting time of the alkali-activated cement. Moreover, alkali-activated cement exhibits significant early setting properties, and its setting time is significantly shorter than that of OPC. It was found that the initial and final setting times of AAM-0 were 70 min and 205 min, respectively, which were 61.1% and 19.6% shorter than those of OPC. The rapid condensation characteristics of alkali-activated materials are mainly due to the strongly alkaline environment created by the two-component activator (water glass + NaOH). Under the action of highly alkaline environment, the active com-ponents in slag powder and fly ash, such as [SiO4]4− and [AlO4]5−, undergo rapid polycondensation reactions, and the hydration reaction rate is much higher than the dissolution rate of C2S and C3S in ordinary Portland cement [36,37], resulting in AAM-0 having a much shorter setting time than OPC.
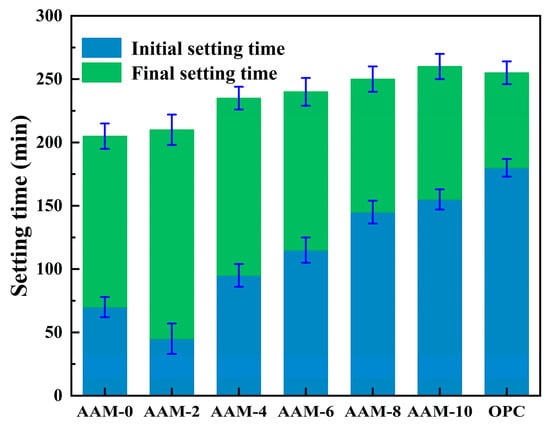
Figure 3.
Setting time of alkali-activated cement.
3.2. Mechanical Property
3.2.1. Compressive Strength
The effect of DG content on the compressive strength of AAM is presented in Figure 4, showing that the compressive strength of the AAM first increased and then decreased with increasing DG content, with the best performance at a DG content of 6.0%. At a DG content of 2.0%, the 28-d compressive strength of AAM-2 reached 58.05 MPa, which was 1.3% higher than that of AAM-0. With an increase in DG content to 6.0%, AAM-6 can achieve a 28-d compressive strength of 63.25 MPa, 10.4% higher than AAM-0. The appropriate addition of DG can significantly increase the compression strength of AAM. The strengthening mechanism mainly includes two aspects: (1) SO42− released from DG dis-solve in water and combine with OH- in the alkali activator to form a composite excitation system, which synergistically promotes the hydration reaction of aluminosilicate precursor, accelerating the formation of C(N)-A-S-H gels, and thereby improving the early-age mechanical properties of the material [38]; (2) SO42− provided by DG can promote the dissolution of Al3+ in fly ash, and then react with Ca2+ to form AFt. The needle-like crystal structure of ettringite can effectively fill the pores of the matrix, improving the compactness of the cement stone and further enhancing the macroscopic mechanical properties [39]. However, when the DG content exceeded 6.0%, the compressive strength of the AAM began to decrease, and the 28-d compressive strength of AAM-10 was 23.9% lower than that of the AAM-0. An increase in the DG content led to a corresponding decrease in the fly ash content in the system, resulting in an increase in the calcium content and a decrease in the silicon and aluminum content of the alkali-activated cementitious material system. When the DG content exceeds a certain content, due to the lack of sufficient active silicates and aluminates to consume the added SO42−, it will not only inhibit the continuous formation of ettringite but also reduce the formation of alkali-activated cement gel, thereby reducing the compressive strength of AAM [40]. In addition, the ettringite formed by DG in alkali-activated cementitious systems has a micro-expansion effect, and large amounts of generated AFt tend to form internal expansion stress, resulting in micro-cracks in the cement matrix, which is detrimental to the mechanical properties of AAM [41]. The data in Figure 4 shows that the mechanical properties of AAM were significantly better than those of OPC. AAM-0 achieved a 28-d compressive strength of 55.3 MPa, 45.9% higher than OPC. The main reason for this is that the porosity of the cementitious system is significantly lower than that of the OPC, leading to better compressive strength [42].
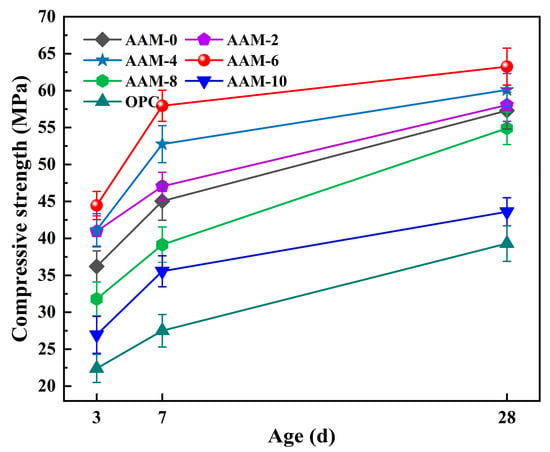
Figure 4.
Compressive strength of AAM.
3.2.2. Flexural Strength
The flexural strength reflects the ability of a material to resist bending loads and is a key indicator for evaluating the mechanical properties of AAM. Figure 5 shows the flexural strength of AAM at different DG contents, indicating that the flexural strength of the AAM increased first and then decreased as the DG content increased, which is consistent with the law of variation in its compressive strength. At 6.0% DG content, the AAM showed the best flexural strength. Of these, AAM-6 achieved a 3-d compressive strength of 7.5 MPa, 8.69% higher than AAM-0. At 28 d, it reached a flexural strength of 10.7 MPa, 12.63% higher than the AAM-0. The incorporation of DG in the alkali-activated cementitious material system can significantly improve the mechanical properties. Its mechanism is mainly reflected in (1) the promotion of early hydration reaction and the improvement in early-age flexural strength, and (2) the catalytic formation of AFt phase enhancing the compactness of the matrix through the filling effect, thereby improving the flexural strength of AAM. However, when the DG content exceeded 6.0%, the flexural strength of the AAM decreased significantly. Specifically, the 3-d flexural strength of the AAM-10 was reduced to 4.2 MPa, which is 39.1% lower than that of the AAM-0. Its 28-d flexural strength was 8.2 MPa, which was 8.42% lower than that of the AAM-0. This is because excessive DG can suppress the hydration reaction rate of alkali-activated cementitious materials and unreacted DG particles aggregate in the matrix, resulting in a stress concentration inside the material that significantly reduces the AAM flexural strength.
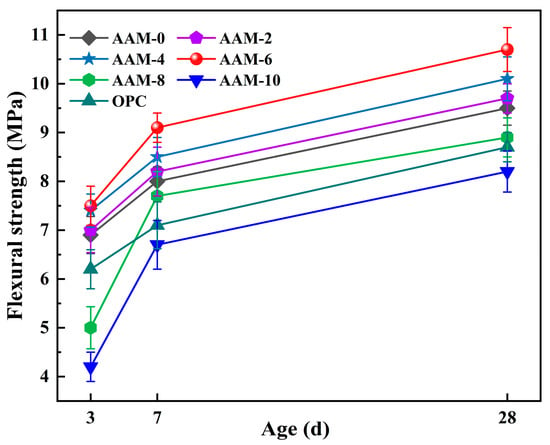
Figure 5.
Flexural strength of AAM.
3.3. Drying Shrinkage
Drying shrinkage is a key factor leading to cracking in cement-based materials, which severely affects their crack resistance, durability, and long-term structural stability. In this study, the drying shrinkage performance of DG-based AAM was systematically studied by measuring the length change rate of AAM specimens under standard curing conditions. The effect of DG content on the drying shrinkage of the AAM is shown in Figure 6, indicating that the drying shrinkage rate of AAM decreased as the DG content increased. At 6% DG content, the 14 d and 56 d shrinkage rates were 13.6% and 12.3% lower, respectively, than for AAM-0. When the DG content was further increased to 10%, the 14-d shrinkage rate was reduced by 26.2%, and the 56-d shrinkage rate was reduced by 20.2%. The improvement effect of DG on drying shrinkage is mainly due to the following mechanisms: (1) In the early stage, SO42− and Ca2+ dissolved from DG are adsorbed on the surface of C-(A)-S-H gel, which reduces the surface tension of pore solution [43] and effectively reduces the early shrinkage of AAM. (2) In the later stage of hydration, SO42− released by continuous dissolution of DG reacts with Ca and Al components in the system to form acicular ettringite with micro-expansion characteristics, which alleviates drying shrinkage through volume compensation effect. (3) With the increase in DG content, the fly ash proportion decreases accordingly, shifting the cementitious material system from low to high calcium. The C-(A)-S-H gel formed by the high Ca/Si ratio system has higher crystallinity and lower sensitivity to drying shrinkage [44], which is beneficial to improve the drying shrinkage performance of the material. The data in Figure 6 also shows that the drying shrinkage rate of AAM is much higher than that of OPC, and the 56-d shrinkage rate of AAM-0 is 30.2% higher than that of OPC. This is because, at the same water–binder ratio, the gel content and free water content are higher in alkali-activated material systems than in ordinary cement-based materials [45]. The evaporation of a large amount of free water significantly enhances the capillary tension, leading to a more significant contraction deformation.
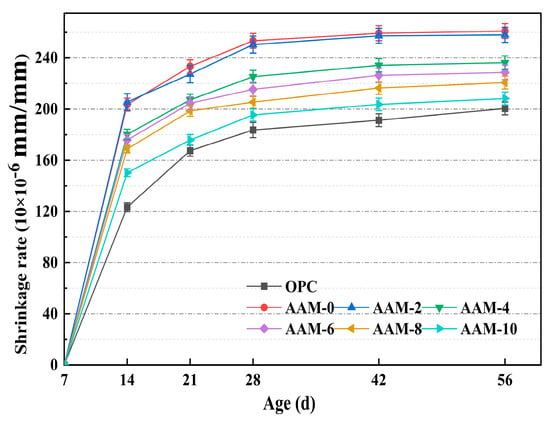
Figure 6.
The drying shrinkage of AAM with different DG content.
3.4. Water Erosion Resistance and Sulfate Resistance
While the introduction of DG can be effective in improving the drying shrinkage of AAM, excessive sulfate and residual gypsum may negatively affect the corrosion resistance of alkali-activated materials. Therefore, this study systematically investigated the changes in the mass and strength of DG-based AAM subjected to water erosion and corrosion by a solution of magnesium sulfate. Figure 7 presents the compressive strength of the DG-based AAM after immersion in tap water and 5% MgSO4 solution for 30 and 60 days. As shown in Figure 7a, compared with AAM-0 specimens without DG addition, the DG-based AAM showed significant strength deterioration in a water environment, and the degree of deterioration increased in line with the increase in DG content. The specific performance was as follows: the strength loss rates were 23.79% and 44.59% for the AAM-4 specimens after 30 d and 60 d of immersion, respectively. The strength loss rates further increased to 44.03% and 52.41% for specimens of AAM-6. The strength loss rates of the control group AAM-0 specimen were 9.24% (30 d) and 22.33% (60 d). This phenomenon can be attributed to the following mechanisms: (1) DG, as a sulfate source, reacts with the aluminum phase and calcium phase dissolved from slag in an alkaline environment to form AFt, and AFt has a crystallization expansion effect, resulting in matrix micro-crack initiation; (2) under the condition of high gypsum content, the continuous water absorption expansion of unreacted gypsum produces additional expansion stress [46,47].
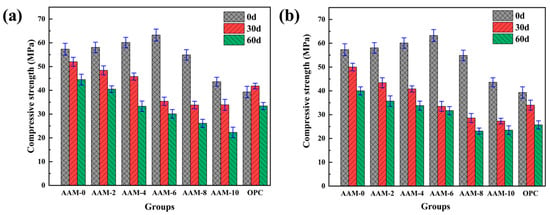
Figure 7.
The compressive strength of AAM with DG after soaking in solution: (a) H2O, (b) 5% MgSO4.
The compressive strength test results of DG-based AAM after soaking in 5% MgSO4 solution for 30 and 60 days are shown in Figure 7b and are similar to the soaking results in water. The DG-based AAM showed significant strength deterioration in the 5% MgSO4 solution. The experimental data show that the strength loss rates of AAM-4 specimens after 30 d and 60 d of immersion were 32.11% and 44.09%, respectively. As the DG content increased to 6%, the strength loss rates increased further to 47.03% and 49.88%, which are significantly higher than the strength loss rates in ordinary water environments. This accelerated deterioration is mainly due to the following synergistic erosion mechanisms: (1) magnesium ion erosion—the replacement of Ca2+ by Mg2+ in C-A-S-H gel forms a non-gel M-S-H phase and precipitates gypsum crystals, leading to expansion stress—and (2) sulfate attack—SO42+ reacts with the active aluminum phase (fly ash) and calcium phase (slag powder) to continuously form AFt, and its crystal expansion leads to the expansion of matrix microcracks [48,49]. In particular, an increase in the DG content will significantly aggravate the above expansion deterioration effects.
Table 3 shows the rates of mass change in the DG-based AAM after immersion in tap water and 5% MgSO4 solution for 30 and 60 days, indicating that the DG-based AAM exhibited an increase in mass in both solutions. After 30 d and 60 d of soaking, the mass growth rate in the 5% MgSO4 solution was significantly higher than in the tap water environment. This is because MgSO4 erosion leads to the conversion of hydration products into gypsum, and its significant water absorption properties directly contribute to the mass increase. Moreover, the continuous generation of AFt easily increases the matrix pore structure rate and increases the water permeability. Therefore, the mass growth of DG-based AAM became more significant with extended soaking time.

Table 3.
The mass change in AAM with DG after soaking in H2O and 5% MgSO4 solution.
By comparing the corrosion resistance of OPC and DG-based AAM, it can be seen that DG-based AAM has a significantly lower mass growth rate than OPC in water, while 5% MgSO4 solution has a higher mass growth rate than OPC. This difference is directly related to the DG content and the chemical erosion of the Mg2+ and SO42−. Combined with the strength test results, after being immersed in water for 60 days, the compressive strength of AAM-4 remained higher than that of OPC, while the compressive strength of AAM-6 was also maintained after being immersed in MgSO4 solution for 60 days. Therefore, the optimal DG content for DG-based AAM in a sulfate-coupled environment is 4.0%.
3.5. Microstructure
The microstructural evolution of the DG-based AAM was comprehensively characterized using XRD, FTIR, and SEM to elucidate the changes in phase composition, molecular structure, and morphology following DG incorporation. These findings provide crucial evidence for understanding the intrinsic mechanisms responsible for the enhancement of its macroscopic properties.
3.5.1. XRD
Figure 8 shows the XRD patterns for alkali-activated materials with different DG contents and 28-day curing ages, indicating the main mineral composition and polymer gel products of DG-based alkali-activated materials. The most significant peaks include mullite, calcite, calcium silicate, quartz, anhydrite, and ettringite. The main gel product of the alkali-activated material was C-S-H, corresponding to peak 2 in the XRD pattern. The characteristic peak is consistent with the characteristic diffraction peak of calcite, suggesting that the system of alkali-activated cementitious materials undergoes a partial carbonization reaction in an alkaline environment to generate calcium carbonate crystals. With increasing DG content, the FA content decreases accordingly, while the calcium content in the system increases significantly, which promotes hydration reactions in alkali-activated cementitious materials. XRD analysis showed that in a high-calcium environment, the hydration reaction in alkali-activated cementitious materials is promoted, as suggested by an increase in the intensity of the characteristic peak of calcium silicate and a gradual decrease in the intensity of the diffraction peak of mullite [50]. The characteristic diffraction peak of AFt gradually appears with increasing SO42− and Ca2+ contents. However, the relatively low diffraction peak intensity of the AFt in this study may be attributed to the partial decomposition of AFt in the alkaline environment of calcite. In addition, the characteristic diffraction peak of calcium sulfate was not detected in samples with 6% DG content, while samples with 10% DG showed a distinct anhydrite diffraction peak. This phenomenon indicates that when the content of DG is less than 6%, CaSO4 in the system reacts more completely with Al2O3 in an alkaline environment (OH-). When the content of DG is higher than 6%, the active aluminosilicate in FA is not sufficient to completely consume Ca2+ and SO42− in the system, resulting in limited formation of the hydration products (AFt and C-(A)-S-H gels). The excessive SO42− residue in the matrix will weaken the mechanical properties of the AAM, and this deterioration effect increases with increasing DG content (Figure 5).
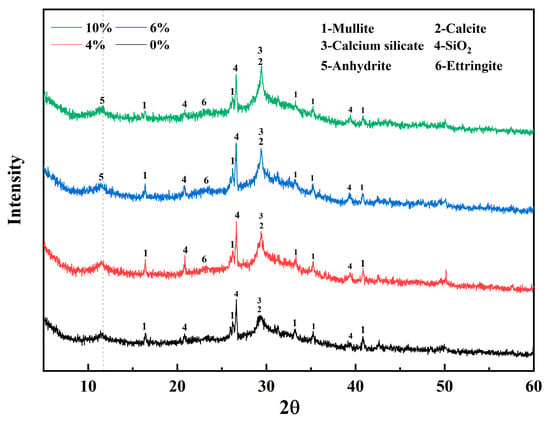
Figure 8.
XRD patterns of alkali-activated materials after 28 d of curing with varying DG content.
The results of the mechanical property tests show that AAM with 6.0% DG content had the best mechanical properties. Figure 9 shows the XRD diffraction patterns of alkali-activated materials with DG content of 6% at different curing ages (1 d, 3 d, 7 d, and 28 d). This shows that as the curing time increased, the intensity of the characteristic peak of mullite in the range of 35–40° gradually decreased, confirming that the active aluminosilicate in FA continued to participate in the hydration reaction. Moreover, the characteristic peaks of anhydrite are clearly visible in the 1-d, 3-d, and 7-d samples, but disappear in the 28-d sample, indicating that SO42− continued to be consumed during the curing process and reacted with Ca2+ and active Al2O3 to form AFt. Correspondingly, the intensity of the AFt diffraction peak increased with age, and this phase evolution had a positive effect on the improvement of the mechanical properties.
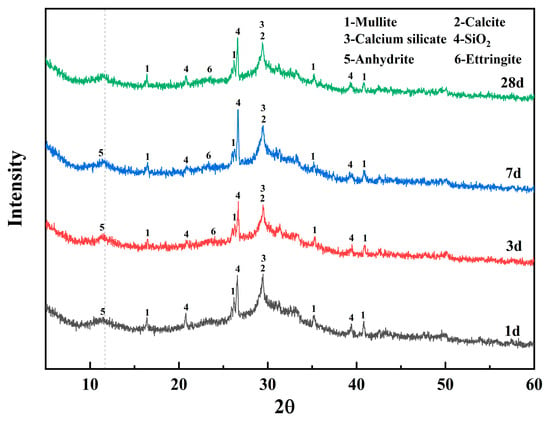
Figure 9.
XRD patterns of alkali-activated materials with 6% DG content after varying curing times.
3.5.2. FTIR
Figure 10 shows the FTIR analysis results of DG on the molecular structure of alkali-activated materials. The main characteristic peaks are in agreement with those reported in the references. The absorption peaks at 3452.0 cm−1 and 3450.81 cm−1 are attributed to the asymmetric stretching of the -OH group in the hydration product, while the absorption peaks at 1640.86 cm−1 and 1640.83 cm−1 correspond to the bending of the -OH group [51]. These characteristic peaks are typical of the vibrational modes of crystalline water in C-A-S-H gels. The peaks at 1418.95 cm−1 and 1491.51 cm−1 correspond to asymmetric stretched vibrational bands of C-O [52], which are attributed to the partial carbonization reaction of the alkali-activated material. Furthermore, the absorption peaks at 874.26 cm−1 and 875.60 cm−1 can be attributed to the Al-O-Si asymmetric stretching vibrations of the AlO4− group in the C-A-S-H gel [53]. The peak at 450.57 cm−1 can be attributed to bending vibrations of the O-Si-O and Si-O-Si bonds, possibly caused by the presence of alkali activators and water molecules [54]. The absorption peak at 709.35 cm−1 originates from the aluminosilicate skeleton structure in the cementitious material. It is worth noting that the AAM-6 sample with DG shows a more significant change in the peak intensity at this wavenumber than the AAM-0 sample, suggesting that the introduction of DG increases the concentration of soluble Ca2+, shifting the characteristic vibrational peak towards lower wavenumbers. In addition, the characteristic peak of the ettringite was not detected in the FTIR analysis, which could be due to the decomposition of the ettringite in the alkaline environment.
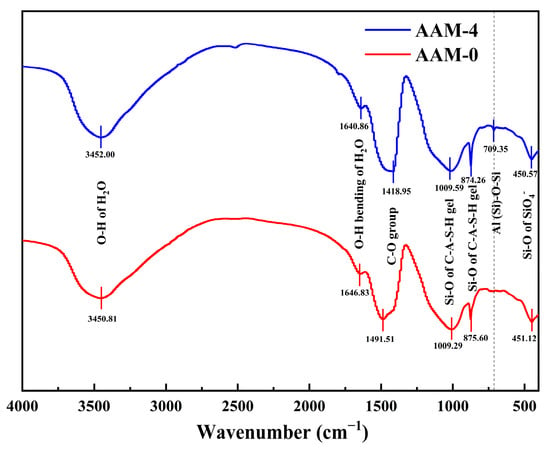
Figure 10.
FTIR spectra of alkali-activated material with varying DG content after a curing time of 28 d.
3.5.3. SEM
In this study, the microscopic morphology of hydration products of alkali-activated materials was characterized through SEM. Figure 11 shows SEM images of the alkali-activated material without DG after 3 d of curing. A two-component alkali activator consisting of water glass and NaOH was used to activate the slag powder and fly ash. The high concentration of OH- promoted the rapid dissolution of the calcium phase in the slag powder, and the silicon–aluminum phase released by the initial hydration reaction formed C-(A)-S-H gel through the polymerization reaction. As shown in Figure 11, hydration products of alkali-activated materials without DG have distinct microcracks with no hydration product filling at the crack. The formation of microcracks can be attributed to the drying shrinkage stress induced by water evaporation during the early hydration phase, which adversely affects the development of mechanical properties. The inclusion of DG significantly improved the compactness of the AAM matrix. Figure 12 shows SEM images of alkali-activated material containing 6% DG after 3 d of curing. Compared with ordinary alkali-activated materials (Figure 11), the micro-cracks in the matrix were significantly reduced, and the compactness of the matrix was significantly enhanced. This suggests that DG can facilitate hydration reactions in cementitious materials at early times, favoring the formation of denser microstructures. High-magnification SEM observation (×20,000) confirmed the existence of AFt phase in hydration products. In the early stages of hydration, AFt exhibited a typical needle-rod like morphology with randomly distributed interstitials among the hydrated products. The formation of AFt helped to improve the drying shrinkage and mechanical properties of the AAM in the early stages. As the curing age increased, the AFt phase continued to grow and promoted further densification of the cement matrix. An SEM image of the alkali-activated material with 6% DG after 28 d of curing is shown in Figure 13, where a well-developed AFt crystal structure can be observed. The AFt crystals effectively fill the microcracks and pores, significantly reducing the porosity of the matrix and forming a denser microstructure. This densification enhances the mechanical properties and reduces the drying shrinkage of the AAM-6. The 28-d SEM results corroborate the findings from the XRD and mechanical tests, confirming that the 6% DG content promoted the formation of a uniform and dense micro-structure, which is crucial for overall performance enhancement.

Figure 11.
SEM images of alkali-activated materials without DG after 3 d of curing.

Figure 12.
SEM images of alkali-activated materials containing 6% DG after 3 d of curing.

Figure 13.
SEM images of alkali-activated materials containing 6% DG after 28 d of curing.
4. Conclusions
In this study, the effects of DG content on the workability, shrinkage performance, mechanical properties, and corrosion resistance of AAM were systematically studied. The phase composition and micro-morphology of DG-based alkali-activated materials were revealed through XRD, FTIR, and SEM analysis. The main conclusions can be highlighted as follows:
- (1)
- The fluidity of the AAM gradually decreased with increasing DG content, while the setting time showed the opposite change rule. When the DG content was 10%, the fluidity of AAM reached 126 mm, and its setting time was equivalent to that of OPC.
- (2)
- DG can effectively improve the shrinkage performance of AAM, while reducing its resistance to water erosion and sulfate erosion. With a DG content of 10%, the 56-d drying shrinkage of AAM was reduced by 20.2%.
- (3)
- The mechanical properties first increased and then decreased with increasing DG content. When the DG content was 6%, the 28-day compressive strength of AAM reached 63.25 MPa and the flexural strength was 10.7 MPa. After 60 days of water corrosion and sulfate corrosion, the compressive strength of AAM-6 was significantly better than that of OPC under the same conditions.
- (4)
- Microscopic analysis shows that DG contributes to the formation of needle-like AFt crystals in alkali-activated systems. AFt grew continuously during the curing process, filling the voids of the hydration product and forming a dense microstructure, which improved the mechanical and contractile properties of the material.
Author Contributions
Resources, Methodology, Funding acquisition, and Writing—Review and Editing, X.Z.; Formal analysis, Writing—Original Draft, and Writing—Review and Editing, X.W.; Methodology and Software, W.Y.; Validation and Software, Y.Z.; Conceptualization, Methodology, and Supervision, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the High-level Talent Scientific Research Project of Qingdao Agricultural University (Grant No. 1120045).
Data Availability Statement
Since the experiment was completed with the support of Oingdao Agricultural University, the data used to support the results of this study are available from the responsible person and the author upon request.
Conflicts of Interest
There is no conflict of interest to declare.
References
- Hou, Z.; Xiong, Y.; Luo, J.; Fang, Y.; Haris, M.; Chen, Q.; Yue, Y. International experience of carbon neutrality and prospects of key technologies: Lessons for China. Pet. Sci. 2023, 20, 893–909. [Google Scholar] [CrossRef]
- Supriya, R.; Chaudhury, U.; Sharma, P.; Thapliyal, L. Singh, Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, S.; Feng, X. Comparative study on the effect of agricultural waste biochar on the properties of cement mortar. Ind. Crops Prod. 2025, 227, 120854. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Fan, C. Research progress on two-dimensional carbon nanomaterials modified alkali-activated cementitious materials: A review. J. Build. Eng. 2024, 92, 109690. [Google Scholar] [CrossRef]
- Zhong, M.; Meng, J.; Ning, B.; Na, F.; Cui, T.; Shi, X.; Cui, T. Preparation and alkali excitation mechanism of coal gangue-iron ore tailings non-sintering ceramsite. Constr. Build. Mater. 2024, 426, 136209. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, S.; Xia, X.; Feng, X. Eco-friendly utilization of plant-derived biochar as partial replacement of sand in alkali-activated mortar. Constr. Build. Mater. 2025, 495, 143723. [Google Scholar] [CrossRef]
- Li, B.; Wu, J.; Song, N.; Zheng, Y.; Lu, Y.; Chi, Y. Time-dependent drying shrinkage model for alkali-activated slag/fly ash-based concrete modified with multi-walled carbon nanotubes. J. Build. Eng. 2025, 111, 113082. [Google Scholar] [CrossRef]
- Nassar, A.; Sivanandam, S.; Saran, D.; Sundar, S.B.S.; Kathirvel, P.; Murali, G.; Dixit, S. Durability assessment of sustainable one-part alkali activated concrete produced from agricultural and industrial waste activators under aggressive environmental conditions. Constr. Build. Mater. 2025, 486, 141823. [Google Scholar] [CrossRef]
- Xi, X.; Zheng, Y.; Zhuo, J.; Zhang, P.; Golewski, G.L.; Du, C. Influence of water glass modulus and alkali content on the properties of alkali-activated thermally activated recycled cement. Constr. Build. Mater. 2024, 452, 138867. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Z.; Sun, X.; Zhu, X. Utilization of surface-modified fly ash cenosphere waste as an internal curing material to intensify concrete performance. J. Clean. Prod. 2022, 358, 132042. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Firouz, R.M.; Illikainen, M. Drying shrinkage in alkali-activated binders-a critical review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, X.; Xiong, D.; Li, Q.; Yang, C.; Yang, K.; Basheer, M. Mitigation of autogenous shrinkage of alkali-activated slag mortar by stearate salts. Constr. Build. Mater. 2023, 384, 131383. [Google Scholar] [CrossRef]
- Tran, N.P.; Nguyen, T.N.; Ngo, T.D. The role of organic polymer modifiers in cementitious systems towards durable and resilient infrastructures: A systematic review. Constr. Build. Mater. 2022, 360, 129562. [Google Scholar] [CrossRef]
- Ma, H.; Fu, C.; Huang, K.; Dai, E.; Zhang, S.; Fang, Y.; Feng, J. Study on the characteristics of alkali-activated fly ash-slag improved by cenosphere: Hydration and drying shrinkage. Constr. Build. Mater. 2023, 372, 130822. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Zhang, E.Q.; Ren, J. Shrinkage behaviour, early hydration and hardened properties of sodium silicate activated slag incorporated with gypsum and cement. Constr. Build. Mater. 2020, 248, 118687. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Lin, H.; Li, Y.; Ye, J.; Zhang, W. New insights on the action mechanisms of anhydrite on autogenous shrinkage and mechanical properties of clinkerless Ultra-high performance concrete. Constr. Build. Mater. 2025, 486, 141949. [Google Scholar] [CrossRef]
- Liu, P.; Gu, Y.; Zhong, J.; Kuang, J.; Huang, X.; Jin, F.; Zhao, M.; Mo, L. Carbonated steel slag powder in cement: Retardation mechanism and triethanolamine-enhanced hydration strategy. Constr. Build. Mater. 2025, 492, 142881. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Cai, Z.; Liu, R.; Zhu, P.; Yan, X.; Yu, J.; He, J. Synergistic mechanism of the performance and recyclability of recycled fine aggregate concrete under chloride salt dry-wet cycles by composite mineral admixtures. Constr. Build. Mater. 2025, 491, 142683. [Google Scholar] [CrossRef]
- An, Q.; Pan, H.; Zhao, Q.; Du, S.; Wang, D. Strength development and microstructure of recycled gypsum-soda residue-GGBS based geopolymer. Constr. Build. Mater. 2022, 331, 127312. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, J.; Zhang, S.; Wang, X.; Huang, X.; Ni, W. Properties and hydration mechanism of eco-friendly binder from circulating fluidized bed bottom ash, carbide slag, and desulfurization gypsum. Constr. Build. Mater. 2024, 457, 139411. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Injorhor, B.; Phoo-ngernkham, T.; Damrongwiriyanupap, N.; Li, L.Y.; Sukontasukkul, P.; Chindaprasirt, P. Drying shrinkage, strength and microstructure of alkali-activated high-calcium fly ash using DSG-gypsum and dolomite as expansive additive. Cem. Concr. Compos. 2020, 114, 103760. [Google Scholar]
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Duan, D.; Liao, H.; Wei, F.; Wang, J.; Wu, J.; Cheng, F. Solid waste-based dry-mix mortar using fly ash, carbide slag, and flue gas desulfurization gypsum. J. Mater. Res. Technol. 2022, 21, 3636–3649. [Google Scholar]
- Ying, J.; Yang, Z.; Xie, Z.; Tian, Z.; Liang, L. Enhancing cement-based grouting materials with water-based three-dimensional porous graphene nanofluid: Effects on pore structure, hydration products, and multi-scale mechanical properties. Constr. Build. Mater. 2025, 493, 143189. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Liu, C.; Wang, L.; Chen, M.; Yue, G. Study of mix design and performance of alkali-activated concrete with recycled concrete aggregate. Constr. Build. Mater. 2023, 400, 132882. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, S.; Feng, X. Study on improving the properties of alkali-activated materials by using corncob ash. ACS Omega 2025, 10, 16291–16299. [Google Scholar] [CrossRef]
- GB/T 2419-2024; Method for Determining the Flowability of Cement Mortar. Cement New Materials Institute, China National Building Materials Academy: Beijing, China, 2024.
- GB/T 1346-2024; Test Methods for Water Requirement of Standard Consistency, Setting Time and Soundness of the Portland Cement. National Technical Committee for Cement Standardization: Beijing, China, 2024.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). General Administration for National Market Supervision: Beijing, China, 2021.
- JGJ/T70-2009; Standard for Test Method of Performance on Building Mortar. Shanxi Academy of Building Research: Shanxi, China, 2009.
- ASTM C267-01; Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacings and Polymer Concretes. Annual Book of ASTM Standard: Philadelphia, PA, USA, 2012.
- Wang, W.; Li, L.; Ren, Z.; Yang, J.; Kong, T. Filling mechanism of coal gasification fly ash in lightweight gypsum blocks with a high water-to-gypsum ratio. Constr. Build. Mater. 2025, 460, 139828. [Google Scholar] [CrossRef]
- Luo, T.; Ma, Y.; Xie, H.; Li, F.; Li, Z.; Fu, J. Piezoresistivity of carbon fiber-reinforced alkali-activated materials: Effect of fly ash microspheres and quartz sands. Constr. Build. Mater. 2024, 425, 136125. [Google Scholar] [CrossRef]
- Nanda, B.; Mishra, J.; Patro, S.K. Synthesis of rice husk ash based alkaline activators for geopolymer binder systems: A review. J. Build. Eng. 2024, 91, 109694. [Google Scholar] [CrossRef]
- Parhizkar, A.; Nazarpour, A.; Khayat, N. Investigation of geotechnical and microstructure characteristics of gypsum soil using ground granulated blast-furnace slag (GGBS), fly ash, and lime. Constr. Build. Mater. 2024, 418, 135358. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, X.; Ye, J.; Luan, Z.; Xiao, Y.; Zhang, W. Performance of a steel slag-based supplementary cementitious material via the synergistic modification of binary fly ash and gypsum system. Constr. Build. Mater. 2023, 405, 133186. [Google Scholar] [CrossRef]
- Mareya, M.; Tchadjie, L.; Sithole, T. Turning fly ash and waste gypsum into a resource for backfilling applications. Case Stud. Constr. Mater. 2024, 20, e02703. [Google Scholar] [CrossRef]
- Luan, Y.; Wang, J.; Ma, T.; Wang, S.; Li, C. Modification mechanism of flue gas desulfurization gypsum on fly ash and ground granulated blast-furnace slag alkali-activated materials: Promoting green cementitious material. Constr. Build. Mater. 2023, 396, 132400. [Google Scholar] [CrossRef]
- Fan, K.; Cui, S.; Yao, Y. Fire resistance performance of clinker-free cementitious materials produced from phosphorus slag, calcium carbide slag, desulfurization gypsum, and metakaolin. Constr. Build. Mater. 2025, 467, 140400. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, X.; Guo, W. Effect of alkaline activator on SIOT-GGBS-MK based alkali-activated mortar. Constr. Build. Mater. 2025, 467, 140322. [Google Scholar] [CrossRef]
- Ma, Y.; Chu, H.; Rong, H.; Ba, M. Influence of sodium silicate on the properties of ground granulated blast furnace slag-desulfurized gypsum (GGBS-DG) based composite cementitious materials and LCA evaluation. Sustain. Chem. Pharm. 2025, 46, 102066. [Google Scholar] [CrossRef]
- Shi, P.; Falliano, D.; Vecchio, F.; Marano, G.C. Investigation on the compressive strength and durability properties of alkali-activated slag mortar: Effect of superabsorbent polymer dosage and water content. Dev. Built Environ. 2024, 17, 100322. [Google Scholar] [CrossRef]
- Lv, F.; Wang, L.; An, H.; Chen, S.; Shu, J.; Kong, D. Effects of hybrid fibers on properties of desulfurized gypsum-based composite cementitious materials. Constr. Build. Mater. 2023, 392, 131840. [Google Scholar] [CrossRef]
- Xue, L.; Ni, Z.; Zhou, Z.; Zhang, Z.; Xiong, H.; Wang, H.; Zhuge, X.; Liu, H. Effects of desulfurized gypsum on shrinkage behavior of alkali-activated slag (AAS) and hybrid alkali-activated cement (HAC). Case Stud. Constr. Mater. 2025, 22, e04320. [Google Scholar]
- Yang, J.; Meng, T.; Han, J.; Qiao, Y.; Chen, X.; Wang, Q.; Zhang, T. Mechanical properties and microstructure of circulating fluidized bed fly ash-based hybrid alkali-activated cement. Constr. Build. Mater. 2025, 486, 142006. [Google Scholar]
- Feng, C.; Wang, Y.; Wang, L.; Zhao, X.; Zhang, W.; Zhu, J.; Du, M. Improving the water resistance of gypsum-based building materials with slag activated by calcium oxide. J. CO2 Util. 2024, 90, 102996. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Gao, F.; Wang, Y.; Li, M.; Wang, J.; Guo, X.; Nikolayevich, K.S.; Nikolaevich, L.S. Characteristics of alkali-activated slag mortar under combined freeze-thaw cycles and sulfate exposure: Microstructural and mechanical insights. Constr. Build. Mater. 2025, 493, 143087. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, S.; Wang, Y.; Wu, W.; Wang, X. Effect of multi-factors on the performance of one-part geopolymer mortar against magnesium sulfate erosion. Adv. Cem. Res. 2025, 37, 199–213. [Google Scholar] [CrossRef]
- Shu, T.; Xu, J.; Guo, P.; Tian, Y.; Liu, D. Experimental study on durability and performance evaluation of alkali activated metakaolin slag concrete under sulphate dry-wet cycle. Case Stud. Constr. Mater. 2025, 23, e05310. [Google Scholar] [CrossRef]
- Ponomar, V.; Kamali, S.; Luukkonen, T.; Hajimohammadi, A.; Kilpimaa, K. The effect of gypsum on reaction kinetics and microstructure of alkali-activated CaO-FeOx-SiO2 slag. Cem. Concr. Compos. 2025, 160, 106033. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Liu, T.; Wan, Q.; Zheng, D. Effect of high alumina-based solid waste on efflorescence behavior of alkali-activated steel slag. Constr. Build. Mater. 2022, 349, 128804. [Google Scholar]
- Pradhan, S.S.; Mishra, U.; Biswal, B.K. Mechanical and microstructural study of slag based alkali activated concrete incorporating RHA. Constr. Build. Mater. 2023, 400, 132685. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, Y.; Li, X.; Yang, X.; Rao, F.; Zhong, L. Durability of alkali-activated materials with different C-S-H and N-AS-H gels in acid and alkaline environment. J. Mater. Res. Technol. 2022, 16, 619–630. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).