1. Introduction
Alkali-resistant (AR) glass fiber has been specifically engineered to enhance the performance of cement-based composites in construction applications. This material plays a key role in glass fiber-reinforced concrete (GFRC) and textile-reinforced concrete (TRC), where its properties help improve durability and mechanical behavior [
1]. In GFRC, short AR-glass fibers are commonly incorporated to mitigate early-age shrinkage cracking and enhance the fracture resistance of the otherwise brittle cement matrix [
2]. Both TRC and GFRC are widely used in lightweight architectural elements and structural strengthening applications. A critical requirement for these materials is maintaining their high tensile strength and toughness over time, ensuring long-term performance in demanding environments [
3].
There are several stages in the reaction process of the glass with alkaline solution. Initially OH- is adsorbed on the glass surface [
4]. Then the following reactions occur:
The final phase, reaction products are desorbed from the glass. The limited availability of active adsorption sites causes the corrosion process to stabilize, after which the rate of glass destruction is determined exclusively by how quickly these products dissolve [
5].
The primary strategies for improving fiber alkali resistance include:
Optimizing the glass composition [
6,
7,
8];
Developing new protective coatings [
9,
10];
Employing additives in the cement or concrete mix [
11].
Basalt fibers demonstrate superior alkali resistance compared to conventional E-glass fibers widely used in construction [
12]. Our previous research has identified zirconium oxide doping as an effective method for enhancing the alkali stability of continuous basalt fibers [
13].
We have thoroughly characterized the mechanism underlying this approach. When exposed to hydroxide ions, zirconium oxides form insoluble hydroxides (primarily Zr(OH)
4) on the fiber surface. This protective layer significantly impedes alkaline penetration, thereby preserving the structural integrity of the fibers [
7,
14].
While zirconium oxide is particularly effective due to the exceptional chemical stability of Zr(OH)4 in alkaline environments, we have observed that its solubility in basalt glasses and fibers is inherently limited. To address this, we developed a novel method to increase zirconium incorporation. The resulting zirconium-enriched basalt fibers achieve performance characteristics comparable to commercial AR-glass fibers. The persistent zirconium hydroxide surface film—owing to its extremely low solubility in alkaline solutions—provides long-term protection by preventing solution infiltration and subsequent fiber degradation.
The exceptional durability of zirconium-enriched basalt fibers in alkaline environments stems from the extremely low solubility of zirconium hydroxide (Zr(OH)4), with a solubility product constant (Ksp)~10−52. This property allows the protective surface film to remain stable for extended periods, effectively blocking alkaline solution penetration and preventing fiber degradation. Comparative analysis of hydroxide solubility products reveals why basalt fibers inherently outperform conventional E-glass fibers: while Ca(OH)2 (Ksp~10−6), Mg(OH)2 (~10−10), and even Ti(OH)4 (~10−29) show progressively better stability, they cannot match the performance of Zr(OH)4. The natural alkali resistance of basalt fibers is further enhanced by their high iron oxide content, as evidenced by the remarkably low Ksp of Fe(OH)3 (~10−38). Interestingly, titanium oxide emerges as a potentially cost-effective alternative to expensive zirconium oxide for fiber doping, given the intermediate but still substantial insolubility of Ti(OH)4 in alkaline solutions. This solubility hierarchy explains both the superior performance of modified basalt fibers compared to E-glass and the potential for optimizing cost/performance ratios in fiber development.
This hypothesis finds experimental confirmation in practice studies [
14,
15], where the addition of 2 wt.% titanium oxide was shown to increase alkali resistance by 50%. However, a higher doping level of 4 wt.% resulted in less significant improvements, suggesting the existence of an optimal concentration range for titanium oxide additives.
The addition of TiO
2 to silicate melts leads to a decrease in their heat capacity and enthalpy, as well as a decrease in the activation energy of viscous flow, which indicates a weakening of the melt network due to the formation of weaker Ti–O bonds compared to Si–O [
16]. The studies demonstrate the potential of TiO
2 as a functional filler not only for improving the mechanical and tribological properties of polymer matrices [
14], but also as a possible candidate for modifying inorganic fibers, such as basalt, to expand their application in seawater [
10].
The application of basalt fiber extends beyond conventional concrete reinforcement: it is successfully employed in self-compacting concretes blended with bagasse ash and metakaolin [
17], in lightweight geopolymer composites based on pumice, and in environmentally conscious solutions where thermal performance and carbon footprint reduction are as critical as mechanical properties [
18]. In all these applications, long-term alkali resistance remains a key factor determining composite durability and service life
In the present work, we conducted the first systematic investigation of titanium oxide doping effects in basalt fibers in wide range of composition. We produced a series of basalt glass samples with titanium oxide content varying up to 8 mol%, significantly expanding the concentration range studied in earlier research.
4. Discussion
The incorporation of titanium dioxide (TiO
2) into the composition of continuous basalt fibers represents a promising strategy for enhancing their chemical and alkali resistance. Basalt fibers inherently exhibit superior durability in alkaline environments compared to conventional E-glass fibers, primarily due to the high iron oxide content in natural basalt and the extremely low solubility of iron hydroxides—particularly Fe(OH)
3 (Ksp~10
−38) [
12]. This natural resistance can be further improved through chemical modification of the glass network. As previously demonstrated in our studies, doping basalt glass with zirconium oxide (ZrO
2) significantly enhances fiber stability in aggressive environments [
7,
13]. The protective mechanism involves the formation of an insoluble zirconium hydroxide layer (Zr(OH)
4, Ksp~10
−52) on the fiber surface upon exposure to alkali, which acts as a diffusion barrier, effectively preventing further penetration of hydroxide ions and subsequent glass degradation [
15].
However, despite its excellent protective properties, zirconium oxide has notable practical limitations. It is a high-melting-point compound, requiring elevated processing temperatures, and is relatively expensive, which may hinder its widespread industrial application [
20]. Moreover, its solubility in basalt melts is limited, restricting the achievable concentration and potentially leading to phase separation or crystallization during fiber production [
21].
In this study, titanium oxide is explored as a cost-effective alternative to zirconium oxide. Although titanium hydroxide (Ti(OH)4, Ksp~10−29) is more soluble than its zirconium counterpart, it still exhibits very low solubility in alkaline media, making it a viable candidate for forming a protective surface layer. The hypothesis is that, similar to Zr4+, Ti4+ ions can integrate into the glass network and, upon alkali exposure, precipitate as insoluble hydroxides, thereby improving long-term chemical stability.
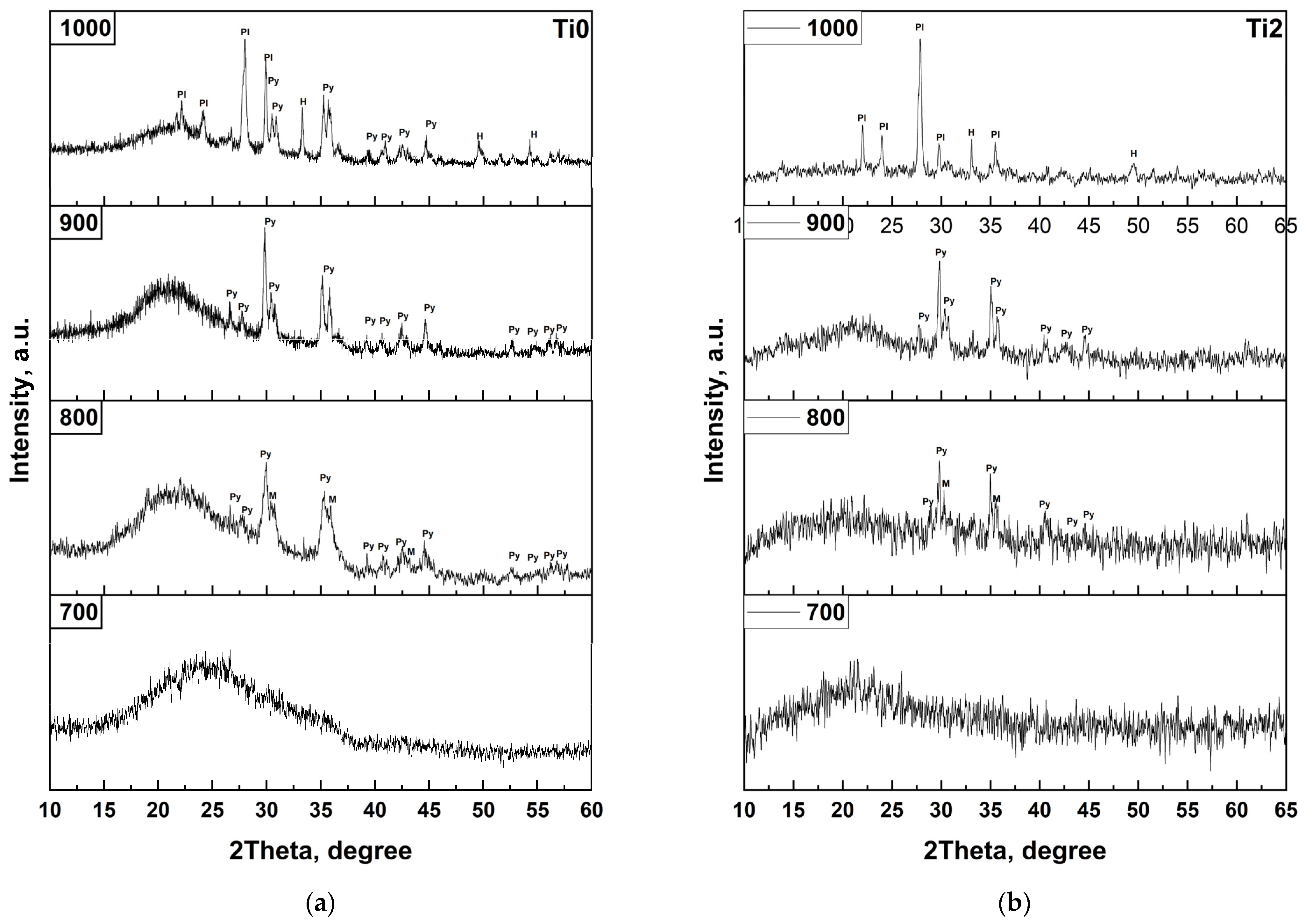
For the first time, this work systematically investigates TiO
2 doping across a broad compositional range—from 2 to 8 mol%—in basalt-based glasses. X-ray fluorescence (XRF) analysis confirms that titanium oxide dissolves completely in the basalt melt within this concentration range. This is further supported by X-ray diffraction (XRD) data, which show no crystalline reflections attributable to free TiO
2 phases (
Figure 5) in the quenched glass samples, indicating full incorporation of Ti
4+ into the amorphous silicate network. The absence of phase segregation suggests that titanium is not merely present as an inert filler but actively participates in the glass structure, likely in both network-forming and modifying roles depending on concentration and local coordination.
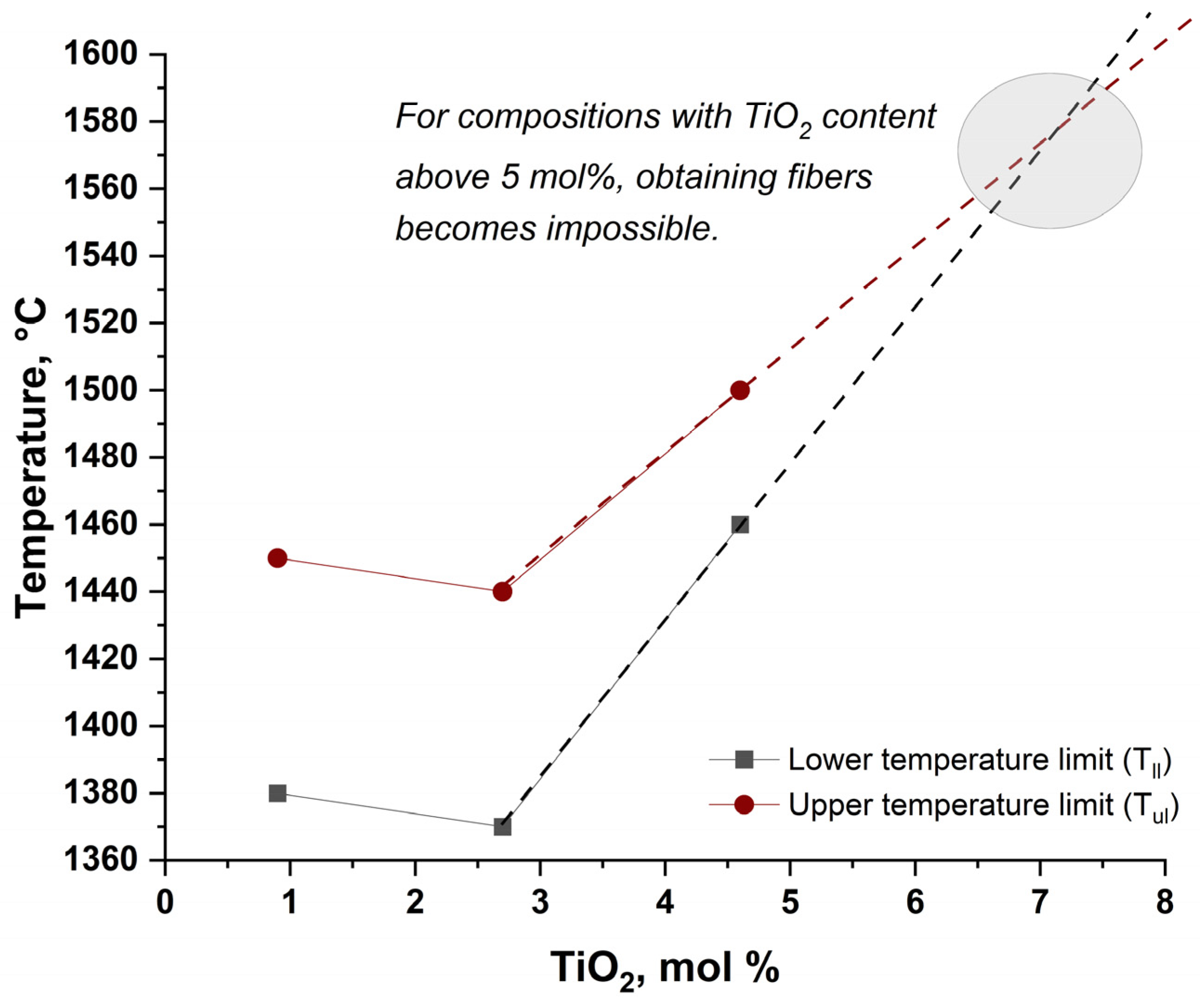
The feasibility of fiber drawing from the prepared glass compositions was evaluated by determining the fiber-forming temperature range (ΔT
ff), defined as the interval between the lower limit (T
ll)—the minimum temperature at which a continuous fiber with diameter below 20 μm could be drawn—and the upper limit (T
ul)—the temperature at which the spinneret begins to flood and fiber formation ceases. As shown in
Figure 6, the fiber-forming window progressively narrows with increasing TiO
2 content more than 2 mol %. In the undoped (Ti0) and low-doped (Ti2) samples, a sufficiently wide ΔT
ff allows stable fiber production. However, with further TiO
2 addition—starting from approximately 4 mol% (Ti4)—the processing window becomes significantly reduced. For compositions with 6 mol% TiO
2 (Ti6) and higher, the temperature interval is so narrow that continuous fiber drawing becomes practically unfeasible under standard conditions.
Moreover, the onset temperature for fiber formation (T
ll) increases with TiO
2 content, more than 2 mol % indicating a rise in the viscosity of the melt at high temperatures. This trend is contrary to earlier experimental findings [
15], where titanium oxide was observed to act as a network modifier, typically associated with a reduction in melting temperature and enhanced glass melt fluidity in silicate systems. For instance, in a study on basalt melts, it was demonstrated that the addition of TiO
2 leads to a significant decrease in viscosity due to the depolymerization of the silicate network, which can effectively lower the processing temperature and reduce production costs [
22]. Within certain concentration limits, this effect is beneficial for fiber manufacturing, as reduced viscosity improves melt homogeneity and drawing stability.
The role of TiO
2 is system-dependent. In simple silicate melts, Ti
4+ predominantly remains in octahedral coordination and acts as a network modifier even at high concentrations. However, in complex multicomponent basalt glass—rich in Si, Al, and Fe—Ti
4+ competes effectively for tetrahedral sites with Fe
3+. Our Mössbauer data (
Table 3) show a clear decrease in tetrahedral Fe
3+ and increase in octahedral Fe
3+ with rising TiO
2 content, indicating Ti
4+ incorporation into [TiO
4] units at higher concentrations (>4 mol%). This aligns with studies on glass-ceramics where TiO
2 acts as a nucleating agent due to its ability to form tetrahedral units [
22], and explains both the improved alkali resistance and the narrowed fiber-forming window observed here. Specifically, when TiO
2 content exceeds approximately 1 wt% (equivalent to 2.33 mol%), the melt viscosity above the crystallization temperature decreases below the optimal range of 10–30 Pa·s required for stable continuous fiber formation. This reduction in viscosity leads to excessive melt fluidity, resulting in frequent fiber breakage during drawing [
21]. Our results extend these observations by systematically investigating higher TiO
2 concentrations up to 8 mol%. We observe that the fiber-forming temperature window progressively narrows with increasing TiO
2 content, starting from approximately 4.6 mol%, and becomes unfeasibly narrow at 5.8 mol% and above, leading to complete loss of spinnability. These findings are consistent with the literature, demonstrating that while low TiO
2 levels act as network modifiers, enhancing melt fluidity, higher concentrations induce structural changes that compromise processability
Our data indicate that, in the basaltic system studied here, higher TiO
2 loading promotes the incorporation of Ti
4+ into tetrahedral network-forming positions, thereby enhancing polymerization and increasing melt rigidity—contrary to the depolymerizing effect observed at lower concentrations in other systems. This network-forming behavior is consistent results reported in other studies on continuous basalt fibers [
14,
22], where an increase in glass transition temperature with TiO
2 addition was observed. Although the authors did not interpret this effect in terms of titanium coordination, we attribute this trend to the incorporation of Ti
4+ ions into the glass network as network formers, which enhances structural rigidity. The combined evidence supports a dual role for TiO
2: as a network modifier at low concentrations, reducing viscosity, and as a network former at higher concentrations, strengthening the glass structure at the expense of processability.
Both alkali resistance and seawater durability improve with increasing TiO
2 content up to 4.6 mol% (Ti4 composition). The higher tensile strength observed at 2 mol% TiO
2 (2345 MPa) compared to 4.6 mol% (2100 MPa) reflects titanium’s concentration-dependent structural role: at low concentrations (≤3 mol%), Ti
4+ in octahedral coordination ([TiO
6]) acts as a network modifier, improving melt homogeneity and reducing flaw density, thereby enhancing mechanical strength. At 4.6 mol%, increased incorporation into tetrahedral sites ([TiO
4]) strengthens the network against chemical attack but may introduce subtle heterogeneities that slightly reduce intrinsic tensile strength. Compared to ZrO
2-doped fibers [
7,
13], where solubility is limited to ~2–3 mol%, TiO
2 offers higher amorphous solubility (up to 8 mol%) but exhibits a similar functional limit (~4–5 mol%) due to induced crystallization—aligning with literature optima around 2 wt.% (~4.7 mol%).
As shown in
Table 1, the mass loss after exposure to NaOH solution decreases from 9.8% (Ti0) to 8.5% (Ti4), while the mass loss in seawater drops from 5.2% to 3.9%. Similarly, the residual tensile strength after seawater exposure increases from 76.6% (Ti0) to 84.4% (Ti4), and after NaOH treatment—from 34.3% to 43.7%. These results indicate that moderate TiO
2 doping enhances the chemical stability of basalt fibers in aggressive environments (
Table 2).
The mechanism of improved alkali resistance is believed to be analogous to that previously observed in zirconium-modified basalt fibers. In this scenario, Ti4+ ions are released from the glass network upon exposure to alkaline solutions and hydrolyze to form an insoluble titanium hydroxide (Ti(OH)4) layer on the fiber surface. This protective film acts as a diffusion barrier, limiting the penetration of OH− ions and slowing down the degradation of the silicate network.
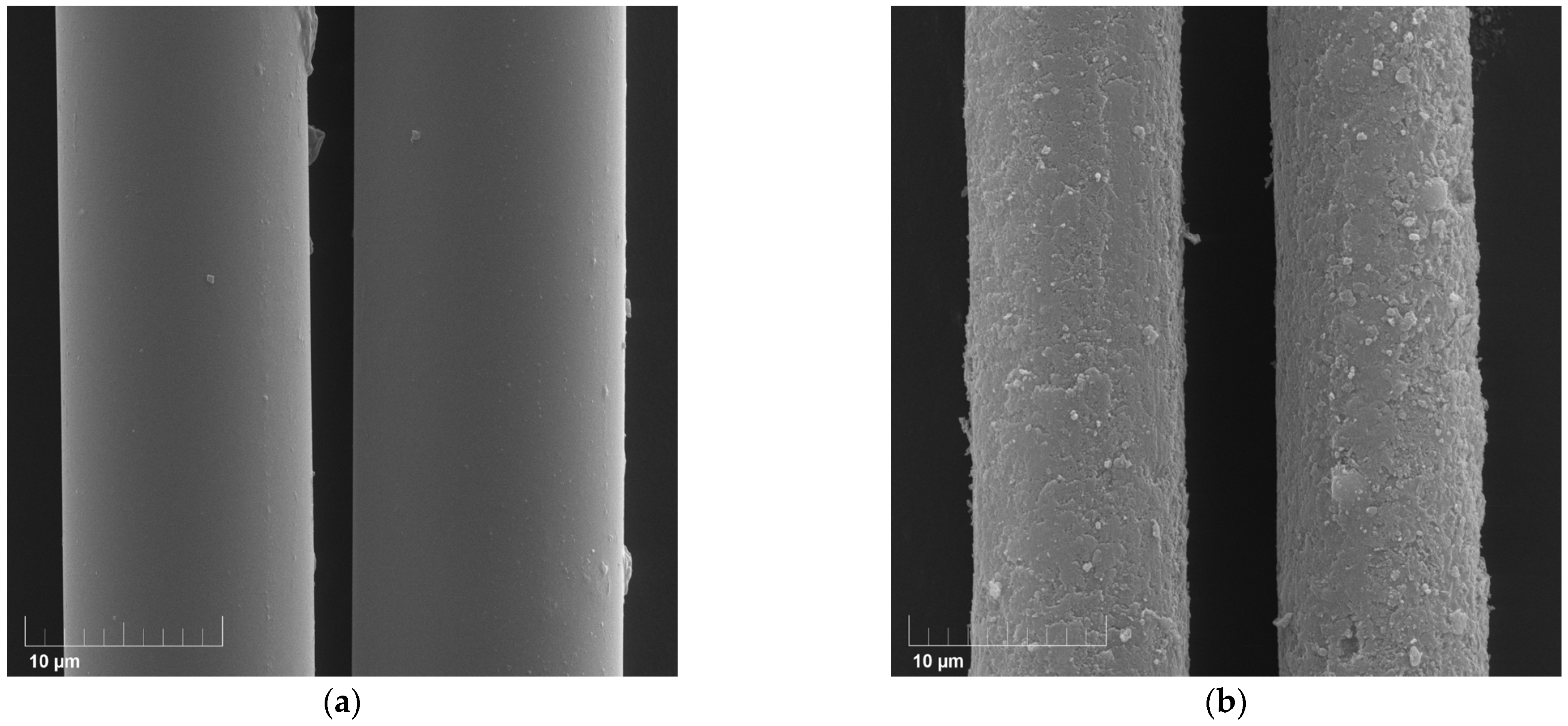
This hypothesis is supported by SEM micrographs (
Figure 7), which reveal the presence of a dense, continuous surface layer on TiO
2-doped fibers after alkali treatment. The morphology of this layer is consistent with the formation of a passivating hydroxide-rich film, similar to the Zr(OH)
4 layers reported in our earlier studies. A similar protective mechanism and results were shown previously [
14].
Thus, while TiO2 doping up to 4 mol% effectively improves both chemical resistance and residual mechanical properties, higher concentrations are not feasible due to processing limitations. This optimal range represents a favorable balance between enhanced functional performance and technological viability in fiber manufacturing.
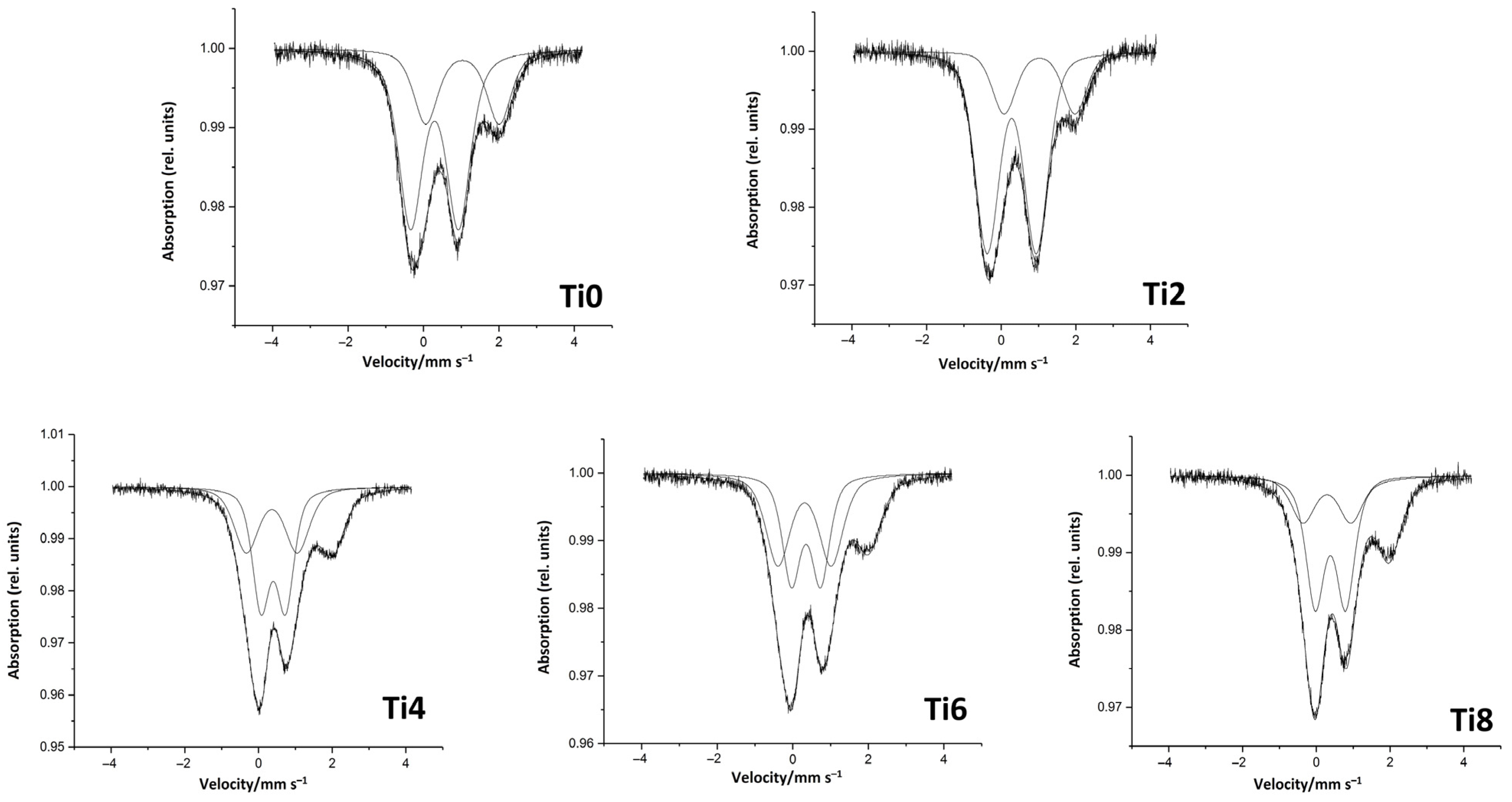
To elucidate the structural origin of the observed changes in fiber performance, the synthesized glasses were systematically investigated using Mössbauer spectroscopy and X-ray diffraction (XRD) after heat treatment at 700, 800, 900, and 1000 °C for 24 h in air. These analyses provide critical insights into the coordination environment of iron, the role of Ti4+ in the glass network, and the crystallization behavior, all of which govern the functional and technological properties of the fibers.
Mössbauer spectra of all TiO
2-modified glass samples exhibit a superposition of contributions from Fe
2+ and Fe
3+ cations, consistent with the redox equilibrium established during high-temperature melting in air. The isomer shift (σ) and quadrupole splitting (Δ) values, along with the relative spectral areas, allow for the identification of the local oxygen coordination of iron species (
Table 3). In the undoped (Ti0) and low-TiO
2 (Ti2) samples, the spectra are well-fitted by two doublets: a Fe
3+ component with σ ≈ 0.28–0.29 mm/s and Δ ≈ 1.27–1.32 mm/s, characteristic of tetrahedrally coordinated Fe
3+ (Fe
3+tet), and a Fe
2+ component with σ ≈ 1.03 mm/s and Δ ≈ 1.90–1.95 mm/s, assigned to octahedrally coordinated Fe
2+ (Fe
2+okt). The Fe
3+tet/Fe
2okt area ratio is approximately 2:1, which aligns well with literature data for natural basaltic glasses [
23], where Fe
2+ content typically does not exceed 30 wt.% of total iron after redox equilibration [
24].
A significant structural transition is observed starting at 4 mol% TiO2 (Ti4). The Mössbauer spectrum of the Ti4 sample reveals the emergence of a third doublet with σ = 0.40 mm/s and Δ = 0.66 mm/s—characteristic of octahedrally coordinated Fe3+ (Fe3+okt). This component becomes more prominent in the Ti6 and Ti8 samples, where the relative area of Fe3+okt increases while the Fe3+tet fraction decreases. This shift indicates a progressive change in the local environment of iron ions, driven by the increasing TiO2 content.
The decrease in Fe
3+tet content suggests that tetrahedral sites are being occupied by another high-field-strength cation—most likely Ti
4+—which competes effectively for these network-forming positions at higher concentrations. This interpretation aligns with the observed evolution of melt properties. At low TiO
2 concentrations (≤2–3 mol%), the deacresing fiber forming temperature (
Figure 6) indicate a network-modifying role of Ti
4+, likely in octahedral coordination ([TiO
6]), leading to depolymerization and reduced viscosity. However, as the TiO
2 content increases beyond ~4 mol%, the Fe
3+tet fraction decreases, and the onset of pseudobrukite crystallization suggests that Ti
4+ begins to incorporate into tetrahedral sites ([TiO
4]), strengthening the network and promoting phase separation. Thus, the dual role of titanium is concentration-dependent: at low levels (≤3 mol%), it acts primarily as a network modifier in octahedral coordination ([TiO
6]), reducing viscosity; at higher concentrations (≥4 mol%), it increasingly occupies tetrahedral sites ([TiO
4]), displacing Fe
3+ into octahedral positions and reinforcing the glass network. This conclusion is supported by convergent evidence: (1) Mössbauer data showing Fe
3+tet → Fe
3+okt redistribution, (2) XRD detection of pseudobrukite (requiring octahedral Ti
4+/Fe
3+) upon heat treatment, and (3) non-monotonic change in fiber-forming temperature. Direct confirmation via
47Ti NMR is planned for future work. This transition explains both the initial improvement in chemical resistance and the subsequent deterioration in spinnability.
The phase transformation processes in basaltic amorphous systems are well-established in the literature. In natural basalt glasses, crystallization typically initiates with the spontaneous formation of magnetite (Fe
3O
4), which is promoted by initial liquid–liquid phase separation [
25]. These magnetite nanocrystals act as heterogeneous nucleation sites for the subsequent precipitation of pyroxene-type silicate phases. The overall crystallization kinetics in bulk basalt glasses have been widely interpreted as a three-dimensional, diffusion-controlled growth of pyroxene-like phases on a fixed number of nuclei. At higher temperatures, further crystallization leads to the formation of plagioclase phase [
26].
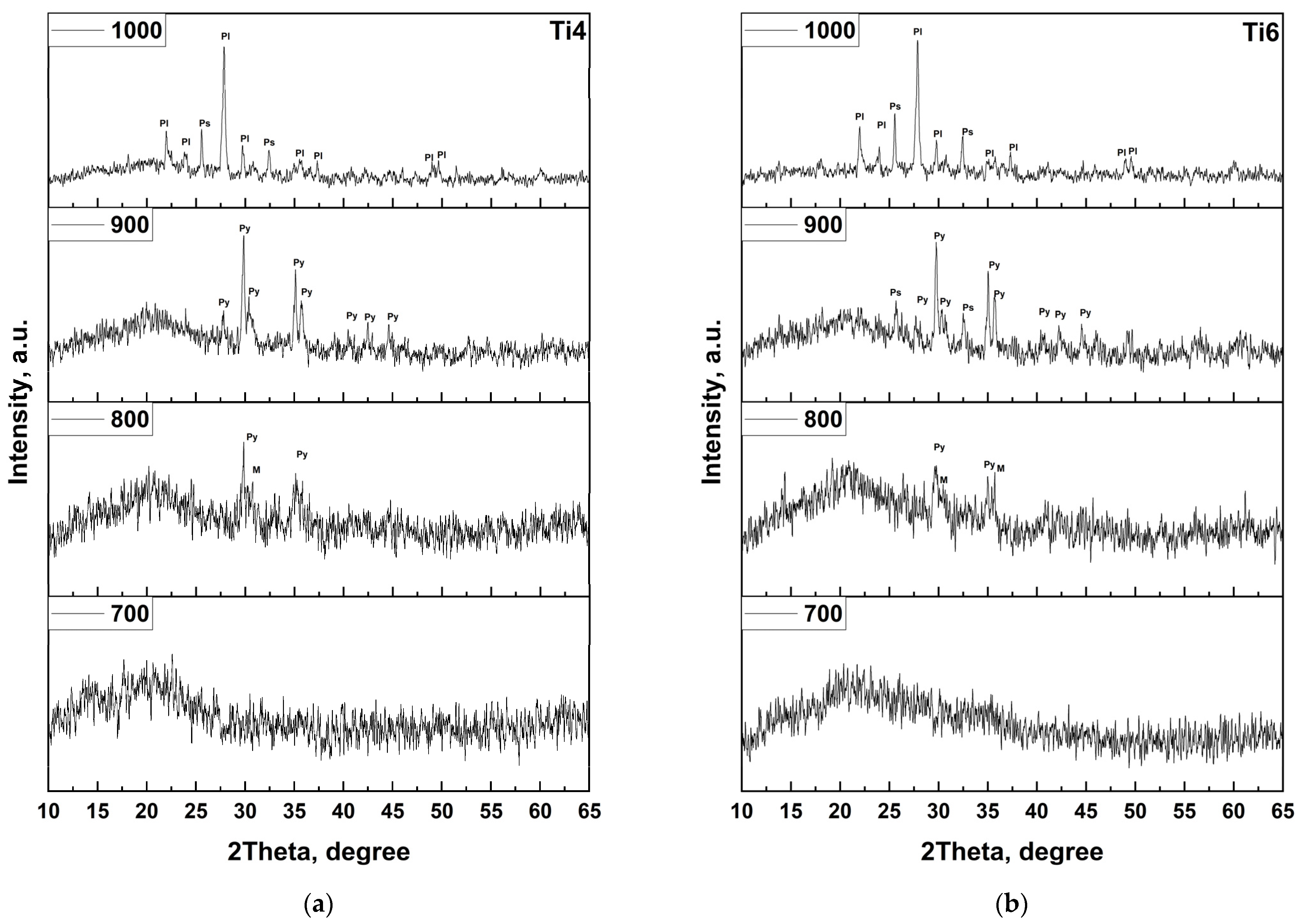
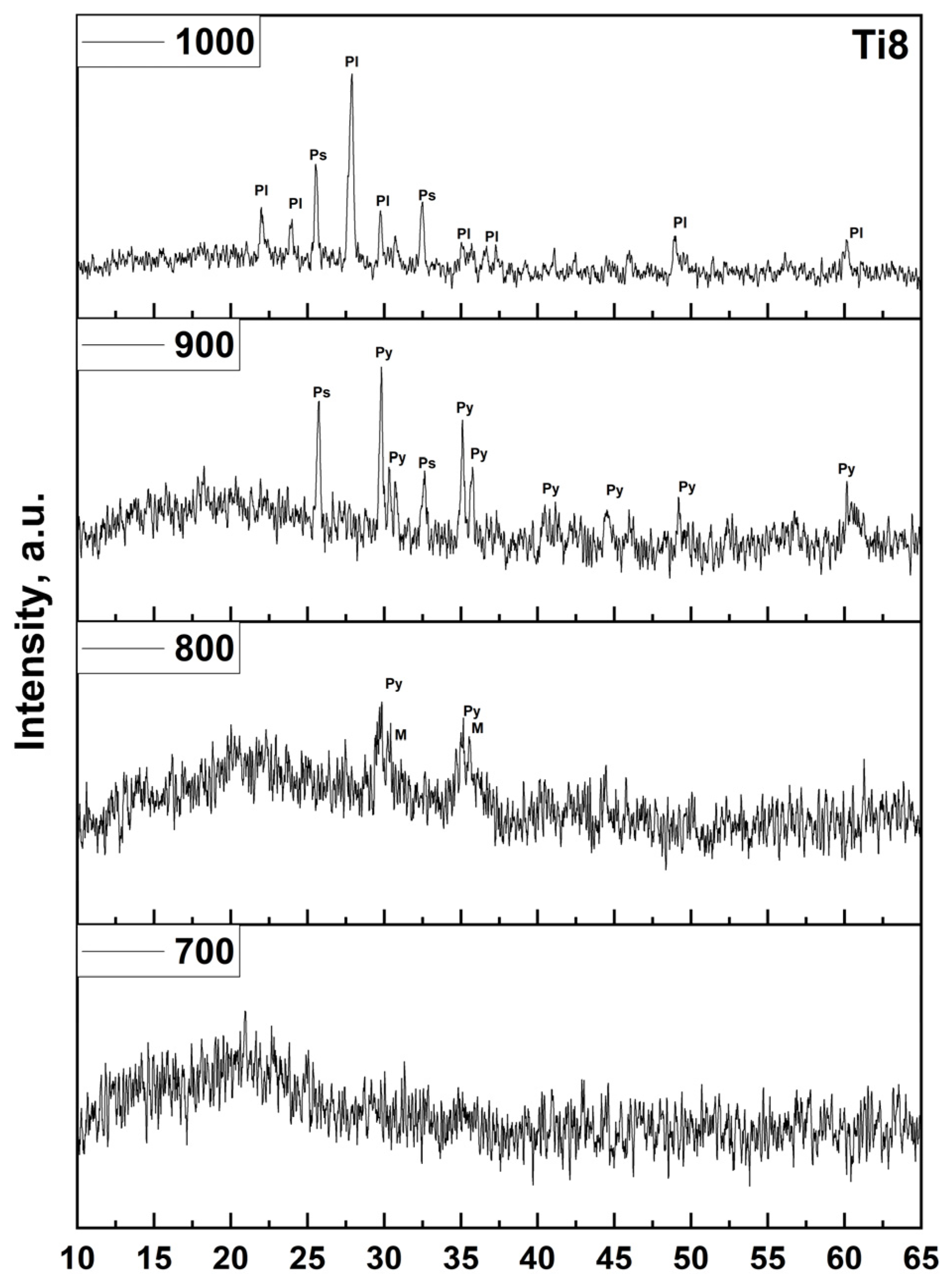
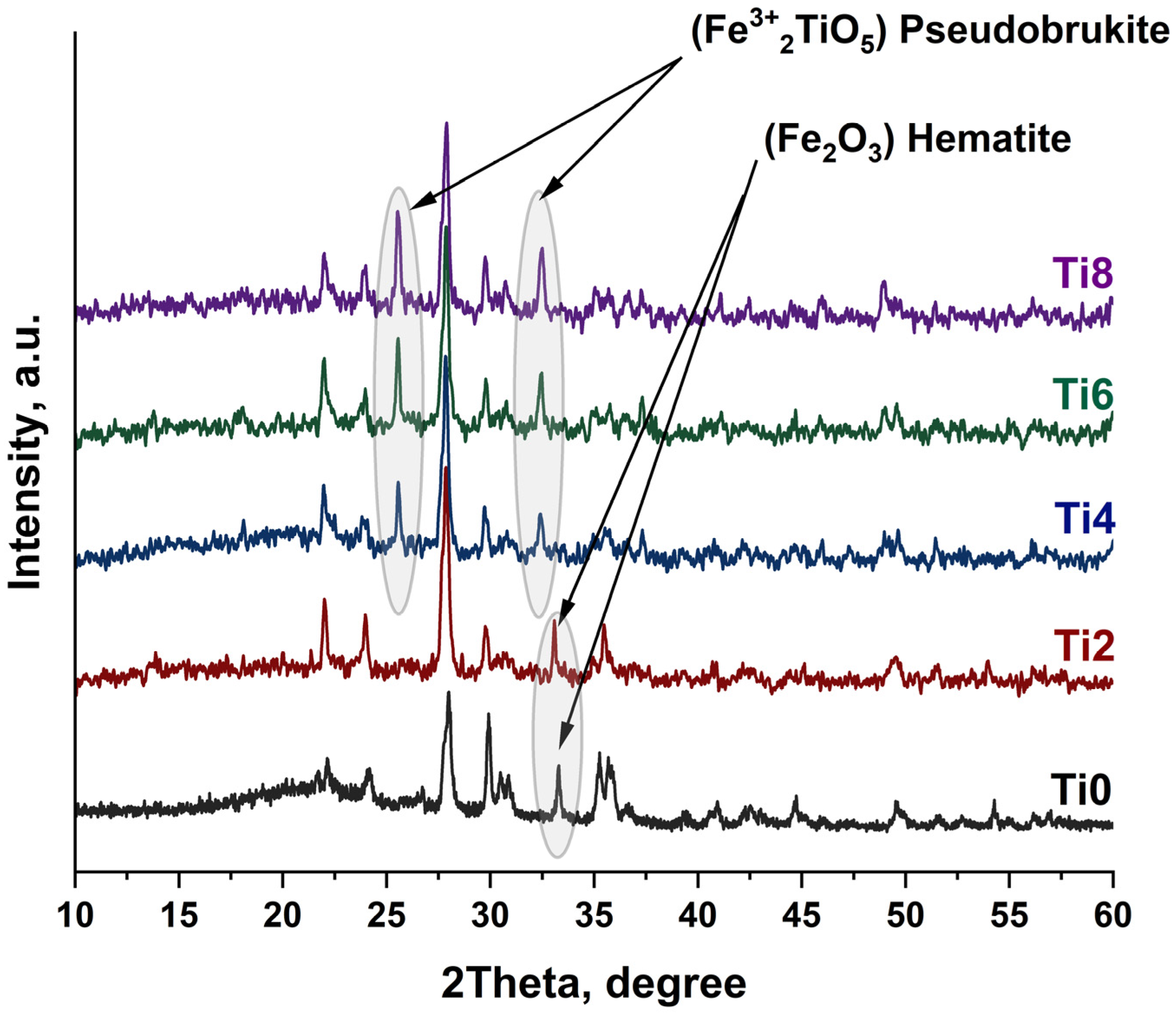
In the present study, this classical crystallization pathway is clearly observed for the undoped (Ti0) and low-TiO
2 (Ti2) samples. XRD patterns after heat treatment reveal the sequential appearance of magnetite, pyroxene, hematite, and plagioclase phases between 700 and 1000 °C, confirming that the base basalt system follows the expected thermodynamic evolution. However, a significant deviation occurs with increasing TiO
2 content. For compositions Ti4, Ti6, and Ti8, a new crystalline phase—pseudobrukite (Fe
2TiO
5)—emerges upon heat treatment. This phase is first detected at 900 °C in the Ti4 sample and becomes increasingly dominant at 1000 °C in Ti6 and Ti8, indicating a strong concentration-dependent crystallization tendency (
Figure 8).
Studies of the CaO–SiO
2–TiO
2 system with the addition of 10 wt.% Al
2O
3 showed that at 1300–1400 °C, solid phases of perovskite (CaO TiO
2), rutile (TiO
2), wollastonite and sphene (CaO SiO
2 TiO
2) exist in equilibrium with the liquid phase, indicating the complex participation of TiO
2 in crystallization processes [
27]. In glass-ceramic systems with zero coefficient of thermal expansion, titanium plays a key role as a crystallization initiator, while its coordination state (4, 5, 6) directly affects the crystal nucleation mechanism and the stability of the amorphous network [
28]. Pseudobrukite, like other TiO
2 polymorphs (rutile, anatase, brukite), is built upon [TiO
6] octahedra. The formation of Fe
2TiO
5 requires the availability of Ti
4+ in octahedral coordination, either residual from the glass structure or formed during heat treatment, as well as local enrichment of Fe
3+ in octahedral sites—consistent with the Mössbauer-observed increase in Fe
3+okt. This structural distortion arises from the partial substitution of Ti
4+ by Fe
3+ ions, which occupy irregular lattice positions due to differences in ionic radius and charge. The formation of Fe
2TiO
5 demonstrates that titanium is not merely a passive network constituent but actively participates in phase separation and crystallization, particularly when its concentration exceeds the network’s capacity for homogeneous dispersion.
The emergence of pseudobrukite at relatively low temperatures (≥900 °C) has critical implications for fiber manufacturing. Although quantitative phase analysis (e.g., Rietveld refinement) was not performed, the strong correlation is evident: pseudobrukite is first detected at 900 °C in Ti4 (where ΔTff begins to narrow significantly) and becomes dominant at 1000 °C in Ti6/Ti8 (where fiber drawing becomes impossible). Since fiber drawing occurs at 1380–1550 °C—well above these crystallization temperatures—pseudobrukite nucleation in the melt at the spinneret inevitably increases viscosity and causes die clogging, directly linking crystallization to loss of spinnability. Since continuous fiber drawing occurs at temperatures between 1380 °C and 1550 °C, any tendency toward early crystallization significantly increases the risk of nucleation and growth of solid phases in the molten glass at the spinneret. This leads to increased melt viscosity, die clogging, and surface defects—ultimately resulting in fiber breakage. This explains the dramatic narrowing of the fiber-forming temperature window (ΔTff) with increasing TiO2 content and the complete inability to produce continuous fibers at Ti6 and Ti8.
Based on the combined Mössbauer and XRD results, we conclude that the structural role of Ti4+ evolves with concentration. At low levels (≤3 mol%), Ti4+ predominantly occupies octahedral sites ([TiO6]), acting as a network modifier that reduces melt viscosity and lowers processing temperatures. At higher concentrations (≥4 mol%), increasing incorporation of Ti4+ into tetrahedral coordination ([TiO4]) strengthens the network and displaces Fe3+ from tetrahedral to octahedral sites. This redistribution promotes local clustering and provides the structural prerequisites for pseudobrukite nucleation, ultimately compromising spinnability. While direct spectroscopic probes of Ti coordination (e.g., Raman, XPS, NMR) were not employed in this study, future work will include 47Ti NMR analysis to directly confirm the tetrahedral/octahedral site occupancy of Ti4+.
To overcome this limitation, we propose two potential strategies. First, following our earlier work with zirconium, titanium could be introduced in the form of a pre-formed silicate (e.g., titanosilicate) to enhance its compatibility with the basalt network and promote tetrahedral coordination. Second, the addition of fluxing agents (e.g., B2O3, F−, or alkali borates) may lower the melting and processing temperatures, thereby suppressing premature crystallization and improving melt homogeneity. The latter approach is particularly promising from a technological standpoint, as it directly addresses the processing challenges identified in this study.
While a full cost–benefit analysis is beyond the scope of this study, the economic advantage of TiO2 is clear: it is 5–10 times cheaper than ZrO2. Although 4.6 mol% TiO2-doped fibers show slightly lower alkali resistance than ZrO2-modified ones—due to the higher solubility of Ti(OH)4 (Ksp~10−29) versus Zr(OH)4 (Ksp~10−52)—the cost difference makes TiO2 doping highly attractive for applications requiring sufficient, not maximum, durability. This is especially relevant in large-scale construction, where material cost dominates decision-making. At scale, the advantage grows: lower raw material cost, compatibility with existing basalt fiber production, and no need for complex processing additives make TiO2 a practical, cost-effective alternative.
These considerations are the subject of our ongoing research. The goal is to extend the solubility limit of Ti4+ in the basalt matrix while maintaining a stable amorphous structure, thereby decoupling functional enhancement from processability constraints.