Analysis on Durability of Bentonite Slurry–Steel Slag Foam Concrete Under Wet–Dry Cycles
Abstract
1. Introduction
2. Experimental Materials and Methodology
2.1. Test Raw Materials
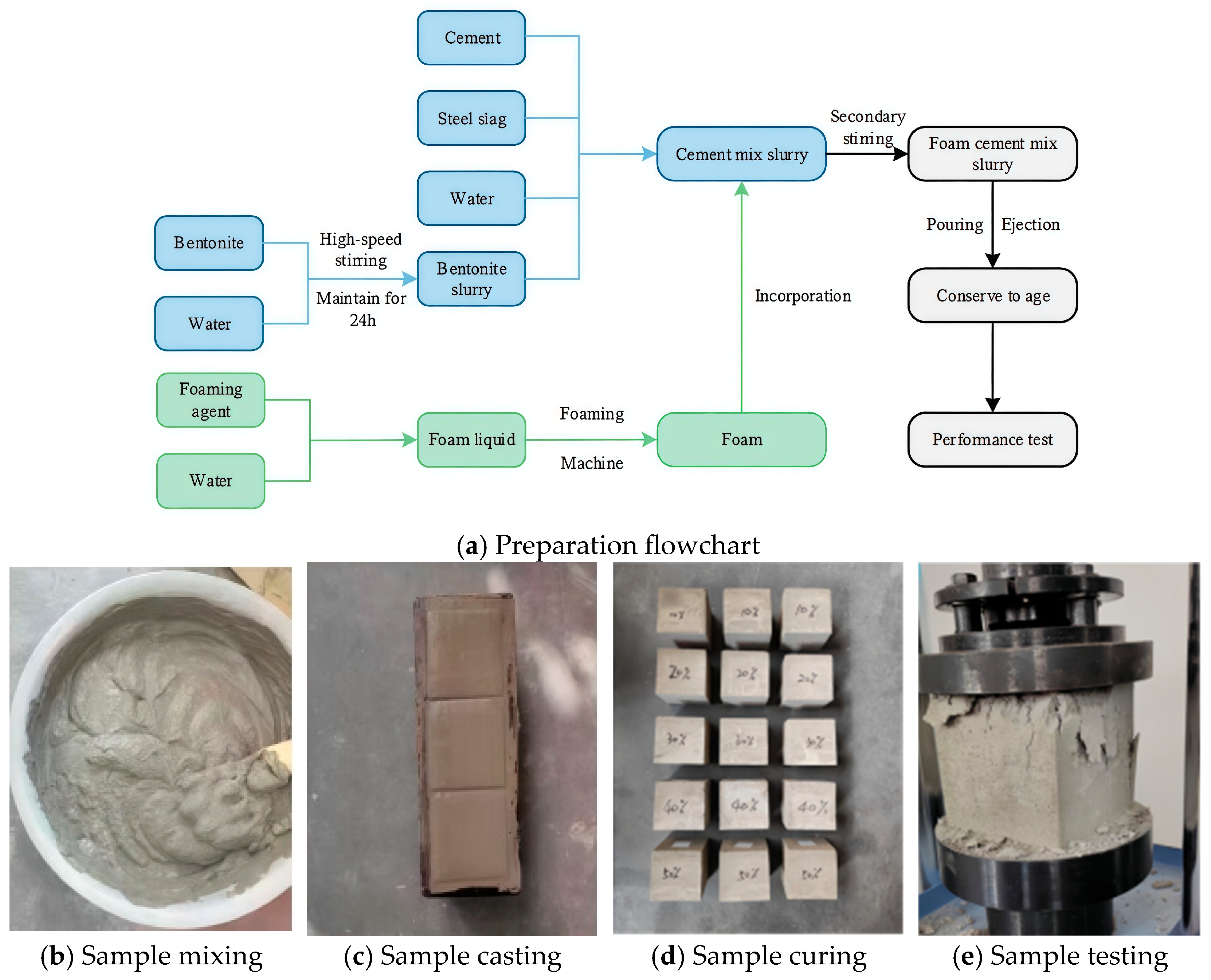
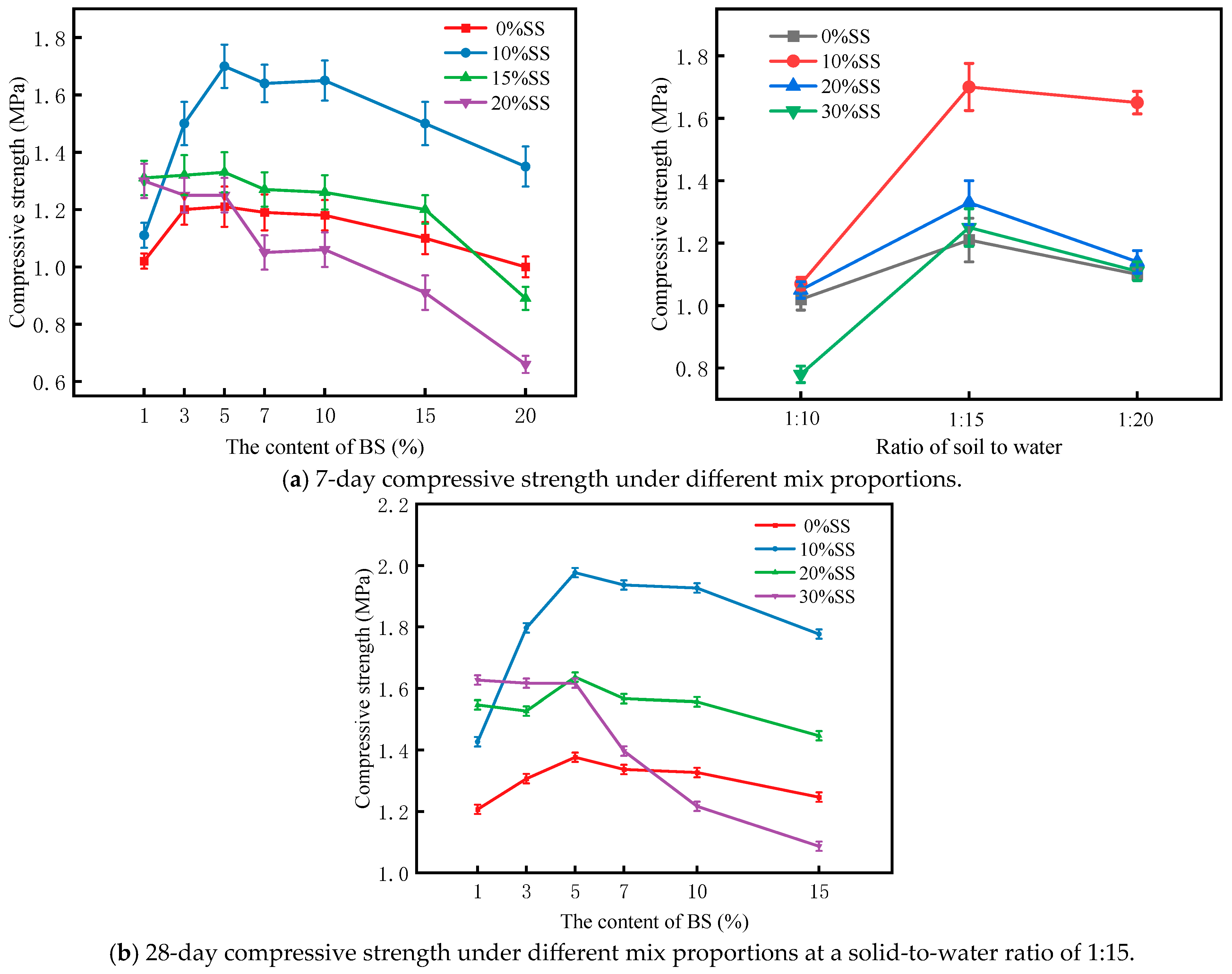
2.2. Preparation of BS-SSFC and Determination of the Optimal Mix Proportion
2.3. Wet–Dry Cycle Experiments
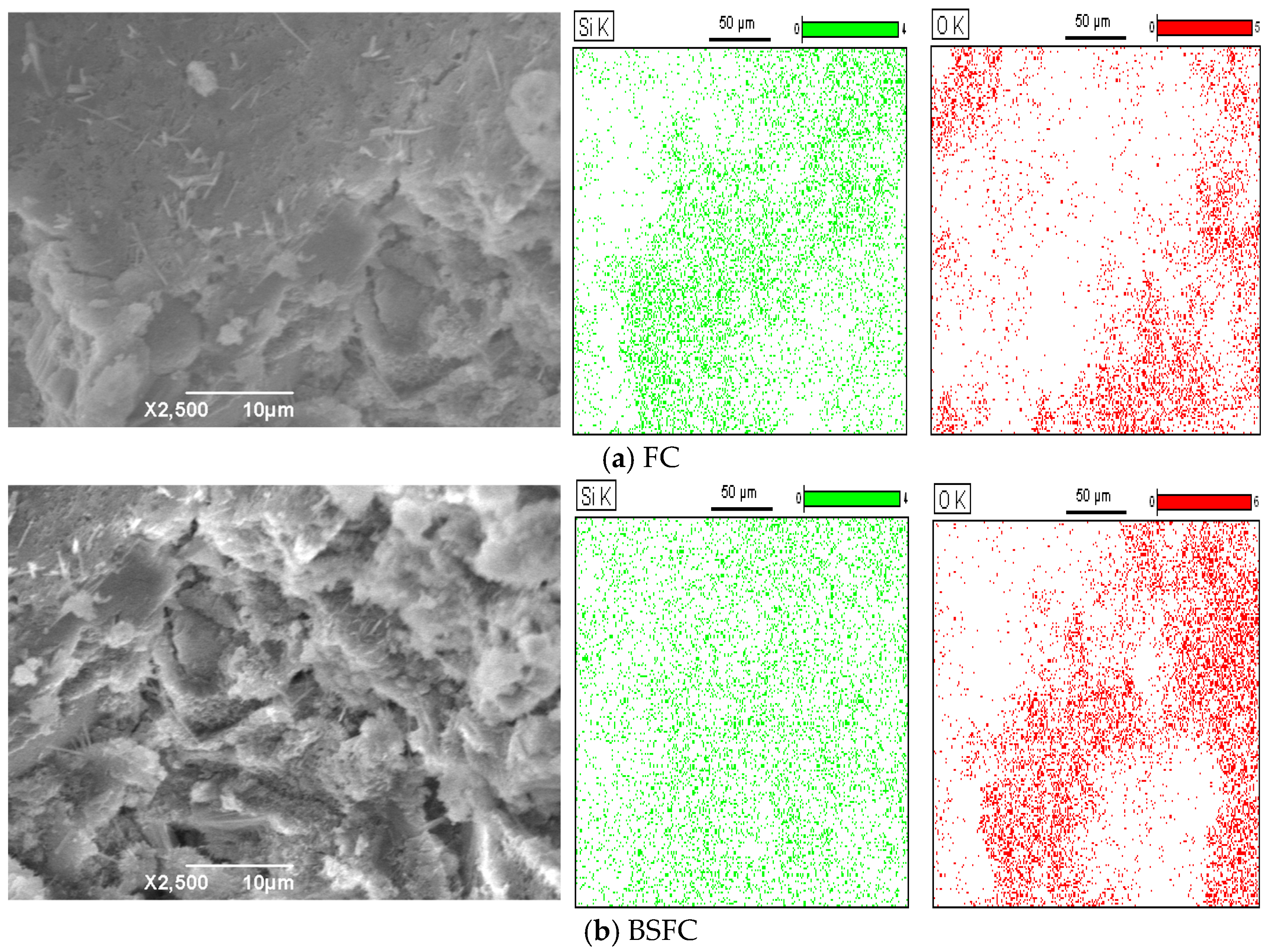
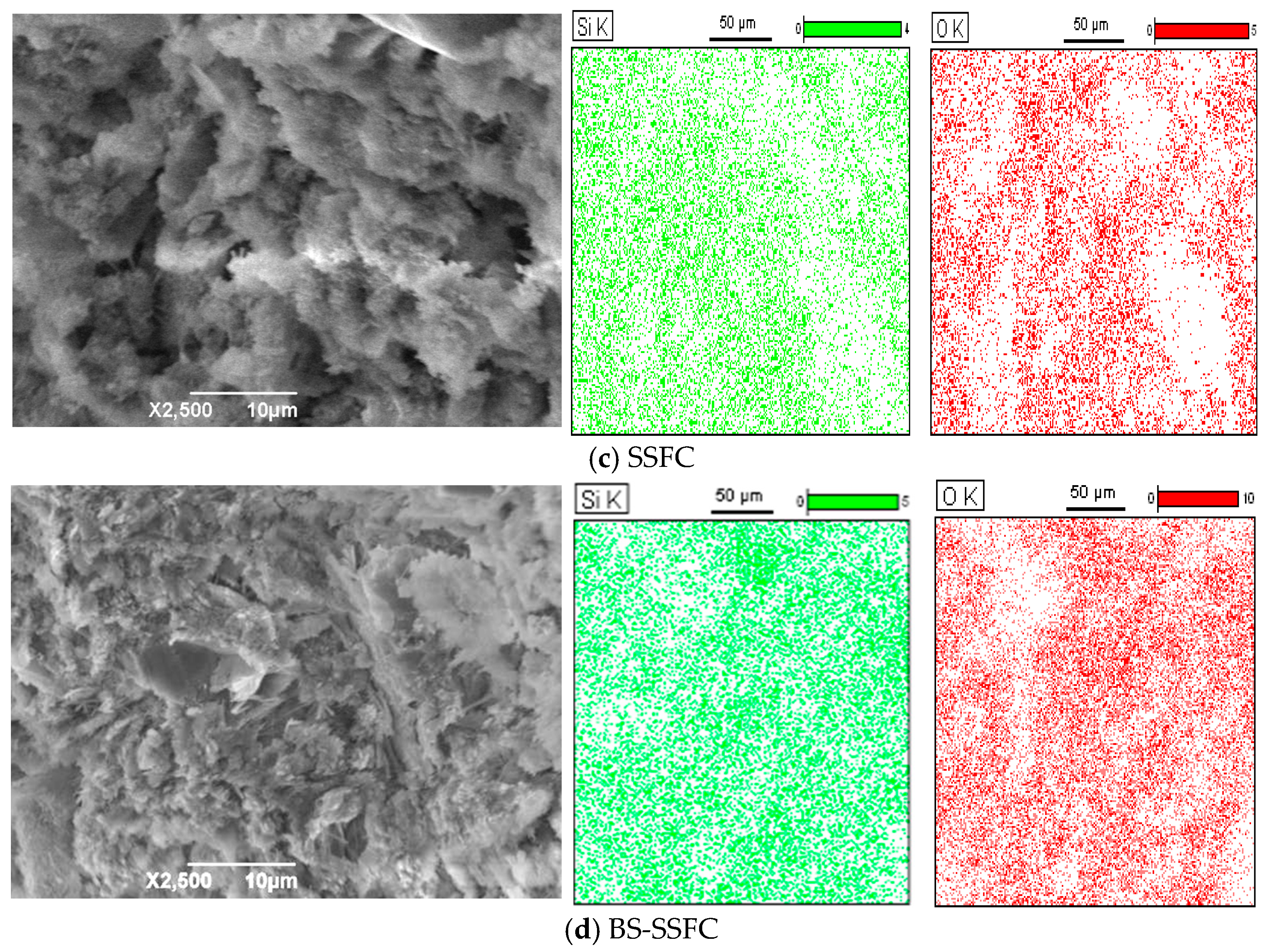
2.4. Scanning Electron Microscopy (SEM)
2.5. Mercury Intrusion Porosimetry (MIP)
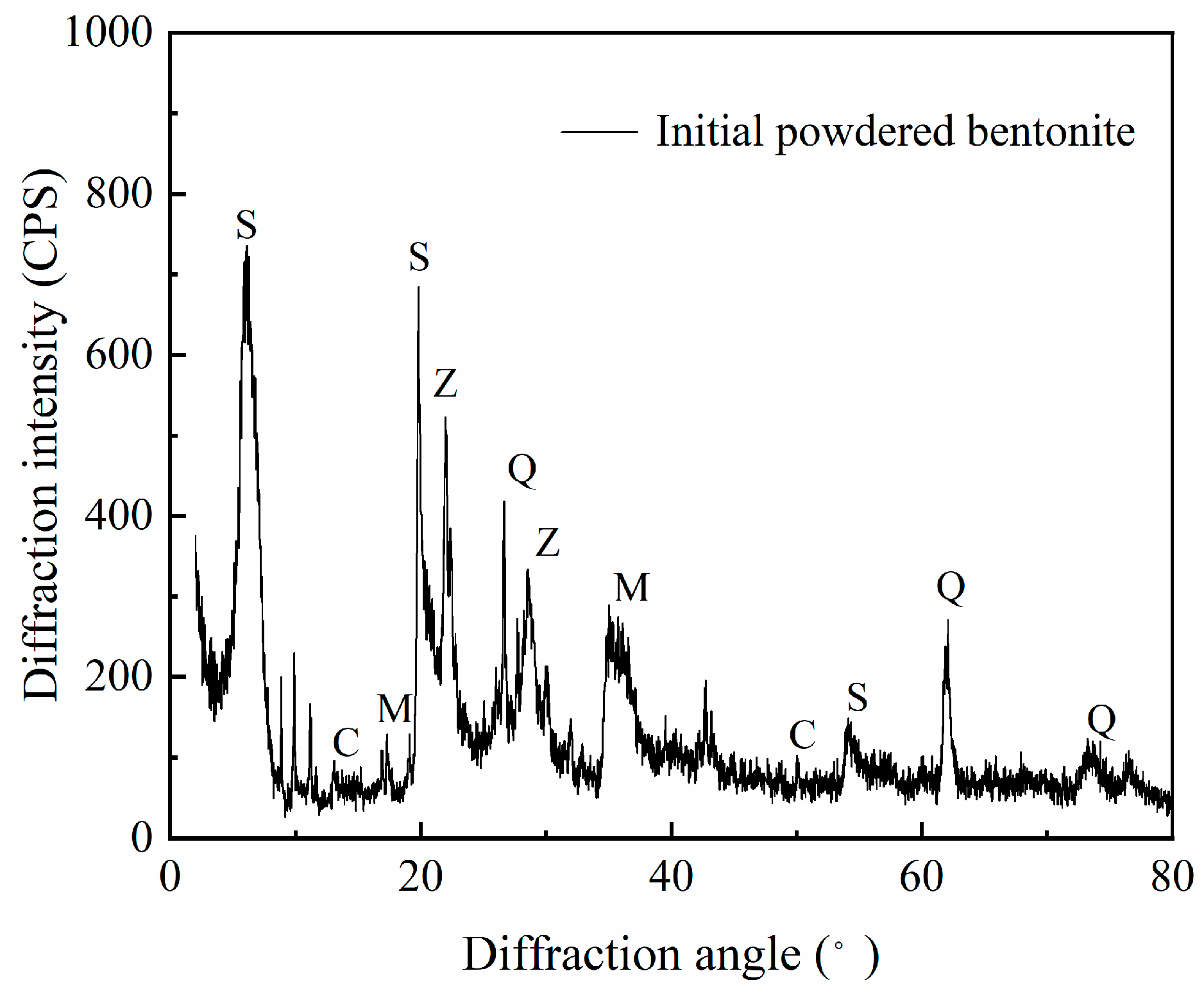
2.6. X-Ray Diffraction (XRD)
3. Experimental Results
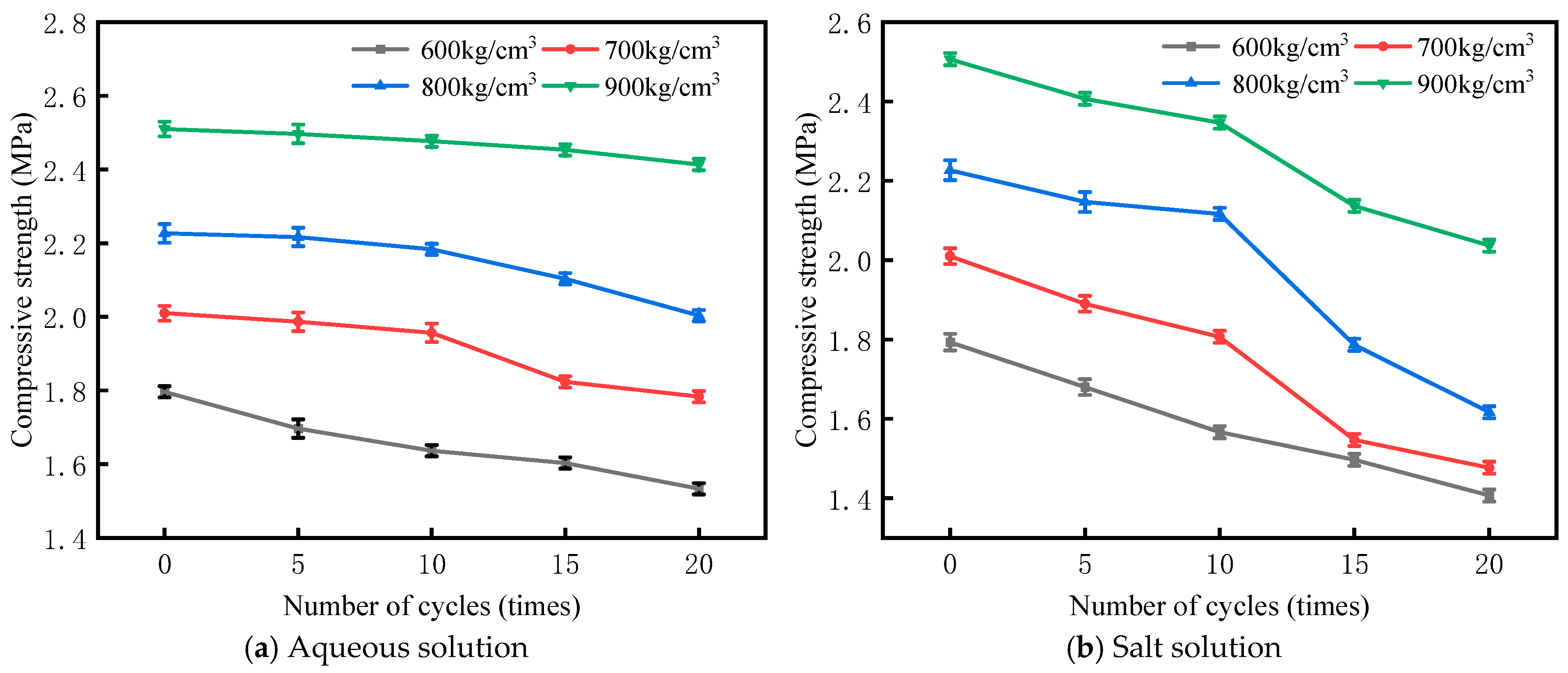
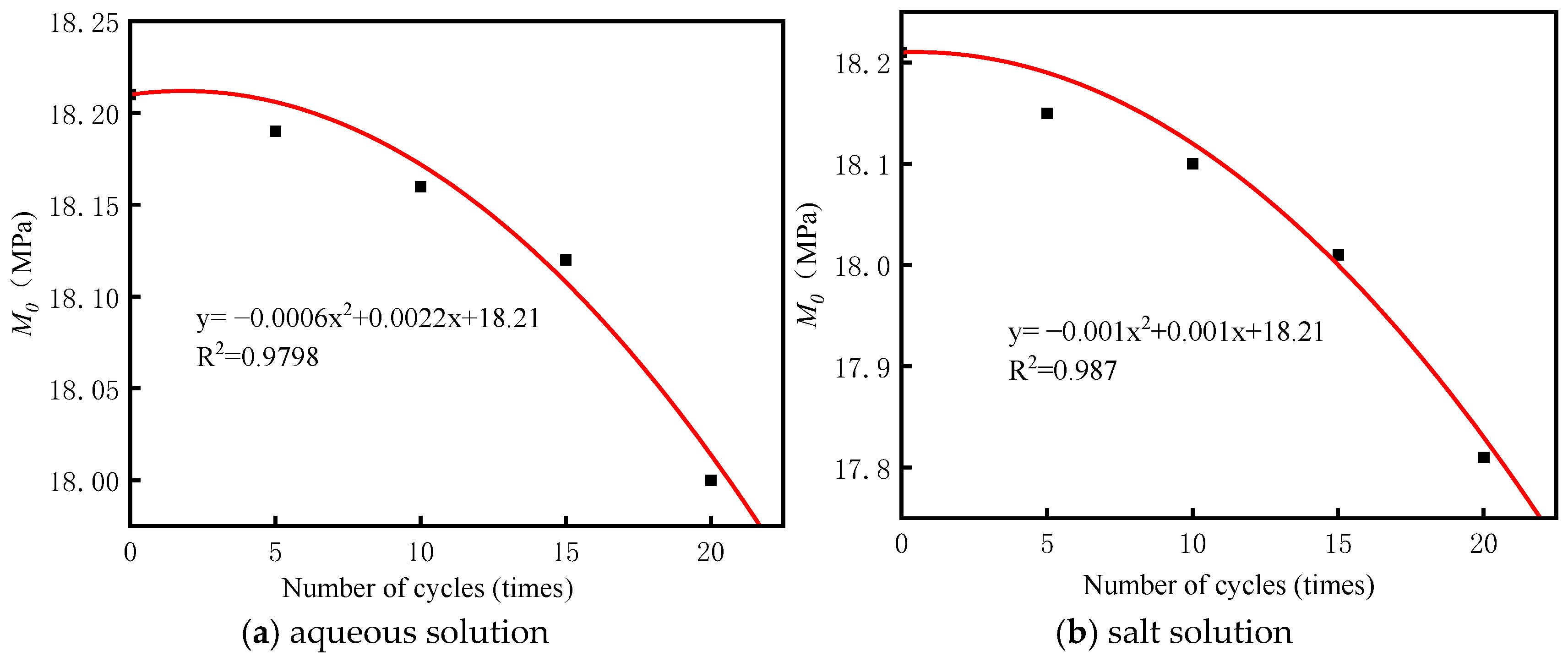
3.1. Wet–Dry Cycle Tests in Different Solutions
3.2. Mechanism of SS and BS for Enhancing the Durability of FC
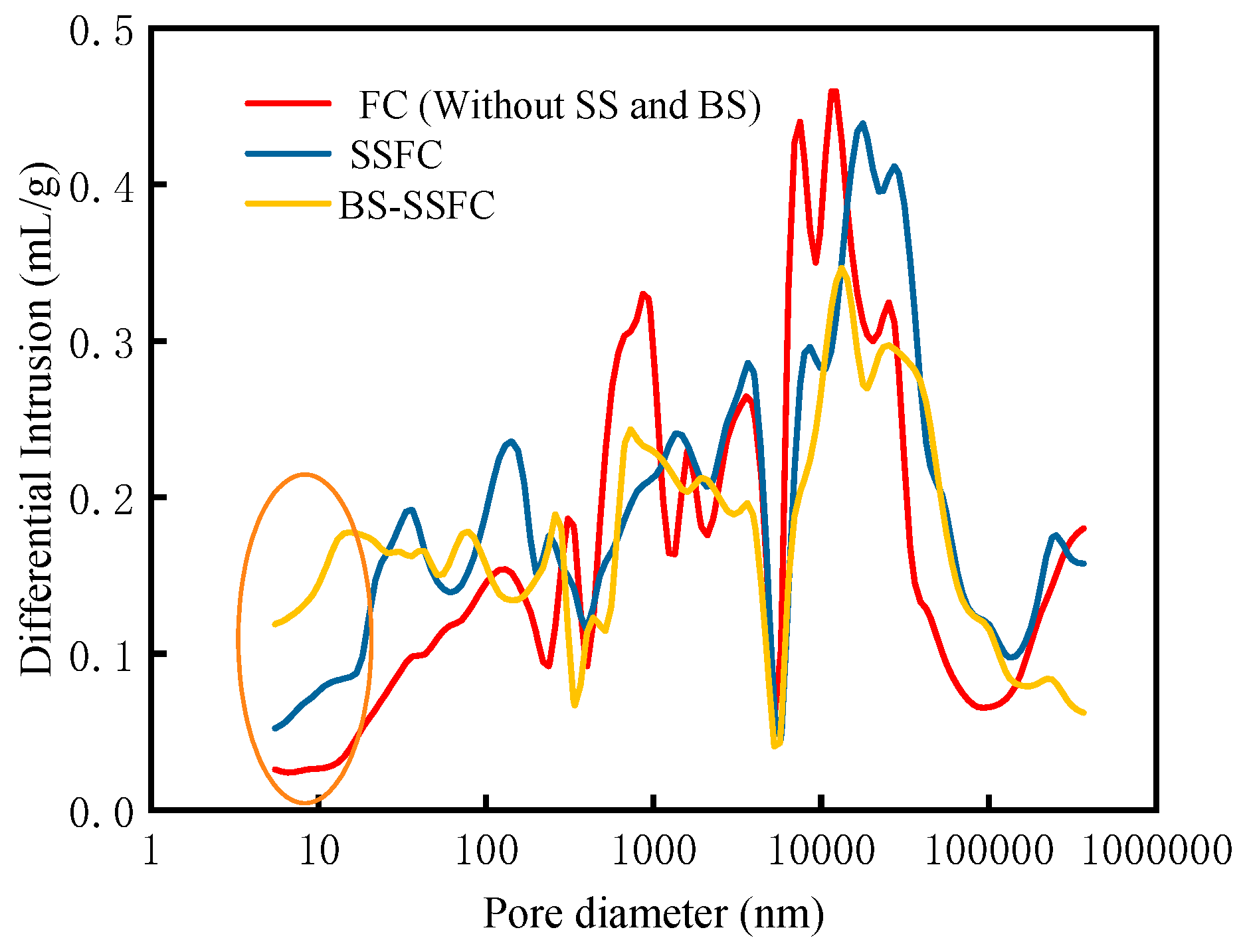
3.2.1. Pore Structure Analysis (MIP)
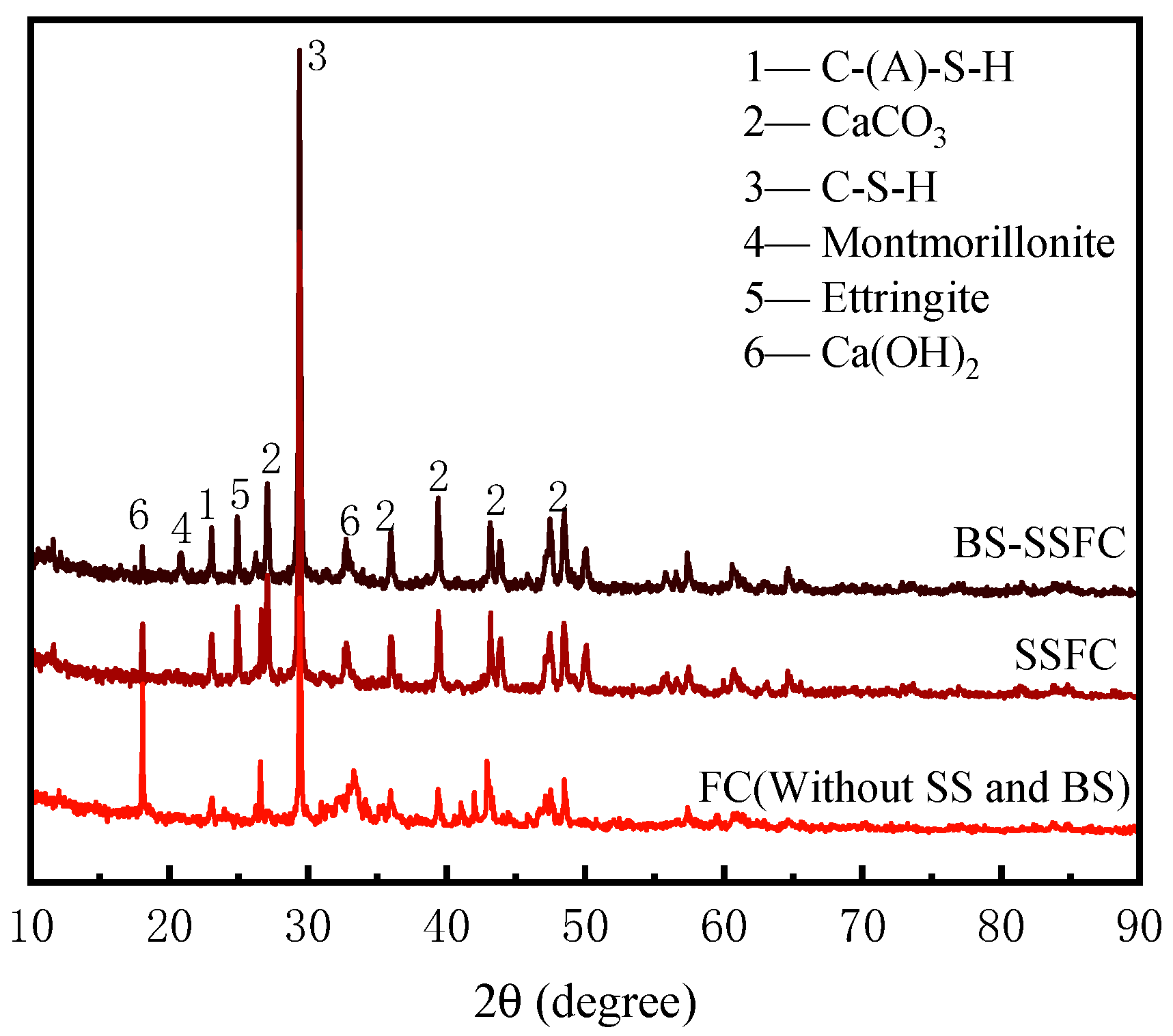
3.2.2. XRD Analysis
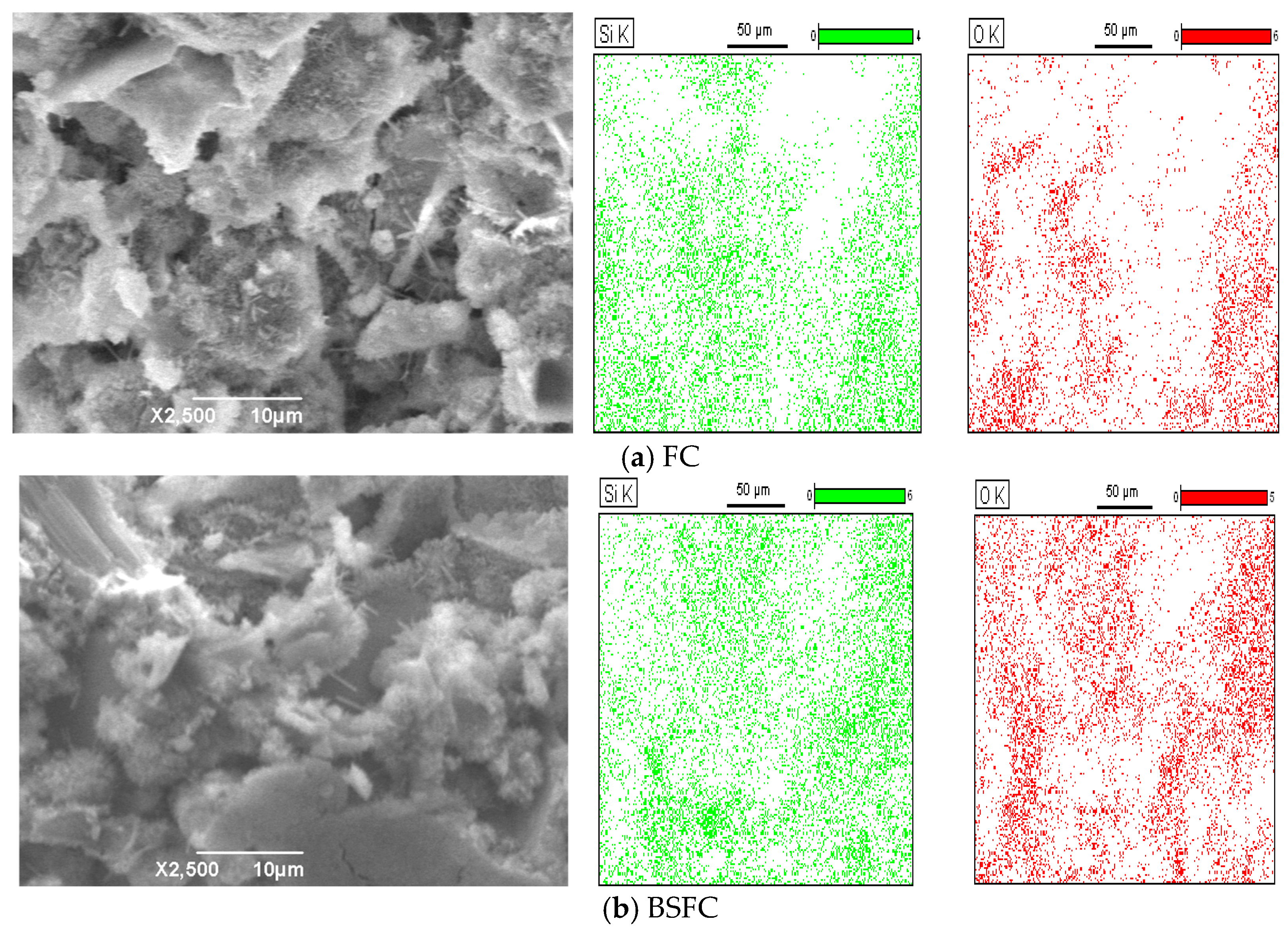
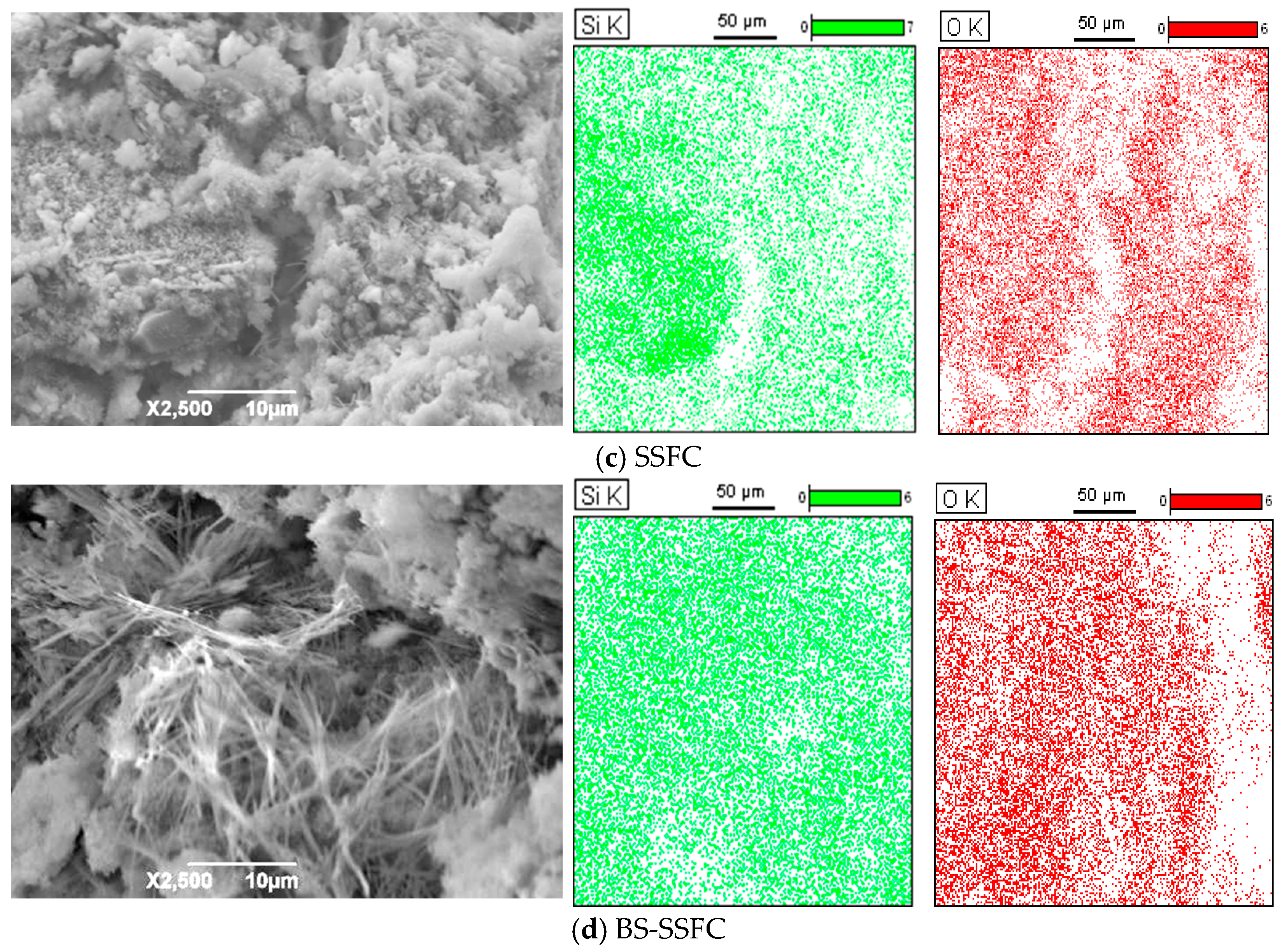
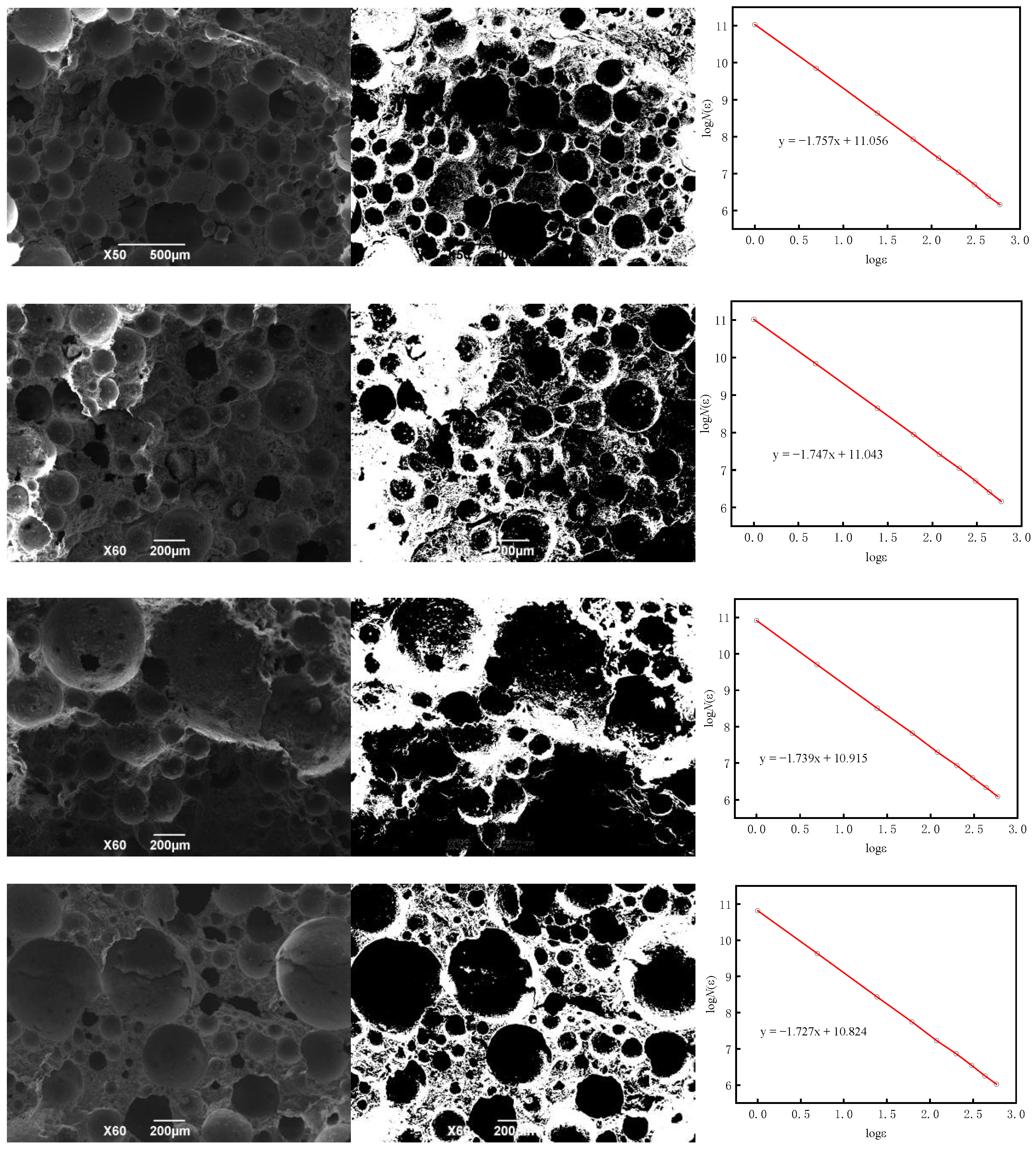
3.2.3. SEM Analysis
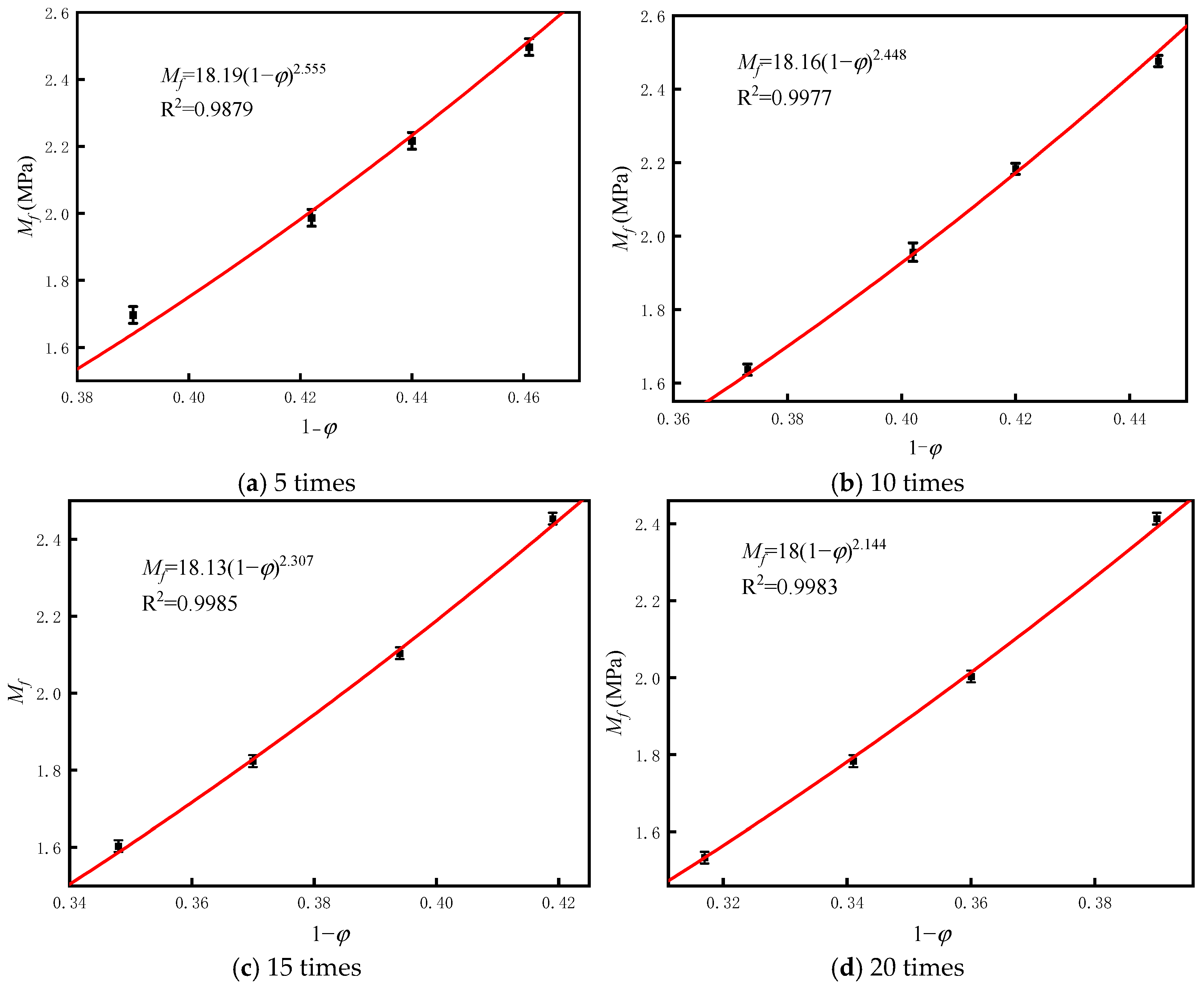
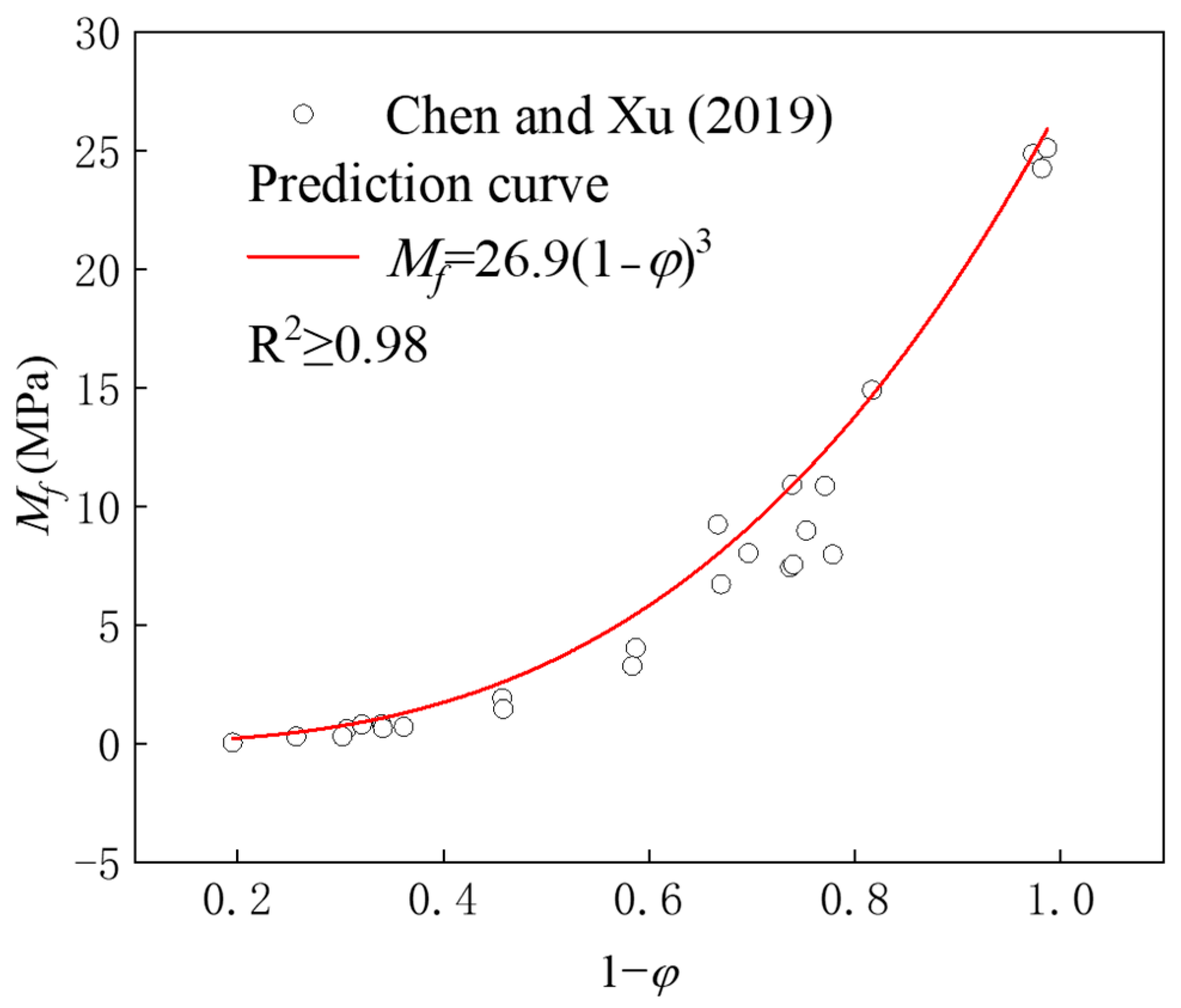
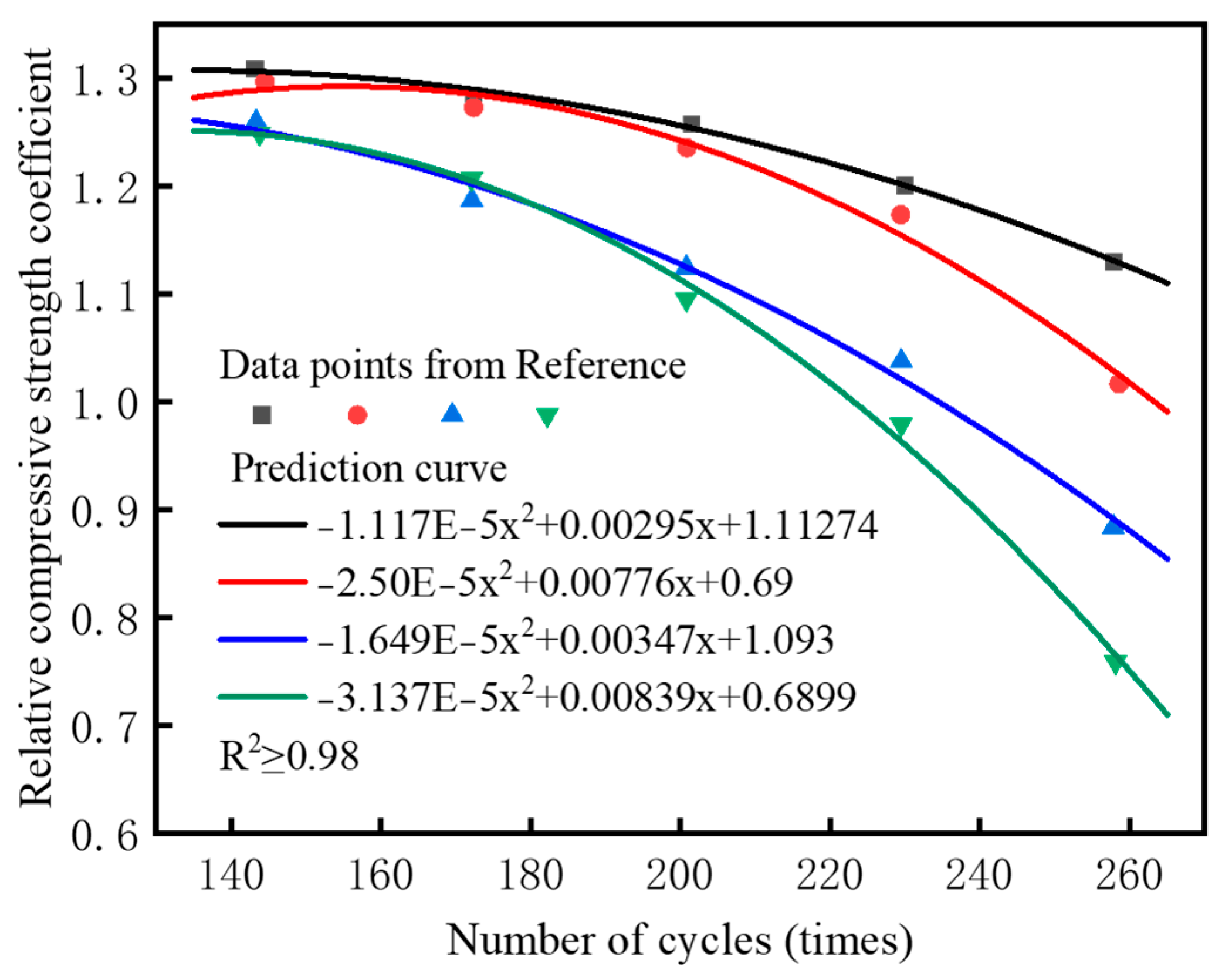
4. Prediction Model for CS Under Wet–Dry Cycles
5. Economic and Environmental Benefit Assessment
6. Conclusions
- (1)
- Under wet–dry cycling conditions, the strength of BS-SSFC with different dry densities gradually decreases as the number of cycles increases; the higher the dry density, the greater the strength retention rate. After wet–dry cycles in different solutions, the order of the CS loss from largest to smallest is as follows: salt solution > aqueous solution.
- (2)
- Incorporating BS and SS into FC can effectively improve its resistance to chemical erosion: as the number of cycles increases, SS can continuously participate in hydration reactions, thereby enhancing the material’s strength; the addition of BS improves the plasticity of the material, enabling it to maintain a high strength retention rate after wet–dry cycles.
- (3)
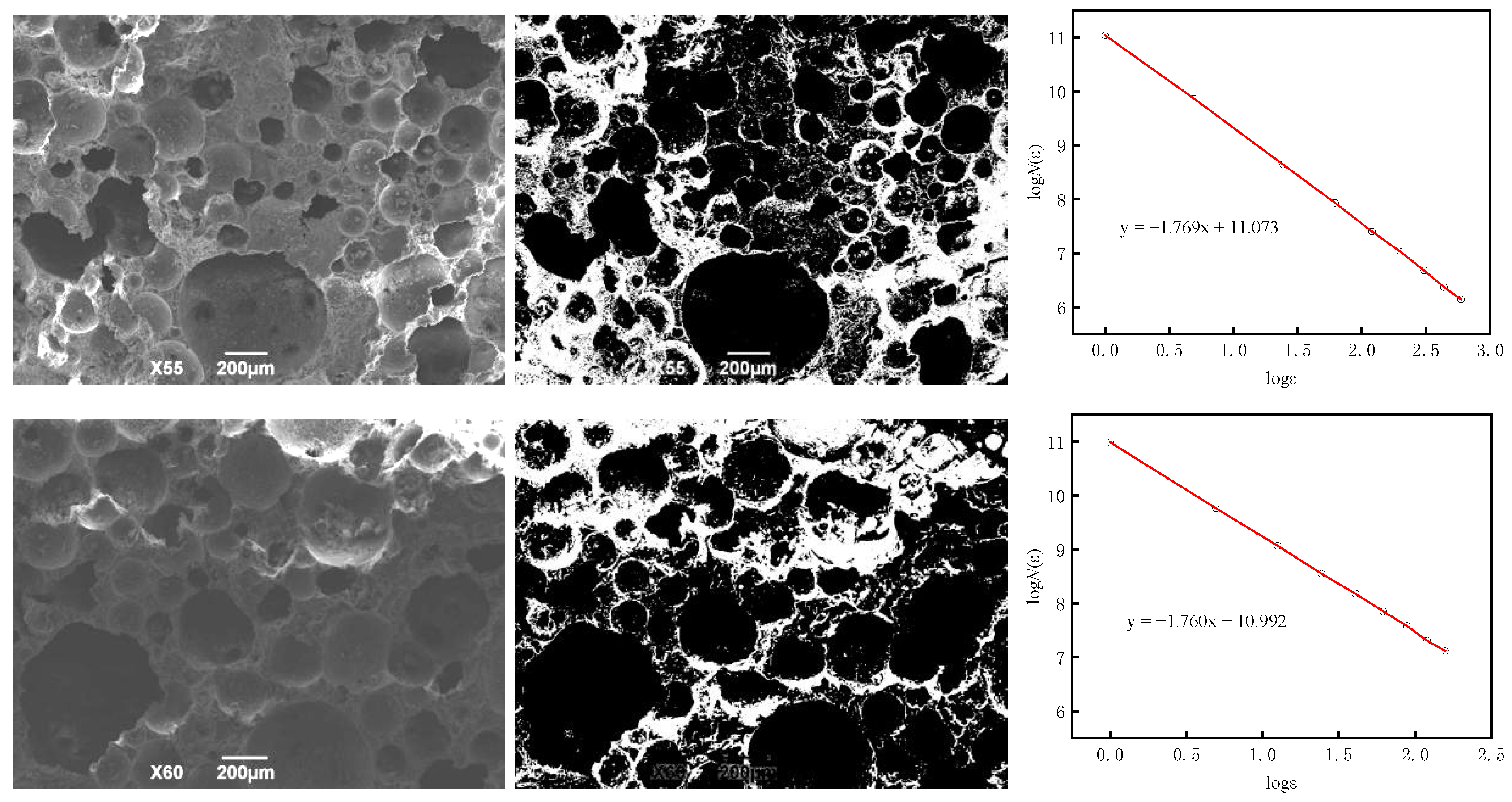
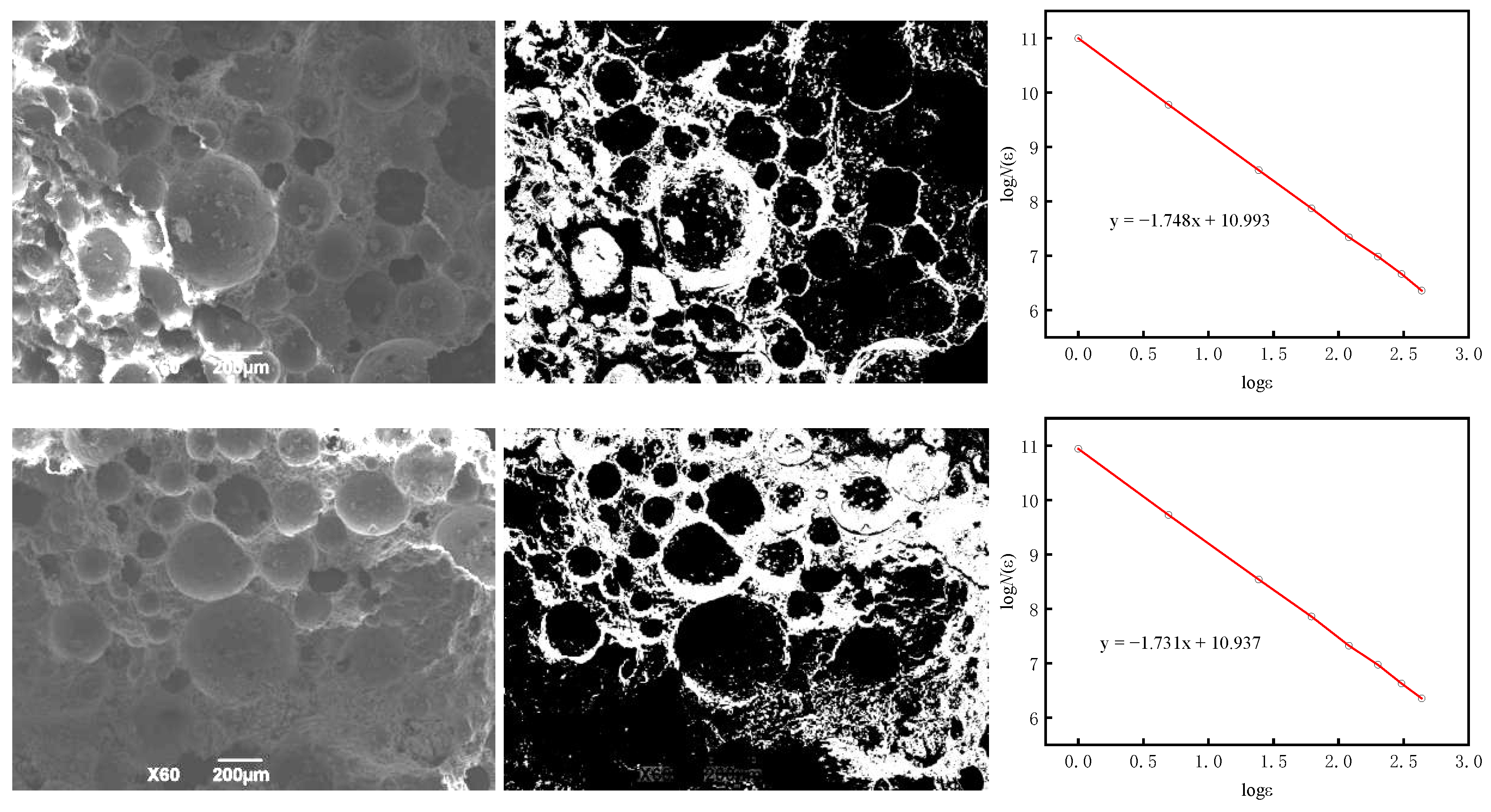
- The fractal dimension of BS-SSFC in different solutions decreases slightly with the increase in the number of wet–dry cycles. By calculating the fractal dimensions under different cycle times using the box-counting method and based on fractal theory, it is found that, in water and salt solutions, the relationship between the compressive strength and porosity of BS-SSFC under different wet–dry cycle times conforms to a specific regularity; meanwhile, the compressive strength at zero porosity has a specific correlation with the number of cycles (n). This regularity is also verified by the experimental data of other researchers.
7. Research Deficiencies and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raj, A.; Sathyan, D.; Mini, K.M. Physical and functional characteristics of foam concrete: A review. Constr. Build. Mater. 2019, 221, 787–799. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Kunhanandan Nambiar, E.K.; Indu Siva Ranjani, G. A classification of studies on properties of foam concrete. Cem. Concr. Compos. 2009, 31, 388–396. [Google Scholar] [CrossRef]
- Lu, J.-X. Recent advances in high strength lightweight concrete: From development strategies to practical applications. Constr. Build. Mater. 2023, 400, 132905. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Li, Y. Deterioration of concrete under the coupling effects of freeze–thaw cycles and other actions: A review. Constr. Build. Mater. 2022, 319, 126045. [Google Scholar] [CrossRef]
- Zhou, G.; Su, R.K.L. A Review on Durability of Foam Concrete. Buildings 2023, 13, 1880. [Google Scholar] [CrossRef]
- Shi, X.; Huang, J.; Su, Q. Experimental and numerical analyses of lightweight foamed concrete as filler for widening embankment. Constr. Build. Mater. 2020, 250, 118897. [Google Scholar] [CrossRef]
- Bader, M.A. Performance of concrete in a coastal environment. Cem. Concr. Compos. 2003, 25, 539–548. [Google Scholar] [CrossRef]
- Choe, G.; Kim, G.; Yoon, M.; Hwang, E.; Nam, J.; Guncunski, N. Effect of moisture migration and water vapor pressure build-up with the heating rate on concrete spalling type. Cem. Concr. Res. 2019, 116, 1–10. [Google Scholar] [CrossRef]
- Mohr, B.J.; Nanko, H.; Kurtis, K.E. Durability of kraft pulp fiber–cement composites to wet/dry cycling. Cem. Concr. Compos. 2005, 27, 435–448. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; Luo, T.; Zhou, M.; Zhang, K.; Ma, W. Study on the Deterioration of Concrete under Dry–Wet Cycle and Sulfate Attack. Materials 2020, 13, 4095. [Google Scholar] [CrossRef] [PubMed]
- Mahzabin, M.S.; Hock, L.J.; Hossain, M.S.; Kang, L.S. The influence of addition of treated kenaf fibre in the production and properties of fibre reinforced foamed composite. Constr. Build. Mater. 2018, 178, 518–528. [Google Scholar] [CrossRef]
- Tang, R.; Wei, Q.; Zhang, K.; Jiang, S.; Shen, Z.; Zhang, Y.; Chow, C.W.K. Preparation and performance analysis of recycled PET fiber reinforced recycled foamed concrete. J. Build. Eng. 2022, 57, 104948. [Google Scholar] [CrossRef]
- Zhou, D.; Gao, H.; Liao, H.; Fang, L.; Cheng, F. Enhancing the performance of foam concrete containing fly ash and steel slag via a pressure foaming process. J. Clean. Prod. 2021, 329, 129664. [Google Scholar] [CrossRef]
- Şeker, B.Ş.; Gökçe, M.; Toklu, K. Investigation of the effect of silica fume and synthetic foam additive on cell structure in ultra-low density foam concrete. Case Stud. Constr. Mater. 2022, 16, e01062. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, J.-X.; Poon, C.S. Enhanced performance and sustainability of foam concrete through shale aggregate integration. Constr. Build. Mater. 2025, 479, 141442. [Google Scholar] [CrossRef]
- Ying, L.; Yu, P.; Wang, F.; Jiang, P. Evaluation of Integral and Surface Hydrophobic Modification on Permeation Resistance of Foam Concrete. Coatings 2025, 15, 854. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, W.; Zhang, Y.; Wang, C. Arresting properties of polypropylene fiber-reinforced foamed concrete under wet-dry cycles. Constr. Build. Mater. 2024, 427, 136233. [Google Scholar] [CrossRef]
- Othuman Mydin, M.A.; Jagadesh, P.; Bahrami, A.; Dulaimi, A.; Onuralp Özkılıç, Y.; Omar, R. Enhanced fresh and hardened properties of foamed concrete modified with nano-silica. Heliyon 2024, 10, e25858. [Google Scholar] [CrossRef]
- Othuman Mydin, M.A.; Mohd Nawi, M.N.; Mohamed, O.; Sari, M.W. Mechanical Properties of Lightweight Foamed Concrete Modified with Magnetite (Fe3O4) Nanoparticles. Materials 2022, 15, 5911. [Google Scholar] [CrossRef] [PubMed]
- Alani, S.; Hassan, M.S.; Jaber, A.A.; Ali, I.M. Effects of elevated temperatures on strength and microstructure of mortar containing nano-calcined montmorillonite clay. Constr. Build. Mater. 2020, 263, 120895. [Google Scholar] [CrossRef]
- Khankhaje, E.; Kim, T.; Jang, H.; Kim, C.-S.; Kim, J.; Rafieizonooz, M. A review of utilization of industrial waste materials as cement replacement in pervious concrete: An alternative approach to sustainable pervious concrete production. Heliyon 2024, 10, e26188. [Google Scholar] [CrossRef]
- Yang, P.; Qi, Y.; Chen, R. Preparation of High-Performance Cementitious Materials from Industrial Solid Wastes. Eng. Proc. 2023, 38, 50. [Google Scholar] [CrossRef]
- Liu, X.; Ni, C.; Meng, K.; Zhang, L.; Liu, D.; Sun, L. Strengthening mechanism of lightweight cellular concrete filled with fly ash. Constr. Build. Mater. 2020, 251, 118954. [Google Scholar] [CrossRef]
- Cai, X.; Cao, Z.; Sun, J.; Wang, H.; Wu, S. Influence of Steel Slag on Properties of Cement-Based Materials: A Review. Buildings 2024, 14, 2985. [Google Scholar] [CrossRef]
- Devi, V.S.; Gnanavel, B.K. Properties of Concrete Manufactured Using Steel Slag. Procedia Eng. 2014, 97, 95–104. [Google Scholar] [CrossRef]
- Andrade, H.D.; de Carvalho, J.M.F.; Costa, L.C.B.; Elói, F.P.d.F.; e Silva, K.D.D.C.; Peixoto, R.A.F. Mechanical performance and resistance to carbonation of steel slag reinforced concrete. Constr. Build. Mater. 2021, 298, 123910. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Jing, W.; Jiang, J.; Ding, S.; Duan, P. Hydration and Microstructure of Steel Slag as Cementitious Material and Fine Aggregate in Mortar. Molecules 2020, 25, 4456. [Google Scholar] [CrossRef]
- Shi, C. Steel Slag—Its Production, Processing, Characteristics, and Cementitious Properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Luo, Z.; He, F.; Zhang, W.; Xiao, Y.; Xie, J.; Sun, R.; Xie, M. Effects of fluoride content on structure and properties of steel slag glass-ceramics. Mater. Chem. Phys. 2020, 242, 122531. [Google Scholar] [CrossRef]
- Jiang, L.; Bao, Y.; Yang, Q.; Chen, Y.; Liu, G.; Han, F.; Wei, J.; Engström, F.; Deng, J. Formation of Spinel Phases in Oxidized BOF Slag under Different Cooling Conditions. Steel Res. Int. 2017, 88, 1700066. [Google Scholar] [CrossRef]
- Lima-Guerra, D.J.; Mello, I.; Resende, R.; Silva, R. Use of Bentonite and Organobentonite as Alternatives of Partial Substitution of Cement in Concrete Manufacturing. Int. J. Concr. Struct. Mater. 2014, 8, 15–26. [Google Scholar] [CrossRef]
- Ashraf, M.; Iqbal, M.F.; Rauf, M.; Ashraf, M.U.; Ulhaq, A.; Muhammad, H.; Liu, Q. Developing a sustainable concrete incorporating bentonite clay and silica fume: Mechanical and durability performance. J. Clean. Prod. 2022, 337, 130315. [Google Scholar] [CrossRef]
- Ahmad, J.; Kontoleon, K.J.; Al-Mulali, M.Z.; Shaik, S.; Hechmi El Ouni, M.; El-Shorbagy, M.A. Partial Substitution of Binding Material by Bentonite Clay (BC) in Concrete: A Review. Buildings 2022, 12, 634. [Google Scholar] [CrossRef]
- Memon, S.A.; Arsalan, R.; Khan, S.; Lo, T.Y. Utilization of Pakistani bentonite as partial replacement of cement in concrete. Constr. Build. Mater. 2012, 30, 237–242. [Google Scholar] [CrossRef]
- Masood, B.; Elahi, A.; Barbhuiya, S.; Ali, B. Mechanical and durability performance of recycled aggregate concrete incorporating low calcium bentonite. Constr. Build. Mater. 2020, 237, 117760. [Google Scholar] [CrossRef]
- Keke, L.; Yong, L.; Liuliu, X.; Junjie, Z.; Kangning, L.; Dingqiang, F.; Rui, Y. Rheological characteristics of Ultra-High performance concrete (UHPC) incorporating bentonite. Constr. Build. Mater. 2022, 349, 128793. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Lu, Z.; Jiang, J.; Niu, Y. Effects of bentonite slurry on air-void structure and properties of foamed concrete. Constr. Build. Mater. 2018, 179, 207–219. [Google Scholar] [CrossRef]
- Fernández, R.; Ruiz, A.I.; Cuevas, J. Formation of C-A-S-H phases from the interaction between concrete or cement and bentonite. Clay Miner. 2016, 51, 223–235. [Google Scholar] [CrossRef]
- GB/T 175-2023; Common Portland Cement. China Building Materials Industry Press: Beijing, China, 2023.
- GB/T 17671-2020; Test Method for Strength of Cement Mortar (ISO Method). China Building Materials Industry Press: Beijing, China, 2020.
- YB/T 4328-2012; Methods for Chemical Analysis of Steel Slag. Metallurgical Industry Press: Beijing, China, 2012.
- GB/T 24763-2009; Steel Slag Used in Cement. China Building Materials Industry Press: Beijing, China, 2009.
- JGJ 63-2006; Standard for Water of Concrete. China Architecture & Building Press: Beijing, China, 2006.
- JG/T 266-2011; Foamed Concrete. China Architecture & Building Press: Beijing, China, 2011.
- Yang, X.; Xu, S.; Zhao, Z.; Lv, Y. Strength, Durability, and Microstructure of Foamed Concrete Prepared Using Special Soil and Slag. Sustainability 2022, 14, 14952. [Google Scholar] [CrossRef]
- Baozhen, S.; Erda, S. Relation between properties of aerated concrete and its porosity and hydrates. In Pore Structure and Construction Materials Properties, Proceedings of the 1st RILEM International Conference on Mineral Carbonation for Cement and Concrete, Aachen, Germany, 16–17 April 2024; Chapman and Hall Press: London, UK; Versailles, France, 1987; pp. 232–237. [Google Scholar]
- Balshin, M.Y. Relation of mechanical properties of powder metals and their porosity and ultimate properties of porous metal-ceramic materials. Dokl. Akad. Nauk. 1949, 67, 831–834. [Google Scholar]
- Xiang, G.; Song, D.; Li, H.; Jalal, F.E.; Wang, H.; Zhou, Y. Investigation on Preparation and Compressive Strength Model of Steel Slag Foam Concrete. J. Build. Eng. 2023, 72, 106548. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.F. Compressive Strength of Fractal-Textured Foamed Concrete. Fractals 2019, 27, 1940003. [Google Scholar] [CrossRef]
- Guo, J.-J.; Wang, K.; Guo, T.; Yang, Z.-Y.; Zhang, P. Effect of Dry–Wet Ratio on Properties of Concrete Under Sulfate Attack. Materials 2019, 12, 2755. [Google Scholar] [CrossRef] [PubMed]
- GB/T 32150-2015; Industrial Enterprises Greenhouse Gas Emissions Accounting and Reporting Guideline. Standards Press of China: Beijing, China, 2015.





| Material | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | SO3 | K2O | P2O5 | MnO | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 57.21 | 23.54 | 6.12 | 4.57 | 2.51 | 0.61 | 2.31 | 0.85 | - | - | 2.28 |
| Steel slag | 58.85 | 12.8 | 5.21 | 14.39 | 2.63 | 0.92 | 0.31 | - | 1.51 | 2.2 | 1.18 |
| Cement | Viscosity | Initial Coagulation/min | Final Condensation/min | Compressive Strength/MPa | Flexural Strength/MPa | ||
|---|---|---|---|---|---|---|---|
| 3d | 28d | 3d | 28d | ||||
| P.O.42.5 | 26.8 | 150 | 248 | 27.1 | 55.3 | 5.5 | 8.6 |
| Number of Dry–Wet Cycles | 5 | 10 | 15 | 20 |
|---|---|---|---|---|
| Two-dimensional fractal dimension (D1) | 1.769 ± 0.002 | 1.760 ± 0.002 | 1.748 ± 0.002 | 1.731 ± 0.002 |
| Three-dimensional fractal dimension D | 2.769 | 2.760 | 2.748 | 2.731 |
| Number of Dry–Wet Cycles | 5 | 10 | 15 | 20 |
|---|---|---|---|---|
| Two-dimensional fractal dimension (D1) | 1.757 ± 0.003 | 1.747 ± 0.002 | 1.739 ± 0.002 | 1.727 ± 0.001 |
| Three-dimensional fractal dimension (D) | 2.757 | 2.747 | 2.739 | 2.727 |
| Solution | Number of Cycles/Times | M0/MPa | Fractal Dimension (D) | b | Mf-φ Relation |
|---|---|---|---|---|---|
| Aqueous solution | 5 | 18.19 | 2.769 | 2.555 | Mf = 18.19(1 − φ)2.555 |
| 10 | 18.16 | 2.760 | 2.448 | Mf = 18.16(1 − φ)2.448 | |
| 15 | 18.12 | 2.748 | 2.307 | Mf = 18.12(1 − φ)2.307 | |
| 20 | 18.00 | 2.731 | 2.144 | Mf = 18.00(1 − φ)2.144 | |
| Salt solution | 5 | 18.15 | 2.757 | 2.414 | Mf = 18.15(1 − φ)2.414 |
| 10 | 18.1 | 2.747 | 2.306 | Mf = 18.1(1 − φ)2.306 | |
| 15 | 18.01 | 2.739 | 2.219 | Mf = 18.01(1 − φ)2.219 | |
| 20 | 17.81 | 2.727 | 2.110 | Mf = 17.81(1 − φ)2.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, G.; Shao, F.; Zhang, H.; Bai, Y.; Fang, Y.; Li, Y.; Li, L.; Ming, Y. Analysis on Durability of Bentonite Slurry–Steel Slag Foam Concrete Under Wet–Dry Cycles. Buildings 2025, 15, 3550. https://doi.org/10.3390/buildings15193550
Xiang G, Shao F, Zhang H, Bai Y, Fang Y, Li Y, Li L, Ming Y. Analysis on Durability of Bentonite Slurry–Steel Slag Foam Concrete Under Wet–Dry Cycles. Buildings. 2025; 15(19):3550. https://doi.org/10.3390/buildings15193550
Chicago/Turabian StyleXiang, Guosheng, Feiyang Shao, Hongri Zhang, Yunze Bai, Yuan Fang, Youjun Li, Ling Li, and Yang Ming. 2025. "Analysis on Durability of Bentonite Slurry–Steel Slag Foam Concrete Under Wet–Dry Cycles" Buildings 15, no. 19: 3550. https://doi.org/10.3390/buildings15193550
APA StyleXiang, G., Shao, F., Zhang, H., Bai, Y., Fang, Y., Li, Y., Li, L., & Ming, Y. (2025). Analysis on Durability of Bentonite Slurry–Steel Slag Foam Concrete Under Wet–Dry Cycles. Buildings, 15(19), 3550. https://doi.org/10.3390/buildings15193550





