Multi-Scale Modification of Sodium Polyacrylate-Modified Cement Grouts: Rheology, Microstructure, and Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cement
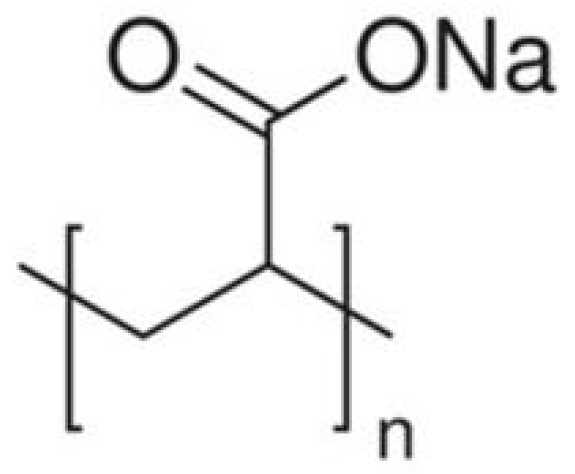
2.1.2. Sodium Polyacrylate
2.1.3. Water
2.2. Preparation of PAAS-Modified Cement-Based Paste
2.3. Experimental Approaches
2.3.1. Fluidity
2.3.2. Rheological Curve
2.3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.4. X-Ray Diffraction (XRD)
2.3.5. Scanning Electron Microscope (SEM)
3. Results and Discussion
3.1. Fluidity and Rheological Properties of PAAS-Modified Composite Grouting
3.1.1. Fluidity
3.1.2. Rheological Curve
3.2. Chemical Characterization of PAAS-Modified Composite Paste
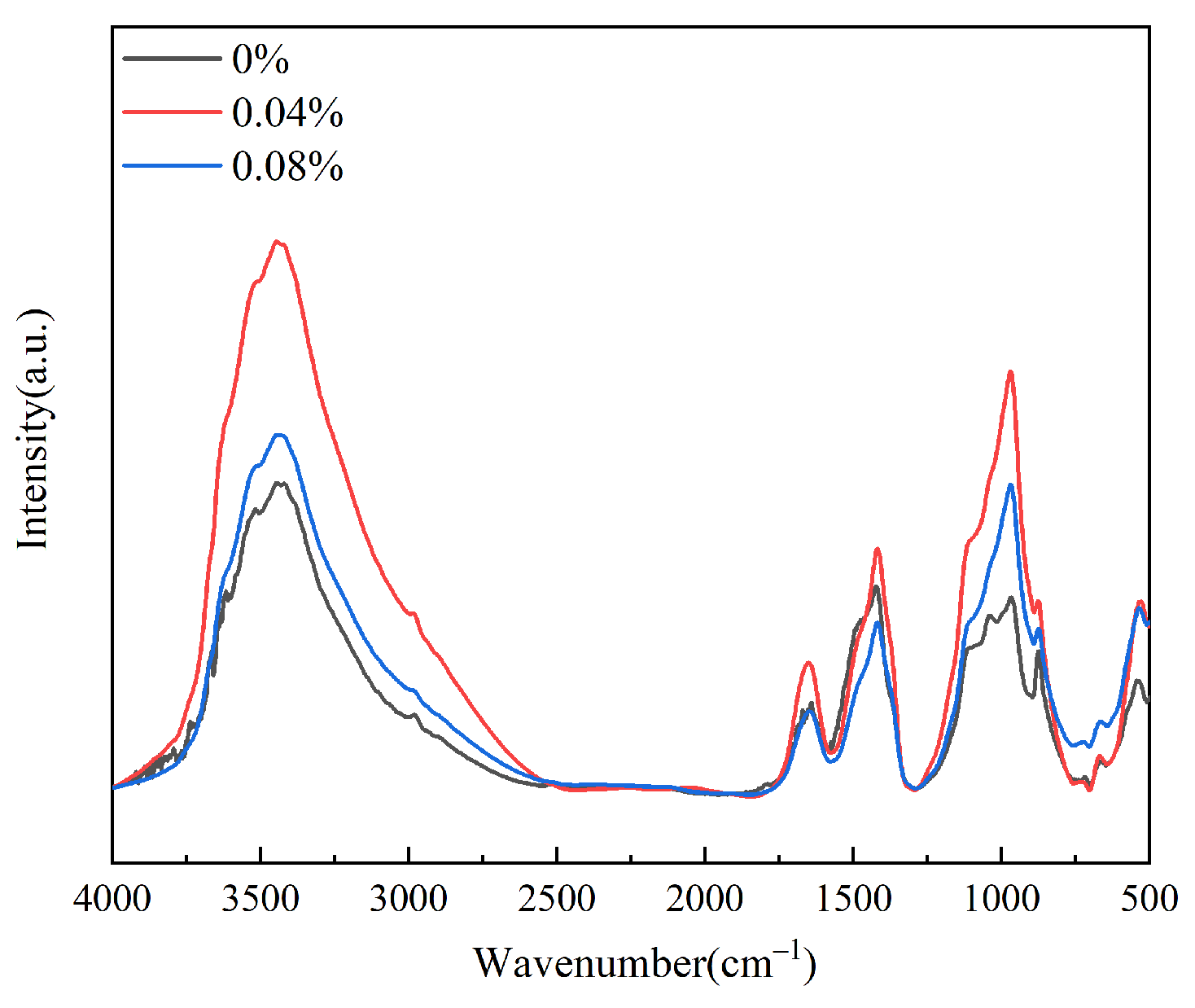
3.2.1. Fourier Transform Infrared Spectroscopy (FT-IR)
3.2.2. X-Ray Diffraction (XRD)
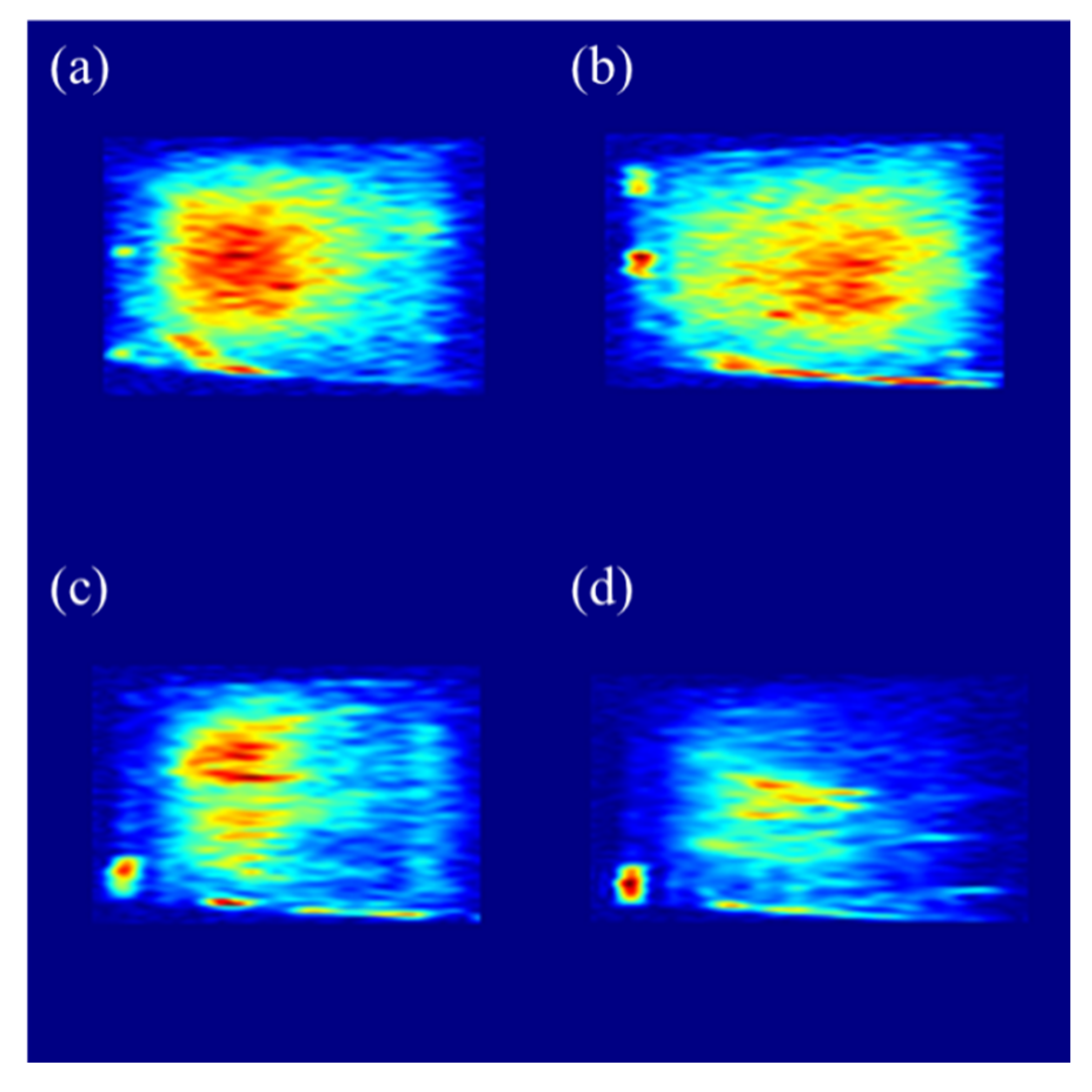
3.2.3. Scanning Electron Microscope (SEM)
3.3. Pore Structure and Mechanical Properties of PAAS-Modified Composite Grouting
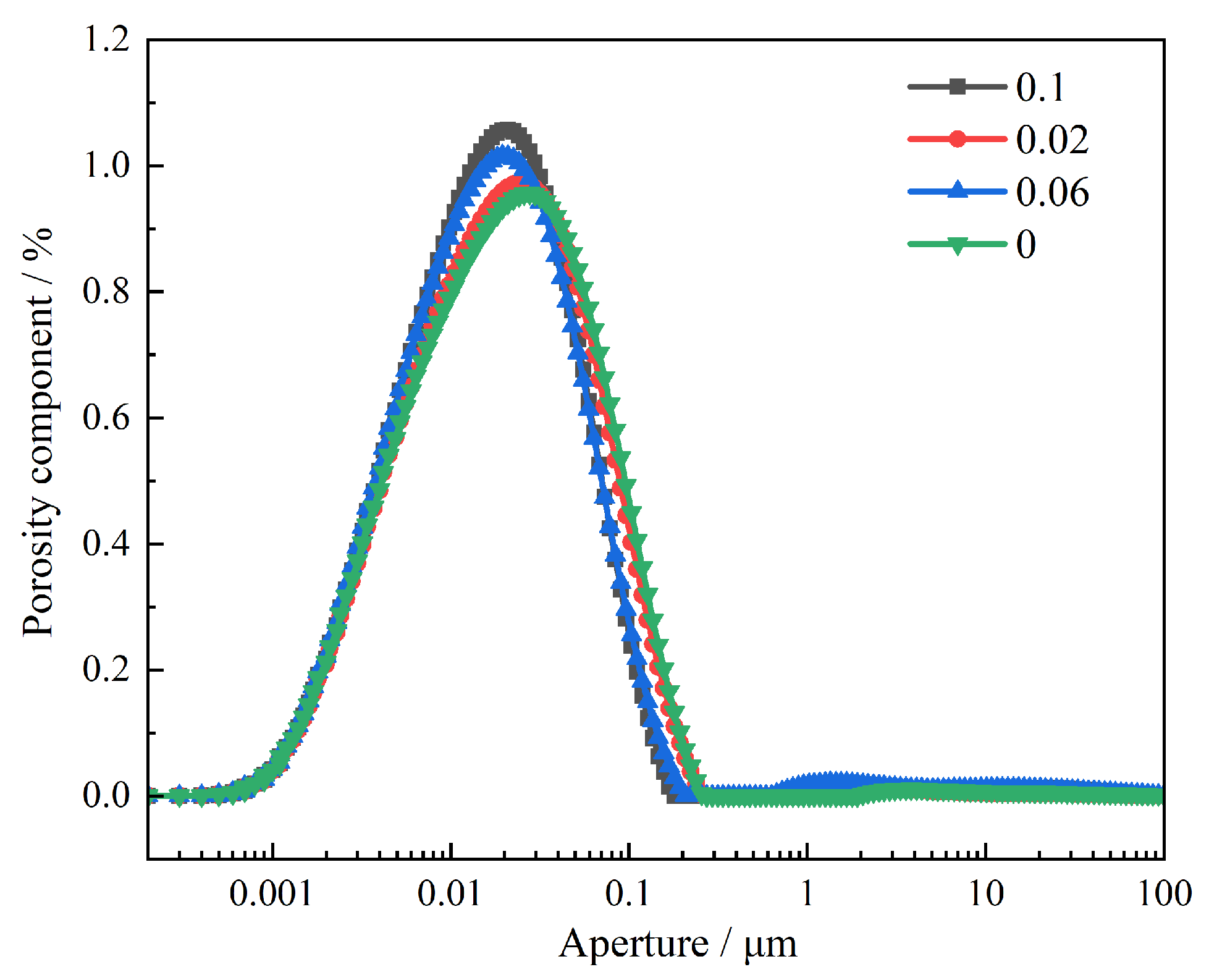
3.3.1. Effect of PAAS on Pore Distribution of Cured Materials
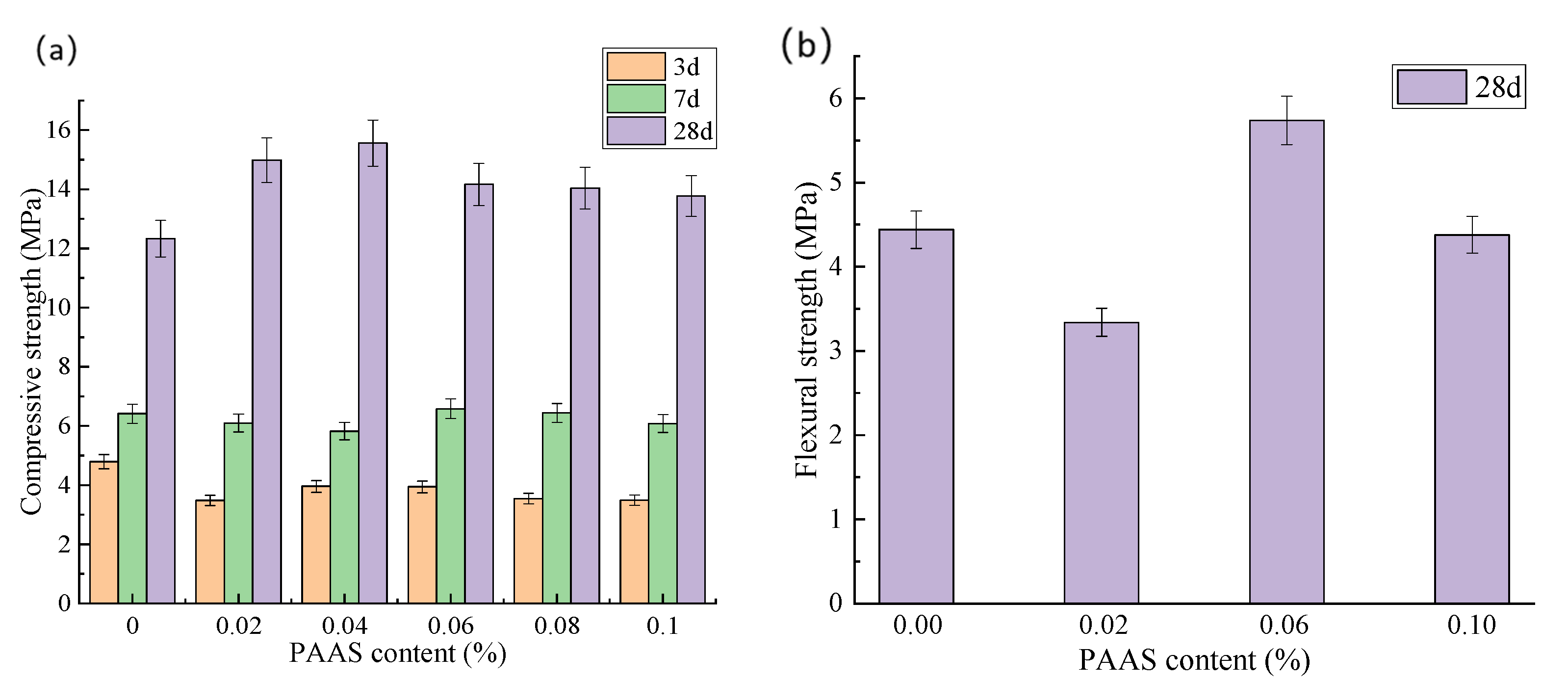
3.3.2. Effect of PAAS on the Mechanical Properties of Cured Materials
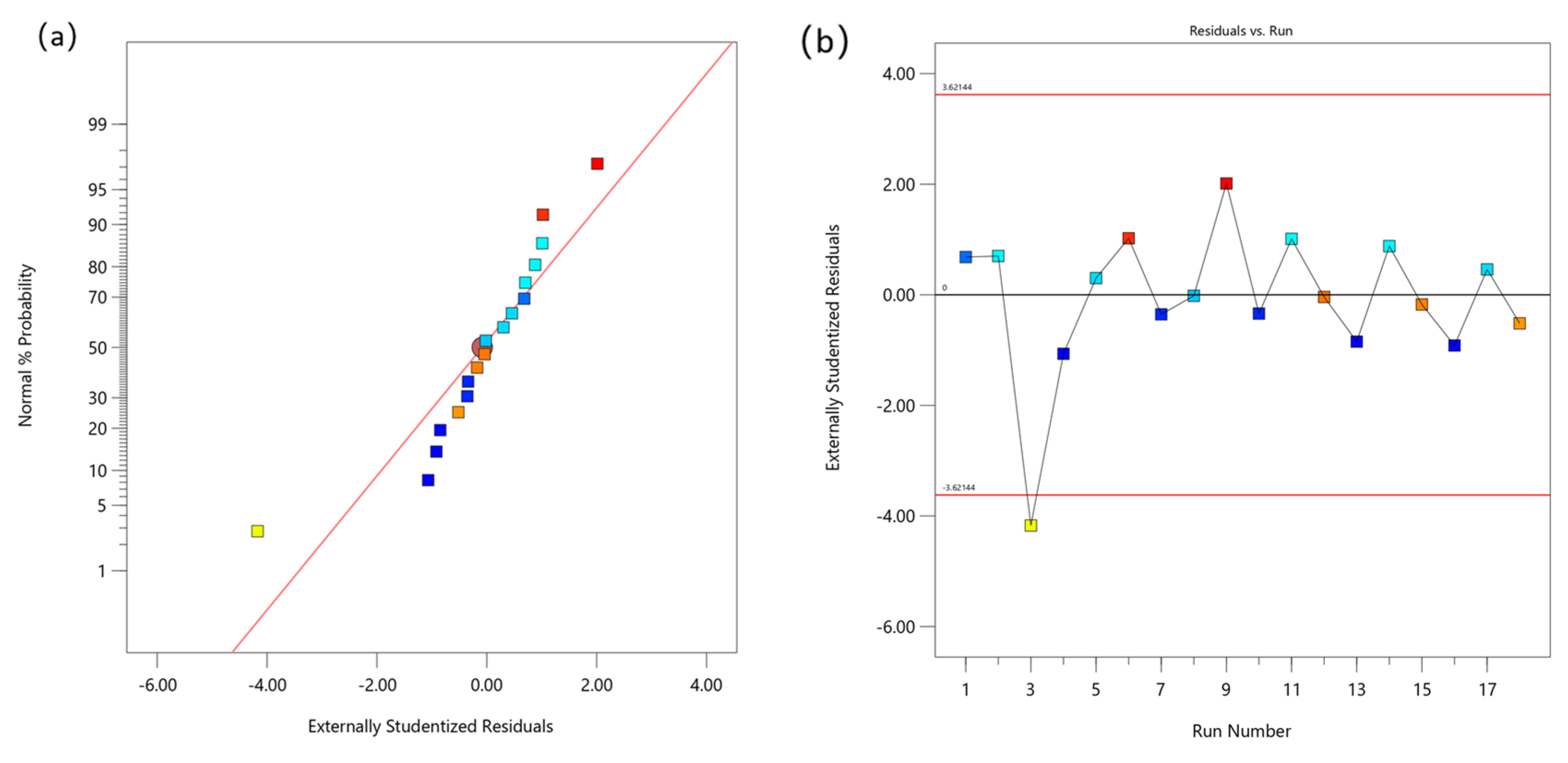
3.3.3. Mechanical Model and Reliability Analysis of PAAS-Modified Composite Paste
3.4. The Modification Mechanism of PAAS-Modified Cement
4. Conclusions
- (1)
- Rheological improvement: PAAS dismantles cement particle flocculation through electrostatic repulsion and steric hindrance synergy. At 0.1% dosage, yield stress reaches τ = 0.95 Pa (80.3% reduction), with plastic viscosity stabilizing at 0.04 Pa·s (23.1% reduction), exhibiting near-Bingham fluid characteristics. Maximum fluidity increases by 30.7% (25.13 cm), significantly improving injectability and fracture diffusion capacity.
- (2)
- Pore-mechanics co-evolution: PAAS-induced microstructural reconstruction follows “macropore elimination-microporous homogenization”. NMR imaging shows >1 μm macropores virtually disappear with increasing dosage, dominant pore size decreases, and submicron pore proportion rises. Despite minor total porosity reduction (0.46%), qualitative pore transformation substantially enhances impermeability. Mechanically, PAAS concurrently suppresses weak-phase formation and promotes C-S-H densification, enabling the 0.04% group to achieve peak 28-day compressive strength (15.56 MPa, +26%), while the 0.06% group shows a dramatic flexural strength increase (5.74 MPa, +29.3%), significantly improving material performance and mitigating brittle failure.
- (3)
- Multi-scale mechanism: PAAS modification originates from chemical bonding and physical dispersion synergy. Molecular: Carboxyl groups (-COO−) selectively chelate Ca2+, directionally inhibiting Ca(OH)2 nucleation and optimizing C-S-H precipitation. Micro: Electrostatic repulsion dismantles flocculates while steric hindrance prevents re-agglomeration, eliminating percolation channels (NMR red zones vanish). Macro: Coordinated enhancement of rheological parameters and mechanical properties.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Burnwal, K.R.; Singh, A. True-triaxial characterisation of the cement-based grout for application in underground space construction. Constr. Build. Mater. 2025, 487, 141887. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Zhang, Q.S.; Wang, T. Long-term properties of grout-soil composite eroded by seawater environment in submarine tunnel. Tunn. Undergr. Space Technol. 2025, 161, 106519. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Liu, Y.; Qu, L.; Liu, Q.; Sun, Y.; Xu, G. Interfacial porosity model and modification mechanism of broken coal grouting: A theoretical and experimental study. Surf. Interfaces 2022, 33, 102286. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, C.; Zhao, C.; Zheng, Z.; Li, W.; Liu, R.; Li, X.; Wang, H. Diffusion Mechanism in Running-Water and CFD-DEM Numerical Simulation of Expandable Particulate Grouting Material. Materials 2025, 18, 1681. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Li, Z.; Wang, A.; Gao, M.; Gao, Y.; Wang, X. Experimental Study on Water-Plugging Performance of Grouted Concrete Crack. Materials 2024, 17, 1568. [Google Scholar] [CrossRef]
- Sha, F.; Zhang, L.; Zhang, M.; Zuo, Y.; Niu, H. Penetration grouting diffusion and strengthening mechanism of sand layer with crucial grout. J. Build. Eng. 2024, 91, 109585. [Google Scholar] [CrossRef]
- Gomes, S.D.R.; Ferrara, L.; Sánchez, L.; Moreno, M.S. A comprehensive review of cementitious grouts: Composition, properties, requirements and advanced performance. Constr. Build. Mater. 2023, 375, 130991. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Guo, Z.C.H. Optimization of the Injection and Physical Properties of Sulfoaluminate Cement via the In Situ Polymerization of Acrylamide. Buildings 2022, 12, 2237. [Google Scholar] [CrossRef]
- Sui, W.; Liu, Z.; Zhang, G.; Mao, D.; Li, R.; Liang, J. Experimental investigation on grout propagation in poured aggregates for controlling water inrush in tunnels with flowing water. Geoenviron. Disasters 2023, 10, 15. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Fu, J.; Wang, S.; Xie, Y. Recycling of discharged soil from EPB shield tunnels as a sustainable raw material for synchronous grouting. J. Clean. Prod. 2020, 268, 121947. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J. Effects of Polymer-Curing Agent Ratio on Rheological, Mechanical Properties and Chemical Characterization of Epoxy-Modified Cement Composite Grouting Materials. Polymers 2024, 16, 2665. [Google Scholar] [CrossRef]
- Mahmood, W.; Mohammed, A.S.; Asteris, P.G.; Kurda, R.; Armaghani, D.J. Modeling Flexural and Compressive Strengths Behaviour of Cement-Grouted Sands Modified with Water Reducer Polymer. Appl. Sci. 2022, 12, 1016. [Google Scholar] [CrossRef]
- Bahraq, A.A.; Al-Osta, M.A.; Al-Amoudi, O.S.B.; Saleh, T.A.; Obot, I.B. Atomistic simulation of polymer-cement interactions: Progress and research challenges. Constr. Build. Mater. 2022, 327. [Google Scholar] [CrossRef]
- Fan, L.; Xu, F.; Wang, S.; Yu, Y.; Zhang, J.; Guo, J. A review on the modification mechanism of polymer on cement-based materials. J. Mater. Res. Technol. 2023, 26, 5816–5837. [Google Scholar] [CrossRef]
- Su, F.; He, T.; He, Z.; Yu, Q.; Wang, H. Mechanism of Acrylate Emulsion-Modified Cement-Based Materials. Molecules 2024, 29, 1260. [Google Scholar] [CrossRef]
- Su, F.; Wang, H.; Ma, X.; He, T.; Lin, Y. Review of the Influence of Acrylate Lotion on the Properties of Cement-Based Materials. Materials 2023, 16, 6597. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, B.; Zhang, Y.; Wang, M.; Miao, G.; Zhou, A. A Review of Waterborne Polymer–Cementitious Composite Repair Materials for Application in Saline Soil Environments: Properties and Progress. Buildings 2024, 14, 848. [Google Scholar] [CrossRef]
- Chen, B.M.; Qiao, G.; Hou, D.S.; Wang, M.H.; Li, Z.J. Cement-based material modified by in-situ polymerization: From experiments to molecular dynamics investigation. Compos. Part B Eng. 2020, 19, 108036. [Google Scholar] [CrossRef]
- Tran, N.P.; Nguyen, T.N.; Ngo, T.D. The role of organic polymer modifiers in cementitious systems towards durable and resilient infrastructures: A systematic review. Constr. Build. Mater. 2022, 360, 129562. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.L.; Xu, T.J. Research on Sodium Polyacrylate and its Effects on Mechanical Properties of Slag-Based Geopolymer. Appl. Mech. Mater. 2014, 638, 1387–1390. [Google Scholar] [CrossRef]
- Robles, P.; Piceros, E.; Leiva, W.H.; Valenzuela, J.; Toro, N.; Jeldres, R.I. Analysis of sodium polyacrylate as a rheological modifier for kaolin suspensions in seawater. Appl. Clay Sci. 2019, 183, 105328. [Google Scholar] [CrossRef]
- Hwang, K.S.; Jung, M.G.; Jang, S.S.; Yong, W.J.; Lee, S.H.; Ha, K.R. Inverse Emulsion Polymerization of Water Absorbent Polymer for Strength Enhancement of Mortars. Polymer 2010, 34, 434–441. [Google Scholar]
- Gu, L.; Liu, T.; Wu, K.; Yang, Z.; Wen, Z.; Zhang, Z.; De, S.G.; Li, H. Comparative study on the role of PAM and PANA on the property of fresh cement paste. Cem. Concr. Compos. 2022, 133, 104701. [Google Scholar] [CrossRef]
- Han, J.; Hu, M.; Ying, Y.; Liu, M.; Yan, X.; Guo, J. Efficient healing of existed cracks in cement via synergistic effects of cement matrix activation and monomer polymerization. Constr. Build. Mater. 2023, 406, 133394. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, W. Carbonation and Electrochemical Corrosion Resistance of Mild Steel Rebar in Concrete Modified by Sodium Polyacrylate as Super Absorbent Polymer exposed to 3.5 wt% NaCl Solution. Int. J. Electrochem. Sci. 2021, 16, 210619. [Google Scholar] [CrossRef]
- Özbakan, N.; Evirgen, B. The effect of sodium polyacrylate gel on the properties of liquefiable sandy soil under seismic condition. J. Eng. Res. 2023, 11, 100006. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Ma, W.; Liu, R.; Liu, Y.; Zhang, C.; Zhu, Z. Comparative study on the properties of cement grout modified with PAM and PANA at elevated temperatures. Powder Technol. 2024, 433, 119256. [Google Scholar] [CrossRef]
- Tang, C.Y.; Dang, Z.H.; Lu, T.L.; Ye, J.D. A novel anti-washout curing solution of calcium phosphate cement prepared via irradiation polymerization. J. Mater. Chem. B 2023, 11, 7410–7423. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Y.; Ming, X.; Liu, Q.; Li, Z.; Chen, B. Yield stress of in-situ polymerization modified cement paste. Cem. Concr. Res. 2023, 174, 107346. [Google Scholar] [CrossRef]
- GB/T 175-2007; Common Portland Cement. National Standards of People’s Republic of China: Beijing, China, 2007.
- ASTM C1602/C1602M; Standard Specification for Mixing Water Used in the Production of Hydraulic Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM C942-2015; Standard Test Method for Compressive Strength of Grouts for Preplaced-Aggregate Concrete in the Laboratory. ASTM International: West Conshohocken, PA, USA, 2015.
- GB/T 50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. National Standards of People’s Republic of China: Beijing, China, 2019.
- GB/T 8077-2012; Methods for Testing Uniformity of Concrete Admixture. National Standards of People’s Republic of China: Beijing, China, 2007.
- GB/T 27788-2020; Microbeam Analysis—Scanning Electron Microscopy—Guidelines for Calibrating Image Magnification. National Standards of People’s Republic of China: Beijing, China, 2020.
- Zhou, Z.; Li, Z.; Gao, X.; Liu, C.; Yang, L. Study on properties of Portland cement-sulfoaluminate cement-based grouting materials modified by in-situ polymerization of calcium acrylate. Constr. Build. Mater. 2023, 409, 134087. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Y.; Peng, Y.; Wang, J.; Zhang, Z.; Gui, Q.; Zeng, Q. Multi-scale structure of in-situ polymerized cementitious composites with improved flowability, strength, deformability and anti-permeability. Compos. Part B Eng. 2022, 245, 110222. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, J.; Yuan, Q.; Que, Y.; Chen, Z.; Zhang, C. Rheological Properties of Fresh Cement Paste Modified by In Situ Polymerization of Acrylamide Monomer. J. Mater. Civ. Eng. 2023, 35, 04023452. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Zhang, C.; Kong, X.; Xie, Y. Effect of latex polymers with different functional monomers on time-dependent rheological properties of fresh cement pastes. Constr. Build. Mater. 2025, 491, 142710. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q. Investigation on fundamental properties and chemical characterization of water-soluble epoxy resin modified cement grout. Constr. Build. Mater. 2021, 299, 123877. [Google Scholar] [CrossRef]
- Yin, B.; Hua, X.; Qi, D.; Wang, P.; Qiao, G.; Fan, F.; Hua, X.; Wang, X.; Hou, D. Performance cement-based composite obtained by in-situ growth of organic–inorganic frameworks during the cement hydration. Constr. Build. Mater. 2022, 336, 127533. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, S.; Zhao, S.; Guan, X. Synthesis of a chitosan-based superabsorbent polymer and its influence on cement paste. Int. J. Biol. Macromol. 2024, 282, 136676. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qiang, S.; Liang, J.; Li, L.; Shi, Y.; Nie, J.; Chen, T.; Yao, S.; Zhang, M. Cellulose nanofibril/mineral composites induced by H-bond/ionic coordination in co-refining system. Carbohydr. Polym. 2022, 289, 119425. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, S.; Liu, S. Effect of different curing temperatures on the hydration of polyacrylate emulsion polymer-modified cement. J. Build. Eng. 2024, 94, 109938. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, L.; Huang, A.; Li, B.; Sun, Y.; Jiang, Z.; Li, W.; Zhu, H. Healing of concrete cracks by in-situ synthesis of ettringite induced by electric field. Constr. Build. Mater. 2022, 352, 128685. [Google Scholar] [CrossRef]
- Liao, J.; Wang, J.; Wang, J.; Chen, W.; Peng, B.; Kong, X. Moisture sorption and mechanical properties of polymer-cement waterproofing membranes investigated by LF NMR. Constr. Build. Mater. 2024, 449, 138188. [Google Scholar] [CrossRef]
- Han, X.; Xu, F.; He, Y.; Qian, W.; Lu, J.; Ge, J.; Li, H.; Meng, X. Research on the improvement of underwater workability of cement paste by composite polymers. Case Stud. Constr. Mater. 2025, 22, e04386. [Google Scholar] [CrossRef]
- Xie, Z.; Yao, H.; Yuan, Q.; Zhong, F. The roles of water-soluble polymers in cement-based materials: A systematic review. J. Build. Eng. 2023, 73, 106811. [Google Scholar] [CrossRef]
- Zhang, X.; Du, M.; Fang, H.; Shi, M.; Zhang, C.; Wang, F. Polymer-modified cement mortars: Their enhanced properties, applications, prospects, and challenges. Constr. Build. Mater. 2021, 299, 124290. [Google Scholar] [CrossRef]



| Density/ (kg/m3) | Chemical Composition/% | |||||
|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | |
| 3150 | 63 | 21.23 | 6.58 | 1.6 | 3.65 | 2.93 |
| No. | Proportion | Cement (g) | Water (g) | PAAS (g) |
|---|---|---|---|---|
| 1 | W/C 0.8, P/C 0% | 100 | 80 | 0 |
| 2 | W/C 0.8, P/C 0.02% | 100 | 80 | 0.02 |
| 3 | W/C 0.8, P/C 0.04% | 100 | 80 | 0.04 |
| 4 | W/C 0.8, P/C 0.06% | 100 | 80 | 0.06 |
| 5 | W/C 0.8, P/C 0.08% | 100 | 80 | 0.08 |
| 6 | W/C 0.8, P/C 0.1% | 100 | 80 | 0.1 |
| PAAS Content/% | Average Diameter (cm) | Coefficient of Variation/% | Increase Compared to the Control Group |
|---|---|---|---|
| 0 | 19.23 ± 1.28 | 6.7 | - |
| 0.02 | 24.30 ± 1.15 | 4.7 | +26.4% |
| 0.04 | 23.75 ± 0.30 | 1.3 | +23.5% |
| 0.06 | 24.55 ± 1.15 | 4.7 | +27.7% |
| 0.08 | 25.13 ± 0.13 | 0.5 | +30.7% |
| 0.10 | 25.06 ± 0.27 | 1.1 | +30.3% |
| PAAS Content/% | Yield Stress τ (Pa) | Plastic Viscosity η (Pa·s) | R2 |
|---|---|---|---|
| 0% | 4.82 | 0.052 | 0.991 |
| 0.02% | 2.37 | 0.041 | 0.998 |
| 0.04% | 1.65 | 0.045 | 0.997 |
| 0.06% | 2.03 | 0.046 | 0.993 |
| 0.08% | 1.28 | 0.043 | 0.999 |
| 0.10% | 0.95 | 0.040 | 0.996 |
| PAAS Content | 0 | 0.02 | 0.06 | 0.1 |
|---|---|---|---|---|
| Porosity | 44.37 | 43.91 | 43.37 | 43.91 |
| Samples | PAAS Content (%) | Curing Age (d) | Compressive Strength (MPa) | Strength Increase (%) | Flexural Strength (MPa) | Strength Increase (%) |
|---|---|---|---|---|---|---|
| A1 | 0 | 3 | 4.79 | 0 | - | - |
| B1 | 0.02 | 3 | 3.48 | −27.3 | - | - |
| C1 | 0.04 | 3 | 3.96 | −17.3 | - | - |
| D1 | 0.06 | 3 | 3.94 | −17.7 | - | - |
| E1 | 0.08 | 3 | 3.54 | −26.1 | - | - |
| F1 | 0.1 | 3 | 3.49 | −27.1 | - | - |
| A2 | 0 | 7 | 6.41 | 0 | - | - |
| B2 | 0.02 | 7 | 6.1 | −4.8 | - | - |
| C2 | 0.04 | 7 | 5.82 | −9.2 | - | - |
| D2 | 0.06 | 7 | 6.58 | 2.7 | - | - |
| E2 | 0.08 | 7 | 6.44 | 0.5 | - | - |
| F2 | 0.1 | 7 | 6.08 | −5.1 | - | - |
| A3 | 0 | 28 | 12.33 | 0 | 4.44 | 0 |
| B3 | 0.02 | 28 | 14.98 | 21.5 | 3.34 | −24.8 |
| C3 | 0.04 | 28 | 15.56 | 26.2 | - | - |
| D3 | 0.06 | 28 | 14.16 | 14.8 | 5.74 | 29.3 |
| E3 | 0.08 | 28 | 14.03 | 13.8 | - | - |
| F3 | 0.1 | 28 | 13.77 | 11.7 | 4.38 | −1.4 |
| Source of Variation | Sum of Squares | Mean Square | F | p | R2 | Adeq Precision |
|---|---|---|---|---|---|---|
| Model | 345.30 | 172.65 | 269.61 | <0.0001 | 0.973 | 31.041 |
| c | 0.0506 | 0.0506 | 0.0838 | 0.7825 | ||
| D | 345.25 | 345.25 | 539.14 | <0.0001 | ||
| Residual | 9.61 | 0.6404 | ||||
| Cor Total | 354.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wang, Y.; Zhang, N.; Yu, Z. Multi-Scale Modification of Sodium Polyacrylate-Modified Cement Grouts: Rheology, Microstructure, and Mechanical Properties. Buildings 2025, 15, 3360. https://doi.org/10.3390/buildings15183360
Yu H, Wang Y, Zhang N, Yu Z. Multi-Scale Modification of Sodium Polyacrylate-Modified Cement Grouts: Rheology, Microstructure, and Mechanical Properties. Buildings. 2025; 15(18):3360. https://doi.org/10.3390/buildings15183360
Chicago/Turabian StyleYu, Hui, Yuxuan Wang, Nianzu Zhang, and Zhiyuan Yu. 2025. "Multi-Scale Modification of Sodium Polyacrylate-Modified Cement Grouts: Rheology, Microstructure, and Mechanical Properties" Buildings 15, no. 18: 3360. https://doi.org/10.3390/buildings15183360
APA StyleYu, H., Wang, Y., Zhang, N., & Yu, Z. (2025). Multi-Scale Modification of Sodium Polyacrylate-Modified Cement Grouts: Rheology, Microstructure, and Mechanical Properties. Buildings, 15(18), 3360. https://doi.org/10.3390/buildings15183360





