Utilising High-Ambient-Temperature Curing in the Development of Low-Calcium Geopolymers
Abstract
1. Introduction
2. Experimental Programme
2.1. Materials
2.2. Sample Preparation
2.3. Curing
2.4. Experimental Methods
3. Results and Discussion
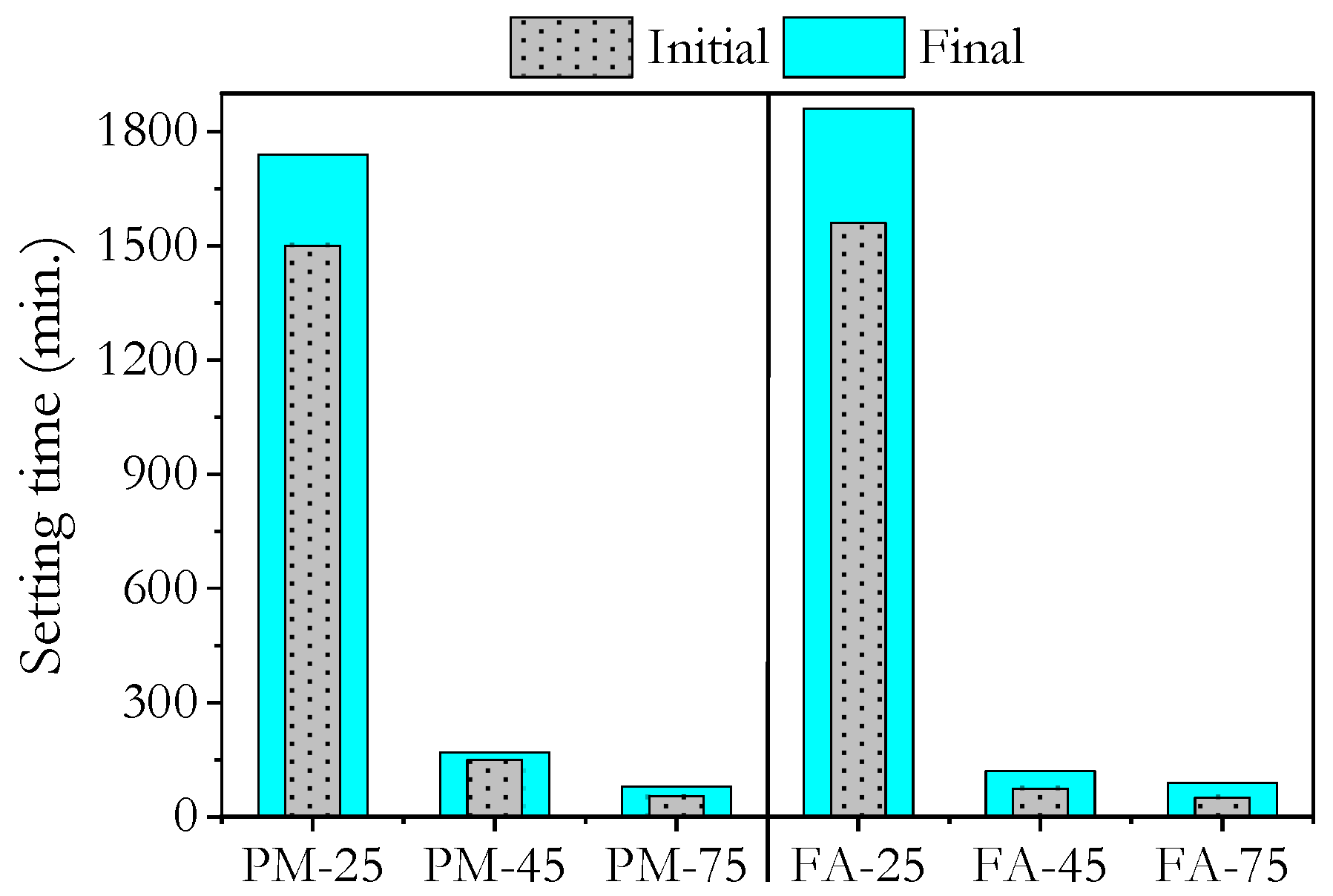
3.1. Setting Times
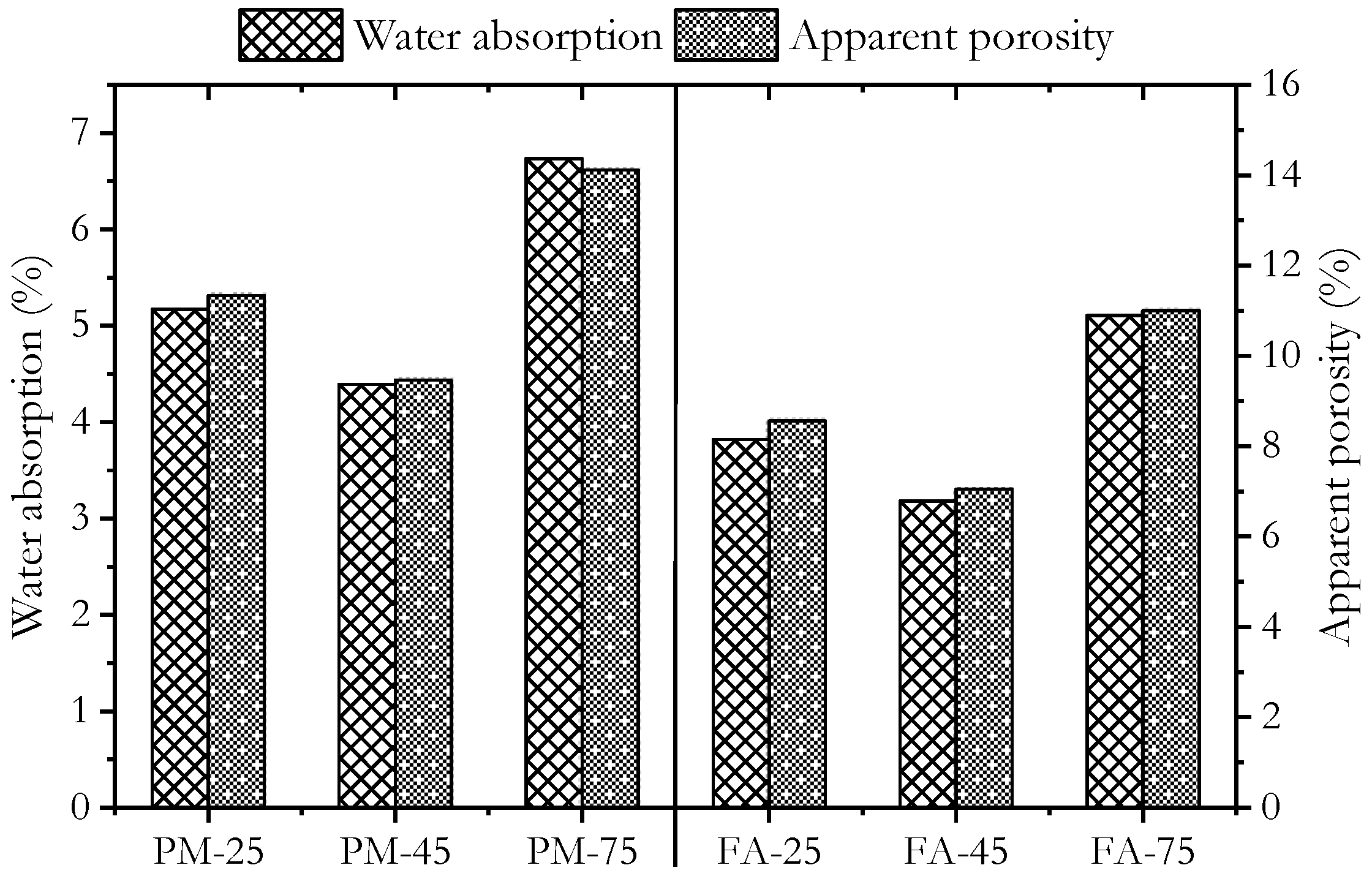
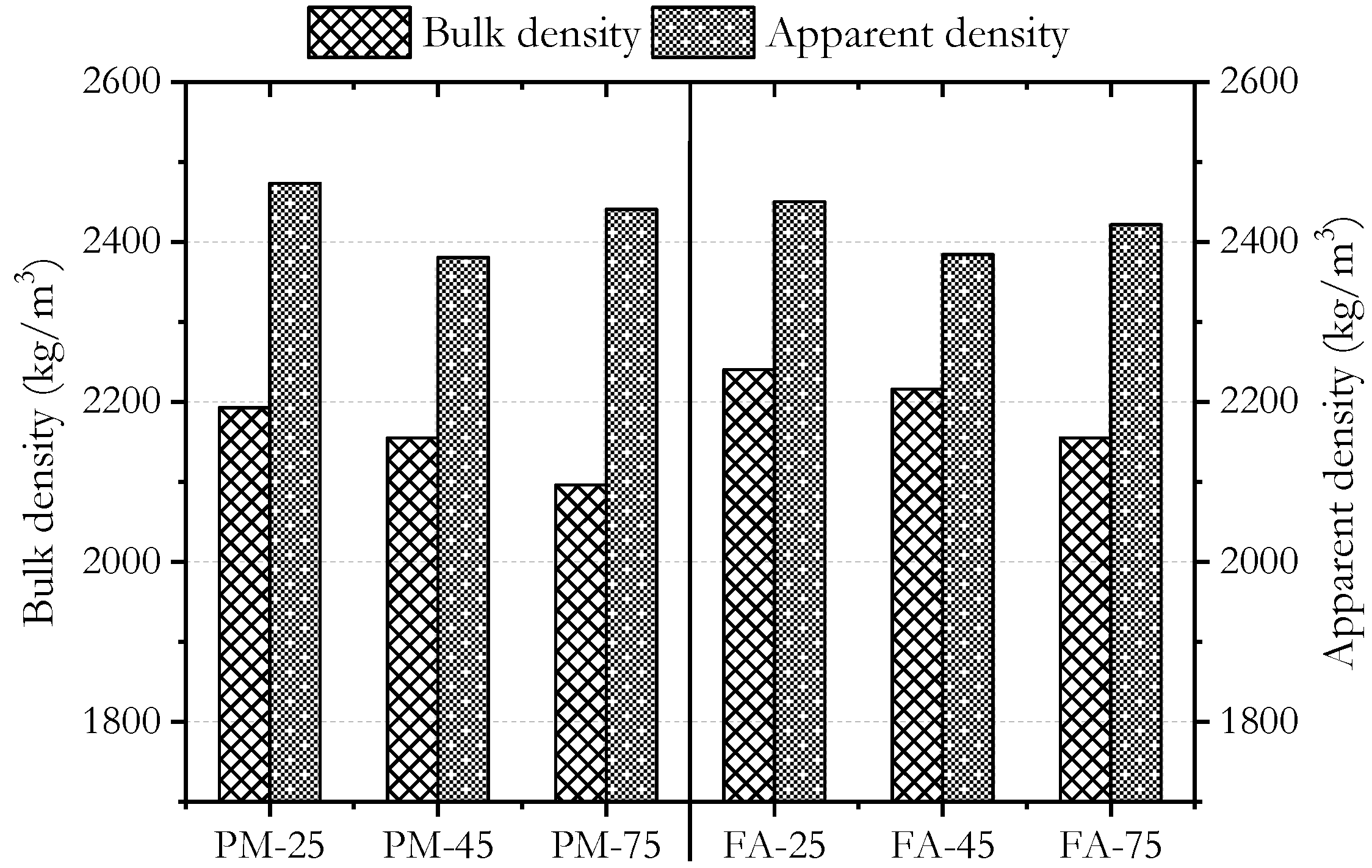
3.2. Physical Properties
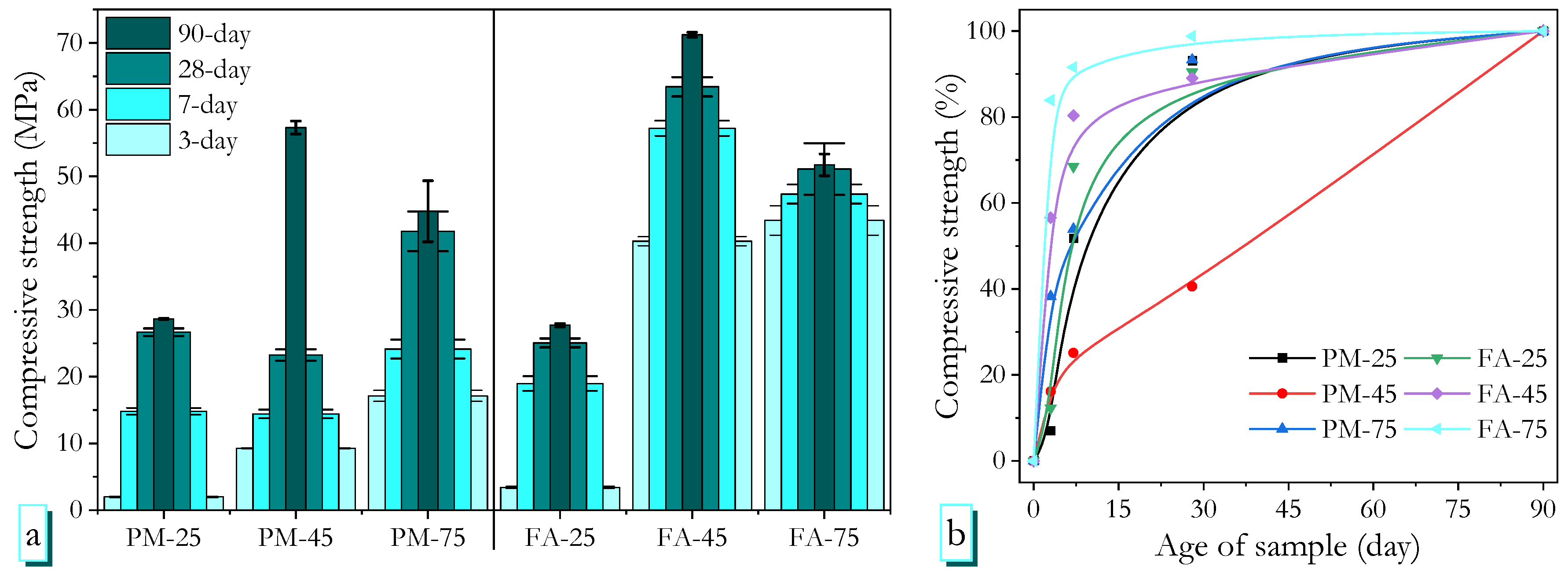
3.3. Compressive Strength Development
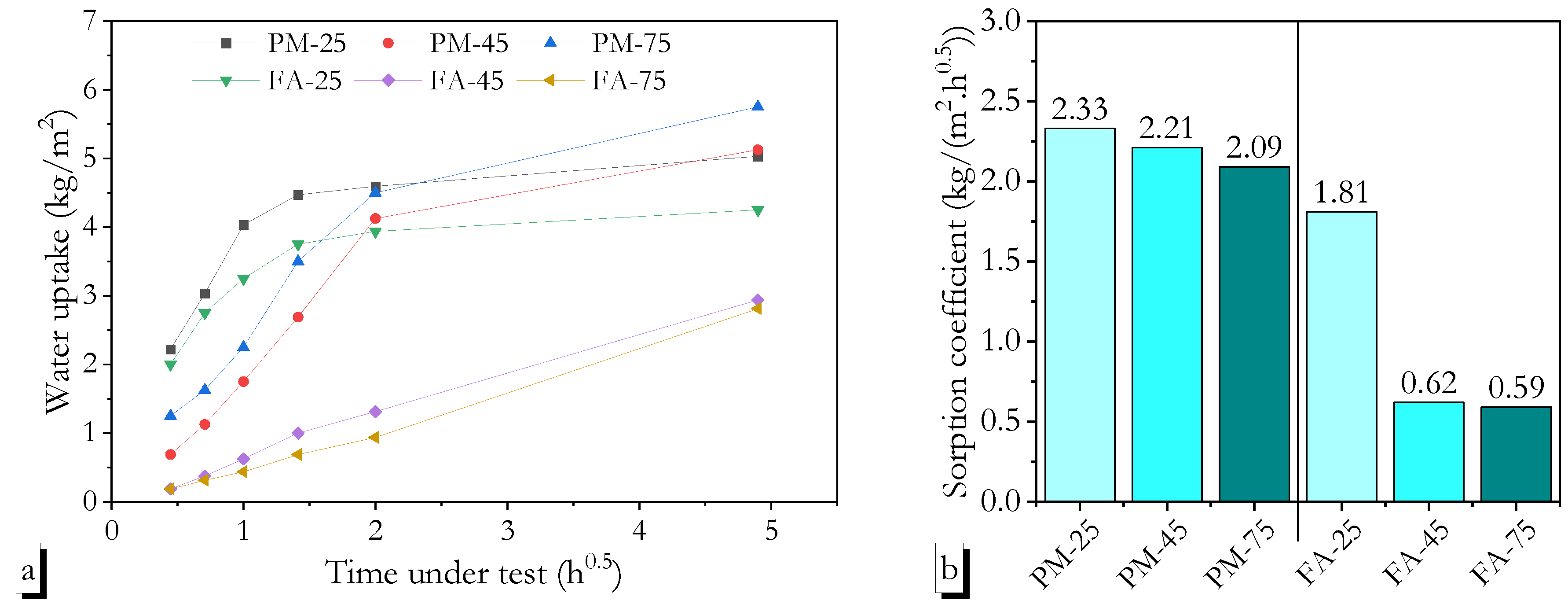
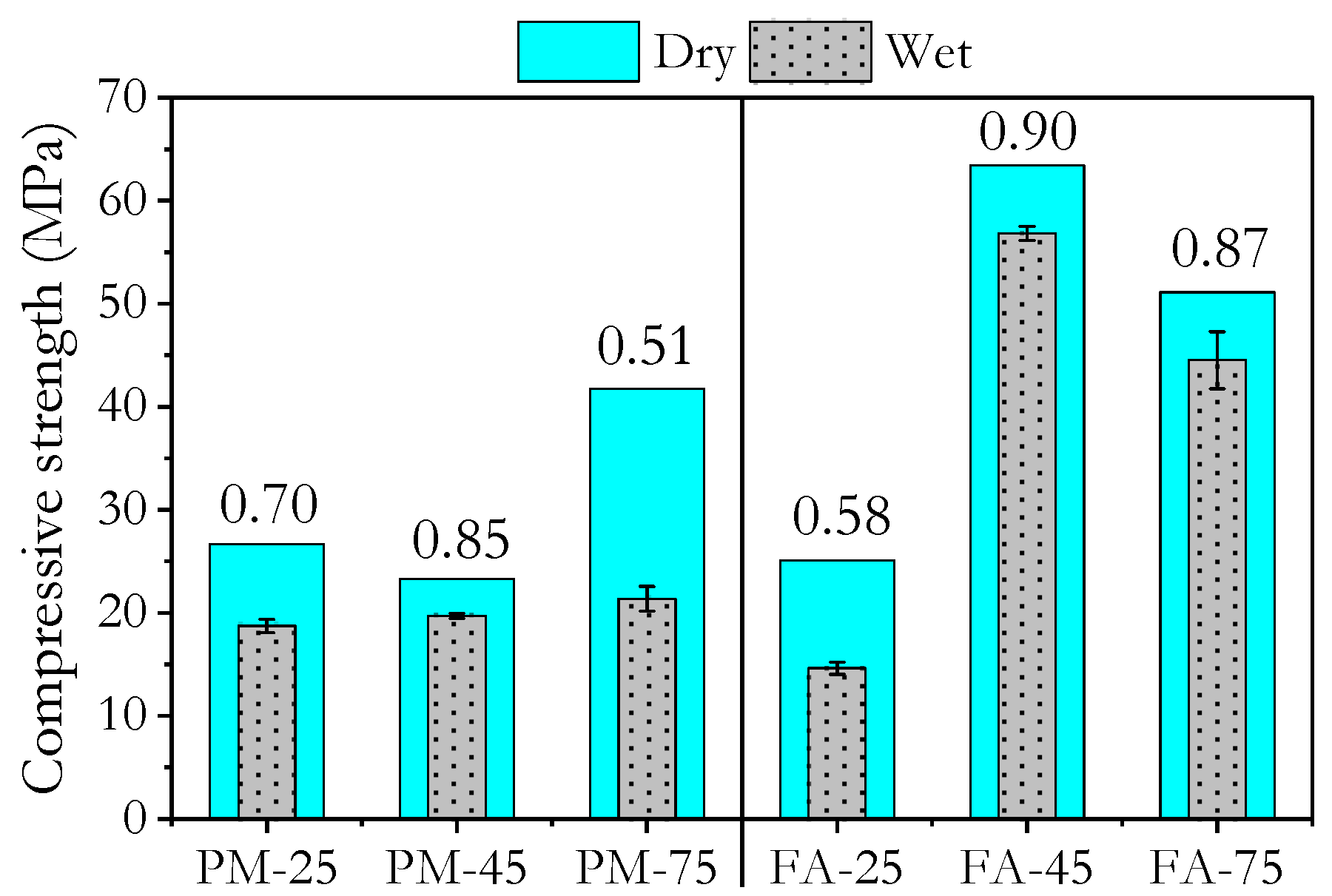
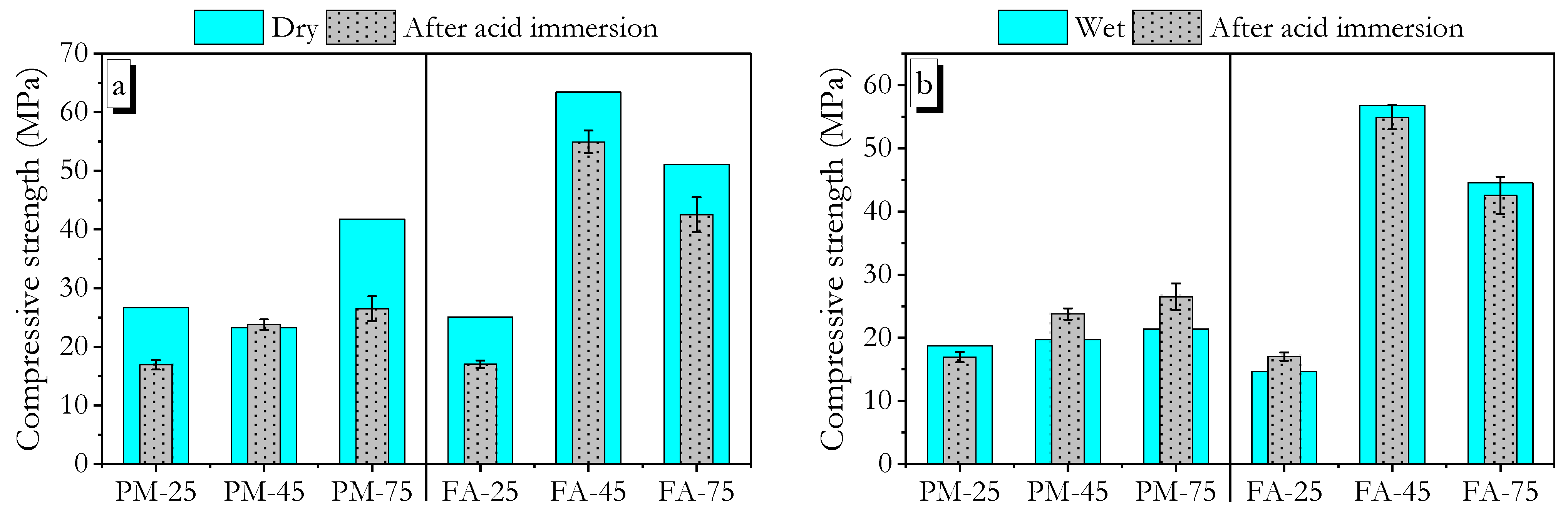
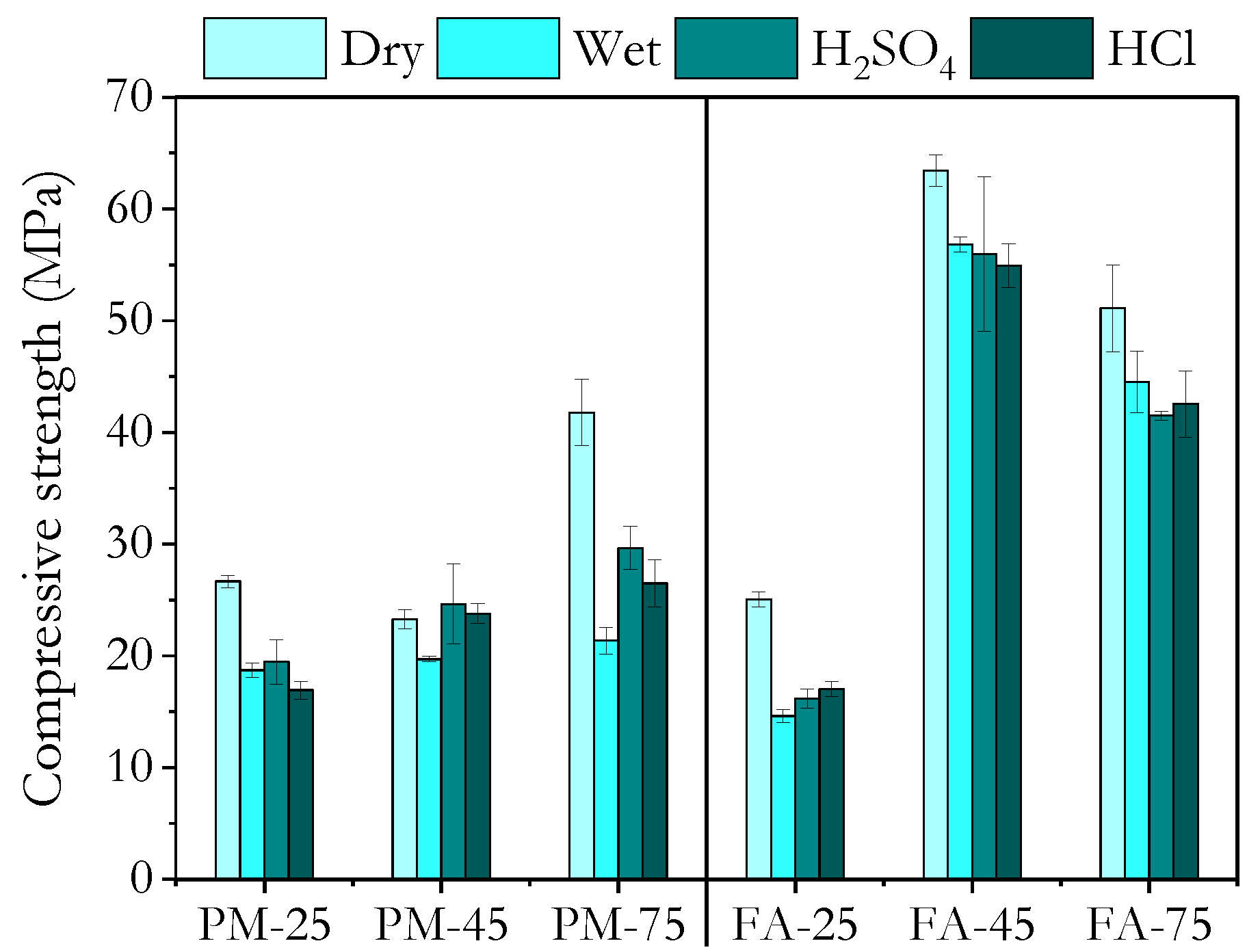
3.4. Water Resistance
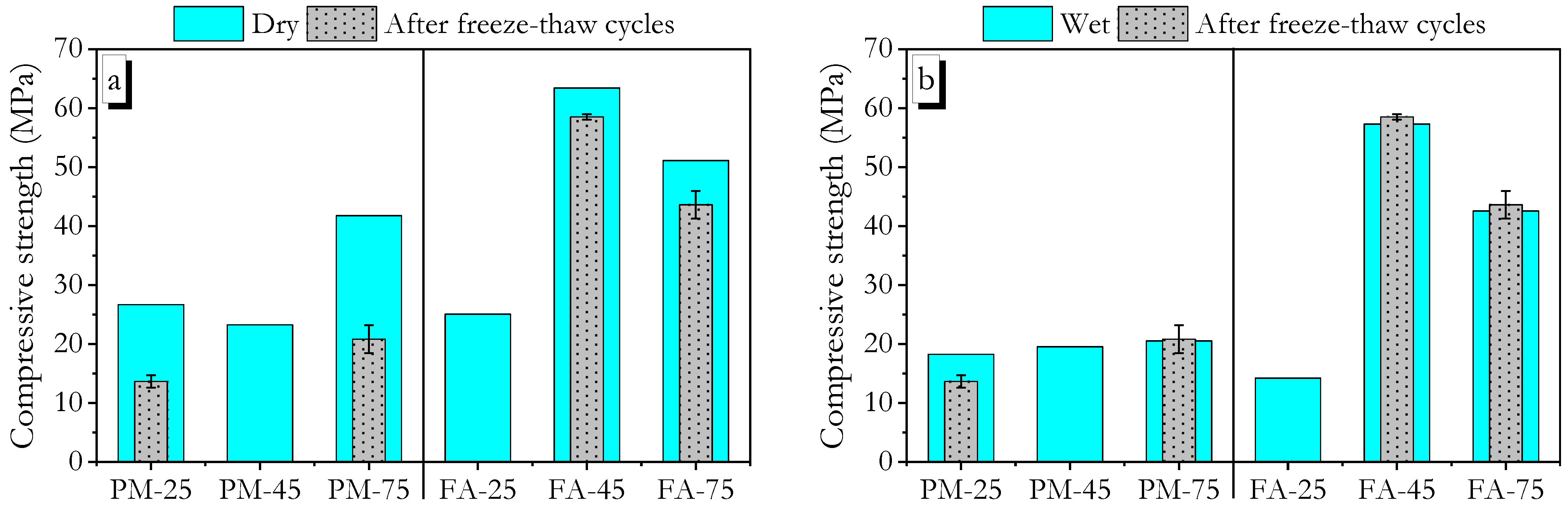
3.5. Freeze–Thaw Durability
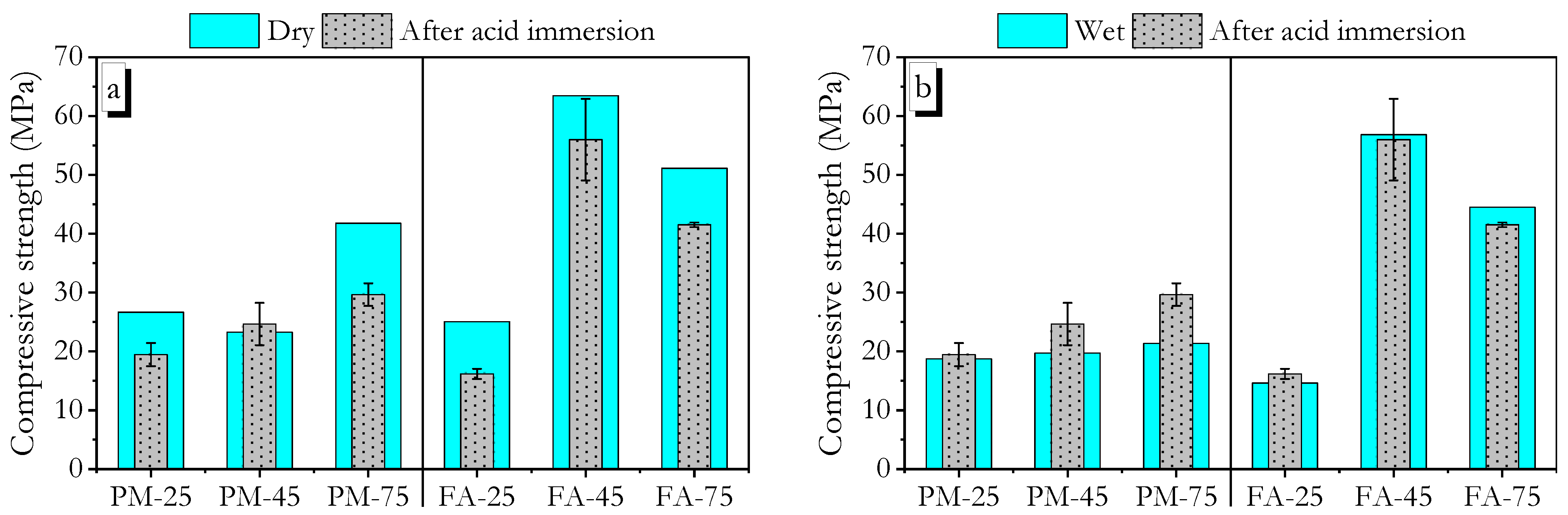
3.6. Acid Resistance
3.6.1. Sulphuric Acid
3.6.2. Hydrochloric Acid
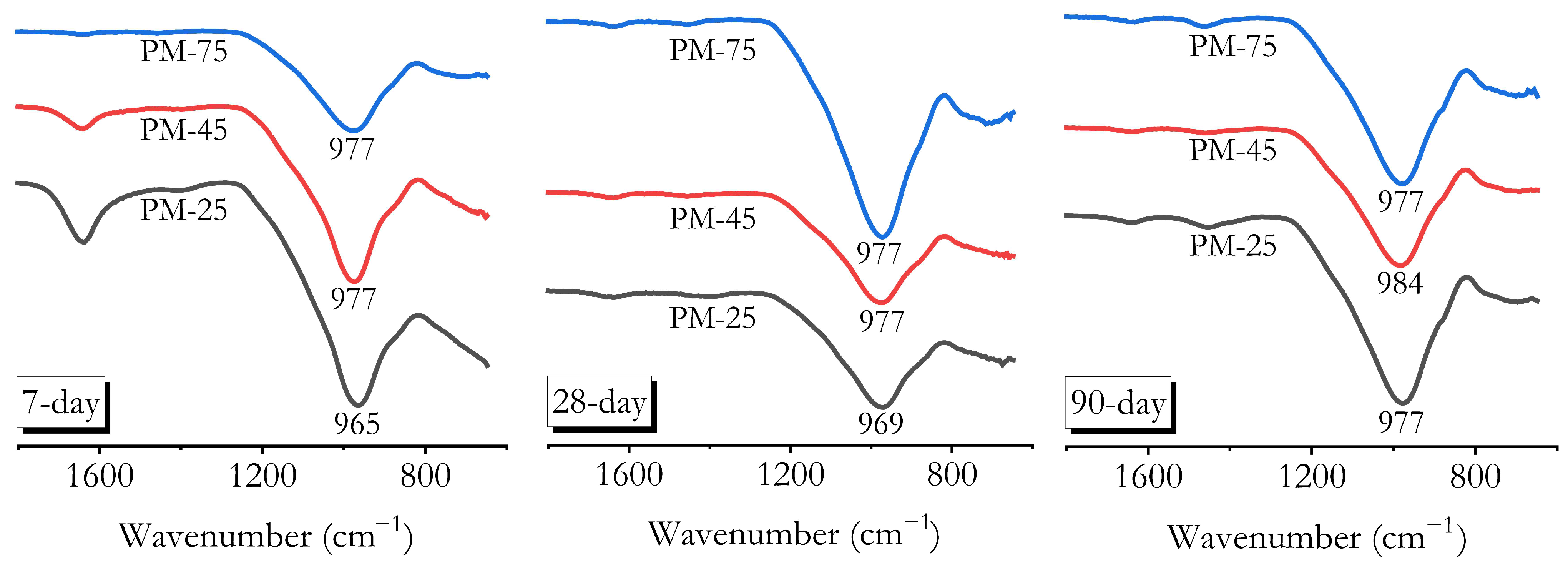
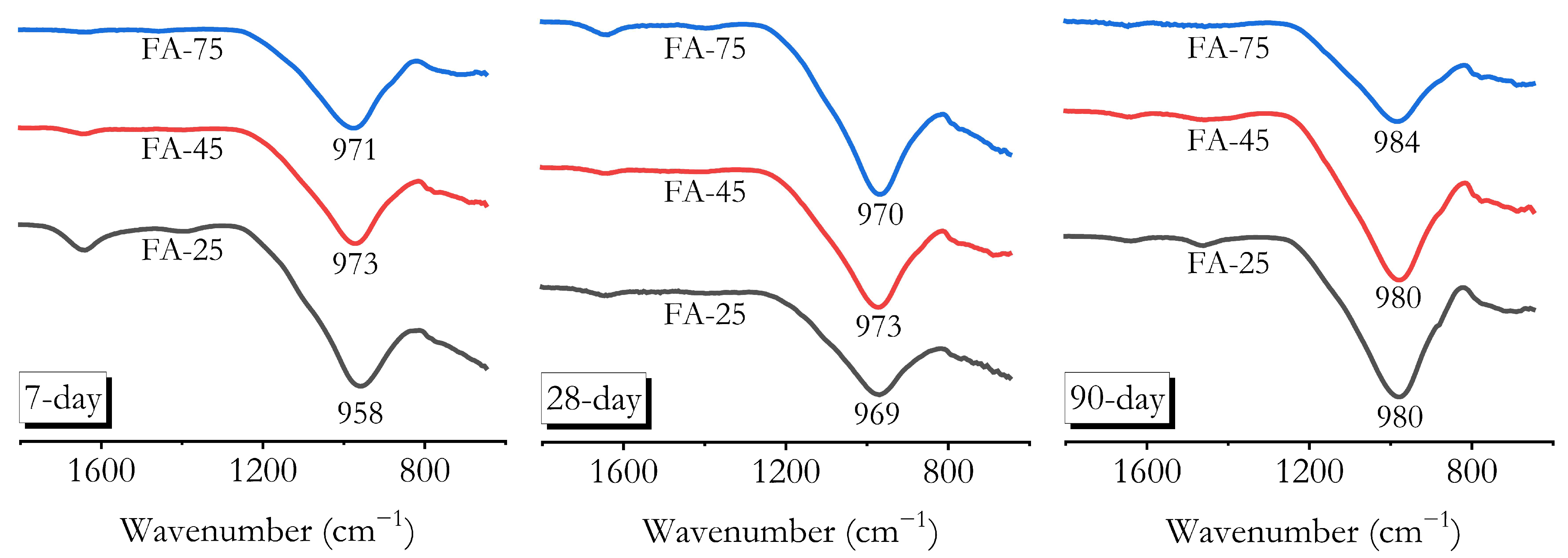
3.7. Chemical Bonding Characterisation by FTIR
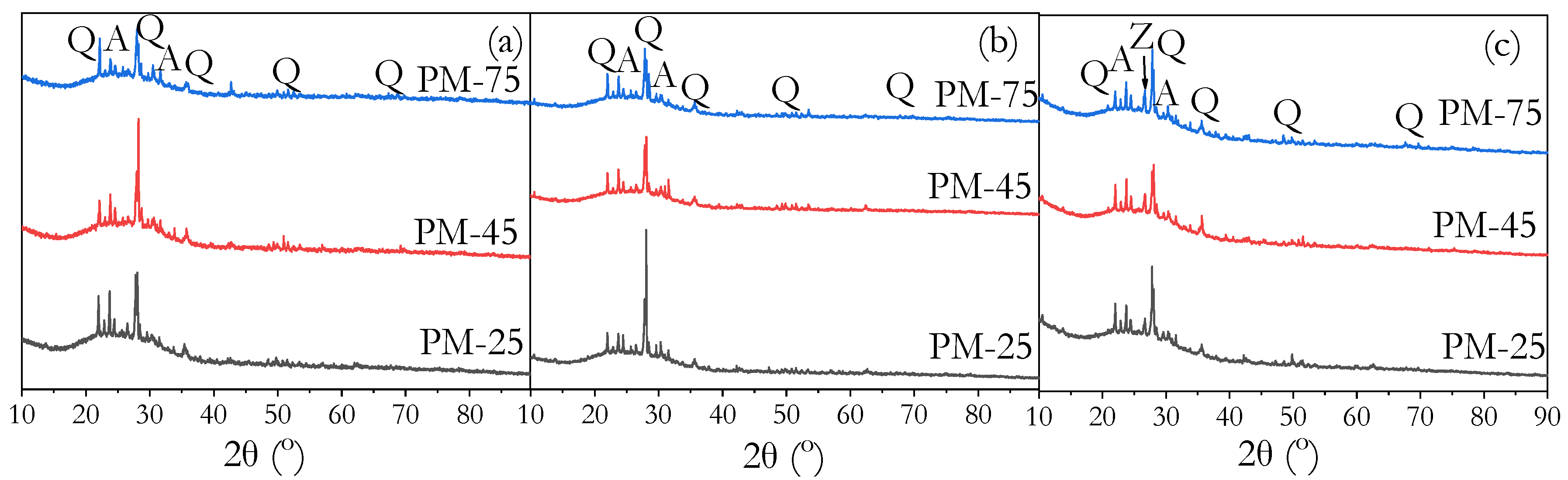
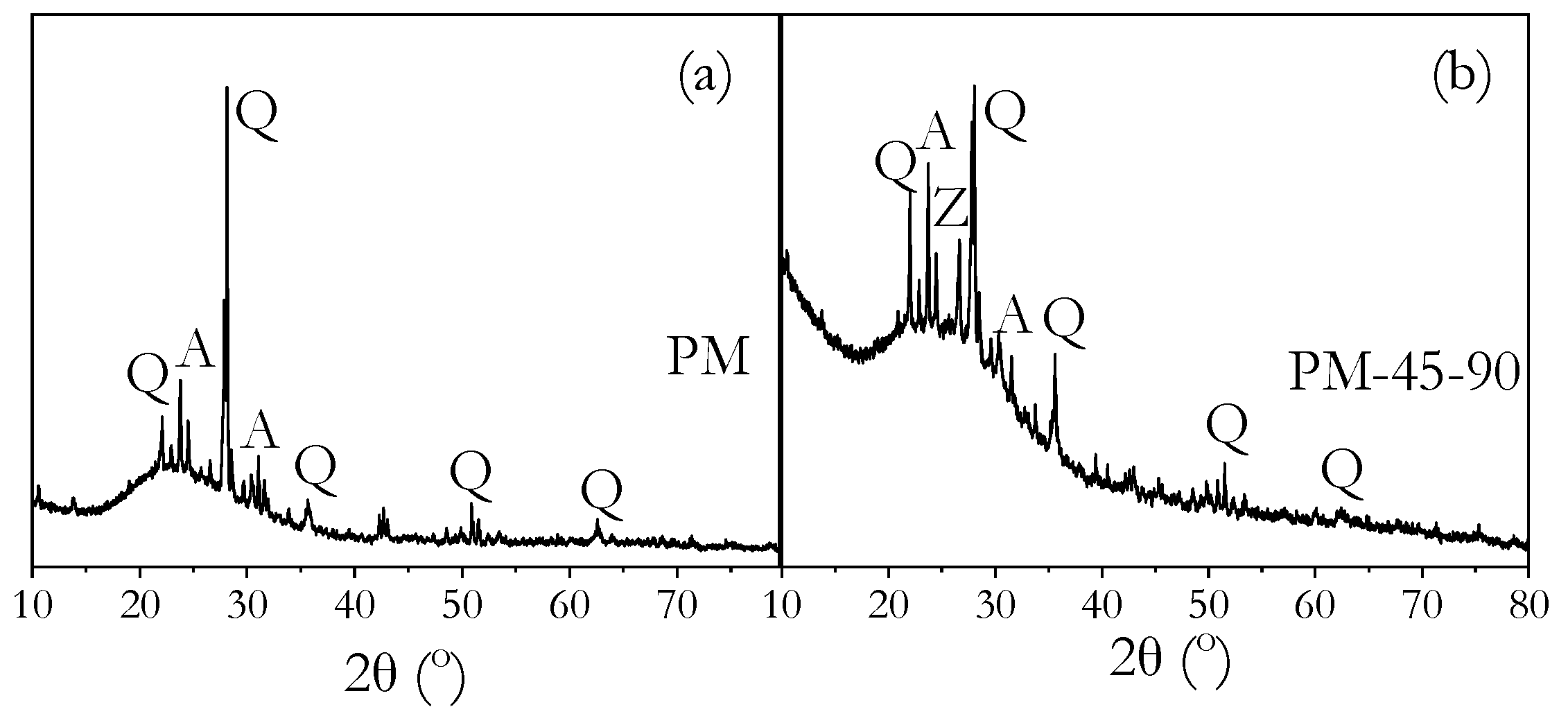
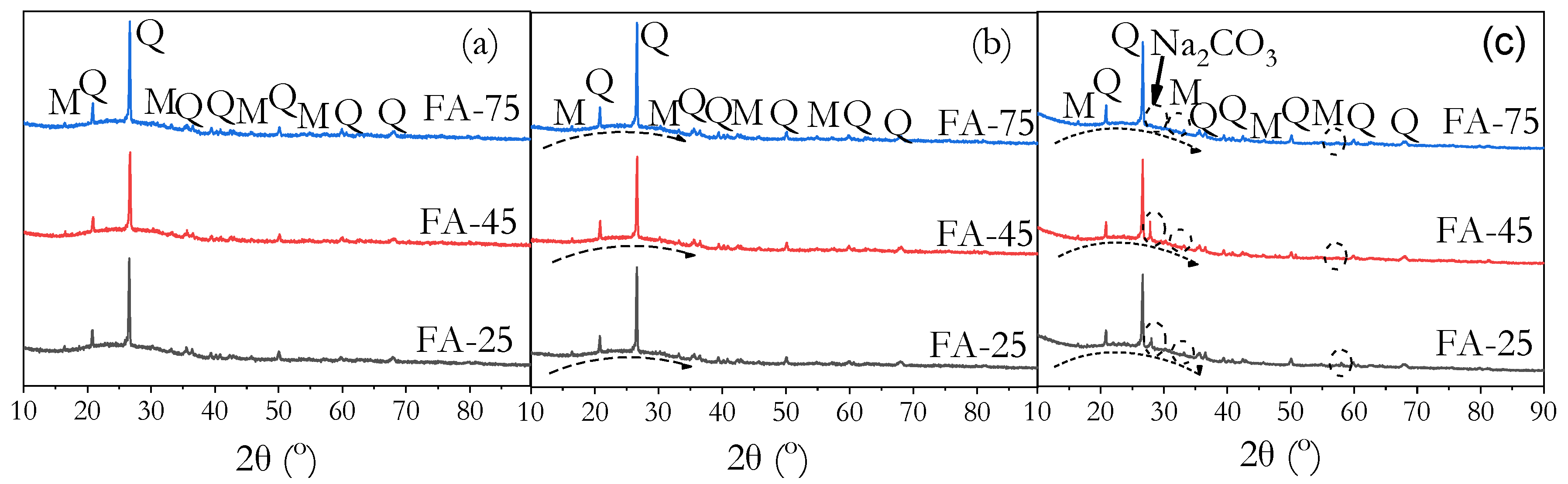
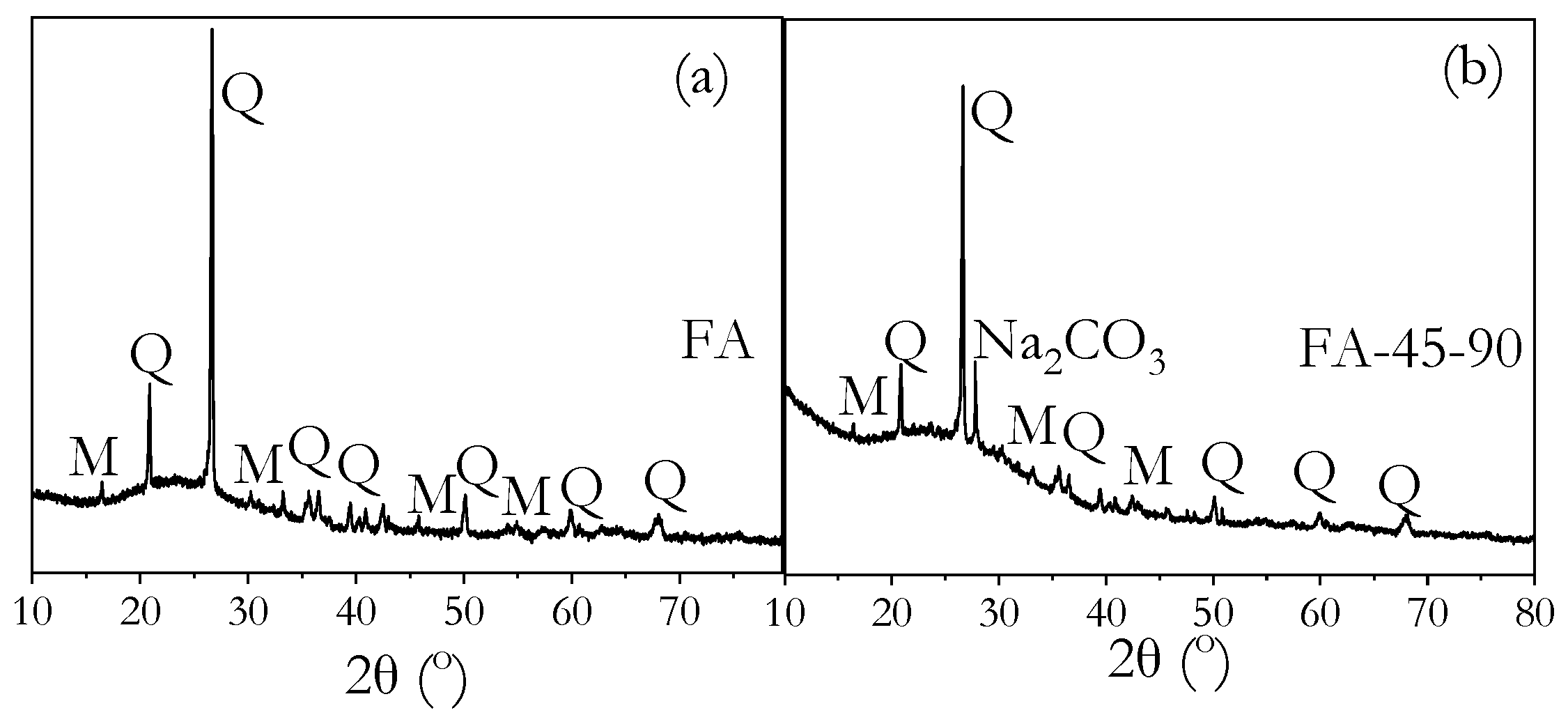
3.8. Phase Identification by XRD
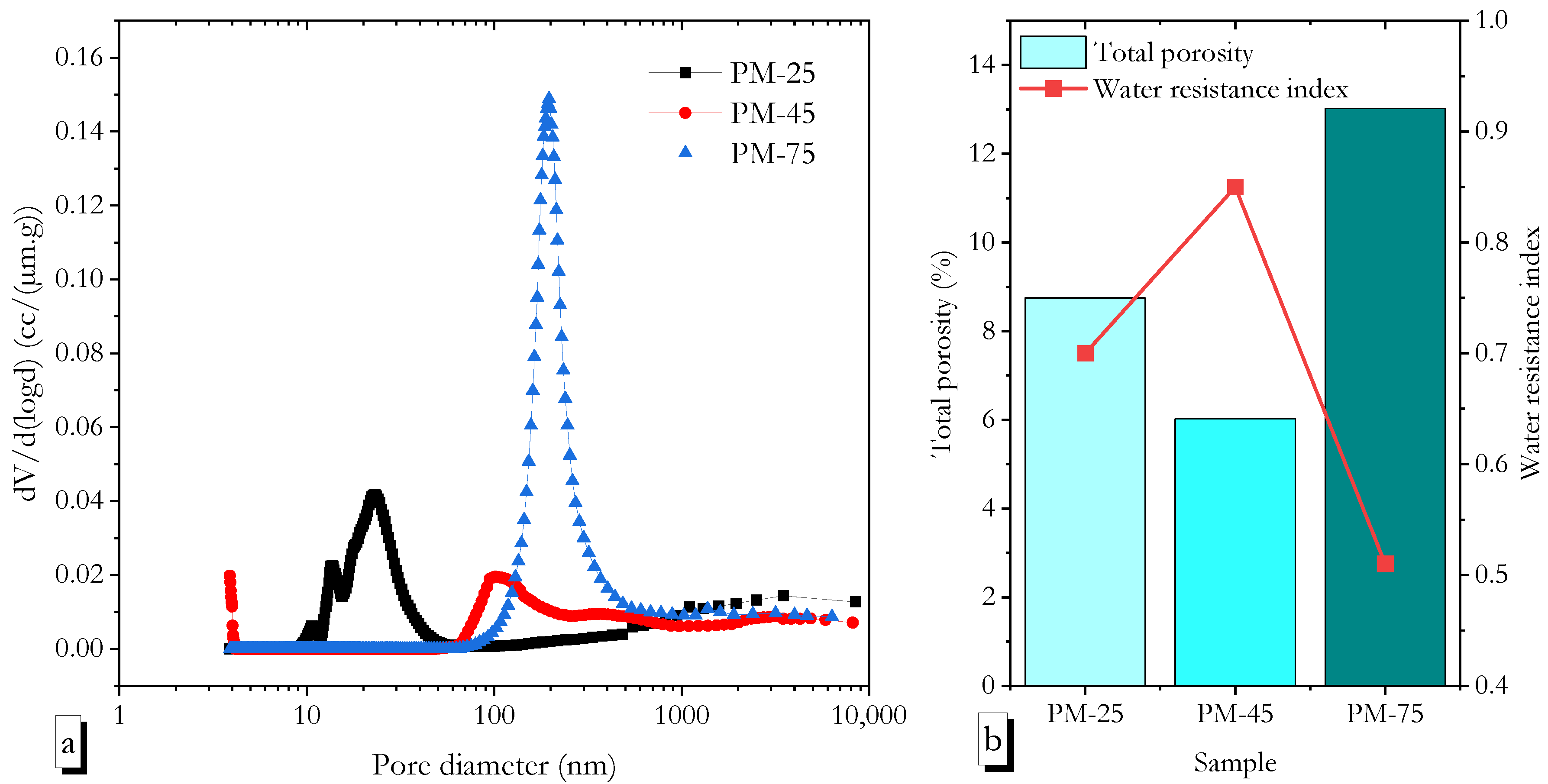
3.9. Pore Structure Evaluation via MIP
4. Conclusions
- High ambient curing allowed low-calcium geopolymers to set within 80–90 min, offering a significant advantage for in situ applications compared to ambient-cured samples, which took over 24 h.
- Low-calcium geopolymers cured at high ambient temperatures exhibited lower water absorption than those cured under other regimes, while their sorptivity was similar to heat-cured specimens.
- High-ambient-temperature curing was more advantageous than heat curing in terms of long-term strength for PMGP, whereas FAGP showed comparable performance even at early ages. PMGP cured at high ambient temperature achieved a 90-day strength of 57.3 MPa, which is 28.0% higher than its heat-cured counterpart. In contrast, FAGP reached 40.3 MPa at 3 days (comparable to heat-cured samples) and 57.2 MPa at 7 days, 24.2% higher than heat-cured FAGP.
- Compressive strength measurements in the wet state after exposure to tap water, 5 wt.% sulphuric acid, and 5 wt.% hydrochloric acid showed that high ambient-cured PMGP and FAGP, as well as heat-cured FAGP, retained strengths close to their dry-state values. This indicates the formation of a robust geopolymer gel.
- Geopolymers with high initial compressive strength generally demonstrated better freeze–thaw resistance. However, high ambient-cured PMGP, which had lower early strength (28-day), was not resistant to freeze–thaw cycles.
- FTIR, XRD, and MIP analyses confirmed that high ambient curing sustained geopolymerisation over 90 days without forming the harmful pores often associated with heat curing. The continuous formation of geopolymer gels filled capillary pores, reducing both their volume and connectivity, leading to a more refined pore structure compared to ambient-cured samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hwang, C.-L.; Vo, D.-H.; Tran, V.-A.; Yehualaw, M.D. Effect of high MgO content on the performance of alkali-activated fine slag under water and air curing conditions. Constr. Build. Mater. 2018, 186, 503–513. [Google Scholar] [CrossRef]
- Balun, B.; Karataş, M. Influence of curing conditions on pumice-based alkali activated composites incorporating Portland cement. J. Build. Eng. 2021, 43, 102605. [Google Scholar] [CrossRef]
- Ayeni, O.; Onwualu, A.P.; Boakye, E. Characterization and mechanical performance of metakaolin-based geopolymer for sustainable building applications. Constr. Build. Mater. 2021, 272, 121938. [Google Scholar] [CrossRef]
- Albidah, A.; Alqarni, A.S.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Behavior of Metakaolin-Based geopolymer concrete at ambient and elevated temperatures. Constr. Build. Mater. 2022, 317, 125910. [Google Scholar] [CrossRef]
- Kürklü, G. The effect of high temperature on the design of blast furnace slag and coarse fly ash-based geopolymer mortar. Compos. Part B Eng. 2016, 92, 9–18. [Google Scholar] [CrossRef]
- Atiş, C.; Görür, E.B.; Karahan, O.; Bilim, C.; İlkentapar, S.; Luga, E. Very high strength (120 MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Zakka, W.P.; Lim, N.H.A.S.; Khun, M.C. A scientometric review of geopolymer concrete. J. Clean. Prod. 2021, 280, 124353. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Hamid, M.A.; Yaltay, N.; Türkmenoğlu, M. Properties of pumice-fly ash based geopolymer paste. Constr. Build. Mater. 2022, 316, 125665. [Google Scholar] [CrossRef]
- Bagci, C.; Kafkas, D.; Samuel, D.M.; Kriven, W.M. Sustainable activation of pumice with partially variable substitutions of metakaolin and/or fumed silica. Int. J. Appl. Ceram. Technol. 2024, 21, 818–828. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Girija, K. Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: A review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. Influence of curing conditions on properties of high calcium fly ash geopolymer containing Portland cement as additive. Mater. Des. 2014, 53, 269–274. [Google Scholar] [CrossRef]
- Karaaslan, C. Enhancing Early Strength Development of Alkali-Activated Slag through Preheating of Materials. Çukurova Univ. Mühendislik Fakültesi Derg. 2023, 38, 1129–1138. [Google Scholar] [CrossRef]
- Yılmaz, A.; Degirmenci, F.N.; Aygörmez, Y. Effect of initial curing conditions on the durability performance of low-calcium fly ash-based geopolymer mortars. Bol. Soc. Esp. Ceram. Y Vidr. 2023, 63, 238–254. [Google Scholar] [CrossRef]
- Safari, Z.; Kurda, R.; Al-Hadad, B.; Mahmood, F.; Tapan, M. Mechanical characteristics of pumice-based geopolymer paste. Resour. Conserv. Recycl. 2020, 162, 105055. [Google Scholar] [CrossRef]
- Mobasheri, F.; Shirzadi Javid, A.A.; Mirvalad, S.; Azizi, S.; Mowlaei, R. Durability and mechanical properties of pumice-based geopolymers: A sustainable material for future. Iran. J. Sci. Technol. Trans. Civ. Eng. 2022, 46, 223–235. [Google Scholar] [CrossRef]
- Yener, E.; Karaaslan, C. Curing Time and Temperature Effect on the Resistance to Wet-Dry Cycles of Fly Ash Added Pumice Based Geopolymer. Cem. Based Compos. 2020, 1, 19–25. [Google Scholar] [CrossRef]
- Sajan, P.; Jiang, T.; Lau, C.; Tan, G.; Ng, K. Combined effect of curing temperature, curing period and alkaline concentration on the mechanical properties of fly ash-based geopolymer. Clean. Mater. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Abdulsahib, S.M.; Zubaidi, S.L.; Almamalachy, Y.; Dulaimi, A. Temperature and precipitation change assessment in the North of Iraq using LARS-WG and CMIP6 models. Water 2024, 16, 2869. [Google Scholar] [CrossRef]
- Rashad, A.M.; Essa, G.M. Effect of ceramic waste powder on alkali-activated slag pastes cured in hot weather after exposure to elevated temperature. Cem. Concr. Compos. 2020, 111, 103617. [Google Scholar] [CrossRef]
- Morsy, M.; Rashad, A.M.; Shoukry, H.; Mokhtar, M.; El-Khodary, S. Development of lime-pozzolan green binder: The influence of anhydrous gypsum and high ambient temperature curing. J. Build. Eng. 2020, 28, 101026. [Google Scholar] [CrossRef]
- Turan, C.; Javadi, A.; Consoli, N.C.C.; Turan, C.; Vinai, R.; Cuisinier, O.; Russo, G. Mechanical properties of calcareous fly ash stabilized soil. In Proceedings of the Eurocoalash 2019, Dundee, Scotland, 10–12 June 2019; pp. 184–194. [Google Scholar]
- Rashad, A.M. An overview of pumice stone as a cementitious material–the best manual for civil engineer. Silicon 2021, 13, 551–572. [Google Scholar] [CrossRef]
- Occhipinti, R.; Stroscio, A.; Finocchiaro, C.; Fugazzotto, M.; Leonelli, C.; Faro, M.J.L.; Megna, B.; Barone, G.; Mazzoleni, P. Alkali activated materials using pumice from the Aeolian Islands (Sicily, Italy) and their potentiality for cultural heritage applications: Preliminary study. Constr. Build. Mater. 2020, 259, 120391. [Google Scholar] [CrossRef]
- Karaaslan, C.; Yener, E.; Bağatur, T.; Polat, R. Improving the durability of pumice-fly ash based geopolymer concrete with calcium aluminate cement. J. Build. Eng. 2022, 59, 105110. [Google Scholar] [CrossRef]
- Karaaslan, C.; Yener, E.; Bağatur, T.; Polat, R.; Gül, R.; Alma, M.H. Synergic effect of fly ash and calcium aluminate cement on the properties of pumice-based geopolymer mortar. Constr. Build. Mater. 2022, 345, 128397. [Google Scholar] [CrossRef]
- Kalatehjari, R.; Najafi, E.K.; Asadi, A.; Brook, M. New Zealand pumicite as a precursor in producing alkaline cement with aluminate-based activators. Case Stud. Constr. Mater. 2024, 21, e04008. [Google Scholar] [CrossRef]
- TS-EN-196-1; Çimento Deney Metotları—Bölüm 1: Dayanım Tayini (Methods of Testing Cement—Part 1: Determination of Strength). TSE: Ankara, Turkey, 2016.
- Karaaslan, C.; Yener, E. The Effect of Alkaline Activator Components on the Properties of Fly Ash Added Pumice Based Geopolymer. J. Inst. Sci. Technol. 2021, 11, 1255–1269. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Salem, H.A. Effect of cement addition, solution resting time and curing characteristics on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 123, 581–593. [Google Scholar] [CrossRef]
- Nagalia, G.; Park, Y.; Abolmaali, A.; Aswath, P. Compressive strength and microstructural properties of fly ash–based geopolymer concrete. J. Mater. Civ. Eng. 2016, 28, 04016144. [Google Scholar] [CrossRef]
- TS-EN-196-3; Çimento Deney Metotları—Bölüm 3: Priz Süreleri ve Genleşme Tayini (Methods of Testing Cement—Part 3: Determination of Setting Times and Sondness). TSE: Ankara, Turkey, 2017.
- TS-EN-1015-10/A1; Kâgir Harcı-Deney Metotları—Bölüm 10: Sertleşmiş Harcın Boşluklu Kuru Birim Hacim Kütlesinin Tayini (Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar). TSE: Ankara, Turkey, 2007.
- TS-EN-13057; Beton Yapılar—Koruma ve Tamir Için Mamul ve Sistemler—Deney Metotları—Kılcal su Emmeye Direncin Tayini (Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Resistance of Capillary Absorption). TSE: Ankara, Turkey, 2002.
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Attenuated total reflectance fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Tchadjié, L.; Djobo, J.; Ranjbar, N.; Tchakouté, H.; Kenne, B.D.; Elimbi, A.; Njopwouo, D. Potential of using granite waste as raw material for geopolymer synthesis. Ceram. Int. 2016, 42, 3046–3055. [Google Scholar] [CrossRef]
- ASTM-666; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. American Society for Testing and Materials: West Conshohocken, PA, USA, 2003.
- Erdoğan, T.Y. Beton (Concrete), 6th ed.; METU Press Publishing Company: Ankara, Turkey, 2016. [Google Scholar]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Kuri, J.C.; Khan, M.N.N.; Sarker, P.K. Fresh and hardened properties of geopolymer binder using ground high magnesium ferronickel slag with fly ash. Constr. Build. Mater. 2021, 272, 121877. [Google Scholar] [CrossRef]
- Hardjito, D.; Cheak, C.C.; Ing, C.H.L. Strength and setting times of low calcium fly ash-based geopolymer mortar. Mod. Appl. Sci. 2008, 2, 3–11. [Google Scholar] [CrossRef]
- Tchakoute, H.; Elimbi, A.; Yanne, E.; Djangang, C. Utilization of volcanic ashes for the production of geopolymers cured at ambient temperature. Cem. Concr. Compos. 2013, 38, 75–81. [Google Scholar] [CrossRef]
- Aldawsari, S.; Kampmann, R.; Harnisch, J.; Rohde, C. Setting time, microstructure, and durability properties of low calcium fly ash/slag geopolymer: A review. Materials 2022, 15, 876. [Google Scholar] [CrossRef]
- Topark-Ngarm, P.; Chindaprasirt, P.; Sata, V. Setting time, strength, and bond of high-calcium fly ash geopolymer concrete. J. Mater. Civ. Eng. 2015, 27, 04014198. [Google Scholar] [CrossRef]
- Heah, C.; Kamarudin, H.; Al Bakri, A.M.; Binhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.; Liew, Y. Effect of curing profile on kaolin-based geopolymers. Phys. Procedia 2011, 22, 305–311. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Mechanical properties and durability of volcanic ash based geopolymer mortars. Constr. Build. Mater. 2016, 124, 606–614. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Sietins, J.M.; Granados-Focil, S.; Pepi, M.S.; Xu, Y.; Tao, M. Reaction kinetics of red mud-fly ash based geopolymers: Effects of curing temperature on chemical bonding, porosity, and mechanical strength. Cem. Concr. Compos. 2018, 93, 175–185. [Google Scholar] [CrossRef]
- TS-EN-1504-3; Beton Yapıların Korunması ve Tamiri Için Mamuller ve Sistemler—Tarifler, Gerekler, Kalite Kontrol ve Uygunluk Değerlendirmesi—Bölüm 3: Yapisal Olan ve Yapısal Olmayan Tamir (Products and Systems for the Protection and Repair of Concrete Structures—Definitions, Requirements, Quality Control and Evaluation of Conformity—Part 3: Structural and Non-Structural Repair). TSE: Ankara, Turkey, 2006.
- Zhao, R.; Yuan, Y.; Cheng, Z.; Wen, T.; Li, J.; Li, F.; Ma, Z.J. Freeze-thaw resistance of Class F fly ash-based geopolymer concrete. Constr. Build. Mater. 2019, 222, 474–483. [Google Scholar] [CrossRef]
- Park, S.; Pour-Ghaz, M. What is the role of water in the geopolymerization of metakaolin? Constr. Build. Mater. 2018, 182, 360–370. [Google Scholar] [CrossRef]
- Karaaslan, C. Unary, binary and ternary use of slag, nano-CaCO3, and cement to enhance freeze-thaw durability in fly ash-based geopolymer concretes. J. Build. Eng. 2025, 99, 111631. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Kuenzel, C.; Ranjbar, N. Dissolution mechanism of fly ash to quantify the reactive aluminosilicates in geopolymerisation. Resour. Conserv. Recycl. 2019, 150, 104421. [Google Scholar] [CrossRef]
- Rabie, M.; Irshidat, M.R.; Al-Nuaimi, N. Ambient and Heat-Cured Geopolymer Composites: Mix Design Optimization and Life Cycle Assessment. Sustainability 2022, 14, 4942. [Google Scholar] [CrossRef]
- Kamwa, R.A.T.; Tome, S.; Nemaleu, J.G.D.; Noumbissie, L.T.; Tommes, B.; Eguekeng, I.; Spieß, A.; Woschko, D.; Chongouang, J.; Janiak, C.; et al. Effect of Curing Temperature on Properties of Compressed Lateritic Earth Bricks Stabilized with Natural Pozzolan-Based Geopolymer Binders Synthesized in Acidic and Alkaline Media. Arab. J. Sci. Eng. 2023, 48, 16151–16165. [Google Scholar] [CrossRef]
- Mo, B.-H.; Zhu, H.; Cui, X.-M.; He, Y.; Gong, S.-Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, D.; Zhou, W.; Lu, H.; Wang, L. Effect of activator and curing mode on fly ash-based geopolymers. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 711–715. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; Van Deventer, J.S. Effect of alumina release rate on the mechanism of geopolymer gel formation. Chem. Mater. 2010, 22, 5199–5208. [Google Scholar] [CrossRef]
- Mindess, S.; Young, F.; Darwin, D. Concrete, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longman: London, UK, 1995; Volume 4. [Google Scholar]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Fu, Y.; Cai, L.; Yonggen, W. Freeze–thaw cycle test and damage mechanics models of alkali-activated slag concrete. Constr. Build. Mater. 2011, 25, 3144–3148. [Google Scholar] [CrossRef]
- Javed, U.; Shaikh, F.U.A.; Sarker, P.K. Corrosive effect of HCl and H2SO4 exposure on the strength and microstructure of lithium slag geopolymer mortars. Constr. Build. Mater. 2024, 411, 134588. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Karaaslan, C.; Yener, E.; Bağatur, T.; Polat, R.; Gül, R. Uçucu kül ve kalsiyum alüminat çimentosu katkılı pomza esaslı geopolimer harçların sülfürik asit direnci. J. Inst. Sci. Technol. 2022, 12, 2302–2312. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A.; Dong, P.; Bassim, N. Acid attack on geopolymer cement mortar based on waste-glass powder and calcium aluminate cement at mild concentration. Constr. Build. Mater. 2018, 193, 363–372. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Zhang, N.; Hedayat, A.; Figueroa, L.; Steirer, K.X.; Li, H.; Sosa, H.G.B.; Bernal, R.P.H.; Tupa, N.; Morales, I.Y.; Loza, R.S.C. Experimental studies on the durability and leaching properties of alkali-activated tailings subjected to different environmental conditions. Cem. Concr. Compos. 2022, 130, 104531. [Google Scholar] [CrossRef]
- Ribeiro, M.G.S.; Ribeiro, M.G.S.; Keane, P.F.; Sardela, M.R.; Kriven, W.M.; Ribeiro, R.A.S. Acid resistance of metakaolin-based, bamboo fiber geopolymer composites. Constr. Build. Mater. 2021, 302, 124194. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Wetzel, A.; Middendorf, B. Influence of silica fume on properties of fresh and hardened ultra-high performance concrete based on alkali-activated slag. Cem. Concr. Compos. 2019, 100, 53–59. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, Á.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative study of the reactivity of fly ash in geopolymerization by FTIR. J. Sustain. Cem. Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef]
- Yusuf, M.O. Bond characterization in cementitious material binders using Fourier-transform infrared spectroscopy. Appl. Sci. 2023, 13, 3353. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a blended slag-fly ash geopolymer concrete in field conditions after 8 years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Nasaeng, P.; Wongsa, A.; Cheerarot, R.; Sata, V.; Chindaprasirt, P. Strength enhancement of pumice-based geopolymer paste by incorporating recycled concrete and calcined oyster shell powders. Case Stud. Constr. Mater. 2022, 17, e01307. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Fly ash-slag interaction during alkaline activation: Influence of activators on phase assemblage and microstructure formation. Constr. Build. Mater. 2016, 122, 594–606. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Mao, Y.; Hu, X.; Shi, C. Characteristics of fly ash-based geopolymer concrete in the field for 4 years. Constr. Build. Mater. 2023, 382, 131222. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Latifi, N.; Meehan, C.L.; Abd Majid, M.Z.; Horpibulsuk, S. Strengthening montmorillonitic and kaolinitic clays using a calcium-based non-traditional additive: A micro-level study. Appl. Clay Sci. 2016, 132–133, 182–193. [Google Scholar] [CrossRef]
- Turan, C.; Javadi, A.A.; Vinai, R.; Russo, G. Effects of fly ash inclusion and alkali activation on physical, mechanical, and chemical properties of clay. Materials 2022, 15, 4628. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Chen, S.; Ruan, S.; Zeng, Q.; Liu, Y.; Zhang, M.; Tian, Y.; Yan, D. Pore structure of geopolymer materials and its correlations to engineering properties: A review. Constr. Build. Mater. 2022, 328, 127064. [Google Scholar] [CrossRef]
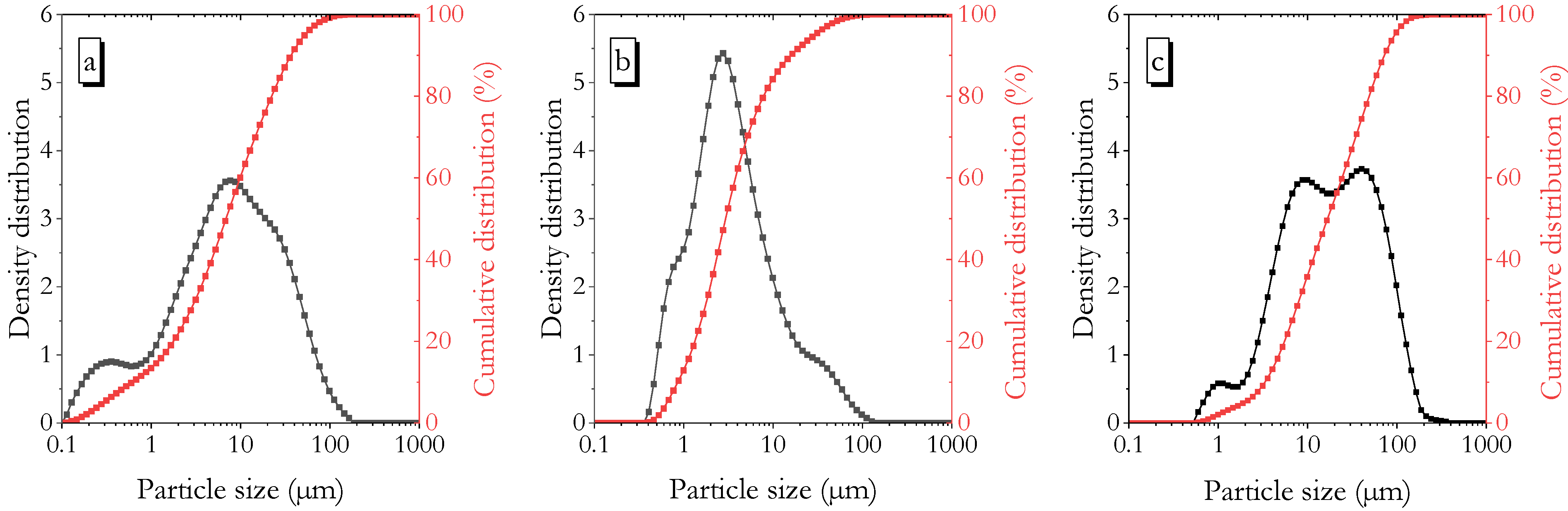
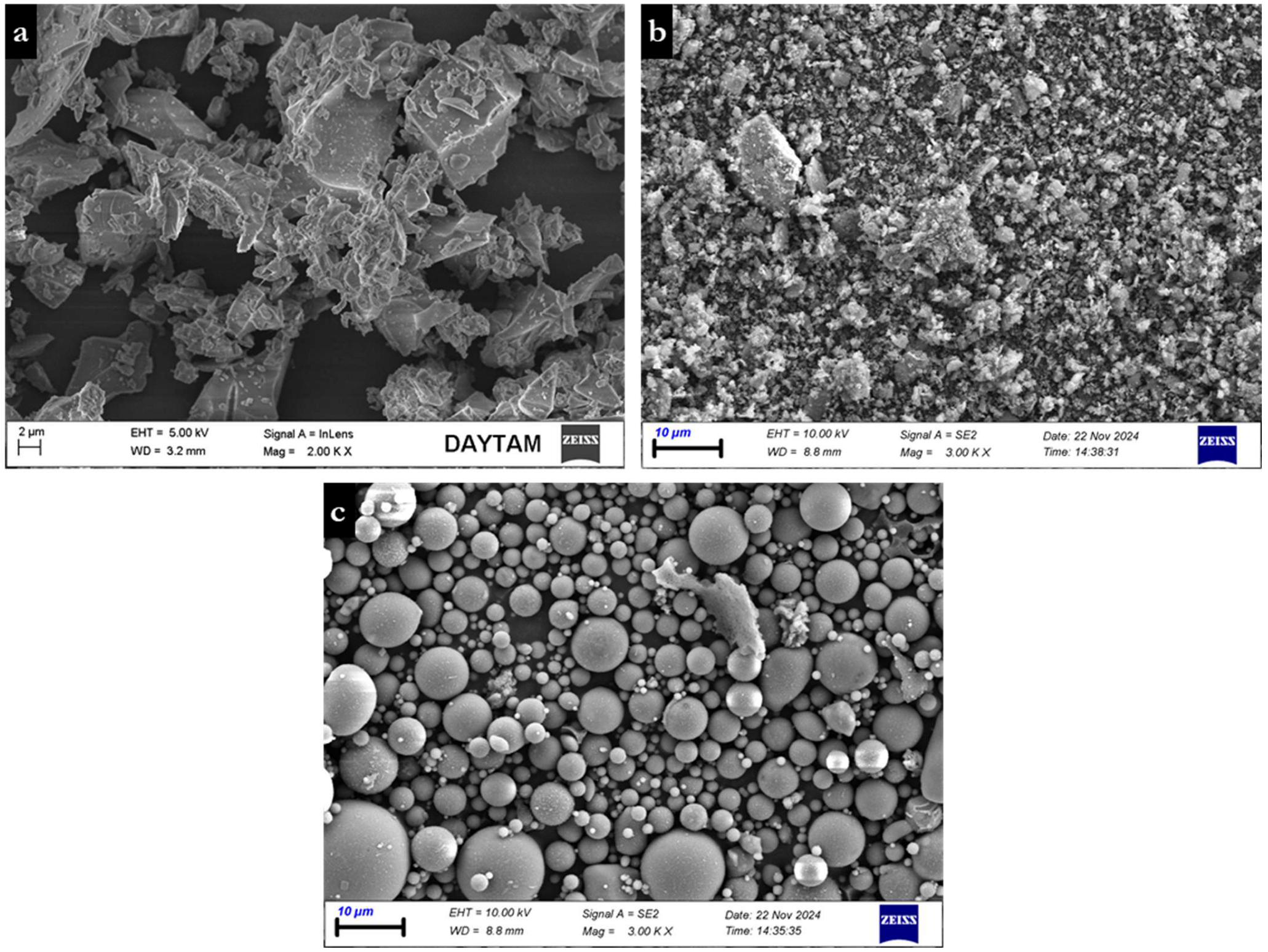
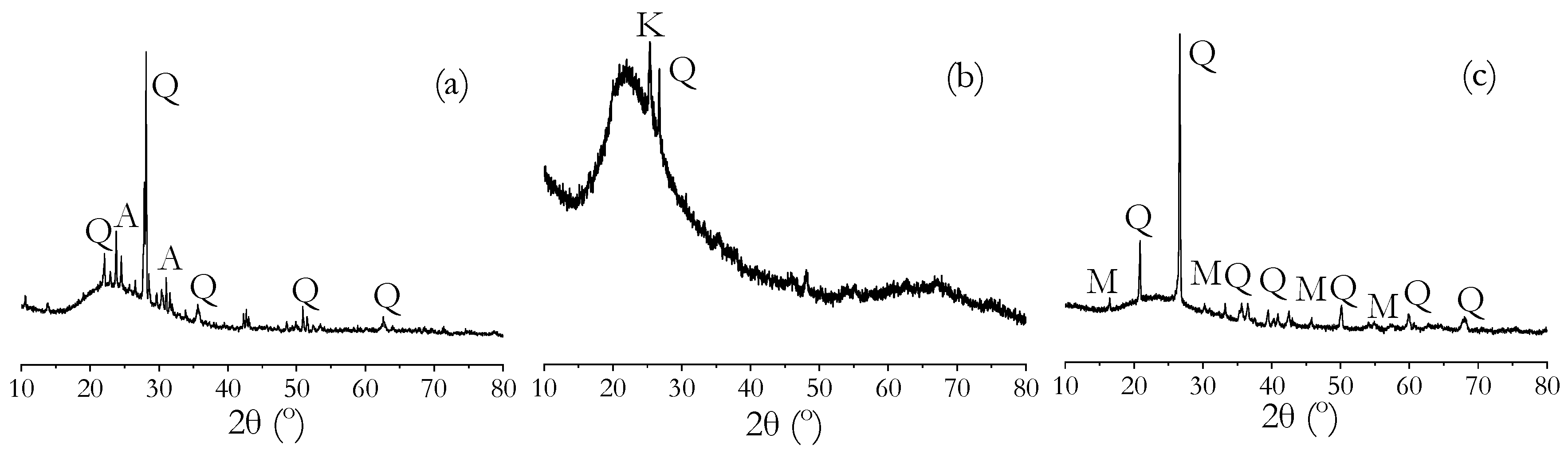
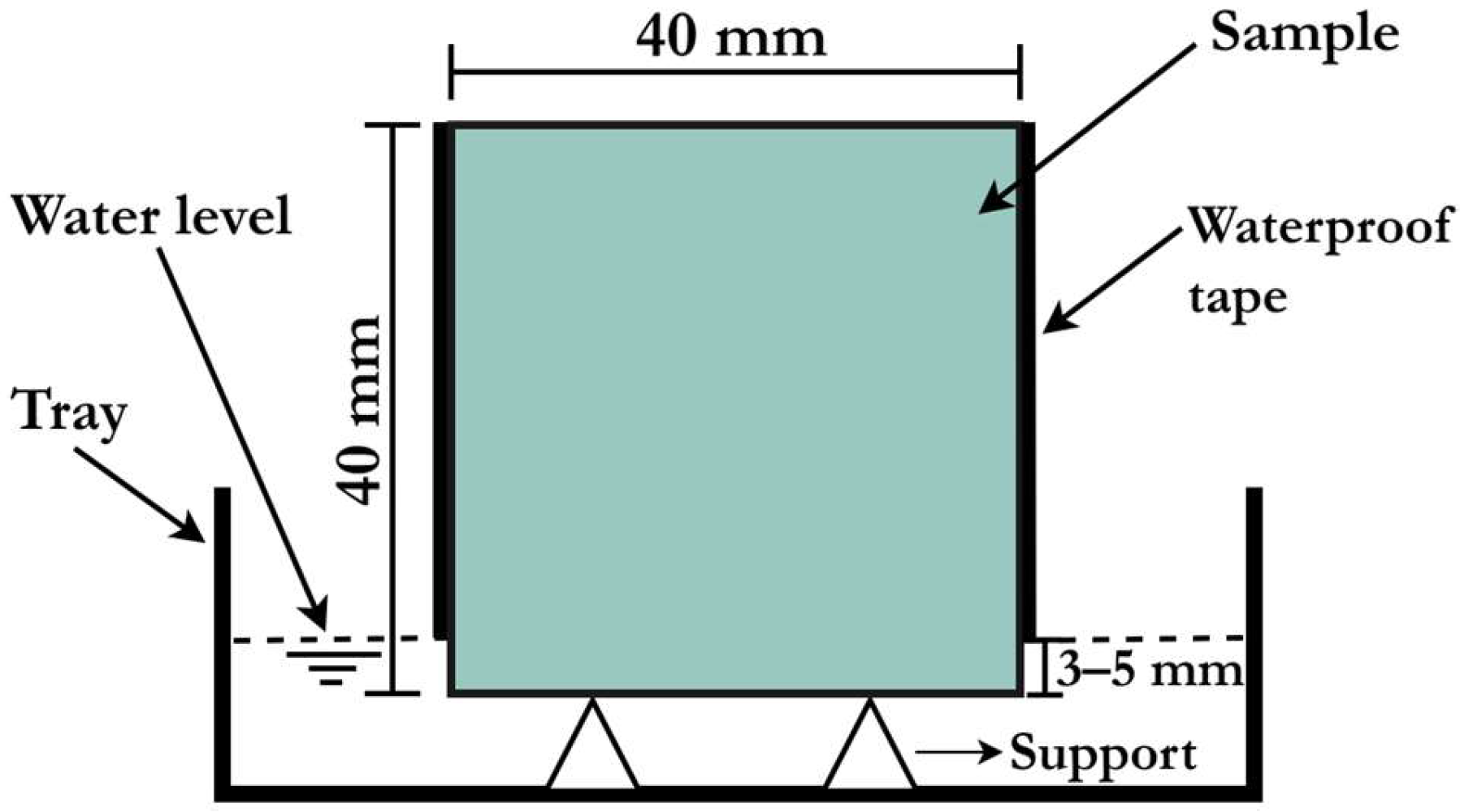
| Powder Binder | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Pumice | 3.56 | 67.49 | 13.07 | 3.98 | 0.69 | 2.63 | 2.36 | 0.18 | 3.69 |
| Metakaolin | 0.38 | 53.29 | 43.18 | 0.62 | 0.22 | 0.34 | 0.25 | 0.16 | 0.80 |
| Fly ash | 3.75 | 55.98 | 21.19 | 6.94 | 2.45 | 1.91 | 2.42 | 0.42 | 2.14 |
| Powder Binder | D10 (μm) | D50 (μm) | D90 (μm) | Surface Area of LPSD (cm2/g) | Blaine Specific Surface Area (cm2/g) | Specific Gravity | Strength Activity Index (28-Day. %) |
|---|---|---|---|---|---|---|---|
| Pumice | 0.70 | 8.04 | 42.0 | 63,550 | 4980 | 2.67 | 74.2 |
| Metakaolin | 0.97 | 3.34 | 18.0 | 25,740 | - | 2.60 | 123.0 |
| Fly ash | 3.87 | 19.00 | 82.10 | 7220 | 3004 | 2.34 | 92.6 |
| Mix Id | NaOH | Na2SiO3 | Pumice | Metakaolin | Fly Ash | Sand |
|---|---|---|---|---|---|---|
| PMGP | 87.4 | 218.6 | 405 | 45 | - | 1350 |
| FAGP | 57.9 | 144.6 | - | - | 450 | 1350 |
| Sample ID | Curing Condition |
|---|---|
| PM-25 | Cured at ambient laboratory conditions until the day of testing. |
| PM-45 | Cured at 45 °C continuously until the day of testing. |
| PM-75 | Initially cured at 75 °C for 24 h, followed by 90 °C for 24 h, and then kept at ambient laboratory conditions until testing. |
| FA-25 | Cured at ambient laboratory conditions until the day of testing. |
| FA-45 | Cured at 45 °C continuously until the day of testing. |
| FA-75 | Initially cured at 75 °C for 24 h, then kept at ambient laboratory conditions until the day of testing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaaslan, C.; Şek, Ş.; Turan, C. Utilising High-Ambient-Temperature Curing in the Development of Low-Calcium Geopolymers. Buildings 2025, 15, 2974. https://doi.org/10.3390/buildings15162974
Karaaslan C, Şek Ş, Turan C. Utilising High-Ambient-Temperature Curing in the Development of Low-Calcium Geopolymers. Buildings. 2025; 15(16):2974. https://doi.org/10.3390/buildings15162974
Chicago/Turabian StyleKaraaslan, Cemal, Şeyda Şek, and Canan Turan. 2025. "Utilising High-Ambient-Temperature Curing in the Development of Low-Calcium Geopolymers" Buildings 15, no. 16: 2974. https://doi.org/10.3390/buildings15162974
APA StyleKaraaslan, C., Şek, Ş., & Turan, C. (2025). Utilising High-Ambient-Temperature Curing in the Development of Low-Calcium Geopolymers. Buildings, 15(16), 2974. https://doi.org/10.3390/buildings15162974





