Abstract
In marine environments, chloride salt ingress into concrete having a porous structure can lead to physical salt attack (PSA) deterioration. However, the slow deterioration of cement-based materials caused by PSA under current laboratory conditions limits the understanding of the mechanisms of PSA. To improve the efficiency of accelerated testing for PSA in a laboratory, this study investigates the accelerated methods for PSA deterioration of cement-based specimens exposed to NaCl solution by adjusting curing ages and water-to-cement ratios. The results indicate that specimens with shorter curing age exhibit accelerated damage due to insufficient hydration, while specimens with higher water-to-cement ratios experience expedited surface scaling due to increased porosity. Reducing curing age from 28 to 7 days shortened the deterioration time of specimens by 50%. For the 28-day-cured specimens, increasing the w/c ratio from 0.4 to 0.5 accelerated the initial damage by 25%. Despite the variations in the curing age and water-to-cement ratio, the fundamental deterioration mechanism remained consistent across specimens. Notably, deterioration predominantly occurred in regions with relatively reduced external salt crystallization, which can serve as an indicator for predicting potential deterioration locations. The findings provide a theoretical basis for enhancing the efficiency of accelerated PSA testing protocols in a laboratory environment.
1. Introduction
Numerous concrete structures in coastal environments, including ports, bridges, and seawalls, are subjected to salt erosion conditions. The durability challenges arising from physical salt attack (PSA) due to salt crystallization from seawater within concrete have garnered considerable attention [1]. This deterioration mechanism is commonly observed in concrete structures partially embedded in salt-affected soils, where saline solutions penetrate the concrete through capillary transport and surface evaporation processes, subsequently precipitating as crystals within the pore network [2]. The crystallization of chloride salts from seawater generates substantial stress that can reach 10–20 MPa, significantly exceeding the tensile strength of concrete and thereby initiating microcrack formation and propagation [3]. This deterioration process ultimately results in reduced structural integrity and shortened service life, leading to substantial economic losses and safety concerns [4,5,6]. Consequently, investigating the PSA behavior of concrete in coastal environments is essential for developing improved erosion-resistant performance.
To efficiently explore the influence mechanism and assess the service life of PSA on concrete, scholars have dedicated efforts to conducting accelerated laboratory testing [7,8]. This testing is designed to reproduce the damage process associated with long-term exposure to saline environments within a relatively short time, serving as a rapid assessment method for evaluating the durability of cement-based materials under salt crystallization pressure. The testing typically involves exposing cement-based material specimens to salt solutions and subjecting them to dry–wet cycles, thereby simulating the repeated dissolution and crystallization of salts within the material’s porous structure to replicate the salt migration and crystal formation processes that occur in natural environments [9,10,11]. This approach can simulate erosion phenomena that would naturally occur over decades within a relatively short period, significantly reducing evaluation time [12] and lowering testing costs [13]. Meanwhile, accelerated laboratory testing can also effectively optimize material selection and structural design, ultimately enhancing the PSA resistance of engineering structures [10,14]. However, for a long time, laboratory PSA testing has predominantly employed Na2SO4 as the primary salt solution [15,16,17]. This preference stems from the fact that Na2SO4 (thenardite) transforms into Na2SO4·10H2O (mirabilite) in the presence of water, generating high saturation levels within porous materials and consequently producing substantial crystallization pressure and destructive forces [18]. Therefore, the PSA deterioration rate induced by Na2SO4 exceeds that of most other salts. In contrast, NaCl, which constitutes the primary component of seawater (accounting for 88% of the total dissolved salts), has received relatively limited attention in previous PSA research [9]. The primary reason for this limitation is that while laboratory studies consistently observe visible salt crystallization of NaCl on concrete or stone surfaces, its low tendency to achieve supersaturation and high propensity for efflorescence formation, rather than sub-florescence crystallization, result in slow deterioration under laboratory conditions [19]. Consequently, testing cycles typically extend from several months to several years [20,21,22,23,24]. For example, Haynes et al. [21] observed only slight deterioration after three years of experimentation. Yang et al. [25] immersed concrete specimens in 3.5% NaCl solution and found no significant physical crystallization deterioration during a 540-day experimental period. Zhang et al. [26] submerged concrete in 5% NaCl solution, with no notable deterioration due to physical crystallization observed within 360 days. Romualdas et al. [27] conducted multi-cycle wet–dry tests using ultra-high concentration solutions (NaCl content of 239.75 kg/m3) to simulate long-term salt crystallization attack coupled with temperature gradient effects, finding a significant reduction in concrete compressive strength after approximately 4 months.
When researchers simulate natural erosion conditions by incorporating humidity changes into wet–dry cycling under semi-immersion conditions, NaCl crystallization beneath the surface was enhanced [28,29]. This approach yields phenomena that more closely align with engineering observations, wherein external NaCl crystallization is less, while internal accumulation of NaCl crystals beneath the surface leads to specimen cracking and spalling. Under these conditions, the deterioration rate induced by NaCl is significantly accelerated, with specimen degradation occurring within approximately 1.5 months [28]. However, the efficiency of the PSA testing in NaCl solution still has room for improvement.
Therefore, this study proposes a PSA accelerated testing strategy based on regulating intrinsic material parameters (curing age and water-to-cement ratio). In fact, the deterioration rate of cement-based materials largely depends on porosity and pore structure [30], while curing age and water-to-cement ratio significantly influence the pore structure of cement-based materials [31,32]. Therefore, it is feasible to consider further accelerating testing rates by adjusting curing age and water-to-cement ratio in addition to introducing humidity cycling. It is noteworthy that cast-in-place concrete structures may be exposed to chloride environments during the early curing stage, and variations in water-to-cement ratio are common in practical engineering applications. Therefore, accelerating NaCl erosion testing through reasonable adjustment of curing age and water–cement ratio also corresponds to actual engineering conditions to some extent.
Based on these considerations, this study investigates the influence of curing age and water-to-cement ratio on specimen performance during accelerated PSA testing, wherein humidity variations are coupled with wet–dry cycling. Through systematic testing, the crystallization behavior and resultant deterioration mechanisms are examined. This research will provide a reference for establishing efficient PSA evaluation systems in laboratory environments. Given the absence of universally accepted standard test methods for evaluating PSA resistance of cement-based materials to date, this study will contribute to the development of standardized testing protocols for assessing PSA resistance in cement-based materials. Furthermore, the findings will facilitate the development of strategies to enhance the durability of concrete structures in marine and coastal environments.
2. Materials and Methods
2.1. Materials
In this study, cement paste was used for the accelerated PSA testing. The cement used in the experiments was 42.5R high sulfate-resistant cement, which is characterized to be low aluminate content, thereby minimizing potential chemical reactions between aluminates and chloride ions in the immersion solution and enabling the research to focus on physical crystallization damage. The cement meets the requirements of Chinese standard GB 748-2005 [33], and its oxide composition is presented in Table 1. Deionized water was used as mixing water throughout the experimental process to eliminate the influence of impurities in water on the experimental results. The NaCl solution for pastes to be immersed is with a mass fraction of 5.32%. The NaCl powder used for solution preparation was of analytical grade with a purity of not less than 99.5%. Deionized water was also used during solution preparation to ensure the repeatability of the experiment.

Table 1.
Oxide composition of cement and content.
2.2. Experimental Settings
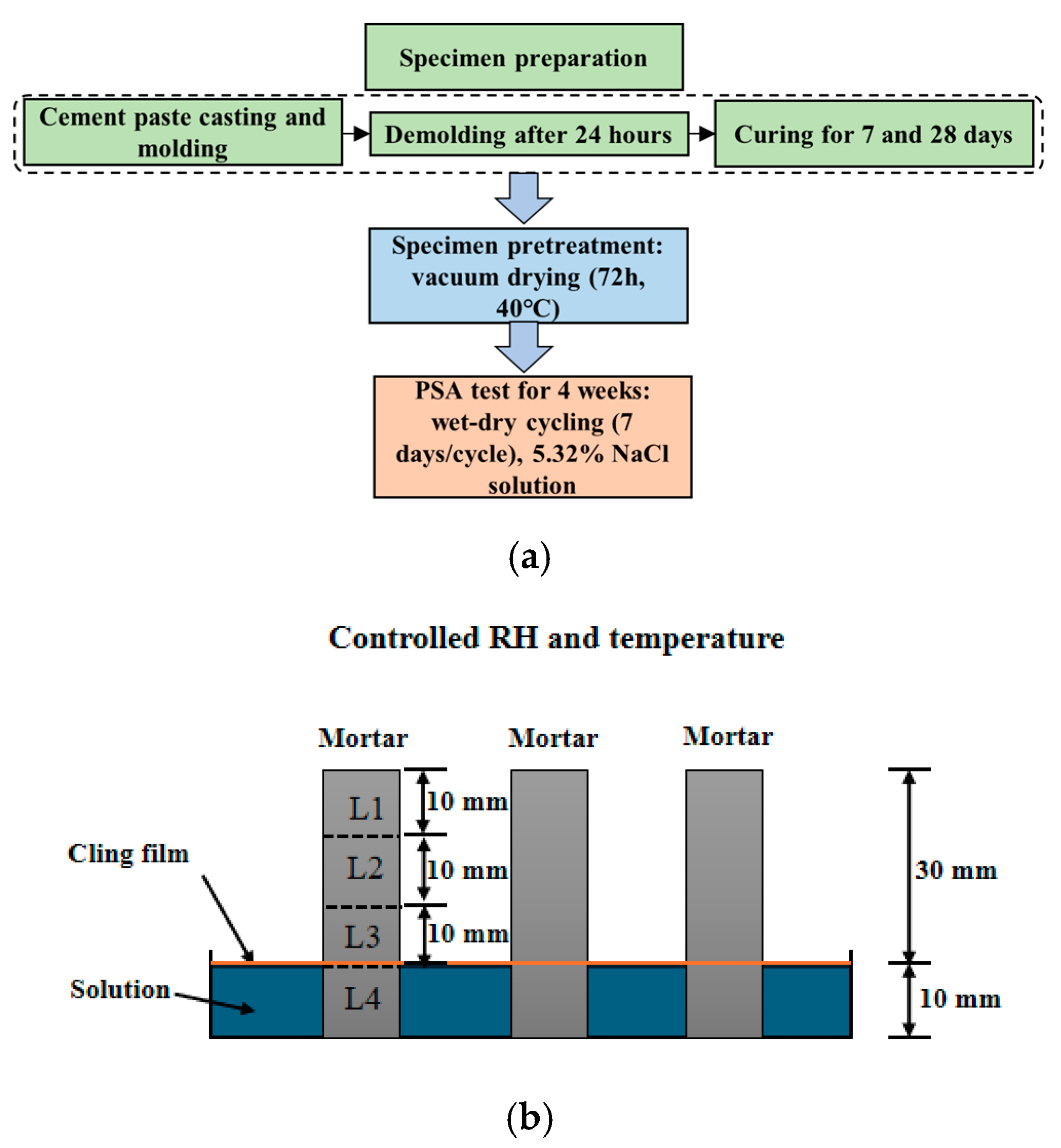
A flowchart for the experiment is shown in Figure 1a. After the cement paste was cast and poured into a mold, specimens with dimensions of 10 × 10 × 40 mm were demolded 24 h after casting and immediately placed in a curing room with a temperature of 20 ± 2 °C and relative humidity of not less than 95%. At 7 days of curing age, 50% of the specimens were randomly selected to terminate curing, while the remaining specimens continued curing to 28 days. This study designed two groups of experiments: the first group maintained a constant water-to-cement ratio of 0.4 with curing ages of 7 days and 28 days, respectively, aimed at investigating the effect of curing age on PSA; the second group had a curing age of 28 days with water–cement ratios of 0.4, 0.45, and 0.5, respectively, used to study the effect of water–cement ratio on PSA. When reaching the designed curing age, the specimens were placed in a vacuum drying oven at 40 °C for 72 h of drying, followed by a PSA test. The semi-immersion setup is shown in Figure 1b, with 1/4 of the specimen immersed in the solution. To ensure solution concentration stability during immersion, the NaCl solution was replaced every 1–2 days, and plastic wrap was placed over the salt solution surface to suppress water evaporation. The dry–wet cycling of the experiment was set based on the accelerated cycling procedure proposed by Lubelli [28] to promote more sub-florescence crystallization and better correspond to actual engineering conditions, with each complete cycle lasting 7 days. The detailed settings of the cycling procedure in each cycle are presented in Table 2.
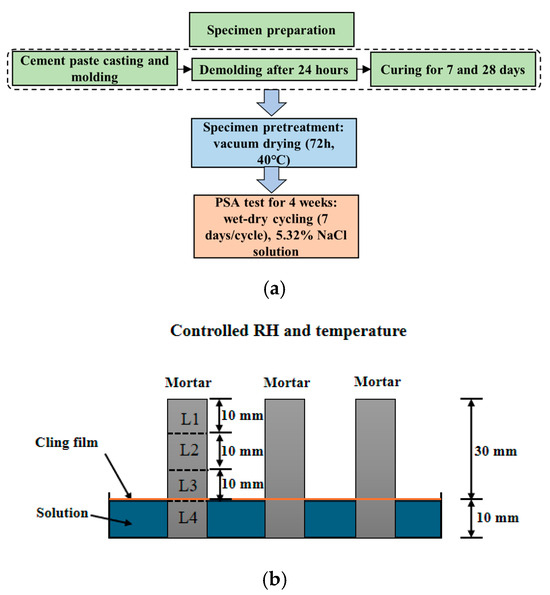
Figure 1.
(a) A flowchart for the experiment; (b) schematic diagram of the accelerated semi-immersion testing in NaCl solution.

Table 2.
Parameter setting for the accelerated semi-immersion test in each cycle.
2.3. Testing
Macroscopic morphology observations were conducted on cement pastes partially immersed in the NaCl solution after the 1st, 2nd, 3rd, and 4th weeks to evaluate the effects of water-to-cement ratio and curing age on paste crystallization and deterioration.
To obtain the distribution and development of internal cracks in the specimens, XCT testing was performed on the cracked and damaged specimens. The XCT testing was employed on a MICROXCT-400 system (Zeiss Group, Oberkochen, Germany) with operating parameters set at 80 kV voltage, 101 μA current, and using a 0.4× magnification lens with an image resolution of 25 μm.
To further observe the influence of internal crystallization distribution on material deterioration, backscattered electron (BSE) and energy dispersive spectroscopy (EDS) techniques in a scanning electron microscope (SEM) were employed to investigate the microscopic morphology and distribution of NaCl within the specimens, thereby clarifying the PSA mechanism. Sample preparation was conducted as follows: according to Figure 1, samples were collected from both near-surface and internal regions of specimen position 1 (damaged area) and dried in a 60 °C oven for 48 h to remove free water completely [34]. Subsequently, the samples were vacuum-impregnated with epoxy resin for curing, and cut using a low-speed saw to obtain fresh cross-sections, which were further polished to achieve a smooth surface. To enhance conductivity and avoid charging effects, the sample surfaces were gold-coated. Testing was performed using a Phenom ProX SEM (Thermo Fisher Scientific Inc., Waltham, MA, USA) with an acceleration voltage set at 10 kV.
3. Results
3.1. Influence of Curing Age on the Accelerated PSA Testing
3.1.1. Macroscopic Morphology
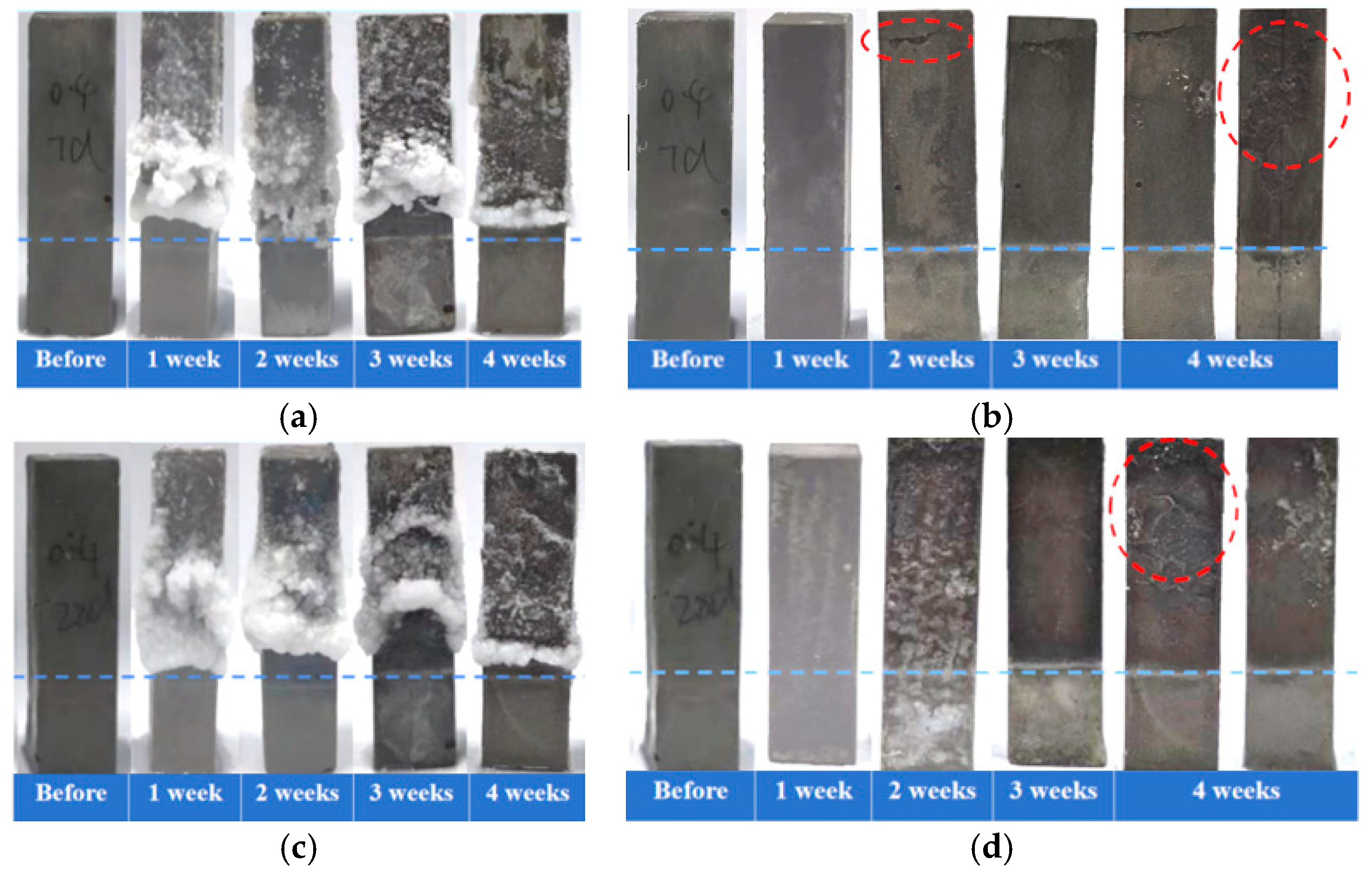
In the accelerated PSA testing, the cement pastes with a water-to-cement ratio of 0.4 were evaluated after curing for 7 days/28 days, followed by a semi-immersion in the NaCl solution. The visual morphology analysis results (Figure 2a,c) indicated that both groups of specimens exhibited obvious salt crystallization accumulation in the region above the immersion line at the end of each cyclic erosion period. With the progress of immersion, surface crystallization gradually decreased in both groups, suggesting that the accumulation of crystalline materials within pores may have formed permeation barriers. Deterioration primarily occurred in regions where external salt crystallization decreased, while no significant deterioration was observed in areas with external salt crystallization accumulation. Morphological observations after removal of surface crystallization (Figure 2b,d) revealed that the 7-day-cured specimens developed initial cracks parallel to the liquid surface in the upper region by the end of the second week. As the erosion process progressed, cracks propagated into the specimen’s interior, accompanied by spalling of the formed surface. In contrast, the 28-day-cured specimens mainly exhibited surface spalling after the fourth week, with only microcracks appearing at edges, and no obvious internal crack propagation was observed. Therefore, the early-age (7-day) specimens demonstrated more severe damage progression during salt crystallization attack, which may be related to higher porosity resulting from lower hydration degree. Furthermore, the reduction in curing age caused specimens to exhibit a more pronounced crack propagation in the accelerated PSA test, further confirming the significant influence of curing age on the resistance of cement-based materials to PSA.
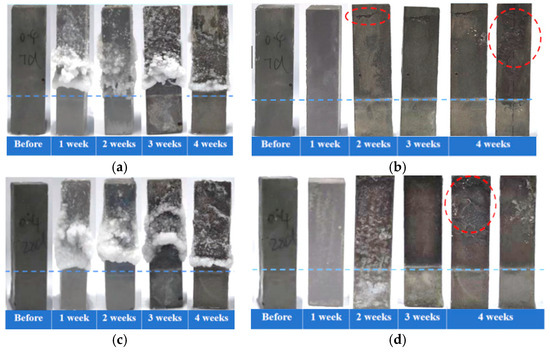
Figure 2.
Macroscopic morphology of cement pastes partially immersed in NaCl solution (blue dashed line indicates the immersion line; red dashed circles indicate the deterioration region). Crystallization development over immersion time for specimens cured for 7 days (a) and for 28 days (c); appearance changes over immersion time after removal of external crystals for specimens cured for 7 days (b) and for 28 days (d).
3.1.2. XCT Analysis
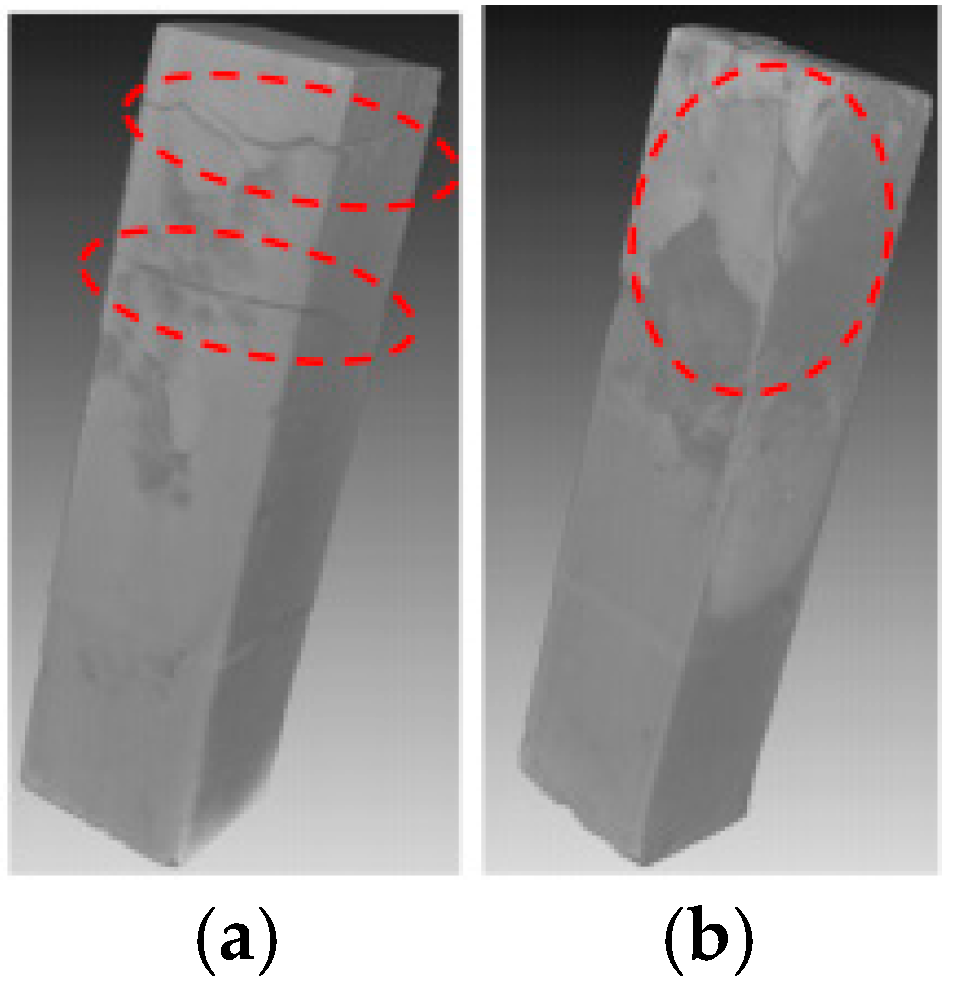
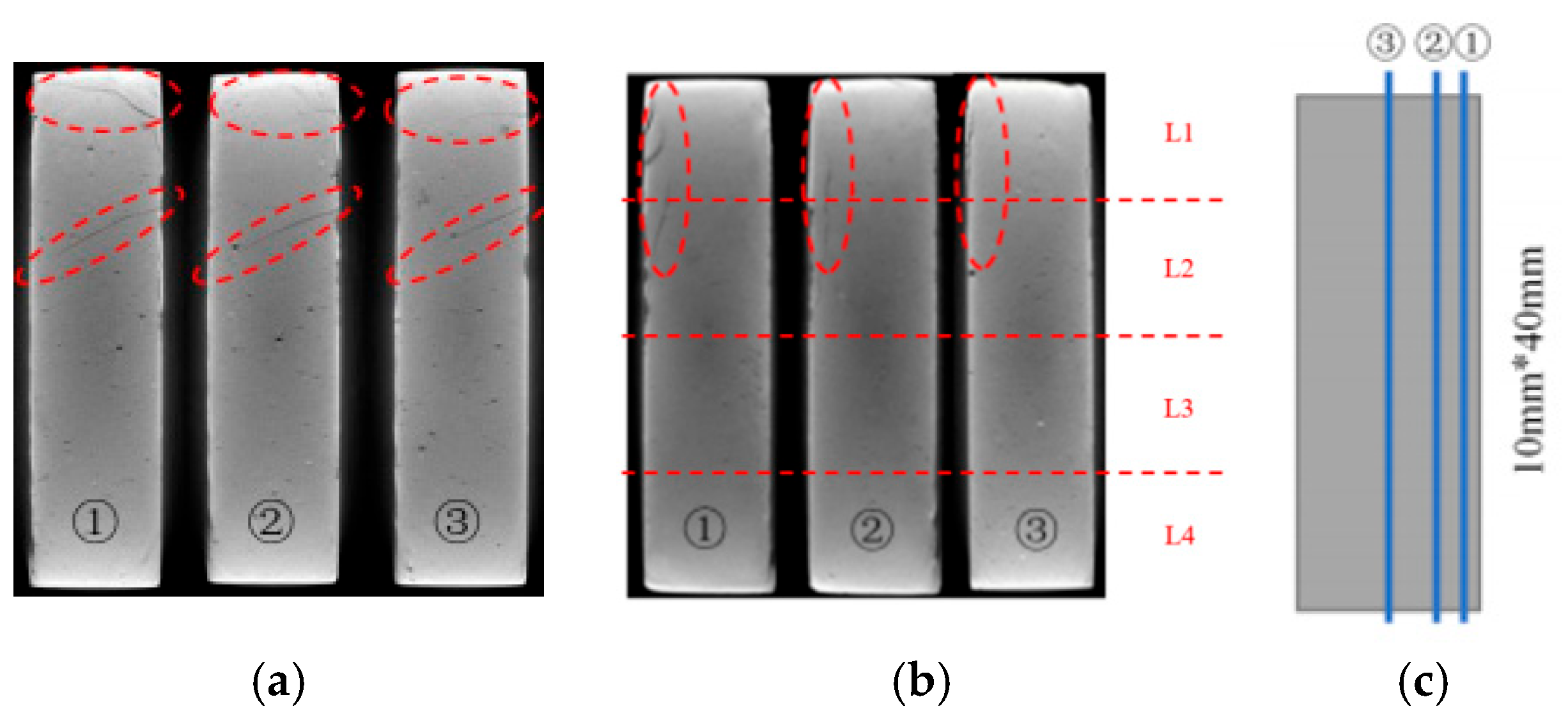
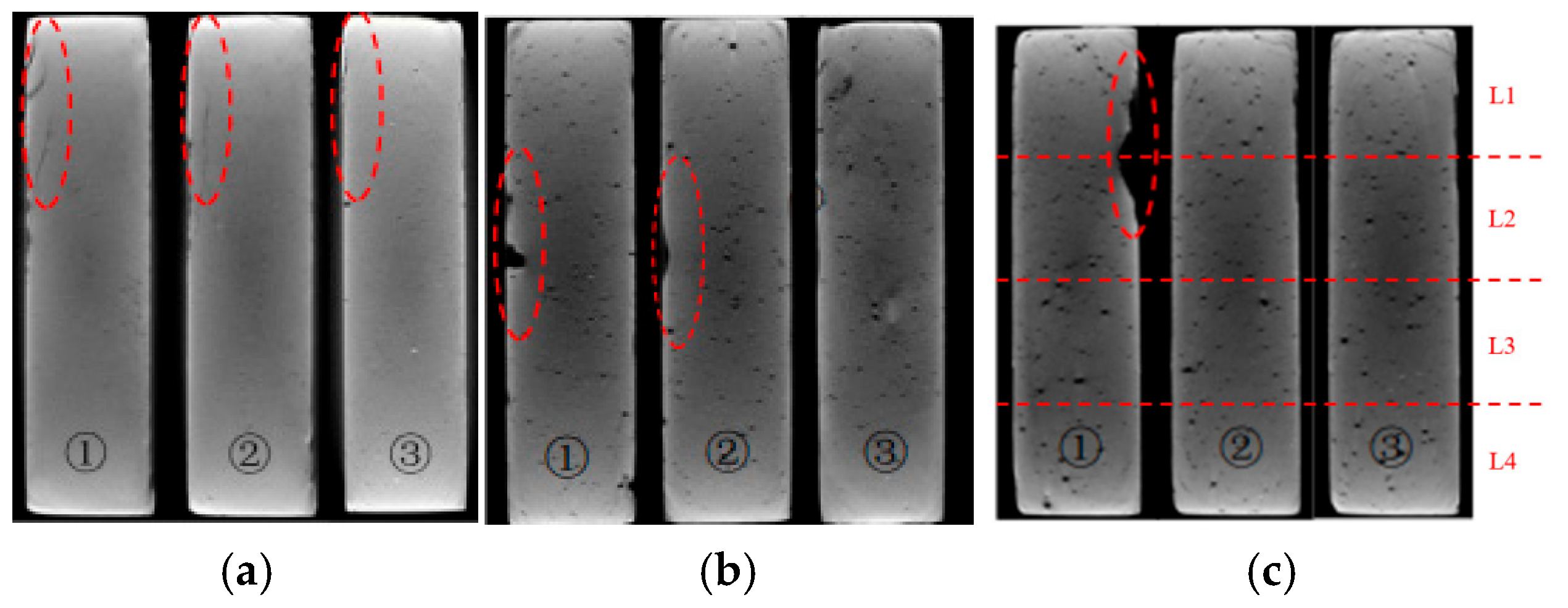
The XCT analysis revealed differences in internal damage characteristics between specimens with curing ages of 7 and 28 days (Figure 3). The experimental results showed that after 28 days of immersion, the 7-day-cured specimens developed obvious horizontal propagating cracks in the L1 and L2 regions, while the 28-day-cured specimens exhibited no significant internal transverse cracks. The cross-sectional analysis results (Figure 4) further revealed that the cracks in the 7-day-cured specimens demonstrated a development trend from surface to interior. In contrast, the damage in the 28-day-cured specimens was primarily confined to surface regions, with no transverse crack propagation observed in the interior. This observation was consistent with the macroscopic deterioration characteristics shown in Figure 2. For the cement paste specimens with a curing age of 7 days, the transverse crack length showed a gradually decreasing trend from outer to inner cross-sections, consistent with the research described by Lubelli [28], further confirming that the 7-day-cured specimens exhibited more severe deterioration than the 28-day-cured specimens during PSA.
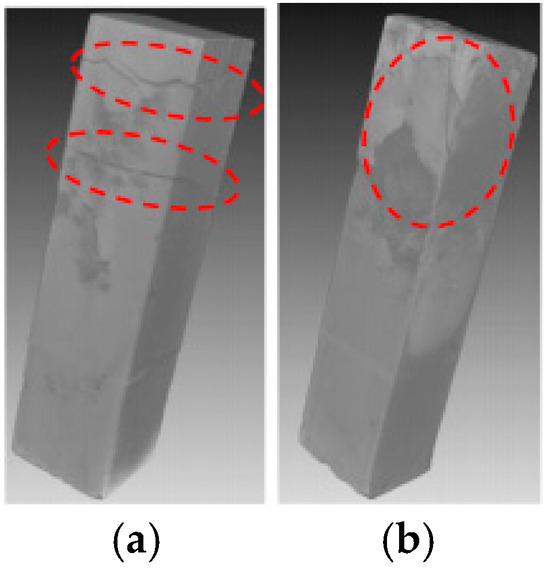
Figure 3.
XCT images of specimens partially immersed in NaCl solution for 4 weeks (the red dashed circles indicate the deterioration region): (a) specimens with a curing age of 7 days; (b) specimens with a curing age of 28 days.
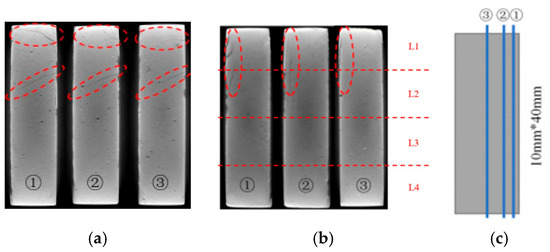
Figure 4.
Internal slice images of the specimen partially immersed in NaCl solution for 4 weeks (red dashed circles indicate the deterioration region): (a) specimens with a curing age of 7 days; (b) specimens with a curing age of 28 days; (c) schematic diagram indicating the positions for the internal slices.
3.1.3. SEM Analysis
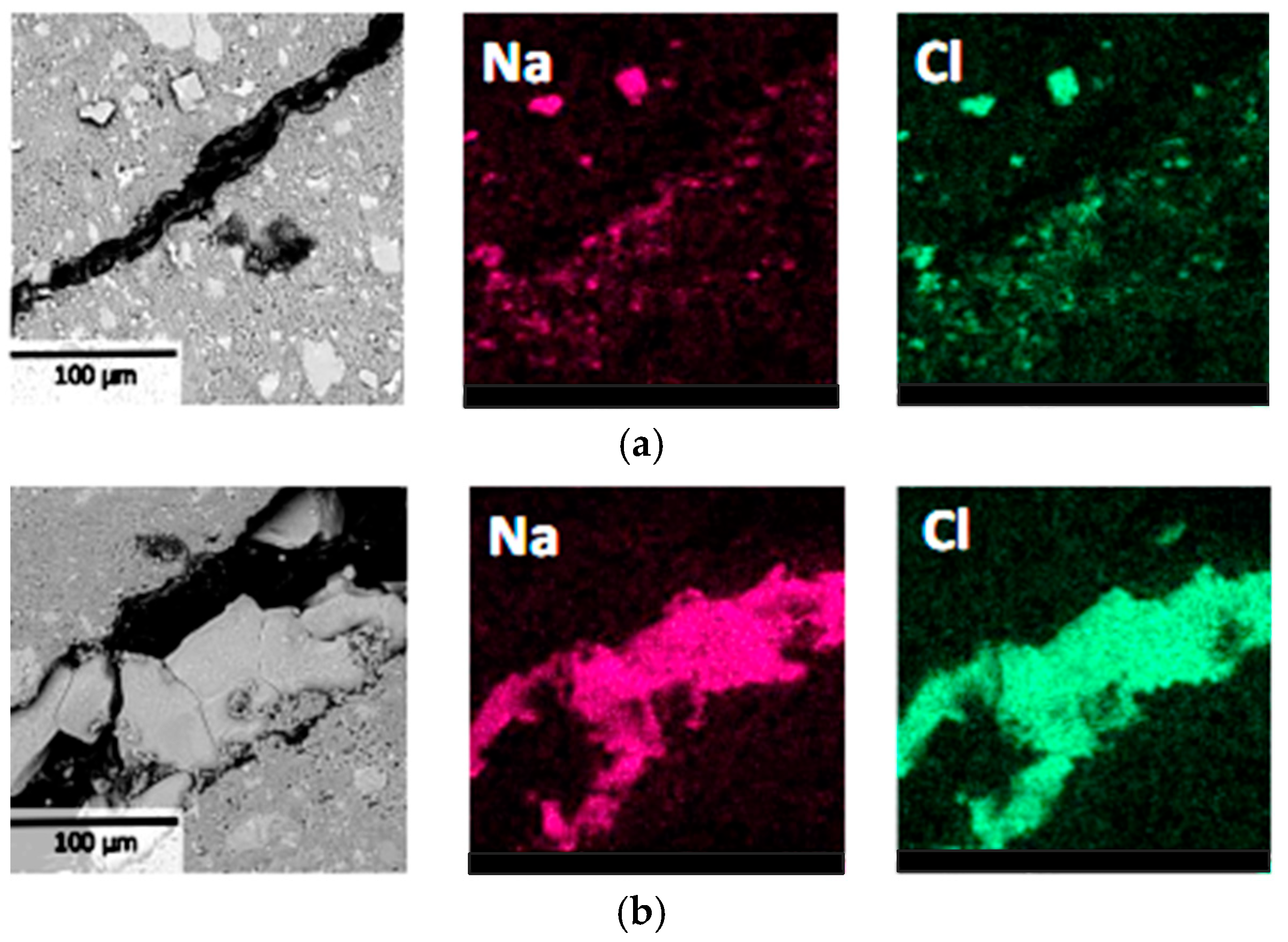
In Figure 5, BSE images and Na, Cl elemental distribution maps in the L1 region (Figure 1) of the specimens were investigated to further observe the influence of NaCl crystallization distribution on material deterioration. It can be seen that obvious deterioration was observed near the surface of the specimens, and the precipitated NaCl crystals exhibited characteristics of relatively large size and concentrated distribution, corresponding to significant damage in the specimen surface layer. In contrast, no large-sized and concentrated NaCl crystals were observed in the internal regions of the specimens, and therefore, no obvious crack structures were found. These experimental results confirmed that during the PSA, when NaCl crystals precipitate at certain locations in specimens that are relatively large and concentrated in distribution, deterioration is prone to occur at those locations. This finding provides microscopic-level evidence for understanding salt crystallization attack mechanisms, indicating that crystal size and distribution density are key factors affecting the deterioration level.
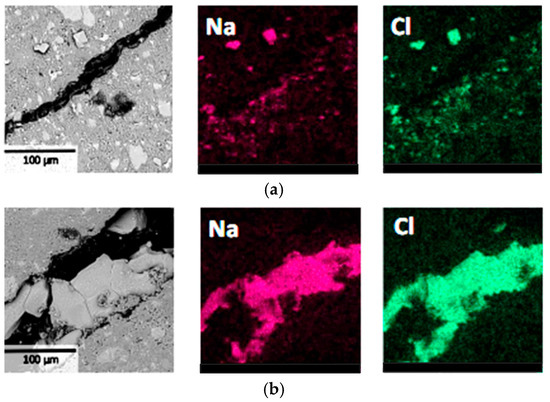
Figure 5.
BSE and EDS results of the surface layer at region L1 of cement pastes: (a) specimen with a curing age of 7 days; (b) specimen with a curing age of 28 days.
3.2. Influence of Water-to-Cement Ratio on PSA
3.2.1. Visual Morphology Analysis
Observation of damage evolution in the 28-day pastes with water-to-cement ratios of 0.4, 0.45, and 0.5 during semi-immersion in Figure 6 revealed that the water-to-cement ratio levels significantly affect the PSA performance of the cement-based materials. All the specimens exhibited crystallization accumulation above the immersion line. After the second weekly cycle, surface crystallization decreased significantly, indicating that crystals gradually accumulated within pores and formed permeation barriers that hindered further salt migration to the surface. Comparing the specimens after removal of surface crystals revealed that deterioration primarily occurred in the regions where salt decreased, while no significant deterioration was observed in the areas with salt accumulation. This finding indicates that potential deterioration locations can be predicted through external crystallization distribution, providing visual indicators for PSA service life assessment. Further observations after surface crystal removal showed that the water-to-cement ratio significantly affected both the deterioration degree and deterioration rate. The specimens with a water-to-cement ratio of 0.5 exhibited severe spalling at edges after the third weekly cyclic erosion (shown in red dashed circles in Figure 6f), the specimens with a water–cement ratio of 0.45 showed similar spalling in the fourth week (shown in red dashed circles in Figure 6d), while the specimens with a water–cement ratio of 0.4 demonstrated the least spalling damage. This indicates that increasing the water–cement ratio significantly aggravated surface spalling in the specimens. Furthermore, the water–cement ratio also influenced the development rate of deterioration, with high water–cement ratio specimens exhibiting faster deterioration rates. Specifically, the specimens with the water–cement ratios of 0.5, 0.45, and 0.4 developed significant deterioration in the second, third, and fourth weeks, respectively. These results further confirmed the significance of reducing the water-to-cement ratio for improving the PSA durability of concrete in salt environments. Based on these findings, it can be inferred that appropriately increasing the water-to-cement ratio can effectively accelerate PSA testing procedures, thereby improving testing efficiency. Additionally, it was also observed that the water-to-cement ratio had minimal influence on the external crystal formation, crystal location, and failure location of cement paste.

Figure 6.
Macroscopic morphology of cement pastes with water-to-cement ratios of 0.4, 0.45, and 0.5 partially immersed in NaCl solution (blue dashed line indicates the immersion line; the red dashed circles indicate the deterioration region). Crystallization on specimens with water-to-cement ratios of 0.4 (a), 0.45 (c), and 0.5 (e); appearance of specimens with water-to-cement ratios of 0.4 (b), 0.45 (d), and 0.5 (f) after removal of external crystallization.
3.2.2. XCT
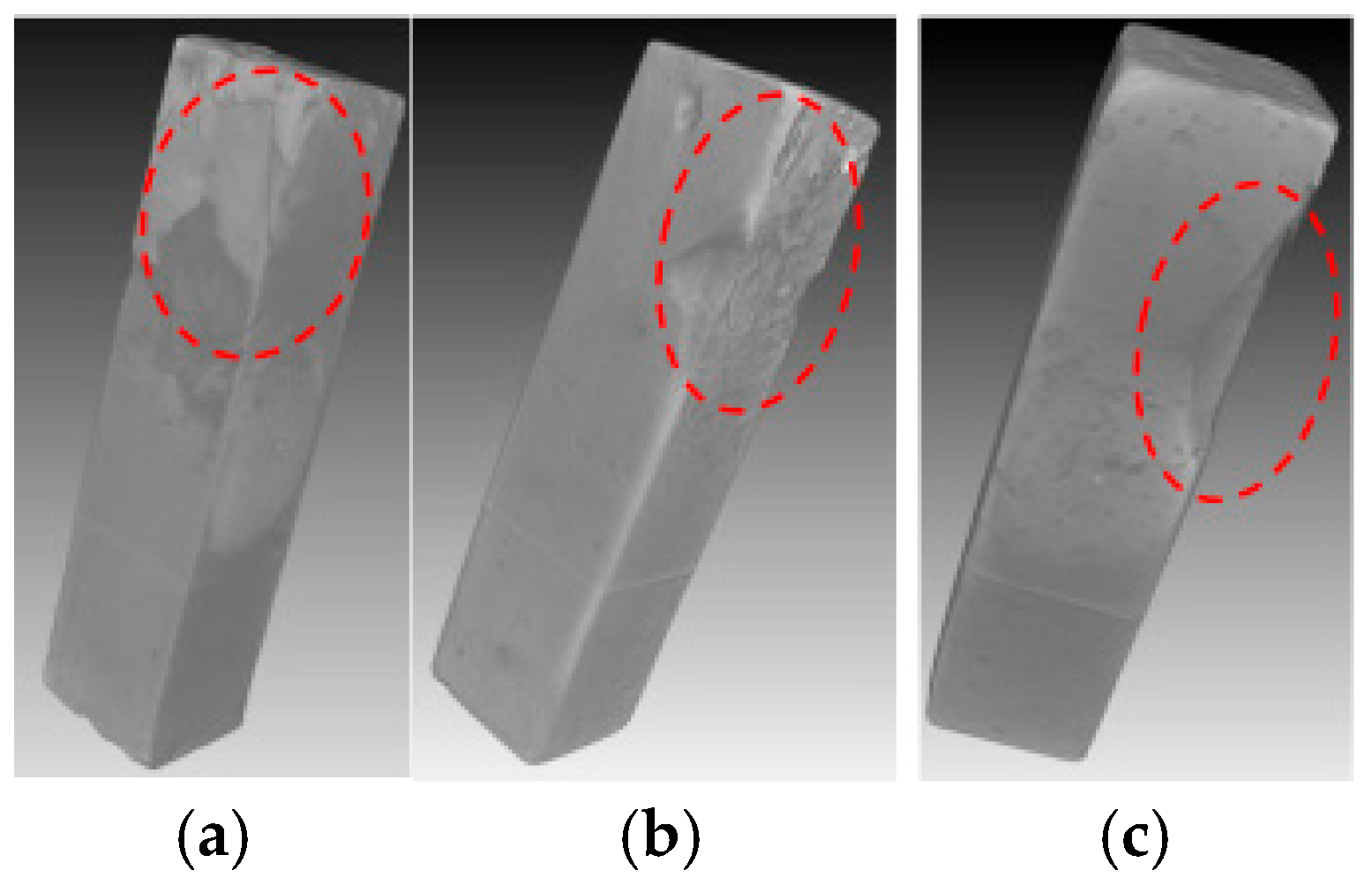
Figure 7 and Figure 8 compared the internal damage characteristics of the cement pastes with water-to-cement ratios of 0.4, 0.45, and 0.5 after 4 weeks of PSA erosion. The XCT results in Figure 7 showed that all three pastes exhibited surface spalling. Analysis of three representative cross-sections from specimen side surfaces to interiors (Figure 8) revealed that although no obvious penetrating cracks were observed inside any of the three paste specimens, the specimens with higher water-to-cement ratios (0.45 and 0.5) experienced severe spalling at edges, resulting in corner loss. Among these, the specimens with a water-to-cement ratio of 0.5 showed the most significant damage. This can be attributed to the higher water-to-cement ratio specimens having higher porosity and larger pore sizes [19], thus allowing NaCl solution to penetrate more easily into the interior of the cement-based materials. With the progress of PSA cycling, crystallization products gradually accumulated in the specimen surface layers, and when the generated crystallization pressure exceeded the local tensile strength of the material, more severe surface spalling ultimately occurred.
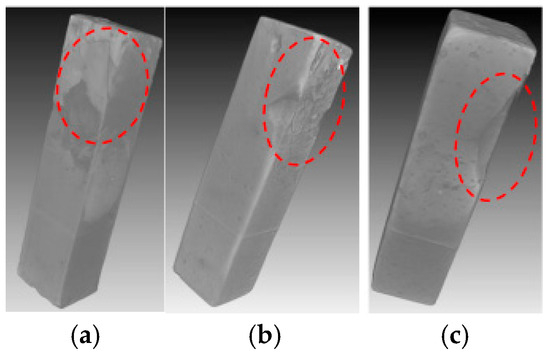
Figure 7.
XCT 3D image of the specimens with water-to-cement ratios of 0.4 (a), 0.45 (b), and 0.5 (c) immersed in NaCl solution for 4 weeks (the red dashed circles indicate the deterioration region).
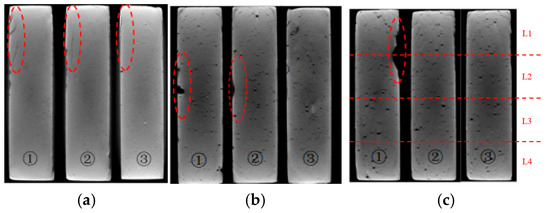
Figure 8.
Internal slices of the specimens with water-to-cement ratios of 0.4 (a), 0.45 (b), and 0.5 (c) semi-immersed in NaCl solution for 4 weeks (the red dashed circles indicate the deterioration region).
3.2.3. SEM
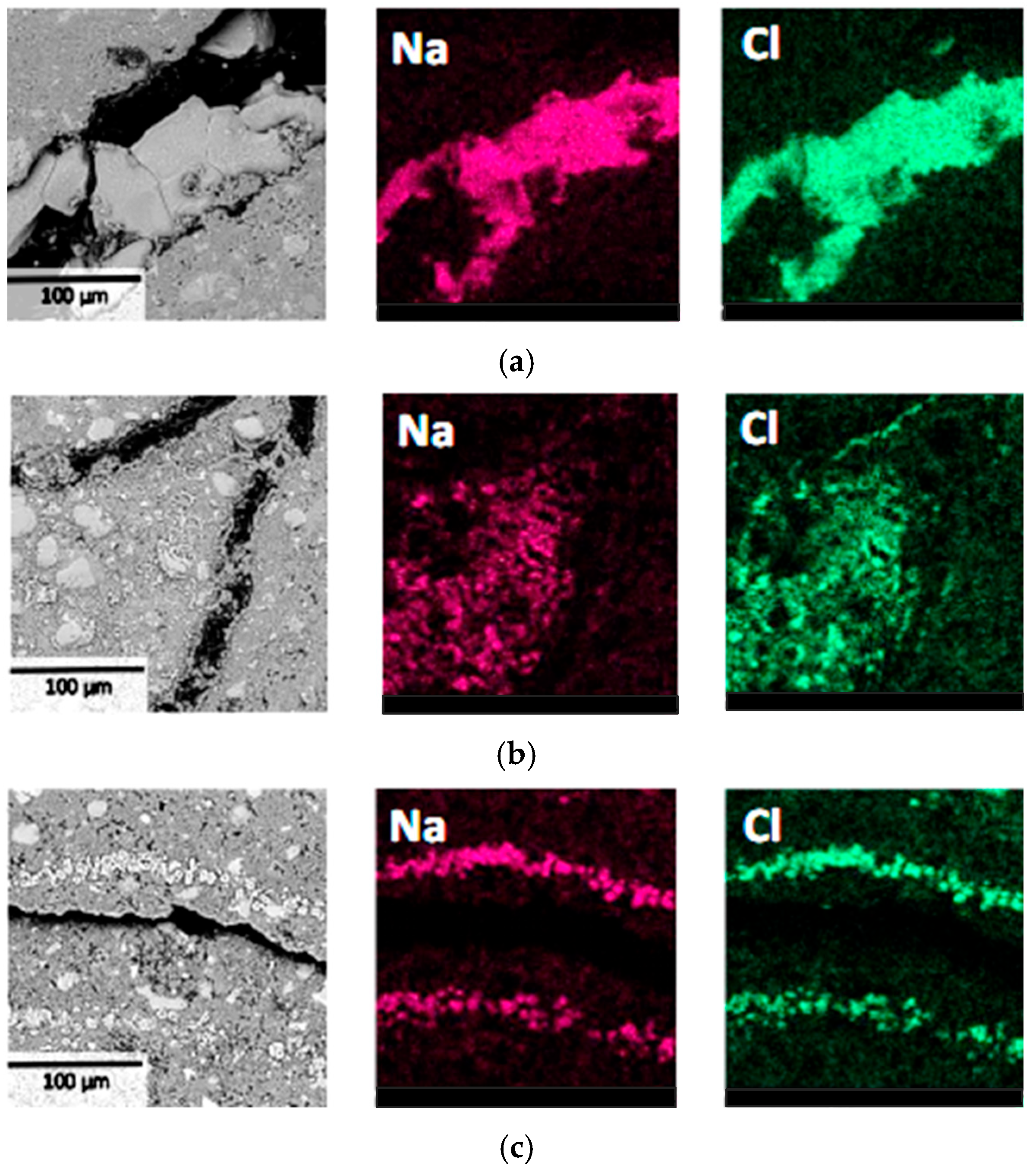
The BSE and EDS results of the specimens are shown in Figure 9. The results indicated that all three specimens with water-to-cement ratios of 0.4, 0.45, and 0.5 exhibited concentrated distribution of NaCl crystals with relatively large crystal sizes near surface deterioration regions. Notably, no NaCl crystallization with similar characteristics was observed in the undamaged internal areas of all the specimens. These results demonstrated that the macroscopic failure of specimens had a significant correlation with NaCl crystal formation and their distribution locations. Although the three groups of specimens had different water-to-cement ratios, the deterioration mechanisms remained essentially consistent [28], all caused by local stress concentration resulting from NaCl crystallization in surface regions. Therefore, it can be inferred that changes in water-to-cement ratio primarily affect the material’s resistance to PSA damage rather than altering its fundamental deterioration mechanism. This provides a theoretical foundation for establishing more rapid and effective laboratory PSA evaluation methods.
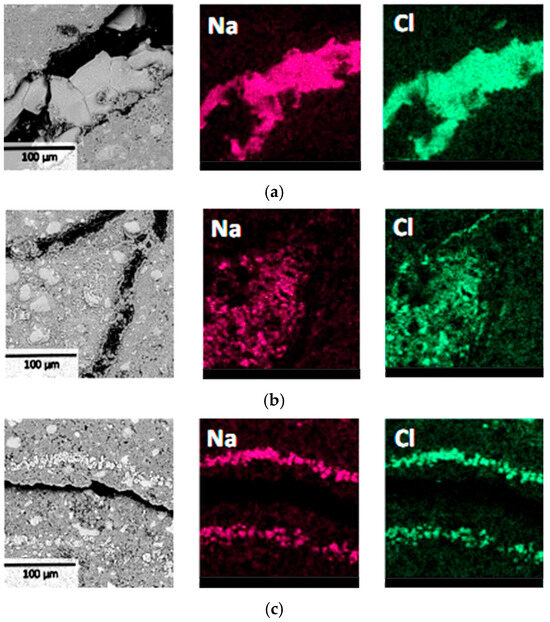
Figure 9.
BSE and EDS results of the surface layer at region L1 of cement pastes with water-to-cement ratios of 0.4 (a), 0.45 (b), and 0.5 (c).
3.3. Mechanism
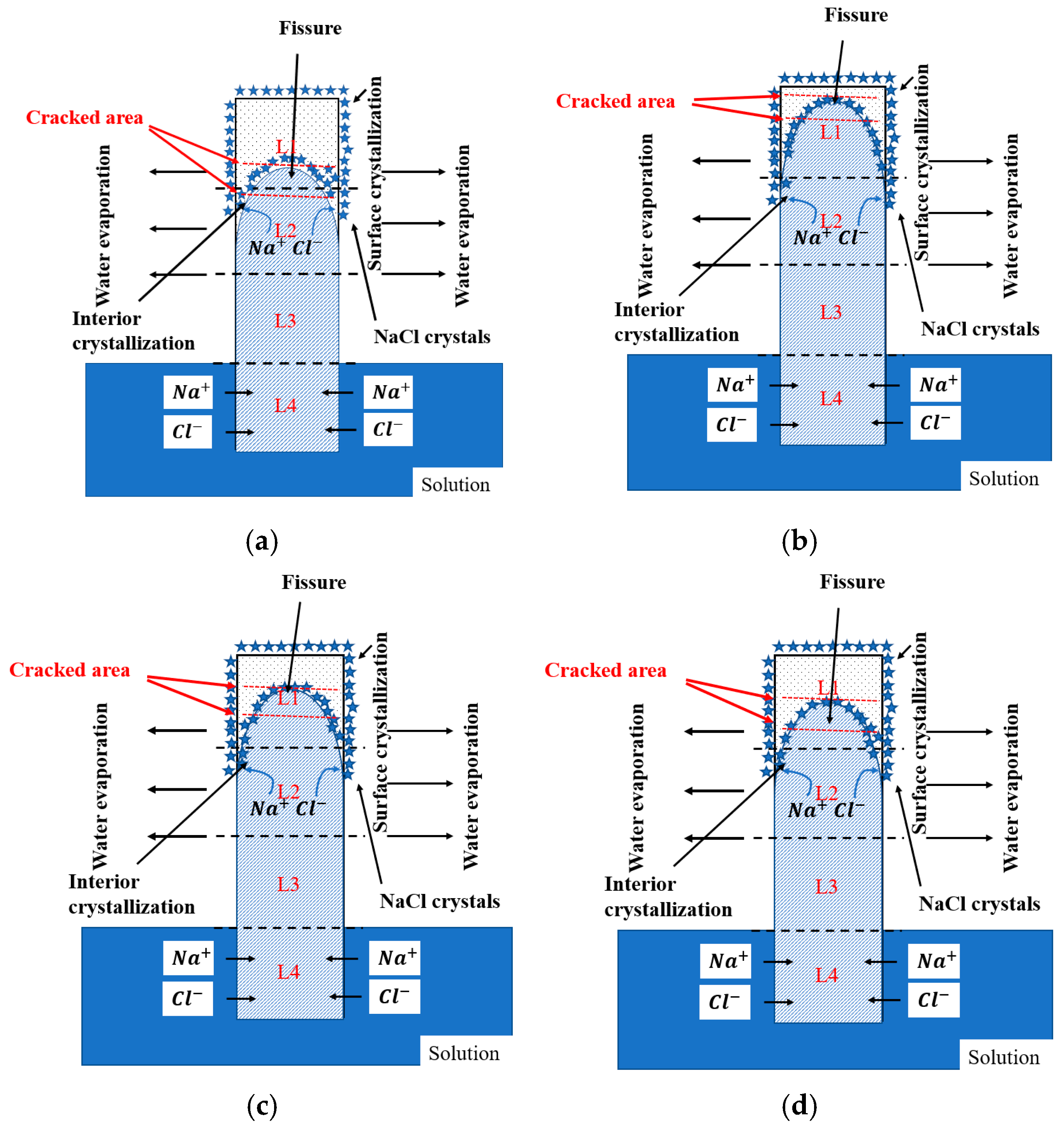
The migration patterns and formation mechanisms of the NaCl solution in the cement paste specimens are illustrated in Figure 10. According to the literature [35], NaCl solution entering the interior of paste specimens through capillary action progresses gradually from surface to interior. NaCl solution exhibits a distribution like a downward-opening parabola in the longitudinal cross-section direction. Specifically, NaCl solution enters the specimen interior through the capillary pore network in the paste. In the initial stage, the rising rate and height reached by the solution in capillary pores are consistent. However, as immersion time increases, when the solution reaches a certain height, its rising rate gradually slows, while simultaneously the water evaporation rate at the paste surface accelerates, leading to the increased solution concentration near the surface. At this point, part of the solution diffuses into the paste interior due to concentration gradients, while another part accumulates at the paste surface. Therefore, solution concentration at the paste surface gradually increases, and when supersaturation is reached, salt crystallization starts. Under the synergistic effects of water evaporation, capillary action, and ion diffusion, NaCl solution forms a characteristic downward-opening parabolic distribution pattern within paste specimens [36]. Furthermore, due to pore structure differences resulting from different specimen curing ages, the specimens cured for 28 days have finer pores compared to those cured for 7 days [37], and capillary forces are stronger in small pores than in large pores; therefore, the specimens with 28-day curing age have higher hydraulic head heights of internal solution.
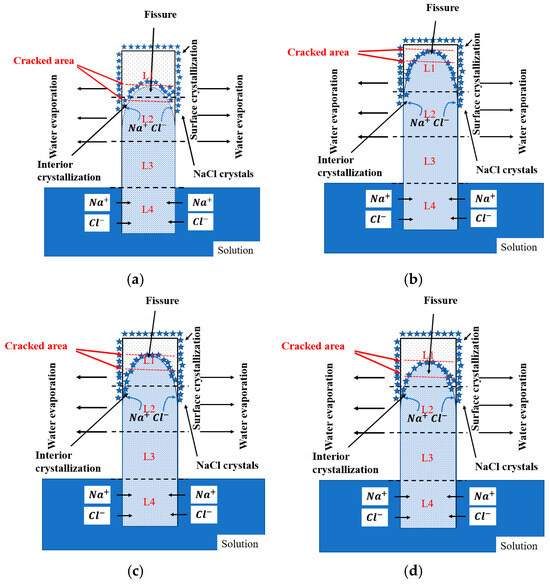
Figure 10.
Schematic illustration of the PSA deterioration process of cement paste specimens partially exposed to NaCl solution: (a) specimen with water-to-cement ratio of 0.4 cured for 7 days; (b) specimen with water-to-cement ratio of 0.4 cured for 28 days; (c) specimen with water-to-cement ratio of 0.45 cured for 28 days; (d) specimen with water-to-cement ratio of 0.5 cured for 28 days.
Additionally, it was also found that paste specimens with short curing ages cracked earlier and more severely than those with long curing ages, and salt crystal spalling was commonly observed at crack locations. This is primarily because paste specimens with short curing ages have higher porosity and lower strength due to lower hydration degree [31]. Higher porosity promotes the accumulation of more crystalline materials on one hand, generating greater crystallization pressure; on the other hand, lower strength makes pore walls in specimens capable of withstanding smaller tensile stresses; therefore, paste specimens with short curing ages exhibit more severe deterioration characteristics. It is noteworthy that the paste specimens cured for 28 days did not exhibit obvious internal cracking even under conditions of relatively high water–cement ratios, indicating that adequate curing can significantly improve specimens’ PSA resistance performance. However, as the water–cement ratio increases, paste surface spalling becomes more severe, and spalling occurrence time is also advanced. This is because larger water–cement ratios result in more loose pore distributions, making NaCl solution more likely to penetrate pore channels in the paste interior, thereby leading to increased crystallization products and making specimen surface spalling more pronounced.
4. Discussion
Curing age and water-to-cement ratio significantly influence the PSA damage behavior of cement paste specimens in semi-immersion NaCl solution environments. The experimental results demonstrate that the specimens cured for 7 days exhibited accelerated deterioration compared to those cured for 28 days, with damage occurring after 2 weeks rather than 4 weeks, thereby reducing testing duration by 50%. Similarly, among the specimens cured for 28 days, those with a water-to-cement ratio of 0.5 demonstrated deterioration approximately one week earlier than specimens with a water-to-cement ratio of 0.4, resulting in a 25% reduction in testing time. Despite these variations in curing age and water-to-cement ratio, the SEM analysis revealed that the fundamental deterioration mechanisms remained consistent across all the specimens.
These findings establish that adjusting the curing age and water-to-cement ratio of cement-based materials constitutes an effective strategy for enhancing the efficiency of accelerated PSA testing in laboratory environments. The proposed methodology offers advantages over conventional approaches, as it does not require high salt concentrations or aggressive environmental conditions, thereby providing a better representation of actual engineering curing conditions. Consequently, this approach is well-suited for rapid durability assessment of marine concrete structures subjected to PSA, including ports and bridges, etc. Implementation of this method enables significant reductions in both time requirements and economic costs associated with material selection for PSA resistance and durability pre-evaluation.
Moreover, it was observed that 28-day curing significantly enhances concrete PSA resistance, while low water-to-cement ratio (0.4) specimens exhibited a reduction in surface spalling compared to high water-to-cement ratio (0.5) specimens. This provides valuable guidance for practical engineering: coastal projects should ensure at least 28 days of curing whenever possible and avoid early exposure to salt environments, such as by using precast concrete structures. Additionally, lower water-to-cement ratios (≤0.45) should be prioritized to enhance structural salt attack resistance and extend service life.
Furthermore, deterioration was observed to occur primarily in areas with reduced surface salt crystallization, providing a visual indicator for durability monitoring in engineering applications. By regularly observing the distribution of salt crystallization on structural surfaces, potential deterioration (such as in crack-prone areas) can be predicted, enabling proactive maintenance strategies (such as locally repairing and surface coating) to reduce engineering downtime and maintenance costs due to sudden deterioration.
Finally, it should be noted that compared to the reported deterioration time ranging from several months to several years in laboratory environments, the PSA deterioration time of specimens in NaCl solution in this work can be significantly reduced to 4 weeks or less. The reduction in deterioration time is attributed not only to adjusting the curing age and water-to-cement ratio, but also probably to the use of smaller specimen dimensions and more effective humidity cycling strategies.
5. Conclusions
This study investigated the PSA damage behavior of cement-based materials with different curing ages and water–cement ratios under NaCl solution semi-immersion conditions. Based on the research results, the main conclusions are as follows:
- (1)
- Specimens with shorter curing ages and higher water-to-cement ratios exhibited accelerated deterioration behavior. By adjusting the curing age and water-to-cement ratio, the PSA deterioration time of specimens in NaCl solution was reduced by 25–50%. Importantly, the SEM analysis revealed that despite these variations in curing age and water-to-cement ratio, the fundamental deterioration mechanisms remained consistent across all the specimens. These findings demonstrate that adjusting the curing age and water-to-cement ratio of cement-based materials constitutes an effective strategy for enhancing the efficiency of accelerated PSA testing in laboratory environments. Implementation of this methodology enables significant reductions in both time requirements and economic costs associated with cement-based material selection for PSA resistance and preliminary durability evaluation.
- (2)
- Adequate curing is crucial for enhancing the early resistance of cement-based materials to physical salt crystallization attack. In practical engineering, particularly for concrete structures in coastal environments, a sufficient curing age is better ensured (at least reaching 28 days of curing) to prevent structures from being exposed to salt erosion environments during early stages when strength is insufficient and porosity is high, thereby effectively delaying the occurrence of cracking failure.
- (3)
- Local crystallization behavior on specimen surfaces during NaCl attack is directly related to macroscopic damage. Monitoring external crystallization distribution patterns can predict potential deterioration locations, providing reliable visual indicators for PSA service life assessment.
- (4)
- It is worth noting that the main conclusions of this study are based on cement paste specimens. When considering PSA on cement-based materials containing aggregates, such as mortar or concrete, the additional effects of PSA on the interfacial transition zone (ITZ) must be taken into account to comprehensively evaluate the overall impact of PSA on these materials, and this will be our future work.
Author Contributions
Conceptualization, H.S.; Investigation, S.L. and Z.L.; Data curation, W.K. and Y.C.; Writing—original draft, W.K. and S.L.; Writing—review & editing, S.Y. and Y.W.; Project administration, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (Grant Nos. 52178230 and 52478269), the Shenzhen Science and Technology Research and Development Fund (Grant No. 20220815102613001), the Guangdong Provincial Key Laboratory of Durability for Marine Civil Engineering (SZU) (Grant No. 2020B1212060074), and the Shenzhen Key Laboratory for Low-carbon Construction Material and Technology (Grant No. ZDSYS20220606100406016).
Data Availability Statement
The data presented in this study are available upon request from the author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sakr, M.; Bassuoni, M.; Hooton, R.; Drimalas, T.; Haynes, H.; Folliard, K. Physical salt attack on concrete: Mechanisms, influential factors, and protection. ACI Mater. J. 2020, 117, 253–268. [Google Scholar] [CrossRef]
- Jiang, X.; Mu, S.; Liu, J. Influence of chlorides and salt concentration on salt crystallization damage of cement-based materials. J. Build. Eng. 2022, 61, 105260. [Google Scholar] [CrossRef]
- Thaulow, N.; Sahu, S. Mechanism of concrete deterioration due to salt crystallization. Mater. Charact. 2004, 53, 123–127. [Google Scholar] [CrossRef]
- Niu, L.; Gao, S.; Guan, J.; Li, L.; Qian, J. Impact of Salt Crystallization on the Mechanical Properties of Masonry. J. Mater. Civ. Eng. 2025, 37, 04025120. [Google Scholar] [CrossRef]
- Alam, M.; Islam, M.; Islam, M. Effect of Pre-curing on Strength Development of Brick Aggregate Concrete in Sea Water Environment. In Advances in Civil Engineering: Select Proceedings of ICACE 2020; Springer: Singapore, 2022; pp. 255–266. [Google Scholar]
- Kong, D.; Xu, L.; Wu, H.; Zeng, F.; Zhou, Y.; Gu, C. Effect of admixture on the properties of marine HPC under crystallization of chloride salt. J. Zhejiang Univ. Technol. 2024, 52, 479–486. [Google Scholar]
- Zanqun, L.; Xiangning, L.; Le, H.; Binlin, S.; Jianhua, D. Accelerating effect of fly ash on damage of concrete partially immersed to sulfate environment. J. Build. Mater. 2017, 20, 439–443+448. [Google Scholar]
- Zhanqun, L.; Min, P.; Fengyan, Z.; Dehua, D. Comparison of chemical attack products in different zones of cement paste partially immersed in Na2SO4 solution. J. Build. Mater. 2020, 23, 485–492. [Google Scholar]
- Lubelli, B.; Cnudde, V.; Diaz-Goncalves, T.; Franzoni, E.; van Hees, R.P.; Ioannou, I.; Menendez, B.; Nunes, C.; Siedel, H.; Stefanidou, M. Towards a more effective and reliable salt crystallization test for porous building materials: State of the art. Mater. Struct. 2018, 51, 55. [Google Scholar] [CrossRef]
- Sakr, M.; Bassuoni, M. Performance of concrete under accelerated physical salt attack and carbonation. Cem. Concr. Res. 2021, 141, 106324. [Google Scholar] [CrossRef]
- Jiang, X.; Mu, S.; Guo, Z.; Liu, G. Effect of temperature on the physical salt attack of cement mortars under repeated partial immersion in sodium sulfate solution. Materials 2022, 15, 6234. [Google Scholar] [CrossRef]
- Newtson, C.; Mousavinezhad, S.; Toledo, W.; Aguayo, F. Accelerated Sulfate Attack Testing for Concrete. 2023. Available online: https://repository.lsu.edu/transet_pubs/155 (accessed on 5 August 2025).
- Bassuoni, M.T.; Rahman, M. Response of concrete to accelerated physical salt attack exposure. Cem. Concr. Res. 2016, 79, 395–408. [Google Scholar] [CrossRef]
- Qin, J.; Jiang, J.; Tao, Y.; Zhao, S.; Zeng, W.; Shi, Y.; Lu, T.; Guo, L.; Wang, S.; Zhang, X. Sunlight tracking and concentrating accelerated weathering test applied in weatherability evaluation and service life prediction of polymeric materials: A review. Polym. Test. 2021, 93, 106940. [Google Scholar] [CrossRef]
- Flatt, R.J. Salt damage in porous materials: How high supersaturations are generated. J. Cryst. Growth 2002, 242, 435–454. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Coussy, O. Deformation and stress from in-pore drying-induced crystallization of salt. J. Mech. Phys. Solids 2006, 54, 1517–1547. [Google Scholar] [CrossRef]
- Chen, Z.; Zou, H.; Liu, Y.; Gai, J.; Basquiroto de Souzae, F.; Sagoe-Crentsil, K.; Neild, A.; Duan, W. Crystallization of sodium sulfate in two-dimensional interconnected pore system: Insights into localized, transient, and anisotropic stress generation and material deterioration. Phys. Rev. Appl. 2024, 22, 034065. [Google Scholar] [CrossRef]
- Abuzeid, M. Physical Salt Attack on Concrete: Field Case Study and Innovative Mitigations. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2022. [Google Scholar]
- Gonçalves, T.D. Salt Crystallization in Plastered or Rendered Walls. Ph.D. Thesis, Universidade Técnica de Lisboa, Lisbon, Portugal, 2007. [Google Scholar]
- Haynes, H.; O’Neill, R.; Neff, M.; Mehta, P.K. Salt Weathering of Concrete by Sodium Carbonate and Sodium Chloride. ACI Mater. J. 2010, 107, 258–266. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Doehne, E. Salt weathering: Influence of evaporation rate, supersaturation and crystallization pattern. Earth Surf. Process. Landf. 1999, 24, 191–209. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—I: The crystallization pressure of large crystals. J. Cryst. Growth 2005, 282, 455–469. [Google Scholar] [CrossRef]
- Kunlin, M. Mechanism and Evaluation Method of Salt Crystallization Attack on Concrete. Ph.D. Thesis, Central South University, Changsha, China, 2009. [Google Scholar]
- Yang, H.; Liu, W.; Yu, H.; Wang, W.; Ma, H. Study on Mass and Performance Deterioration of Concrete Under Multiple Corrosive Environments. Materials 2025, 18, 1931. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, Z.; Wei, F.; Wen, X.; Li, X.; Dai, L.; Liu, L. Study on concrete deterioration in different NaCl-Na2SO4 solutions and the mechanism of Cl− diffusion. Materials 2021, 14, 5054. [Google Scholar] [CrossRef]
- Kliukas, R.; Jaras, A.; Lukoševičienė, O. The Impact of Long-Term Physical Salt Attack and Multicycle Temperature Gradient on the Mechanical Properties of Spun Concrete. Materials 2021, 14, 4811. [Google Scholar] [CrossRef] [PubMed]
- Lubelli, B.A. Sodium Chloride Damage to Porous Building Materials. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2006. [Google Scholar]
- Gonçalves, T.D.; Pel, L.; Rodrigues, J.D. Influence of paints on drying and salt distribution processes in porous building materials. Constr. Build. Mater. 2009, 23, 1751–1759. [Google Scholar] [CrossRef]
- Tran, D.L.; Mouret, M.; Cassagnabère, F.; Phung, Q.T. Effects of intrinsic granular porosity and mineral admixtures on durability and transport properties of recycled aggregate concretes. Mater. Today Commun. 2022, 33, 104709. [Google Scholar] [CrossRef]
- Peng, L.; Zhiwu, Y.; Lingkun, C. Influence of curing age on properties and micro structure of concrete. J. Build. Mater. 2012, 15, 717–723. [Google Scholar]
- Wei, L.; Feng, X.; Youjun, X. Effect of water to cement ratio and mineral admixtures on the porosity of concrete. Low Temp. Archit. Technol. 2006, 1, 9–11. [Google Scholar]
- GB 748-2005; Sulfate Resistance Portland Cement. Standardization Administration of China: Beijing, China, 2005.
- Sun, H.; Liu, S.; Cao, K.; Yu, D.; Memon, S.A.; Liu, W.; Zhang, X.; Xing, F.; Zhao, D. Degradation mechanism of cement mortar exposed to combined sulfate–chloride attack under cyclic wetting–drying condition. Mater. Struct. 2021, 54, 138. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, F.; Wu, X.J.; Hu, Z.P.; Li, X.G.; Gao, L.L. Study on Concrete Deterioration and Chloride Ion Diffusion Mechanism by Different Aqueous NaCl-MgSO4 Concentrations. Buildings 2022, 12, 1843. [Google Scholar] [CrossRef]
- Chen, S.J.; Ren, J.X.; Ren, X.; Li, Y.G. Deterioration laws of concrete durability under the coupling action of salt erosion and drying-wetting cycles. Front. Mater. 2022, 9, 1003945. [Google Scholar] [CrossRef]
- Shunli, B.; Song, G.; Yaoyao, G.; Jian, C.; Lei, Z. Fractal evaluation of hydration age and admixtures on concrete porosity. Concrete 2023, 1, 13–16. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).