Experimental Evaluation of Thermal and Moisture Behavior of Stearic Acid-Coated Expanded Perlite for Sustainable Insulation Mortars
Abstract
1. Introduction
2. Materials and Methods
- Manual sieving of EP (ECP and EFP);
- Coating of ECPs with different SA coating/EP ratios (Group 1 and Group 2);
- Coating of EFPs with different SA coating/EP ratios (Group 3 and Group 4);
- Water-repellent performance analysis for Groups 1, 2, 3, and 4;
- Preparation of mortars with ECP (MC);
- Preparation of mortars with EFP (MF);
- Compressive strength tests for MCs and MFs;
- Capillary water-absorption tests for MCs and MFs;
- Thermal conductivity measurements of MCs and MFs under dry conditions and different moisture contents.
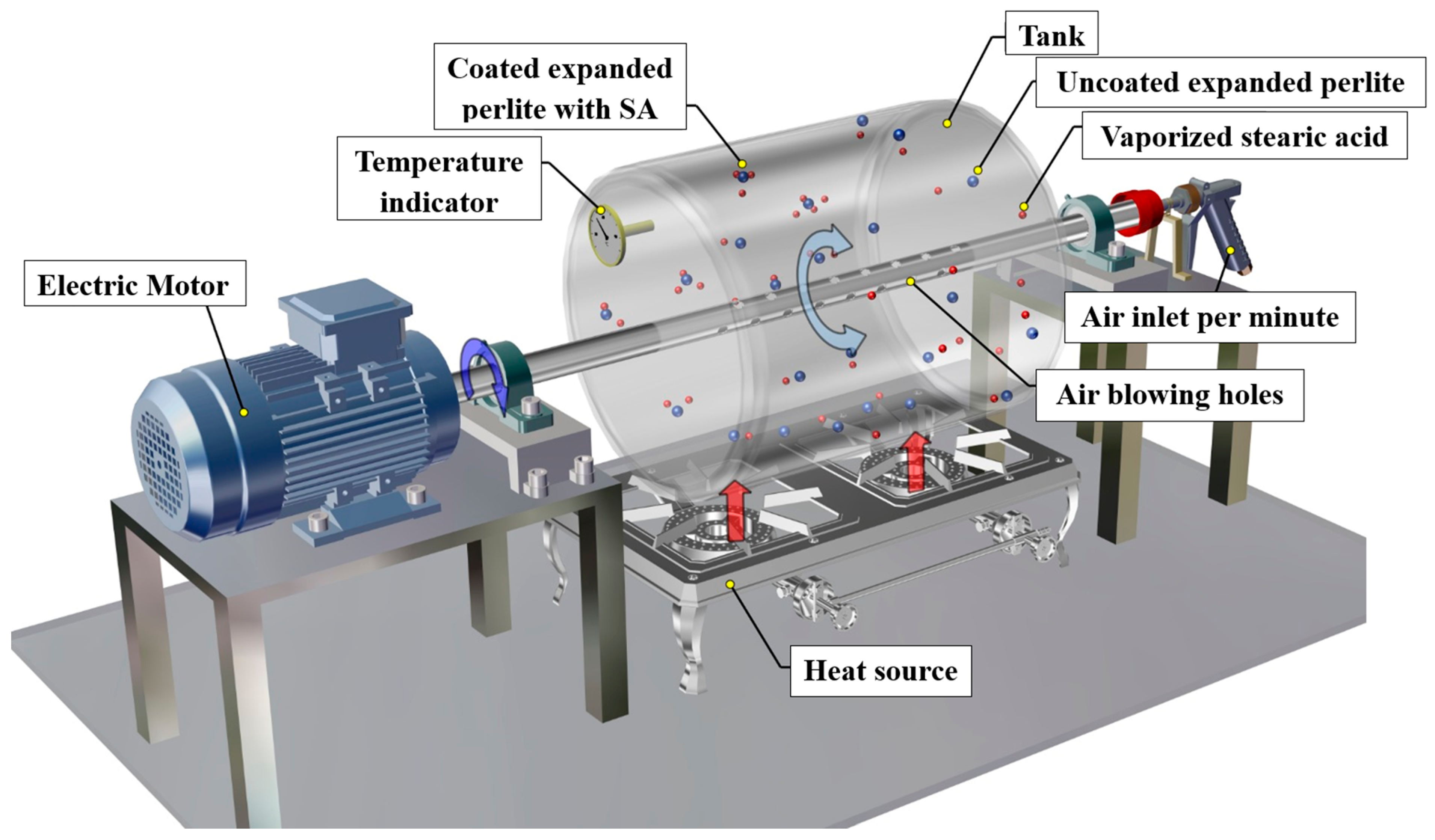
2.1. Coating Procedure of EP Particles
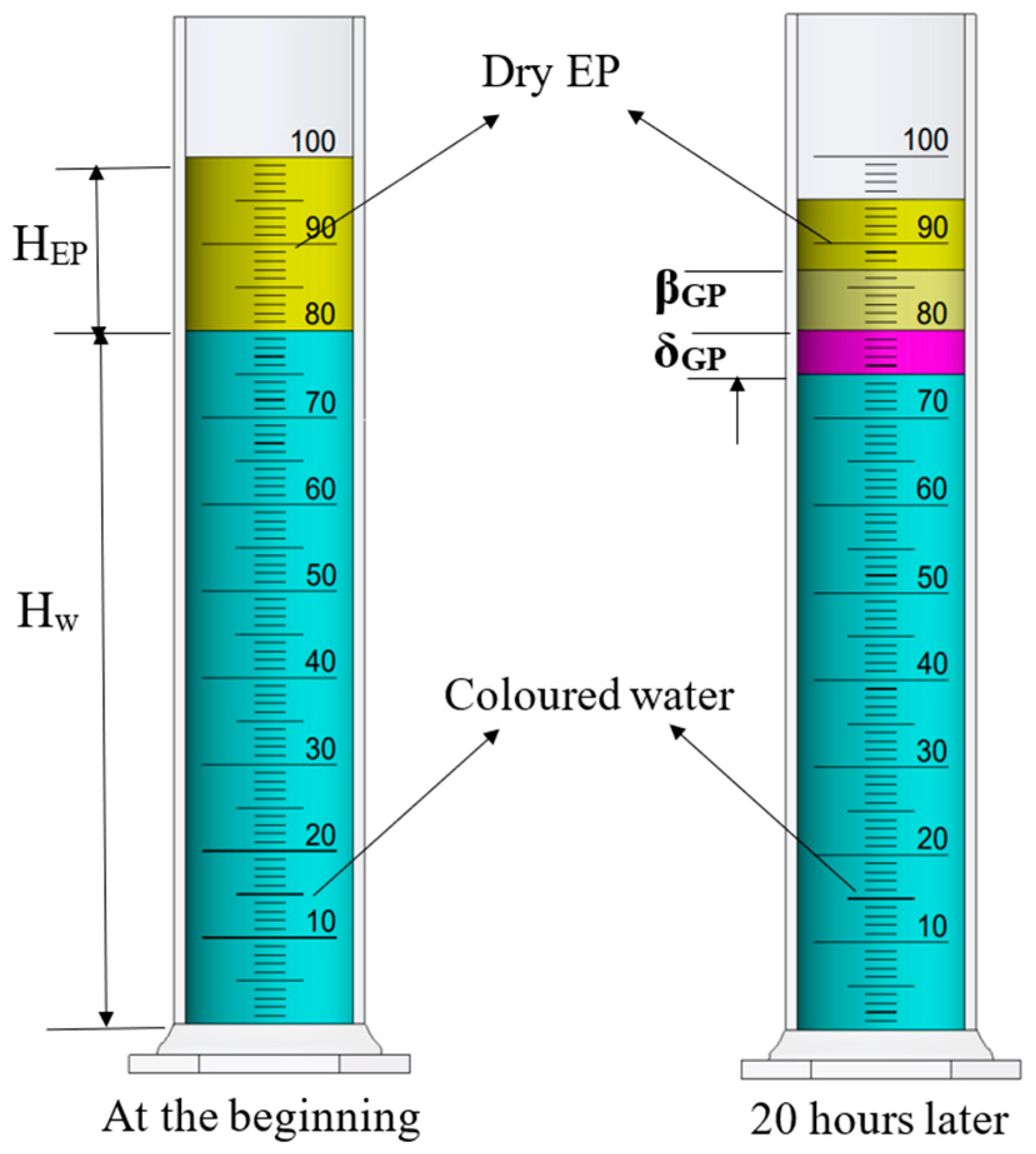
2.2. Water-Repellent Performance Analysis
2.3. Mixture Design for Mortar
2.3.1. Determination of Fresh Mortar Consistency
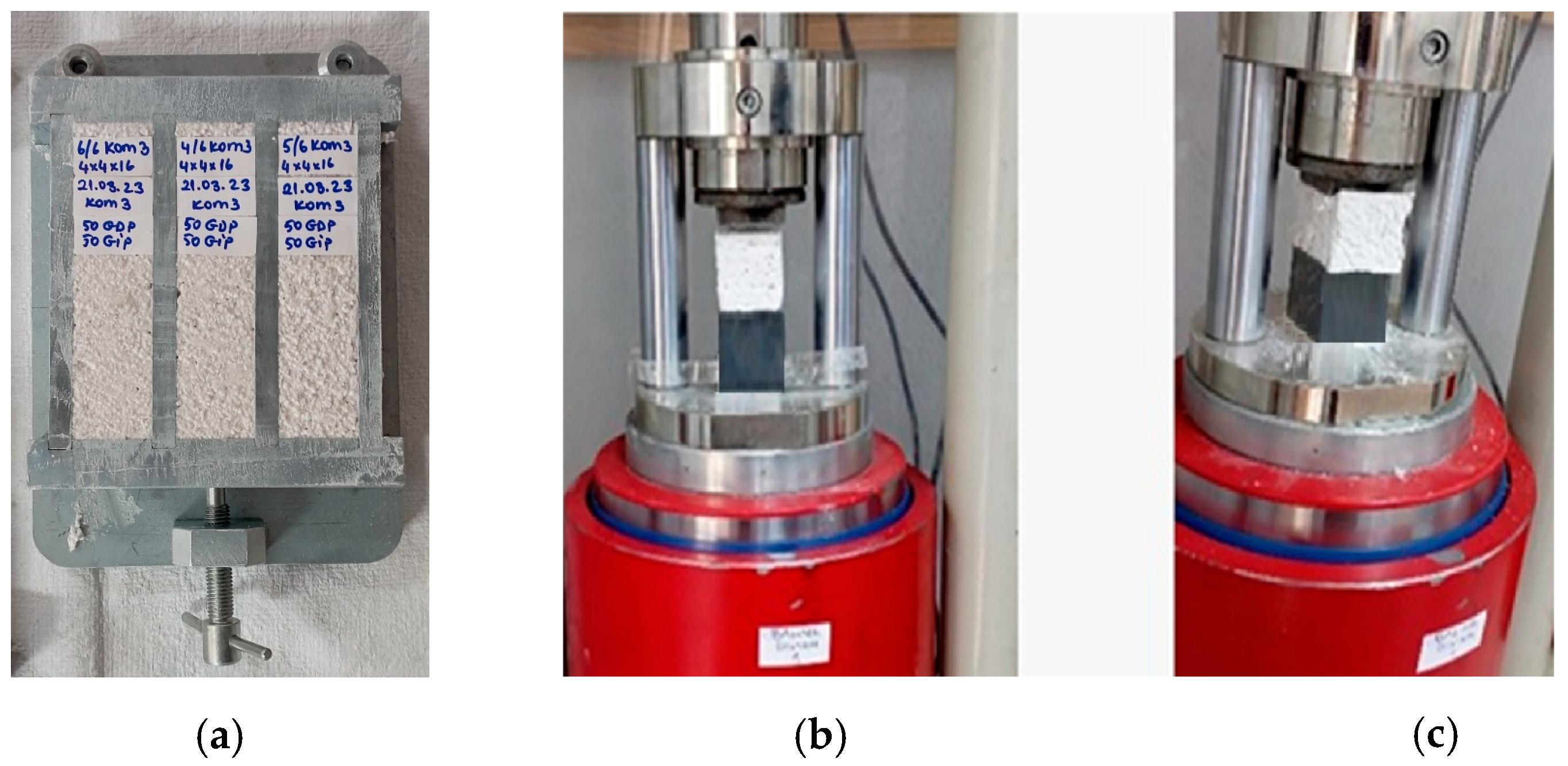
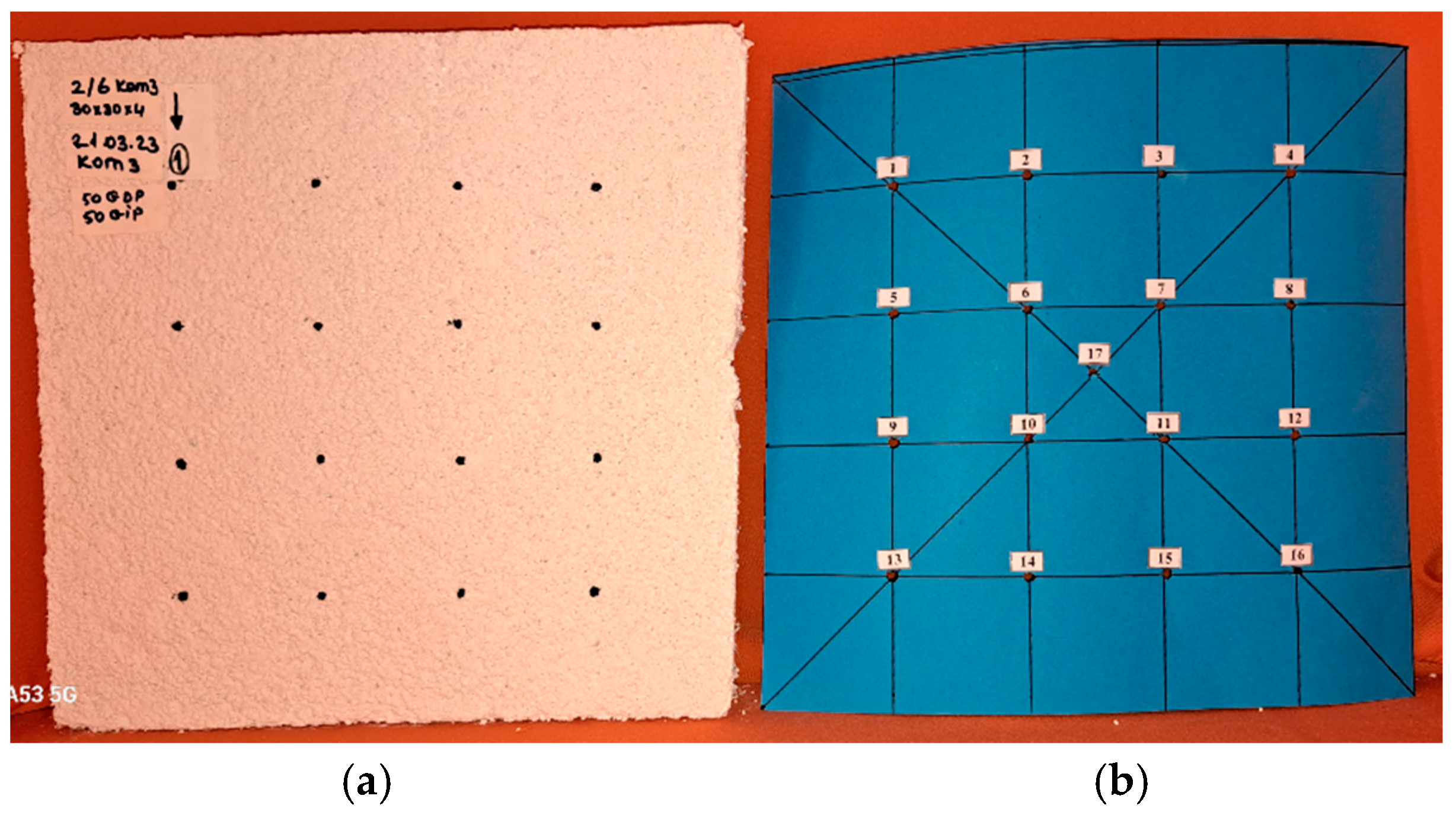
2.3.2. Determination of the Compressive Strength
2.3.3. Capillary Water-Absorption Test
2.3.4. Thermal Conductivity Measurement
3. Results and Discussion
3.1. Performance Analysis for Coated EP
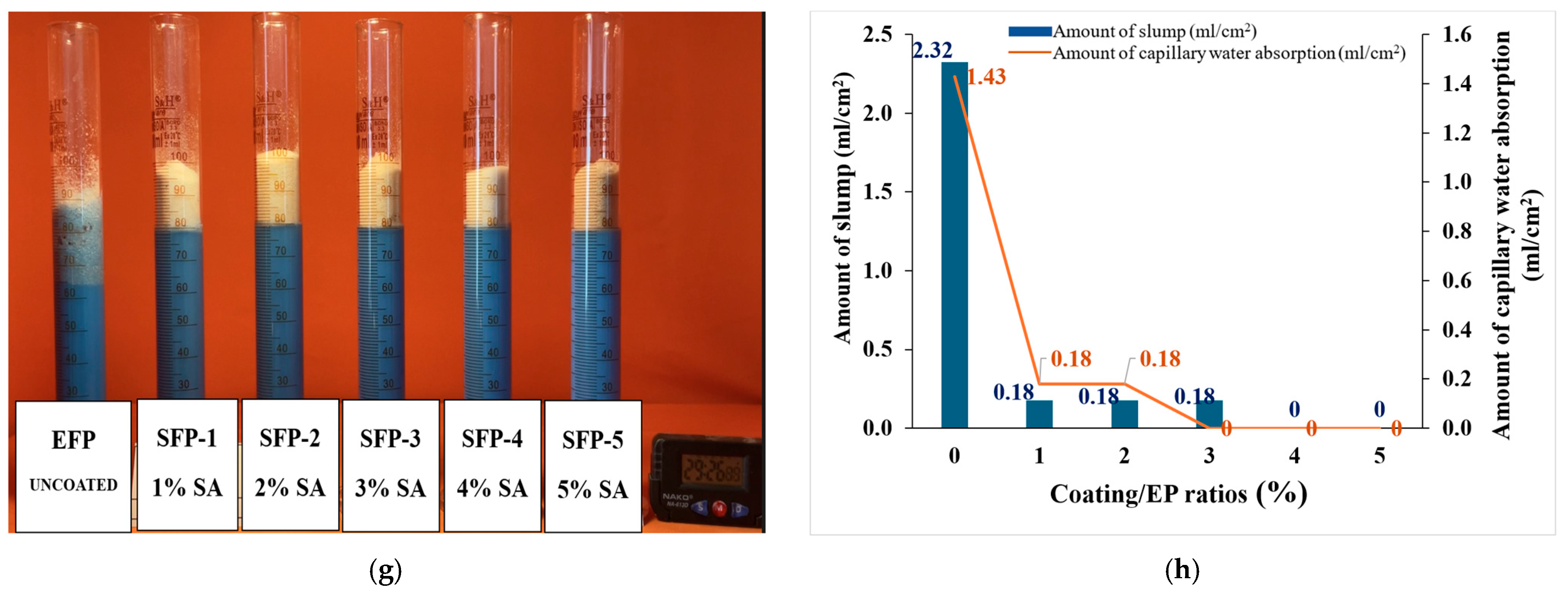
3.1.1. Comparative Analysis of Slump and Capillary Water Absorption Amounts for Coated and Uncoated EP Particles
3.1.2. Comparative Analysis of Slump and Capillary Water Absorption Amounts Between Coarse and Fine EP Particles
3.2. Analysis of Mixture Compositions of Coarse and Fine Mortars
3.2.1. Effect of SA Coating on Compressive Strength
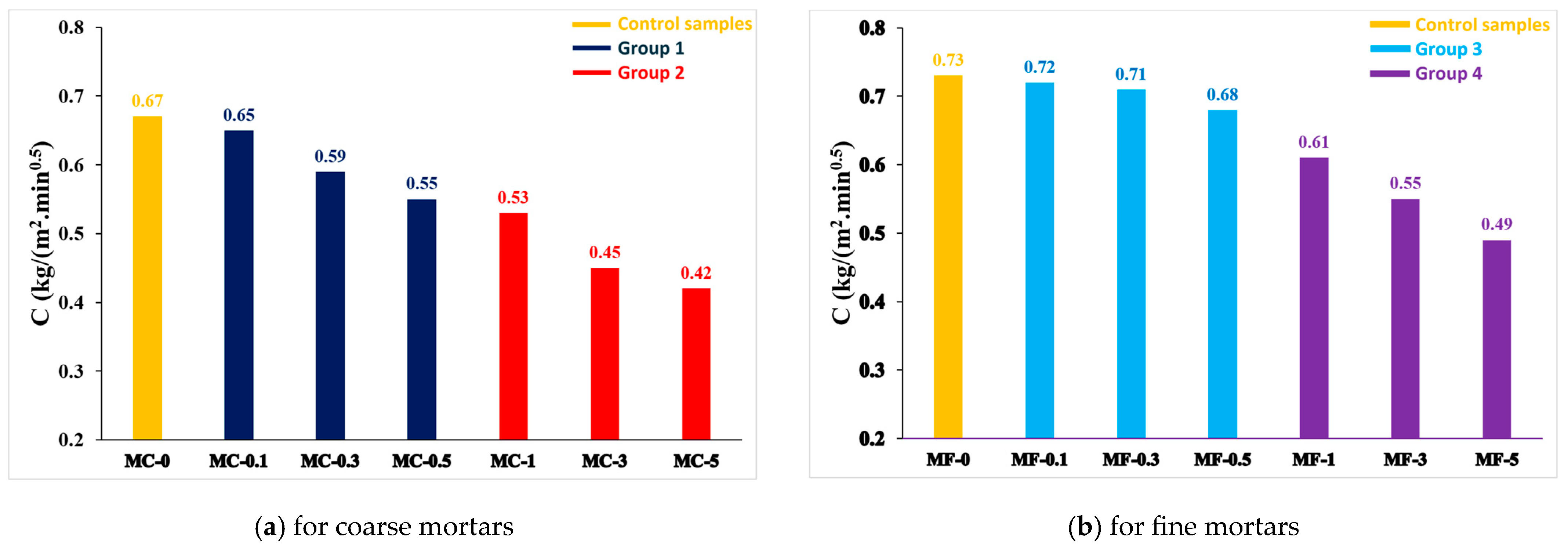
3.2.2. Performance Analysis of Water-Repellent Properties
- Comparative analysis of capillary water-absorption behavior for coated and uncoated mortars
- Comparative analysis of capillary water-absorption behavior between coarse and fine mortars
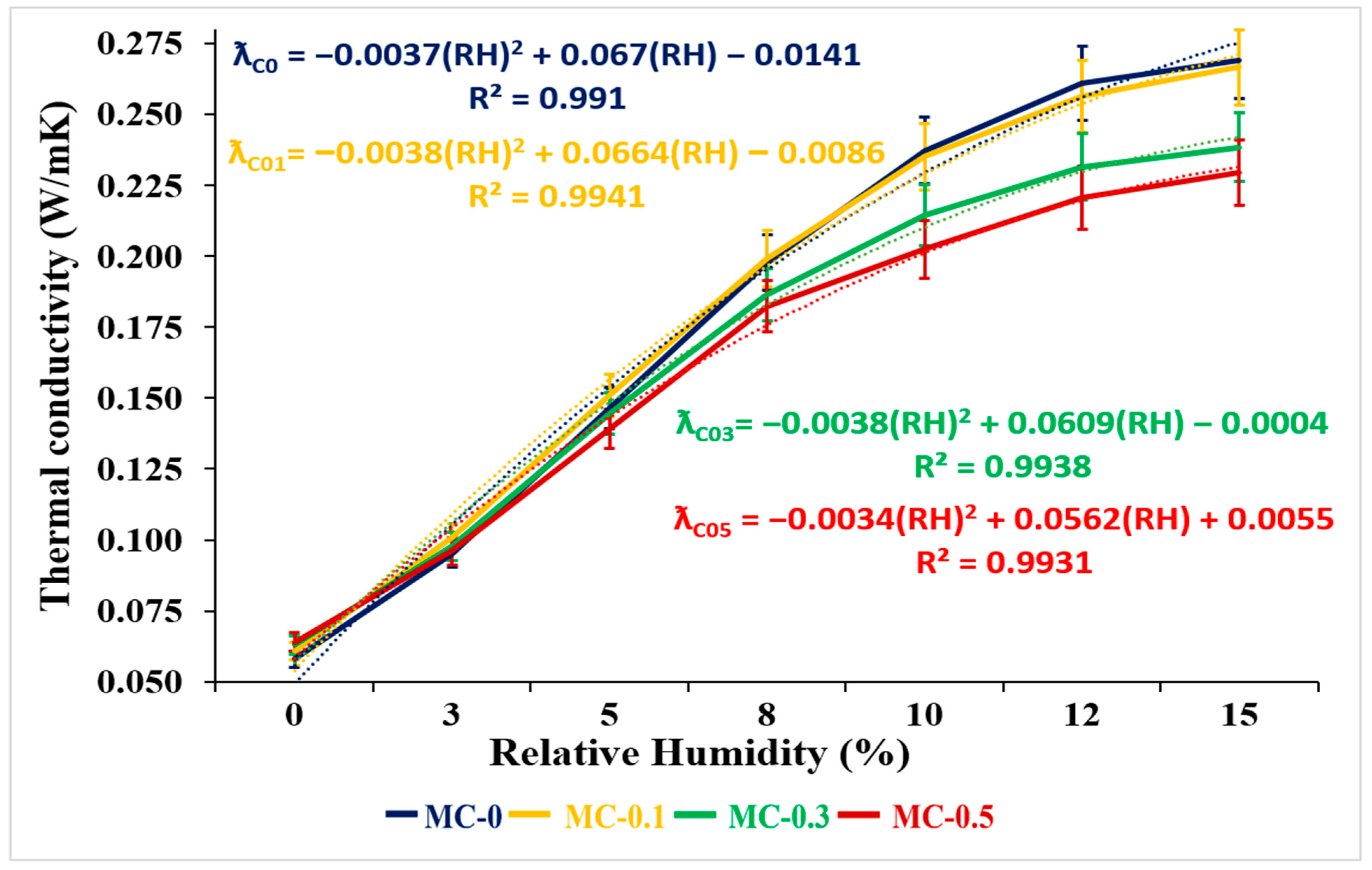
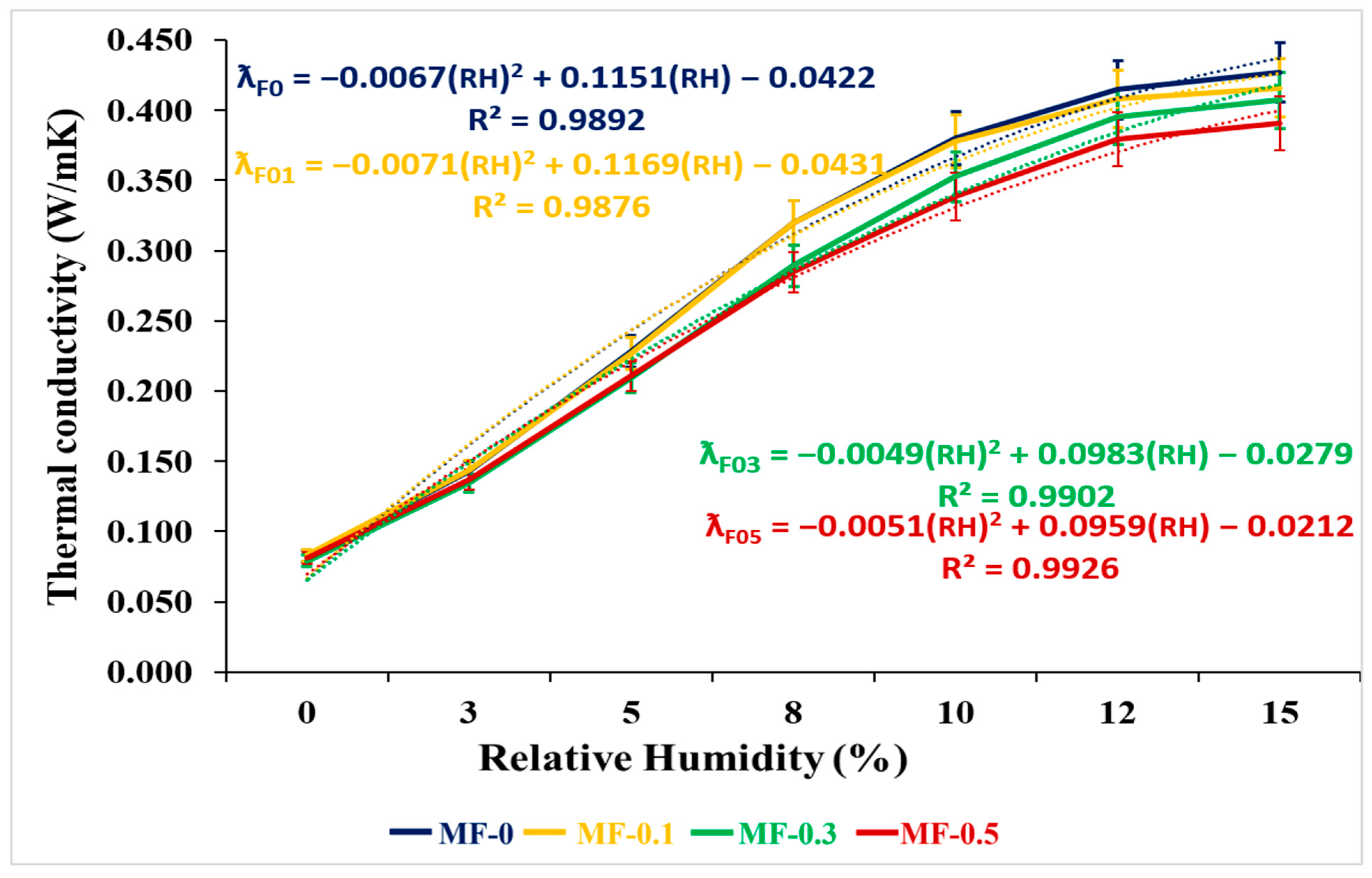
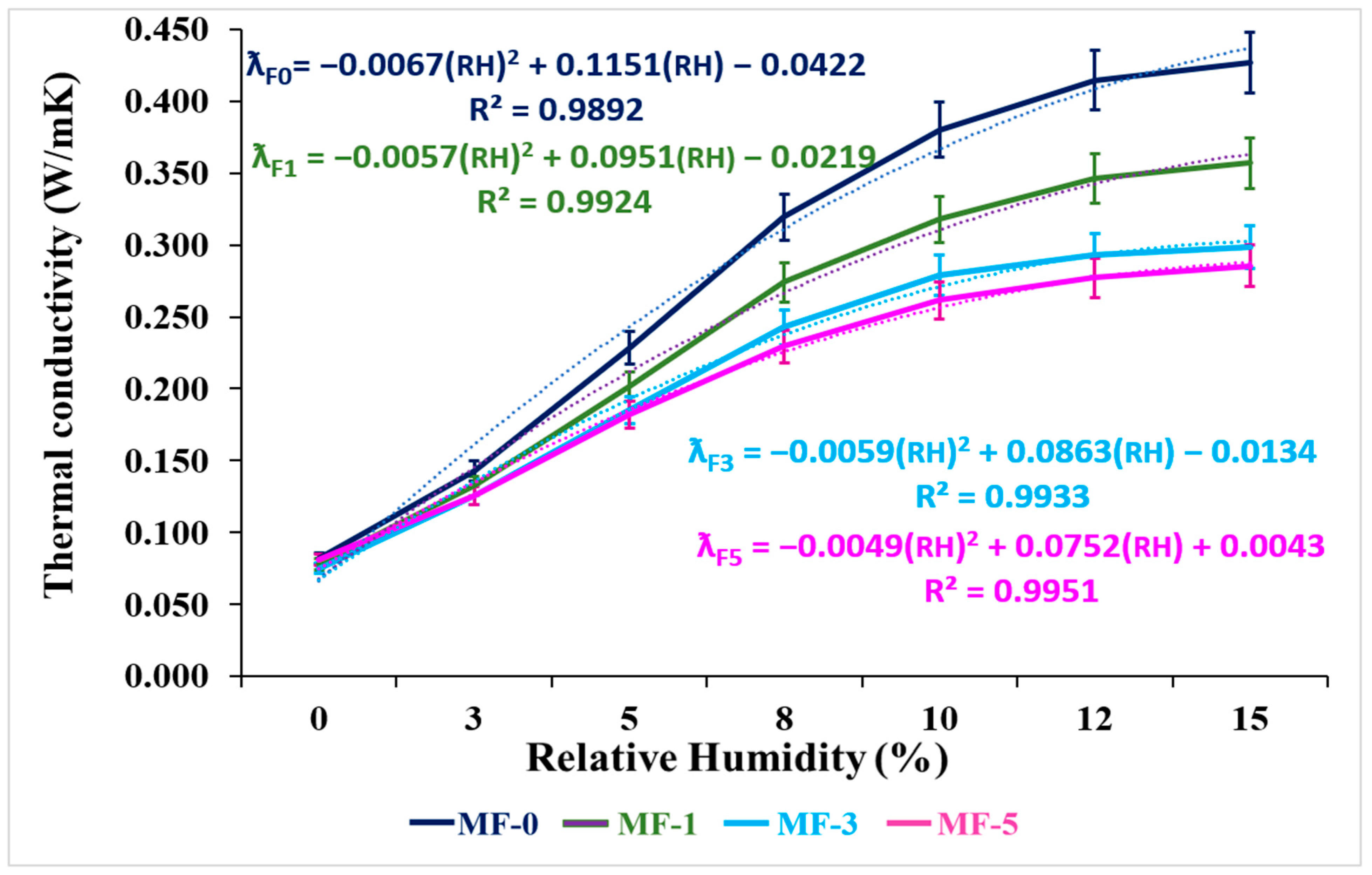
3.2.3. Thermal Conductivity Analysis
4. Conclusions
- (1)
- Both the slump and the capillary water absorption amounts decreased in SA-coated EP particles compared to the uncoated ones.
- (2)
- Water absorption, which is an undesirable property, was significantly reduced when coated with SA, and even SCP showed almost no water absorption behavior at coatings above 2%. These findings will provide valuable contributions to both industrial applications and the literature on SEP.
- (3)
- A significant improvement in compressive strength was observed at coating/EP ratios of 3% and above. In addition, higher strength values were obtained in mortars prepared using coated fine perlite.
- (4)
- When MC-0 was compared with the samples of MC-0.1, -0.3, -0.5 and MC-1, -3, -5, the capillary water-absorption coefficient decreased with the increase in the coating/EP ratio, and thus the water-repellent property improved. In addition, a similar behavior was observed when MF-0 was compared with the samples of MF-0.1, -0.3, -0.5 and MF-1, -3, -5.
- (5)
- The capillary water-absorption coefficient decreased, and therefore the water-repellent performance improved as the SA coating/EP ratio increased. Although it is stated in the literature that water-repellent performance is not optimal at a coating/EP ratio of over 3%, it was observed that the water-repellent performance increased at a coating/EP ratio of 5% with the coating method used in this study.
- (6)
- The thermal conductivity values of the uncoated samples were higher. Additionally, the STP value of MF-0.1 increased by a factor of 3.73 compared to MC-0.1, while the STP value of MC increased 3.24-, 1.78-, 1.44-, 1.23-, and 1.30-fold for coating/EP ratios of 0.3%, 0.5%, 1%, 3%, and 5%, respectively. This situation shows that MC mortars are better than MF mortars in terms of sustainability.
- (7)
- As the coating/EP ratio increased, STP values increased for both MCs and MFs. That is, with the increase in the coating/EP ratio, the mixed mortars became more sustainable.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Symbols
| A0 | Area for test sample with %0 coverage ratio (uncoated) (m2) |
| Ai | Area for test sample with %i coverage ratio (coated) (m2) |
| C | Capillary water-absorption coefficient (kg/(m2.min0.5) |
| Hw | Beginning amount of colored water (80 mL/5.60 cm2) |
| HEP | Beginning amount of dry EP (20 mL/5.60 cm2) |
| I | Heater current (A) |
| M1 | Mass of the sample after 10 min (g) |
| M2 | Mass of the sample after 90 min (g) |
| STP | Sustainable thermal performance (%) |
| t1 | Time after heating started (t1 = 30 s) |
| t2 | Time after heating started (t2 = 60 s) |
| T1 | Temperature at t1 (°C) |
| T2 | Temperature at t2 (°C) |
| δGP | Slump amount of EP (mL/cm2) |
| βGP | Capillary water absorption amount of EP (mL/cm2) |
| λ | Thermal conductivity coefficient (W/mK) |
Abbreviations
| EP | Expanded perlite |
| SA | Stearic acid |
| ECP | Expanded coarse perlite |
| EFP | Expanded fine perlite |
| SCP | SA-coated expanded coarse perlite |
| SFP | SA-coated expanded fine perlite |
| MC | Coarse mortar |
| MF | Fine mortar |
| STP | Sustainable thermal performance |
References
- Topcu, I.B.; Isikdag, B. Effect of expanded perlite aggregate on the properties of lightweight concrete. J. Mater. Process. Technol. 2008, 204, 34–38. [Google Scholar] [CrossRef]
- Sengul, O.; Azizi, S.; Karaosmanoglu, F.; Tasdemir, M. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 43, 671–676. [Google Scholar] [CrossRef]
- Demirboga, R.; Orun, I.; Gul, R. Effects of expanded perlite aggregate and mineral admixtures on the compressive strength of low-density concretes. Cem. Concr. Res. 2001, 31, 1627–1632. [Google Scholar] [CrossRef]
- Demirboga, R.; Gul, R. The effects of expanded perlite aggregate, silica fume and fly ash on the thermal conductivity of lightweight concrete. Cem. Concr. Res. 2003, 33, 723–727. [Google Scholar] [CrossRef]
- Türkmen, İ.; Kantarcı, A. Effects of expanded perlite aggregate and different curing conditions on the physical and mechanical properties of self-compacting concrete. Build. Environ. 2007, 42, 2378–2383. [Google Scholar] [CrossRef]
- Kalkan, Ş.O.; Gündüz, L. Effect of porous aggregate size on the techno-mechanical properties of cementless lightweight mortars. El-Cezerî J. Sci. Eng. 2018, 5, 168–175. [Google Scholar] [CrossRef]
- Pramusanto, P.; Nurrochman, A.; Mamby, H.E.; Nugraha, P. High strength lightweight concrete with expandable perlite as the aggregate. Mater. Sci. Eng. 2020, 830, 042040. [Google Scholar] [CrossRef]
- Pichor, W.; Kamiński, A.; Szołdra, P.; Fra, M. Lightweight cement mortars with granulated foam glass and waste perlite addition. Adv. Civ. Eng. 2019, 2019, 1705490. [Google Scholar] [CrossRef]
- Vyšvařil, M.; Pavlíková, M.; Záleská, M.; Pivák, A.; Žižlavský, T.; Rovnaníková, P.; Bayer, P.; Pavlík, Z. Non-Hydrophobized perlite renders for repair and thermal insulation purposes: Influence of different binders on their properties and durability. Constr. Build. Mater. 2020, 263, 120617. [Google Scholar] [CrossRef]
- Ragul, P.; Naga Theera Hari, M.; Arunachelam, N.; Chellapandian, M. An experimental study on the partial replacement of fine aggregate with perlite in cement concrete. Mater. Today Proc. 2022, 68, 1219–1224. [Google Scholar] [CrossRef]
- Karakaş, H.; İlkentapar, S.; Durak, U.; Örklemez, E.; Özuzun, S.; Karahan, O.; Atiş, C.D. Properties of fly ash-based lightweight-geopolymer mortars containing perlite aggregates: Mechanical, microstructure, and thermal conductivity coefficient. Constr. Build. Mater. 2023, 362, 129717. [Google Scholar] [CrossRef]
- Szymczak-Graczyk, A.; Gajewska, G.; Laks, I.; Kostrzewski, W. Influence of Variable Moisture Conditions on the Value of the Thermal Conductivity of Selected Insulation Materials Used in Passive Buildings. Energies 2022, 15, 2626. [Google Scholar] [CrossRef]
- Gündüz, L.; Kalkan, Ş.O. The effect of different natural porous aggregates on thermal characteristic feature in cementitious lightweight mortars for sustainable buildings. Iran. J. Sci. Technol. Trans. Civ. Eng. 2023, 47, 843–861. [Google Scholar] [CrossRef]
- Slavica, R.M.; Sekulić, Z.; Daković, A.; Jovanović, V. Surface properties of natural calcite filler treated with stearic acid. Ceram. Silik. 2009, 53, 84–106. [Google Scholar]
- Gürsoy, M.; Karaman, M. Hydrophobic coating of expanded perlite particles by plasma polymerization. Chem. Eng. J. 2016, 284, 343–350. [Google Scholar] [CrossRef]
- Pichor, W.; Janiec, A. Thermal stability of expanded perlite modified by mullite. Ceram. Int. 2009, 35, 527–530. [Google Scholar] [CrossRef]
- Alazhari, M.; Sharma, T.; Heath, A.; Cooper, R.; Paine, K. Application of expanded perlite encapsulated bacteria and growth media for self-healing concrete. Constr. Build. Mater. 2018, 160, 610–619. [Google Scholar] [CrossRef]
- Jia, G.; Li, Z.; Liu, P.; Jing, Q. Preparation and characterization of aerogel/expanded perlite composite as building thermal insulation material. J. Non-Cryst. Solids 2018, 482, 192–202. [Google Scholar] [CrossRef]
- Jia, G.; Li, Z. Influence of the aerogel/expanded perlite composite as thermal insulation aggregate on the cement-based materials: Preparation, property, and microstructure. Constr. Build. Mater. 2021, 273, 121728. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, J.; Liu, J.; Ma, B.; Zhou, W.; Li, R.; Qin, W. Microstructure and characterization of capric-stearic acid/modified expanded vermiculite thermal storage composites. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2018, 33, 296–304. [Google Scholar] [CrossRef]
- Feng, J.; Liu, M.; Fu, L.; Zhang, K.; Xie, Z.; Shi, D.; Ma, X. Enhancement and mechanism of vermiculite thermal expansion modified by sodium ions. R. Soc. Chem. Adv. 2020, 10, 7635–7642. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, L.; Kalkan, Ş.O. Effects of exfoliation temperature for vermiculate aggregates modified by sodium ions on thermal and comfort properties of a new generation cementitious mortar. J. Sustain. Constr. Mater. Technol. 2022, 7, 266–281. [Google Scholar] [CrossRef]
- Akyuncu, V.; Sanliturk, F. Investigation of physical and mechanical properties of mortars produced by polymer coated perlite aggregate. J. Build. Eng. 2021, 38, 102182. [Google Scholar] [CrossRef]
- Myronyuk, O.; Baklan, D.; Zilong, J.; Sokolova, L. Obtaining water-repellent coatings based on expanded perlite materials. Mater. Today Proc. 2022, 62, 7720–7725. [Google Scholar] [CrossRef]
- Qi, P.; Lin, N.; Liu, Y.; Zhao, J. Improvement of oil/water selectivity by stearic acid modified expanded perlite for oil spill cleanup. J. Shanghai Jiaotong Univ. (Sci.) 2013, 18, 500–507. [Google Scholar] [CrossRef]
- Vogt, E.; Plachta, L. The new method of modifying the hydrophobic properties of expanded perlite. Energy Fuels 2017, 14, 02034. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Z.; Zhou, Z.; Xu, Q.; Yan, J.; Li, Q. Study on sustained-release pesticides blended with fosthiazate-stearic acid/expanded perlite. J. Renew. Mater. 2021, 11, 256–272. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, W.; Wang, J.; Yu, Y.; Liu, M.; Lv, Y. Preparation and properties of capric acid: Stearic acid/hydrophobic expanded perlite-aerogel composite phase change materials. Renew. Energy 2021, 179, 1027–1035. [Google Scholar] [CrossRef]
- Deepika; Hait, S.K.; Chen, Y. Optimization of milling parameters on the synthesis of stearic acid coated CaCO3 nanoparticles. J. Coat. Technol. Res. 2014, 11, 273–282. [Google Scholar] [CrossRef]
- Karim, A.N.; Johansson, P.; Sasic Kalagasidis, A. Knowledge gaps regarding the hygrothermal and long-term performance of aerogel-based coating mortars. Constr. Build. Mater. 2022, 314, 125602. [Google Scholar] [CrossRef]
- Becker, P.; Effting, C.; Schackow, A. Lightweight thermal insulating coating mortars with aerogel, EPS, and vermiculite for energy conservation in buildings. Cem. Concr. Compos. 2022, 125, 104283. [Google Scholar] [CrossRef]
- Yoon, H.; Lim, T.; Jeong, S.; Yang, K. Thermal transfer and moisture resistances of nano-aerogel-embedded foam concrete. Constr. Build. Mater. 2020, 236, 117575. [Google Scholar] [CrossRef]
- Karim, A.N.; Johansson, P.; Sasic Kalagasidis, A. Increasing Water Absorptivity of an Aerogel-Based Coating Mortar in Subsequent Wetting and Drying. Gels 2022, 8, 764. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Z.; Zhou, Y.; Du, J.; Lai, J.; Qian, K.; Ruan, S.; Bi, Y.; Qian, X. The influence of nano-silica composite superabsorbent polymer on the autogenous shrinkage of mortar. Constr. Build. Mater. 2024, 442, 137683. [Google Scholar] [CrossRef]
- Tian, J.; Yang, Y.; Xue, T.; Chao, G.; Fan, W.; Liu, T. Highly flexible and compressible polyimide/silica aerogels with integrated double network for thermal insulation and fire-retardancy. J. Mater. Sci. Technol. 2022, 105, 194–202. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wang, Y.; Malfait, W.; Zhao, S.; Tian, Y.; Liu, T.; Zhang, X.; Du, A.; Shen, J. Flexible, high-temperature-resistant silica-polymer aerogel hybrids by templating polymethylsilsesquioxane microstructure with trace polyimide. Adv. Compos. Hybrid Mater. 2023, 6, 32. [Google Scholar] [CrossRef]
- Valdez-Cano, R.; González-López, J.; Guerra-Cossío, M. Effects on the Mechanical and Thermal Behaviors of an Alternative Mortar when Adding Modified Silica Aerogel with Aminopropyl Triethoxysilane and PEG-PPG-PEG Triblock Copolymer Additives. Silicon 2023, 15, 5353–5366. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Xie, X.; Zuo, J.; Lyu, K.; Shah, S. Chloride corrosion resistance of cement mortar with recycled concrete powder modified by nano-silica. Constr. Build. Mater. 2023, 364, 129907. [Google Scholar] [CrossRef]
- Muthalvan, R.; Selvaraj, L.; Avudaiappan, S.; Liseitsev, Y. Performance evaluation of super absorbent polymer modified cement mortar with nano-silica/GGBS. Case Stud. Constr. Mater. 2023, 19, e02359. [Google Scholar] [CrossRef]
- Yildirim Erbil, H. Practical applications of superhydrophobic materials and coatings: Problems and perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef]
- Perlite: The Most Sustainable Insulation Solution for Buildings. Available online: https://www.perlite.org/wp-content/uploads/2024/01/Perlite-Sustainable-Insulation.pdf (accessed on 17 July 2025).
- Embodied Carbon Inventory Data for Construction Materials. Available online: https://www.cidb.gov.my/wp-content/uploads/2022/11/V4_EMBODIED-CARBON-INVENTORY-TEMPLATE-final-1.pdf (accessed on 17 July 2025).
- Carbon Footprint for Building Products. Available online: https://publications.vtt.fi/pdf/technology/2013/T115.pdf (accessed on 17 July 2025).
- EN 13139; Aggregates for Mortar. Turkish Standard Institute: Ankara, Turkey, 2013.
- EN 197-1; Cement—Part 1: Compositions and Conformity Criteria for Common Cements. Turkish Standard Institute: Ankara, Turkey, 2002.
- EN 1015-3; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). Turkish Standard Institute: Ankara, Turkey, 2000.
- EN 1015-11; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. Turkish Standard Institute: Ankara, Turkey, 2020.
- EN 1015-18; Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar. Turkish Standard Institute: Ankara, Turkey, 2014.
- EN 1745; Masonry and Masonry Products—Methods for Determining Design Thermal Values. Turkish Standard Institute: Ankara, Turkey, 2020.
- QTM-500; Quick Thermal Conductivity Meter User’s Guide. Available online: https://cdn2.hubspot.net/hubfs/105273/Manuals/QTM-500_Operation_Manual_Ver08.pdf (accessed on 29 July 2025).
- EN 998-1; Specification for Mortar for Masonry—Part 1: Rendering and Plastering Mortar. Turkish Standard Institute: Ankara, Turkey, 2011.
- Rochester Institute of Technology (R.I.T.). Areas by Integration. Available online: https://www.rit.edu/academicsuccesscenter/ (accessed on 5 June 2025).
- Cankaya University. Math 111-11 Integral Techniques. Available online: https://math111.cankaya.edu.tr (accessed on 5 June 2025).
- Yue, J.; Liu, J.; Song, X.; Yan, C. Research on the design and thermal performance of vacuum insulation panel composite insulation materials. Case Stud. Therm. Eng. 2024, 64, 105437. [Google Scholar] [CrossRef]
- Hung Anh, L.D.; Pásztory, Z. An overview of factors influencing thermal conductivity of building insulation materials. J. Build. Eng. 2021, 44, 102604. [Google Scholar] [CrossRef]
- TS EN ISO 6946; Building Components and Building Elements—Thermal Resistance and Thermal Transmittance—Calculation Methods (ISO 6946:2017). Turkish Standards Institute: Ankara, Turkey, 2017; p. 57.
- Qin, Z.; Li, M.; Flohn, J.; Hu, Y. Thermal management materials for energy-efficient and sustainable future buildings. Chem. Commun. 2021, 57, 12236–12253. [Google Scholar] [CrossRef] [PubMed]
- Revuelta-Acosta, J.D.; García-Díaz, A.; Soto-Zarazúa, G.M.; Rico-García, E. Adobe as a sustainable material: A thermal performance. J. Appl. Sci. 2010, 10, 2211–2216. [Google Scholar] [CrossRef]
- Kendzierawska, W.; Trochonowicz, M. Effect of temperature and humidity on the thermal conductivity of insulation materials. Civ. Environ. Eng. Rep. 2023, 33, 42–49. [Google Scholar] [CrossRef]
- Gbekou, F.K.; Benmahiddine, F.; Boudenne, A.; Belarbi, R.; Eddhahak, A.; Benzarti, K. Hygrothermal characterization of cement mortar composites incorporating micronized miscanthus fibers. Case Stud. Constr. Mater. 2024, 21, e04004. [Google Scholar] [CrossRef]
- Baptista da Silva, F.S. Thermal Conductivity of Thermal Mortars with EPS and Silica Aerogel; Tecnico Lisboa: Lisboa, Portugal, 2017; Available online: https://fenix.tecnico.ulisboa.pt/downloadFile/1407770020545701/ExtendedAbstractFilipaFinal.pdf (accessed on 29 July 2025).
- Gawin, D.; Kosny, J.; Desjarlais, A. Effect of moisture on thermal performance and energy efficiency of buildings with lightweight concrete walls. Proc. ACEEE Summ. Stud. Energy Eff. Build. 2000, 3, 3149–3160. [Google Scholar]
- Pessoa, S.; Jesus, M.; Guimaraes, A.S.; Lucas, S.S.; Simoes, N. Experimental characterisation of hygrothermal properties of a 3D printed cementitious mortar. Case Stud. Constr. Mater. 2023, 19, e02355. [Google Scholar] [CrossRef]












| General Feature | |
|---|---|
| Color | White solid |
| Smell | Sharp, oil-like |
| Bulk density | 0.9408 g/cm3 |
| Boiling point | 361 °C |
| Melting point | 69.3 °C |
| Vapor pressure | 0.01 kPa (158 °C) |
| Solubility (in water) | 0.00029 g/100 g (20 °C) |
| Group Name | Control Samples | Coated Coarse and Fine EP Particles | ||||
|---|---|---|---|---|---|---|
| Group 1 | ECP | SCP-0.1 | SCP-0.2 | SCP-0.3 | SCP-0.4 | SCP-0.5 |
| Group 2 | SCP-1 | SCP-2 | SCP-3 | SCP-4 | SCP-5 | |
| Group 3 | EFP | SFP-0.1 | SFP-0.2 | SFP-0.3 | SFP-0.4 | SFP-0.5 |
| Group 4 | SFP-1 | SFP-2 | SFP-3 | SFP-4 | SFP-5 | |
| Mixture Mortar Types | Coating/EP Ratios (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 1 | 2 | 3 | 4 | 5 | |
| Sample Codes | |||||||||||
| Samples with EFP | MF-0 | MF-0.1 | MF-0.2 | MF-0.3 | MF-0.4 | MF-0.5 | MF-1 | MF-2 | MF-3 | MF-4 | MF-5 |
| Samples with ECP | MC-0 | MC-0.1 | MC-0.2 | MC-0.3 | MC-0.4 | MC-0.5 | MC-1 | MC-2 | MC-3 | MC-4 | MC-5 |
| Coarse Mortars (MCs) | Coarse Mortar Components | Fine Mortars (MFs) | Fine Mortar Components | Percentage by Weight (%) |
|---|---|---|---|---|
| MC-0 | ECP | MF-0 | EFP | 30 |
| MC-0.1 | SCP-0.1 | MF-0.1 | SFP-0.1 | |
| MC-0.3 | SCP-0.3 | MF-0.3 | SFP-0.3 | |
| MC-0.5 | SCP-0.5 | MF-0.5 | SFP-0.5 | |
| MC-1 | SCP-1 | MF-1 | SFP-1 | |
| MC-3 | SCP-3 | MF-3 | SFP-3 | |
| MC-5 | SCP-5 | MF-5 | SFP-5 | |
| For all mortars | Cement | For all mortars | Cement | 30 |
| Powder Lime | Powder Lime | 4 | ||
| Powder polymer | Powder polymer | 2.5 | ||
| Glass fiber | Glass fiber | 1.50 | ||
| Filling material | Filling material | 11 | ||
| Superplasticizer | Superplasticizer | 0.10 | ||
| Calcite + quartz | Calcite + quartz | 20.90 |
| Sample Codes | Coarse Mortar Properties | |||
|---|---|---|---|---|
| Water/Solid | Bulk Density of Powder (kg/m3) | Bulk Density of Fresh Mortar (kg/m3) | Dry Bulk Density of Hardened Mortar (kg/m3) | |
| MC-0 | 1.88 | 200 | 489 | 245 |
| MC-0.1 | 1.88 | 200 | 491 | 248 |
| MC-0.3 | 1.85 | 200 | 496 | 251 |
| MC-0.5 | 1.80 | 201 | 491 | 259 |
| MC-1 | 1.75 | 202 | 489 | 265 |
| MC-3 | 1.63 | 204 | 473 | 274 |
| MC-5 | 1.58 | 210 | 481 | 281 |
| Sample Codes | Fine Mortar Properties | |||
| Water/Solid | Bulk density of powder (kg/m3) | Bulk density of fresh mortar (kg/m3) | Dry bulk density of hardened mortar (kg/m3) | |
| MF-0 | 1.38 | 333 | 679 | 339 |
| MF-0.1 | 1.38 | 333 | 679 | 345 |
| MF-0.3 | 1.33 | 334 | 664 | 346 |
| MC-0.5 | 1.33 | 334 | 641 | 346 |
| MF-1 | 1.30 | 336 | 617 | 350 |
| MF-3 | 1.30 | 339 | 613 | 356 |
| MF-5 | 1.28 | 347 | 603 | 366 |
| Coarse Mortars (MCs) | Relative Humidity (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0 (dry) | 3 | 5 | 8 | 10 | 12 | 15 | |
| Thermal Conductivity (W/mK) | |||||||
| MC-0 | 0.045 | 0.095 | 0.146 | 0.198 | 0.237 | 0.261 | 0.269 |
| MC-0.1 | 0.045 | 0.101 | 0.151 | 0.199 | 0.235 | 0.256 | 0.267 |
| MC-0.3 | 0.046 | 0.098 | 0.145 | 0.186 | 0.214 | 0.232 | 0.238 |
| MC-0.5 | 0.048 | 0.096 | 0.139 | 0.182 | 0.202 | 0.221 | 0.229 |
| MC-1 | 0.049 | 0.095 | 0.136 | 0.178 | 0.202 | 0.221 | 0.230 |
| MC-3 | 0.052 | 0.086 | 0.113 | 0.133 | 0.144 | 0.148 | 0.153 |
| MC-5 | 0.054 | 0.082 | 0.097 | 0.107 | 0.115 | 0.119 | 0.122 |
| Fine Mortars (MFs) | Relative Humidity (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0 (Dry) | 3 | 5 | 8 | 10 | 12 | 15 | |
| Thermal Conductivity (W/mK) | |||||||
| MF-0 | 0.069 | 0.143 | 0.228 | 0.320 | 0.380 | 0.415 | 0.427 |
| MF-0.1 | 0.070 | 0.144 | 0.227 | 0.320 | 0.377 | 0.408 | 0.416 |
| MF-0.3 | 0.071 | 0.134 | 0.210 | 0.289 | 0.353 | 0.395 | 0.407 |
| MC-0.5 | 0.070 | 0.096 | 0.139 | 0.182 | 0.202 | 0.221 | 0.229 |
| MF-1 | 0.071 | 0.133 | 0.202 | 0.274 | 0.318 | 0.347 | 0.357 |
| MF-3 | 0.073 | 0.126 | 0.185 | 0.243 | 0.279 | 0.293 | 0.299 |
| MF-5 | 0.076 | 0.126 | 0.182 | 0.229 | 0.261 | 0.277 | 0.285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asan, B.T.; Gündüz, L.; Yakar, G. Experimental Evaluation of Thermal and Moisture Behavior of Stearic Acid-Coated Expanded Perlite for Sustainable Insulation Mortars. Buildings 2025, 15, 2749. https://doi.org/10.3390/buildings15152749
Asan BT, Gündüz L, Yakar G. Experimental Evaluation of Thermal and Moisture Behavior of Stearic Acid-Coated Expanded Perlite for Sustainable Insulation Mortars. Buildings. 2025; 15(15):2749. https://doi.org/10.3390/buildings15152749
Chicago/Turabian StyleAsan, Betül Tülin, Lütfullah Gündüz, and Gülay Yakar. 2025. "Experimental Evaluation of Thermal and Moisture Behavior of Stearic Acid-Coated Expanded Perlite for Sustainable Insulation Mortars" Buildings 15, no. 15: 2749. https://doi.org/10.3390/buildings15152749
APA StyleAsan, B. T., Gündüz, L., & Yakar, G. (2025). Experimental Evaluation of Thermal and Moisture Behavior of Stearic Acid-Coated Expanded Perlite for Sustainable Insulation Mortars. Buildings, 15(15), 2749. https://doi.org/10.3390/buildings15152749






