1. Introduction
Against the backdrop of sustainable development and the national “dual carbon” strategy, the research and application of green and low-carbon construction materials have been increasingly regarded as a key focus in the field of engineering materials [
1,
2,
3,
4]. As a novel inorganic cementitious material, geopolymers have been widely attracted in recent years due to their excellent mechanical properties, high corrosion resistance, and environmental sustainability [
4,
5,
6]. The concept of geopolymers was first introduced by the French scholar Davidovits in the 1970s [
7]. Essentially, geopolymers are aluminosilicate materials, in which a 3D amorphous to semi-crystalline network structure is formed through alkali activation. These materials can be synthesized from industrial by-products such as fly-ash and slag, thereby not only alleviating the environmental burden associated with industrial waste disposal, but also significantly reducing carbon emissions and energy consumption.
From an ecological perspective, geopolymers have been reported to reduce carbon footprint by up to 50% and energy demand by approximately 25% [
8,
9,
10]. Studies have demonstrated that geopolymer materials exhibit excellent thermal resistance. Wastiels et al. [
11] observed that fly-ash derived from coal combustion possesses a certain degree of alkali reactivity, suggesting its potential for geopolymer synthesis. This finding has accelerated the resource utilization of industrial solid wastes in geopolymer production. Furthermore, when immersed in 5% acetic and sulfuric acid solutions for 28 days, geopolymers were found to exhibit significantly lower mass and compressive strength losses compared to ordinary Portland cement (OPC) materials, indicating superior acid resistance [
12]. These results suggest that geopolymers outperform OPC in acid environments. In addition, geopolymers have demonstrated enhanced resistance to sulfate attack, chloride ion penetration, and carbonation in comparison to conventional cement-based materials [
13,
14,
15].
However, underground infrastructure is not only exposed to the long-term attack from corrosive agents such as chlorides and sulfates present in groundwater, but its service life may also be affected by stray-currents [
16,
17,
18,
19]. Stray-currents refer to unintended electrical currents that deviate from their designated pathways and are commonly encountered in urban subway systems, light rail networks, and other direct current (DC) electrified railway systems [
20,
21,
22]. Among these, DC stray-currents are particularly harmful, with corrosion capabilities reported to be 20 to 100 times greater than those of alternating current stray-currents of equivalent intensity [
23]. Through electrolysis action, stray-currents accelerate both the corrosion of steel reinforcement and the dissolution of surrounding materials by forming electrolytic corrosion cells, thereby significantly reducing the durability and safety of concrete structures. When coupled with corrosive ions in groundwater, the degradation process is further intensified, as ion migration and diffusion within the concrete matrix are enhanced, posing a serious threat to the service life of underground infrastructure [
24,
25].
Aghajani et al. [
26] employed a stray-current simulation apparatus to investigate the combined effects of stray-current and soft-water on the permeability of concrete with varying water-to-binder (W/B) ratios and silica fume contents. Experimental results indicated that silica fume incorporation significantly increased the electrical resistivity of concrete, leading to a reduction in electric flux. Moreover, the resistivity degradation and the increase in electric flux after current application were both mitigated in concrete specimens containing silica fume. Meanwhile, other researchers [
27,
28,
29] have reported that the presence of stray-currents can deteriorate the pore structure of concrete, reduce its electrical resistivity, and facilitate the ingress of corrosive ions—particularly chloride and sulfate—which are highly detrimental and to both reinforcement and the cementitious matrix, causing severe damage. Chloride ions are known to break down the passive film on steel surfaces, triggering localized corrosion, while sulfate ions tend to react with hydration products to form expansive corrosion compounds, thereby exacerbating cracking. In addition, under the influence of an applied electric field, cations such as Na
+, K
+, and Ca
2+ migrate toward the cathode, while anions including OH
−, Cl
−, and
migrate toward the anode [
30]. This ion migration accelerates the electrolytic process and alters the spatial distribution of corrosive species, thereby impacting the overall performance and durability of the material.
To address corrosion issues caused by stray-current, several studies have attempted to improve the durability of cement-based materials by incorporating mineral admixtures such as fly-ash, slag, and silica fume [
31,
32,
33]. While these approaches have shown some improvement, most existing studies focus primarily on individual corrosion factors and rarely consider their combined effects under realistic service conditions. In this study, FAG was selected as the target material due to its potential for enhanced durability and environmental benefits. The coupled deterioration effects of stray-current and soft-water were experimentally reproduced to simulate actual underground service conditions. The evolution of mechanical properties and associated microstructural degradation mechanisms were systematically investigated. This work fills a critical research gap by elucidating the coupled erosion behavior of FAG under dual chemical–electrical attack. It offers theoretical and experimental support for applying geopolymer materials in underground infrastructure, thereby promoting the engineering implementation of green, environmentally friendly, and durable alternative cementitious materials in infrastructure development.
2. Raw Materials and Test Methods
2.1. Raw Materials
The Class I fly-ash used was obtained from the Huaneng Power Plant in Qingdao, Shandong Province. It had a density of 2300 kg/m
3, a moisture content of 0.7%, a water demand ratio of 0.72%, and a strength activity index of 0.72. The chemical composition of the fly-ash is presented in
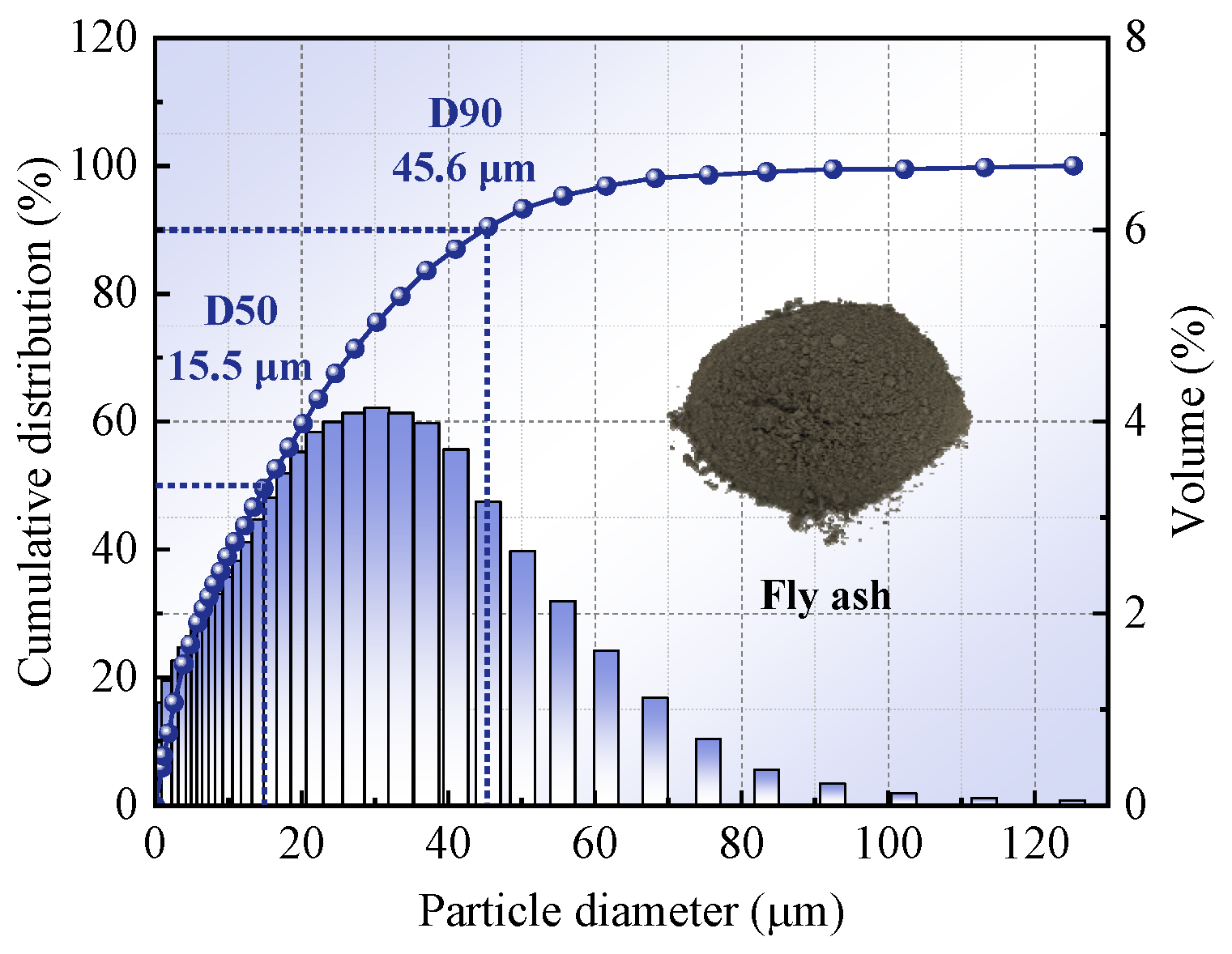
Table 1. Its particle size distribution was analyzed using a laser particle size analyzer (Dandong Bettersize Instruments Co., Ltd., Dandong, China), and the results are shown in
Figure 1. The microstructure characteristics of the fly-ash were observed using a field-emission scanning electron microscope (JEOL Ltd., Akishima, Japan), as illustrated in
Figure 2.
Water glass (Na2O·nSiO2) and sodium hydroxide (NaOH) were mixed to produce the alkali activator. The liquid sodium silicate was supplied by Lvsan Chemical Co., Ltd., Linyi, China, with a density of 1.38 g/mL, a modulus of 3.3, Na2O content of 8.82%, a SiO2 content of 28.26%, and a Baumé degree of 39.9 Bé. The sodium hydroxide used was analytical-grade granular NaOH, supplied by Comeo Chemical Reagent Co., Ltd., Tianjin, China.
Deionized water, provided by Dalian Jinke Chemical Reagent Co., Ltd., Dalian, China. was used throughout the experiments. The metal electrodes consisted of 304 stainless steel mesh with a wire diameter of 0.6 mm and a mesh size of 8. The graphite electrodes were made from GHSM-5 graphite (Guanghui brand), with an electrical resistivity ranging from 8 to 10 μΩ·m.
2.2. Preparation and Curing of FAG
Five influencing factors were investigated: W/B, Ms, alkali content (AC, defined as Na
2O-to-fly-ash mass ratio), curing temperature, and high-temperature curing duration. The designed mix proportions of FAG are listed in
Table 2. Based on the mix design, the required quantities of sodium silicate solution, water, and sodium hydroxide were calculated. Initially, sodium silicate solution and water were mixed in a beaker and stirred uniformly using a glass rod. Sodium hydroxide was then added, and the mixture was stirred using a magnetic stirrer for 10 min until a clear and transparent activator solution was obtained. After stirring, the activator was allowed to stand at room temperature for more than 12 h. Subsequently, the prepared activator and pre-weighed fly-ash were mixed using a JJ-5 cement mortar mixer (Wuxi Jianyi Instrument & Machinery Co., Ltd., Wuxi, China) to form a uniform slurry. Two layers of slurry were sequentially cast into molds measuring 40 mm × 40 mm × 40 mm. The first half was added into the mold and vibrated on a vibrating table for 30 s. The remaining half was then added and vibrated again for 30 s. Excess paste on the surface was leveled, and the molds were wrapped in plastic wrap to evaporation moisture loss. In total, 348 specimens were prepared following this procedure.
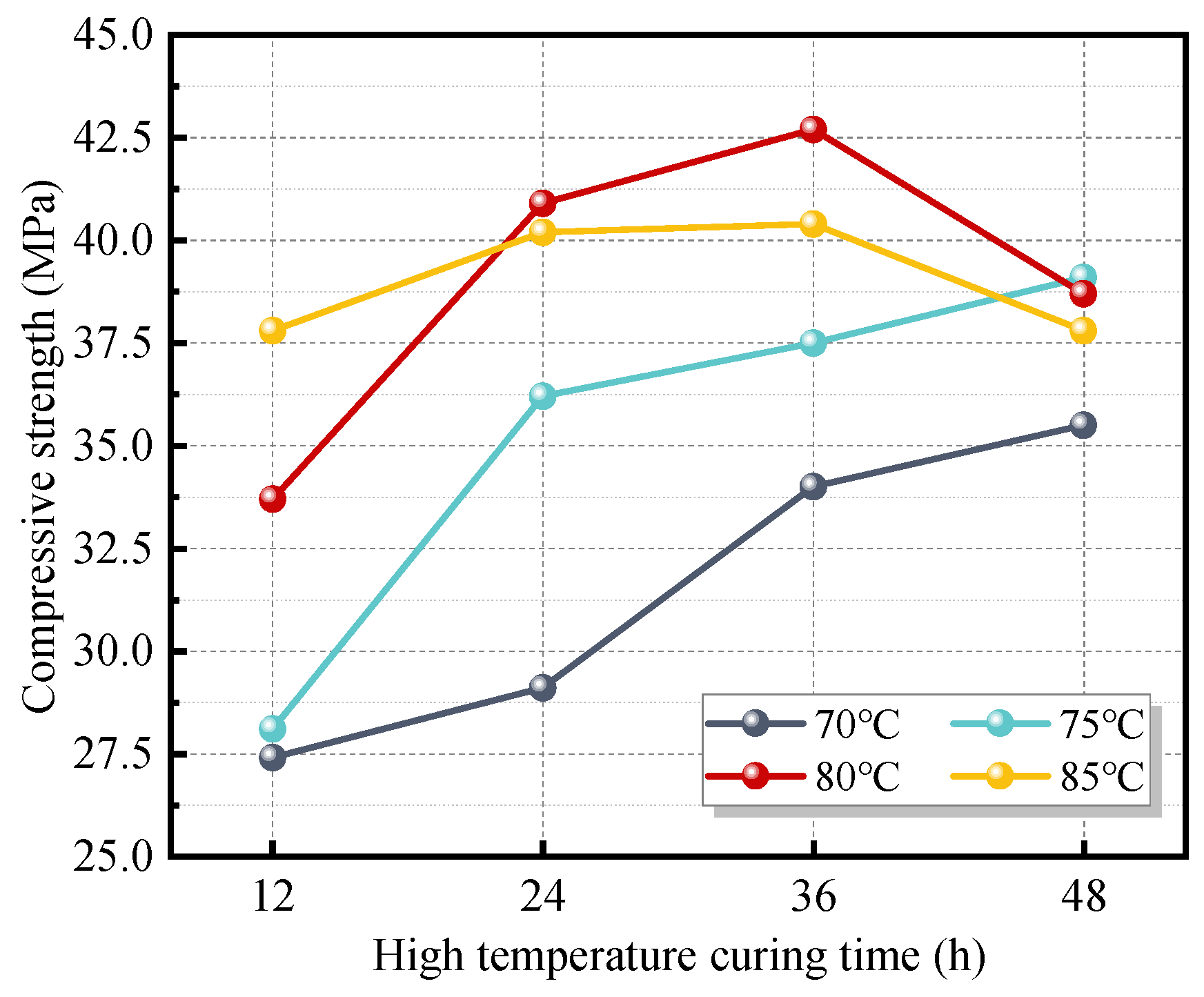
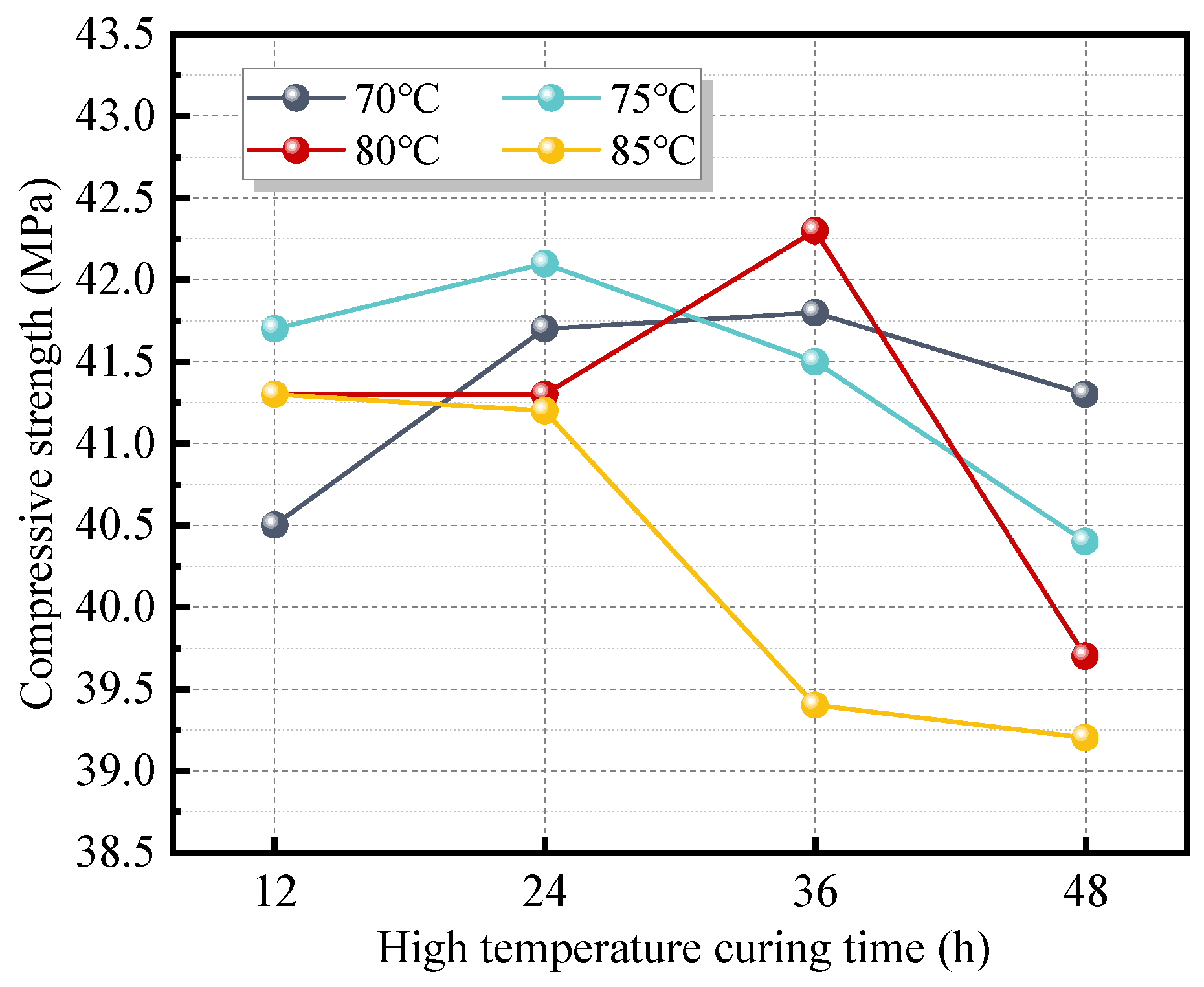
Two curing regimes were employed in this study. The first was ambient temperature curing. After being covered with plastic wrap, the freshly mixed paste was placed in a room maintained at a temperature of 20 ± 5 °C and a relative humidity of 70 ± 5%. Samples were demolded after 3 days and then continuously cured under the same conditions until the specified age. To accelerate the hydration reaction and promote early strength development, the second regime involved initial high-temperature curing followed by ambient curing. For this regime, the freshly cast paste, covered with plastic wrap, was sealed in a zip-lock bag and placed in a high-temperature curing chamber under the preset temperature and duration. As the strength of the FAG developed rapidly under elevated temperature, specimens requiring high-temperature curing durations exceeding 6 h were demolded after 6 h. The demolded specimens were then resealed in zip-lock bags and returned to the curing chamber to complete the scheduled curing period. Subsequently, the specimens were subjected to ambient curing identical to that used in the first regime until the designated testing age. The curing conditions for high-temperature treatment are presented in
Table 3.
2.3. Test Methods
An L
16 (4
5) orthogonal experimental design was utilized to evaluate the resistance of FAG to the coupled erosion of stray-current (20 V) and soft-water over a period of 90 days. Two evaluation indices were considered: compressive strength and compressive strength loss rate. The three factors included in the orthogonal design were W/B, Ms, and AC. Based on the results obtained in
Section 2.2, four levels were selected for each factor. The specific factor-level arrangement is shown in
Table 4. FAG specimens in each group were cured for 28 days according to the optimal curing regime identified previously, and their compressive strengths were tested and recorded as the pre-erosion compressive strength. Subsequently, another set of specimens from the same group was subjected to the coupled effect of 20 V stray-current and soft-water for 90 days, after which compressive strength was measured and recorded as the post-erosion compressive strength.
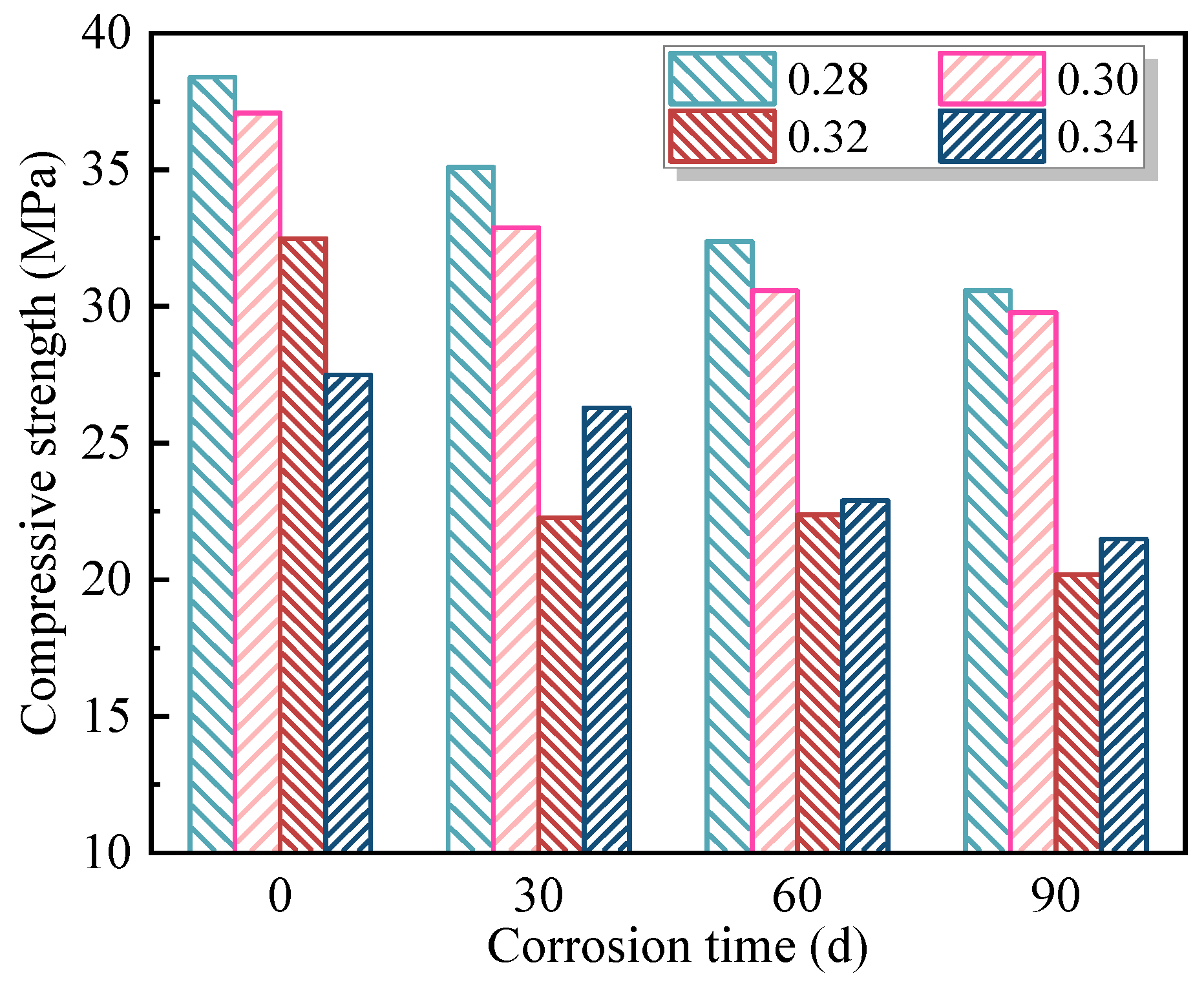
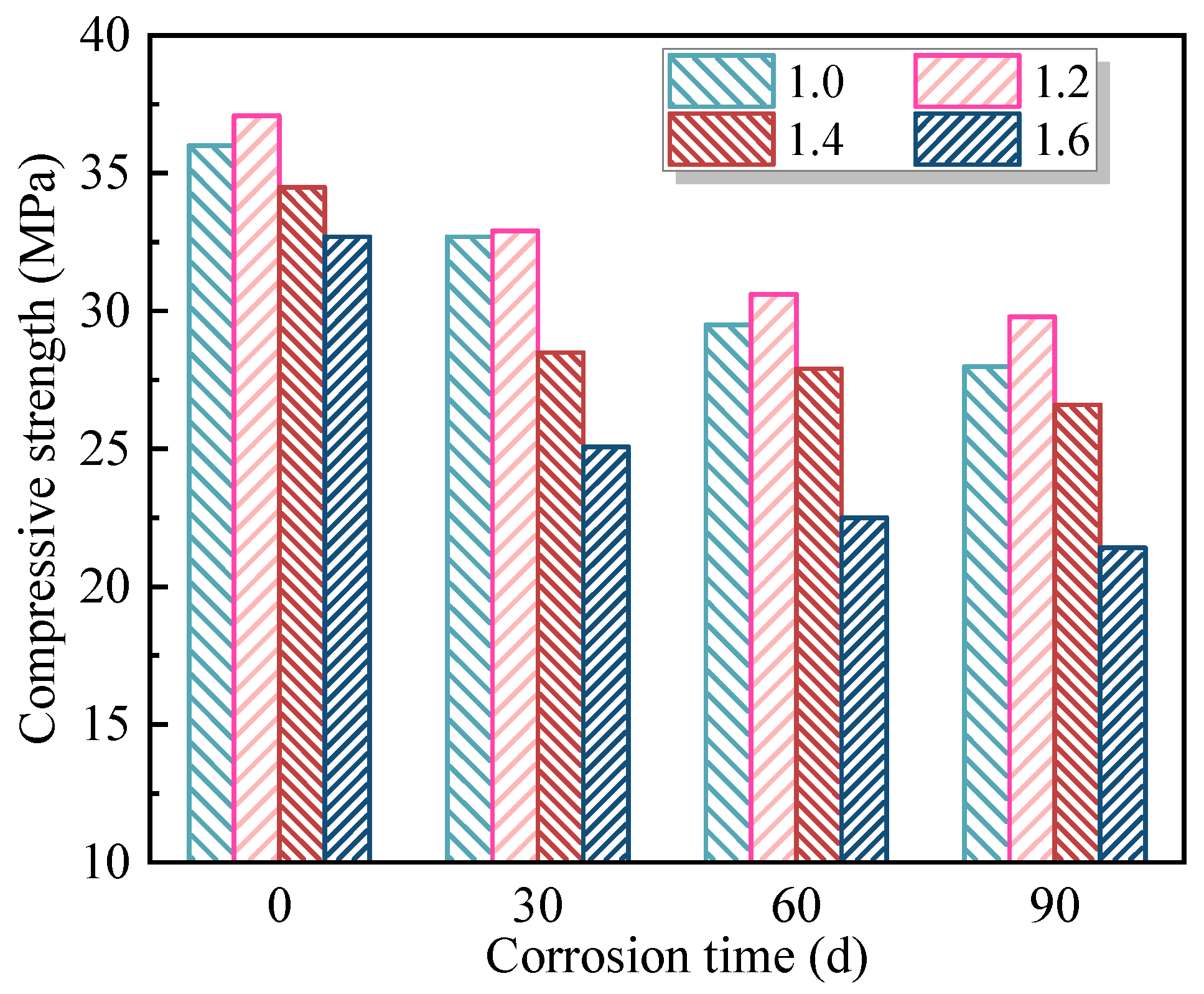
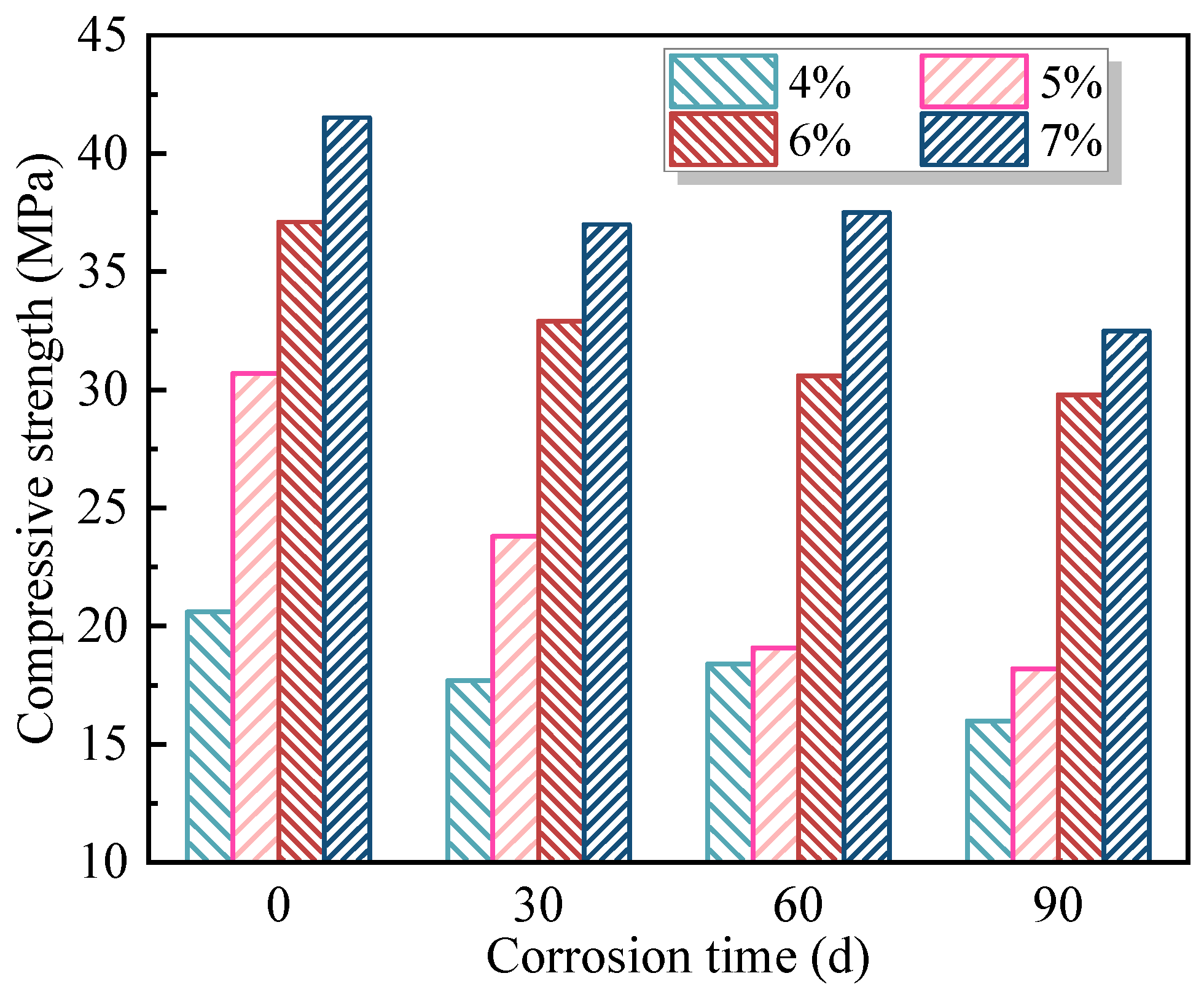
Based on the mix group that exhibited the lowest compressive strength loss rate after 90 days of coupled erosion by soft-water and 20 V stray-current, three sets of single-factor experiments were designed to investigate the influence of individual parameters—namely W/B, Ms, and AC—on the erosion behavior of FAG under stray-current. For the reference mix, additional tests were conducted under a standard curing environment and under coupled erosion environments involving 0 V, 20 V, and 40 V stray-currents combined with soft-water exposure for 30, 60, and 90 days. These tests aimed to assess the impact of stray-current intensity and erosion duration on the degradation of FAG.
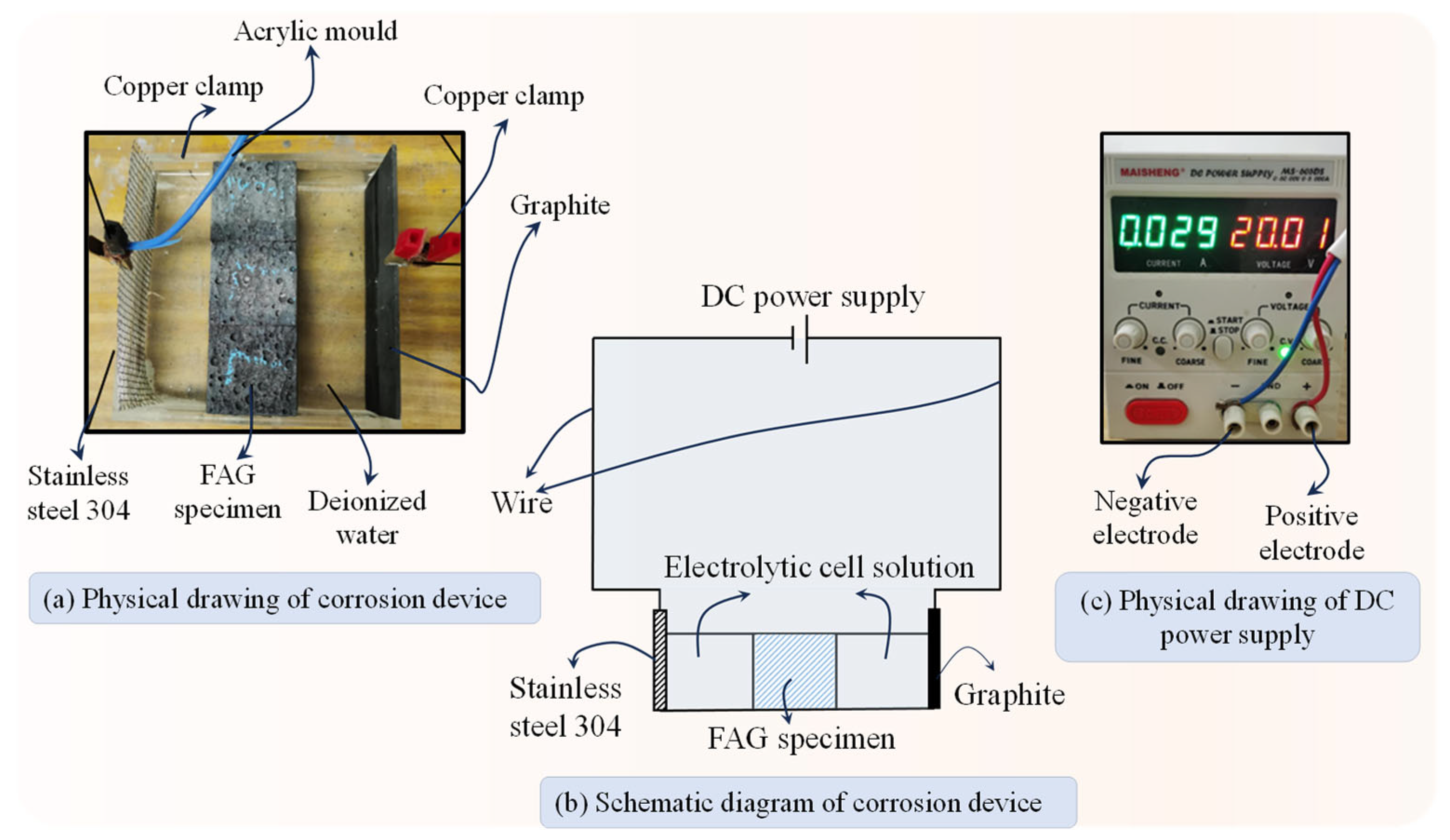
The apparatus used for the coupled erosion tests is shown in
Figure 3. A mold with internal dimensions of 120 mm × 120 mm × 40 mm was fabricated using acrylic. Graphite was employed as the anode and 304 stainless steel as the cathode, which were linked to the positive and negative terminals of a DC power supply via copper wires, respectively. Deionized water was used as the electrolyte solution in the electrochemical cell.
4. Conclusions
This investigation focused on the effects of W/B, Ms, and AC on the mechanical properties of FAG at different ages. The influence of curing temperature and duration of high-temperature curing on the early and later development of FAG mechanical performance was also examined. Furthermore, the influence of stray-current coupled with soft-water erosion on FAG, under varying W/B, Ms, and AC, were explored to identify the optimal mix proportion resistant to the coupled erosion. The main conclusions are as follows:
- (1)
After 90 days of curing, the compressive strength of FAG displayed an initial increase followed by a decrease with the increase in W/B, Ms, and AC. The reasonable ranges for W/B, Ms, and AC were determined to be 0.28–0.34, 1.0–1.6, and 4–7%, respectively. Under normal-temperature curing, the strength development of FAG was slow. A moderate increase in early curing temperature and an extension of the high-temperature curing duration significantly accelerated strength development. However, excessively high curing temperatures (>80 °C) and prolonged high-temperature curing (>36 h) were found to adversely affect the mechanical performance of FAG. The optimal high-temperature curing regime was identified as 80 °C for 24 h.
- (2)
The erosive impact of soft-water on FAG was limited, while the application of stray-current markedly enhanced the erosion caused by soft-water. Compared to soft-water alone, the erosion rate increased by factors of 4.1 and 7.2 when stray-currents of 20 V and 40 V were applied, respectively. Under varying current intensities, the coupled erosion effect weakened continuously with increasing erosion time, and the erosion rate exhibited a linearly decreasing trend over time.
- (3)
The influence degree of stray-current coupled with soft-water erosion on FAG compressive strength and strength loss rate was ranked as AC > W/B > Ms. Both AC and W/B demonstrated significant or highly significant effects on these two indices. The mix proportion of W/B = 0.30, Ms = 1.2, and AC = 7% resulted in the highest post-erosion compressive strength of 32.5 MPa, with a strength loss rate of 21.7%. Meanwhile, the mix with W/B = 0.30, Ms = 1.2, and AC = 6% exhibited the lowest strength loss rate of 19.7%, with a residual compressive strength of 29.8 MPa after erosion.
Future research could focus on the coupling degradation effects of stray-current with multiple corrosive media such as chloride and sulfate ions, which are commonly present in subway groundwater environments. Additionally, the potential of mineral admixtures to enhance the durability of FAG under such complex conditions warrants further investigation.