Experimental Study on the Adsorption Performance of Metal–Organic Framework MIL-101 (Cr) for Indoor Toluene
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Material
2.2. Toluene Adsorption Experiments
3. Results and Discussion
3.1. Characteristics of MIL-101 (Cr)
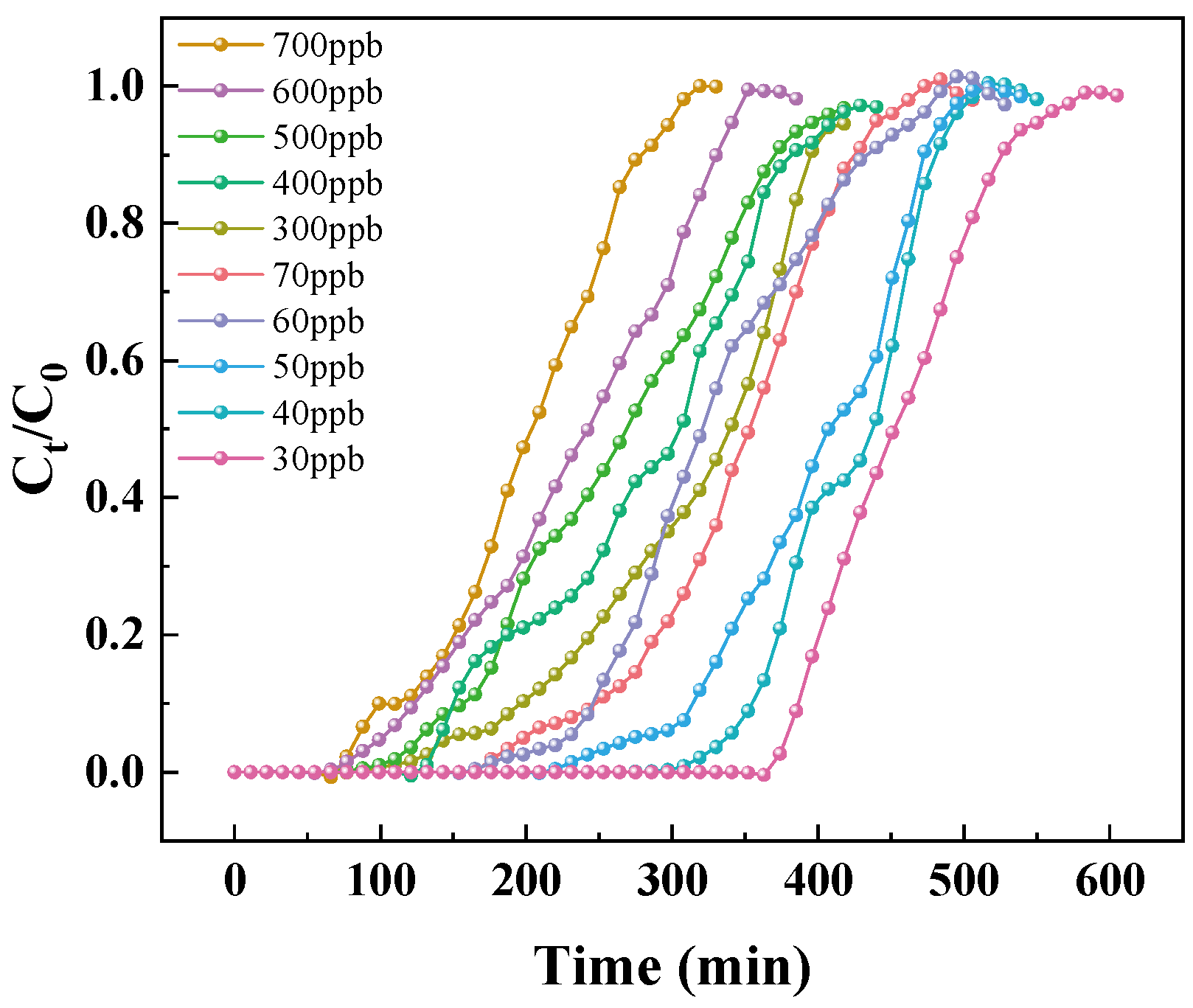
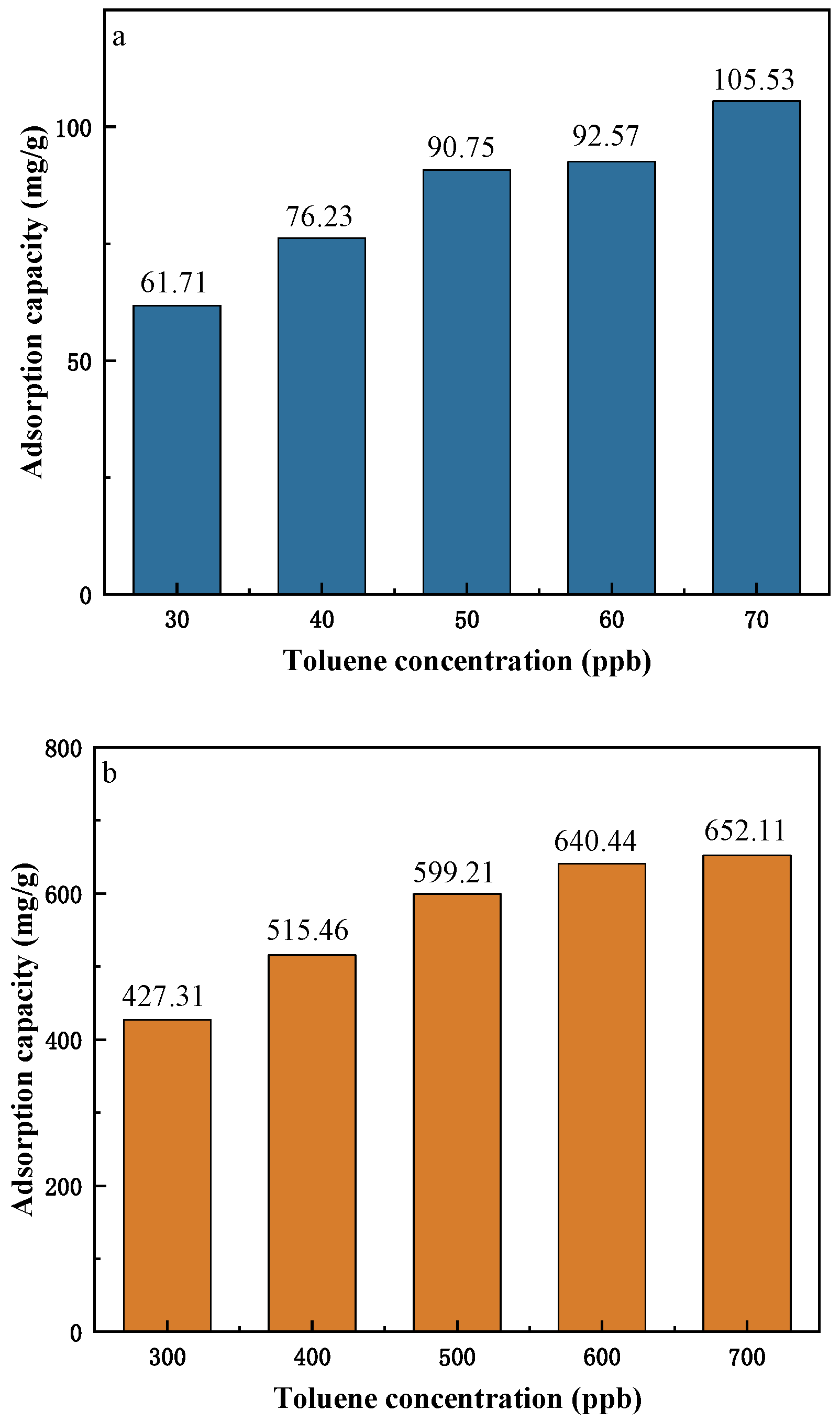
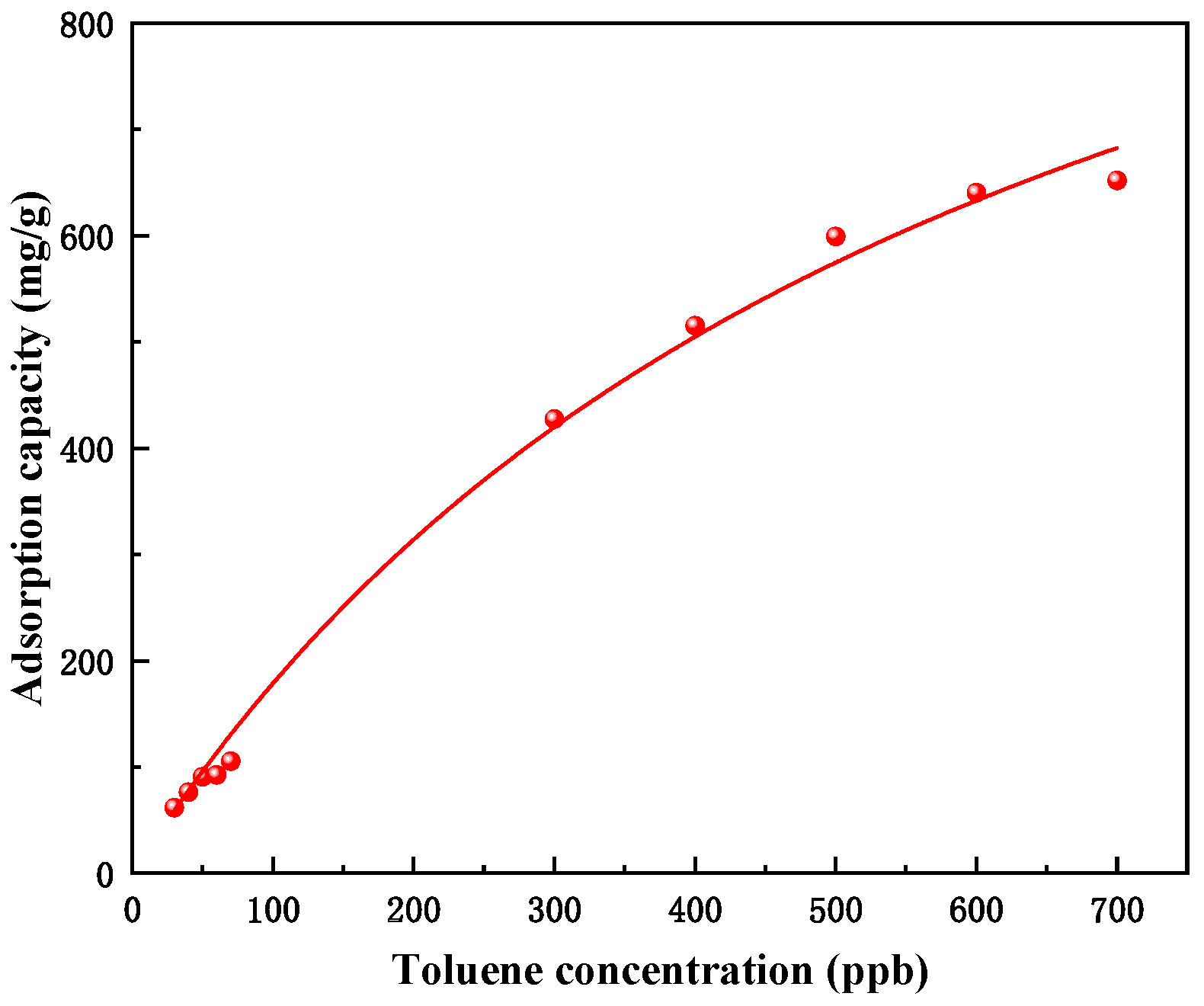
3.2. Effect of Initial Concentration of Toluene on Adsorption
3.3. Effect of Temperature on Adsorption
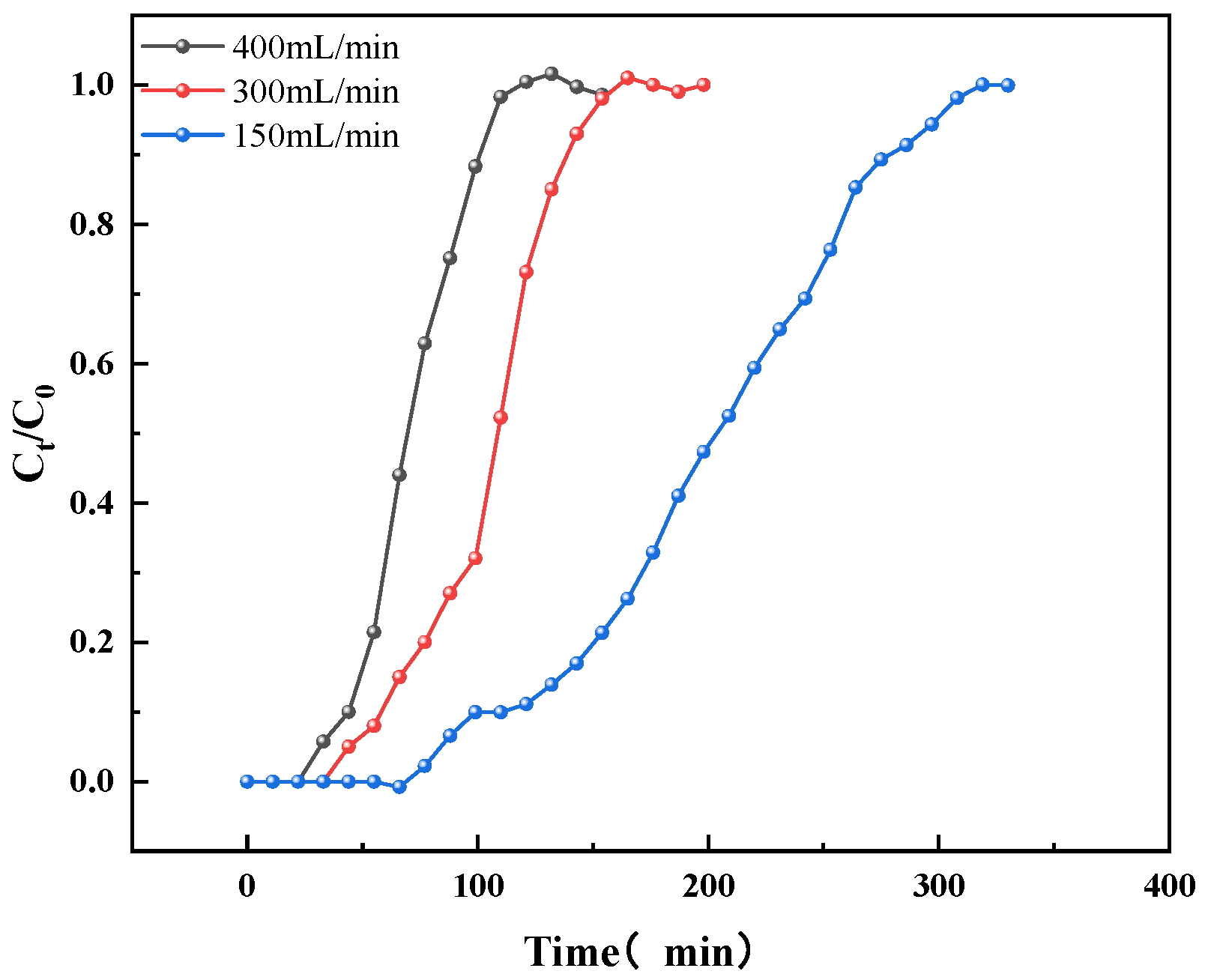
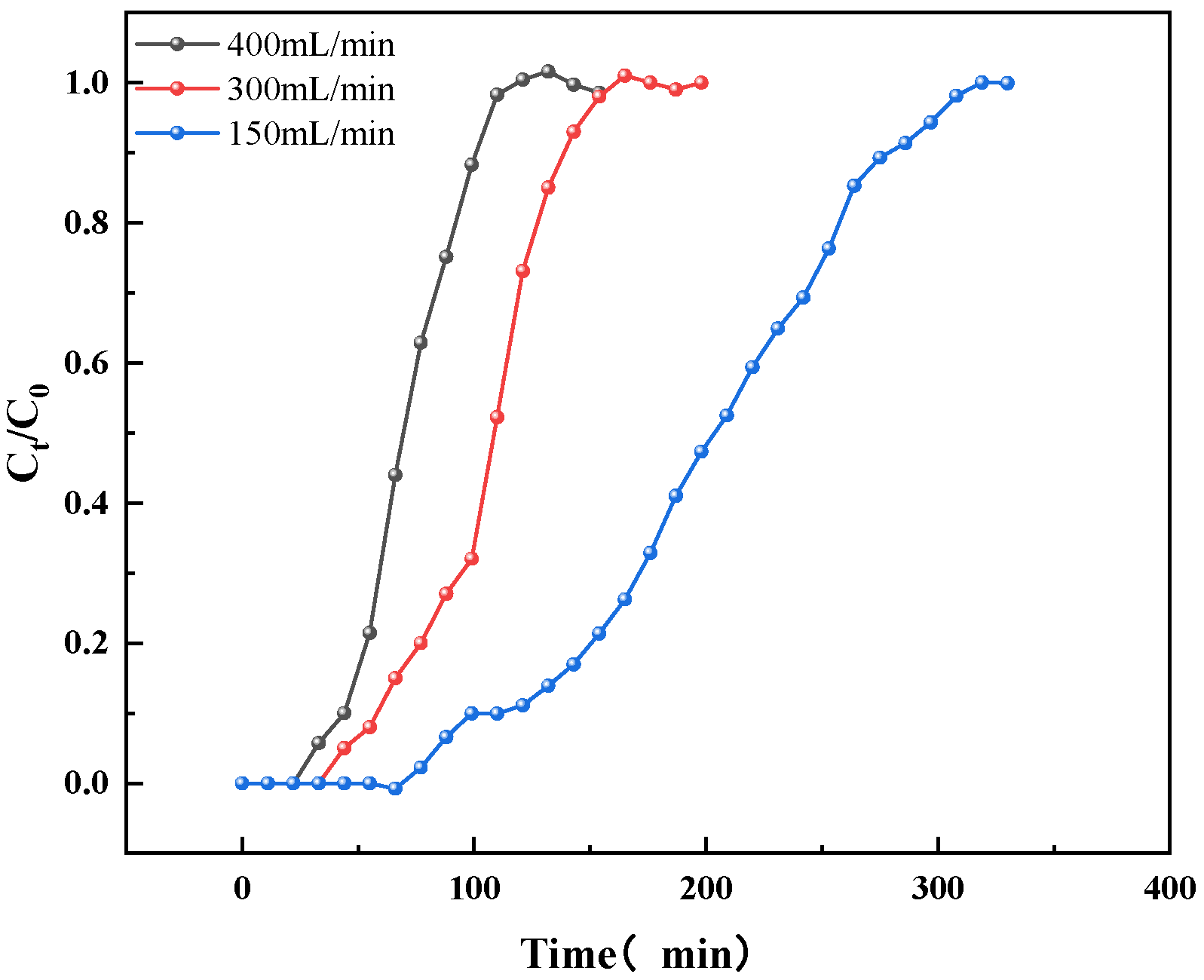
3.4. Effect of Gas Flow Rate on Adsorption
3.5. Adsorption Mechanism
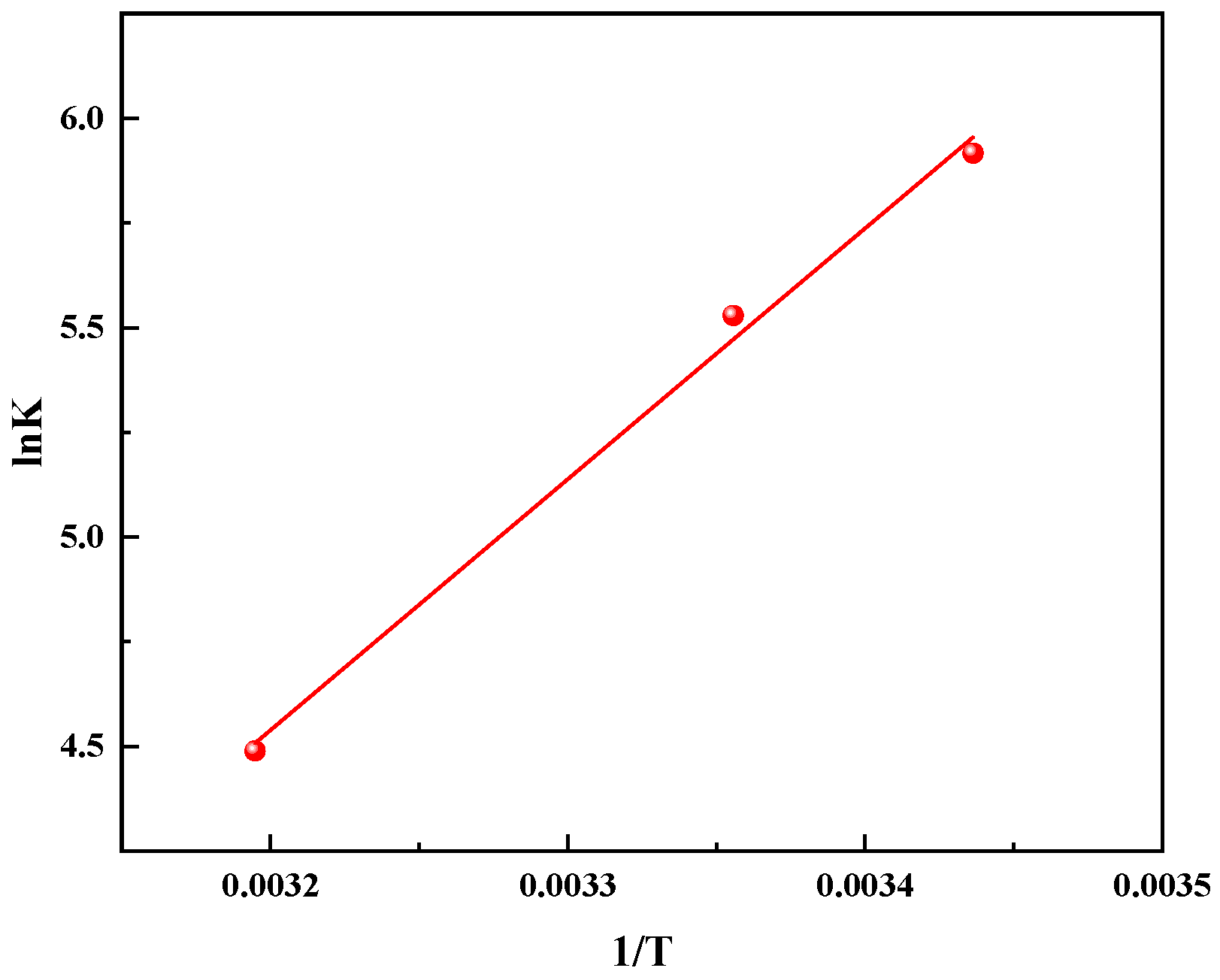
3.6. Thermodynamic Analysis of Toluene Adsorption on MIL-101 (Cr)
3.7. Discussion of Cycling Stability and Effect of Relative Humidity
4. Conclusions
- As the concentration of toluene at indoor levels increases, the adsorption breakthrough time of MIL-101 (Cr) to toluene gradually shortens, the adsorption capacity gradually increases, but the growth rate of the adsorption capacity gradually decreases. The adsorption isotherm is generally in line with the Langmuir single-layer adsorption model.
- The adsorption capacity of MIL-101 (Cr) for low concentrations of toluene is five–eight times that of silica gel. It could be a material with great potential for indoor air purification. It is expected to become a good substitute for silica gel in the application of purification air conditioning systems.
- Although the increase in temperature will shorten the breakthrough time and saturation time, the adsorption capacity decreases significantly. The adsorption capacity at 40 °C is only 25.8% of that at 18 °C. In practical applications, it is better to control the temperature below 25 °C.
- As the gas flow rate increases, the saturation time and breakthrough time are shortened, and the adsorption capacity is slightly reduced. The influence of flow rate on the adsorption capacity is negligible. Increasing the flow rate appropriately can enhance the adsorption process and reduce the saturation time. However, excessive flow rate can affect the penetration of adsorbate molecules, making it easier for the adsorbent to be broken through. In actual application, it is necessary to balance the relationship between adsorption rate and flow rate to achieve the best working conditions of the equipment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOC | Volatile Organic Compound |
| MOF | Metal–Organic Framework |
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, J.; Cantera, S.; Muñoz, R.; Lebrero, R. Indoor air VOCs biofiltration by bioactive coating packed bed bioreactors. J. Environ. Manag. 2024, 349, 119362. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Yin, Y.; Liu, J.; Dai, X. An eight-city study of volatile organic compounds in Chinese residences: Compounds, concentrations, and characteristics. Sci. Total Environ. 2020, 698, 134137. [Google Scholar] [CrossRef] [PubMed]
- Enesca, A.; Cazan, C. Volatile organic compounds (VOCs) removal from indoor air by heterostructures/composites/doped photocatalysts: A mini-review. Nanomaterials 2020, 10, 1965. [Google Scholar] [CrossRef]
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A state–of–the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef]
- Rouhani, S.; Taghipour, F. Modeling of UV-LED photocatalytic reactors for the degradation of gaseous volatile organic compounds (VOCs) in indoor environments. J. Environ. Chem. Eng. 2022, 10, 107657. [Google Scholar] [CrossRef]
- Cui, P.; Schito, G.; Cui, Q. VOC emissions from asphalt pavement and health risks to construction workers. J. Clean. Prod. 2020, 244, 118757. [Google Scholar] [CrossRef]
- Elliott, E.G.; Trinh, P.; Ma, X.; Leaderer, B.P.; Ward, M.H.; Deziel, N.C. Unconventional oil and gas development and risk of childhood leukemia: Assessing the evidence. Sci. Total Environ. 2017, 576, 138–147. [Google Scholar] [CrossRef]
- Elia, E.; Stylianou, M.; Agapiou, A. Investigation on the source of VOCs emission from indoor construction materials using electronic sensors and TD-GC-MS. Environ. Pollut. 2024, 348, 123765. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Lin, C.-C.; Hsu, S.-C. Comparison of conventional and green building materials in respect of VOC emissions and ozone impact on secondary carbonyl emissions. Build. Environ. 2015, 87, 274–282. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Castillo, A.-S.R.; Biard, P.-F.; Guihéneuf, S.; Paquin, L.; Amrane, A.; Couvert, A. Assessment of VOC absorption in hydrophobic ionic liquids: Measurement of partition and diffusion coefficients and simulation of a packed column. Chem. Eng. J. 2019, 360, 1416–1426. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Z.; Wang, X.; Wang, F.; Zeng, L.; Li, Z. Adsorption equilibrium of activated carbon amid fluctuating benzene concentration in indoor environments. Build. Environ. 2023, 245, 110964. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Yang, L.; Su, M.; Niu, Y.; Liu, Y.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Highly efficient removal of PM and VOCs from air by a self-supporting bifunctional conjugated microporous polymers membrane. J. Membr. Sci. 2022, 659, 120728. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X.; Chen, L.; Guo, J.; Wang, J. A complementary VOCs recovery system based on cryogenic condensation and low-temperature adsorption. Int. J. Refrig. 2023, 153, 222–230. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Sui, H.; Liu, H.; An, P.; He, L.; Li, X.; Cong, S. Application of silica gel in removing high concentrations toluene vapor by adsorption and desorption process. J. Taiwan Inst. Chem. Eng. 2017, 74, 218–224. [Google Scholar] [CrossRef]
- Ligotski, R.; Gilles, K.-D.; Roehnert, M.; Sager, U.; Asbach, C.; Schmidt, F. In-situ-desorption of indoor relevant VOC toluene and limonene on activated carbon based filter media using high relative humidity. Build. Environ. 2021, 191, 107556. [Google Scholar] [CrossRef]
- Feng, A.; Mi, L.; Yu, Y.; Cao, Y.; Yu, Y.; Song, L. Development of intracrystalline mesoporosity in NH4HF2-etched NaY zeolites by surfactant-templating and its effect on toluene adsorption. Chem. Eng. J. 2020, 390, 124529. [Google Scholar] [CrossRef]
- Jafari, S.; Ghorbani-Shahna, F.; Bahrami, A.; Kazemian, H. Adsorptive removal of toluene and carbon tetrachloride from gas phase using Zeolitic Imidazolate Framework-8: Effects of synthesis method, particle size, and pretreatment of the adsorbent. Microporous Mesoporous Mater. 2018, 268, 58–68. [Google Scholar] [CrossRef]
- Kraus, M.; Trommler, U.; Holzer, F.; Kopinke, F.-D.; Roland, U. Competing adsorption of toluene and water on various zeolites. Chem. Eng. J. 2018, 351, 356–363. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Q.; Ding, Z.; Jiang, H.; Yang, H.; Du, W.; Zheng, Y.; Huo, K.; Shaw, L.L. MOFs-based materials for solid-state hydrogen storage: Strategies and perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Zhao, Q.; Lian, S.; Li, R.; Yu, Z.; Liu, Q.; Zang, G.-L.; Song, C. Architecting MOFs-based mixed matrix membrane for efficient CO2 separation: Ameliorating strategies toward non-ideal interface. Chem. Eng. J. 2022, 443, 136290. [Google Scholar] [CrossRef]
- Li, Y.; Yao, B.; Chen, Y.; Zhou, Y.; Duan, X. Metal-organic frameworks (MOFs) as efficient catalysts for electro-Fenton (EF) reactions: Current progress and prospects. Chem. Eng. J. 2023, 463, 142287. [Google Scholar] [CrossRef]
- Li, X.-M.; Jia, J.; Yang, D.; Jin, J.; Gao, J. Construction of biomimetic proton transport channels in metal-organic framework. Chin. Chem. Lett. 2024, 35, 108474. [Google Scholar] [CrossRef]
- Kritskiy, I.; Volkova, T.; Sapozhnikova, T.; Mazur, A.; Tolstoy, P.; Terekhova, I. Methotrexate-loaded metal-organic frameworks on the basis of γ-cyclodextrin: Design, characterization, in vitro and in vivo investigation. Mater. Sci. Eng. C 2020, 111, 110774. [Google Scholar] [CrossRef]
- Huang, L.; Shen, R.; Shuai, Q. Adsorptive removal of pharmaceuticals from water using metal-organic frameworks: A review. J. Environ. Manag. 2021, 277, 111389. [Google Scholar] [CrossRef]
- Amador, R.N.; Cirre, L.; Carboni, M.; Meyer, D. BTEX removal from aqueous solution with hydrophobic Zr metal organic frameworks. J. Environ. Manag. 2018, 214, 17–22. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, S.; Li, N.; Cui, P.; Wang, H.; Amirkhanian, S.; Zhao, Z. Performance and VOCs emission inhibition of environmentally friendly rubber modified asphalt with UiO-66 MOFs. J. Clean. Prod. 2023, 385, 135633. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Zorainy, M.Y.; Alalm, M.G.; Kaliaguine, S.; Boffito, D.C. Revisiting the MIL-101 metal–organic framework: Design, synthesis, modifications, advances, and recent applications. J. Mater. Chem. A 2021, 9, 22159–22217. [Google Scholar] [CrossRef]
- Lim, J.H.; Goh, K.; Ng, D.Y.F.; Chew, J.; Wang, R. Layer-by-layer hierarchically structured nanofibrous membrane scaffolds incorporating metal-organic framework and carbon nanotube adsorbents for high-performance versatile organic solvent recovery. J. Clean. Prod. 2023, 404, 136925. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Synergistic ionic liquid encapsulated MIL-101 (Cr) metal-organic frameworks for an innovative adsorption desalination system. J. Clean. Prod. 2024, 474, 143565. [Google Scholar] [CrossRef]
- Ehrenmann, J.; Henninger, S.K.; Janiak, C. Water adsorption characteristics of MIL-101 for heat-transformation applications of MOFs. Eur. J. Inorg. Chem. 2011, 2011, 471–474. [Google Scholar] [CrossRef]
- Ma, L.; Rui, Z.; Wu, Q.; Yang, H.; Yin, Y.; Liu, Z.; Cui, Q.; Wang, H. Performance evaluation of shaped MIL-101–ethanol working pair for adsorption refrigeration. Appl. Therm. Eng. 2016, 95, 223–228. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; He, J.; He, Y.; Luo, L.; Wang, L.; Chen, C.; Shen, F.; Deng, S.; Zhang, Y. A core–shell Fe3O4@ NH2-MIL-101 (Cr) composite material for efficient removal of formaldehyde. J. Mater. Res. 2022, 37, 1739–1749. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Fu, X.; Pan, T.-T.; Ma, X.-B.; Cao, H.-X.; Jiang, W.-C. Stable effect on MIL-101 (Cr) with Cu2+ for the toluene adsorption. J. Solid State Chem. 2022, 316, 123633. [Google Scholar] [CrossRef]
- Jangodaz, E.; Alaie, E.; Safekordi, A.A.; Tasharrofi, S. Adsorption of ethylbenzene from air on metal–organic frameworks MIL-101 (Cr) and MIL-53 (Fe) at room temperature. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2090–2099. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Huang, S.; Xia, Q.; Li, Z. Adsorption and diffusion of benzene on chromium-based metal organic framework MIL-101 synthesized by microwave irradiation. Ind. Eng. Chem. Res. 2011, 50, 2254–2261. [Google Scholar] [CrossRef]
- Wang, J.; Muhammad, Y.; Gao, Z.; Shah, S.J.; Nie, S.; Kuang, L.; Zhao, Z.; Qiao, Z.; Zhao, Z. Implanting polyethylene glycol into MIL-101 (Cr) as hydrophobic barrier for enhancing toluene adsorption under highly humid environment. Chem. Eng. J. 2021, 404, 126562. [Google Scholar] [CrossRef]
- Rico-Barragán, A.A.; Álvarez, J.R.; Ovando-Medina, V.M.; Rojas-Mayorga, C.K.; Aguayo-Villarreal, I.A.; Luna-Triguero, A.; Dávila-Guzmán, N.E. Unraveling the toluene adsorption mechanism of MIL-101 (Cr) derived from PET waste for COV removal applications. Mater. Today Sustain. 2024, 27, 100925. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Song, M.; Gu, Z.-Y.; Wang, H.-F.; Yan, X.-P. Probing the adsorption characteristic of metal–organic framework MIL-101 for volatile organic compounds by quartz crystal microbalance. Environ. Sci. Technol. 2011, 45, 4490–4496. [Google Scholar] [CrossRef]
- Du, Z.; Mo, J.; Zhang, Y.; Xu, Q. Benzene, toluene and xylenes in newly renovated homes and associated health risk in Guangzhou, China. Build. Environ. 2014, 72, 75–81. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines for Europe; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- GB 50325-2020; Standard for Indoor Environmental Pollution Control of Civil Building Engineering. China Standards Press: Beijing, China, 2020.
- Liu, Q.; Liu, Y.; Zhang, M. Personal exposure and source characteristics of carbonyl compounds and BTEXs within homes in Beijing, China. Build. Environ. 2013, 61, 210–216. [Google Scholar] [CrossRef]
- Kamani, H.; Baniasadi, M.; Abdipour, H.; Mohammadi, L.; Rayegannakhost, S.; Moein, H.; Azari, A. Health risk assessment of BTEX compounds (benzene, toluene, ethylbenzene and xylene) in different indoor air using Monte Carlo simulation in zahedan city, Iran. Heliyon 2023, 9, e20294. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of volatile organic compounds by metal–organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef]
- Sheng, Y.; Dong, Q.; Ren, Q.; Wang, M. Prediction for the Adsorption of Low-Concentration Toluene by Activated Carbon. Sustainability 2023, 15, 1555. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lin, W.H. Dynamic Adsorption and Desorption of Toluene on Carbon Nanotubes Grafted Activated Carbon Fibers. Appl. Mech. Mater. 2013, 284, 72–76. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, M.; Dong, Q. Gas-particle two-phase adsorption of toluene and ultrafine particles on activated carbon studied by molecular simulation. Sci. Total Environ. 2023, 891, 164591. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, X.; Chen, J.; Yang, Y.; Lu, G. The preparation of defective UiO-66 metal organic framework using MOF-5 as structural modifier with high sorption capacity for gaseous toluene. Environ. Chem. Eng. 2019, 7, 103405. [Google Scholar] [CrossRef]
- Liu, C.; Cai, W.; Liu, L. Hydrothermal carbonization synthesis of Al-pillared montmorillonite@ carbon composites as high performing toluene adsorbents. Appl. Clay Sci. 2018, 162, 113–120. [Google Scholar] [CrossRef]
- Du, Y.; Xiao, G.; Guo, Z.; Lin, B.; Fu, M.; Ye, D.; Hu, Y. A high-performance and stable Cu/Beta for adsorption-catalytic oxidation in-situ destruction of low concentration toluene. Sci. Total Environ. 2022, 833, 155288. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Liu, Q.; Hu, X.; Wang, Y.; Liu, F. Study on the adsorption performance of toluene by bimetallic doped MOFs with high adsorption capacity. J. Mol. Struct. 2025, 1324, 140912. [Google Scholar] [CrossRef]
- Chen, F.-F.; Li, H.-F.; Jia, X.-R.; Wang, Z.-Y.; Qin, Y.-Y.; Liang, X.; Chen, W.-Q.; Ao, T.-Q. A quantitative prediction model for the phosphate adsorption capacity of carbon materials based on pore size distribution. Electrochim. Acta 2020, 331, 135377. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, L.; Wang, F.; Li, X.; Li, Z. Influence of pore volume and surface area on benzene adsorption capacity of activated carbons in indoor environments. Build. Environ. 2022, 216, 109011. [Google Scholar] [CrossRef]
- Cao, J.; Li, Y.; Ma, X.; Qi, M.; Liu, B.; Zhao, D.; Wang, Y. Constructing binuclear sites to modulate the charge distribution of MIL-101 for enhanced toluene adsorption performance: Experimental and theoretical studies. Sep. Purif. Technol. 2025, 354, 129400. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhou, S.-Y.; Xu, Z.; Ding, L.; Cheng, Y.-H. Metal-organic frameworks of MIL-100(Fe, Cr) and MIL-101(Cr) for aromatic amines adsorption from aqueous solutions. Molecules 2019, 24, 3718. [Google Scholar] [CrossRef]
- Li, K.; Wang, X. Adsorptive removal of Pb (II) by activated carbon prepared from Spartina alterniflora: Equilibrium, kinetics and thermodynamics. Bioresour. Technol. 2009, 100, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Q.; Wang, Z.; Wu, T.; Zhang, M. Synthesis of MIL-101(Cr) and its water adsorption performance. Microporous Mesoporous Mater. 2020, 297, 110044. [Google Scholar] [CrossRef]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Tehrani, N.H.M.H.; Alivand, M.S.; Kamali, A.; Esrafili, M.D.; Shafiei-Alavijeh, M.; Ahmadi, R.; Samipoorgiri, M.; Tavakoli, O.; Rashidi, A. Seed-mediated synthesis of a modified micro-mesoporous MIL-101(Cr) for improved benzene and toluene adsorption at room conditions. J. Environ. Chem. Eng. 2023, 11, 109558. [Google Scholar] [CrossRef]
- Xian, S.; Yu, Y.; Xiao, J.; Zhang, Z.; Xia, Q.; Wang, H.; Li, Z. Competitive adsorption of water vapor with VOCs dichloroethane, ethyl acetate and benzene on MIL-101(Cr) in humid atmosphere. RSC Adv. 2015, 5, 1827–1834. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Mi, J.; Jin, J.; Meng, H. Dual hydrophobic modification on MIL-101(Cr) with outstanding toluene removal under high relative humidity. Chem. Eng. J. 2023, 451, 139000. [Google Scholar] [CrossRef]
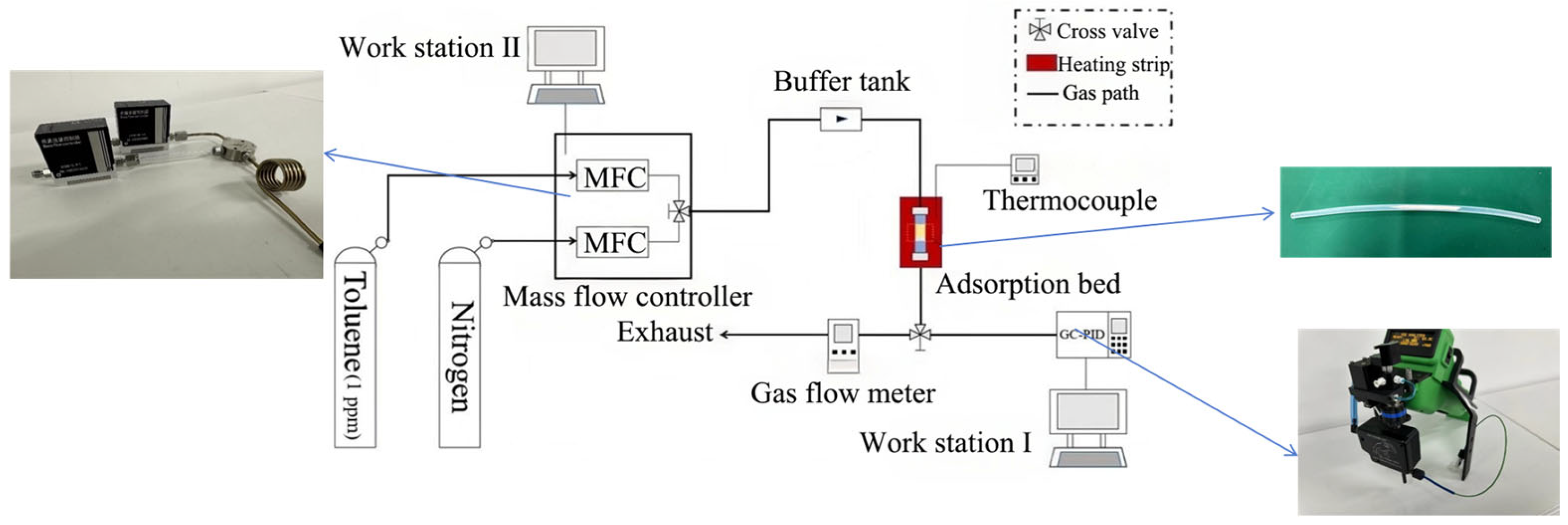
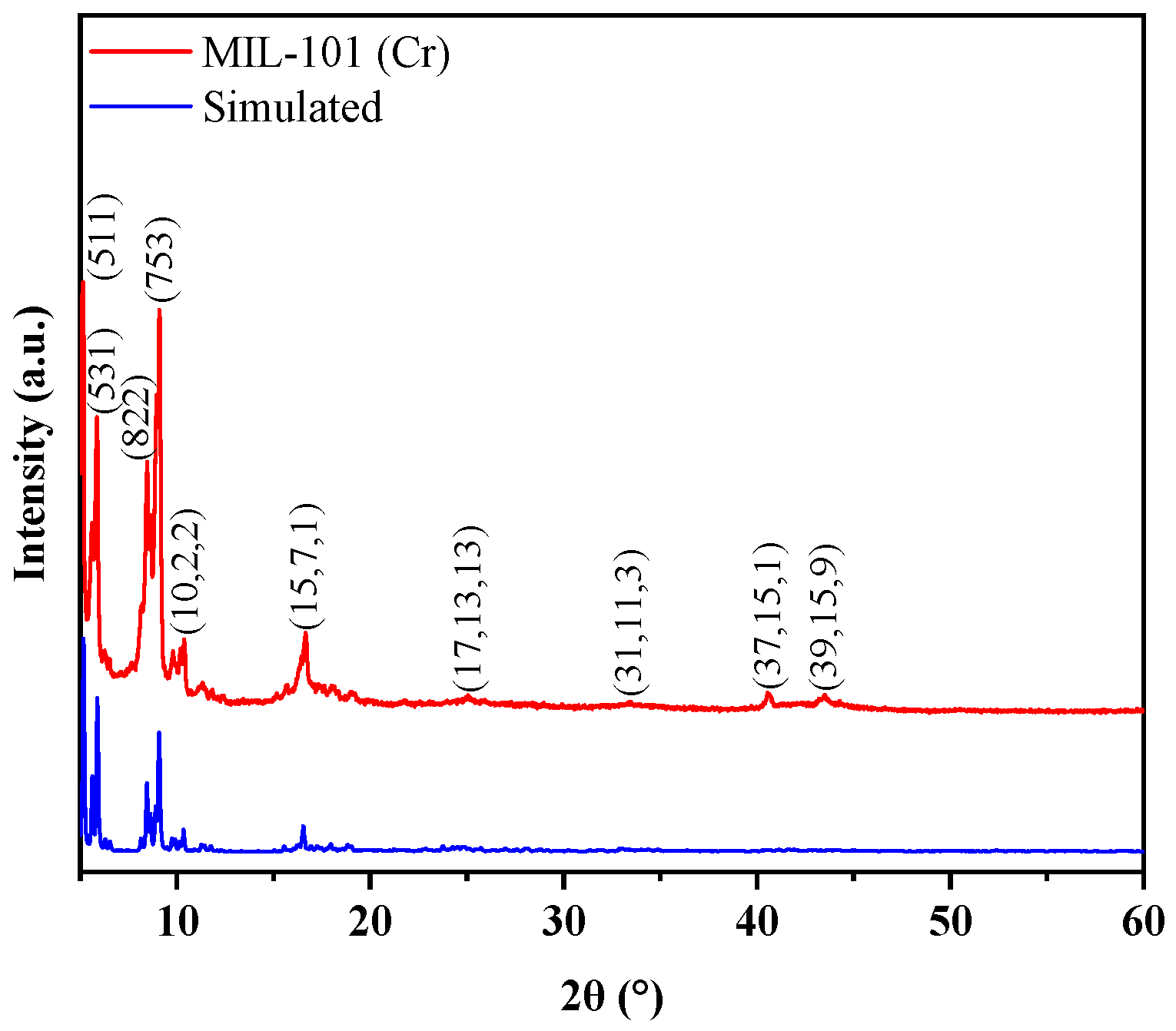
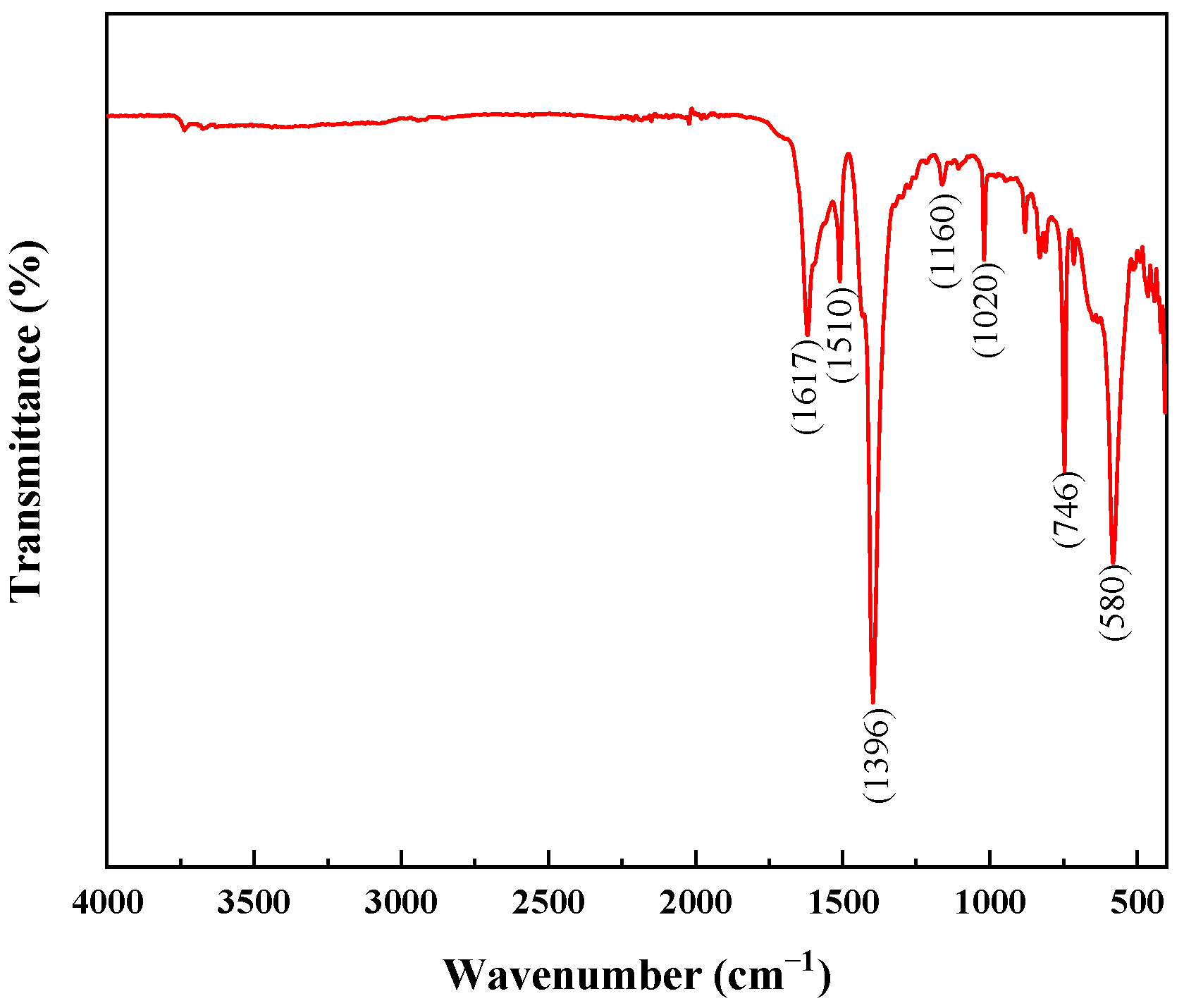

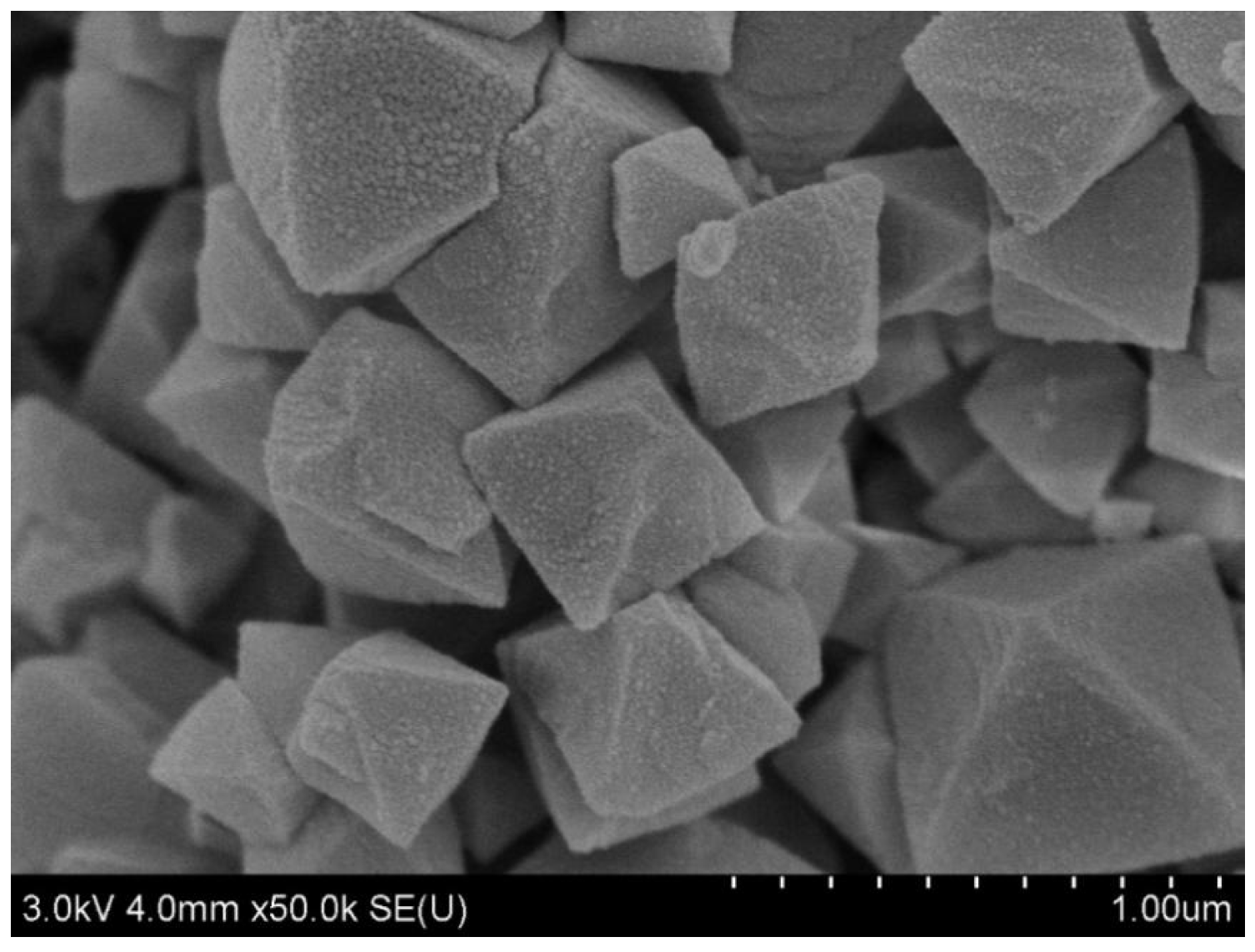

| Equipment | Model | Manufacturer |
|---|---|---|
| Electronic Balance | FA1024E | Changzhou Nebula Electronic Equipment Co., Ltd. (Changzhou, China) |
| Programmable Dynamic Dilution Apparatus | GT-310A-2 | Tianjin Geist Instrument Co., Ltd. (Tianjin, China) |
| Gas Chromatograph-Photoionization Detector (GC-PID) | FROG5000 | Defiant Inc. (Seattle, WA, USA) |
| Electronic Flowmeter | Bios Defender 520H | MesaLabs Inc. (Lakewood, CO, USA) |
| Constant Temperature Heating Tape | HX-W2078G | Dechuang Keyi (Beijing) Technology Co., Ltd. (Beijing, China) |
| Sample | Pore Volume (cm3/g) | Specific Surface Area (m2/g) | Average Pore Size (nm) | |||
|---|---|---|---|---|---|---|
| Total Pore Volume | Micropore Volume | Mesopore Volume | Macropore Volume | |||
| MIL-101 (Cr) | 1.58 | 1.22 (52%) | 0.97 (42%) | 0.14 (6%) | 2781.49 | 2.82 |
| Silica gel | 0.97 | 0.0044 (0.43%) | 1.00 (98.66%) | 0.0092 (0.91%) | 355.65 | 7.85 |
| Adsorbent | qm (mg/g) | K | R2 |
|---|---|---|---|
| MIL-101 (Cr) | 1285.77 | 0.00162 | 0.99 |
| Toluene Concentration (ppb) | Breakthrough Time (min) | Saturation Time (min) | Adsorption Capacity (mg/g) | |||
|---|---|---|---|---|---|---|
| Silica Gel | MIL-101 (Cr) | Silica Gel | MIL-101 (Cr) | Silica Gel | MIL-101 (Cr) | |
| 30 | 121 | 465 | 451 | 561 | 10.17 | 61.71 |
| 40 | 105 | 341 | 407 | 495 | 11.30 | 76.23 |
| 50 | 88 | 275 | 341 | 484 | 12.65 | 90.75 |
| 60 | 77 | 242 | 319 | 473 | 13.33 | 92.57 |
| 70 | 68 | 198 | 297 | 440 | 14.01 | 105.53 |
| Sorbent | Parameters | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| ACF | 407 ppm, 200 mL/min, 298 K | 160 | [51] |
| AC/ZnO | 10 mg/m3, 50 mL/min, 298 K | 68 | [52] |
| AC/ZrO2 | 60 | [52] | |
| AC/MgO | 56 | [52] | |
| AC/CuO | 46 | [52] | |
| AC | 41 | [52] | |
| AC | 500 ppb | 55 | [53] |
| UiO-66 | 100 ppm, 50 mL/min, 298 K | 166 | [54] |
| UiO-66(NH2) | 252 | [54] | |
| MOF-199 | 159 | [54] | |
| ZIF-67 | 224 | [54] | |
| MIL-101(Fe) | 98.3 | [54] | |
| MOF-5 | 32.9 | [54] | |
| M-U-0.01 | 1000 ppm,50 mL/min | 257 | [55] |
| Al-Mt@C(3/5) | 1000 ppm,100 mL/min 298 K | 39.9 | [56] |
| AC | 200 ppm, 400 mL/min 303 K | 184 | [11] |
| AC | 500 ppb, 2.5 L/min | 69 | [50] |
| Cu/Beta | 50 ppm | 77 | [57] |
| MIL-101 (Cr) | 500 ppm, 30 mL/min 298 K | 331.46 | [58] |
| Silica gel | 50 ppb, 150 mL/min 298 K | 12.65 | This study |
| MIL-101 (Cr) | 50 ppb, 150 mL/min, 298 K | 90.75 | This study |
| MIL-101 (Cr) | 500 ppb, 150 mL/min, 298 K | 599.21 | This study |
| Nut shell AC | 500 ppb, 150 mL/min, 298 K | 21.5 | This study |
| Temperature (°C) | Breakthrough Time (min) | Saturation Time (min) | Adsorption Capacity (mg/g) |
|---|---|---|---|
| 18 | 154 | 407 | 961.46 |
| 25 | 88 | 308 | 652.11 |
| 40 | 40 | 121 | 230.57 |
| Flow Rate (mL/min) | Breakthrough Time (min) | Saturation Time (min) | Adsorption Capacity (mg/g) |
|---|---|---|---|
| 150 | 88 | 308 | 652.11 |
| 300 | 46 | 153 | 645.63 |
| 400 | 33 | 110 | 590.92 |
| Temperature (°C) | Flow Rate (mL/min) | Toluene Concentration (ppb) | ΔG (kJ/mol) |
|---|---|---|---|
| 25 | 150 | 30 | −15.66 |
| 25 | 150 | 40 | −15.47 |
| 25 | 150 | 50 | −15.35 |
| 25 | 150 | 60 | −14.95 |
| 25 | 150 | 70 | −14.89 |
| 25 | 150 | 300 | −14.75 |
| 25 | 150 | 400 | −14.50 |
| 25 | 150 | 500 | −14.32 |
| 25 | 150 | 600 | −14.03 |
| 25 | 150 | 700 | −13.69 |
| 18 | 150 | 700 | −14.66 |
| 25 | 150 | 700 | −13.70 |
| 40 | 150 | 700 | −11.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Nie, J.; Huang, H.; He, F.; Wang, K.; Yang, P. Experimental Study on the Adsorption Performance of Metal–Organic Framework MIL-101 (Cr) for Indoor Toluene. Buildings 2025, 15, 2506. https://doi.org/10.3390/buildings15142506
Zhao Z, Nie J, Huang H, He F, Wang K, Yang P. Experimental Study on the Adsorption Performance of Metal–Organic Framework MIL-101 (Cr) for Indoor Toluene. Buildings. 2025; 15(14):2506. https://doi.org/10.3390/buildings15142506
Chicago/Turabian StyleZhao, Zirong, Jinzhe Nie, Honghao Huang, Fuqun He, Kaiqiao Wang, and Pu Yang. 2025. "Experimental Study on the Adsorption Performance of Metal–Organic Framework MIL-101 (Cr) for Indoor Toluene" Buildings 15, no. 14: 2506. https://doi.org/10.3390/buildings15142506
APA StyleZhao, Z., Nie, J., Huang, H., He, F., Wang, K., & Yang, P. (2025). Experimental Study on the Adsorption Performance of Metal–Organic Framework MIL-101 (Cr) for Indoor Toluene. Buildings, 15(14), 2506. https://doi.org/10.3390/buildings15142506





