Valorization of Alkali–Thermal Activated Red Mud for High-Performance Geopolymer: Performance Evaluation and Environmental Effects
Abstract
1. Introduction
2. Experimental Program
2.1. Raw Materials
2.2. Material Design and Preparation
2.3. Testing Methods
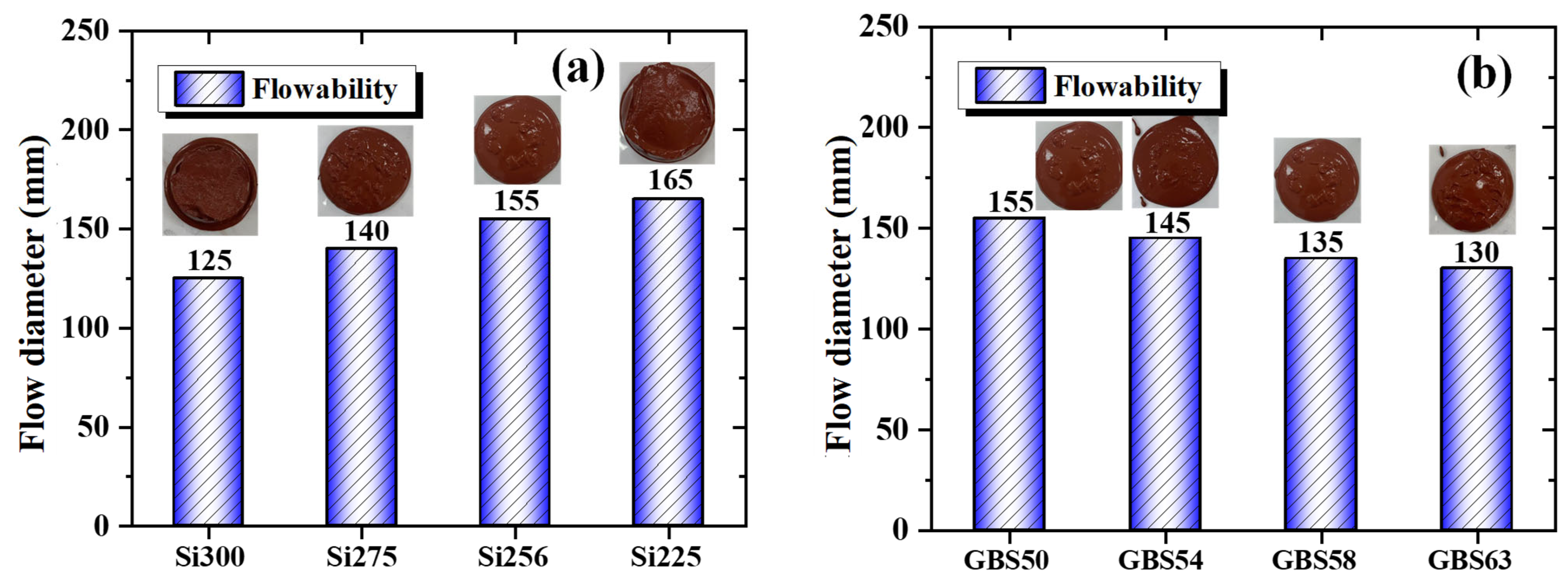
2.3.1. Flowability
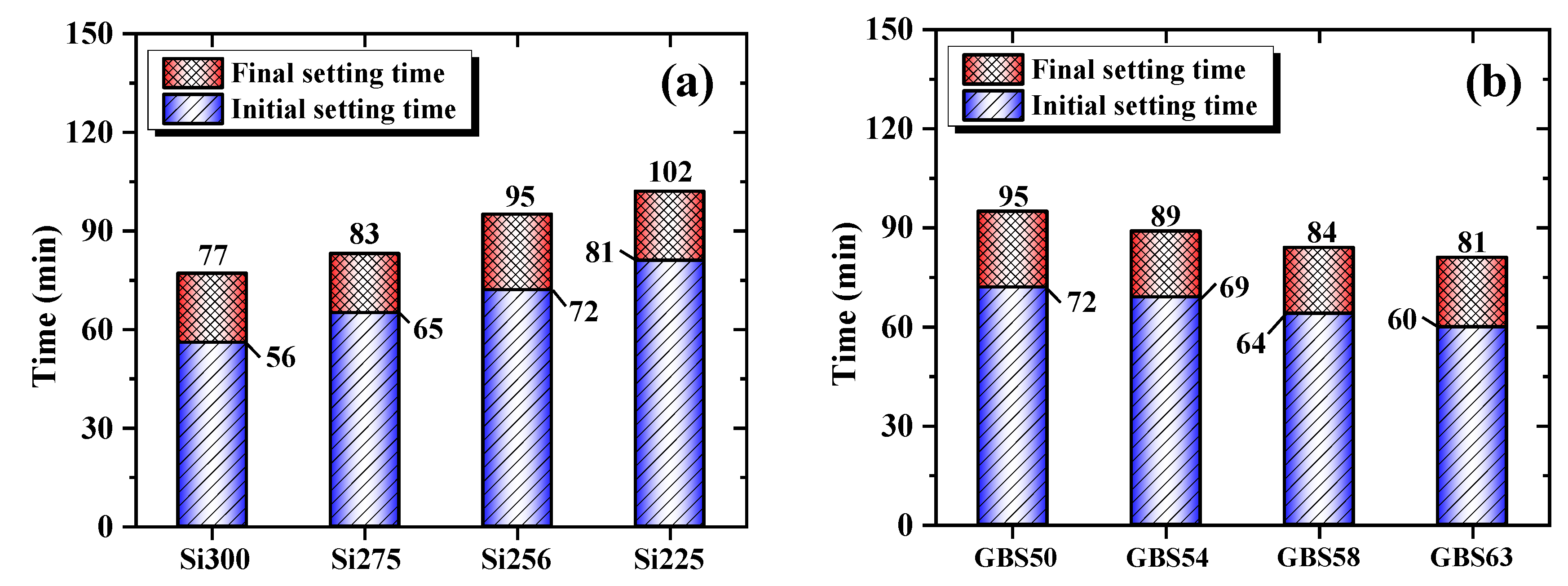
2.3.2. Setting Time
2.3.3. Compressive Strength
2.3.4. Drying Shrinkage
2.3.5. Water Permeability
2.3.6. SEM-EDS Analysis
2.3.7. FTIR Analysis
2.3.8. XRD Analysis
2.3.9. TG-DTG Analysis
3. Results and Discussion
3.1. Fresh Properties and Hardened Properties
3.1.1. Flowability
3.1.2. Setting Time
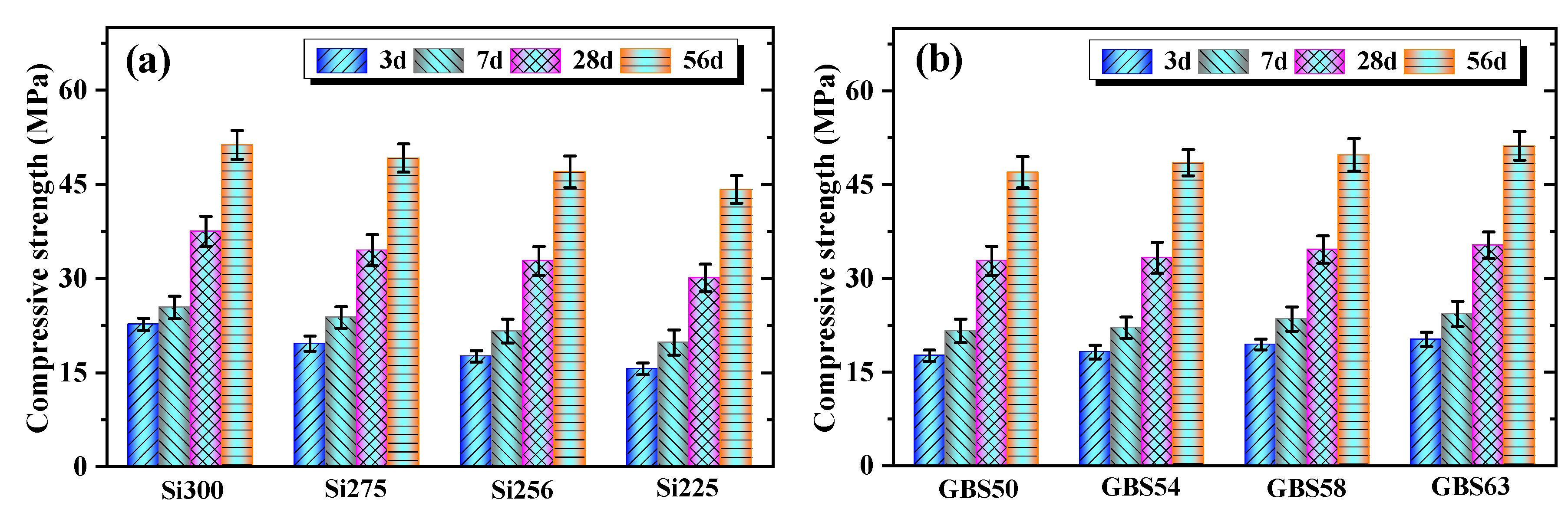
3.1.3. Compressive Strength
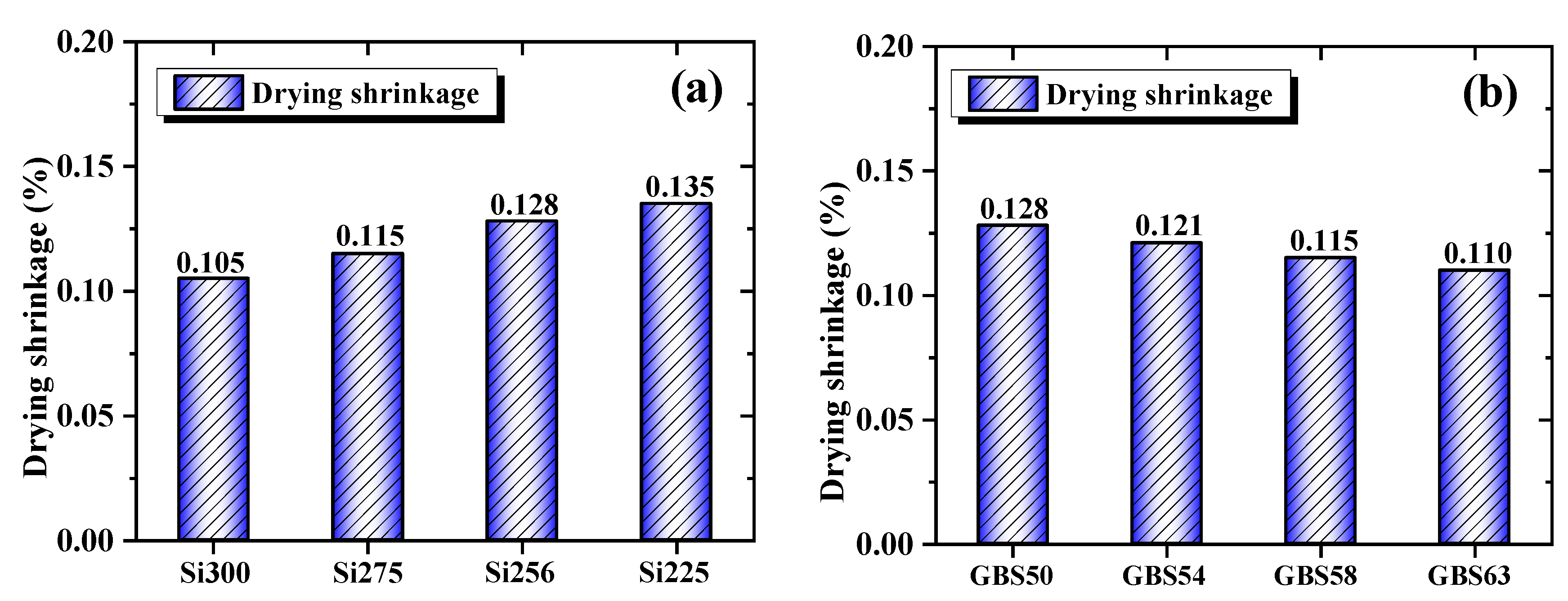
3.1.4. Drying Shrinkage
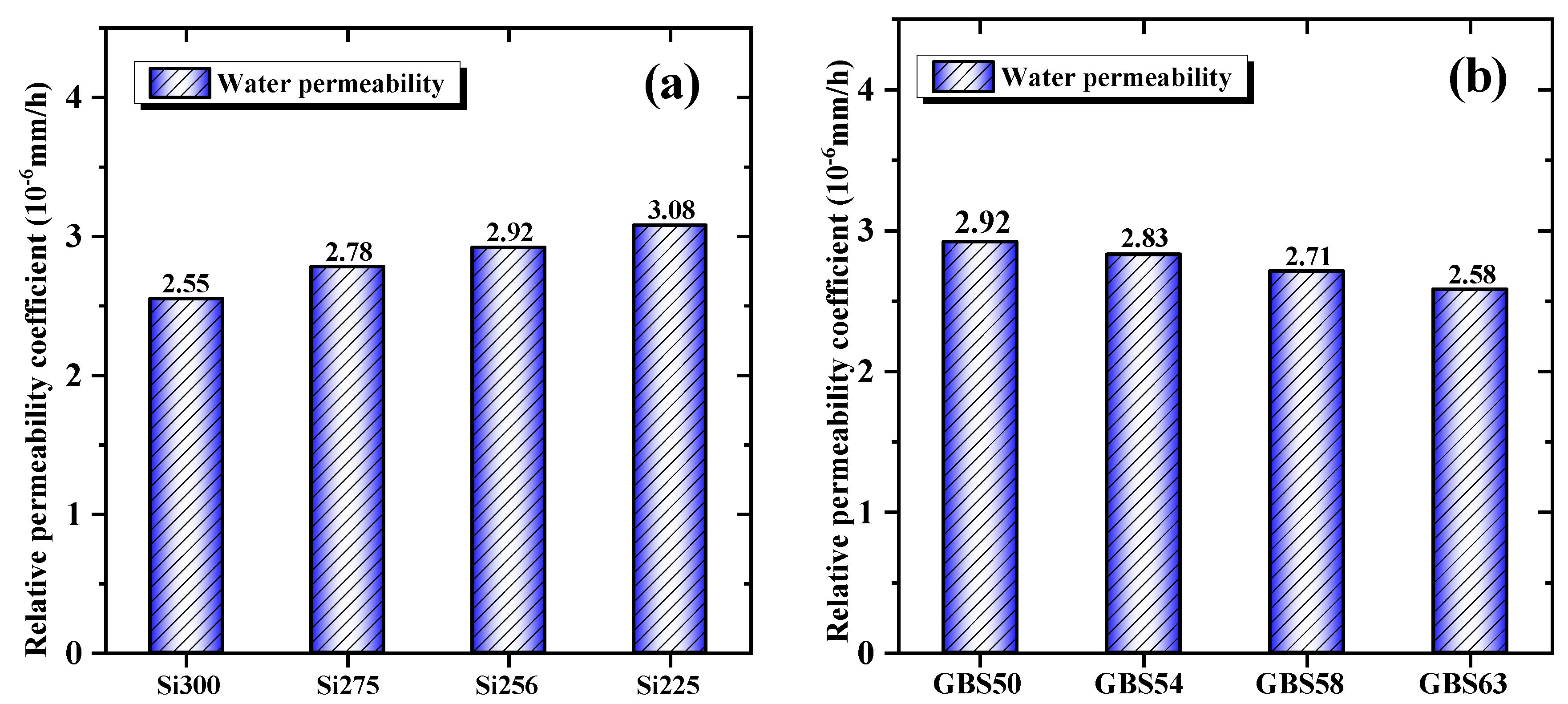
3.1.5. Water Permeability
3.2. Microstructure
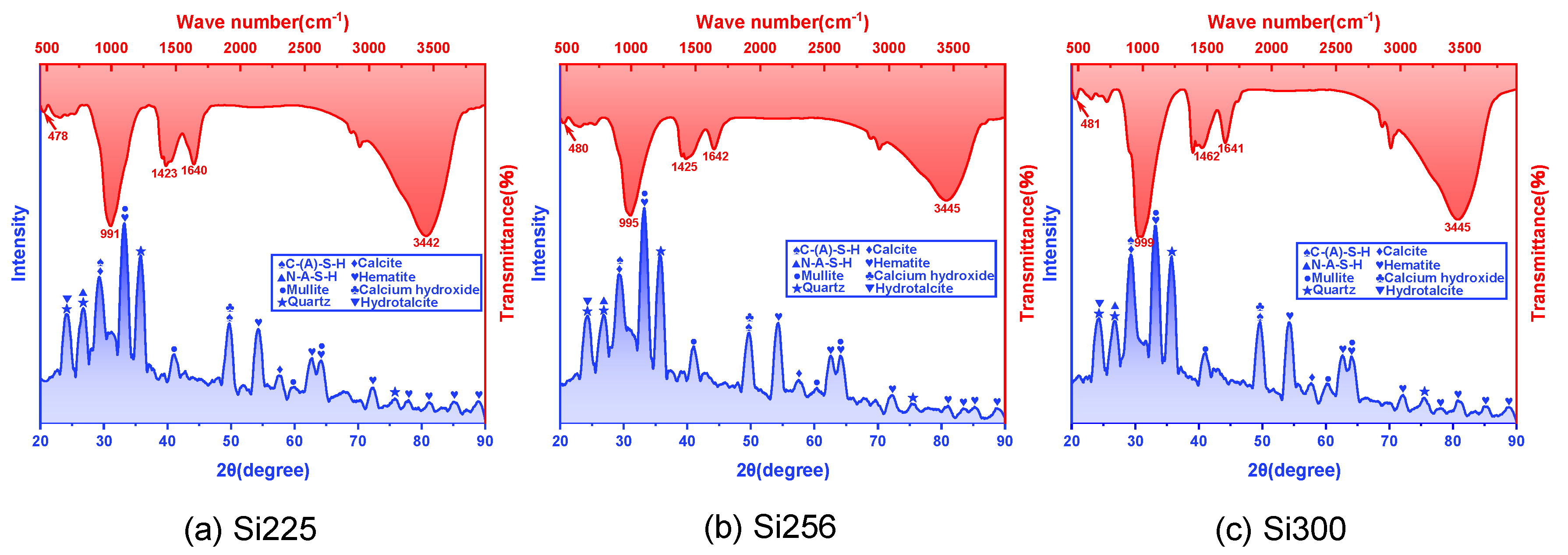
3.2.1. FTIR and XRD Analyses
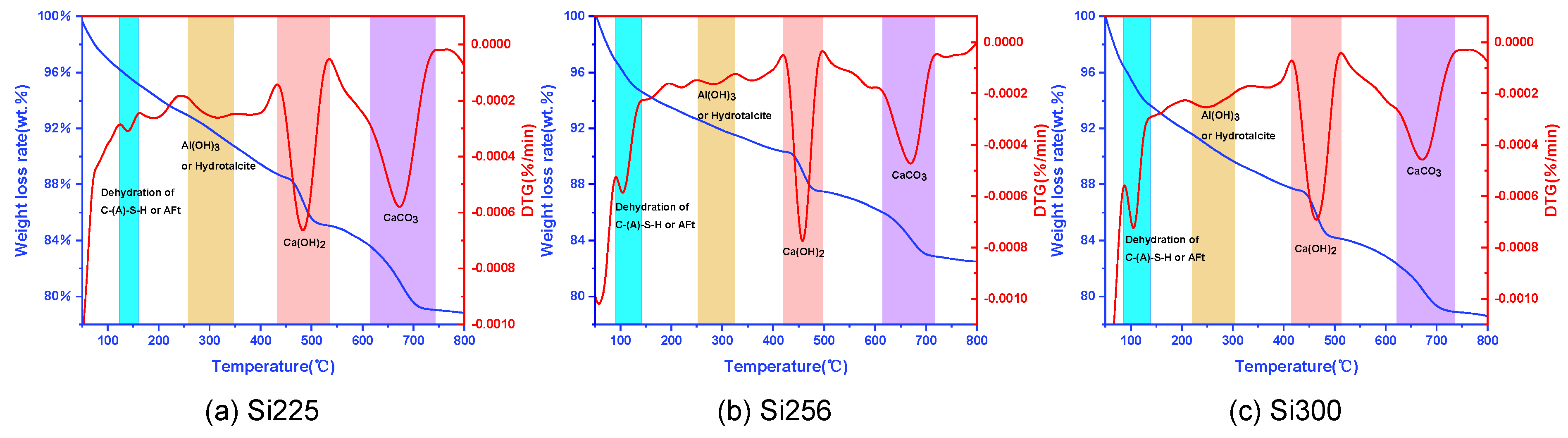
3.2.2. TG-DTG Analysis
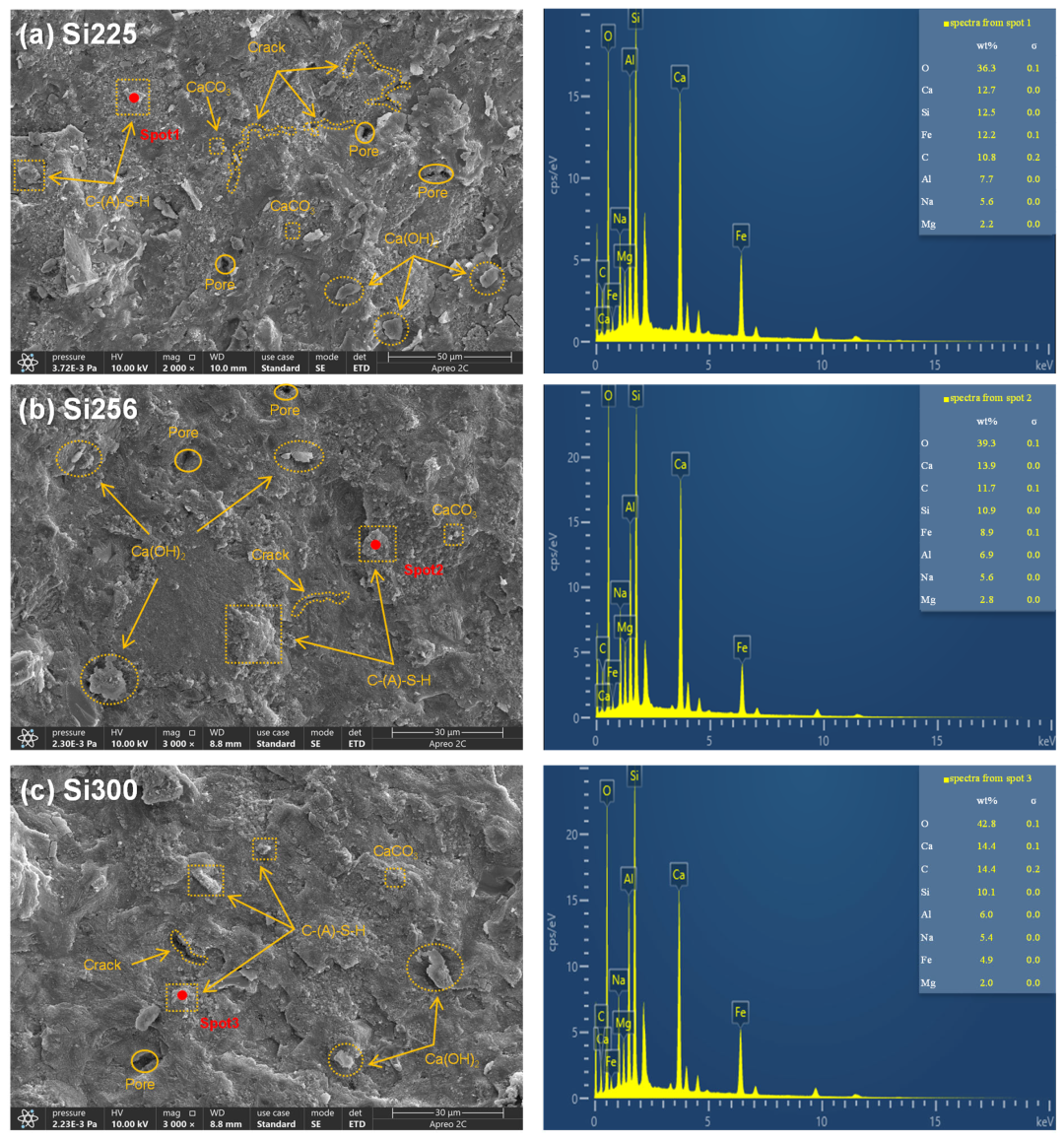
3.2.3. SEM-EDS Analysis
3.3. Sustainability Analysis
4. Conclusions
- (1)
- The high SiO2/Al2O3 ratio and increased GGBS addition raised the concentrations of SiO2 and Ca2+ in the system, respectively. This accelerated the formation of a three-dimensional gel network, resulting in reduced setting time and flowability of RM-based geopolymers;
- (2)
- Increasing the SiO2/Al2O3 ratio and GGBS addition enhanced the 56-day compressive strength by 6.3–16.1% and 3.2–8.9%, respectively. The higher SiO2/Al2O3 ratio increased the concentration of [SiO4]4− units and facilitated the dissolution of Si and Al. GGBS promoted the release of Ca2+ and exothermic reactions, thereby improving the strength of RM-based geopolymers;
- (3)
- When the SiO2/Al2O3 ratio increased, the drying shrinkage was reduced by 22.2% due to the enhanced formation of C-(A)-S-H/N-A-S-H gels and a decrease in mesopore content. High GGBS addition reduced the shrinkage by 14.1%, primarily promoting C-(A)-S-H gel formation, facilitating CaCO3 crystallization, and reducing the evaporation of free water. Both approaches reduced water permeability by optimizing the pore structure and enhancing the densification of the gel network;
- (4)
- The primary hydration products of RM-based geopolymers included C-(A)-S-H, N-A-S-H, calcite, hydrotalcite, and calcium hydroxide. These products effectively filled the pores, leading to a more compact microstructure. An SEM-EDS analysis further showed that raising the SiO2/Al2O3 ratio reduced the crack length and pore quantity. CaCO3, Ca(OH)2, and C-(A)-S-H formed an interpenetrating gel–crystal network structure. The Ca/Si ratio in the C-(A)-S-H gel increased from 1.02 to 1.43, and the Si/Al ratio rose from 1.62 to 1.68;
- (5)
- In comparison to the referenced RM-based geopolymers, the CO2 emission and costs in this study were reduced by 13.13–44.33% and 3.64–39.68%, respectively. The CO2 emissions of RM-based geopolymers were closely influenced by the SiO2/Al2O3 ratio. Adjusting the SiO2/Al2O3 ratio effectively reduced CO2 emissions, thereby promoting sustainability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Zheng, Y.; He, J.; Cui, K.; Shen, P.; Peng, G.; Guo, R.; Xia, D.; Poon, C.S. Production of carbonates calcined clay cement composites via CO2-assisted vigorous stirring. Cem. Concr. Compos. 2025, 163, 106181. [Google Scholar] [CrossRef]
- Li, Z.; Dong, H.F.; Zhao, X.S.; Wang, K.; Gao, X.J. Utilization of bayer red mud for high-performance geopolymers: Competitive roles of different activators. Case Stud. Constr. Mater. 2025. [Google Scholar] [CrossRef]
- Ghafoor, M.T.; Rakhimova, N.; Shi, C. One part geopolymers: A comprehensive review of advances and key challenges. J. Build. Eng. 2025, 111, 113112. [Google Scholar] [CrossRef]
- Hassan, A.; Zhang, C. Dynamic and static behaviour of geopolymer concrete for sustainable infrastructure development: Prospects, challenges, and performance review. Compos. Struct. 2025, 359, 118984. [Google Scholar] [CrossRef]
- Guo, K.; Dong, H.; Zhang, J.; Zhang, L.; Li, Z. Experimental study of Alkali-Activated cementitious materials using thermally activated red mud: Effect of the Si/Al ratio on fresh and mechanical properties. Buildings 2025, 15, 565. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, R.; Zhang, M.M.; Hu, W.; Bai, Y.; Huang, B.S. A laboratory investigation of steel to fly ash-based geopolymer paste bonding behavior after exposure to elevated temperatures. Constr. Build. Mater. 2020, 254, 119267. [Google Scholar] [CrossRef]
- Karthik, S.; Mohan, K.S.R.; Murali, G.; Abid, S.R.; Dixit, S. Impact of various fibers on mode I, III and I/III fracture toughness in slag, fly Ash, and silica fume-based geopolymer concrete using edge-notched disc bend specimen. Theor. Appl. Fract. Mech. 2024, 134, 104751. [Google Scholar] [CrossRef]
- Bilal, H.; Chen, T.F.; Ren, M.; Gao, X.J.; Su, A.S. Influence of silica fume, metakaolin & SBR latex on strength and durability performance of pervious concrete. Constr. Build. Mater. 2021, 275, 122124. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; El-Reash, Y.G.A.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R.M. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, A.; Zhu, Y.; Dai, J.; Xu, Q.; Liu, K.; Hao, F.; Sun, D. Manufacturing ultra-high performance geopolymer concrete (UHPGC) with activated coal gangue for both binder and aggregate. Compos. Part B: Eng. 2024, 284, 111723. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Al-Fakih, A.; Al-Osta, M.A.; Gondal, M.A. Intelligent optimization for modeling carbon dioxide footprint in fly ash geopolymer concrete: A novel approach for minimizing CO2 emissions. J. Environ. Chem. Eng. 2024, 12, 111835. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.J. Properties of coal gangue-portland cement mixture with carbonation. Fuel 2019, 245, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, hazard and iron recovery technology of red mud—A critical review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef]
- Li, Y.; Min, X.; Ke, Y.; Liu, D.; Tang, C. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef]
- Zakira, U.; Zheng, K.; Xie, N.; Birgisson, B. Development of high-strength geopolymers from red mud and blast furnace slag. J. Clean. Prod. 2023, 383, 135439. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Chen, X.; Duan, W.; Fan, R.; Outhred, L.; Wang, L. Micro-chemomechanical properties of red mud binder and its effect on concrete. Compos. Part B Eng. 2023, 258, 110688. [Google Scholar] [CrossRef]
- Qin, T.; Luo, H.; Han, R.; Zhao, Y.; Chen, L.; Liu, M.; Gui, Z.; Xing, J.; Chen, D.; He, B. Red mud in combination with construction waste red bricks for the preparation of Low-Carbon binder materials: Design and material characterization. Buildings 2024, 14, 3982. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.; Dong, B.; Fang, G.; Xing, F. Engineering and micro-properties of alkali-activated slag pastes with Bayer red mud. Constr. Build. Mater. 2022, 351, 128869. [Google Scholar] [CrossRef]
- Nikbin, I.M.; Aliaghazadeh, M.; Charkhtab, S.; Fathollahpour, A. Environmental impacts and mechanical properties of lightweight concrete containing bauxite residue (red mud). J. Clean. Prod. 2018, 172, 2683–2694. [Google Scholar] [CrossRef]
- Luo, Z.; Ge, M.; Liu, L.; Liu, X.; Zhang, W.; Ye, J.; Gao, M.; Yang, Y.; Zhang, M.; Liu, X. Desulfurization-modified red mud for supersulfated cement production: Insights into hydration kinetics, microstructure, and mechanical properties. Compos. Part B Eng. 2025, 297, 112340. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Liu, Z.; Wang, F.; Hu, S. Dealkalization behavior and leaching mechanisms of red mud with simulated aluminum profile wastewater. Dev. Built. Environ. 2025, 21, 100613. [Google Scholar] [CrossRef]
- Xi, X.; Jin, K.A.; Niu, X.; Zhang, S. A novel tandem pyrolysis-catalytic upgrading strategy for plastic waste to high-value products with zeolite and red mud. Energy 2025, 330, 136921. [Google Scholar] [CrossRef]
- Li, X.; Jin, S.; Yan, T.; Qiao, X.; Yuan, J. Unraveling the interactive effects of Na2O/Al2O3, SiO2/Al2O3 and calcium on the properties of geopolymers from circulating fluidized bed fly ashes. Case Stud. Constr. Mater. 2024, 21, e3798. [Google Scholar] [CrossRef]
- Khan, M.I.; Azizli, K.; Sufian, S.; Man, Z. Sodium silicate-free geopolymers as coating materials: Effects of Na/Al and water/solid ratios on adhesion strength. Ceram. Int. 2015, 41 Pt B, 2794–2805. [Google Scholar] [CrossRef]
- Kim, B.; Kang, J.; Shin, Y.; Yeo, T.; Heo, J.; Um, W. Effect of Si/Al molar ratio and curing temperatures on the immobilization of radioactive borate waste in metakaolin-based geopolymer waste form. J. Hazard. Mater. 2023, 458, 131884. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Lei, Z.; Gao, M.; Sun, J.; Tong, L.; Chen, S.; Wang, Y. Designing low-carbon fly ash based geopolymer with red mud and blast furnace slag wastes: Performance, microstructure and mechanism. J. Environ. Manag. 2024, 354, 120362. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Doh, J.; Ong, D.E.L.; Dinh, H.L.; Podolsky, Z.; Zi, G. Investigation on red mud and fly ash-based geopolymer: Quantification of reactive aluminosilicate and derivation of effective Si/Al molar ratio. J. Build. Eng. 2023, 71, 106559. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Kim, G.; Cho, S.; Im, S.; Yoon, J.; Suh, H.; Kanematsu, M.; Machida, A.; Shobu, T.; Bae, S. Evaluation of the thermal stability of metakaolin-based geopolymers according to Si/Al ratio and sodium activator. Cem. Concr. Compos. 2024, 150, 105562. [Google Scholar] [CrossRef]
- Pimraksa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K.; Sato, T. Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios. Mater. Sci. Eng. A 2011, 528, 6616–6623. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Souayfan, F.; Roziere, E.; Justino, C.; Paris, M.; Deneele, D.; Loukili, A. Comprehensive study on the reactivity and mechanical properties of alkali-activated metakaolin at high H2O/Na2O ratios. Appl. Clay Sci. 2023, 231, 106758. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Saini, G.; Vattipalli, U. Assessing properties of alkali activated GGBS based self-compacting geopolymer concrete using nano-silica. Case Stud. Constr. Mater. 2020, 12, e352. [Google Scholar] [CrossRef]
- Zhou, X.; Geng, Z.; Shi, J. Enhanced passivity of reinforcing steel in cementitious materials with thermally-activated red mud. Cem. Concr. Compos. 2024, 153, 105696. [Google Scholar] [CrossRef]
- Kopecskó, K.; Hajdu, M.; Khalaf, A.A.; Merta, I. Fresh and hardened properties for a wide range of geopolymer binders—An optimization process. Clean. Eng. Technol. 2024, 21, 100770. [Google Scholar] [CrossRef]
- Churata, R.; Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Torres-Almirón, J.; Ortiz-Valdivia, Y.; Velasco, F. Study of geopolymer composites based on volcanic ash, fly ash, pozzolan, metakaolin and mining tailing. Buildings 2022, 12, 1118. [Google Scholar] [CrossRef]
- El-Hassan, H.; Kianmehr, P.; Tavakoli, D.; El-Mir, A.; Dehkordi, R.S. Synergic effect of recycled aggregates, waste glass, and slag on the properties of pervious concrete. Dev. Built Environ. 2023, 15, 100189. [Google Scholar] [CrossRef]
- Hou, D.; Wu, D.; Wang, X.; Gao, S.; Yu, R.; Li, M.; Wang, P.; Wang, Y. Sustainable use of red mud in ultra-high performance concrete (UHPC): Design and performance evaluation. Cem. Concr. Compos. 2021, 115, 103862. [Google Scholar] [CrossRef]
- Emminger, Y.H.; Ladner, L.; Ruiz-Agudo, C. Comparative study of the early stages of crystallization of calcium silicate hydrate (C-S-H) and calcium aluminate silicate hydrate (C-A-S-H). Cem. Concr. Res. 2025, 193, 107873. [Google Scholar] [CrossRef]
- Chen, X.; Niu, Z.; Wang, J.; Zhu, G.R.; Zhou, M. Effect of sodium polyacrylate on mechanical properties and microstructure of metakaolin-based geopolymer with different SiO2/Al2O3 ratio. Ceram. Int. 2018, 44, 18173–18180. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. Part B Eng. 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Ai, T.; Zhong, D.; Zhang, Y.; Zong, J.; Yan, X.; Niu, Y. The Effect of Red Mud Content on the Compressive Strength of Geopolymers under Different Curing Systems. Buildings 2021, 11, 298. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Z.; Zhu, H.; Zhang, B.; Dong, Z.; Zhan, X. Efficient utilization of coral waste for internal curing material to prepare eco-friendly marine geopolymer concrete. J. Environ. Manag. 2024, 368, 122173. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, C.; Rong, H.; Liu, S.; Shi, Y.; Wang, H.; Wang, Q.; Zhang, X.; Han, J.; Jing, K.; et al. The shrinkage and durability performance evaluations for concrete exposed to temperature variation environments at early-age. J. Build. Eng. 2025, 104, 112457. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, C.; Wang, Y.; Zou, Y.; Zhao, J. Experimental study on mechanical properties and drying shrinkage compensation of solidified Ultra-Fine dredged sand blocks made with GGBS-Based geopolymer. Buildings 2023, 13, 1750. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, H.; Li, Y.; Yang, L.; Cheng, S.; Yu, H. Engineered cementitious composites using blended limestone calcined clay and fly ash: Mechanical properties and drying shrinkage modeling. Case Stud. Constr. Mater. 2024, 20, e2960. [Google Scholar] [CrossRef]
- Wang, P.; Yin, Z.Y.; Hicher, P.Y.; Cui, Y.J. Micro-mechanical analysis of one-dimensional compression of clay with dem. Int. J. Numer. Anal. Methods Geomech. 2023, 47, 2706–2724. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. The effect of silica availability on the mechanism of geopolymerisation. Cem. Concr. Res. 2011, 41, 210–216. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Daluee, M.A.; Amirriahi, M.; Eslamieh, M.A.; Rajaee, K.; Zaresefat, M. Durability assessment of clay soils stabilised with geopolymers based on recycled glass powder in various corrosive environments. Results Eng. 2025, 27, 105691. [Google Scholar] [CrossRef]
- Song, S.X.; Wang, P.; Yin, Z.Y.; Cheng, Y.P. Micromechanical modeling of hollow cylinder torsional shear test on sand using discrete element method. J. Rock Mech. Geotech. Eng. 2024, 16, 5193–5208. [Google Scholar] [CrossRef]
- Zhang, M.; He, M.; Pan, Z. Inhibition of efflorescence for fly ash-slag-steel slag based geopolymer: Pore network optimization and free alkali stabilization. Ceram. Int. 2024, 50 Pt C, 48538–48550. [Google Scholar] [CrossRef]
- Hu, Z.; Wyrzykowski, M.; Lura, P. Estimation of reaction kinetics of geopolymers at early ages. Cem. Concr. Res. 2020, 129, 105971. [Google Scholar] [CrossRef]
- Raza, A.; Selmi, A.; Elhadi, K.M.; Ghazouani, N.; Chen, W. Strength analysis and microstructural characterization of calcined red mud based-geopolymer concrete modified with slag and phosphogypsum. J. Mater. Res. Technol. 2024, 33, 6168–6181. [Google Scholar] [CrossRef]
- Gaifutdinov, A.M.; Andrianova, K.A.; Amirova, L.M.; Amirov, R.R. Optimizing the manufacturing technology of high-strength fiber reinforced composites based on aluminophosphates. Compos. Part A Appl. Sci. Manuf. 2024, 185, 108310. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Lei, Z.; Tong, L.; Sun, J.; Wang, Y.; Wang, X.; Jiang, X. Ternary cementless composite based on red mud, ultra-fine fly ash, and GGBS: Synergistic utilization and geopolymerization mechanism. Case Stud. Constr. Mater. 2023, 19, e2410. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Yong, C.L.; Jan, R.J.S.; Tan, T.H.; Lin, J.; Mo, K.H. Utilization of waste glass as precursor material in one-part alkali-activated aggregates. J. Mater. Res. Technol. 2024, 33, 5551–5558. [Google Scholar] [CrossRef]
- Yang, Z.; Li, R.; Zhu, H.; Zhang, B.; Dong, Z.; Zhan, X.; Zhang, G.; Zhang, H. Synthesis of eco-sustainable seawater sea-sand geopolymer mortars from ternary solid waste: Influence of microstructure evolution on mechanical performance. Sustain. Mater. Technol. 2024, 41, e1056. [Google Scholar] [CrossRef]
- Cioffi, R.; Maffucci, L.; Santoro, L. Optimization of geopolymer synthesis by calcination and polycondensation of a kaolinitic residue. Resour. Conserv. Recycl. 2003, 40, 27–38. [Google Scholar] [CrossRef]
- Costa, L.M.; Souza, T.D.C.C.; Oliveira, R.K.F.G.; Almeida, N.G.S.; Houmard, M. Effects of silicas and aluminosilicate synthesized by sol-gel process on the structural properties of metakaolin-based geopolymers. Appl. Clay Sci. 2025, 267, 107743. [Google Scholar] [CrossRef]
- Chen, K.; Lin, W.; Liu, Q.; Chen, B.; Tam, V.W.Y. Micro-characterizations and geopolymerization mechanism of ternary cementless composite with reactive ultra-fine fly ash, red mud and recycled powder. Constr. Build. Mater. 2022, 343, 128091. [Google Scholar] [CrossRef]
- Lyu, B.; Guo, L.; Fei, X.; Wu, J.; Bian, R. Preparation and properties of green high ductility geopolymer composites incorporating recycled fine brick aggregate. Cem. Concr. Compos. 2023, 139, 105054. [Google Scholar] [CrossRef]
- He, M.; Yang, Z.; Li, N.; Zhu, X.; Fu, B.; Ou, Z. Strength, microstructure, CO2 emission and economic analyses of low concentration phosphoric acid-activated fly ash geopolymer. Constr. Build. Mater. 2023, 374, 130920. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.J.; Li, Q.Y. Upcycling carbon dioxide to improve mechanical strength of portland cement. J. Clean. Prod. 2018. [Google Scholar] [CrossRef]
- Bahmani, H.; Mostafaei, H.; Santos, P.; Fallah Chamasemani, N. Enhancing the mechanical properties of Ultra-High-Performance concrete (UHPC) through silica sand replacement with steel slag. Buildings 2024, 14, 3520. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, Q.; Xakalashe, B.S.; Fan, X.; Gan, M.; Chen, X.; Ji, Z.; Huang, X.; Friedrich, B. Mechanical and environmental characteristics of red mud geopolymers. Constr. Build. Mater. 2022, 321, 125564. [Google Scholar] [CrossRef]
- Bayat, A.; Hassani, A.; Azami, O.A. Thermo-mechanical properties of alkali-activated slag–Red mud concrete. Road Mater. Pavement Des. 2020, 21, 411–433. [Google Scholar] [CrossRef]
- Bayat, A.; Hassani, A.; Yousefi, A.A. Effects of red mud on the properties of fresh and hardened alkali-activated slag paste and mortar. Constr. Build. Mater. 2018, 167, 775–790. [Google Scholar] [CrossRef]
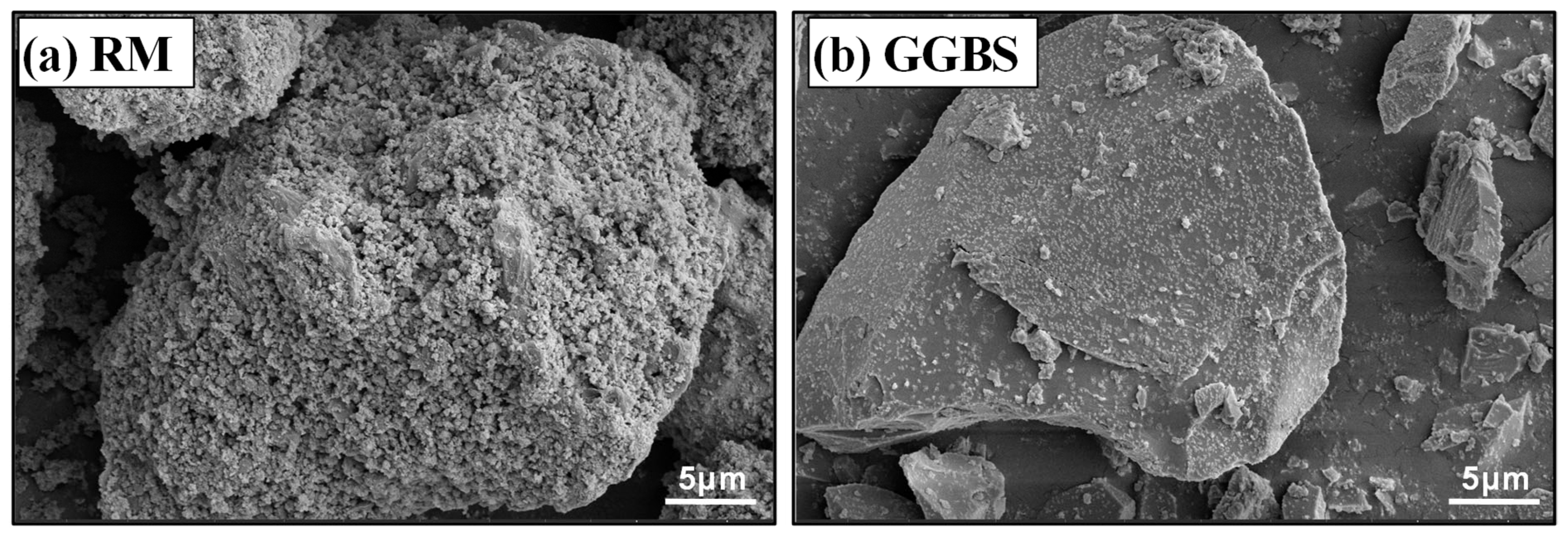
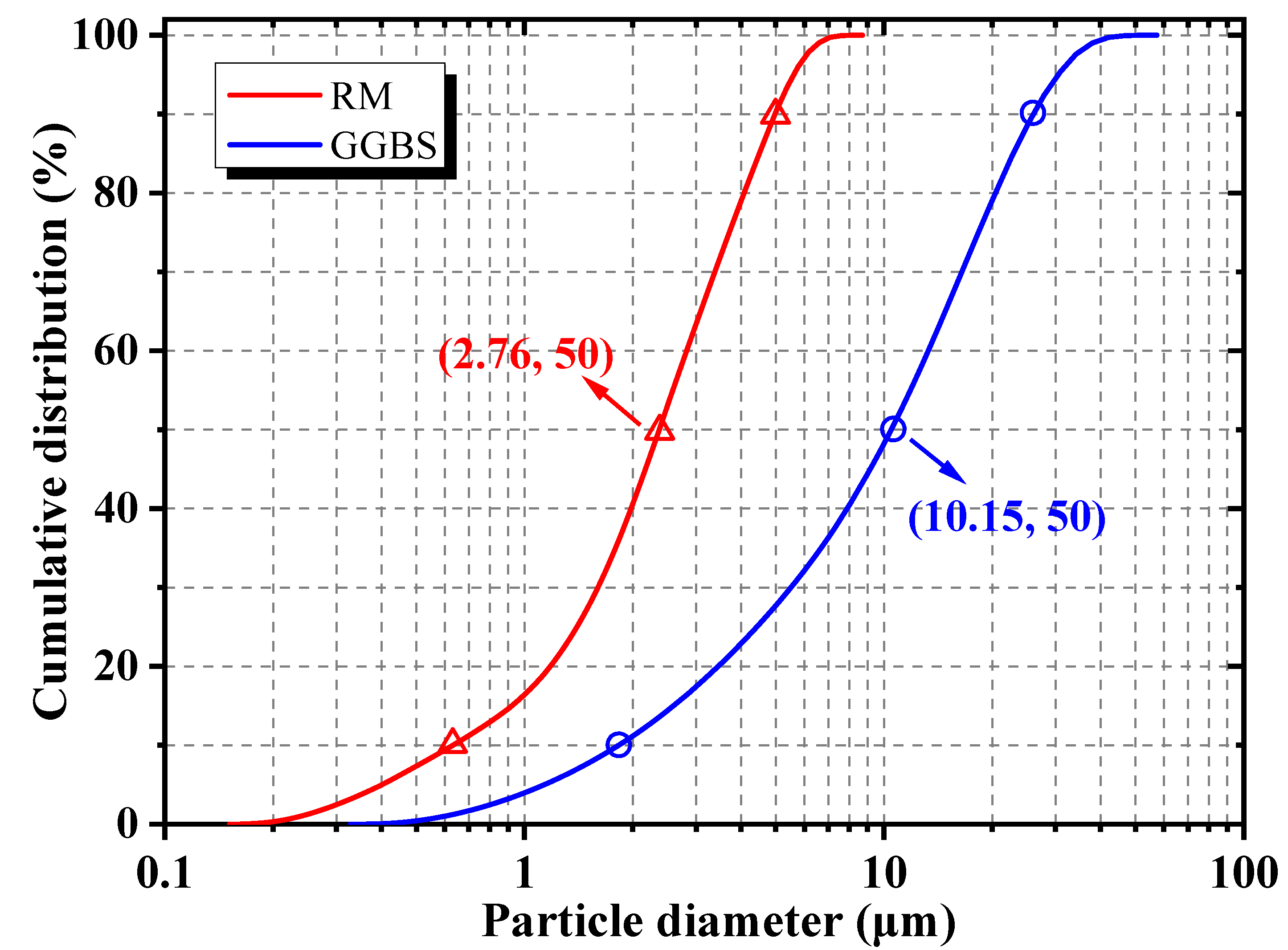

| Raw Material | Al2O3 | SiO2 | CaO | Na2O | Fe2O3 | TiO2 | Others | LOI |
|---|---|---|---|---|---|---|---|---|
| RM | 25.11 | 16.93 | 6.02 | 11.60 | 36.43 | 1.54 | 1.70 | - |
| GGBS | 13.70 | 31.10 | 40.90 | 0.38 | 0.65 | 1.26 | 2.85 | 0.96 |
| No. | Precursors | Activators a | SiO2/Al2O3 | Na2O/Al2O3 | H2O/Na2O | Water a | Remarks | ||
|---|---|---|---|---|---|---|---|---|---|
| RM | GGBS | NaOH | Na2O·nSiO2 | ||||||
| Si300 | 36 | 64 | 0.035 | 0.074 | 3.00 | 0.85 | 17.24 | 0.363 | Group 1 |
| Si275 | 43 | 57 | 0.032 | 0.069 | 2.75 | 0.85 | 17.24 | 0.390 | |
| Si256 | 50 | 50 | 0.026 | 0.072 | 2.56 | 0.85 | 17.24 | 0.406 | |
| Si225 | 56 | 44 | 0.031 | 0.048 | 2.25 | 0.85 | 17.24 | 0.457 | |
| GBS50 | 50 | 50 | 0.026 | 0.072 | 2.56 | 0.85 | 17.24 | 0.406 | Group 2 |
| GBS54 | 46 | 54 | 0.029 | 0.080 | 2.72 | 0.87 | 17.24 | 0.395 | |
| GBS58 | 42 | 58 | 0.032 | 0.088 | 2.90 | 0.89 | 17.24 | 0.385 | |
| GBS63 | 37 | 63 | 0.035 | 0.098 | 3.12 | 0.92 | 17.24 | 0.372 | |
| Material Type | CO2 Emissions | Cost a |
|---|---|---|
| (kg CO2•eq/kg) | (CNY/kg) | |
| RM [57,62] | 0.303 | 0.22 |
| GGBS [57,63] | 0.067 | 0.5 |
| NaOH [57,63] | 3.2 | 7.53 |
| Na2O·nSiO2 b [57,64] | 0.4 | 4.67 |
| Water c [65] | 0 | 0.0083 |
| Type | Mixtures in References | Mixtures in This Study | |||||
|---|---|---|---|---|---|---|---|
| [67] | [68] | [69] | Si225 | Si256 | Si275 | Si300 | |
| RM (kg/m3) | 714.05 | 519.56 | 471.05 | 613.00 | 551.40 | 484.83 | 413.31 |
| GGBS (kg/m3) | 714.05 | 779.35 | 706.57 | 481.64 | 551.40 | 642.68 | 734.78 |
| NaOH (kg/m3) | 95.25 | 58.45 | 52.99 | 33.93 | 28.67 | 36.08 | 40.18 |
| Na2O·nSiO2 (kg/m3) | 48.41 | 55.20 | 101.28 | 52.54 | 79.40 | 77.80 | 84.96 |
| Water (kg/m3) | 428.43 | 487.09 | 500.49 | 569.21 | 551.40 | 541.21 | 528.12 |
| CO2 emissions (kg/m3) | 588.38 | 418.77 | 400.16 | 347.62 | 327.53 | 336.54 | 337.03 |
| Cost (CNY/kg) | 1461.03 | 1205.96 | 1333.07 | 881.30 | 988.30 | 1019.21 | 1162.04 |
| Compressive strength (28 MPa) | 36 | 34 | 37 | 30.1 | 32.8 | 34.5 | 37.5 |
| CO2 intensity (kg/m3/MPa) | 16.34 | 12.32 | 10.82 | 11.55 | 9.99 | 9.75 | 8.99 |
| Cost intensity (CNY/m3/MPa) | 40.58 | 35.47 | 36.03 | 29.28 | 30.13 | 29.54 | 30.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Dong, H.; Wang, Y.; Men, J.; Wang, J.; Zhao, X.; Zou, S. Valorization of Alkali–Thermal Activated Red Mud for High-Performance Geopolymer: Performance Evaluation and Environmental Effects. Buildings 2025, 15, 2471. https://doi.org/10.3390/buildings15142471
Li Z, Dong H, Wang Y, Men J, Wang J, Zhao X, Zou S. Valorization of Alkali–Thermal Activated Red Mud for High-Performance Geopolymer: Performance Evaluation and Environmental Effects. Buildings. 2025; 15(14):2471. https://doi.org/10.3390/buildings15142471
Chicago/Turabian StyleLi, Zhiping, Haifeng Dong, Yuwen Wang, Jianbing Men, Junqiang Wang, Xiushao Zhao, and Sikai Zou. 2025. "Valorization of Alkali–Thermal Activated Red Mud for High-Performance Geopolymer: Performance Evaluation and Environmental Effects" Buildings 15, no. 14: 2471. https://doi.org/10.3390/buildings15142471
APA StyleLi, Z., Dong, H., Wang, Y., Men, J., Wang, J., Zhao, X., & Zou, S. (2025). Valorization of Alkali–Thermal Activated Red Mud for High-Performance Geopolymer: Performance Evaluation and Environmental Effects. Buildings, 15(14), 2471. https://doi.org/10.3390/buildings15142471









