Laboratory Investigation and Mechanical Evaluation on Xanthan Gum-Reinforced Clay: Unconfined Compression Test, Triaxial Shear Test, and Microstructure Characterization
Abstract
1. Introduction
2. Experiment Method
2.1. Test Material
2.2. Test Scheme
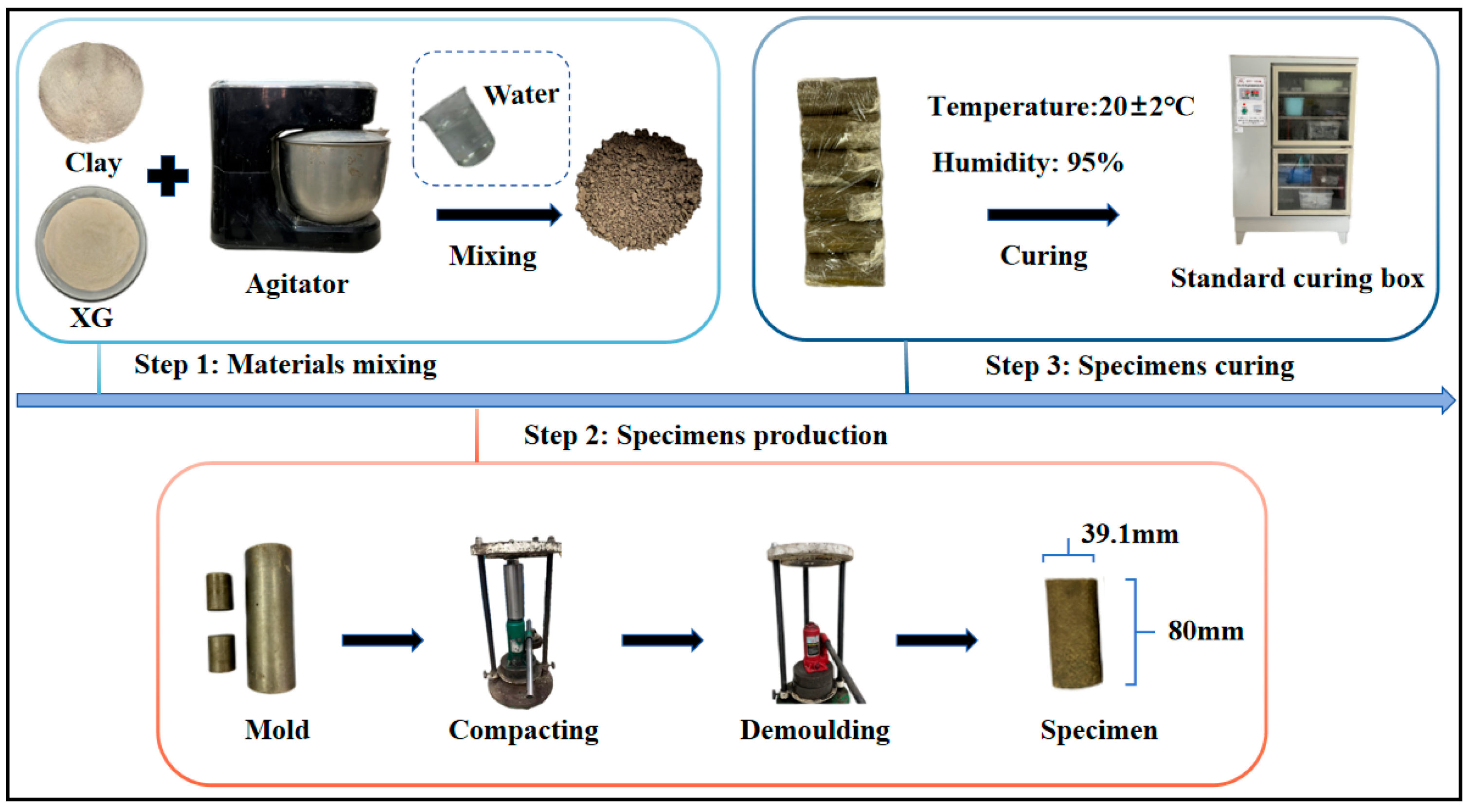
2.3. Sample Preparation
3. Test Results and Analysis
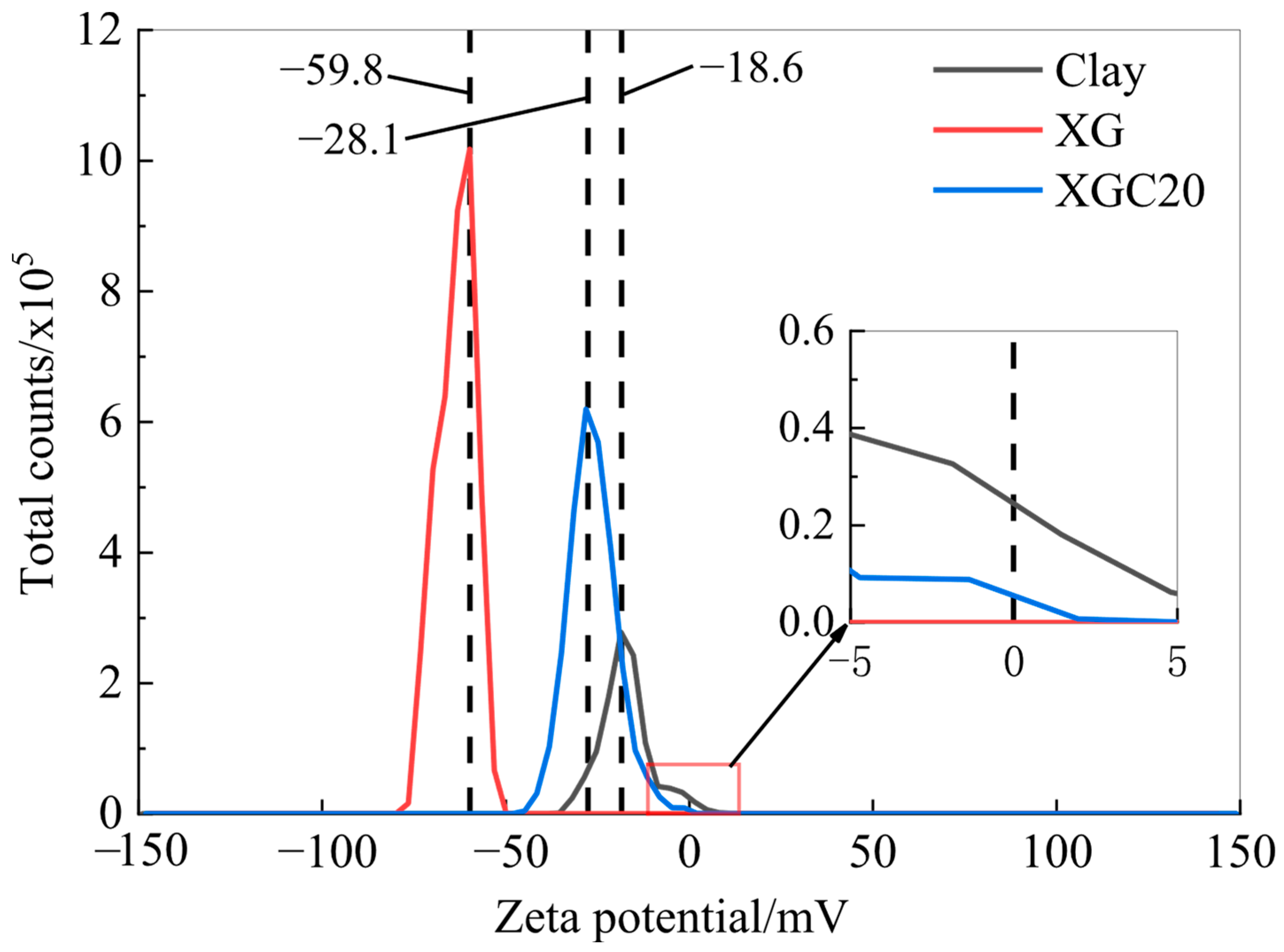
3.1. Zeta Potential Test Results and Analysis
3.2. Infrared Spectroscopy Test Results and Analysis
3.3. pH Test Results and Analysis
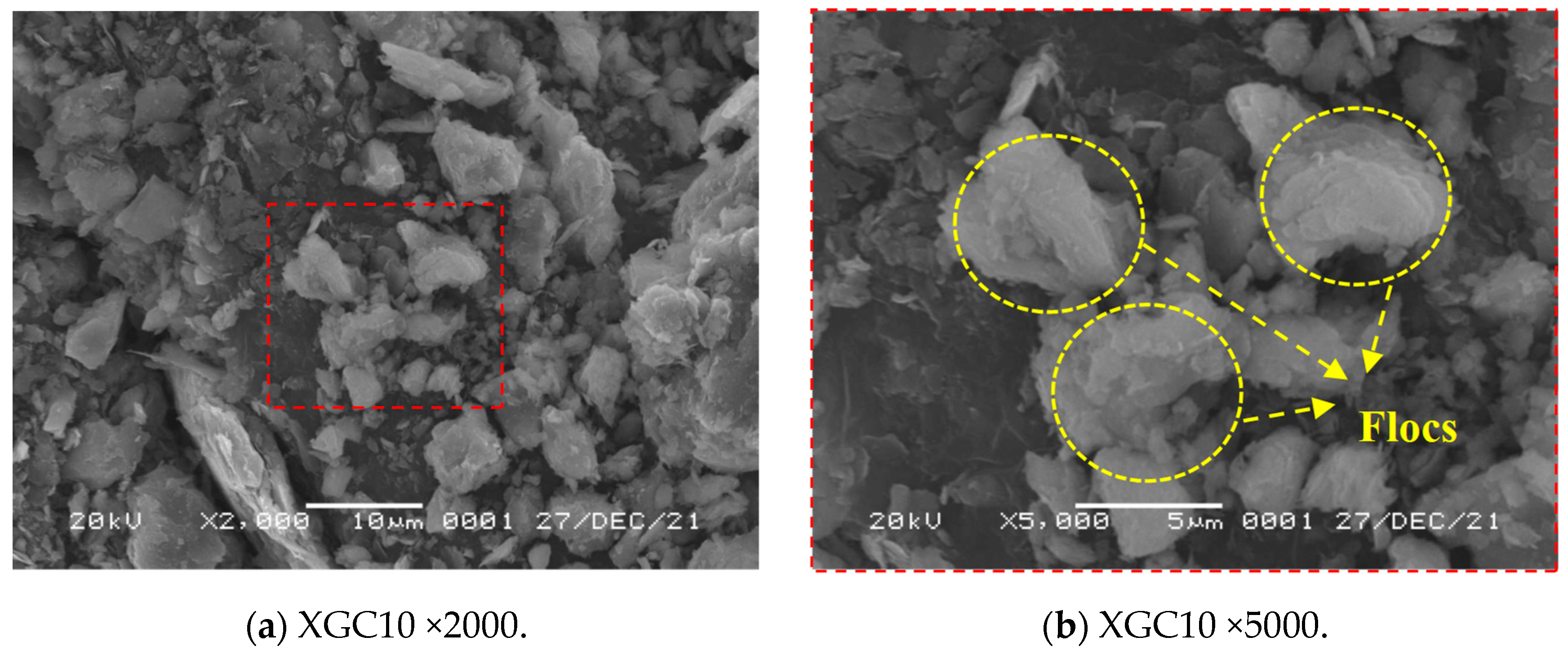
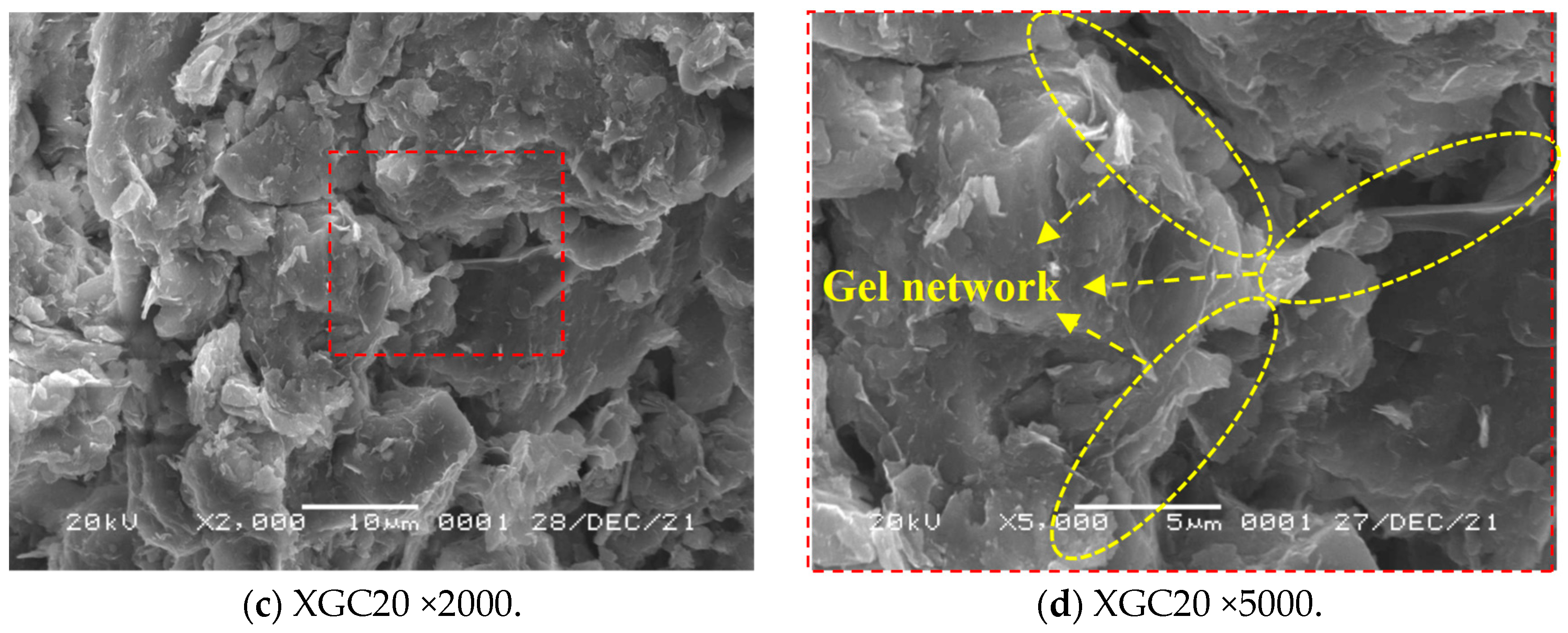
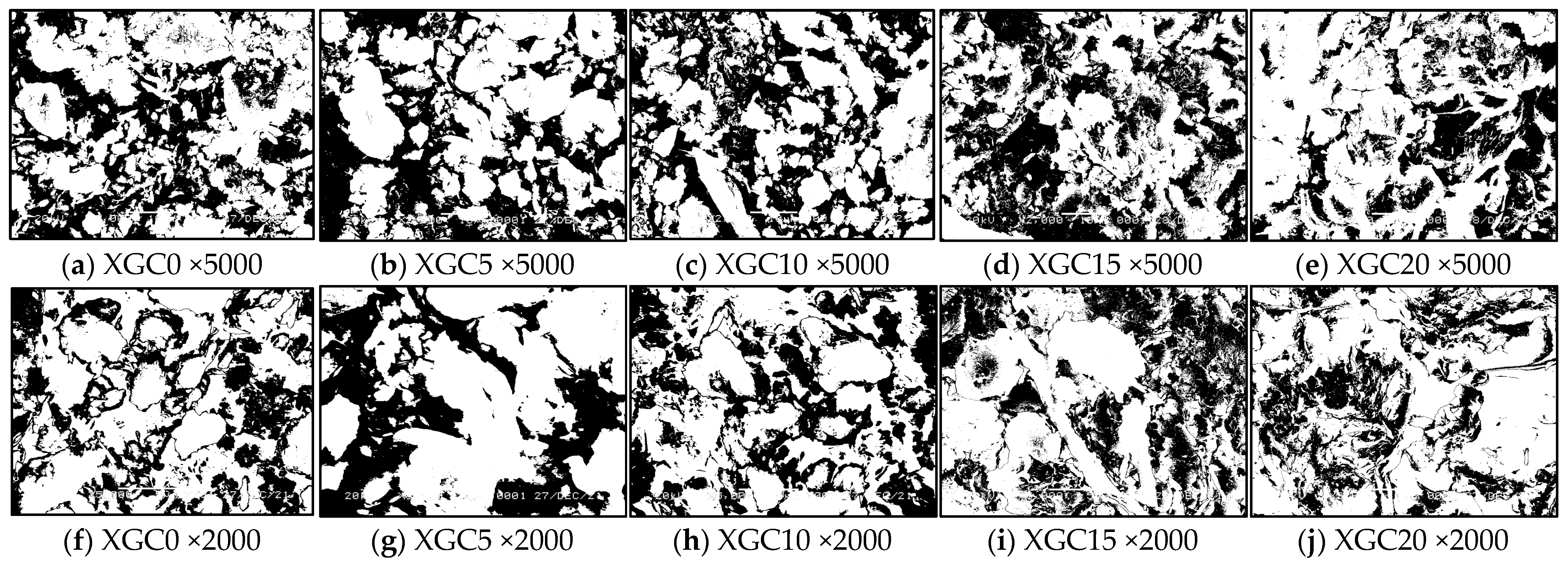
3.4. SEM Test Results and Analysis
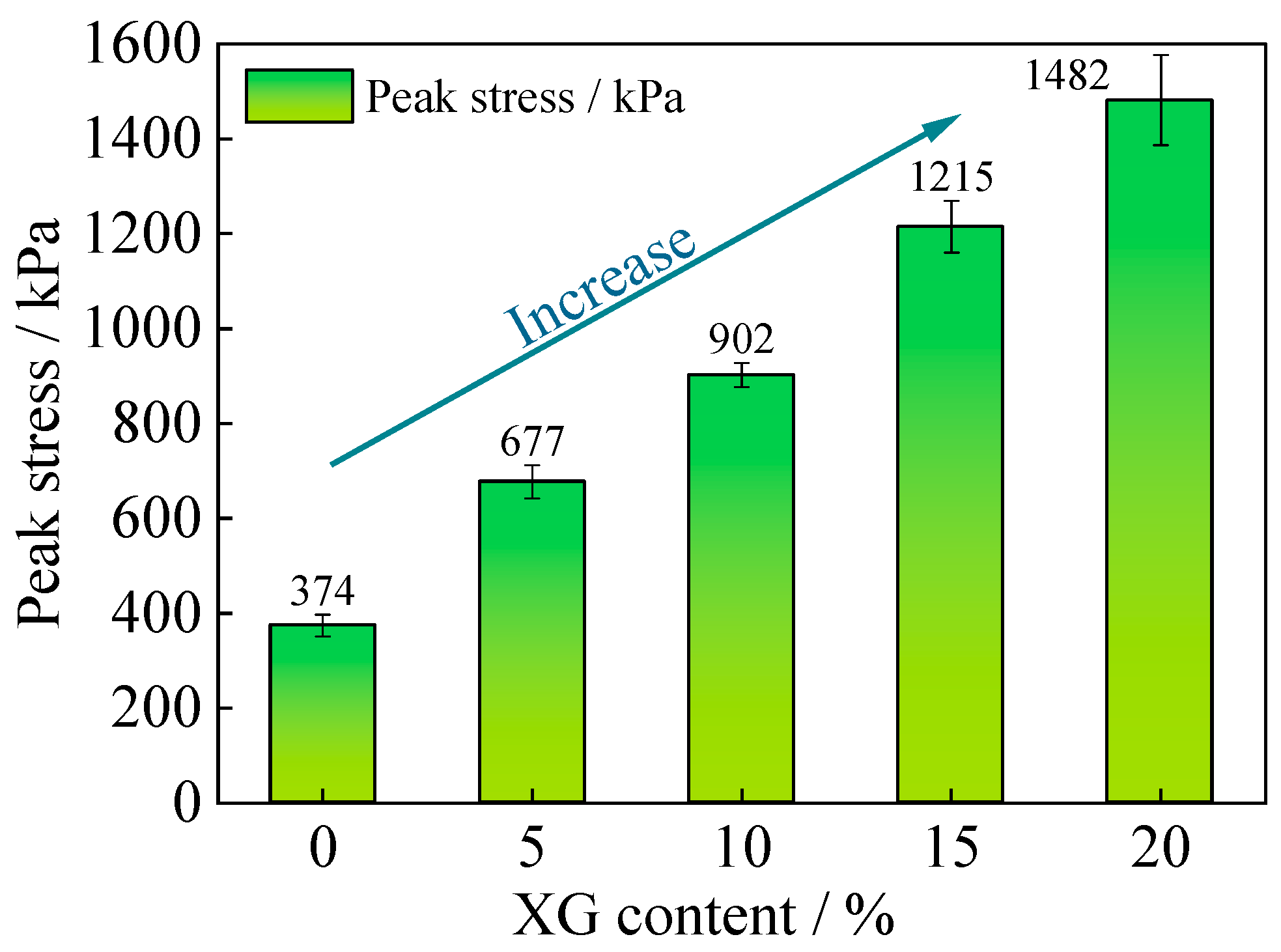
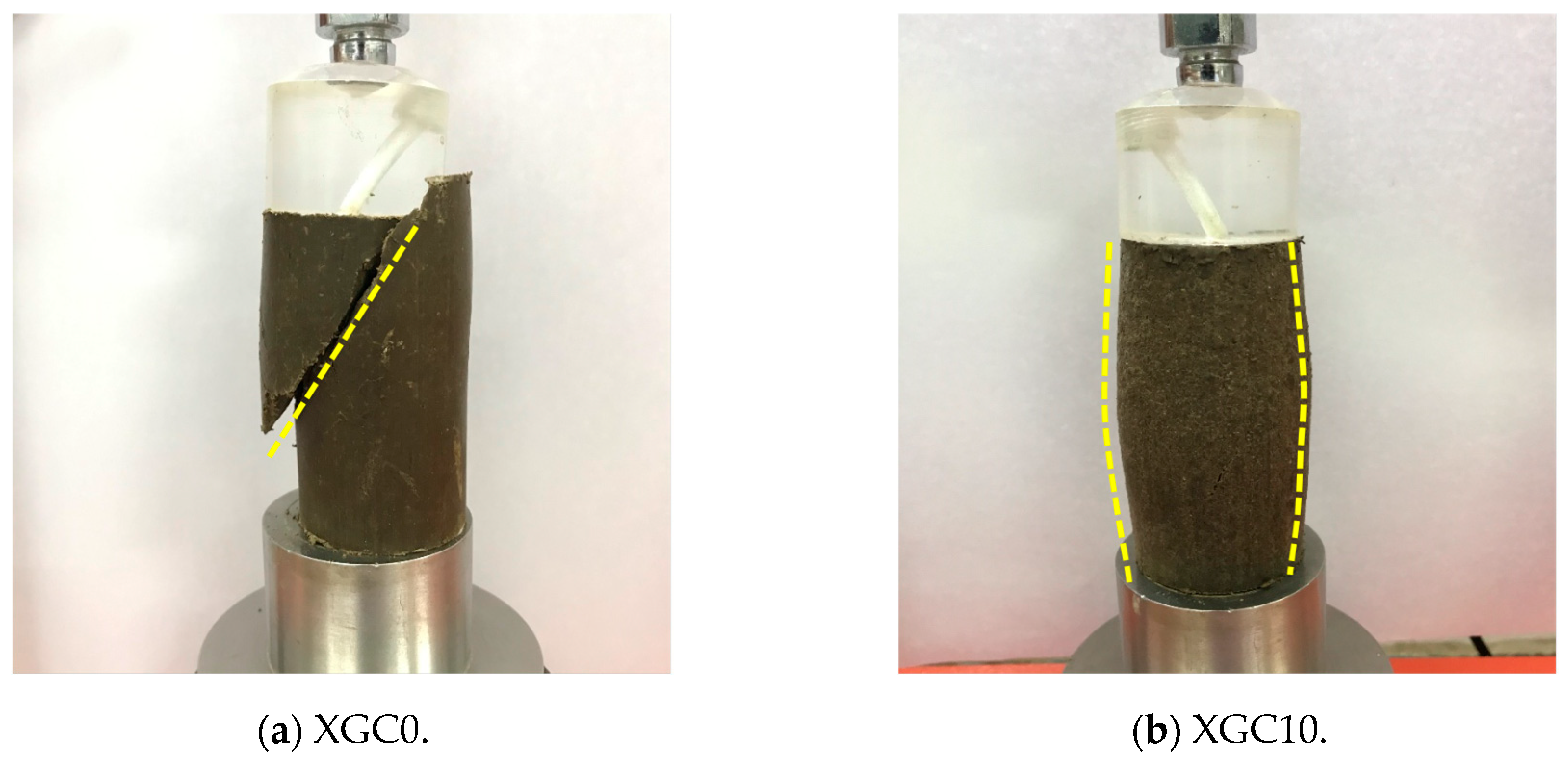
3.5. Results and Analysis of Unconfined Compression Test
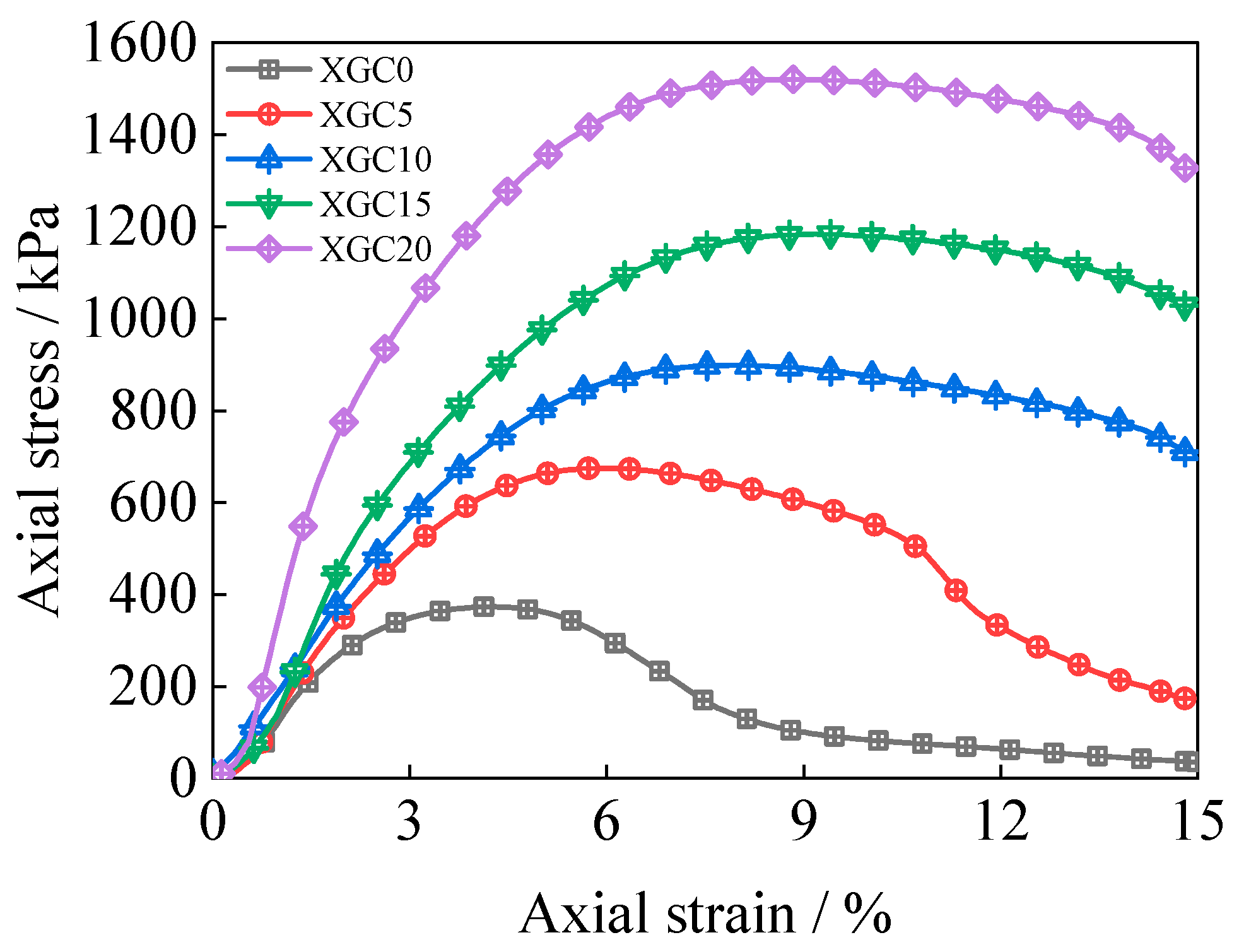
3.6. Triaxial Shear Test Results and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Wu, E.; Huang, S.; Song, X.; Li, N.; Jiang, P. Experimental Investigation on Static and Dynamic Properties of Nanosilica Modified Cement Soil. Constr. Build. Mater. 2024, 412, 134746. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, T.; Xiao, J.; Xie, Q.; Zong, Z.; Yu, Z. Mechanical Properties and Field Test of Lime-Soda Residue Stabilized Soil for Subgrade. Constr. Build. Mater. 2024, 419, 135489. [Google Scholar] [CrossRef]
- Kim, D.; Park, K. Evaluation of the Grouting in the Sandy Ground Using Bio Injection Material. Geomech. Eng. 2017, 12, 739–752. [Google Scholar] [CrossRef]
- Proaño, L.; Sarmiento, A.T.; Figueredo, M.; Cobo, M. Techno-Economic Evaluation of Indirect Carbonation for Co2 Emissions Capture in Cement Industry: A System Dynamics Approach. J. Clean Prod. 2020, 263, 121457. [Google Scholar] [CrossRef]
- Semieniuk, G.; Yakovenko, V.M. Historical Evolution of Global Inequality in Carbon Emissions and Footprints Versus Redistributive Scenarios. J. Clean Prod. 2020, 264, 121420. [Google Scholar] [CrossRef]
- Loh, P.Y.; Shafigh, P.; Ibrahim, Z. The Ph Measurement of Cement-Based Materials: Effect of Leaching Time, Leaching Technique and Water-to-Solid Ratio. Constr. Build. Mater. 2024, 411, 134525. [Google Scholar] [CrossRef]
- González, A.; Jameson, G.; Carteret, R.; Yeo, R. Laboratory Fatigue Life of Cemented Materials in Australia. Road Mater. Pavement Des. 2013, 14, 518–536. [Google Scholar] [CrossRef]
- Fedrigo, W.; Heller, L.F.; Brito, L.A.T.; Núñez, W.P. Fatigue of Cold Recycled Cement-Treated Pavement Layers: Experimental and Modeling Study. Sustainability 2023, 15, 7816. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Kodikara, J. Shrinkage Behaviour of Crushed Basaltic Rock and Residual Clay Mixture Stabilized with Cementitious Binders. Int. J. Pavement Eng. 2005, 6, 27–37. [Google Scholar] [CrossRef]
- Mendonça, A.; Morais, P.V.; Pires, A.C.; Chung, A.P.; Oliveira, P.V. A Review on the Importance of Microbial Biopolymers Such as Xanthan Gum to Improve Soil Properties. Appl. Sci. 2020, 11, 170. [Google Scholar] [CrossRef]
- Li, J.; Wen, K.; Zhang, B. Investigation of Mechanical Behavior of Sustainable Construction Materials: Recycled Glass Sand Stabilized with Natural Binder Material—Biopolymers. Constr. Build. Mater. 2024, 418, 135359. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, J.; Gu, J.; Liu, S.; Huang, Y.; Sadeghi, H. Influence of Biopolymer-Vegetation Interaction on Soil Hydro-Mechanical Properties under Climate Change: A Review. Sci. Total Environ. 2024, 954, 176535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Sujatha, E.R. An Appraisal of the Hydro-Mechanical Behaviour of Polysaccharides, Xanthan Gum, Guar Gum and Β-Glucan Amended Soil. Carbohydr. Polym. 2021, 265, 118083. [Google Scholar] [CrossRef]
- Jang, J. A Review of the Application of Biopolymers on Geotechnical Engineering and the Strengthening Mechanisms between Typical Biopolymers and Soils. Adv. Mater. Sci. Eng. 2020, 2020, 1465709. [Google Scholar] [CrossRef]
- Fatehi, H.; Ong, D.E.; Yu, J.; Chang, I. Sustainable Soil Treatment: Investigating the Efficacy of Carrageenan Biopolymer on the Geotechnical Properties of Soil. Constr. Build. Mater. 2024, 411, 134627. [Google Scholar] [CrossRef]
- Park, D.Y.; Chang, I.; Lee, M.; Cho, G.C. Enhancing Soil Liquefaction Resistance and Small-Strain Dynamic Properties Using Cation-Crosslinked Biopolymer Hydrogel. Soil Dyn. Earthq. Eng. 2025, 190, 109212. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.C. Effects of Xanthan Gum Biopolymer on Soil Strengthening. Constr. Build. Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Sujatha, E.R.; Atchaya, S.; Sivasaran, A.; Keerdthe, R. Enhancing the Geotechnical Properties of Soil Using Xanthan Gum—An Eco-Friendly Alternative to Traditional Stabilizers. Bull. Eng. Geol. Environ. 2021, 80, 1157–1167. [Google Scholar] [CrossRef]
- Li, P.; Li, T.; Zeng, Y.; Li, X.; Jiang, X.; Wang, Y.; Xie, T.; Zhang, Y. Biosynthesis of Xanthan Gum by Xanthomonas Campestris Lrelp-1 Using Kitchen Waste as the Sole Substrate. Carbohydr. Polym. 2016, 151, 684–691. [Google Scholar] [CrossRef]
- Jensen, P.F.; Kadziola, A.; Comamala, G.; Segura, D.R.; Anderson, L.; Poulsen, J.C.N.; Rasmussen, K.K.; Agarwal, S.; Sainathan, R.K.; Monrad, R.N. Structure and Dynamics of a Promiscuous Xanthan Lyase from Paenibacillus Nanensis and the Design of Variants with Increased Stability and Activity. Cell Chem. Biol. 2019, 26, 191–202. [Google Scholar] [CrossRef]
- Han, G.; Chen, Q.; Liu, F.; Cui, Z.; Shao, H.; Liu, F.; Ma, A.; Liao, J.; Guo, B.; Guo, Y. Low Molecular Weight Xanthan Gum for Treating Osteoarthritis. Carbohydr. Polym. 2017, 164, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, Z.; Jin, W.; Li, P.; Li, Y.; Liang, H.; Li, Y.; Li, B. Structural and Rheological Properties of Xanthan Gum/Lysozyme System Induced by in Situ Acidification. Food Res. Int. 2016, 90, 85–90. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Jiang, B.; Chen, J. A Review of the Enzymatic, Physical, and Chemical Modification Techniques of Xanthan Gum. Int. J. Biol. Macromol. 2021, 186, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Elella, M.H.A.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.; Yoon, K.R. Xanthan Gum-Derived Materials for Applications in Environment and Eco-Friendly Materials: A Review. J. Environ. Chem. Eng. 2021, 9, 104702. [Google Scholar] [CrossRef]
- Wu, M.; Qu, J.; Shen, Y.; Dai, X.; Wei, W.; Shi, Z.; Li, G.; Ma, T. Gel Properties of Xanthan Containing a Single Repeating Unit with Saturated Pyruvate Produced by an Engineered Xanthomonas Campestris Cgmcc 15155. Food Hydrocoll. 2019, 87, 747–757. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M. Study on Shear Strength of Xanthan Gum-Amended Soil. Sustainability 2019, 11, 6142. [Google Scholar] [CrossRef]
- Ni, J.; Hao, G.L.; Chen, J.Q.; Ma, L.; Geng, X.Y. The Optimisation Analysis of Sand-Clay Mixtures Stabilised with Xanthan Gum Biopolymers. Sustainability 2021, 13, 3732. [Google Scholar] [CrossRef]
- Lee, S.; Chang, I.; Chung, M.K.; Kim, Y.; Kee, J. Geotechnical Shear Behavior of Xanthan Gum Biopolymer Treated Sand from Direct Shear Testing. Geomech. Eng. 2017, 12, 831–847. [Google Scholar] [CrossRef]
- Dehghan, H.; Tabarsa, A.; Latifi, N.; Bagheri, Y. Use of Xanthan and Guar Gums in Soil Strengthening. Clean Technol. Environ. Policy 2019, 21, 155–165. [Google Scholar] [CrossRef]
- Chen, C.; Wu, L.; Perdjon, M.; Huang, X.; Peng, Y. The Drying Effect on Xanthan Gum Biopolymer Treated Sandy Soil Shear Strength. Constr. Build. Mater. 2019, 197, 271–279. [Google Scholar] [CrossRef]
- Bonal, N.S.; Prasad, A.; Verma, A.K. Effect of Microbial Biopolymers on Mechanical Properties of Bauxite Residue. KSCE J. Civ. Eng. 2021, 25, 2437–2450. [Google Scholar] [CrossRef]
- GB/T 50145-2007; Standard for Engineering Classification of Soil. China Planning Publishing House: Beijing, China, 2008.
- Sulaiman, H.; Taha, M.R.; Abd Rahman, N.; Taib, A.M. Performance of Soil Stabilized with Biopolymer Materials–Xanthan Gum and Guar Gum. Phys. Chem. Earth 2022, 128, 103276. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, M.; Jiang, T.; Kato, S.; Sun, D.A.; Gao, Y.; Yang, Z. Dynamic Deformation Characteristics and Microscopic Analysis of Xanthan Gum-Treated Silty Soil During Wetting Process. J. Rock Mech. Geotech. Eng. 2025, 17, 1149–1162. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; González López, Ó. Water-Soluble Polymers in Agriculture: Xanthan Gum as Eco-Friendly Alternative to Synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- GB/T50123-2019; Standard for Geotechnical Testing Methods. Ministry of Water Resources and Hydropower Planning and Design Institute: Beijing, China, 2019.
- Maierdan, Y.; Zhao, D.; Chokshi, P.H.; Garmonina, M.; Kawashima, S. Rheology, 3d Printing, and Particle Interactions of Xanthan Gum-Clay Binder for Earth Concrete. Cem. Concr. Res. 2024, 182, 107551. [Google Scholar] [CrossRef]
- Fatehi, H.; Ong, D.E.; Yu, J.; Chang, I. Biopolymers as Green Binders for Soil Improvement in Geotechnical Applications: A Review. Geosciences 2021, 11, 291. [Google Scholar] [CrossRef]
- Duong, L.H.; Nguyen, M.N. Colloidal Interaction of Fly Ash and Soil Clay. Colloid Surf. A-Physicochem. Eng. Asp. 2024, 692, 133944. [Google Scholar] [CrossRef]
- Ko, M.S.; Jeon, Y.J.; Kim, K.W. Novel Application of Xanthan Gum-Based Biopolymer for Heavy Metal Immobilization in Soil. J. Environ. Chem. Eng. 2022, 10, 108240. [Google Scholar] [CrossRef]
- Chatterji, J.; Borchardt, J. Applications of Water-Soluble Polymers in the Oil Field. J. Pet. Technol. 1981, 33, 2042–2056. [Google Scholar] [CrossRef]
- Tinti, A.; Tugnoli, V.; Bonora, S.; Francioso, O. Recent Applications of Vibrational Mid-Infrared (Ir) Spectroscopy for Studying Soil Components: A Review. J. Cent. Eur. Agric. 2015, 16, 141299. [Google Scholar] [CrossRef]
- Anandha Kumar, S.; Sujatha, E. Assessing the Potential of Xanthan Gum to Modify in-Situ Soil as Baseliners for Landfills. Int. J. Environ. Sci. Technol. 2022, 19, 10613–10624. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wan, J.L.; Jin, F.; Reddy, K.R.; Jiang, N.J.; Du, Y.J. Gas Permeability and Microscopic Mechanisms of Xanthan Gum-Amended Compacted Lean Clay as a Gas Barrier Material. Eng. Geol. 2025, 353, 108095. [Google Scholar] [CrossRef]
- Bozyigit, I.; Javadi, A.; Altun, S. Strength Properties of Xanthan Gum and Guar Gum Treated Kaolin at Different Water Contents. J. Rock Mech. Geotech. Eng. 2021, 13, 1160–1172. [Google Scholar] [CrossRef]
- Baldovino, J.d.J.A.; de la Rosa, Y.E.N.; Calabokis, O.P.; Vergara, J.A.A.; López, L.C.S. Geotechnical Behavior of Xanthan Gum-Stabilized Clay Reinforced with Polypropylene Fibers. Polymers 2025, 17, 363. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weng, Z.; Liu, Q.; Wang, T.; Pan, X.; Li, G.; Wang, Z. Improving the Mechanical Properties of Red Clay Using Xanthan Gum Biopolymer. Int. J. Polym. Sci. 2021, 2021, 1535772. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Li, N.; Ma, L. Mechanical Properties and Micro Mechanism of Nano-Sio2 Modified Coastal Cement Soil at Short Age. Acta Mater. Compos. Sin 2022, 39, 1701–1714. [Google Scholar] [CrossRef]
- Bağrıaçık, B.; Mahmutluoğlu, B. Model Experiments on Coarse-Grained Soils Treated with Xanthan Gum Biopolymer. Arab. J. Geosci. 2021, 14, 1621. [Google Scholar] [CrossRef]
- Wang, W.; Wu, H.; Feng, T.; Jiang, P.; Sun, M.; Yang, S.; Liu, Y.; Li, N.; Song, Y. Study on the Small Strain Dynamic Characteristics and Microscopic Mechanism of Nano-Silica Modified Lime Soil. J. Mater. Res. Technol. 2025, 37, 851–869. [Google Scholar] [CrossRef]
- Benmounah, A.; Benyounes, K.K.; Chelah, K.; Djamal, E.D. Effect of Xanthan Gum and Sodium Carboxymetylcellulose on the Rheological Properties and Zeta Potential of Bentonite Suspensions. In Proceedings of the CFM 2017-23ème Congrès Français de Mécanique, Lille, France, 28 August–1 September 2017; AFM, Maison de la Mécanique: Courbevoie, France, 2017. [Google Scholar]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan Gum in Aqueous Solutions: Fundamentals and Applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M.; Auad, M.L. Biopolymers as a Sustainable Solution for the Enhancement of Soil Mechanical Properties. Sci Rep. 2020, 10, 267. [Google Scholar] [CrossRef]
- Bagheri, P.; Gratchev, I.; Rybachuk, M. Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties. Appl. Sci. 2023, 13, 887. [Google Scholar] [CrossRef]
- Rashid, A.S.A.; Tabatabaei, S.; Horpibulsuk, S.; Mohd Yunus, N.Z.; Hassan, W.H.W. Shear Strength Improvement of Lateritic Soil Stabilized by Biopolymer Based Stabilizer. Geotech. Geol. Eng. 2019, 37, 5533–5541. [Google Scholar] [CrossRef]
- Cabalar, A.F.; Awraheem, M.H.; Khalaf, M.M. Geotechnical Properties of a Low-Plasticity Clay with Biopolymer. J. Mater. Civ. Eng. 2018, 30, 04018170. [Google Scholar] [CrossRef]
- JTG/T F20-2015; Technical Guidelines for Construction of Highway Roadbases. Ministry of Transport of the People’s Republic of China: Beijing, China, 2015.
- JTG D30-2015; Specifications for Design of Highway Subgrades. Ministry of Transport of the People’s Republic of China: Beijing, China, 2015.
- Zhang, J.; Meng, Z.; Jiang, T.; Wang, S.; Zhao, J.; Zhao, X. Experimental Study on the Shear Strength of Silt Treated by Xanthan Gum During the Wetting Process. Appl. Sci. 2022, 12, 6053. [Google Scholar] [CrossRef]
- Ren, F.; Ding, H.; Dong, B.; Qian, X.; Liu, J.; Tan, J. Study on the Improvement of Soil Properties Using Hydrophilic-Hydrophobic Biopolymer Crosslinking. Constr. Build. Mater. 2024, 415, 135101. [Google Scholar] [CrossRef]
- Bonal, N.; Prasad, A.; Verma, A. Use of Biopolymers to Enhance the Geotechnical Properties of Coal Mine Overburden Waste. Géotech. Lett. 2020, 10, 179–185. [Google Scholar] [CrossRef]




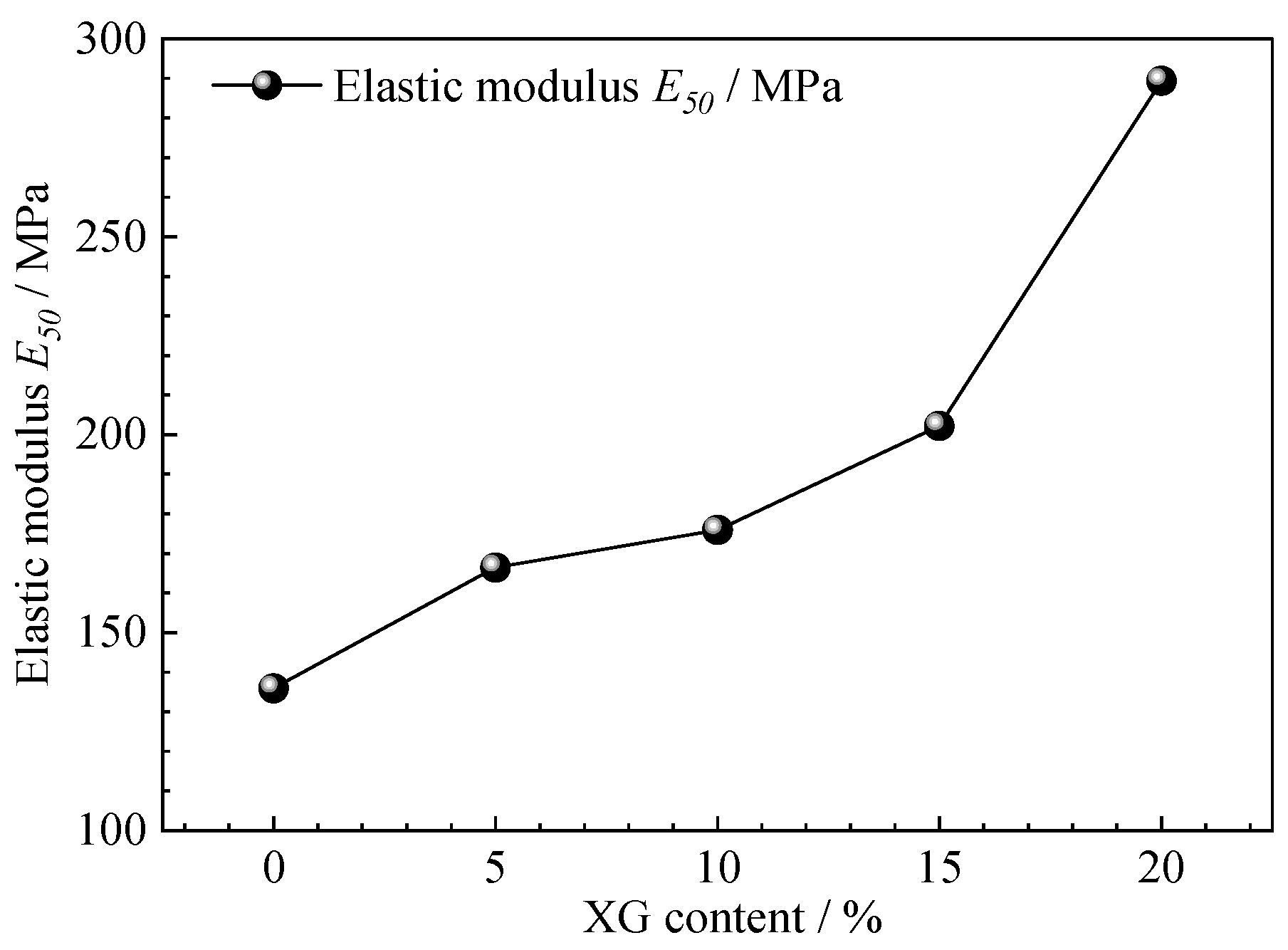
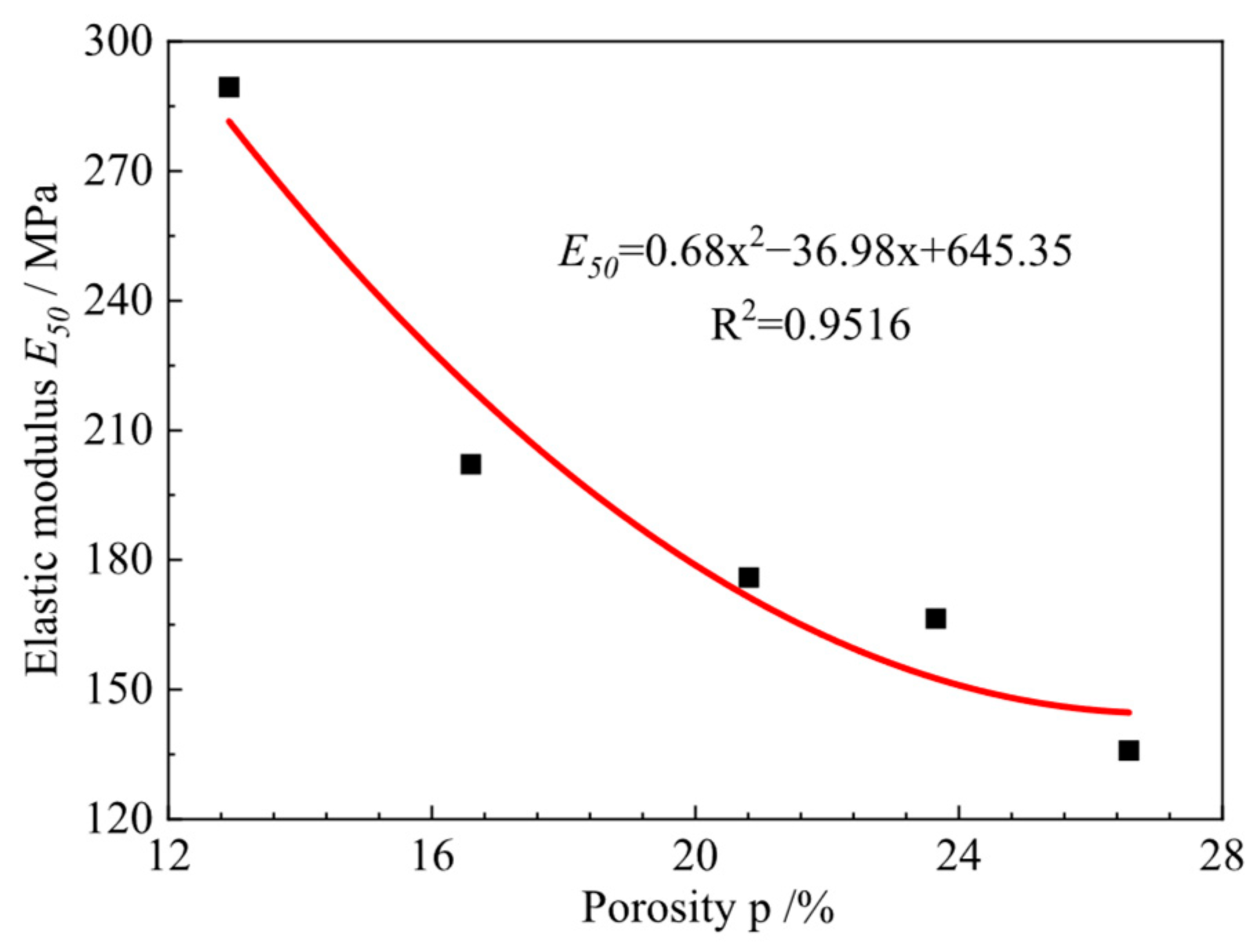



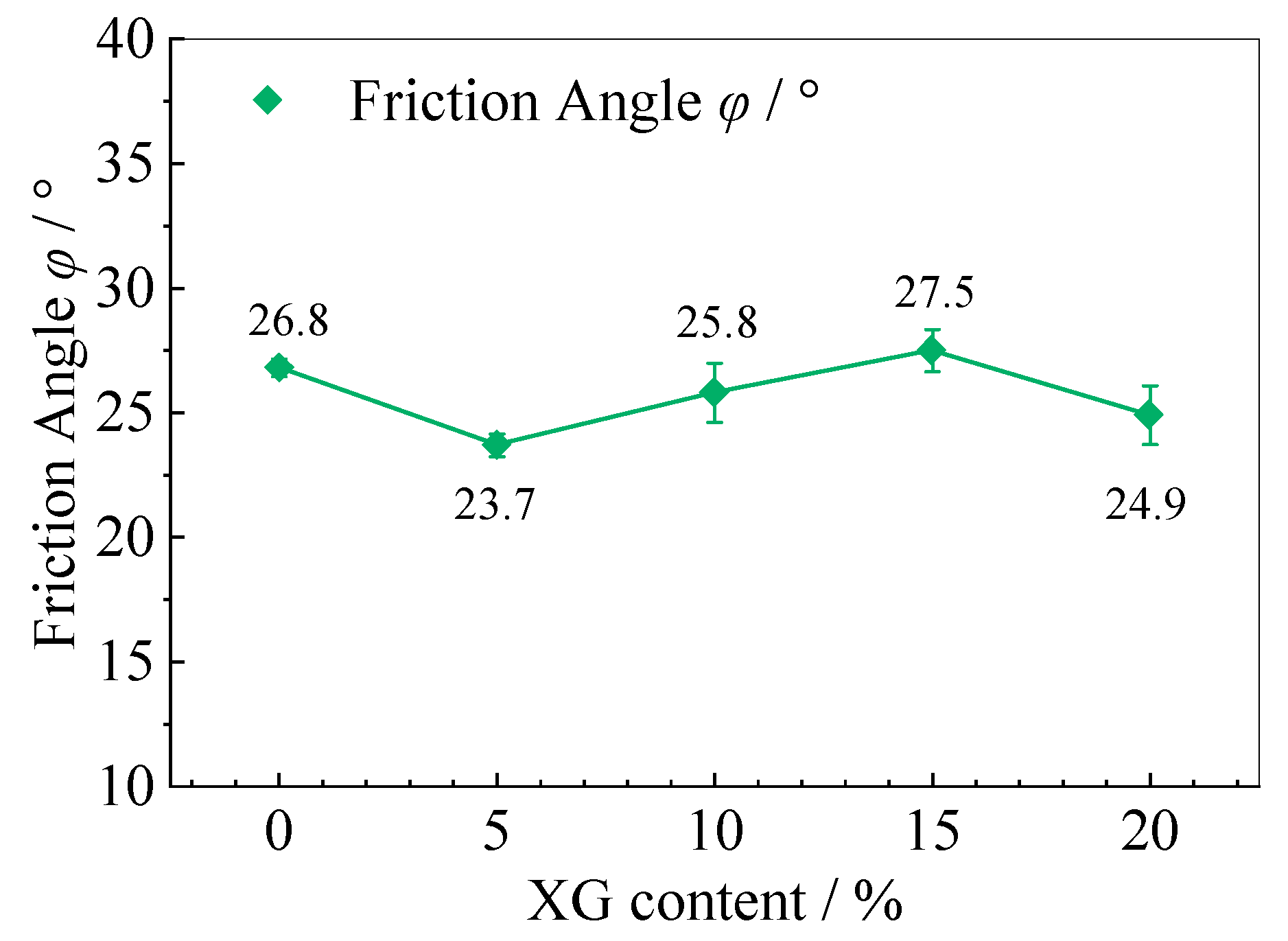
| Soil Category | Plastic Limit (%) | Liquid Limit (%) | Plasticity Index | Maximum Dry Density (g·cm−3) | Optimal Moisture Content (%) | Internal Friction Angle φ (°) | Cohesion C (kPa) |
|---|---|---|---|---|---|---|---|
| Low liquid limit clay | 15.15 | 39.52 | 24.37 | 1.85 | 16.00 | 27.02 | 91.49 |
| Sample Number | Moisture Content (%) | XG Content (%) | Test Method | |||||
|---|---|---|---|---|---|---|---|---|
| Zeta Potential Test | Infrared Spectroscopy Test | Scanning Electron Microscopy (SEM) Test | pH Test | Unconfined Compressive Strength (UCS) Test | Triaxial Shear Test | |||
| XGC0 | 16 | 0 | √ | √ | √ | √ | √ | √ |
| XGC5 | 16 | 5 | √ | √ | ||||
| XGC10 | 16 | 10 | √ | √ | √ | |||
| XGC15 | 16 | 15 | √ | √ | ||||
| XGC20 | 16 | 20 | √ | √ | √ | √ | √ | √ |
| Sample Name | XGC0 | XGC5 | XGC10 | XGC15 | XGC20 |
|---|---|---|---|---|---|
| pH | 7.95 | 7.92 | 7.96 | 7.94 | 7.96 |
| Sample Name | XGC0 | XGC5 | XGC10 | XGC15 | XGC20 | |
|---|---|---|---|---|---|---|
| Porosity | 5000 times | 26.80 | 23.80 | 20.90 | 16.67 | 13.14 |
| 2000 times | 26.05 | 23.27 | 20.60 | 16.40 | 12.38 | |
| Average porosity | 26.58 | 23.65 | 20.81 | 16.59 | 12.92 | |
| Soil Type | Silty Sand | Silty Soil | Red Soil | Kaolin | Clay | Clay |
|---|---|---|---|---|---|---|
| XG content (%) | 0, 1, 2, 4 | 0, 0.5, 1, 2 | 0, 0.5, 1, 1.5 | 0, 0.5, 1, 1.5, 2 | 0, 0.5, 1, 1.5, 2, 3 | 0, 5, 10, 15, 20 |
| Moisture content (%) | 16.5 | 21.7 | 35 | 25 | 21 | 16 |
| Control group strength (kPa) | 1197 | 1200 | 150 | 76 | 421 | 374 |
| Optimal content (%) | 2 | 2 | 1.5 | 2 | 1.5 | 20 |
| Maximum improvement range (%) | 400 | 133 | 113 | 276 | 31 | 296 |
| Curing time (d) | 5 | 7 | 7 | 7 | 7 | 7 |
| References | [53] | [54] | [55] | [45] | [56] | this test |
| Sample Value | 26.8 | 23.7 | 25.8 | 27.5 | 24.9 |
|---|---|---|---|---|---|
| Median | 25.8 | ||||
| Average | 25.7 | ||||
| Variance | 2.28 | ||||
| Standard deviation | 1.51 | ||||
| Soil Type | Loess | Sand | Bauxite Slag | Clay |
|---|---|---|---|---|
| XG content (%) | 0, 0.5, 1, 2 | 0, 0.5, 1, 2 | 0, 0.25, 0.5, 1, 1.5 | 0, 5, 10, 15, 20 |
| Peak strength (kPa) | 350–550 | 600–1050 | 1200–1600 | 1127–3071 |
| Cohesion (kPa) | 100–200 | 140–220 | 221–275 | 263–864 |
| Friction angle (°) | 13.9–15 | 35–40 | 33.8–39.3 | 23.2–28.3 |
| References | [29] | [61] | [31] | this test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, L.; Xu, M.; Luo, J.; Wang, W. Laboratory Investigation and Mechanical Evaluation on Xanthan Gum-Reinforced Clay: Unconfined Compression Test, Triaxial Shear Test, and Microstructure Characterization. Buildings 2025, 15, 2438. https://doi.org/10.3390/buildings15142438
Ying L, Xu M, Luo J, Wang W. Laboratory Investigation and Mechanical Evaluation on Xanthan Gum-Reinforced Clay: Unconfined Compression Test, Triaxial Shear Test, and Microstructure Characterization. Buildings. 2025; 15(14):2438. https://doi.org/10.3390/buildings15142438
Chicago/Turabian StyleYing, Liangbo, Mengqi Xu, Jiale Luo, and Wei Wang. 2025. "Laboratory Investigation and Mechanical Evaluation on Xanthan Gum-Reinforced Clay: Unconfined Compression Test, Triaxial Shear Test, and Microstructure Characterization" Buildings 15, no. 14: 2438. https://doi.org/10.3390/buildings15142438
APA StyleYing, L., Xu, M., Luo, J., & Wang, W. (2025). Laboratory Investigation and Mechanical Evaluation on Xanthan Gum-Reinforced Clay: Unconfined Compression Test, Triaxial Shear Test, and Microstructure Characterization. Buildings, 15(14), 2438. https://doi.org/10.3390/buildings15142438







