3.1. Influence of VAE Dosage on the Fluidity of Repair Mortar
The impact of varying VAE dosages on the fluidity of OPC-SF repair mortar is depicted in
Figure 2. The findings indicate that the mortar’s fluidity is trending upward as the VAE dosage is increased. The fluidities of the specimens with VAE dosages of 2.5 wt.%, 5.0 wt.%, 7.5 wt.%, and 10.0 wt.% increased by 1.95%, 4.34%, 13.02%, and 14.97%, respectively, in comparison to the reference group (0 wt.% VAE). A maximum fluidity increase of 14.97% is observed at the VAE dosage of 10.0 wt.%. This result demonstrates that a reasonable amount of VAE integration can successfully boost the fluidity of the repair mortar.
The following synergistic effects are responsible for VAE’s improvement of fluidity [
23,
24,
25]: (1) The micro-filling and lubricating effect: VAE particles, which typically range in size from 50 to 500 nm, can fill the cement matrix’s microporous structure, lowering internal frictional resistance and improving the relative sliding ability between particles. (2) Interfacial activity regulation: VAE copolymer stabilizes micro-bubbles by reducing solid–gas interfacial energy. Unlike entrapped or mechanically entrained air, these bubbles originate from gaseous products of chemical reactions between active mortar components. Normally unstable and susceptible to collapse, the bubbles are stabilized by VAE’s amphiphilic polymer structure which acts as a surfactant. The hydrophobic segments reduce surface tension, while mechanical shear organizes VAE particles and micro-bubbles into ball-bearing-like structures that enhance paste fluidity.
It should be mentioned, though, that the polymer film that results during VAE hydration [
26] will strengthen the bonds between cement particles, raising the slurry’s viscosity. Existing studies have shown that when the VAE dosage continues to increase, the increase in viscosity will become the dominant factor, leading to an insignificant increase in fluidity [
24]. The viscosity characteristics of the repair materials are another key index affecting their performance. Therefore, the effect of further increasing the VAE dosage is not significant from the perspective of fluidity. The dosage should be optimized by comprehensively considering other indicators to achieve a synergistic improvement in mortar performance.
3.2. Influence of VAE Dosage on the Flexural and Compressive Strengths of Repair Mortar
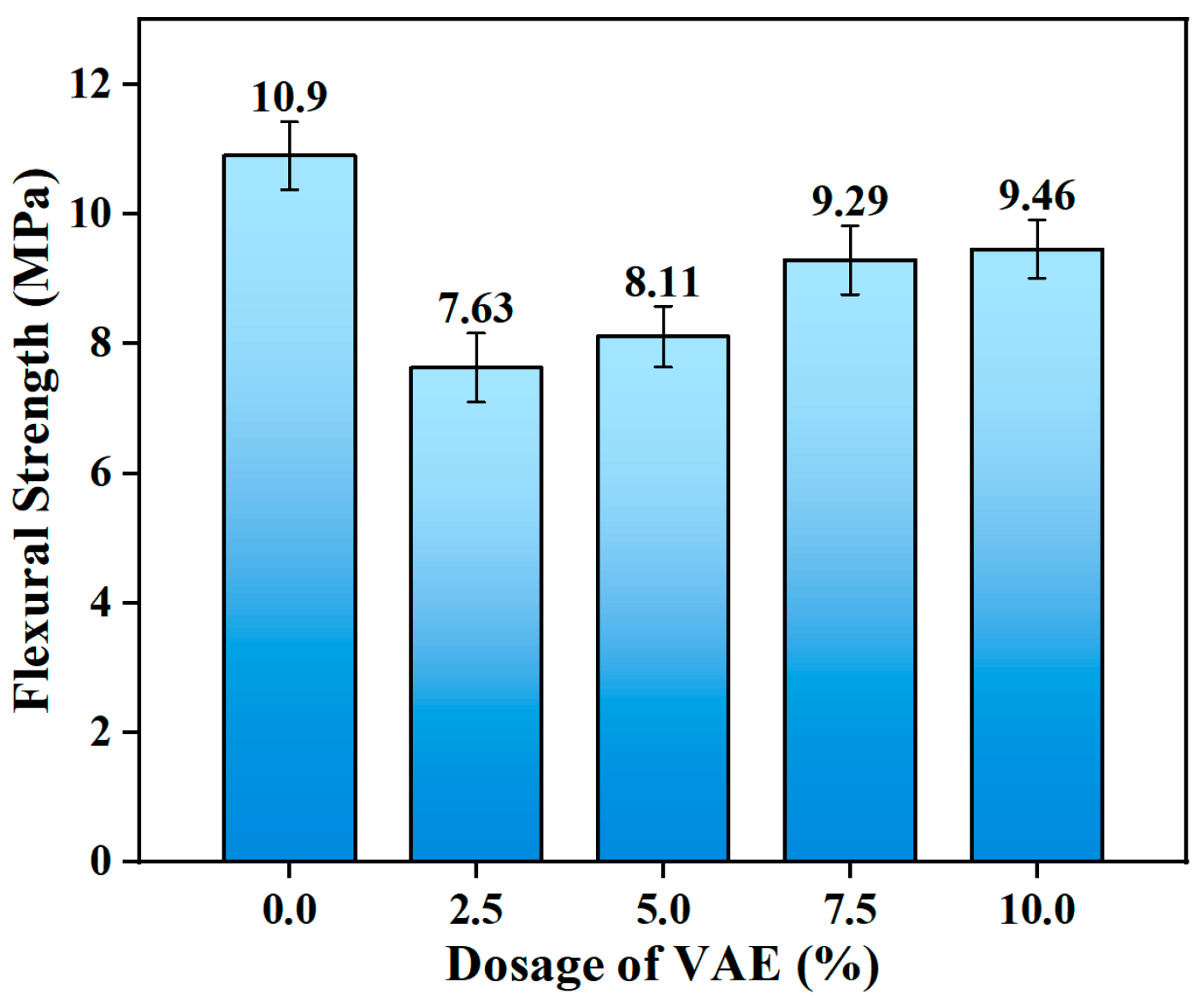
As shown in
Figure 3, different VAE dosages have a nonlinear influence on the 28-day flexural strength of OPC-SF repair mortar. The findings indicate that as the VAE dosage increases from 0 wt.% to 10.0 wt.%, the flexural strength shows a non-monotonic evolution trend of first decreasing and then increasing. All modified groups’ flexural strengths, however, fall short of the reference group’s (0 wt.% VAE). The specimens with VAE dosages of 2.5 wt.%, 5 wt.%, 7.5 wt.% and 10.0 wt.% have flexural strengths of 7.63 MPa (a decrease of 30.0%), 8.11 MPa (a decrease of 25.6%), 9.29 MPa (a decrease of 14.8%), and 9.46 MPa (a decrease of 13.2%), respectively. It is important to note that at low dosages (2.5 wt.%), the strength loss is most noticeable, but at high dosages (10.0 wt.%), the reduction slows down.
The dynamic rivalry mechanism between the “defect effect” and “enhancement effect” that VAE introduced is responsible for this phenomenon: (1) Effect of pore-inducing: The mechanical continuity of the interfacial transition zone (ITZ) [
9,
27] is greatly weakened by VAE, which, due to its surface-active nature, entrains excessive micro-bubbles during mixing, increasing the number of stress-concentration spots. This effect dominates at the low-dosage stage, leading to a sharp decrease in flexural strength. (2) Polymer bridging effect: The polymer film created during hydration can create a three-dimensional interpenetrating network structure when the VAE dosage is ≥7.5% (see
Section 3.4). Through physical anchoring and chemical bonding, it improves the interfacial interaction between aggregates and C-S-H gel. Furthermore, the hydration induction period is prolonged by the retarding impact of VAE [
28], which encourages the uniform distribution of hydration products and helps to partially offset the strength loss brought on by pore defects.
The findings show a substantial nonlinear relationship between the mortar’s compressive strength and the VAE dosage (see
Figure 4). The reference group (0 wt.% VAE) has a 28-day compressive strength of 58.7 MPa. The compressive strengths exhibit a pattern known as “decrease-increase-decrease again” [
9] when the VAE dosages are doped from 2.5 wt.% to 10.0 wt.%. These reduce to 44.2 MPa (a 24.7% decrease), 50.2 MPa (a 14.5% decrease), 50.5 MPa (a 14.0% decrease), and 48.3 MPa (a 17.7% decrease), respectively. There is an ideal dosage range since the compressive strength loss is lowest at the critical dosage (7.5 wt.%).
Due to differences in interfacial compatibility, the dispersed VAE particles decrease the stress transfer efficiency at low dosages (<5.0 wt.%). Furthermore, they are unable to properly bridge load-induced microcracks since no continuous polymer film is created. Through the energy-dissipation mechanism, the continuous polymer network slows the spread of cracks at medium doses (5.0 wt.% to 7.5 wt.%). In the meantime, the subsequent hydration reaction is encouraged by VAE’s water-retaining property. Together, the two effects raise the compressive strength. At large dosages (>7.5 wt.%), excessive VAE significantly elevates slurry viscosity. This viscosity surge hinders dense packing of cement particles. Simultaneously, it disrupts the topological connectivity of C-S-H gel. These combined effects ultimately result in strength degradation [
29].
3.3. Influence of VAE Dosage on the Tensile Bond Strength of Repair Mortar
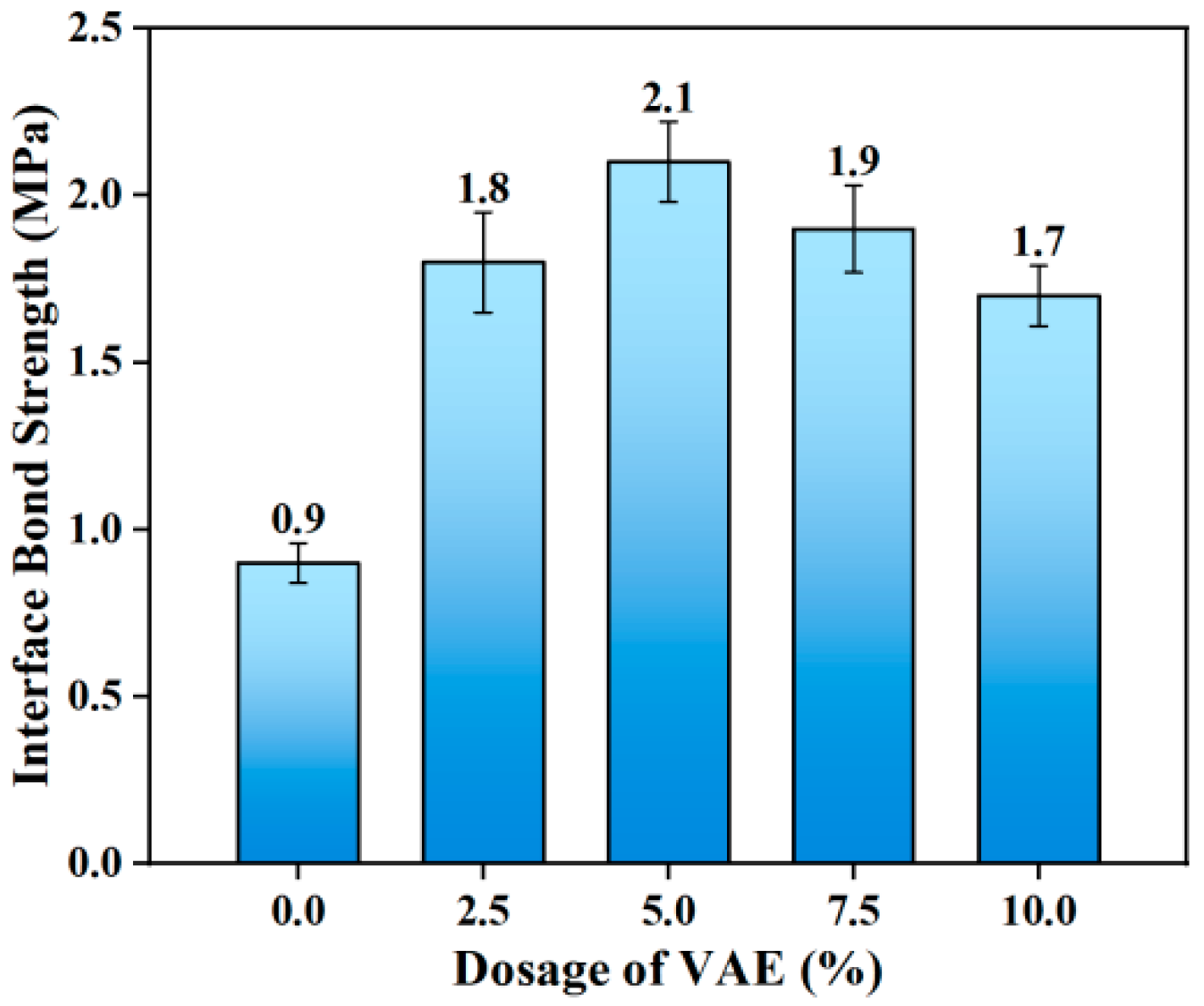
The impact of varying VAE dosages on the interfacial bond strength of OPC-SF repair mortar is shown in
Figure 5. The findings demonstrate that there is a notable two-stage response in the interfacial bond strength as the VAE dosage increases. The 28-day interfacial bond strength improves by 133.3% from 0.9 MPa to 2.1 MPa when the VAE dosage is increased from 0 wt.% to 5.0 wt.%. This suggests that VAE has a large dose-dependent strengthening effect on interfacial characteristics in the low-dosage range. However, the bond strength exhibits a reversed attenuation pattern when the dosage surpasses 5.0 wt.%. The strengths of the 7.5 wt.% and 10.0 wt.% groups decreased to 1.9 MPa and 1.7 MPa, respectively, with strength loss rates of 9.5% and 19.0% when compared to the group that received the optimal dosage.
VAE’s synergistic effect with cement hydration products is responsible for improving the interfacial bond performance of repair mortar. The calcium silicate hydrate gel (C-S-H) and the continuous polymer film created by the VAE emulsion in the cement paste interpenetrate in a staggered fashion to create a composite three-dimensional network structure. This structure uses two mechanisms to optimize the interfacial properties: (1) Physical filling effect: By efficiently filling the nanoscale defects (<100 nm) in the interfacial transition zone (ITZ) and the micron-scale pores (50–200 μm) inside the mortar, the polymer–gel composite lowers porosity and increases interfacial density [
30,
31,
32]. (2) Stress-buffering effect: The interface has a great energy-dissipation capacity due to the high ductility of the polymer film (elongation upon break > 200%). By uniformizing the stress distribution, it inhibits the development and spread of microcracks, postponing the failure of interfacial debonding [
33].
It is worth noting that an excessive VAE dosage (>5.0 wt.%) will cause a degradation of interfacial properties. Research shows the following: (1) The mechanical interlocking action of hydration products is weakened at super-critical doses because an excessively thick polymer film (thickness > 1 μm) creates a dense barrier layer in the ITZ, limiting direct contact between cement particles and the substrate [
34]. (2) High-concentration polymer adsorbed on cement particle surfaces causes a loose microstructure of hydration products, limits the bridging growth of C-S-H gel, and delays the hydration kinetic process of mineral phases such C
3S and C
2S [
9]. The aforementioned findings suggest that in order to maximize the performance of the interfacial tensile bond strength, the VAE dosage must be kept below 5.0 wt.%.
3.4. Microscopic Morphology Analysis
For each group, we performed microscopic morphology analysis by keeping the water–cement ratio and sand–cement ratio constant.
Figure 6 shows the SEM images of the hydration products of the reference group and the 5.0 wt.% VAE-modified group after 28-day hydration.
The compactness of the hydration products’ microstructure can be shown by contrasting
Figure 6(a1,b1). The reference group exhibits many pores with interconnected microcracks with diameters ranging from 10 to 100 μm. The 5.0 wt.% VAE-modified group, on the other hand, has a very compact and evenly distributed microstructure, with a notable decrease in the quantity of big pores and interconnected microcracks. This suggests that VAE can efficiently fill the pores in the cement microstructure. Simultaneously, the reference group’s hydration products have relatively large crystal sizes and needle-rod-shaped AFt crystals and lamellar C-S-H crystals that mostly form in loosely structured places like pores and fractures. On the other hand, the AFt crystals developing in the pores are more uniformly distributed and considerably smaller in the microstructure of the 5.0 wt.% VAE-modified group. This suggests that the polymer contributes to the cement paste’s grain refinement. The polymer’s filling impact on the cement’s internal pores and the polymer film covering the hydration products’ surface are the two causes of this occurrence. Together, these two elements restrict the hydration products’ development range, which reduces the size of the crystal. This is also supported by Ma’s research [
35].
3.5. EDS Spectrum Analysis
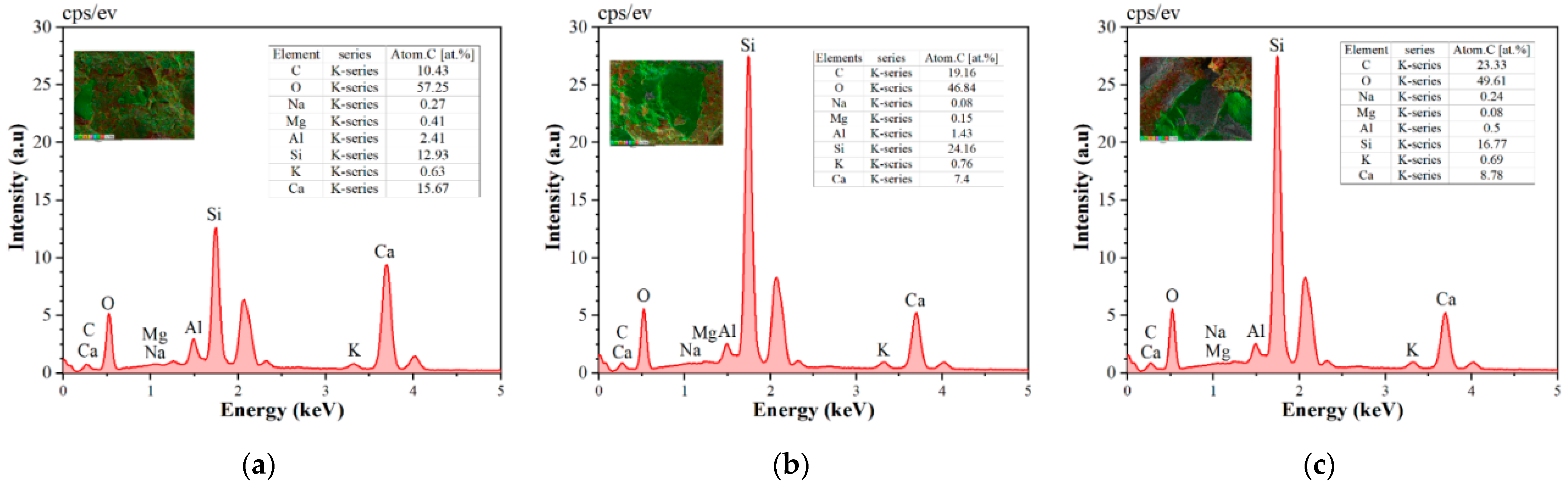
The elemental distribution of the reference group and VAE-modified cement mortar was characterized using EDS (see
Figure 7). According to the analysis, the specimens’ primary constituents include carbon (C), oxygen (O), silicon (Si), and calcium (Ca). A quantitative comparison shows that the fraction of oxygen elements in the VAE-modified mortar considerably drops, the relative amount of carbon elements increases, and the silicon elements exhibit an enrichment trend.
In the cement matrix, carbon components mostly exist as carbonate phases (such as CaCO
3), depending on the phase occurrence state. Through interfacial interactions, it chemically interacts with calcium silicate hydrate gel (C-S-H) to promote the heterogeneous nucleation of secondary calcium carbonate [
36,
37]. The matrix’s compactness is improved by the combined actions of pore filling and chemical bonding.
The cement matrix contains silicon elements primarily in the form of silicon oxide (SiO2). Nanoscale silicon dioxide (SiO2) particles, typically sourced from silica fume, are associated with the enrichment of silicon components. Through the physical filling mechanism, these particles maximize the cement paste’s pore structure. The aforementioned microstructural evolution can significantly increase the material’s durability and impermeability. The physical filling action of nanoscale SiO2 particles is responsible for the enrichment of silicon elements. Their specific surface area (>200 m2/g) contributes to the formation of a tight packing structure between cement particles. Consequently, the material’s durability and impermeability are significantly enhanced.
The decrease in oxygen element content, a characteristic of hydration products (such Ca(OH)2, C-S-H, etc.), suggests that the VAE polymer somewhat interferes with the cement-based material’s hydration process. This could be a result of the organic long chains of VAE forming an adsorption layer on cement particle surfaces, which slows down the rate at which Ca2+ dissolves. The EDS spectrum shows reduced Ca element content, confirming fewer early-stage hydration products. This reduction stems from interfacial control action.
According to the elemental composition and calcium–silicon ratio analysis, a low calcium–silicon ratio usually corresponds to a denser gel structure, higher gelling activity and stronger interfacial bonding ability. VAE can optimize the interfacial bonding performance by regulating the calcium–silicon ratio and microstructure of cement hydration products (densification of the C-S-H gel and refinement of the AFt crystals).
Compared with the reference (VAE-0) group, the increase in VAE content significantly reduces the calcium–silicon ratio, and the microstructure becomes more abundant while the gel particles are insufficiently closely connected [
38], leading to a decrease in compressive strength relative to the reference group. However, with the increase in VAE content, the silicon–calcium ratio shows an upward trend. The C-S-H gel generated by hydration has a more dense structure [
39]. Under the synergy with the polymer film, the compressive strength shows an upward trend, and the bond strength also increases. In the high-dosage (VAE-10) group, the balance of the calcium–silicon ratio is disrupted [
40]. Although the amount of hydration products may relatively increase, the excessive polymer film forms a barrier, leading to the slowdown of the hydration process. Meanwhile, the hydration products fail to form strong chemical bonds due to the lack of effective contact, and the compactness decreases. In addition, the increase in fluidity hinders the close packing of cement particles, which corresponds to the deterioration of compressive strength and may also be one of the reasons for the decrease in bond strength. This is also supported by Wei’s research [
38].