Mix Design-Driven Control of Carbonation and Hydration in CO2-Mixed Cement Pastes: Effects of Water, Slag, and Surfactant
Abstract
1. Introduction
2. Experiment
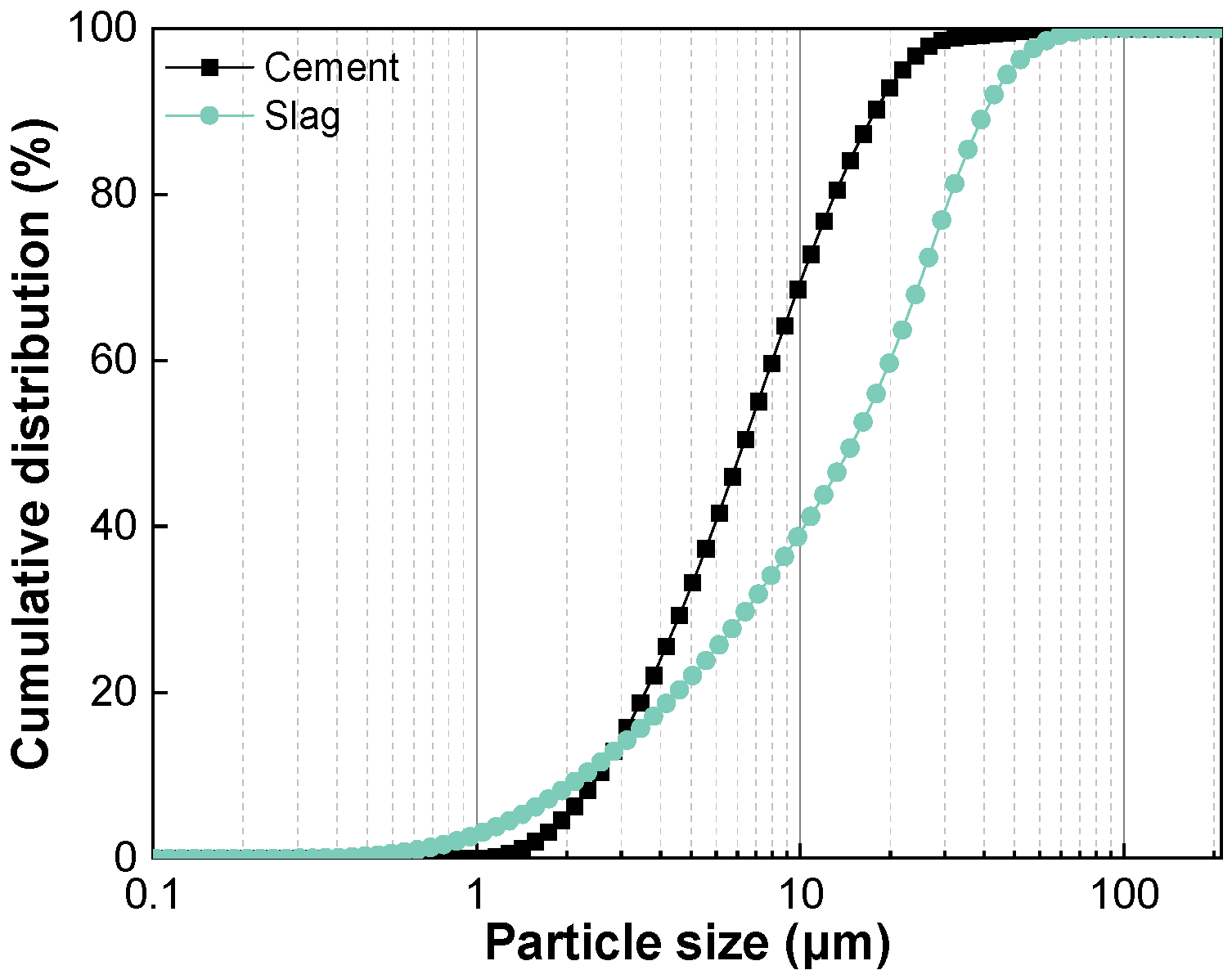
2.1. Materials
2.2. Mix Proportions and Sample Preparation
2.3. Testing Method
2.3.1. XRD
2.3.2. TGA
2.3.3. Nitrogen Adsorption
2.3.4. CO2 Uptake
3. Results
3.1. Mineral Composition
3.1.1. XRD Analysis
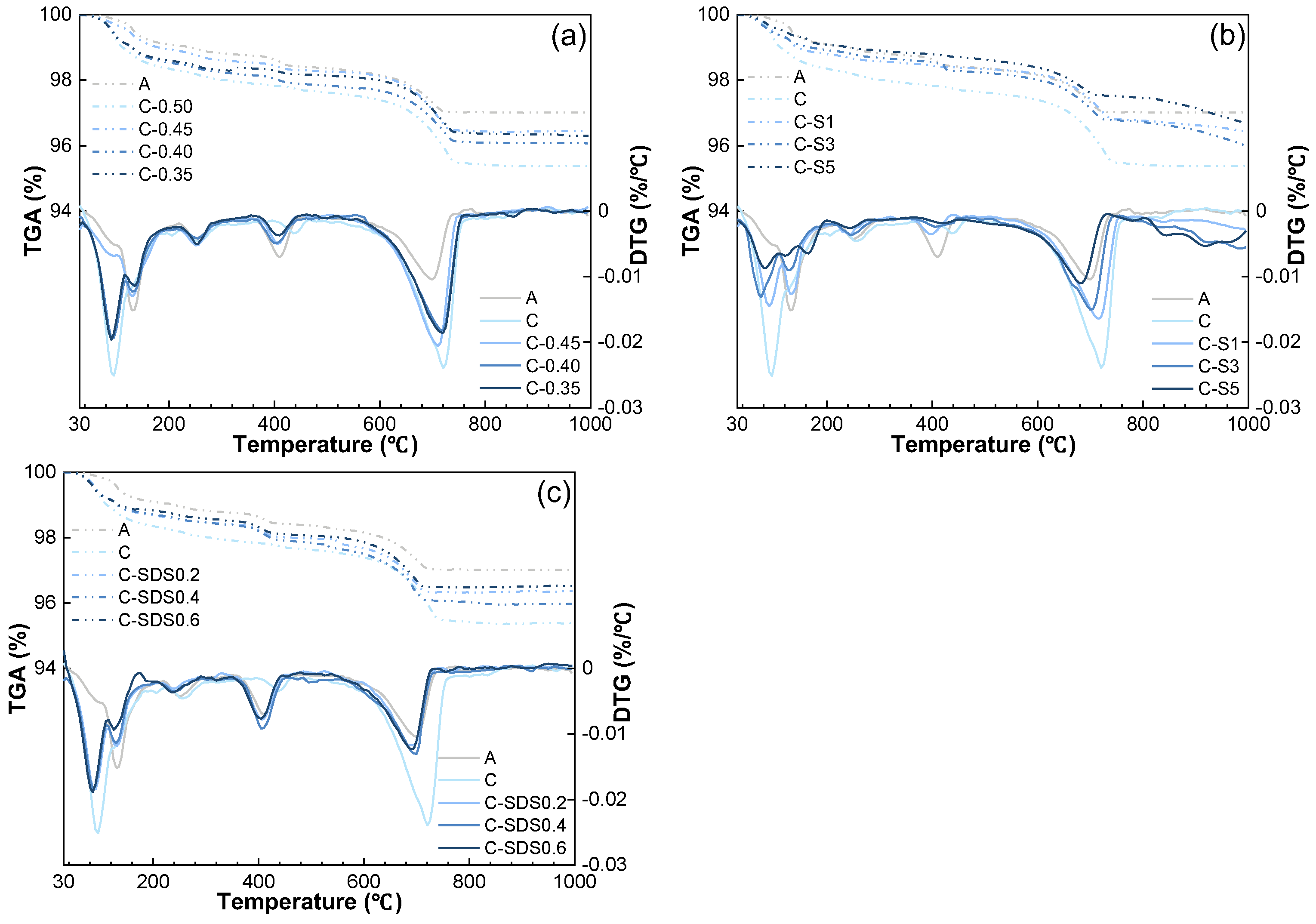
3.1.2. TGA/DTG Analysis
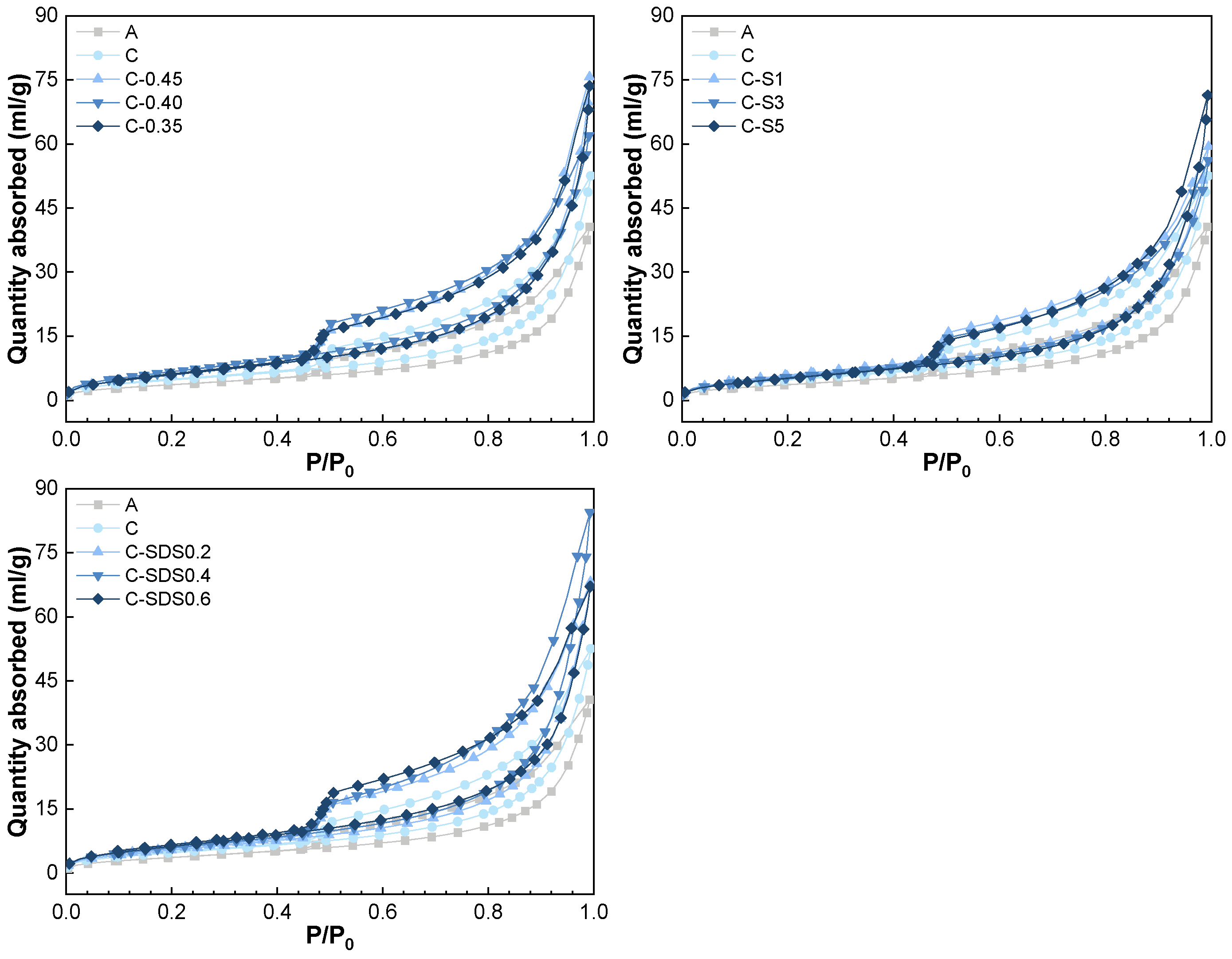
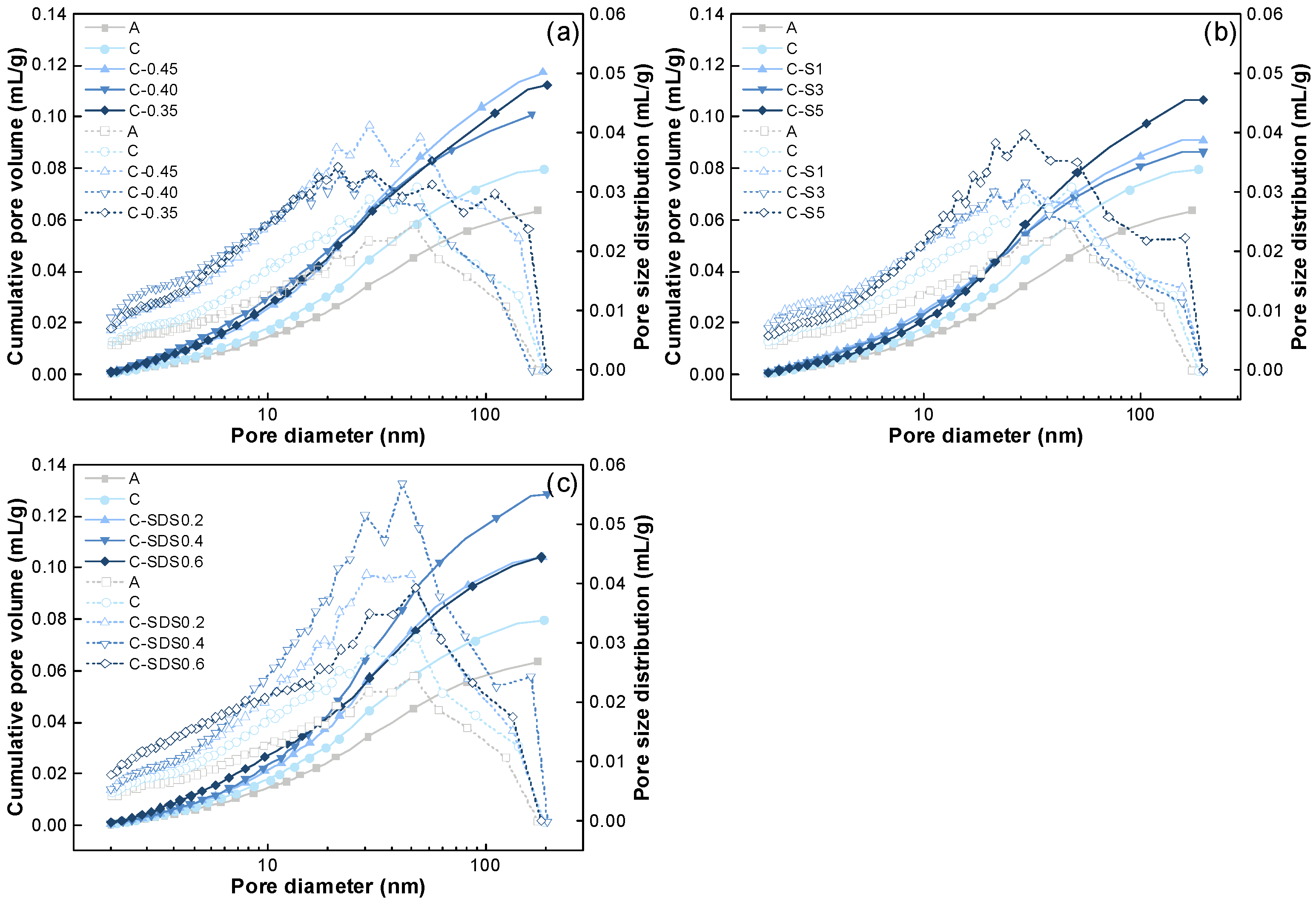
3.2. Pore Structure Analysis
3.3. CO2 Uptake
4. Discussion
4.1. Effect of Water-to-Binder Ratio
4.2. Effect of Slag Incorporation
4.3. Effect of AEA Incorporation
5. Conclusions
- (1)
- Lower w/b ratios limit CO2 dissolution and mass transport, shifting the reaction pathway toward hydration. This results in reduced carbonate formation and coarser pore structures. At moderate w/b ratios, concurrent carbonation and hydration are promoted, but excessively low water content hinders both reaction kinetics and pore refinement.
- (2)
- Incorporating slag increases the amorphous content and influences early-age reaction rates. Up to 30% slag causes minimal reductions in carbonation and hydration. However, higher contents significantly delay both processes and lead to wider capillary pores due to the dilution of reactive phases and reduced alkalinity.
- (3)
- Increasing SDS dosage stabilizes CO2 bubbles but significantly reduces immediate CO2 dissolution and associated reaction products. Entrained gas increases porosity, particularly in the 20 nm–200 nm range, and promotes an interconnected pore network if overdosed.
- (4)
- The early-age performance of CO2-mixed cement paste depends on a well-balanced mix design. A moderate w/b ratio, slag content below 30%, and restrained AEA dosage collectively enhance carbonation–hydration synergy, improve pore structure, and support effective CO2 sequestration.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- Churkina, G.; Organschi, A.; Reyer, C.P.O.; Ruff, A.; Vinke, K.; Liu, Z.; Reck, B.K.; Graedel, T.E.; Schellnhuber, H.J. Buildings as a global carbon sink. Nat. Sustain. 2020, 3, 269–276. [Google Scholar] [CrossRef]
- Cheng, D.; Reiner, D.M.; Yang, F.; Cui, C.; Meng, J.; Shan, Y.; Liu, Y.; Tao, S.; Guan, D. Projecting future carbon emissions from cement production in developing countries. Nat. Commun. 2023, 14, 8213. [Google Scholar] [CrossRef] [PubMed]
- Rostami, V.; Shao, Y.; Boyd, A.J.; He, Z. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.-C.; Eggermont, S. Combining hydration and carbonation of cement using super-saturated aqueous CO2 solution. Constr. Build. Mater. 2019, 229, 116825. [Google Scholar] [CrossRef]
- He, Z.; Li, Z.; Shao, Y. Effect of Carbonation Mixing on CO2 Uptake and Strength Gain in Concrete. J. Mater. Civ. Eng. 2017, 29, 04017176. [Google Scholar] [CrossRef]
- Monkman, S.; Lee, B.E.J.; Grandfield, K.; MacDonald, M.; Raki, L. The impacts of in-situ carbonate seeding on the early hydration of tricalcium silicate. Cem. Concr. Res. 2020, 136, 106179. [Google Scholar] [CrossRef]
- Rashid, S.; Singh, M. An Investigation on Carbon Dioxide Incorporated Sustainable Ready-Mix Concrete Using OPC and PPC. Arab. J. Sci. Eng. 2023, 48, 14213–14236. [Google Scholar] [CrossRef]
- Monkman, S.; Sargam, Y.; Naboka, O.; Lothenbach, B. Early age impacts of CO2 activation on the tricalcium silicate and cement systems. J. CO2 Util. 2022, 65, 102254. [Google Scholar] [CrossRef]
- Monkman, S.; Hwang, S.D.; Khayat, K. Rheology modification of flowable mortar with CO2. Cem. Concr. Compos. 2024, 151, 105584. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, Q.; Ni, J.; Shi, C.; Zheng, K.; Tan, J. Study of CO2 Injection Timing within the Mixing Process of Ready-Mix Concrete for Win–Win Improvements of Mechanical Properties and CO2 Sequestration. ACS Sustain. Chem. Eng. 2024, 12, 1480–1492. [Google Scholar] [CrossRef]
- Wang, M.; Luo, S.; Pham, B.T.; Ling, T.-C. Effect of CO2-mixing dose and prolonged mixing time on fresh and hardened properties of cement pastes. J. Zhejiang Univ. Sci 2023, 24, 886–897. [Google Scholar] [CrossRef]
- Wang, Y.; He, F.; Yang, L. Influence of dry ice on the performance of Portland cement and its mechanism. Constr. Build. Mater. 2018, 188, 898–904. [Google Scholar] [CrossRef]
- Xuan, M.-Y.; Lee, S.-H.; Hu, H.-Q.; Wang, X.-Y. Adding dry ice into ultra-high-performance concrete to enhance engineering performances and lower CO2 emissions. Constr. Build. Mater. 2023, 392, 131858. [Google Scholar] [CrossRef]
- Kwasny, J.; Basheer, P.A.M.; Russell, M.; Doherty, W.; Owens, K.; Ward, N. CO2 sequestration in cement-based materials during mixing process using carbonated water and gaseous CO2. In Proceedings of the 4th International Conference on the Durability of Concrete Structures, ICDCS, West Lafayette, IN, USA, 24–26 July 2014; pp. 72–79. [Google Scholar] [CrossRef]
- Fan, D.; Lu, J.-X.; Lv, X.-S.; Noguchi, T.; Yu, R.; Poon, C.S. Carbon capture and storage CO2 foam concrete towards higher performance: Design, preparation and characteristics. Cem. Concr. Compos. 2025, 157, 105925. [Google Scholar] [CrossRef]
- Liu, G.; Lu, W.; Liu, H.; Wen, X. Performance and mechanism of carbon sequestration of air-entraining wet shotcrete. J. Build. Eng. 2024, 96, 110623. [Google Scholar] [CrossRef]
- GB 175; Common Portland Cement. Standardization Administration of China: Beijing, China, 2023.
- GB/T 18046; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. Standardization Administration of China: Beijing, China, 2017.
- Li, C.; Zhang, Y.; Ren, Q.; Jiang, Z. Early-age hydration of cement paste prepared with CO2 mixing under varying pressures. Constr. Build. Mater. 2025, 472, 140948. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Qian, X.; Wang, J.; Fang, Y.; Wang, L. Carbon dioxide as an admixture for better performance of OPC-based concrete. J. CO2 Util. 2018, 25, 31–38. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- De Weerdt, K.; Plusquellec, G.; Belda Revert, A.; Geiker, M.R.; Lothenbach, B. Effect of carbonation on the pore solution of mortar. Cem. Concr. Res. 2019, 118, 38–56. [Google Scholar] [CrossRef]
- Zajac, M.; Irbe, L.; Bullerjahn, F.; Hilbig, H.; Ben Haha, M. Mechanisms of carbonation hydration hardening in Portland cements. Cem. Concr. Res. 2022, 152, 106687. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Bai, G.; Liu, H.; Meng, Y.; Wang, Z. 3D printed concrete with recycled sand: Pore structures and triaxial compression properties. Cem. Concr. Compos. 2023, 139, 105048. [Google Scholar] [CrossRef]
- Dong, Y.; Shen, K.; Zhu, H.; Hu, C.; Wang, F. Unveiling the synergistic effect of in-situ generated calcium carbonate by CO2 mixing on the reaction of calcined clay. Cem. Concr. Compos. 2024, 148, 105490. [Google Scholar] [CrossRef]
- Liu, L.; Ji, Y.; Gao, F.; Zhang, L.; Zhang, Z.; Liu, X. Study on high-efficiency CO2 absorption by fresh cement paste. Constr. Build. Mater. 2021, 270, 121364. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, M.G.; Wang, Y.C.; Su, Y.M.; Deng, J.L. Preliminary Study on Reaction of Fresh Concrete with Carbon Dioxide. Defect Diffus. Forum 2018, 382, 230–234. [Google Scholar] [CrossRef]
- Davolio, M.; Muciaccia, G.; Ferrara, L. Concrete carbon mixing—A systematic review on the processes and their effects on the material performance. Clean. Mater. 2025, 15, 100292. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C–S–H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lee, M.-G.; Wang, W.-C.; Kan, Y.-C.; Kao, S.-H.; Chang, H.-W. CO2 Curing on the Mechanical Properties of Portland Cement Concrete. Buildings 2022, 12, 817. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Bucher, R.; Cyr, M.; Escadeillas, G. Carbonation of Blended Binders Containing Metakaolin. In Calcined Clays for Sustainable Concrete; Scrivener, K., Favier, A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 27–33. [Google Scholar] [CrossRef]
- Mutti, M.; Joseph, S.; Snellings, R.; Cizer, Ö. Effect of slag pre-carbonation on its early-age reactivity in alkali activated binder. Constr. Build. Mater. 2024, 411, 134413. [Google Scholar] [CrossRef]
- Ke, G.; Zhang, J.; Tian, B.; Wang, J. Characteristic analysis of concrete air entraining agents in different media. Cem. Concr. Res. 2020, 135, 106142. [Google Scholar] [CrossRef]
- Cao, L.; Shi, F.; Qiu, M.; Chen, W.; Cao, P.; Zhou, C. Effects of air entraining agent on the rheological properties and electrochemical parameters of cement mortar. Constr. Build. Mater. 2022, 344, 128233. [Google Scholar] [CrossRef]
- Wong, H.S.; Pappas, A.M.; Zimmerman, R.W.; Buenfeld, N.R. Effect of entrained air voids on the microstructure and mass transport properties of concrete. Cem. Concr. Res. 2011, 41, 1067–1077. [Google Scholar] [CrossRef]


| CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO | K2O | LOI | |
|---|---|---|---|---|---|---|---|---|
| Cement | 64.6 | 18.3 | 4.6 | 3.6 | 3.5 | 3.2 | 0.8 | 1.4 |
| Slag | 51.3 | 21.4 | 14.1 | 1.7 | 1.3 | 6.3 | 0.5 | 1.3 |
| Sample | Mixing Atmosphere | Water-to-Binder Ratio | Slag Content | AEA Dosage |
|---|---|---|---|---|
| A | Air | 0.50 | / | / |
| C | CO2 | 0.50 | / | / |
| C-0.45 | CO2 | 0.45 | / | / |
| C-0.40 | CO2 | 0.40 | / | / |
| C-0.35 | CO2 | 0.35 | / | / |
| C-S1 | CO2 | 0.50 | 10% | / |
| C-S3 | CO2 | 0.50 | 30% | / |
| C-S5 | CO2 | 0.50 | 50% | / |
| C-SDS0.2 | CO2 | 0.50 | / | 0.2% |
| C-SDS0.4 | CO2 | 0.50 | / | 0.4% |
| C-SDS0.6 | CO2 | 0.50 | / | 0.6% |
| Sample | Relative Mass at Different Temperature/% | ||
|---|---|---|---|
| 350 °C | 550 °C | 1000 °C | |
| A | 98.76 | 98.27 | 97.01 |
| C | 97.91 | 97.53 | 95.39 |
| C−0.45 | 98.16 | 97.76 | 95.84 |
| C−0.40 | 98.55 | 98.23 | 96.36 |
| C−0.35 | 98.37 | 98.09 | 96.30 |
| C−S1 | 98.52 | 98.29 | 96.43 |
| C−S3 | 98.62 | 98.15 | 96.00 |
| C−S5 | 98.84 | 98.53 | 96.70 |
| C−SDS0.2 | 98.41 | 97.85 | 96.38 |
| C−SDS0.4 | 98.38 | 97.66 | 95.98 |
| C−SDS0.6 | 98.52 | 98.01 | 96.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Li, C.; Ma, H.; Ren, Q. Mix Design-Driven Control of Carbonation and Hydration in CO2-Mixed Cement Pastes: Effects of Water, Slag, and Surfactant. Buildings 2025, 15, 2116. https://doi.org/10.3390/buildings15122116
Xia J, Li C, Ma H, Ren Q. Mix Design-Driven Control of Carbonation and Hydration in CO2-Mixed Cement Pastes: Effects of Water, Slag, and Surfactant. Buildings. 2025; 15(12):2116. https://doi.org/10.3390/buildings15122116
Chicago/Turabian StyleXia, Jingliang, Chunjin Li, Haoyuan Ma, and Qiang Ren. 2025. "Mix Design-Driven Control of Carbonation and Hydration in CO2-Mixed Cement Pastes: Effects of Water, Slag, and Surfactant" Buildings 15, no. 12: 2116. https://doi.org/10.3390/buildings15122116
APA StyleXia, J., Li, C., Ma, H., & Ren, Q. (2025). Mix Design-Driven Control of Carbonation and Hydration in CO2-Mixed Cement Pastes: Effects of Water, Slag, and Surfactant. Buildings, 15(12), 2116. https://doi.org/10.3390/buildings15122116






