Alkali-Activated Slag–Fly Ash–Desert Sand Mortar for Building Applications: Flowability, Mechanical Properties, Sulfate Resistance, and Microstructural Analysis
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Solid Precursors
2.2. Alkali Activating Solution
2.3. Test Scheme and Sample Production
2.4. Testing and Characterization
2.4.1. Flowability
2.4.2. Flexural and Compressive Strength
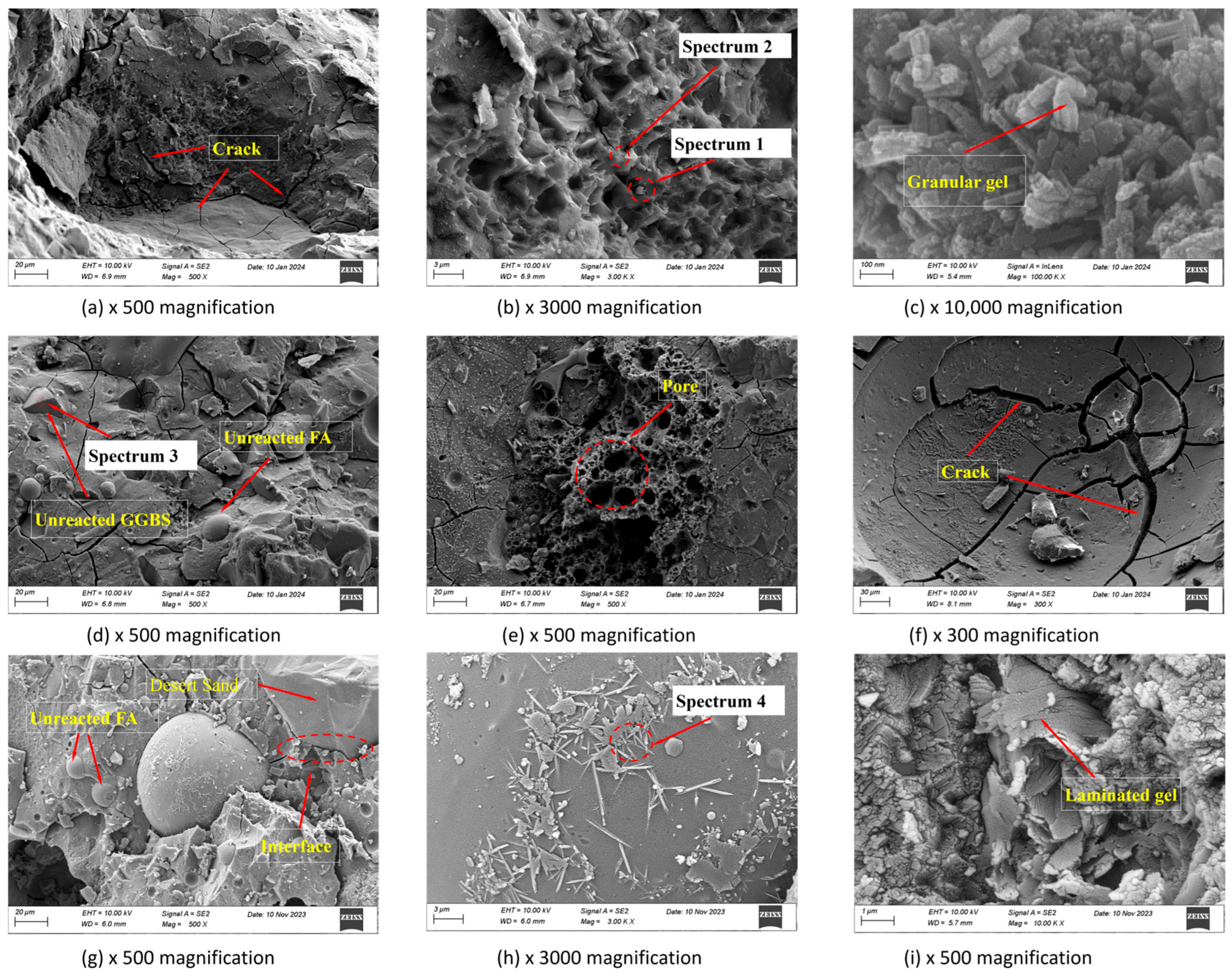
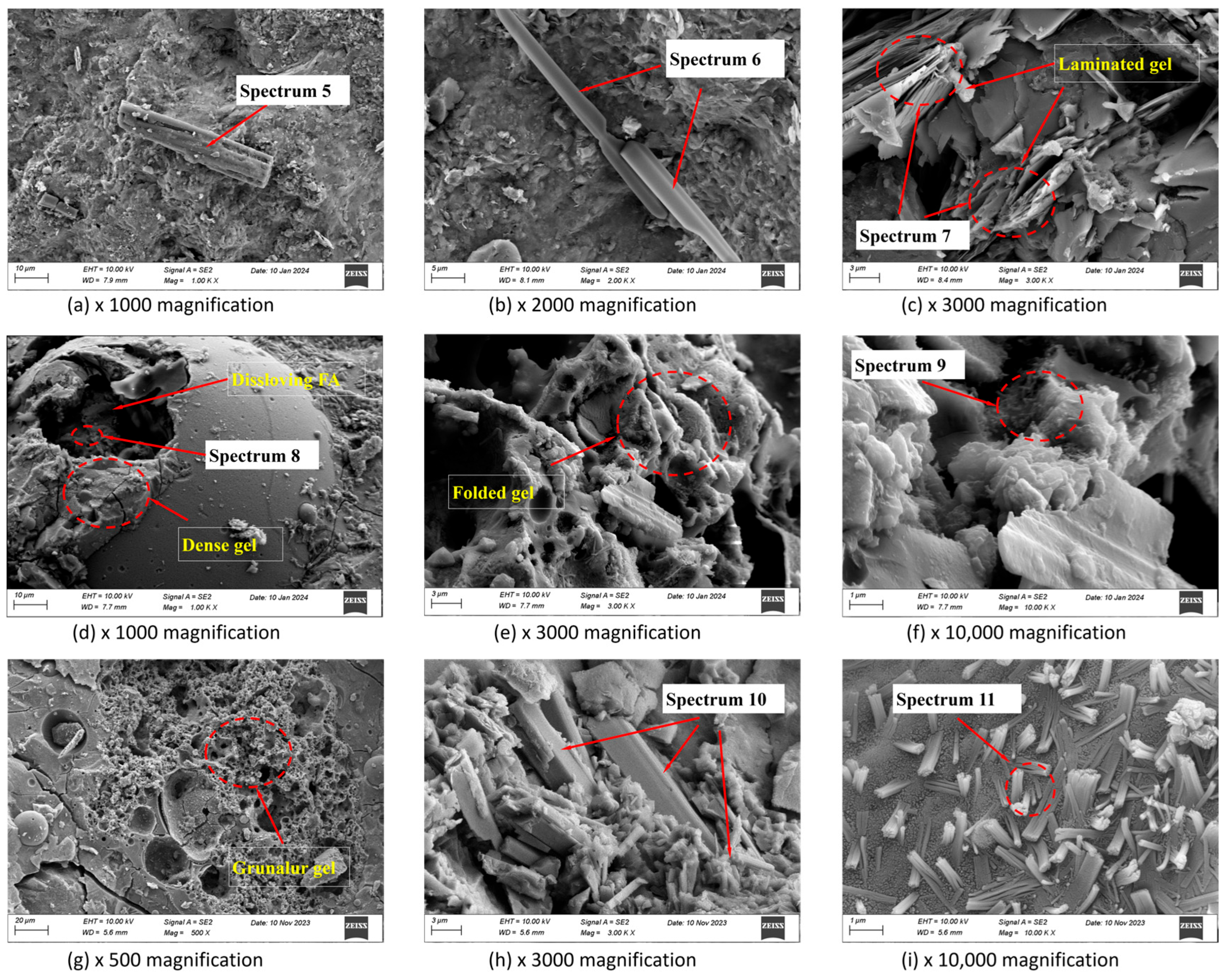
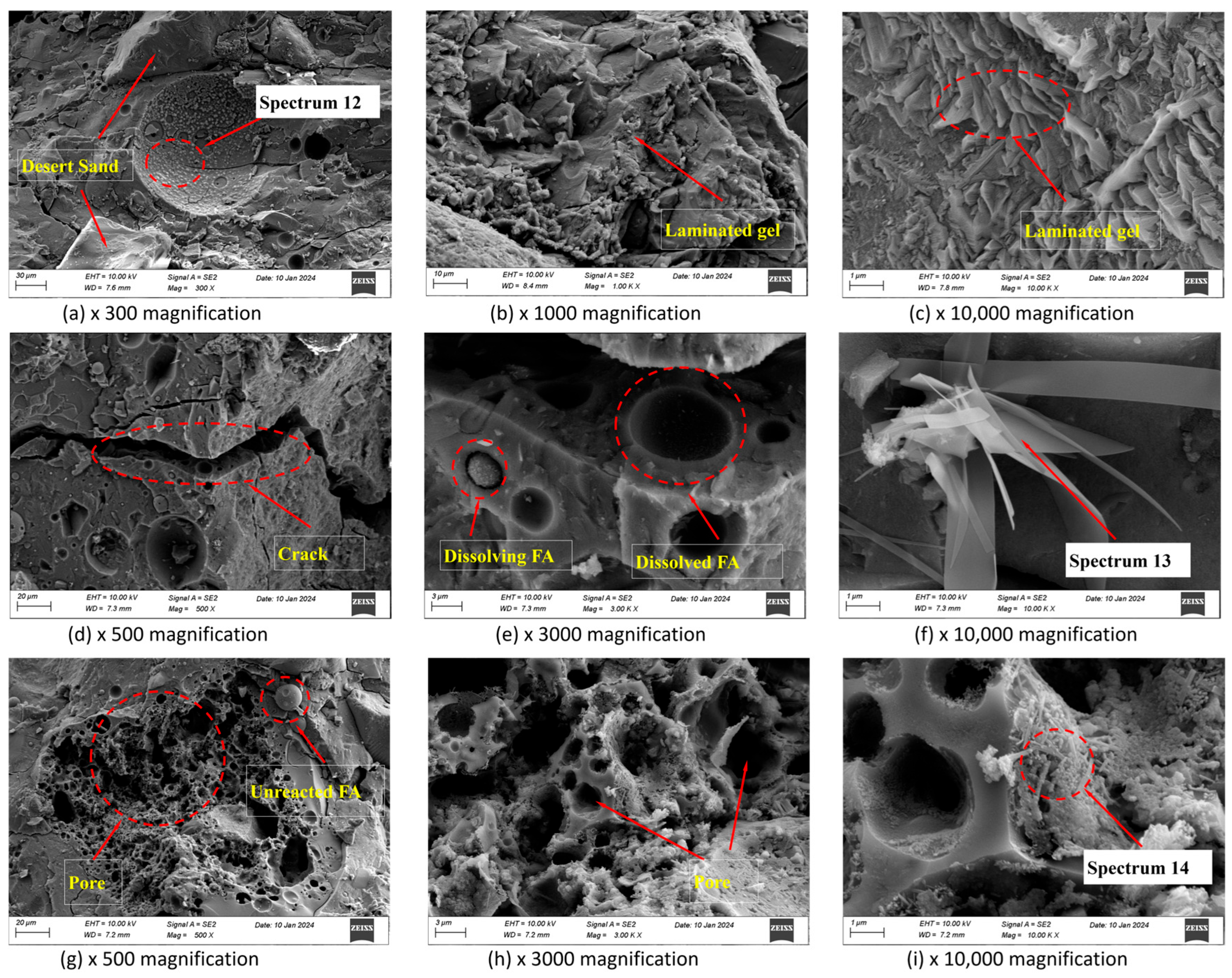
2.4.3. Microstructural Features
3. Results and Discussion
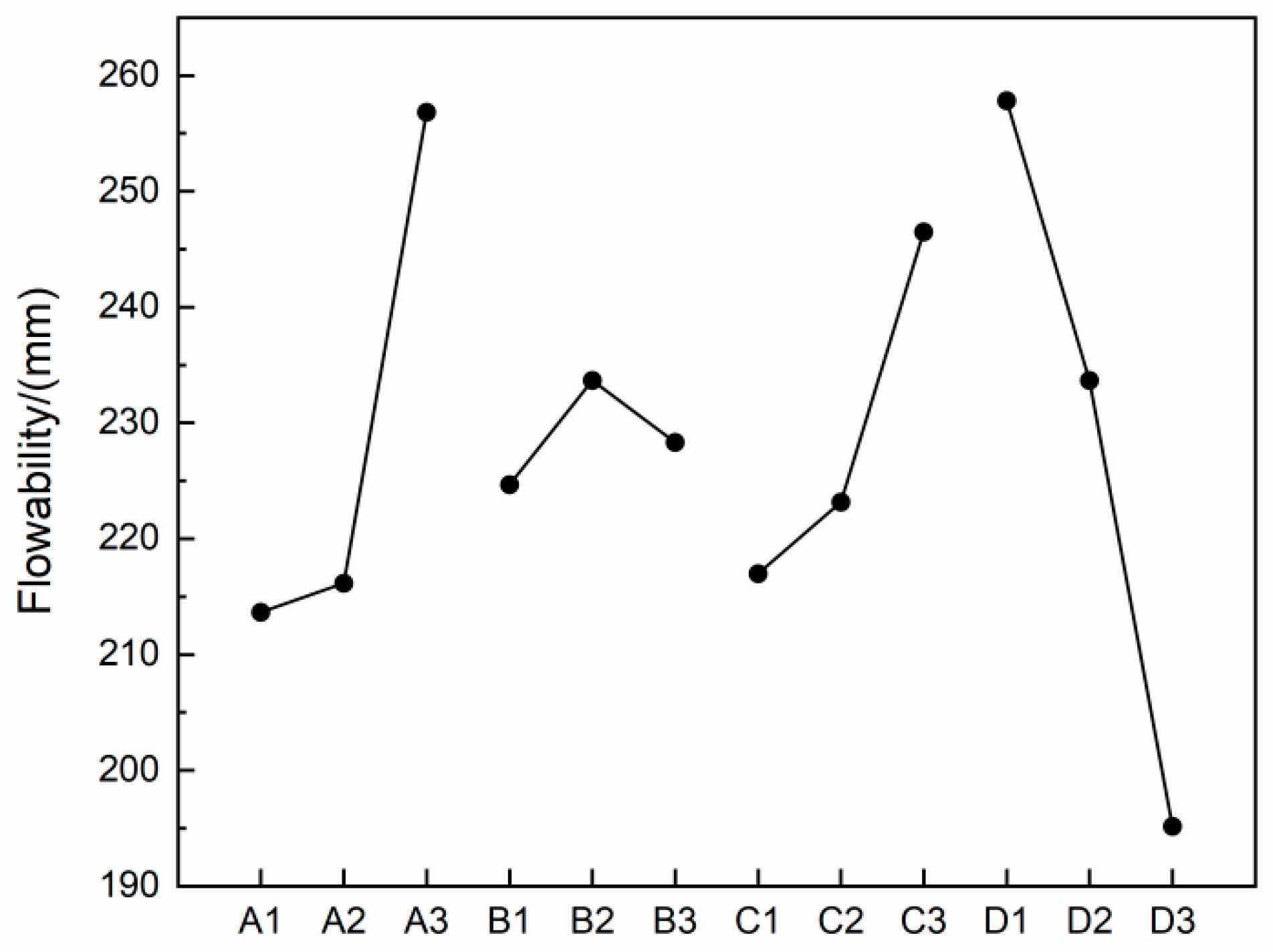
3.1. Flowability
3.2. Flexural Strength and Compressive Strength
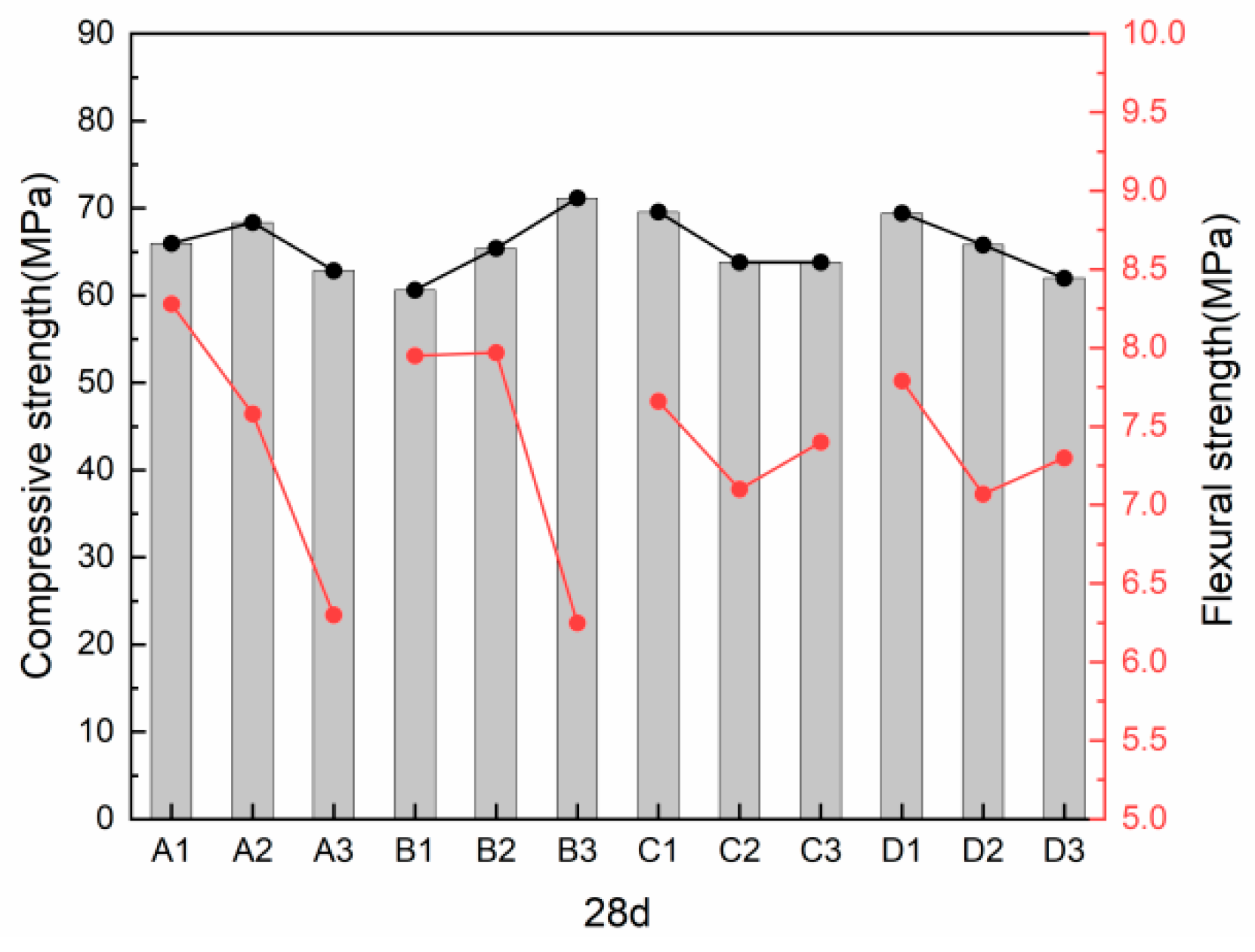
3.2.1. Twenty-Eight-Day Compressive and Flexural Strength
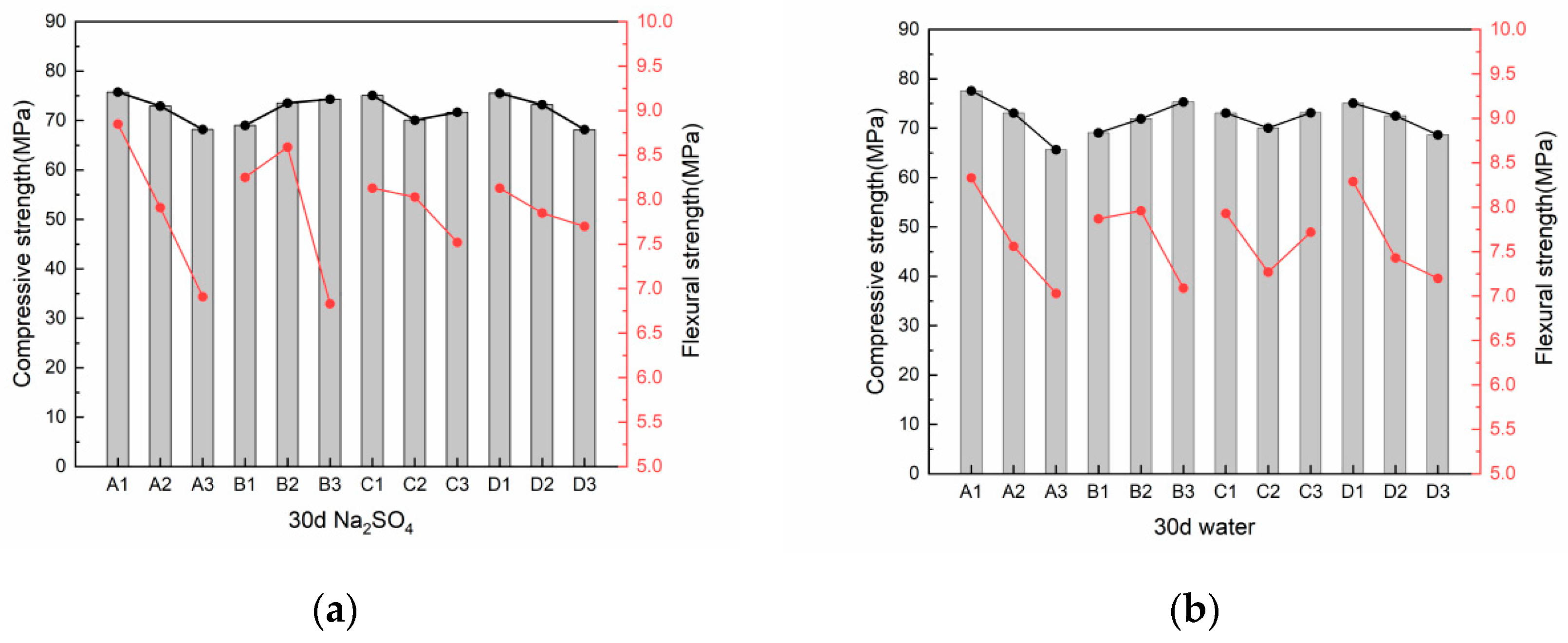
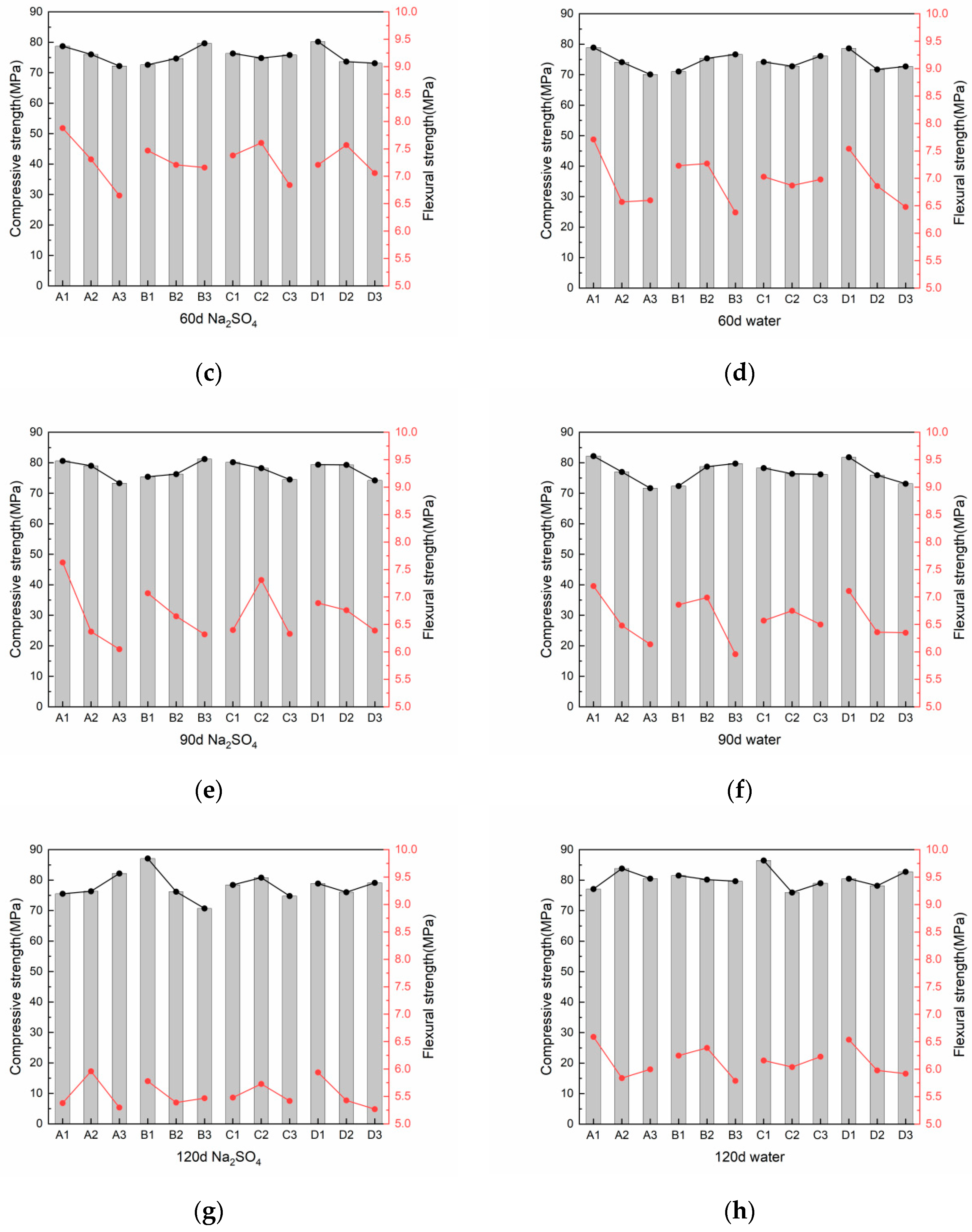
3.2.2. Compressive and Flexural Strength After Sulfate Attack
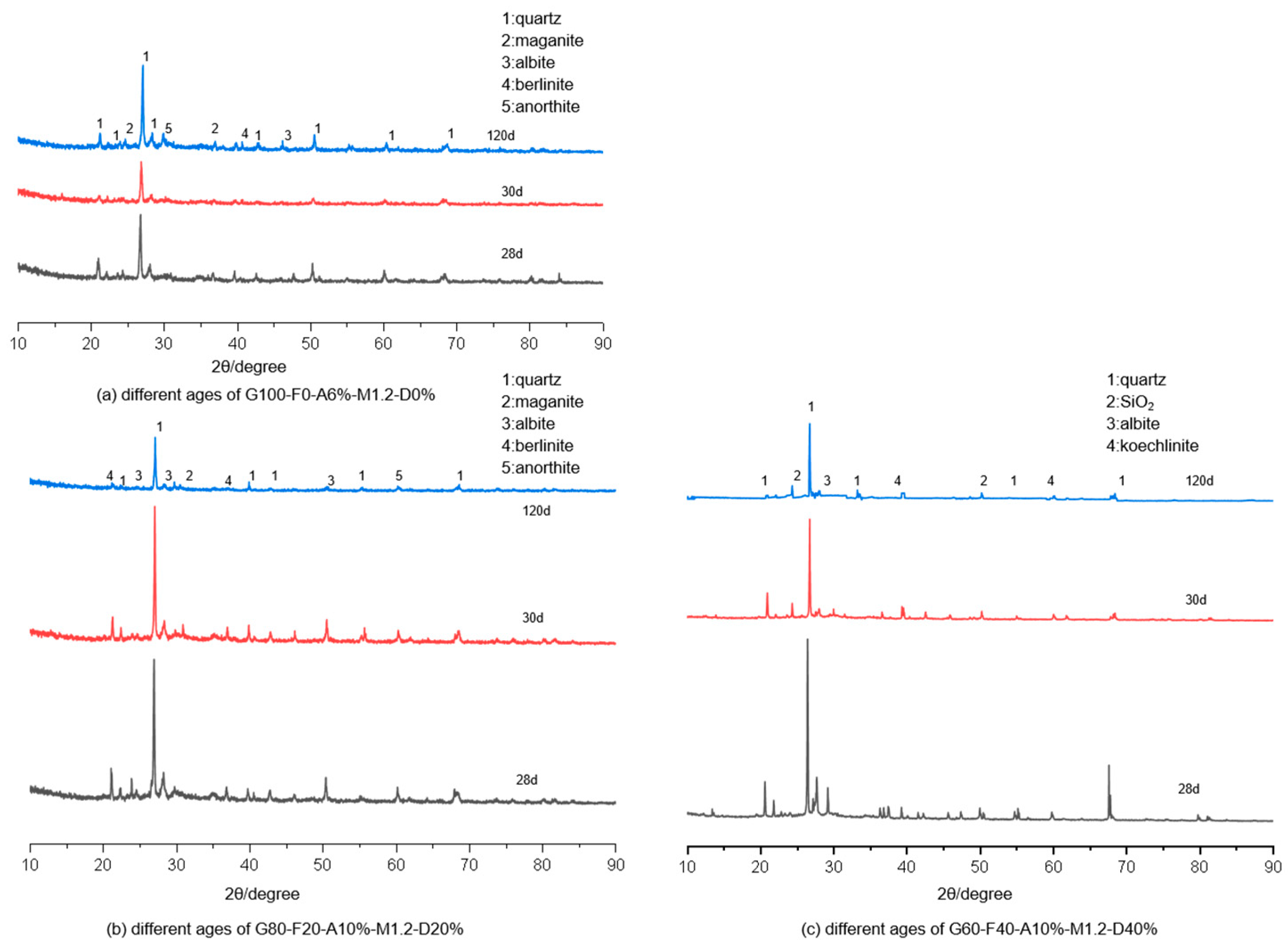
3.3. X-Ray Diffraction Analysis
3.4. SEM Analysis and EDS Tests
3.5. Pore Structure
4. Conclusions
- (1)
- The replacement rate of natural sand by desert sand had the most significant impact on the flow properties, followed by slag content, while alkali content and alkali excitation modulus had a relatively minor effect on the flow properties.
- (2)
- The factors influencing the 28 d flexural strength, in descending order, were slag, alkali content, desert sand content, and alkali excitation modulus. At a slag content of 100%, alkali content of 6%, alkali excitation modulus of 1.2, and a desert sand substitution rate of 0%, the maximum flexural strength reached 9.52 MPa. Conversely, the factors affecting the 28 d compressive strength were, in sequence, alkali content, desert sand content, alkali excitation modulus, and slag content. The highest 28 d compressive strength of 77.7 MPa was achieved with an alkali content of 10%, a fly ash substitution rate of 20%, and a desert sand substitution rate of 20%.
- (3)
- As the erosion duration lengthens, fly ash and desert sand contribute favorably to the late-stage increase in compressive strength. Furthermore, higher levels of slag and alkali content correspond to accelerated reaction rates and increased production of expansion products in the presence of sulfates, ultimately resulting in a reduction in compressive strength.
- (4)
- Through microscopic analysis methods such as XRD, SEM/EDS, and MIP, it was demonstrated that the slag exhibited high activity and a rapid reaction dissolution rate. However, the fly ash and certain portions of the desert sand continued to dissolve under sulfate exposure, forming C (N)-A-S-H gel that ultimately enhanced strength in the later stages. The voids created by alkali-excited desert sand mortar primarily consist of harmless pores, which are filled by expansion products generated during sulfate attack, leading to a reduction in cumulative porosity. Nonetheless, as the reaction progresses, the mortar continuously produces expansion products like gypsum calcium alumina, causing the internal structure to deteriorate and the cumulative porosity to increase.
- (5)
- Combining desert sand with an alkali-activated slag–fuel ash system can leverage the benefits of a well-performing, high-strength, and durable alkali-activated slag–fuel ash system. Nevertheless, challenges persist for alkali-activated desert sand, including the procurement, preparation, and use of slag in the mass production of concrete, and the high transportation costs associated with desert sand and large-scale production of concrete.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Tan, Y.; Wang, Y.; Liu, C. Mechanical Properties and Chloride Permeability of Green Concrete Mixed with Fly Ash and Coal Gangue. Constr. Build. Mater. 2020, 233, 117166. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Z.; Sui, T.; Akbarnezhad, A.; Duan, Z. Mechanical Properties of Concrete Mixed with Recycled Powder Produced from Construction and Demolition Waste. J. Cleaner Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Wang, J.; Msigwa, G.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Circular Economy Strategies for Combating Climate Change and Other Environmental Issues. Environ. Chem. Lett. 2023, 21, 55–80. [Google Scholar] [CrossRef]
- Kai, L.; Liu, X.; Xie, X.; Liu, R.; Li, T.; Shah, S.P. The Feasibility of Utilizing Sifted Desert Sand (<75 μm) as Sustainable Supplementary Cementitious Materials (SCM). Constr. Build. Mater. 2023, 406, 133375. [Google Scholar]
- Liu, M.; Liu, E.; Hao, J.L.; Sarno, L.D.; Xia, J. Hydration and Material Properties of Blended Cement with Ground Desert Sand. Constr. Build. Mater. 2023, 389, 131624. [Google Scholar] [CrossRef]
- Guettala, S.; Mezghiche, B. Compressive Strength and Hydration with Age of Cement Pastes Containing Dune Sand Powder. Constr. Build. Mater. 2011, 25, 1263–1269. [Google Scholar] [CrossRef]
- Hamada, H.M.; Abed, F.; Al-Sadoon, Z.A.; Elnassar, Z.; Hassan, A. The Use of Treated Desert Sand in Sustainable Concrete: A Mechanical and Microstructure Study. J. Build. Eng. 2023, 79, 107843. [Google Scholar] [CrossRef]
- Luo, F.J.; He, L.; Pan, Z.; Duan, W.H.; Zhao, X.L.; Collins, F. Effect of Very Fine Particles on Workability and Strength of Concrete Made with Dune Sand. Constr. Build. Mater. 2013, 47, 131–137. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-Activated Binders: A Review Part 1. Historical Background, Terminology, Reaction Mechanisms and Hydration Products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, T.; Qiao, L.; Yu, F.; Zhang, L.; Sun, H.; Li, C. Alkali-Activated Metakaolin-Blast Furnace Slag Blends as an Alternative Inorganic Adhesive for FRP-Based Structural Rehabilitation. J. Build. Eng. 2025, 103, 112163. [Google Scholar] [CrossRef]
- Terzi, C.; Kutuk, S.; Kutuk-Sert, T. Influence of Processed Tea Waste Ash on the Hydration Products and Mechanical Property of Hybrid Cement as an Eco-Friendly Solution. Waste Manag. 2025, 191, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, J.; Lin, H.; Li, Y. Optimization Design for Alkali-Activated Slag-Fly Ash Geopolymer Concrete Based on Artificial Intelligence Considering Compressive Strength, Cost, and Carbon Emission. J. Build. Eng. 2023, 75, 106929. [Google Scholar] [CrossRef]
- Bawab, J.; El-Hassan, H.; El-Dieb, A.; Khatib, J. Effect of Mix Design Parameters on the Properties of Cementitious Composites Incorporating Volcanic Ash and Dune Sand. Dev. Built Environ. 2023, 16, 100258. [Google Scholar] [CrossRef]
- Ye, H.; Chen, Z.; Huang, L. Mechanism of Sulfate Attack on Alkali-Activated Slag: The Role of Activator Composition. Cem. Concr. Res. 2019, 125, 105868. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Hu, X. Reaction Mechanism of Sulfate Attack on Alkali-Activated Slag/Fly Ash Cements. Constr. Build. Mater. 2022, 318, 126052. [Google Scholar] [CrossRef]
- Xia, D.; Chen, R.; Cheng, J.; Tang, Y.; Xiao, C.; Li, Z. Desert Sand-High Calcium Fly Ash-Based Alkali-Activated Mortar: Flowability, Mechanical Properties, and Microscopic Analysis. Constr. Build. Mater. 2023, 398, 131729. [Google Scholar] [CrossRef]
- GB/T 1596-2017; Fly Ash Used in Cement and Concrete. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2017.
- Kutuk, S. Morphology, Crystal Structure and Thermal Properties of Nano-Sized Amorphous Colemanite Synthesis. Arab. J. Sci. Eng. 2024, 49, 11699–11716. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Method of Testing Cement Strength. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2021.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2005.
- Kuang, F.; Long, Z.; Kuang, D.; Guo, R.; Sun, J. Experimental Study on Preparation and Properties of Low Content Rubberized Geopolymer Mortar. Constr. Build. Mater. 2022, 352, 128980. [Google Scholar] [CrossRef]
- Kwan, A.; Fung, W. Packing Density Measurement and Modelling of Fine Aggregate and Mortar. Cem. Concr. Compos. 2009, 31, 349–357. [Google Scholar] [CrossRef]
- Lai, M.H.; Chen, Z.H.; Wang, Y.H.; Ho, J.C.M. Effect of Fillers on the Mechanical Properties and Durability of Steel Slag Concrete. Constr. Build. Mater. 2022, 335, 127495. [Google Scholar] [CrossRef]
- De La Varga, I.; Castro, J.; Bentz, D.P.; Zunino, F.; Weiss, J. Evaluating the Hydration of High Volume Fly Ash Mixtures Using Chemically Inert Fillers. Constr. Build. Mater. 2018, 161, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Gu, X.; Wang, Z.; Xu, S.; Jiang, H.; Yilmaz, E. Admixture Effects on the Rheological/Mechanical Behavior and Micro-Structure Evolution of Alkali-Activated Slag Backfills. Minerals 2022, 13, 30. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and Acid Resistance of Ambient-Cured Geopolymer Mortars Containing Nano-Silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef]
- Huseien, G.F.; Tahir, M.M.; Mirza, J.; Ismail, M.; Shah, K.W.; Asaad, M.A. Effects of POFA Replaced with FA on Durability Properties of GBFS Included Alkali Activated Mortars. Constr. Build. Mater. 2018, 175, 174–186. [Google Scholar] [CrossRef]
- Yuan, Q.; Huang, Y.; Huang, T.; Yao, H.; Wu, Q. Effect of Activator on Rheological Properties of Alkali-Activated Slag-Fly Ash Pastes. J. Cent. South Univ. 2022, 29, 282–295. [Google Scholar] [CrossRef]
- Sun, B.; Sun, Y.; Ye, G.; De Schutter, G. A Mix Design Methodology of Slag and Fly Ash-Based Alkali-Activated Paste. Cem. Concr. Compos. 2022, 126, 104368. [Google Scholar] [CrossRef]
- El-Hassan, H.; Ismail, N. Effect of Process Parameters on the Performance of Fly Ash/GGBS Blended Geopolymer Composites. J. Sustain. Cem.-Based Mater. 2018, 7, 122–140. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on Setting, Workability and Early Strength Properties of Fly Ash Geopolymer Concrete Cured in Ambient Condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Nedeljković, M.; Li, Z.; Ye, G. Setting, Strength, and Autogenous Shrinkage of Alkali-Activated Fly Ash and Slag Pastes: Effect of Slag Content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and Mechanical Properties of Alkali-Activated Fly Ash/Slag Concrete Manufactured at Room Temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z.; Zhu, D.; Hwang, H.-J.; Zhu, Y.; Sun, T. A Mixture Proportioning Method for the Development of Performance-Based Alkali-Activated Slag-Based Concrete. Cem. Concr. Compos. 2018, 93, 163–174. [Google Scholar] [CrossRef]
- Atabey, İ.İ.; Karahan, O.; Bilim, C.; Atiş, C.D. The Influence of Activator Type and Quantity on the Transport Properties of Class F Fly Ash Geopolymer. Constr. Build. Mater. 2020, 264, 120268. [Google Scholar] [CrossRef]
- Tian, L.; Chen, X.; Liu, X.; Li, H.; Ge, Y. Mechanisms of Recycled Fine Brick Aggregate in Autogenous Shrinkage Mitigation, Mechanical Properties Enhancement and Microstructure Improvement of Alkali-Activated Slag-Fly Ash Mortar. Constr. Build. Mater. 2023, 373, 130755. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Van Deventer, J.S.J. Microscopy and Microanalysis of Inorganic Polymer Cements. 2: The Gel Binder. J. Mater. Sci. 2009, 44, 620–631. [Google Scholar] [CrossRef]
- Kara, C.; Kutuk, S.; Kutuk-Sert, T. Improvement of the Durability of Concrete by Substitution of Raw Ground Colemanite. Case Stud. Constr. Mater. 2023, 19, e02393. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of Slag in Microstructural Development and Hardening of Fly Ash-Slag Geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Mastali, M.; Woof, B.; Illikainen, M. High Strength Fiber Reinforced One-Part Alkali Activated Slag/Fly Ash Binders with Ceramic Aggregates: Microscopic Analysis, Mechanical Properties, Drying Shrinkage, and Freeze-Thaw Resistance. Constr. Build. Mater. 2020, 241, 118129. [Google Scholar] [CrossRef]
- Veiga, K.K.; Gastaldini, A.L.G. Sulfate Attack on a White Portland Cement with Activated Slag. Constr. Build. Mater. 2012, 34, 494–503. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, L.; Guo, M.; Chen, Y.; Song, Z.; Bian, R. Evaluation of Sulfate Resistance of Slag Contained Concrete under Steam Curing. Constr. Build. Mater. 2019, 195, 231–237. [Google Scholar] [CrossRef]
- Škvára, F.; Šmilauer, V.; Hlaváček, P.; Kopecký, L.; Zuzan, K. A Weak Alkali Bond in (n, k)–a–s–h Gels: Evidence from Leaching and Modeling. Ceram-Silik. 2012, 56, 374–382. [Google Scholar]
- Jia, Z.; Cao, R.; Chen, C.; Zhang, Y. Using In-Situ Observation to Understand the Leaching Behavior of Portland Cement and Alkali-Activated Slag Pastes. Composites Part B 2019, 177, 107366. [Google Scholar] [CrossRef]
- Fang, G.; Wang, Q.; Zhang, M. In-Situ X-Ray Tomographic Imaging of Microstructure Evolution of Fly Ash and Slag Particles in Alkali-Activated Fly Ash-Slag Paste. Composites Part B 2021, 224, 109221. [Google Scholar] [CrossRef]
- Fu, Q.; Bu, M.; Zhang, Z.; Xu, W.; Yuan, Q.; Niu, D. Hydration Characteristics and Microstructure of Alkali-Activated Slag Concrete: A Review. Engineering 2023, 20, 162–179. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Xu, X.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Multi-Technical Characterization and Correlations between Properties of Standard Cured Alkali-Activated High-Calcium FA Binders with GGBS as Additive. Constr. Build. Mater. 2020, 241, 117996. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Zhang, W.; Ning, Y.; Casas, J.R. Utilization of Ultra-Fine Dredged Sand from the Yangtze River in Alkali-Activated Slag/Fly Ash Mortars: Mechanical Properties, Drying Shrinkage and Microstructure. Case Stud. Constr. Mater. 2023, 19, e02264. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Wang, J.; Thom, N. Effects of Alkali Dosage and Silicate Modulus on Autogenous Shrinkage of Alkali-Activated Slag Cement Paste. Cem. Concr. Res. 2021, 141, 106322. [Google Scholar] [CrossRef]
- Li, W.G.; Huang, Z.; Zu, T.; Shi, C.; Duan, W.H.; Shah, S.P. Influence of Nanolimestone on the Hydration, Mechanical Strength, and Autogenous Shrinkage of Ultrahigh-Performance Concrete. J. Mater. Civ. Eng. 2016, 28, 04015068. [Google Scholar] [CrossRef]
- Craeye, B.; De Schutter, G.; Desmet, B.; Vantomme, J.; Heirman, G.; Vandewalle, L.; Cizer, Ö.; Aggoun, S.; Kadri, E.H. Effect of Mineral Filler Type on Autogenous Shrinkage of Self-Compacting Concrete. Cem. Concr. Res. 2010, 40, 908–913. [Google Scholar] [CrossRef]
- Vance, K.; Aguayo, M.; Oey, T.; Sant, G.; Neithalath, N. Hydration and Strength Development in Ternary Portland Cement Blends Containing Limestone and Fly Ash or Metakaolin. Cem. Concr. Compos. 2013, 39, 93–103. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Hydration of Portland Cement with High Replacement by Siliceous Fly Ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
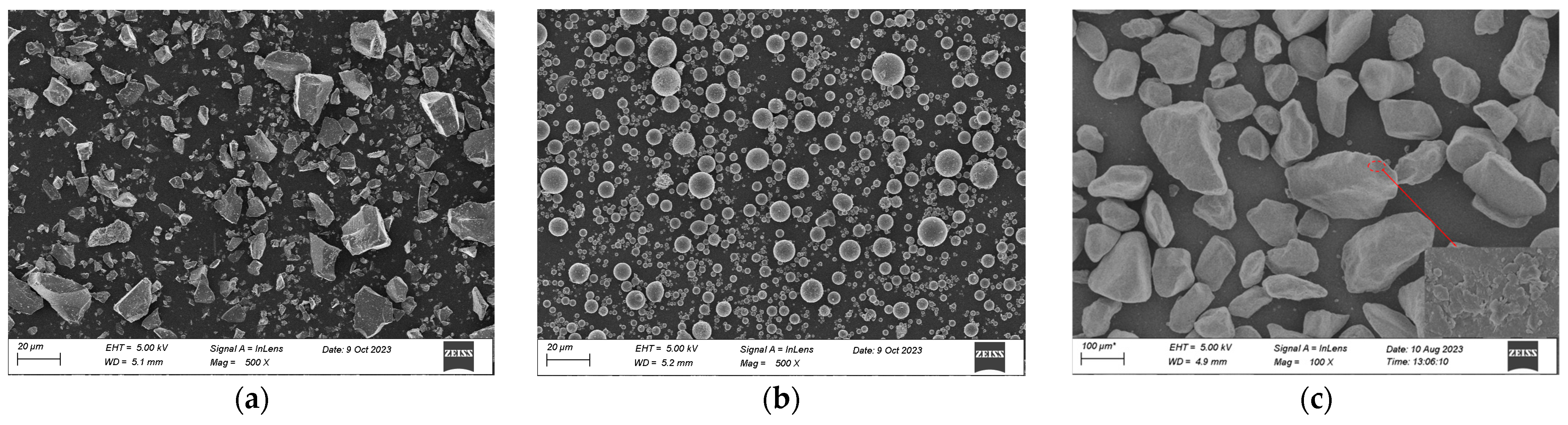
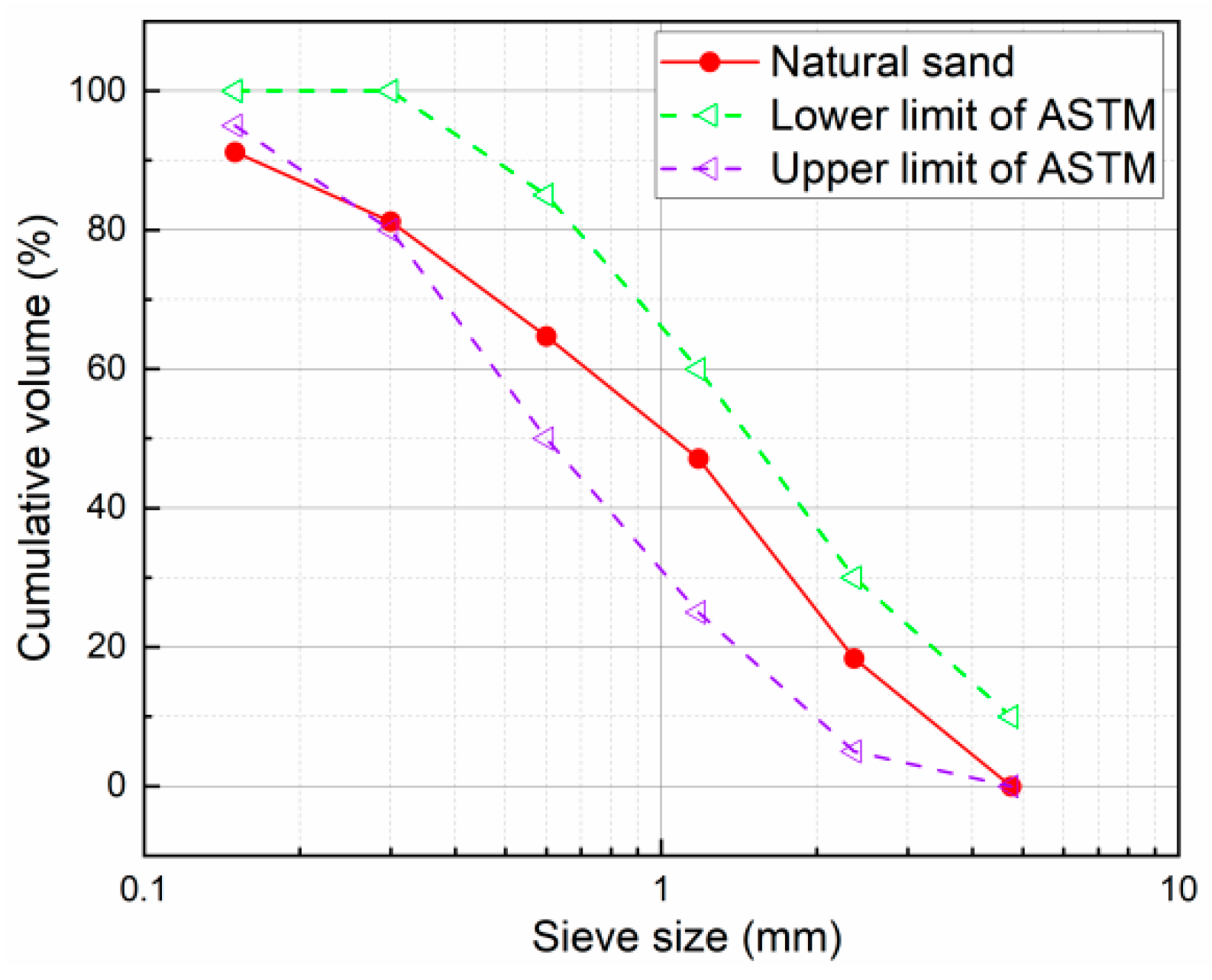

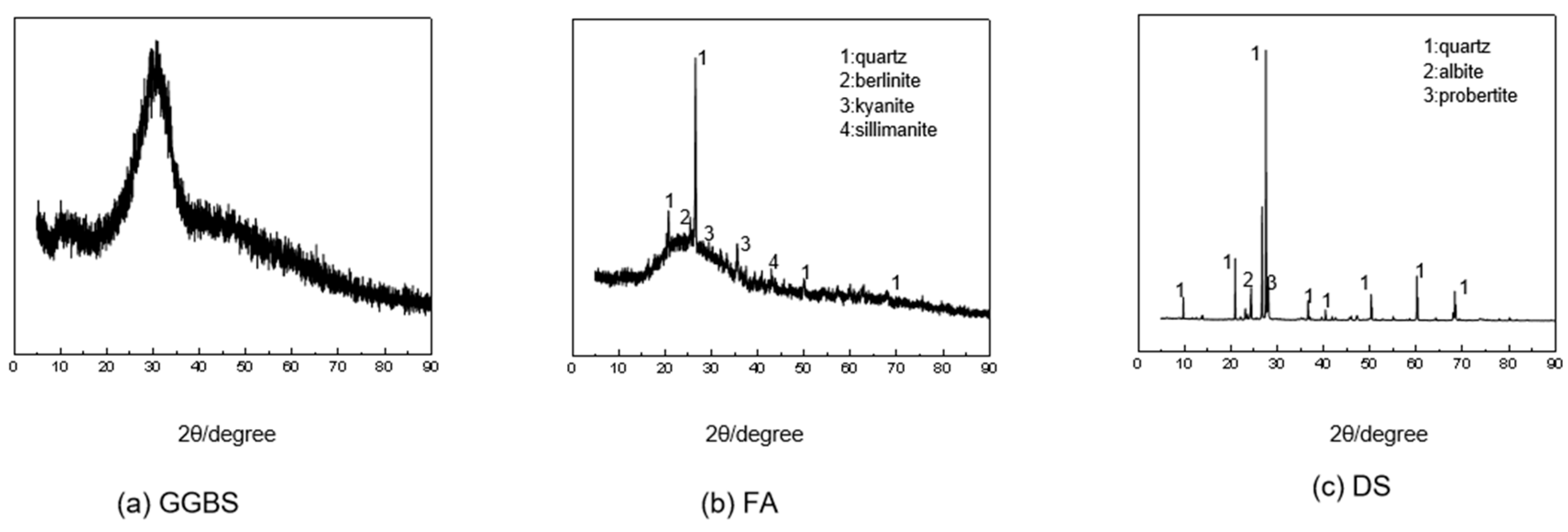


| Materials | Mass Fraction | LOI | ||||||
|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | SO3 | Fe2O3 | Cl− | ||
| GGBS | 41.83 | 36.77 | 10.07 | 9.78 | 0.53 | 1.89 | 0.023 | 0.9 |
| FA | 5.18 | 53.51 | 30.84 | 1.68 | 1.46 | 4.81 | — | 1.0 |
| DS | 15.08 | 65.43 | 12.39 | — | 0.46 | 3.16 | — | — |
| Levels | Factors | |||
|---|---|---|---|---|
| FA Replacement of Slag Rate (A) | Na2O/b (B) | Sodium Silicate Modulus (C) | Substitution Rates of Desert Sand (D) | |
| 1 | 0 | 6% | 1.2 | 0 |
| 2 | 20% | 8% | 1.4 | 20% |
| 3 | 40% | 10% | 1.6 | 40% |
| Group | Factors | |||
|---|---|---|---|---|
| FA Replacement of Slag Rate (A) | Na2O/b (B) | Sodium Silicate Modulus (C) | Substitution Rates of Desert Sand (D) | |
| G1-A1B1C1D1 | 0 | 6% | 1.2 | 0 |
| G2-A1B2C2D2 | 0 | 8% | 1.4 | 20% |
| G3-A1B3C3D3 | 0 | 10% | 1.6 | 40% |
| G4-A2B1C2D3 | 20% | 6% | 1.4 | 40% |
| G5-A2B2C3D1 | 20% | 8% | 1.6 | 0 |
| G6-A2B3C1D2 | 20% | 10% | 1.2 | 20% |
| G7-A3B1C3D2 | 40% | 6% | 1.6 | 20% |
| G8-A3B2C1D3 | 40% | 8% | 1.2 | 40% |
| G9-A3B3C2D1 | 40% | 10% | 1.4 | 0 |
| Group | Factors | Flowability/(mm) | S/N Ratio | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| G1 | 1 | 1 | 1 | 1 | 226.5 | −47.10 |
| G2 | 1 | 2 | 2 | 2 | 217.5 | −46.75 |
| G3 | 1 | 3 | 3 | 3 | 197.0 | −45.89 |
| G4 | 2 | 1 | 2 | 3 | 172.5 | −44.74 |
| G5 | 2 | 2 | 3 | 1 | 267.5 | −48.55 |
| G6 | 2 | 3 | 1 | 2 | 208.5 | −46.38 |
| G7 | 3 | 1 | 3 | 2 | 275.0 | −48.79 |
| G8 | 3 | 2 | 1 | 3 | 216.0 | −46.69 |
| G9 | 3 | 3 | 2 | 1 | 279.5 | −48.93 |
| K1 | 641.00 | 674.00 | 651.00 | 773.50 | ||
| K2 | 648.50 | 701.00 | 669.50 | 701.00 | ||
| K3 | 770.50 | 685.00 | 739.50 | 585.50 | ||
| k1 | 213.67 | 224.67 | 217.00 | 257.83 | ||
| k2 | 216.17 | 233.67 | 223.17 | 233.67 | ||
| k3 | 256.83 | 228.33 | 246.50 | 195.17 | ||
| R | 43.17 | 9.00 | 29.50 | 62.67 | ||
| Factor order | D > A > C > B | |||||
| Superior level | A3 | B2 | C3 | D1 | ||
| Excellent combination | A3 B2 C3 D1 | |||||
| Indicator | 28 d Flexural Strength | 28 d Compressive Strength | ||||||
|---|---|---|---|---|---|---|---|---|
| factors | A | B | C | D | A | B | C | D |
| K1 | 24.84 | 23.84 | 22.99 | 23.38 | 197.98 | 181.88 | 208.78 | 208.29 |
| K2 | 22.75 | 23.92 | 21.30 | 21.21 | 205.08 | 196.25 | 191.44 | 197.38 |
| K3 | 18.91 | 18.75 | 22.21 | 21.91 | 188.59 | 213.51 | 191.43 | 185.98 |
| k1 | 8.28 | 7.95 | 7.66 | 7.79 | 65.99 | 60.63 | 69.59 | 69.43 |
| k2 | 7.58 | 7.97 | 7.10 | 7.07 | 68.36 | 65.42 | 63.81 | 65.79 |
| k3 | 6.30 | 6.25 | 7.40 | 7.30 | 62.86 | 71.17 | 63.81 | 61.99 |
| R | 1.98 | 1.72 | 0.57 | 0.72 | 5.50 | 10.55 | 5.78 | 7.44 |
| Factor order | A > B > D > C | B > D > C > A | ||||||
| Superior level | A1 | B2 | C1 | D1 | A2 | B3 | C1 | D1 |
| Excellent combination | A1 B2 C1 D1 | A2 B3 C1 D1 | ||||||
| Element (wt%) | O | Si | Ca | Na | Mg | Al | S | Ca/Si | Na/Si | Al/Si | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 d | Spectrum1 | 53.46 | 37.89 | 4.40 | 2.24 | 0.46 | 1.06 | 0.44 | 0.12 | 0.06 | 0.03 |
| Spectrum2 | 22.20 | 24.86 | 41.22 | 4.29 | 1.55 | 4.37 | 1.04 | 1.66 | 0.17 | 0.18 | |
| Spectrum3 | 22.94 | 21.34 | 40.01 | 1.26 | 5.11 | 6.40 | 2.07 | 1.87 | 0.06 | 0.30 | |
| Spectrum4 | 28.54 | 27.24 | 1.58 | 0.54 | 1.06 | 28.14 | 12.90 | 0.06 | 0.02 | 1.03 | |
| 30 d | Spectrum5 | 69.85 | 8.09 | 5.59 | 3.18 | 0.89 | 0.00 | 12.40 | 0.69 | 0.39 | 0.00 |
| Spectrum6 | 41.86 | 38.76 | 0.97 | 11.32 | 1.19 | 4.53 | 38.76 | 0.03 | 0.29 | 0.12 | |
| Spectrum7 | 37.89 | 29.88 | 0.34 | 0.24 | 1.22 | 19.78 | 0.53 | 0.01 | 0.01 | 0.66 | |
| Spectrum8 | 29.08 | 32.15 | 16.35 | 3.48 | 0.98 | 12.23 | 32.15 | 0.51 | 0.11 | 0.38 | |
| Spectrum9 | 35.07 | 31.56 | 1.43 | 2.23 | 0.93 | 25.32 | 0.98 | 0.05 | 0.07 | 0.80 | |
| Spectrum10 | 33.17 | 28.93 | 23.67 | 2.34 | 6.37 | 1.88 | 1.36 | 0.82 | 0.08 | 0.06 | |
| Spectrum11 | 34.23 | 10.98 | 8.13 | 7.13 | 0.63 | 2.90 | 20.26 | 0.74 | 0.65 | 0.26 | |
| 120 d | Spectrum12 | 46.56 | 20.60 | 21.69 | 3.31 | 2.34 | 3.63 | 1.58 | 1.05 | 0.16 | 0.18 |
| Spectrum13 | 35.70 | 23.17 | 26.18 | 4.28 | 1.92 | 5.10 | 3.10 | 1.13 | 0.18 | 0.22 | |
| Spectrum14 | 46.40 | 24.97 | 0.64 | 1.76 | 0.22 | 21.61 | 2.80 | 0.03 | 0.07 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Cheng, H.; Zhang, M.; Qin, Y.; Cao, J.; Cao, X. Alkali-Activated Slag–Fly Ash–Desert Sand Mortar for Building Applications: Flowability, Mechanical Properties, Sulfate Resistance, and Microstructural Analysis. Buildings 2025, 15, 2069. https://doi.org/10.3390/buildings15122069
Yan W, Cheng H, Zhang M, Qin Y, Cao J, Cao X. Alkali-Activated Slag–Fly Ash–Desert Sand Mortar for Building Applications: Flowability, Mechanical Properties, Sulfate Resistance, and Microstructural Analysis. Buildings. 2025; 15(12):2069. https://doi.org/10.3390/buildings15122069
Chicago/Turabian StyleYan, Wenlong, Haoran Cheng, Meng Zhang, Yongjun Qin, Jianqing Cao, and Xuyang Cao. 2025. "Alkali-Activated Slag–Fly Ash–Desert Sand Mortar for Building Applications: Flowability, Mechanical Properties, Sulfate Resistance, and Microstructural Analysis" Buildings 15, no. 12: 2069. https://doi.org/10.3390/buildings15122069
APA StyleYan, W., Cheng, H., Zhang, M., Qin, Y., Cao, J., & Cao, X. (2025). Alkali-Activated Slag–Fly Ash–Desert Sand Mortar for Building Applications: Flowability, Mechanical Properties, Sulfate Resistance, and Microstructural Analysis. Buildings, 15(12), 2069. https://doi.org/10.3390/buildings15122069







