Abstract
The protective materials for cultural relic buildings generally have a deficiency of relatively shallow penetration depth. Based on the principle of changing the permeability coefficient of cultural relic buildings by “water blocking water” and considering the characteristics of magnesium acrylate polymer and the requirement of extending the curing time, a method of modifying magnesium acrylate polymer with glycerol and sodium methyl silicate is proposed. Experimental studies on magnesium acrylate, glycerol–magnesium acrylate, and sodium methyl silicate—glycerol–magnesium acrylate polymers were carried out, and tests and analyses on curing time, swelling performance, water loss rate, and soil sample protection were conducted. The results show that the initiator concentration is a key factor affecting the curing rate of magnesium acrylate polymers. When the initiator content is ≥4%, the curing time is significantly shortened to 20–67 min, and the incorporation of glycerol prolongs the curing time by more than 100 min through the dilution reaction system. Glycerol modification significantly enhanced the swelling capacity of the polymer, with the swelling rate increasing by approximately 15–20% compared to the unmodified system. Sodium methyl silicate effectively improved the construction performance of magnesium acrylate and prevented the occurrence of bubbles. The optimal formula of magnesium acrylate polymer is 25% magnesium acrylate, 40% glycerol, and 2% sodium methyl silicate. While maintaining curing for 120 min, it features a high swelling rate (equilibrium swelling ratio Ew ≈ 0.32) and a low dehydration rate (dehydration rate ≤ 35% after 48 h), and has volume stability after interaction with soil samples.
1. Introduction
Groundwater erosion has become an important factor threatening the safety of earthen sites [1,2]. There is an urgent need to conduct in-depth research [3], build waterproof and water-stop curtains [4], lay waterproof and water-stop materials [5], implement vegetation control, and control the surrounding water usage. This study closely combines the actual needs of soil site protection and widely draws on various technologies and cutting-edge research ideas. Based on the capillary water absorption characteristics of soil itself, the water-blocking material—modified polymer—is adopted to introduce the water-blocking material into the soil, exploring effective methods to resist the damage to soil sites caused by the capillary rise of groundwater.
Many scholars have reinforced and protected the earthen sites by using different materials. Zhu Feiqing et al. [6,7,8] reinforced the soil around the Suoyang City site using Staphylococcus basiensis and urease and found that these two materials could undergo mineralization and improve the mechanical properties of the soil. Du Zhilin et al. [9] selected four materials to conduct experiments on soil sites in the Lintong area of Shaanxi Province and concluded that the M-CMC material reinforced the samples with good mechanical properties, and the SH material could improve the durability of the soil [8]. Zhu Jie et al. [10] took the Guangfulin Cultural Site in Shanghai as the object to study the reinforcement effect of ethyl silicate reinforcement materials in different proportions. They found that ethyl silicate could enhance the anti-powderization ability of soil and inhibit the growth of mold [11]. The effect was relatively the best when the proportion was 2:1. Wang Jia Kun et al. [12,13] conducted research on non-water dispersion materials of acrylic resin, improved the preparation process, explored the factors affecting the permeability performance, and found that this material mainly reinforces soil through film-forming bonding, providing a theoretical basis for the application of the material. Wang Youwei et al. [14] screened four types of alkyl reinforcing agents for the reinforcement of the Tanshishan Site in Fujian Province. The results showed that reinforcing agents with basic components such as long-chain alkyl groups and alkoxysiloxane small molecules had better effects and could be used for the reinforcement and protection of the site itself. Jianlin Luo et al. [15] A three-factor and three-level orthogonal test was adopted, and the test parameters for the repair performance of PVA fiber-reinforced waterborne epoxy resin cement mortar (PVA-FPCM) were optimized through mean and variance analysis. Through further process tests, the optimal process parameter combination for high-performance repair mortar was determined as P/C = 0.20, W/C = 0.35, and wPVA = 1%. Micaela Mercuri et al. [16] A systematic study was conducted on the newly developed lime-based mortar. Polyvinyl alcohol (PVA) fibers were used to reinforce the mortar, which was tested in a three-point bending configuration to calculate the flexural strength and fracture energy. The newly proposed mortar is an effective system for strengthening masonry structures. Yang Haijun et al. [17] conducted experiments on the soil of the Jinyang Ancient City Ruins using waterborne polyurethane polyacrylate materials and lime to study the properties of the soil under different ratios. The proposed ratio scheme of raw soil + lime (10:0.7) + 3%PUA has a better effect and can provide a reference for on-site reinforcement projects. At present, certain phased achievements have been made in the research on the protection and reinforcement materials for soil sites that prevent groundwater erosion. All kinds of inorganic, organic, and inorganic–organic composite reinforcement materials have demonstrated their respective advantages in different aspects. However, the penetration depth of these materials is limited. They only deform within the surface layer of the Earth’s crust under the stress caused by surface temperature, resulting in the “empty shell” effect.
Glycerol has a strong hygroscopicity and can be mixed with various solvents in any proportion, improving the stability and uniformity of the product [18,19]. Magnesium acrylate has excellent water resistance. The acrylic groups and magnesium ions in its molecular structure can form stable chemical bonds, which makes it have good stability in water and not easily dissolved or decomposed by water. It can be used to prepare various water-resistant coatings, waterproof materials, etc., to improve the waterproof performance and weather resistance of materials [20,21]. Under certain conditions, it can form a uniform and dense acrylic polymer film, which has good flexibility and adhesion, and can firmly adhere to the surface of objects, playing a protective and waterproof role. It has good resistance to many chemical substances and can resist the erosion of acids, alkalis, salts, and other chemical substances, protecting the surface of objects from damage by chemical substances. Therefore, experiments will be conducted to study the composites of these three materials to prevent the damage caused by the upward infiltration of groundwater.
Currently, most materials struggle to strike a balance between protective performance and material compatibility, resulting in irreversible damage to cultural relics. Moreover, traditional coating materials have insufficient durability and are unable to provide long-term, effective protection in complex environments. In this study, three materials, namely magnesium acrylate gel, glycerin, and sodium methyl silicate, were used to conduct various performance tests on repair materials such as magnesium acrylate gel, glycerin–magnesium acrylate gel, and sodium methyl silicate–glycerin–magnesium acrylate gel composites. These tests included measurements of curing time, swelling properties, water loss rate, etc., and repair tests and analyses were also carried out on these materials. Through the comparison and analysis of experimental results, the optimal polymer ratio for restoring cultural heritage buildings was determined. In practical applications, the modified magnesium acrylate gel polymer is injected into the interior of the heritage site from the bottom up through capillary rise. It forms a gel within the soil pores, effectively reducing the soil’s permeability coefficient, preventing groundwater from infiltrating upward into the upper part of the soil heritage site, and thus weakening the weathering process of the soil heritage. This polymer repair method not only enhances soil stability but also aims to prevent the diffusion of groundwater to the upper part of the soil heritage site, providing new materials and methods for the restoration and protection of cultural heritage buildings.
2. Experimental Principles and Testing Methods
2.1. Experimental Principle
- (1)
- Experimental materials: The main raw materials for the experiment include distilled water, acrylic acid, magnesium oxide, ammonium persulfate, triethanolamine, and glycerol. The acrylic acid manufacturer is Jinan Mingwei Chemical Co., Ltd. (Jinan, China), the magnesium oxide manufacturer is Tianjin Zhiyuan Chemical Reagent Co., Ltd. (Tianjin, China), the ammonium persulfate manufacturer is Tianjin Zhiyuan Chemical Reagent Co., Ltd., the triethanolamine manufacturer is Tianjin Zhonglian Chemical Reagent Co., Ltd. (Tianjin, China), and the glycerol manufacturer is Tianjin Zhonglian Chemical Reagent Co., Ltd.
- (2)
- Preparation principle: Based on the acid-base neutralization reaction, acrylic acid is mixed with MgO and reacted to prepare the magnesium acrylate monomer solution. The reaction formula is as follows:
MgO + 2CH2=CH-COOH → (CH2=CHCOO)2Mg + H2O
Mg(OH)2 + 2CH2=CH-COOH → (CH2=CHCOO)2Mg + 2H2O
- (3)
- Magnesium acrylate exhibits excellent water resistance. The acrylic groups and magnesium ions in its molecular structure can form stable chemical bonds, endowing it with good stability in water and making it less prone to dissolution or decomposition by water. It can be used to prepare various water-resistant coatings, waterproof materials, etc., enhancing the waterproof performance and weather resistance of materials.
2.2. Experimental Test Methods
- (1)
- Curing time test
The curing is determined by the hardness of the topmost surface of the liquid in the mold. Mix all the parts to make the magnesium acrylate solution evenly, pour it into the mold, and then start timing. The measurement continued until the glass rod gently touched the liquid surface and felt a gel-like solid, and the glass rod did not penetrate deeply into the interior of the liquid surface. The recorded time represents the non-adhesive curing time and indicates the curing rate. The experiment was carried out at room temperature. Three measurements were made for each group, and the average time was taken as the curing time.
- (2)
- Swelling performance test
The swelling performance of the polymer was tested by the weighing method. First, wash the original sample with ultrapure water, soak it, and change the water three times to remove surface residues, which is recorded as ; Secondly, immerse it in ultrapure water, dry the surface moisture regularly with dust-free filter paper, and then weigh it. This is recorded as . The swelling equilibrium is reached when the weight remains constant. The calculation formula of the swelling rate, , is shown in (3), and the calculation formula of the equilibrium swelling ratio, , is shown in (4):
In the formula: represents the mass of the initial sample (g), represents the mass of the sample at different times (g), and represents the mass of the sample at the swelling equilibrium (g).
- (3)
- Water loss rate test
The polymer is placed in a vacuum oven at 60 °C for constant temperature storage. The sample is taken out and weighed every 24 h, denoted as . The initial mass is recorded as . Then calculate the dehydration rate of the sample in each time period based on the mass of the sample and reflect its thermal stability through the dehydration rate. The calculation formula of dehydration rate is shown as Equation (5):
In the formula: represents the weight of the sample before heating (g), and represents the weight of the sample at a certain moment during heating (g).
- (4)
- Soil sample immersion test analysis
Ring knife soil samples were prepared through compaction experiments. Then, 10 mL of polymer was dropped into the samples to observe the reaction of the soil samples under the interaction of the polymer, so as to determine the stability under the interaction of the two. By observing the conditions of the soil sample after dripping, such as color changes, the initially determined permeability performance, and the volume stability of the soil sample.
3. Results and Discussion
3.1. Analysis of the Properties of Magnesium Acrylate Polymer
3.1.1. Preparation of Magnesium Acrylate Solution
This experiment mainly prepared magnesium acrylate solutions of different concentrations. The experimental process involves the mixing of liquids and the dissolution of solids. It is necessary to strictly control the reaction rate and conditions to prevent the occurrence of self-polymerization. First, 17.32 g, 21.65 g, and 25.98 g of acrylic acid, a certain amount of water, and polymerization inhibitor were added to the three-necked flask. Then, 4.84 g, 6.05 g, and 7.26 g of magnesium oxide powder were weighed, respectively. The addition should be completed within 30 min, and the reaction conditions should be controlled to prevent self-polymerization. The final obtained solutions of magnesium acrylate with different concentrations of 20%, 25%, and 30% are shown in Figure 1.
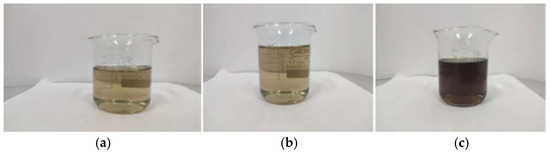
Figure 1.
Schematic diagrams of the appearance of solutions with different concentrations: (a) 20% magnesium acrylate solution; (b) 25% magnesium acrylate solution; (c) 30% magnesium acrylate solution.
In the pre-experiment, 20%, 25%, and 30% magnesium acrylate solutions were explored. It was found that the 20% concentration magnesium acrylate solution required a high-concentration initiator to trigger the reaction and a high-concentration accelerator to accelerate the reaction. The water polymer generated during curing was less, and there was a large amount of water in the upper layer. A 30% concentration of magnesium acrylate solution only requires a high concentration of initiator to initiate a reaction and solidify into a water polymer, which appears blackish-brown. In accordance with the requirement of “restoring the old to its original state” for cultural relics restoration, this experiment demands the generation of colorless or light-colored water polymers. The hydrogel formed by a 25% magnesium acrylate solution is colorless and meets the requirements of this experiment.
3.1.2. Curing Time Analysis
The key to polymer restoration of cultural relic buildings lies in the depth to which the polymer penetrates the cultural relic itself. According to Darcy’s Law, the penetration depth is not only related to the material of the cultural relic itself and the pressure gradient of the polymer, but also closely related to the time of polymer penetration. Therefore, developing polymers with a longer curing time is an important indicator to ensure the quality of cultural relic restoration. It is necessary to further explore the influence of the initiator concentration of 25% magnesium acrylate solution in different ratios on the solidification time.
To explore the curing time of magnesium acrylate polymers, when the dosages of crosslinking agent and reducing agent are determined, an increase in the dosage of initiator shortens the curing time. This is because an increase in the dosage of initiator generates more active types of free radicals, which enhances the polymerization reaction rate and shortens the curing time. When the initiator dosage exceeds 4%, this promoting effect is very obvious. However, when its content is less than 4%, the curing time is longer, and the curing effect is poor. As shown in Figure 2, the initiator content is 1–6%. When the initiator content is 1%, 2%, and 3%, the magnesium acrylate polymer shows incomplete curing, with a layer of uncured magnesium acrylate solution on the surface. It indicates that the curing effect becomes worse as the content of the initiator decreases. It can be seen from Table 1 that when the amount of initiator is controlled above 4%, it is best for the polymer to cure and form well. The curing time shortens as the content of the initiator increases. The specific experimental plan is shown in Table 1.
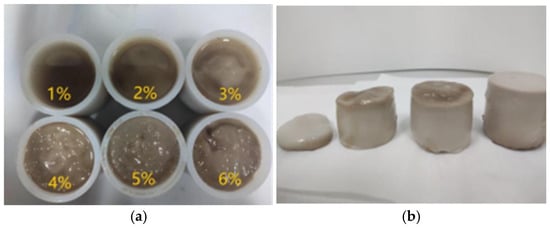
Figure 2.
Fully cured and partially cured polymers. (a) Polymer curing and molding; (b) polymer solidified body.

Table 1.
Experimental Scheme Table.
3.1.3. Analysis Swelling Performance Analysis
The swelling properties of six groups of magnesium acrylate polymers with different initiator ratios were tested. The changes in volume and mass within seven days were tested. Three parallel samples were set for each group of samples and placed in lattice containers, and deionized water was added for soaking. During the swelling process, take out the swollen magnesium acrylate polymer sample at the predetermined time interval of 24 h, gently absorb the deionized water on the surface of the sample with filter paper (be careful not to squeeze the sample to avoid affecting the measurement of the swelling degree), then quickly weigh the mass of the swollen sample with an electronic balance, record the data, and put the sample back into the grid container to continue the swelling experiment.
As shown in Figure 3a, the mass changes in magnesium acrylate polymers with six different initiator ratios Z1–Z6, after water absorption within 1–7 days were studied and observed. From the perspective of the curve slope, the curve slope of magnesium acrylate polymer in the first two days is significantly greater than that at other times, indicating that it has a relatively fast speed in the initial stage of water absorption and can absorb a large amount of water. The slopes of the curves of materials Z1, Z3, and Z4 are relatively close, and the water absorption rates are relatively moderate. The curve slope of Z2 material is the smallest, indicating that its water absorption process is slow. As time increases, the slopes of the curves of each material gradually decrease, indicating that the water absorption rate gradually slows down and tends to stabilize. The swelling curve should be able to clearly show the changing trend of the swelling process of the sample over time. At the beginning stage of the curve, the swelling degree increases rapidly over time, which is due to the rapid diffusion of solvent molecules into the interior of the sample. As time goes by, the swelling rate gradually slows down. When the swelling equilibrium is reached, the degree of swelling no longer changes with time.
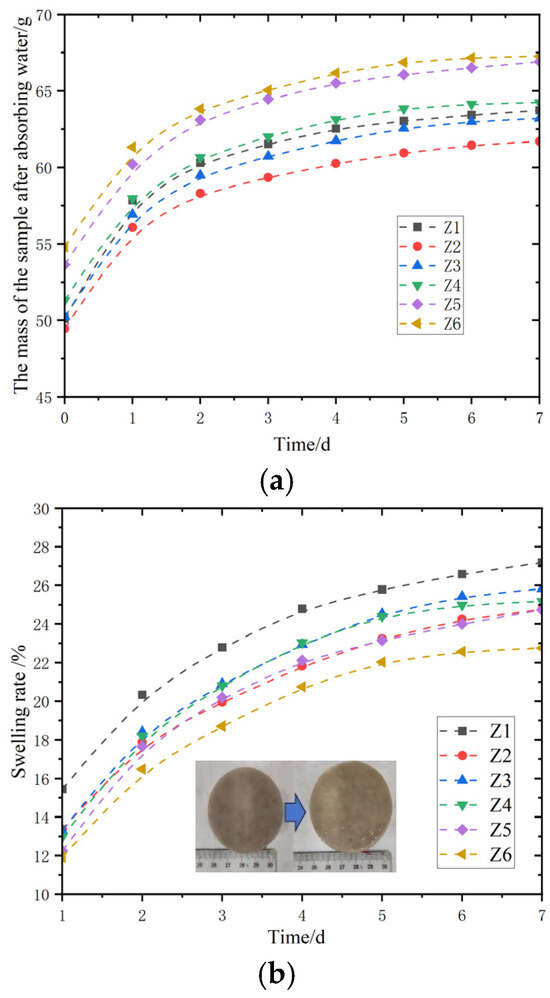
Figure 3.
Swelling test of magnesium acrylate gel. (a) Mass change graph; (b) variation of swelling rate.
Figure 3b shows the changes in the swelling rate of the same material within the corresponding time. Among them, the curve slope of the magnesium acrylate polymer was prominent in the first three days, indicating that its swelling rate rose rapidly in the initial stage of swelling, showing a strong swelling ability. In the later stage of the experiment, the slope of the material swelling rate curve decreased, indicating that the swelling rate slowed down and the swelling of the magnesium acrylate polymer basically reached the saturated state.
It can be seen that the differences in water absorption and swelling characteristics of magnesium acrylate polymers with different initiator ratios are not obvious. The change in the slope of the curve directly reflects the dynamic characteristics of these materials during the water absorption and swelling process. The water absorption and swelling characteristics of the polymer are determined by the properties of the magnesium acrylate polymer itself. Initiators of different dosages did not have a significant impact on the swelling performance of magnesium acrylate polymers.
3.1.4. Analysis of Water Loss Rate
To study the water retention performance and drying characteristics of the material, it is necessary to accurately determine the water loss of the sample under specific conditions. The applicability and stability of the material in different environments are evaluated by calculating the water loss rate.
The water loss performance of six groups of magnesium acrylate polymers with different initiator ratios was tested, and the mass change at a temperature of 60 °C within 48 h was measured. Place the Petri dishes containing the samples in a drying oven at a room temperature of 60 °C for drying. Take out the samples at certain time intervals (such as every 6 h) and then weigh their mass with an electronic balance.
Figure 4a shows the variation of the mass of the samples after drying over time. Within the initial 6 h, the absolute values of the slopes of each curve are relatively large. The mass of the six samples, Z1 to Z6, decreased rapidly in the early stage of drying. During the 6–24 h period in the middle stage, the absolute value of the curve slope gradually decreases, the rate of sample mass decline slows down, the slope differences among different samples narrow, and the drying process enters a relatively stable deceleration stage. After 24 h in the later stage, the slopes of each curve approach 0, indicating that the quality of the sample tends to stabilize after drying and no longer shows obvious changes.
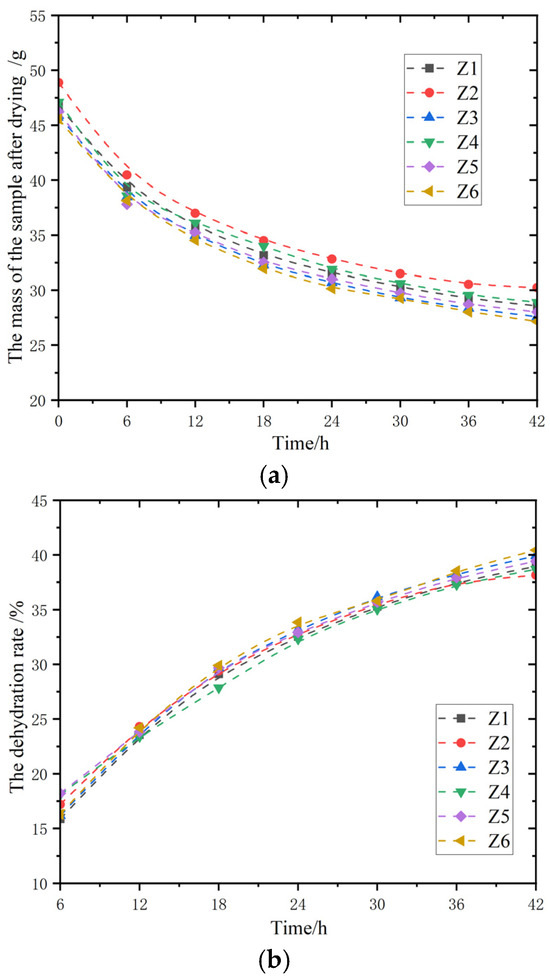
Figure 4.
Dehydration test of magnesium acrylate gel. (a) The curves of water loss mass change in the samples; and (b) the curves of the dehydration rate change in the samples.
Figure 4b shows the variation of dehydration rate with time. Within the initial stage of 6 h, the slopes of each curve are small and relatively close, indicating that the dehydration rate of the six samples Z1 to Z6 increases relatively slowly and the rate differences are not significant during this period. During the intermediate stage from 6 to 24 h, the slope of the curve increased and remained similar, indicating that the growth rate of the dehydration rate accelerated, and the dehydration rates of different samples increased basically simultaneously. After 24 h in the later stage, the slopes of each curve decreased but were still greater than those in the initial stage, indicating that the dehydration rate was still rising, but the growth rate slowed down.
Both the mass change and the dehydration rate reflect the loss of moisture during the drying process, but the dehydration rate, compared with the mass change, better reflects the degree of drying. The mass change shows the remaining mass of the sample after drying, while the dehydration rate indicates the proportion of water removed, which can more intuitively reflect the dryness degree of the sample relative to its initial state, facilitating the comparison of drying effects among samples of different initial masses. By analyzing the change in dehydration rate over time, the efficiency of water loss per unit time can be seen more clearly. At the initial stage of drying, if the dehydration rate rises rapidly, it indicates that the efficiency of moisture loss is high. If the growth rate slows down in the later stage, it indicates that the difficulty of water loss increases. The changing trend of the dehydration rate can reflect the binding state of water in the sample to a certain extent. The dehydration rate increases slowly in the final stage, meaning that this part of the water is closely bound to the sample and is difficult to remove.
During the drying process, there are numerous influencing factors, mainly including temperature, humidity, air flow rate, sample characteristics, and drying equipment, etc. Temperature is the key factor affecting the drying process. An increase in temperature provides more energy for water evaporation, accelerates the thermal motion of water molecules, makes it easier for water to move from the polymer surface and diffuse into the surrounding environment, thereby significantly accelerating the drying speed. In the early stage of drying, a higher temperature can cause the moisture on the surface of the polymer to evaporate rapidly, and the drying rate will increase rapidly. As the drying process proceeds, the speed at which the internal moisture of the polymer migrates to the surface gradually becomes a limiting factor, and the enhancing effect of temperature on the drying rate will gradually weaken. The pore structure, specific surface area, and chemical composition of magnesium acrylate polymers can affect the migration of water within the polymer and the evaporation on its surface.
3.1.5. Testing and Analysis of Polymer Soil Samples
Whether the penetration process of magnesium acrylate solution causes damage to cultural relics, such as site soil, is an important indicator for evaluating the quality of polymers. Therefore, the main purpose of this experiment is to verify whether the magnesium acrylate solution causes expansive damage to the soil structure. Silty clay was used as the preparation soil sample, and the density and porosity were basically kept consistent. Deionized water was used as the control group, and magnesium acrylate solution was used as the experimental group. The experimental operation process is to drop the solution evenly onto the surface of the soil block (5 drops each time, with an interval of 5 min), observe, and record the experimental phenomena. In the experimental group, the magnesium acrylate solution selected a ratio with better swelling and water loss rate performance. The initiator content was 5%, and three parallel samples were set up to reduce random errors. Only deionized water was used as the benchmark for comparative tests to eliminate the interference of the soil’s own characteristics. The result is shown in Figure 5.
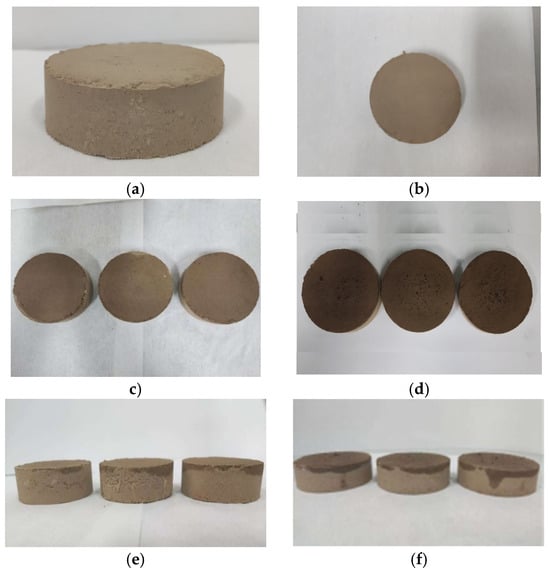
Figure 5.
Comparison of drip and drip polymer samples. (a) The initial front view of the sample; (b) The initial top view of the sample; (c) Top view of the water drop sample; (d) Top view of the polymer drop sample; (e) Front view of the water drop sample; (f) Front view of the polymer drop sample.
As shown in Figure 5d, deionized water and magnesium acrylate solution were, respectively, dropped on the samples. It was found that the surface of the sample with magnesium acrylate solution added had small holes, and compared with the sample with deionized water added, the surface of the soil sample later showed phenomena of expansion, bubbles, and a honeycomb structure.
The main reasons for this are:
- (1)
- Magnesium acrylate solution is weakly acidic (acrylic acid is an organic acid, approximately pH = 6.8), and it will undergo soil carbonate reaction. If the soil contains calcium carbonate (commonly found in alkaline soil), the acid reacts with the carbonate to produce carbon dioxide gas, and the release of the gas causes bubbles to appear on the surface of the soil sample [22].
- (2)
- Precipitation and structural remodeling produce a honeycomb structure, generating insoluble salts. If calcium ions are present in the soil, the magnesium acrylate solution may react with Ca2+ to form slightly soluble calcium acrylate, which precipitates in the pores of the soil sample to form a network structure, supporting the honeycomb surface [23,24].
- (3)
- Compared with the experiment with deionized water, the osmotic pressure of the magnesium acrylate solution is higher due to the influence of high-concentration solutions, which may promote the migration of water on the surface of the soil sample, intensify expansion, and form a porous structure [25].
3.1.6. Summary
The dual role of initiator concentration: when the concentration is ≥4%, the curing effect is significantly optimized without significant impact on the swelling performance; when the concentration is <4%, the curing is insufficient. Acrylic acid magnesium gels with different initiator ratios exhibit similar trends during the swelling process. Dynamic characteristics: In the initial stage (first 2–3 days), the swelling rate is rapid as solvent molecules quickly penetrate into the gel interior; the rate then gradually slows down until swelling equilibrium is finally reached. The initiator concentration has no significant effect on the water absorption and swelling characteristics of the gel, as the swelling performance is mainly determined by the inherent properties of the acrylic acid magnesium gel itself, with no obvious differences in swelling behavior observed across different initiator dosages. Under a 60 °C drying condition, the dehydration process of the acrylic acid magnesium gel can be divided into three stages: Initial stage (0–6 h): The sample mass decreases rapidly, but the dehydration rate grows slowly with little difference between samples of different ratios. Middle stage (6–24 h): The mass decline rate slows down, while the dehydration rate increases rapidly, and the dehydration rates of different samples tend to synchronize. Later stage (after 24 h): The mass is basically stable, and the dehydration rate still rises slowly, indicating that some water is tightly bound to the gel and difficult to remove. Acrylic acid magnesium solution can cause expansion damage to the soil surface through chemical action, with a significant effect upon first contact. This issue requires further research and resolution in subsequent studies.
3.2. Performance Analysis of 4-Glycerol-Modified Magnesium Acrylate Polymer
3.2.1. Curing Time Analysis
From the experiments of pure acrylic polymers, it can be known that the solidification time of 25% magnesium acrylate solution under different initiator concentrations is different. Then, according to the needs of the subsequent experiments, the solidification time is controlled at about 40 min, and the initiator concentration is selected at 5%. Study the influence of glycerol content on the solidification time and solidification effect of magnesium acrylate polymers. The specific experimental plan is shown in Table 2.

Table 2.
Experimental scheme of glycerol-modified acrylic polymer.
After adding different amounts of glycerol, both the curing time and curing effect of magnesium acrylate polymer changed. The addition of glycerol prolonged the curing time of magnesium acrylate polymer. With the increase in glycerol content, the curing time also increased. The additional amount of glycerol also directly affected the curing of magnesium acrylate polymer. As shown in Figure 6 and Figure 7, when the glycerol content exceeds 55%, the magnesium acrylate polymer undergoes incomplete curing. After 6 h, it basically stops curing, with a layer of transparent liquid on the surface and curing at the bottom. The overall mass of the sample after demolding is relatively small, and it is relatively soft after curing. Based on the analysis of its curing time and effect, in the subsequent experiments, the dosage of glycerol should be controlled below 55% to avoid the negative impact of excessive glycerol incorporation on the curing time and curing effect of the magnesium acrylate polymer.

Figure 6.
Curing experiment of Pre-test samples.
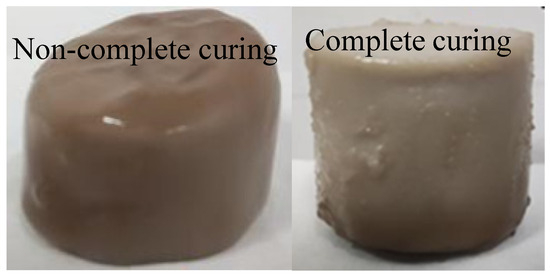
Figure 7.
Curing shapes of different specimens.
3.2.2. Swelling Performance Analysis
Magnesium acrylate polymers with an initiator concentration of 5% were subjected to 7-day swelling performance tests for six groups of glycerol-modified magnesium acrylate polymers with different content ratios when different contents of glycerol were added. Similarly, three parallel samples were set for each group.
In Figure 8a, the change in the mass of the sample after absorbing water over time, that is, the water absorption process of six different glycerol contents of magnesium acrylate polymers Y1–Y6. At the beginning of the experiment, the slope of the curve is the largest, indicating that the mass increases after absorbing water per unit time is the greatest, that is, the water absorption rate is the fastest. As time goes on, the slopes of the curves of glycerol-modified magnesium acrylate polymers gradually decrease. In the later stage of the experiment, although each group of materials was still absorbing water, the rate was slowing down, and the slope was relatively small. Observe Figure 8b. At the initial stage of swelling, the slope of the Y3 curve is significantly greater than that of other curves, indicating that the swelling rate of the glycerol-modified magnesium acrylate polymer is extremely fast. The molecular structure rapidly expands or spreads out after absorbing water, resulting in a rapid increase in the swelling rate. Subsequently, the slope of the curve is relatively small, and its swelling process is rather slow. In the middle and later stages of the experiment, the slope differences of most material curves gradually narrowed, indicating that the swelling rate tended to stabilize.
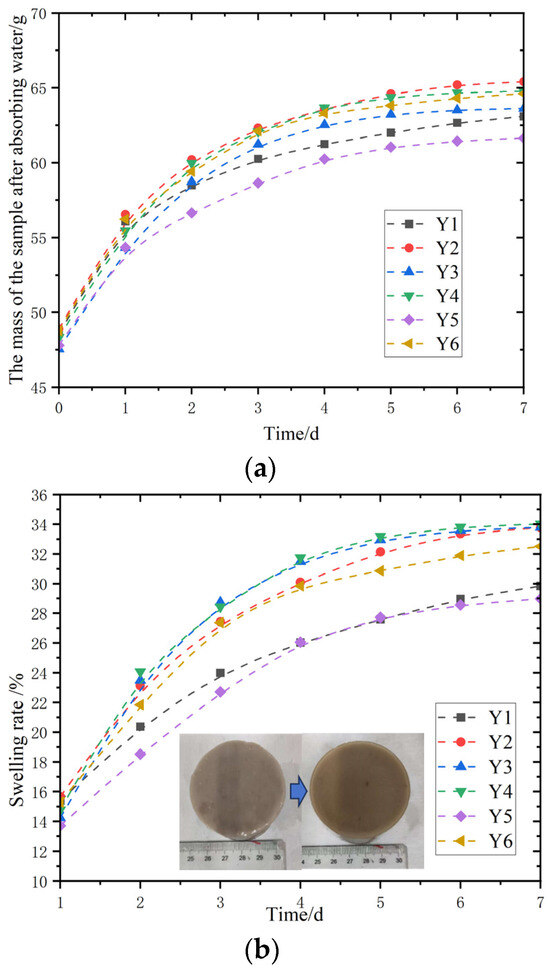
Figure 8.
Swelling test of glycerol-modified gel. (a) Mass change curves; (b) curves of swelling rate change.
Thus, through the analysis results, it is concluded that adding glycerol to the magnesium acrylate polymer for modification will increase the swelling rate of the magnesium acrylate polymer, increase the mass of water absorption, and also improve the initial water absorption rate. The influence of glycerol dosage alone on the swelling performance of magnesium acrylate polymers is not very obvious.
3.2.3. Analysis of Water Loss Rate
The water loss performance of magnesium acrylate polymers under six different glycerol dosage ratios was tested, and the mass change at a temperature of 60 °C within 48 h was also tested. The sample is placed in a drying oven at a room temperature of 60 °C for drying. The sample is taken out at certain time intervals (such as every 6 h), and then its mass is measured with an electronic balance.
The horizontal axis of Figure 9a represents time (h), and the vertical axis represents the mass of the sample after drying (g). The variation trends of the mass of the six different samples Y1–Y6 after drying with time show that as time increases, the mass of each sample gradually decreases after drying, and the decline is relatively large in the initial stage and tends to be stable in the later stage. Figure 9b shows that the horizontal axis also represents time (h), and the vertical axis represents the dehydration rate. The changes in the dehydration rates of the six samples Y1–Y6, over time. As time goes by, the dehydration rates of each sample gradually increase, and the rate is relatively fast in the early stage and gradually stabilizes in the later stage. The growth trends of the dehydration rates of different samples are slightly different.
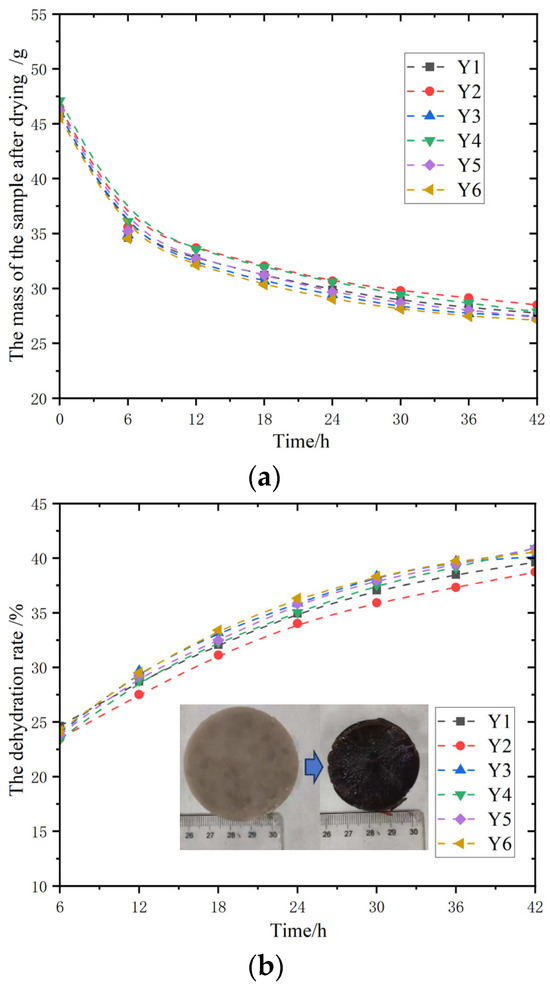
Figure 9.
Dehydration test of glycerol-modified gel. (a) The curves of water loss mass change in the samples; (b) the curves of the dehydration rate change in the samples.
From this, it can be concluded that the addition of glycerol has an impact on the water loss rate of magnesium acrylate polymers, which is related to the properties of glycerol itself. Glycerol appears as a clear and viscous liquid, can be mixed with water in any proportion, has strong hygroscopicity, and can absorb moisture from the air. When making glycerol-modified magnesium acrylate polymers, it makes the polymers have better water retention. Therefore, when conducting the water loss performance test, the glycerol-modified magnesium acrylate polymer will lose most of its water in the initial stage, and the quality will decline rapidly in the early stage. As shown in the picture, the slope of the decline is relatively large.
3.2.4. Testing and Analysis of Soil Samples Modified with Glycerol Polymer
Combining the swelling and water loss rate performance tests of six groups of magnesium acrylate polymers with different glycerol contents, it was determined that the modified magnesium acrylate polymer with a glycerol content of 40% was used for soil sample immersion tests. The experimental operation procedure involves uniformly dropping the modified magnesium acrylate polymer solution onto the surface of the soil block (five drops each time, with an interval of 5 min). The initiator content is 5%. Three parallel samples are set up to reduce random errors. The experimental phenomena are observed and recorded, as shown in Figure 10.

Figure 10.
Comparison of the surfaces of the polymer samples with water drops and glycerol drops. (a) Samples with water drops; (b) samples with glycerol drops.
As shown in Figure 10, compared with the single magnesium acrylate solution, the modified magnesium acrylate polymer did not show obvious expansion or honeycomb formation, indicating that the incorporation of glycerol changed the workability of the solution and made it more compatible with soil samples. The analysis of the possible reasons might be:
- (1)
- The complexation effect of glycerol on metal ions [26], the polyhydroxyl structure of glycerol (glycerol) can form stable polymers with Mg2+ (such as [Mg(glycerol)]2+), significantly reducing the concentration of free Mg2+ in the solution. The exchange between Mg2+ and surface cations (such as Ca2+ and Na+) of clay minerals has been reduced, thereby weakening the driving force for the water absorption and expansion of soil samples.
- (2)
- Glycerol’s inhibition of acid-base reactions [27,28]: Glycerol is a neutral substance. After addition, it may dilute the acidity of the magnesium acrylate solution, reduce the intensity of the reaction with soil carbonates (such as CaCO3), and decrease the release of CO2 gas.
- (3)
- Changes in the physical properties of the solution [29,30]: The high viscosity of glycerol (approximately 945 mPa·s) reduces the fluidity of the solution, hindering the rapid release and diffusion of CO2 bubbles, resulting in a reduction or even disappearance of the bubbles. Glycerol reduces the surface tension of the solution, making bubbles more prone to rupture and difficult to form a stable honeycomb structure on the surface of the soil sample.
This phenomenon indicates that glycerol can regulate the reaction between magnesium acrylate and soil through multiple mechanisms such as chemical complexation, physical dilution, and interfacial interaction, providing new ideas for soil treatment or material modification.
3.2.5. Summary
When the initiator concentration is 2%, the swelling behavior of glycerol-modified magnesium acrylate gel is a dynamic process: the water absorption rate is rapid in the early stage, slows down over time, and eventually stabilizes. Glycerol dosage enhances the swelling rate, but the difference between different dosages is not significant, indicating that glycerol can improve the hydrophilicity of the gel but is not a decisive factor for swelling performance. Under 60 °C drying conditions, the dehydration process of the glycerol-modified gel shows rapid mass loss and a high dehydration rate in the early stage (0–6 h), followed by a gentle trend in the later stage. The higher the glycerol dosage, the stronger the water retention capacity of the gel. The polyhydroxy structure of glycerol locks water through physical adsorption and chemical complexation, causing loosely bound water to dissipate rapidly in the early stage of dehydration, while tightly bound water is difficult to remove in the later stage, leading to a slowdown in the rate of dehydration. Compared with pure magnesium acrylate solution, the modified gel does not exhibit obvious expansion, bubbles, or honeycomb structures after being dropped into soil samples, indicating that glycerol can improve the compatibility between the solution and soil. Glycerol dosage has a bidirectional effect: when the dosage is ≤55%, it can regulate the gel forming speed by prolonging the curing time, but a balance with the curing effect is required; when the dosage is ≥40%, it significantly enhances the water retention and swelling rate of the gel, but the impact on swelling performance is limited.
3.3. Performance Analysis of Composite Modified Magnesium Acrylate Polymer
3.3.1. Curing Time Analysis
Based on the solidification time, solidification effect, improvement of the polymer’s own properties, and the influence on the volume stability when immersed in soil samples of 25% concentration magnesium acrylate solution under different glycerol concentrations, the glycerol ratio with the best performance after improvement was selected. The final step was to study the influence of adding different contents of sodium methyl silicate solution on the curing performance of the modified magnesium acrylate polymer. First, determine the influence of adding sodium methyl silicate solution with different contents on the curing time. The specific experimental plan is shown in Table 3.

Table 3.
Experimental scheme for composite modified magnesium acrylate polymer.
Through experiments on adding different contents of sodium methyl silicate solution to the modified magnesium acrylate polymer, it was obtained that the curing time of the original modified magnesium acrylate polymer did not change with the increase in the content of sodium methyl silicate solution. However, when the content of sodium methyl silicate solution added to the modified magnesium acrylate polymer reaches 5%, a trace amount of white precipitate will occur at the bottom.
The reasons for the formation of white precipitate when sodium methyl silicate is added to magnesium acrylate solution mainly involve chemical reactions and the properties of substances. The possible reasons for the formation of precipitate are analyzed as follows:
- (1)
- Magnesium acrylate is a weak acid salt. In aqueous solution, it undergoes a certain degree of hydrolysis, giving the solution a certain degree of alkalinity. Sodium methyl silicate will ionize into silicate ions and so on in water. When magnesium acrylate solution is mixed with sodium methyl silicate, magnesium ions (Mg2+) react with silicate ions (SiO32−) to form magnesium silicate (MgSiO3).
Its chemical reaction equation can be expressed as:
Mg(OH)2 + 2CH2=CH-COOH → (CH2=CH-COO)2Mg + 2H2O
Magnesium silicate is a poorly soluble substance that will precipitate from the solution, thus forming a white precipitate.
- (2)
- Sodium methyl silicate has a certain surface activity effect. When added to magnesium acrylate solution, it may change the surface tension and colloidal properties of the solution. Magnesium acrylate solution may have certain colloidal properties. The addition of sodium methyl silicate may disrupt the stability of this colloid, causing some of its components to coagulate or precipitate.
- (3)
- From the perspective of ionic reactions, the ion concentration in the solution and the interactions between ions have changed. After adding sodium methyl silicate, the types and concentrations of ions in the solution change, causing the ion product of magnesium ions and silicate ions to exceed the solubility product constant of magnesium silicate, thereby promoting the precipitation of magnesium silicate.
3.3.2. Swelling Performance Analysis
Magnesium acrylate polymer with an initiator concentration of 5% was mixed with 40% glycerol. Six groups of modified magnesium acrylate polymer composites with different contents of sodium methyl silicate solution ratios were subjected to 7-day swelling performance tests. Similarly, three parallel samples were set for each group. The mass changes over seven days of swelling were obtained, as shown in Figure 11.
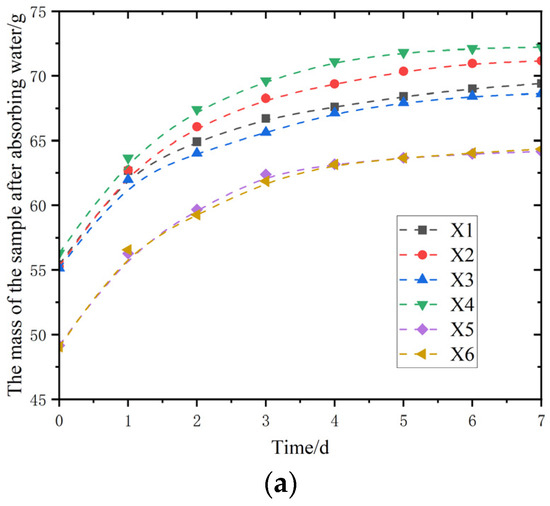
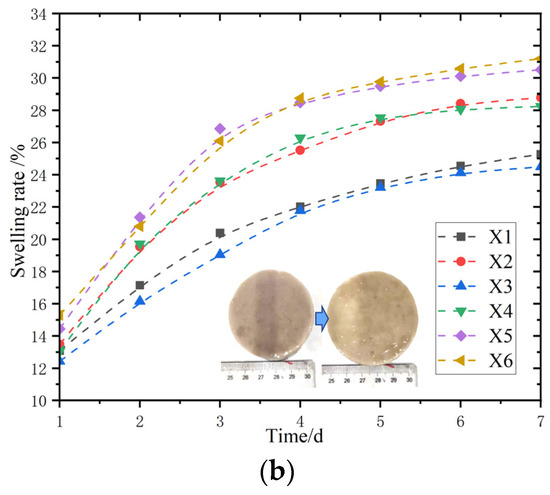
Figure 11.
Swelling test of modified polymer. (a) Mass change curve; (b) curves of swelling rate change.
As shown in Figure 11a,b, the mass changes and swelling rate changes in different samples (X1–X6) after water absorption over a period of time are presented, respectively. Figure 11 shows the mass change in the samples after absorbing water. As the time extends from 0 days to 7 days, each sample shows a trend of increasing mass after absorbing water, and the slope represents the water absorption rate of the samples. The slope of the curve is relatively large in the early stage and remains at a high level all the time, indicating that it can absorb water rapidly in the early stage of the experiment and maintain a relatively fast water absorption rate continuously. The material has a strong affinity for water or has a microstructure that is conducive to the rapid entry of water. As time goes by, the slopes of each curve gradually decrease and approach a flat state, indicating that the water absorption process gradually reaches saturation. The slope of the swelling rate change curve in Figure 11 reflects the swelling rate of the sample. By comparing the swelling result change graphs of acrylic polymers and glycerol acrylic polymers, it was found that the incorporation of sodium methyl silicate solution had no effect on the swelling performance of the modified polymer composites.
3.3.3. Analysis of Water Loss Rate
The water loss performance of the modified magnesium acrylate polymer under six different ratios of sodium methyl silicate content was tested, and the mass change at a temperature of 60 °C within 48 h was also tested. The sample is placed in a drying oven at a room temperature of 60 °C for drying. The sample is taken out at certain time intervals (such as every 6 h), and then its mass is measured with an electronic balance.
Figure 12a shows the mass–time relationship graph of the samples after drying. Within the initial 6 h, the absolute values of the slopes of each curve are relatively large, indicating that in the initial stage of drying, the mass of all samples decreases at a relatively fast rate, and during the initial stage of drying, moisture rapidly detaches from the samples. During the intermediate stage of 6–24 h, the absolute values of the slopes of each curve decreased, indicating that the rate of decline in the sample mass slowed down. For example, in the X1 curve, the slope changes significantly, indicating that the rate at which moisture detaches from the sample slows down at this time, and the sample gradually enters a slow drying process. After 24 h in the later stage, the slopes of each curve tend to level off and approach 0, indicating that the sample quality tends to stabilize, the moisture content basically no longer changes, and the drying process is approaching the end.
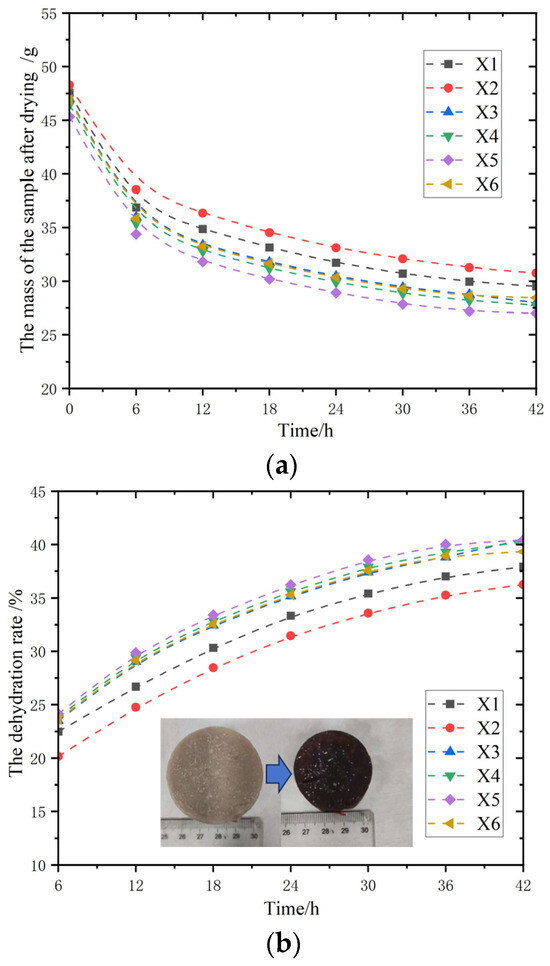
Figure 12.
Dehydration test of modified polymer. (a) The curves of water loss mass change in the samples; (b) the curves of the dehydration rate change in the samples.
Figure 12b shows the dehydration rate—time relationship graph. In the initial stage, the large slope of each curve indicates that the dehydration rate of the sample is rapid, and a large amount of water is removed during this period. In the middle stage, the slope of most curves slightly decreases, and the growth rate of dehydration slows down, suggesting that a large amount of water is still being removed at this stage. In the final stage, the slope of the curve becomes smaller, the dehydration rate slows down, and the dehydration rate tends to stabilize, meaning that the sample gradually reaches a saturated state of dehydration, and it is difficult to remove a large amount of water anymore.
3.3.4. Testing and Analysis of Soil Samples Modified with Composite Polymer
After the above multiple sets of experimental tests, it was finally explored whether the addition of sodium methyl silicate solution would change the original properties. It is known that the addition of sodium methyl silicate solution did not cause any change in the properties of the modified magnesium acrylate polymer. Therefore, the soil sample immersion test was conducted on the composite magnesium acrylate polymer with a glycerol content of 40% and a sodium methyl silicate solution content of 2%. The experimental operation procedure involves uniformly dropping the composite magnesium acrylate polymer solution onto the surface of the soil block (five drops each time, with an interval of 5 min). The initiator content is 5%. Three parallel samples are set up to reduce random errors. Observe and record the experimental phenomena [31,32].
As shown in Figure 13, the composite magnesium acrylate polymer did not show obvious expansion or honeycomb formation, indicating that the addition of sodium methyl silicate solution improved the compatibility of the polymer with soil samples and enhanced the water-blocking capacity of the polymer-modified soil samples.
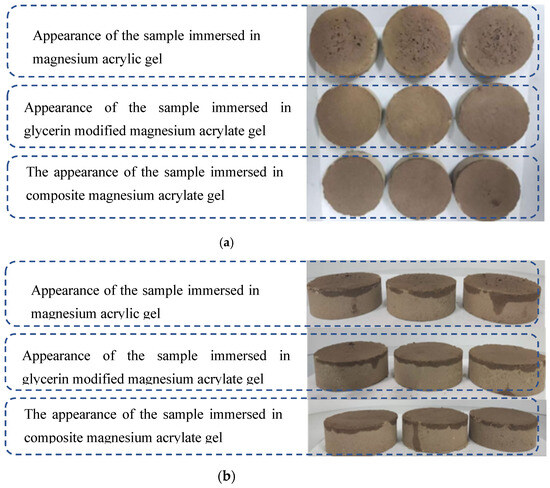
Figure 13.
The appearance conditions of the samples treated with three polymers. (a) Frontal display comparison; (b) side view comparison.
3.3.5. Summary
When the initiator concentration is 5% and the glycerol dosage is 40%, the swelling process of sodium methyl silicate-modified gel exhibits rapid water absorption at the initial stage, which gradually slows down and tends to equilibrium over time, consistent with the trend of pure glycerol-modified gel. Swelling rates and water absorption masses show no significant differences across different sodium methyl silicate dosages, indicating that it does not significantly alter the gel’s hydrophilicity or microstructure. Under 60 °C drying conditions, the dehydration process of sodium methyl silicate-modified gel shares similar characteristics with glycerol-modified gel. The effect of sodium methyl silicate is limited: At dosages ≤ 4%, it has no significant impact on curing time, swelling performance, or dehydration rate, and no precipitation occurs. At dosages ≥ 5%, chemical precipitation reactions cause gel heterogeneity, necessitating avoidance of high dosages. The composite-modified gel polymer demonstrates synergistic advantages: Glycerol (40%) primarily improves soil compatibility (inhibiting expansion) and water retention. Sodium methyl silicate (≤2%) auxiliary enhances water resistance without interfering with primary properties, achieving multiple effects of “anti-expansion + water retention + stability”.
4. Conclusions
In this study, through experiments on curing time, swelling performance, water loss rate, and soil sample immersion, the effects of glycerol and sodium methyl silicate composite modification on the properties of magnesium acrylate polymers were systematically explored. The main conclusions are as follows:
- (1)
- The concentration of the initiator is a key factor affecting the curing rate of magnesium acrylate polymers. When the initiator content is ≥4%, the curing time is significantly shortened to 20–67 min, and the curing effect is stable. The incorporation of glycerol prolonged the curing time (100–140 min) by diluting the reaction system, but the dosage should be controlled at ≤55% to avoid incomplete curing. The addition of sodium methyl silicate does not change the curing time, but an excess (≥5%) will cause magnesium silicate precipitation, affecting the stability of the system.
- (2)
- Glycerol modification significantly enhanced the swelling capacity of the polymer, with the swelling rate increasing by approximately 15% to 20% compared to the unmodified system, and the initial water absorption rate also accelerated. The introduction of sodium methyl silicate did not have a significant effect on the swelling performance, but the high hygroscopicity of glycerol enhanced the water retention capacity of the composite polymer at 60 °C, reducing the dehydration rate by approximately 10–15%.
- (3)
- The composite modification of glycerol and sodium methyl silicate achieves complementary performance: Glycerol improves the flexibility and water retention of the polymer, while sodium methyl silicate enhances the durability of the water-blocking barrier. The optimal formula of magnesium acrylate polymer was obtained: 25% magnesium acrylate + 40% glycerol + 2% sodium methyl silicate. While maintaining curing for 120 min, it has a high swelling rate (equilibrium swelling ratio Ew ≈ 0.32) and a low dehydration rate (dehydration rate ≤ 35% after 48 h), and has volume stability after interaction with soil samples.
- (4)
- Polymers with excellent volume stability can form stable complexes with soil samples, preventing soil expansion and contraction caused by environmental humidity and temperature changes. Such materials reduce the need for repeated repairs, eliminating the requirement for frequent maintenance and avoiding secondary interventions on cultural relics. For non-renewable cultural heritage resources, this directly reflects the “minimum intervention” protection principle. Volume stability is often associated with a material’s environmental adaptability. Stable polymers can prevent soil disintegration caused by salt migration while avoiding the release of harmful substances due to water absorption and swelling, achieving the dual goals of “protection-compatibility”.
Author Contributions
This article is the result of the joint efforts of all authors, and I will explain and thank them for their contributions. The corresponding author J.Y. is responsible for ensuring the accuracy of the description and obtaining the consent of all authors. T.H. and X.W. participated in the experimental operation, data processing, and analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Henan Provincial Department of Science and Technology] grant number [221111320200-01].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We acknowledge the contributions of our research assistants and participants who devoted their time and effort to this study. Their dedication and commitment were invaluable to the success of this research.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Burri, N.M.; Weatherl, R.; Moeck, C.; Schirmer, M. A review of threats to groundwater quality in the anthropocene. Sci. Total Environ. 2019, 684, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Xu, S.; Lei, Y.; Yue, M.; Zhu, X. Macroscopic compressive strength study of historical grey bricks based on microscopic scale. Constr. Build. Mater. 2024, 421, 135634. [Google Scholar] [CrossRef]
- Li, B.; Jia, C.; Wang, G.; Ren, J.; Lu, G.; Liu, N. Numerical Analysis on the Performance of the Underwater Excavation. Adv. Civ. Eng. 2020, 8894138. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Fan, X. Causes Analysis and Reinforcement Techniques for 21 Horse Wall Cracks of Xi’an Ancient City Wall. J. Water Resour. Archit. Eng. 2017, 218, 012019. [Google Scholar]
- Yun, W. Comparison and selection of consolidation materials for the earthen sites and experimental study. J. Shaanxi Univ. Technol. Nat. Sci. Ed. 2010, 26, 36–39. [Google Scholar]
- Zhu, F.; Li, J.; Guo, Q.; Chen, Y.; Zhang, G.; Li, P.; Shang, D.; Yu, J.; Shan, Z.; Wu, F. Biomineralization technology of evaluation of soil reinforcement SuoYangCheng. J. Civ. Environ. Eng. 2025, 1–13, (In English and Chinese). Available online: http://kns.cnki.net/kcms/detail/50.1218.TU.20241008.1516.006.html (accessed on 9 October 2024).
- Sánchez-Calvillo, A.; Guzmán, E.M.A.; Ruvalcaba-Sil, J.L.; Molina, W.M.; Garcia, H.L.C.; Bedolla-Arroyo, J.A.; Navarro-Mendoza, E.G.; Blancas-Herrera, V.H.; Perez, J.A.V. Colorimetry of Clays as a Tool to Identify Soil Materials for Earthen Buildings Restoration. Key Eng. Mater. 2020, 862, 56–60. [Google Scholar] [CrossRef]
- Yoshihiro, H.; Mamoru, F. Study on a Construction Method of Earthen Walls Using Soil Cement. J. Struct. Constr. Eng. 2007, 72, 95–101. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, J.; Ma, T.; Zhao, X.; Luo, H.; Liu, S. A Comparative Study on Reinforcement Materials for Earth Sites in Semi-Arid Areas: A Case Study of a Large Earth Site in Lintong Area, Shaanxi Province. Mater. Rev. 2025, 39, 24030010-9. [Google Scholar]
- Zhu, J.; Cai, L.; Guo, G. Research on the Application of Ethyl Silicate Reinforcement Materials in the Reinforcement and Protection of Soil Sites in Damp Environments. Cult. Relics Prot. Archaeol. Sci. 2015, 27, 7–14. [Google Scholar]
- Zhang, Y.; Shao, S.; Yin, X.; Guo, S.; Han, S. Evolution of the anti-weathering characteristics of clay at earthen sites treated with K2SiO3 solutions: Micromechanism analysis based on SEM tests. Bull. Eng. Geol. Environ. 2022, 81, 439. [Google Scholar] [CrossRef]
- Wang, J.K.; Zhou, S. Research on the Permeability Performance of Non-water Bulk Materials for Reinforcement of Earthen Sites. Res. Grottoes Earthen Site Conserv. 2022, 1, 93–101. [Google Scholar]
- Chai, X.J.; He, C.F.; Yu, J.W.; Gao, Y.S.; Rao, C.X. Unconfined Compression Strength of Tianluoshan Relic Soils Solidified by Methyl Acrylic Acid Resin. Adv. Mater. Res. 2012, 594–597, 343–346. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G. Experimental Study on the Screening of Reinforcement and Protection Materials for Earth Sites in Damp Environments: A Case Study of the Tanshishan Site in Fujian Province. Cult. Relics Prot. Archaeol. Sci. 2014, 26, 8–21. [Google Scholar]
- Luo, J.; Sun, S.; Zhao, T.; Li, Q.; Gao, S. Bonding and toughness properties of PVA fibre reinforced aqueous epoxy resin cement repair mortar. Constr. Build. Mater. 2013, 49, 766–771. [Google Scholar] [CrossRef]
- Mercuri, M.; Vailati, M.; Gregori, A. Lime-based mortar reinforced with randomly oriented polyvinyl-alcohol (PVA) fibers for strengthening historical masonry structures. Dev. Built Environ. 2023, 14, 100152. [Google Scholar] [CrossRef]
- Yang, H.; Liu, P.; Sun, B.; Zhou, A.; PAN, D. Experimental Study on Screening and Proportion of Reinforcement Materials for the Ruins of Jinyang Ancient City. Sci. Technol. Eng. 2017, 17, 24–34. [Google Scholar]
- Hasbi Ab Rahim, M.; He, Q.; Lopez-Sanchez, J.A.; Hammond, C.; Dimitratos, N.; Sankar, M.; Carley, A.F.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J.; et al. Gold, palladium and gold–palladium supported nanoparticles for the synthesis of glycerol carbonate from glycerol and urea. Catal. Sci. Technol. 2012, 2, 1914–1924. [Google Scholar] [CrossRef]
- Sun, C.; Wu, N.; Kou, S.; Wu, H.; Liu, Y.; Pei, A.; Li, Q. Occurrence, formation mechanism, detection methods, and removal approaches for chloropropanols and their esters in food: An updated systematic review. Food Chem. X 2023, 17, 10. [Google Scholar] [CrossRef]
- Guan, B. Effect of Acrylate Emulsion on the Mechanical and Microscopic Properties of Straw Fiber-Reinforced Cement-Magnesium Slag Stabilized Soil. Polymers 2024, 16, 3462. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, J.; Lyu, B.; Ma, J.; Yang, Z. Polyacrylate crosslinked with furyl alcohol grafting bismaleimide: A self-healing polymer coating. Prog. Org. Coat. 2020, 139, 105475. [Google Scholar] [CrossRef]
- Ahmad, W.; Singh, B.; Dalal, R.C.; Dijkstra, F.A. Carbon dynamics from carbonate dissolution in Australian agricultural soils. Soil Res. 2015, 53, 144–153. [Google Scholar] [CrossRef]
- Zha, F.; Liu, C.; Kang, B.; Yang, X.; Zhou, Y.; Yang, C. Acid rain leaching behavior of Zn-contaminated soils solidified/stabilized using cement–soda residue. Chemosphere 2021, 281, 130916. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Nie, J.; Hu, N.; Ding, D.; Han, F.X. Microbially Induced Carbonate Precipitation Techniques for the Remediation of Heavy Metal and Trace Element–Polluted Soils and Water. Water Air Soil Pollut. 2021, 232, 268. [Google Scholar] [CrossRef]
- Yue, J.; Huang, X.; Zhao, L.; Wang, Z. Study on the factors affecting cracking of earthen soil under dry shrinkage and freeze–thaw conditions. Sci. Rep. 2022, 12, 1816. [Google Scholar] [CrossRef]
- Weng, L.; Elliott, G.D. Polymerization Effect of Electrolytes on Hydrogen-Bonding Cryoprotectants: Ion–Dipole Interactions between Metal Ions and Glycerol. J. Phys. Chem. B 2014, 118, 14546–14554. [Google Scholar] [CrossRef]
- Villa, A.; Campisi, S.; Mohammed KM, H.; Dimitratos, N.; Vindigni, F.; Manzoli, M.; Jone, W.; Bowker, M.; Hutchings, G.J. Tailoring the selectivity of glycerol oxidation by tuning the acid–base properties of Au catalysts. Catal. Sci. Technol. 2015, 5, 1126–1132. [Google Scholar] [CrossRef]
- Lauriol-Garbey, P.; Postole, G.; Loridant, S.; Auroux, A.; Belliere-Baca, V.; Rey, P.; Millet, J.M.M. Acid–base properties of niobium-zirconium mixed oxide catalysts for glycerol dehydration by calorimetric and catalytic investigation. Appl. Catal. B Environ. 2011, 106, 94–102. [Google Scholar] [CrossRef]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98, 50–60. [Google Scholar] [CrossRef]
- Oss, C.V.; Good, R.J.; Busscher, R.J. Estimation of the polar surface tension parameters of glycerol and formamide, for use in contact angle measurements on polar solids. J. Dispers. Sci. Technol. 1990, 11, 75–81. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Chen, G.; Wang, L. Experimental study of the feasibility of using anhydrous sodium metasilicate as a geopolymer activator for soil stabilization. Eng. Geol. 2020, 264, 105316. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, S. Effect on Silt Capillary Water Absorption upon Addition of Sodium Methyl Silicate (SMS) and Microscopic Mechanism Analysis. Coatings 2020, 10, 724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).