Microstructural Evolution and Rheological Enhancement of Asphalt–Rubber Binders: Unveiling the Role of Morphology in Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
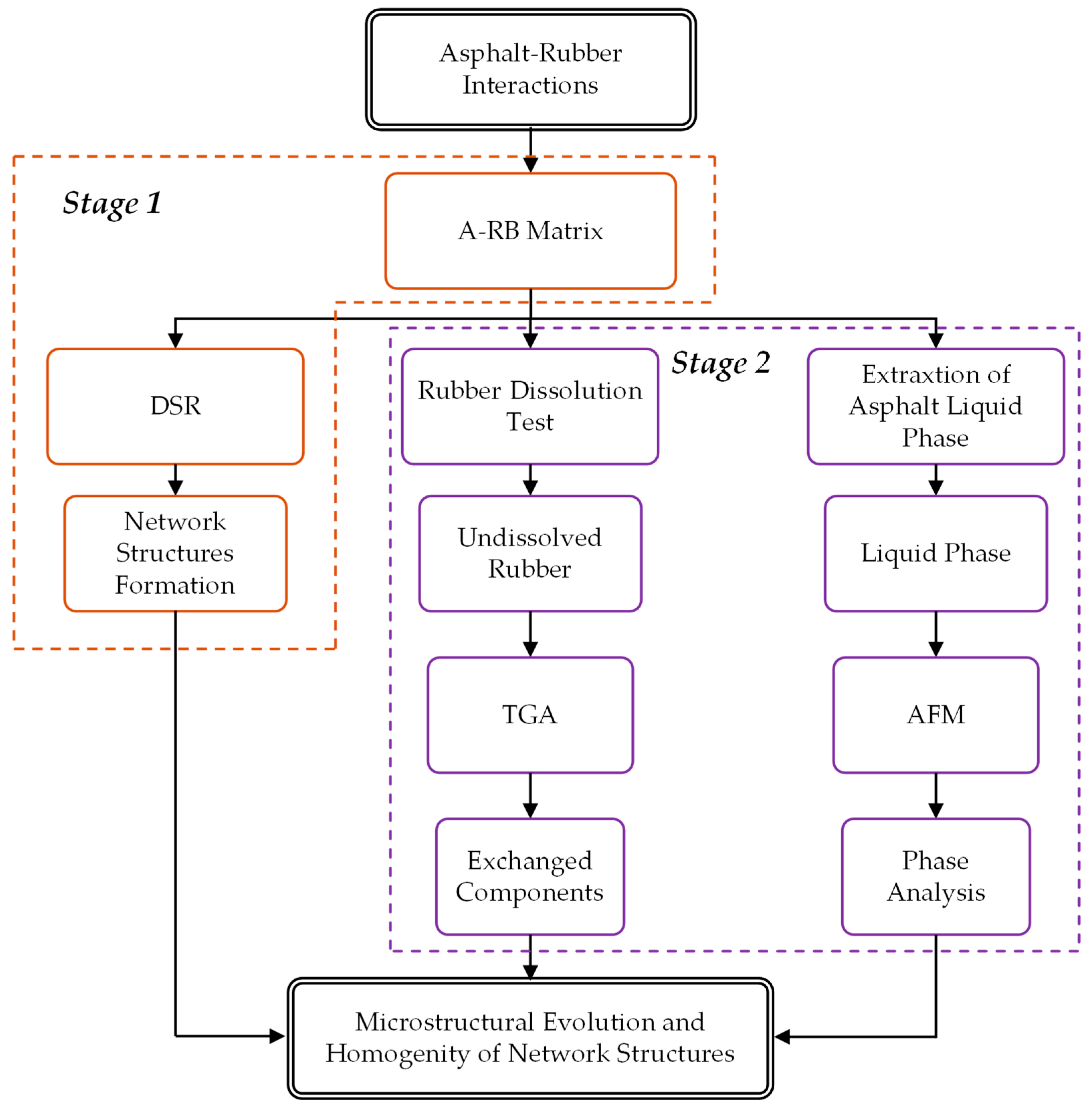
2.2.1. Asphalt–Rubber Interactions
2.2.2. Stage 1: Asphalt–Rubber Matrix
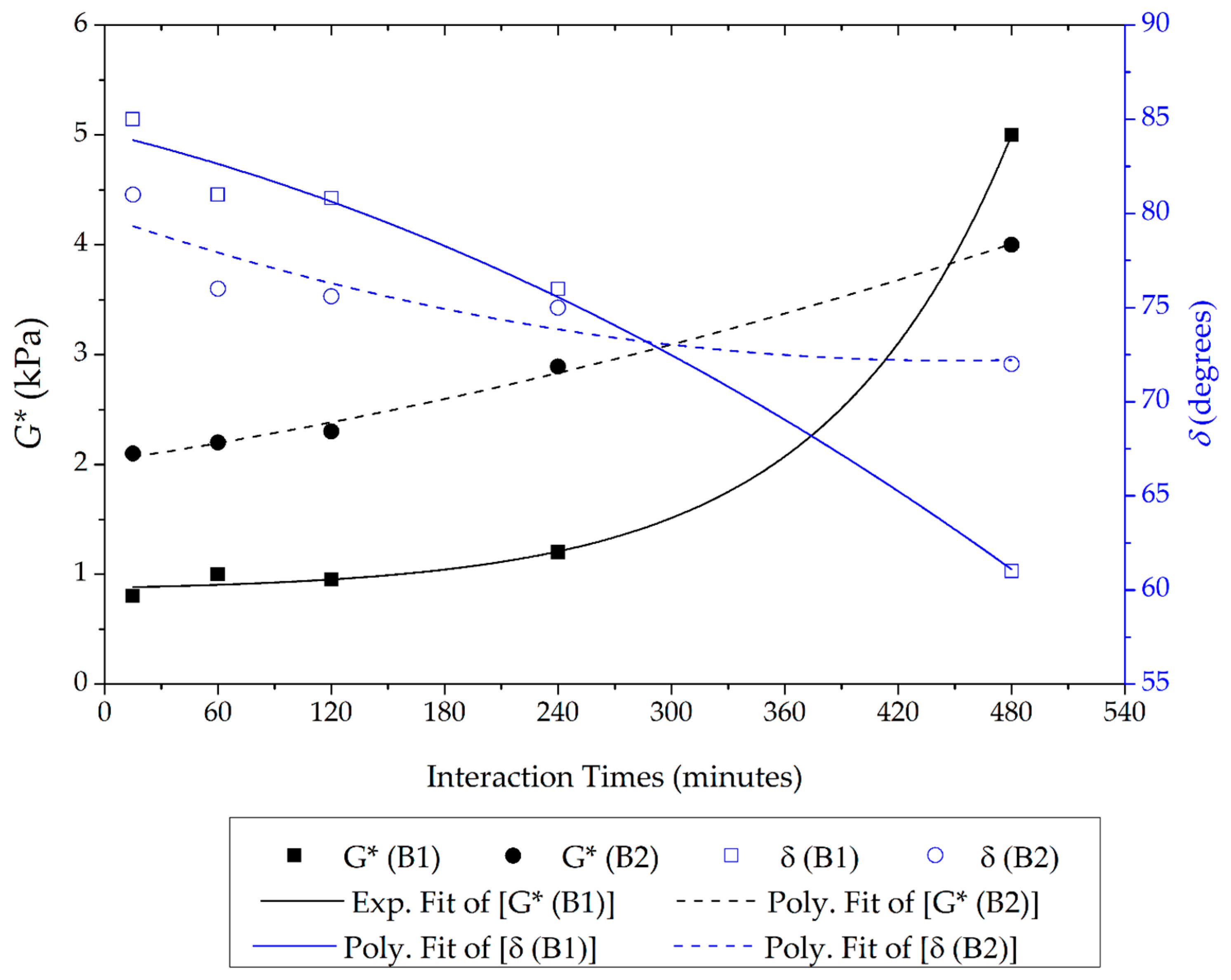
Single-Point Test
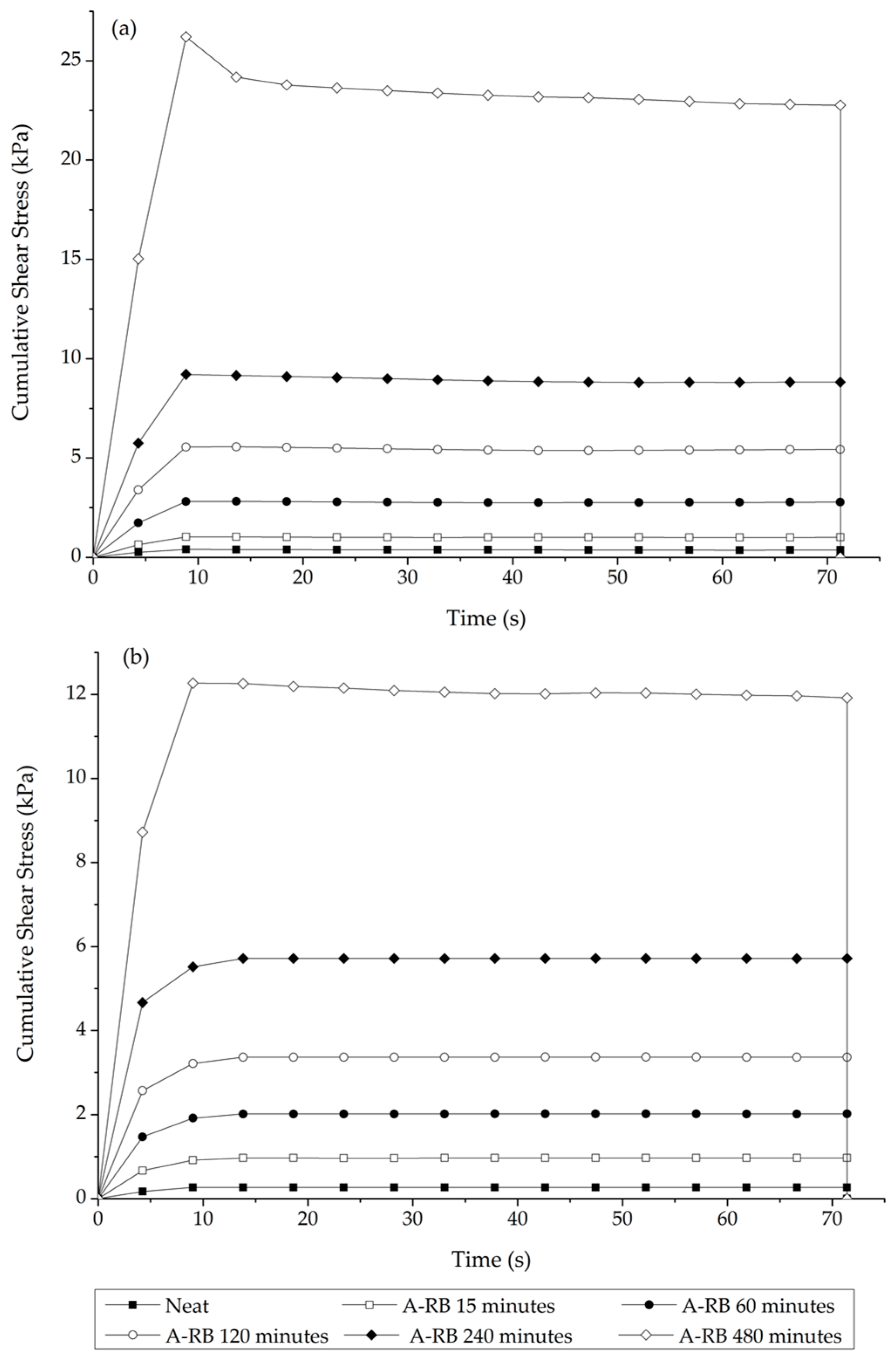
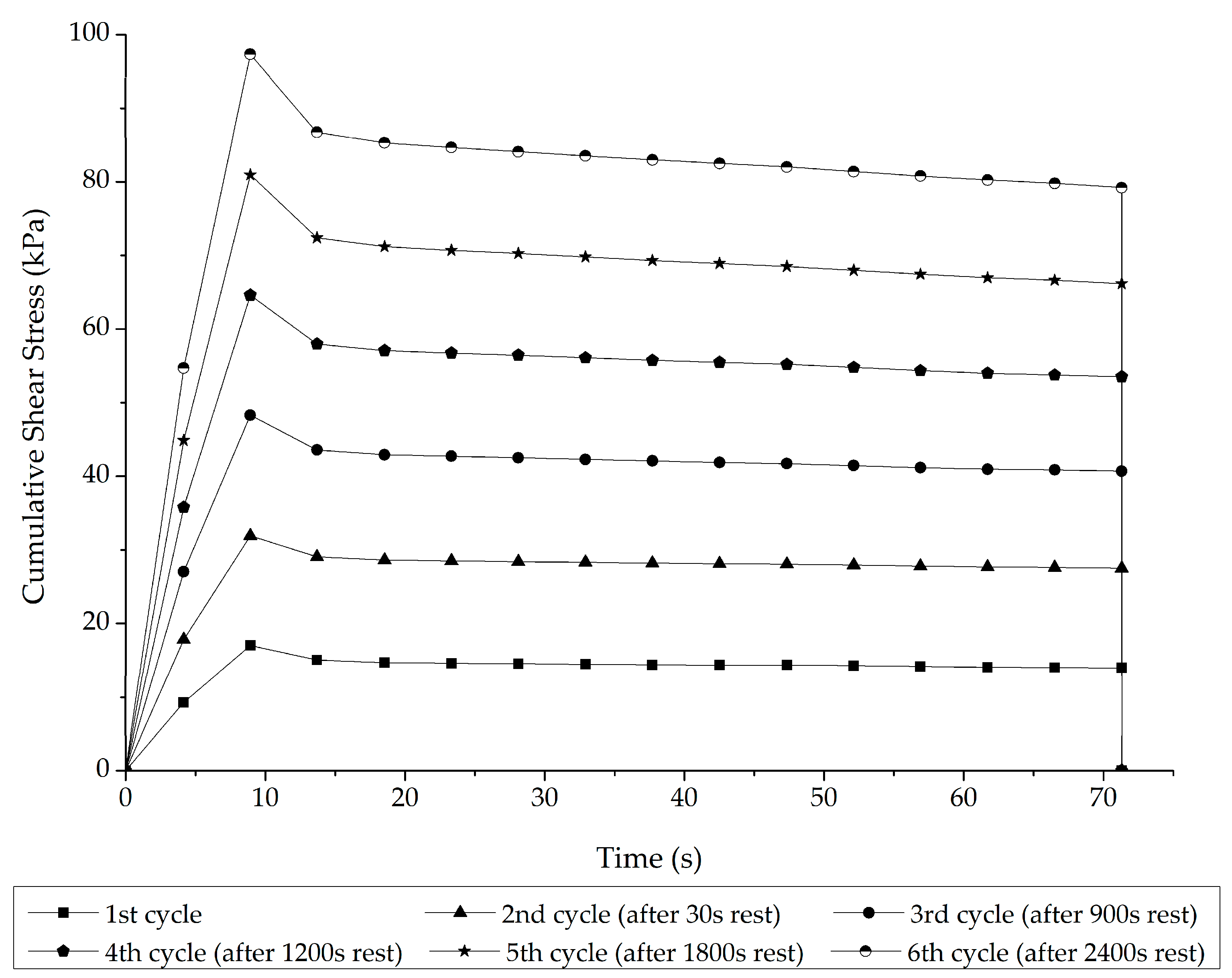
Interrupted Shear Flow Test
2.2.3. Stage 2: Rubber and Liquid Phase
Rubber Dissolution Test
- DR: percentage of dissolved rubber;
- A: total weight of rubber expected in an A-RB sample;
- B: total weight of rubber extracted from an A-RB sample.
Rubber Thermogravimetric Analysis
- RC%: percentage of rubber components;
- C: weight of a rubber component in TGA;
- D: weight of a rubber sample in TGA.
Extraction of Asphalt Liquid Phase
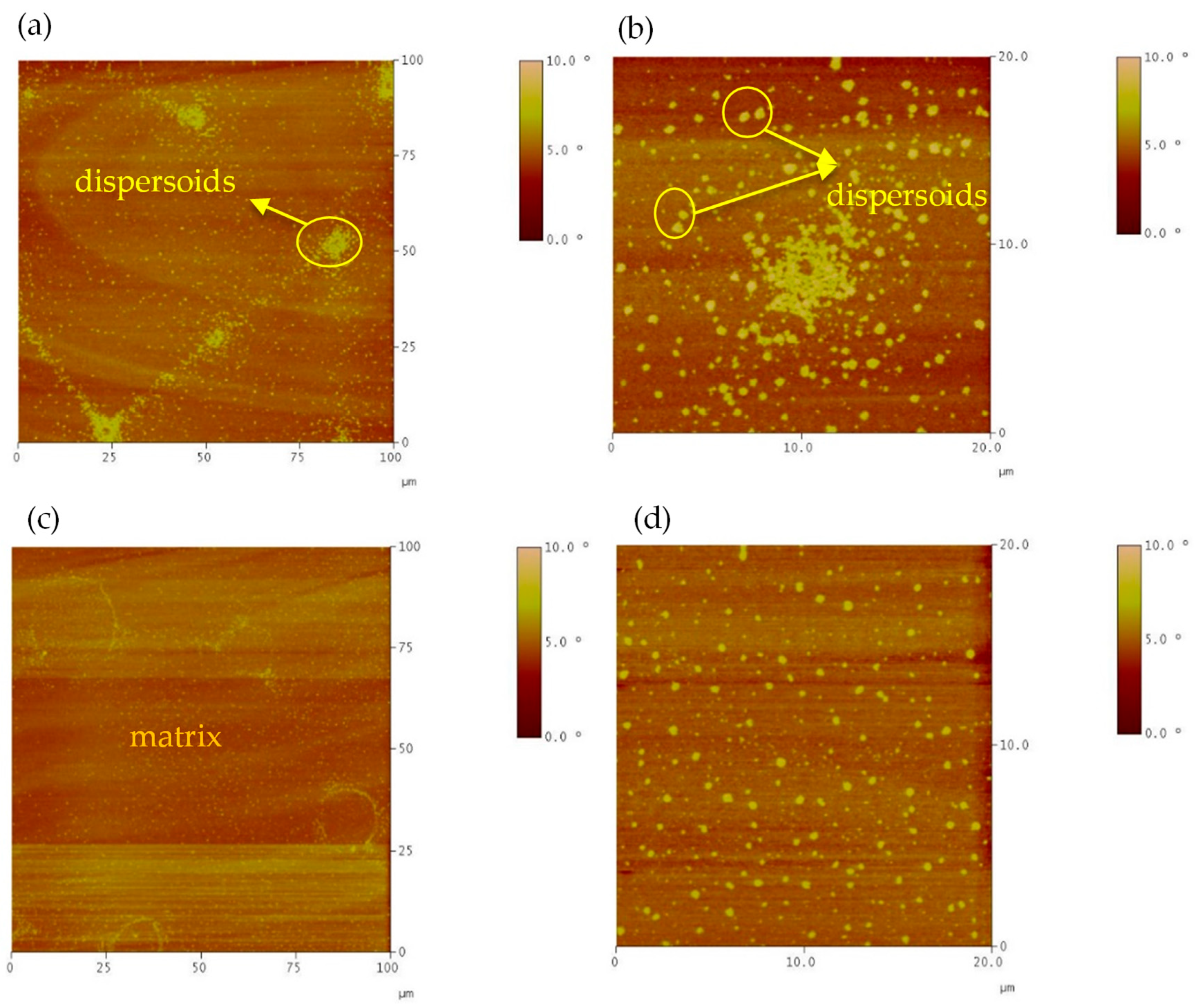
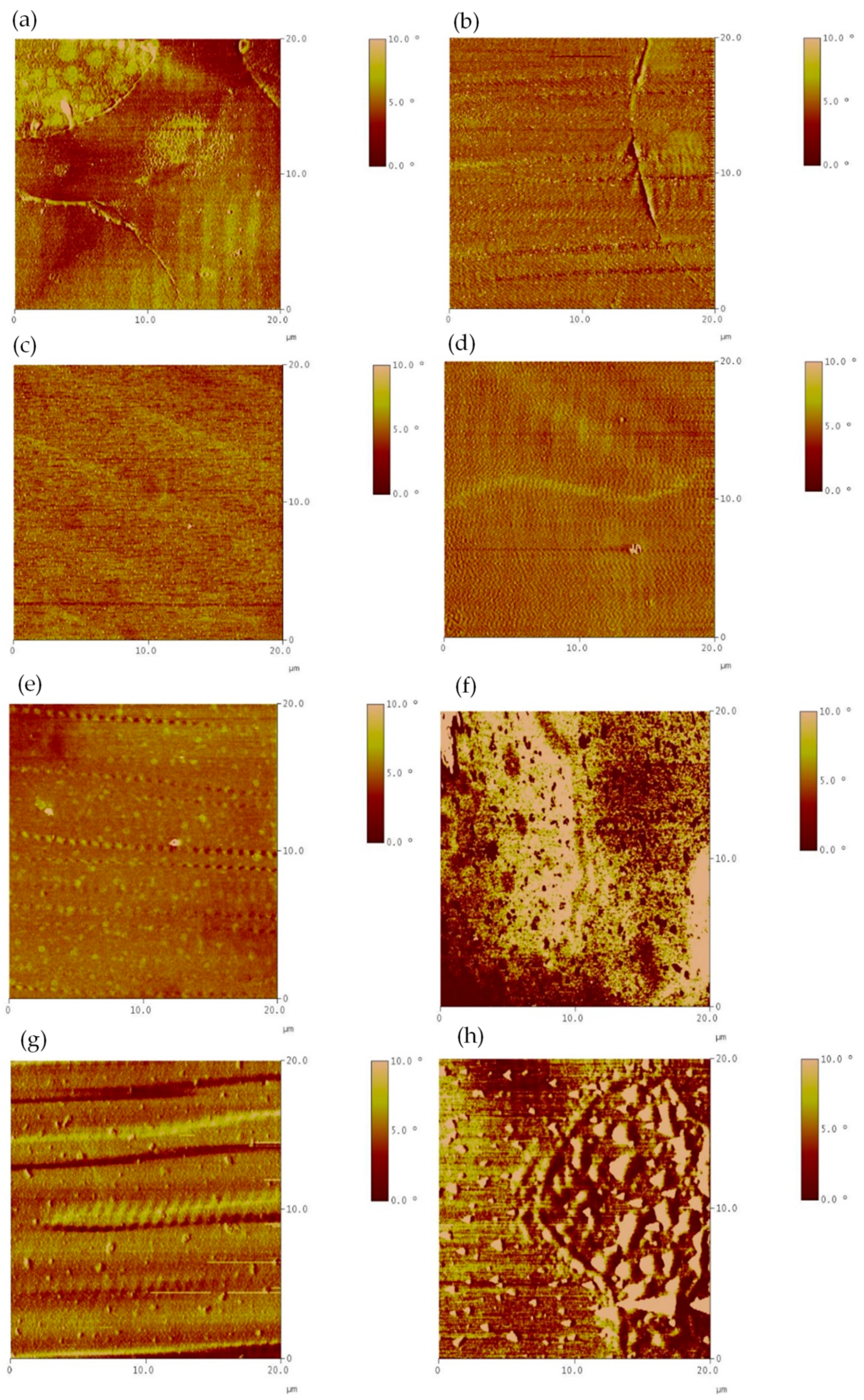
Atomic Force Microscopy Test
3. Results and Discussion
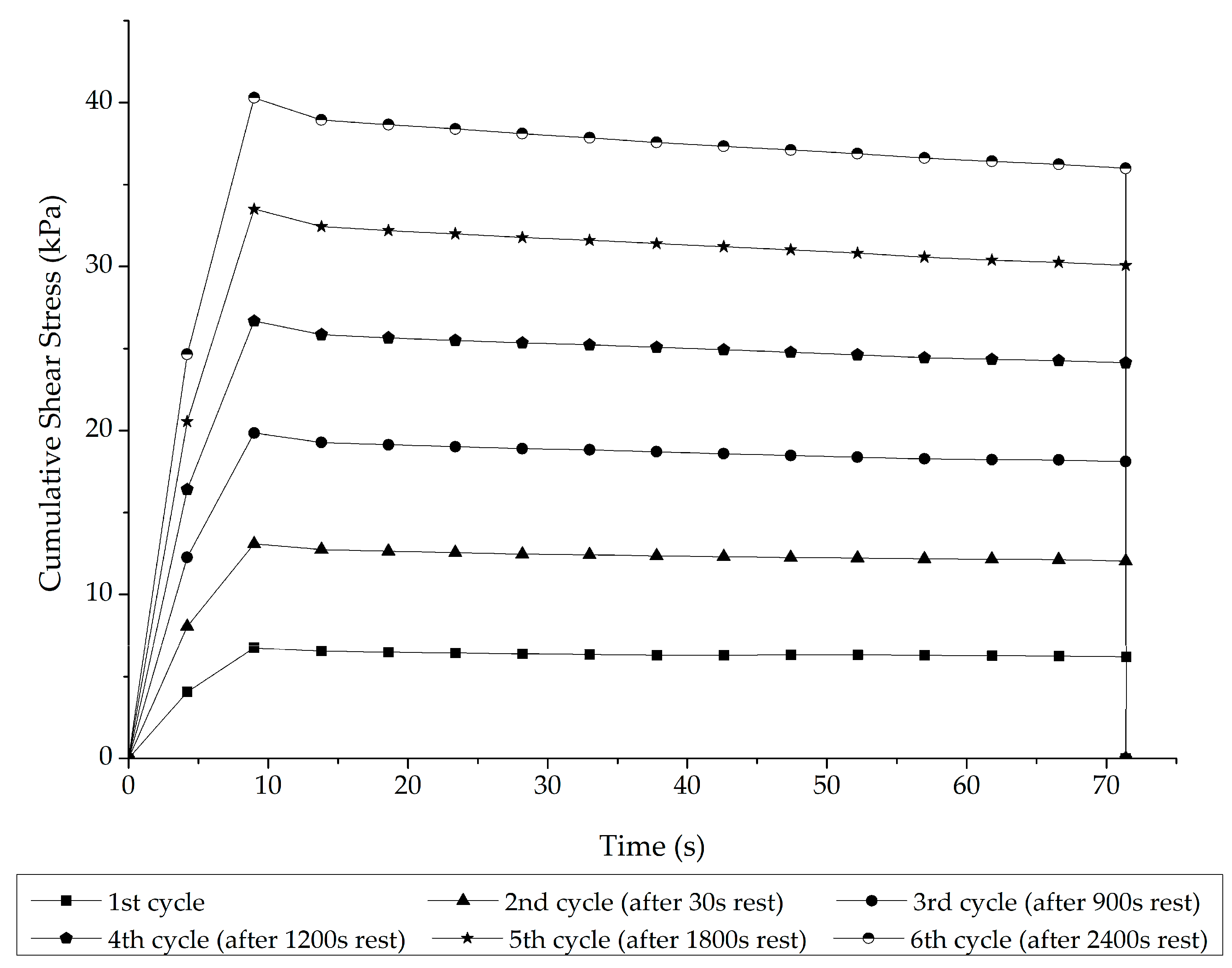
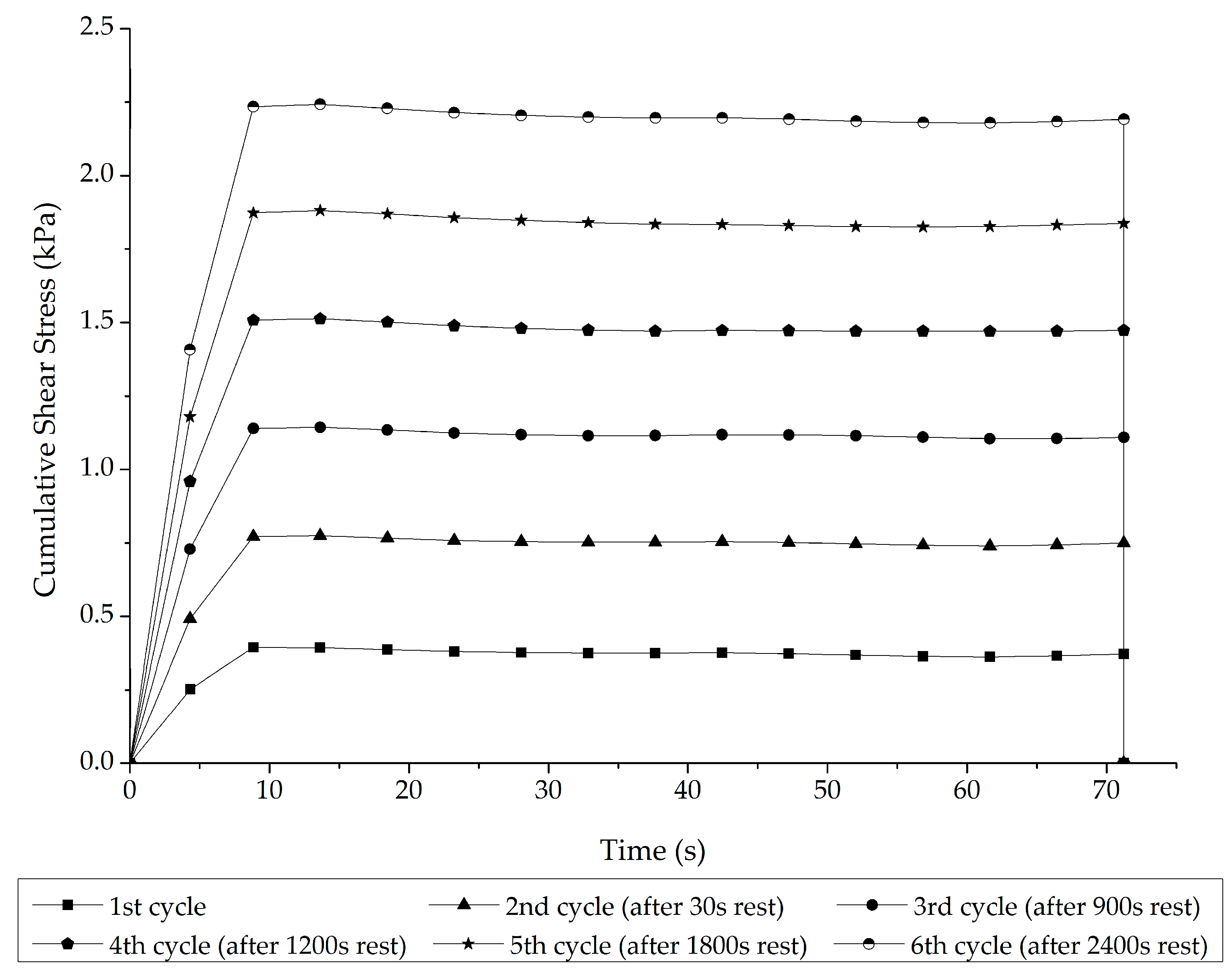
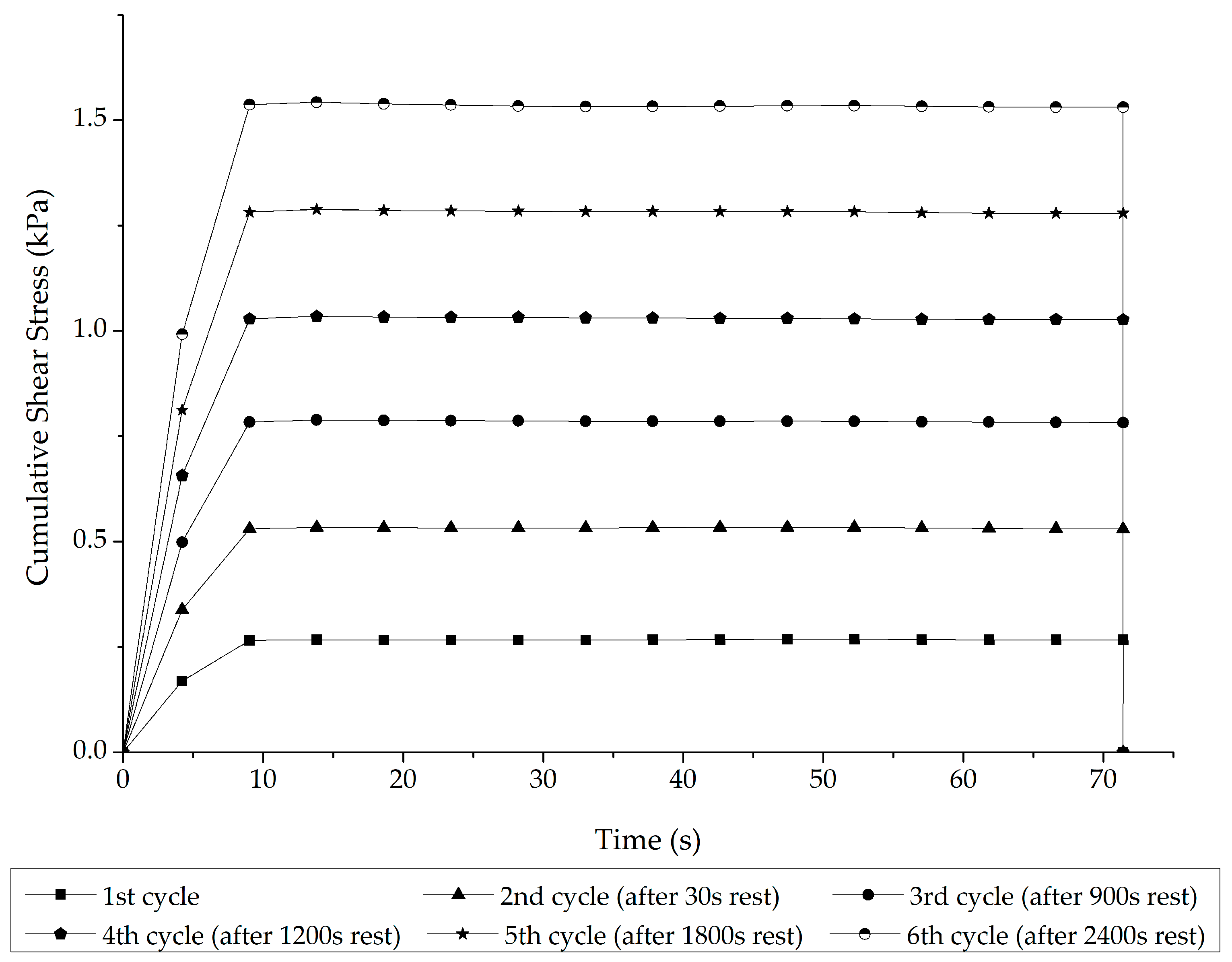
3.1. Stage 1 Analysis: Asphalt–Rubber Matrix Analysis
3.2. Stage 2 Analysis: Rubber and Liquid Phase Analysis
3.2.1. Analysis of Rubber
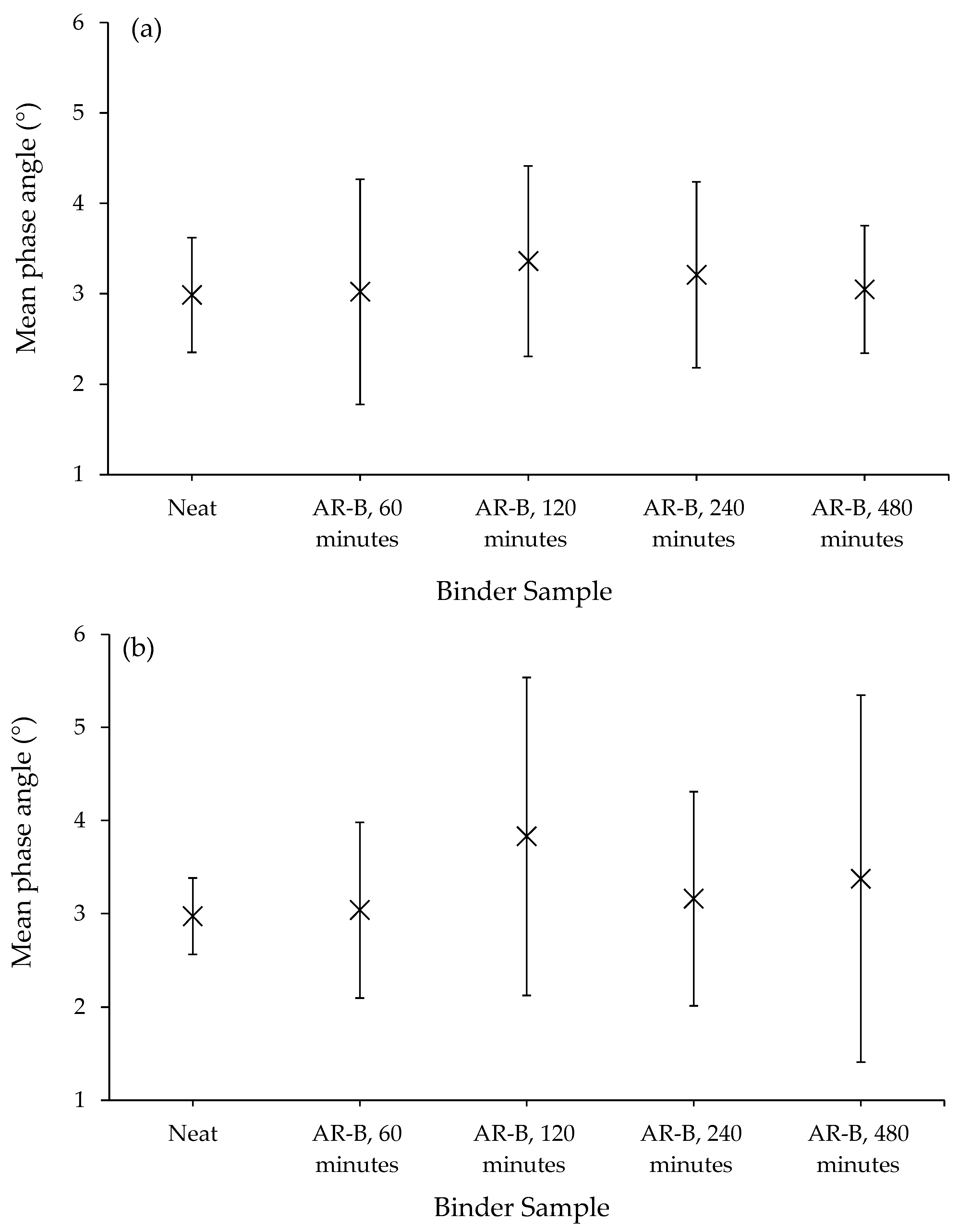
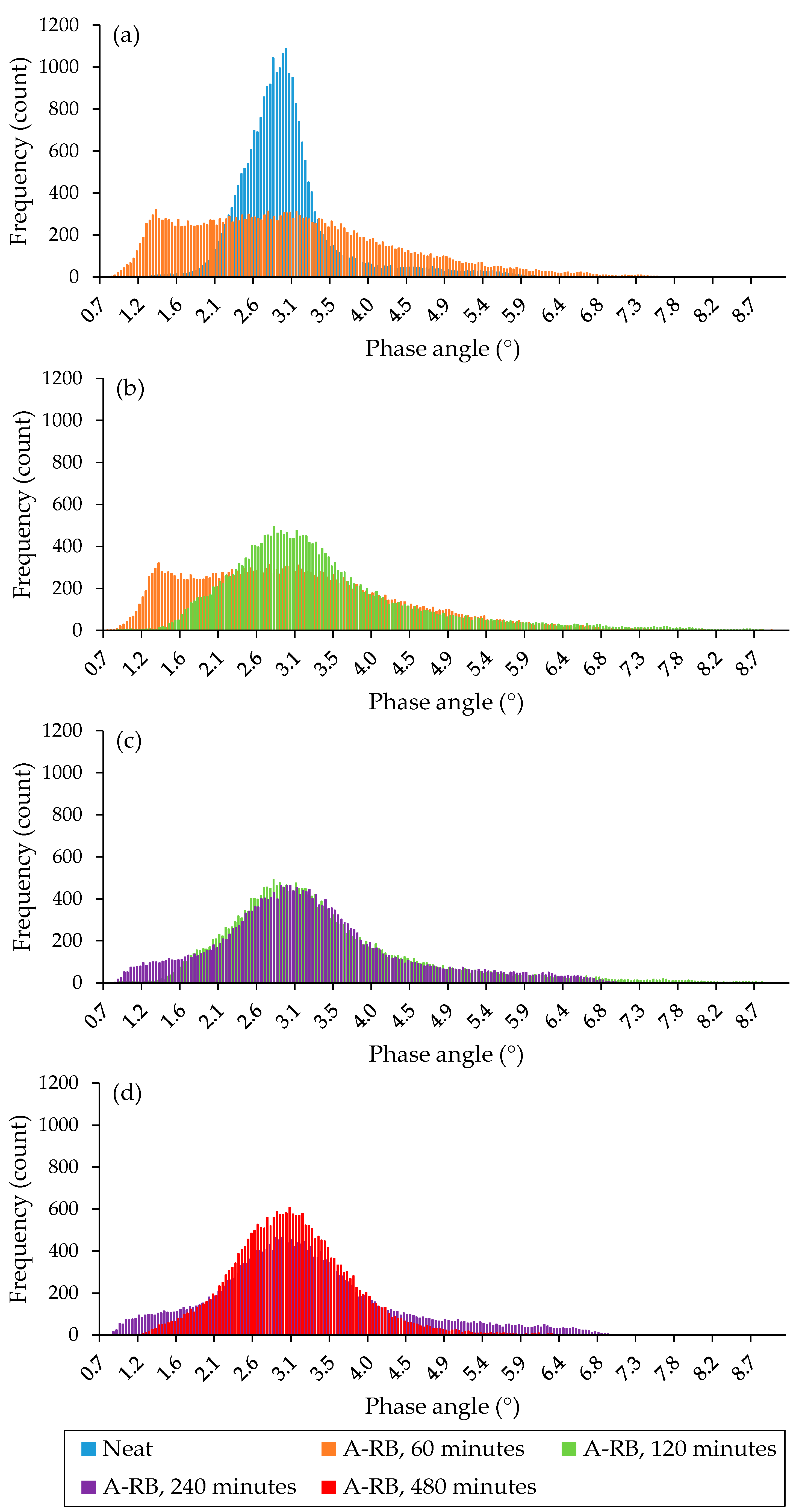
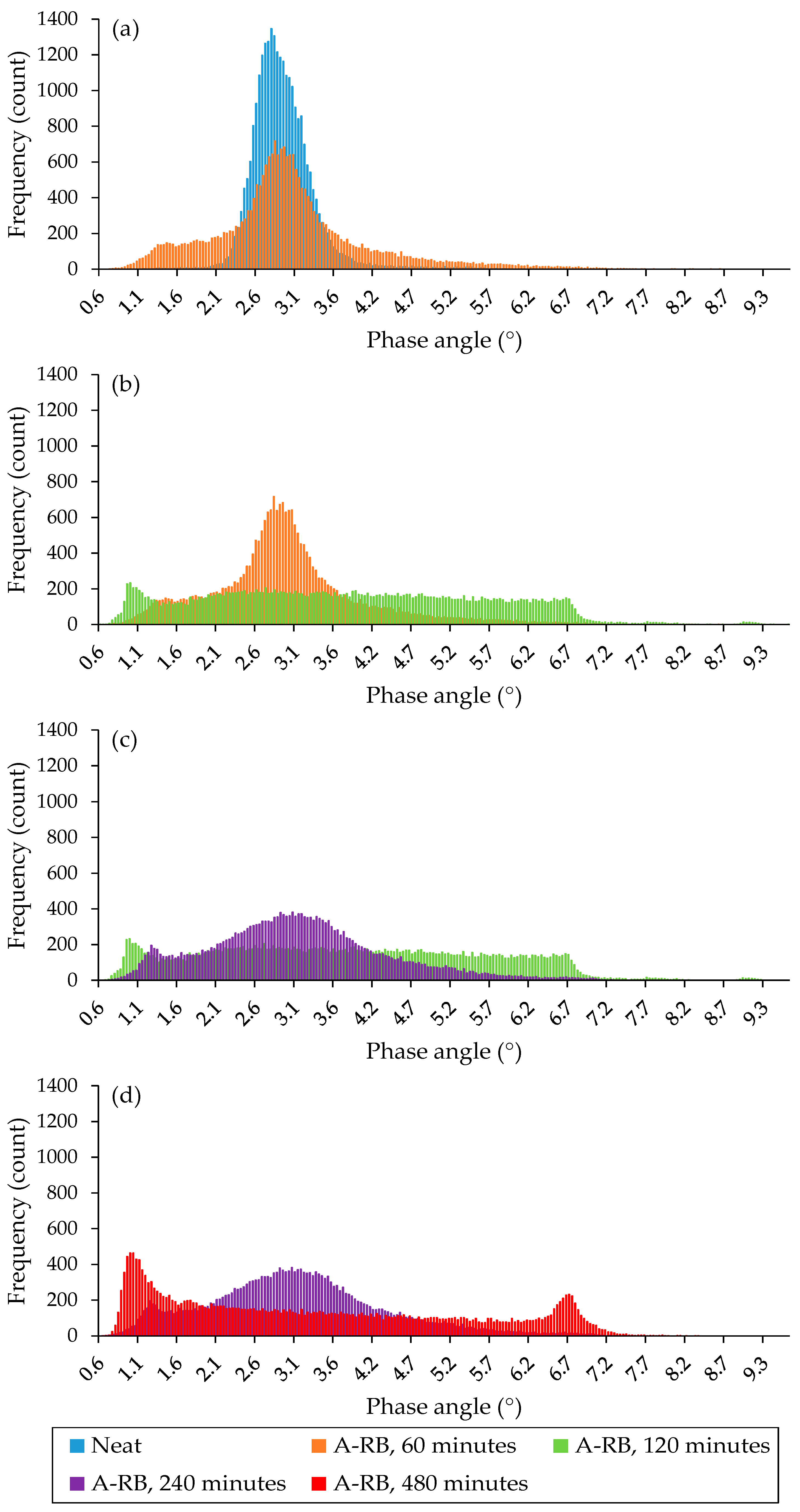
3.2.2. Morphology Analysis of Liquid Phases
4. Conclusions
- Time-driven morphological evolution: the morphology of the liquid phase evolved from agglomerated domains to a well-organized lamellar structure as the interaction time between soft asphalt and rubber increased after 4 h, especially after 8 h, due to the rubber dissolution and the exchange of its components with the asphalt. Extending the interaction time from 4 to 8 h increased rubber dissolution by 5–23%, depending on the binder type.
- Influence of binder stiffness on network uniformity: the homogeneous network structures formed in A-RBs interacted with the rubber and soft binder. However, heterogeneous network structures were detected in the A-RBs that interacted with the same rubber and a stiffer binder.
- Microstructural homogeneity was confirmed by AFM phase quantitative analysis: after 8 h of interaction between rubber and the soft binder, the A-RB displayed the lowest average phase angle and standard deviation, with a 10% decrease in the mean phase angle and a 64% reduction in the standard deviation, indicating a more homogeneous network with a smoother surface. These nanoscale findings validate the visual observations of the well-organized lamellar structures.
- Rheological indicators of network formation: the stress overshoot effects were more pronounced, specifically by 150%, in the A-RBs that interacted with the soft binder after 8 h than those that interacted with the stiffer one.
- Optimized blending guidelines for A-RBs: this study showed A-RB production with improved blending conditions (190 °C, 50 Hz, and 8 h) to gain uniform internal network development and maximize performance in A-RBs.
5. Recommendations and Future Work
- The interaction conditions, 50 Hz speed, 8 h duration, and 190 °C temperature, were determined to be effective in inducing the formation of the internal network structures of the A-RBs.
- For the enhancement of asphalt morphology understanding, AFM analysis should be combined with other methods, such as advanced chemical analyses.
- Future studies must examine the influence of different rubber sources and their corresponding proportions on A-RB morphology.
- Further studies should take into consideration aging, exposure to the environment, and different storage periods as variables affecting A-RB morphologies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| A-RB | Asphalt–Rubber Binder |
| DTG | Derivative of Thermograph |
| F | Filler |
| ISF | Interrupted Shear Flow |
| NR | Natural Rubber |
| OC | Oily Components |
| PDM | Phase Detection Mode |
| PG | Performance Grade |
| SR | Synthetic Rubber |
| TGA | Thermogravimetric Analysis |
References
- Branthaver, J.F.; Petersen, J.; Robertson, R.; Duvall, J.; Kim, S.; Harnsberger, P.; Mill, T.; Ensley, E.; Barbour, F.; Scharbron, J. Binder Characterization and Evaluation Volume 2: Chemistry; SHRP-A-368; National Academy of Sciences: Washington, DC, USA, 1993. [Google Scholar]
- Lamontagne, J.; Dumas, P.; Mouillet, V.; Kister, J. Comparison by Fourier transform infrared (FTIR) spectroscopy of different ageing techniques: Application to road bitumens. Fuel 2001, 80, 483–488. [Google Scholar] [CrossRef]
- Martínez-Estrada, A.; Chávez-Castellanos, A.E.; Herrera-Alonso, M.; Herrera-Nájera, R. Comparative study of the effect of sulfur on the morphology and rheological properties of SB-and SBS-modified asphalt. J. Appl. Polym. Sci. 2010, 115, 3409–3422. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, M.; Zhang, D.; Zhang, H.; Zhang, B. The effect of styrene–butadiene–rubber/montmorillonite modification on the characteristics and properties of asphalt. Constr. Build. Mater. 2009, 23, 3112–3117. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Ali, M.F. Studies on the aging behavior of the Arabian asphalts. Fuel 1999, 78, 1005–1015. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Ali, M.F.; Shirokoff, J. Use of X-ray diffraction in assessing the aging pattern of asphalt fractions. Fuel 2002, 81, 51–58. [Google Scholar] [CrossRef]
- Trejo, F.; Ancheyta, J.; Morgan, T.; Herod, A.; Kandiyoti, R. Characterization of asphaltenes from hydrotreated products by SEC, LDMS, MALDI, NMR, and XRD. Energy Fuel 2007, 21, 2121–2128. [Google Scholar] [CrossRef]
- De Moraes, M.; Pereira, R.; Simão, R.; Leite, L. High temperature AFM study of CAP 30/45 pen grade bitumen. J. Microsc. 2010, 239, 46–53. [Google Scholar] [CrossRef]
- Petersen, J.; Robertson, R.; Branthaver, J.; Harnsberger, P.; Duvall, J.; Kim, S.; Anderson, D.; Christiansen, D.; Bahia, H. Binder Characterization and Evaluation Volume 1; SHRP-A-367, Strategic Highways Research Program; National Research Council: Washington, DC, USA, 1994. [Google Scholar]
- Wekumbura, C.; Stastna, J.; Zanzotto, L. Destruction and recovery of internal structure in polymer-modified asphalts. J. Mater. Civ. Eng. 2007, 19, 227–232. [Google Scholar] [CrossRef]
- Deef-Allah, E.; Abdelrahman, M.; Ragab, M. Components’ exchanges between recycled materials and asphalt binders in asphalt mixes. Adv. Civ. Eng. Matls 2022, 11, 94–114. [Google Scholar] [CrossRef]
- Green, E.; Tolonen, W.J. The Chemical and Physical Properties of Asphalt Rubber Mixtures, Part I. Basic Material Behavior; FHWA-AZ-HPR14-162; Arizona DOT: Phoenix, AZ, USA, 1977. [Google Scholar]
- Airey, G.D.; Rahman, M.M.; Collop, A.C. Absorption of bitumen into crumb rubber using the basket drainage method. Int. J. Pavement Eng. 2003, 4, 105–119. [Google Scholar] [CrossRef]
- Zanzotto, L.; Kennepohl, G.J. Development of rubber and asphalt binders by depolymerization and devulcanization of scrap tires in asphalt. Transp. Res. Rec. 1996, 1530, 51–58. [Google Scholar] [CrossRef]
- Billiter, T.; Davison, R.; Glover, C.; Bullin, J. Production of asphalt-rubber binders by high-cure conditions. Transp. Res. Rec. 1997, 1586, 50–56. [Google Scholar] [CrossRef]
- Deef-Allah, E.; Abdelrahman, M. Effect of used motor oil as a rejuvenator on crumb rubber modifier’s released components to asphalt binder. Prog. Rubber Plast. Recycl. Technol. 2020, 37, 87–114. [Google Scholar] [CrossRef]
- Deef-Allah, E.; Abdelrahman, M.; Hemida, A. Improving asphalt binder’s elasticity through controlling the interaction parameters between CRM and asphalt binder. Adv. Civ. Eng. Mater. 2020, 9, 262–282. [Google Scholar] [CrossRef]
- Abdelrahman, M. Controlling performance of crumb rubber-modified binders through addition of polymer modifiers. Transp. Res. Rec. 2006, 1962, 64–70. [Google Scholar] [CrossRef]
- Gawel, I.; Stepkowski, R.; Czechowski, F. Molecular interactions between rubber and asphalt. Ind. Eng. Chem. Res. 2006, 45, 3044–3049. [Google Scholar] [CrossRef]
- Billiter, T.; Chun, J.; Davison, R.; Glover, C.; Bullin, J. Investigation of the curing variables of asphalt-rubber binder. Pet. Sci. Technol. 1997, 15, 445–469. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Carpenter, S. Mechanism of interaction of asphalt cement with crumb rubber modifier. Transp. Res. Rec. 1999, 1661, 106–113. [Google Scholar] [CrossRef]
- Wang, H.; Apostolidis, P.; Zhu, J.; Liu, X.; Skarpas, A.; Erkens, S. The role of thermodynamics and kinetics in rubber–bitumen systems: A theoretical overview. Int. J. Pavement Eng. 2021, 22, 1785–1800. [Google Scholar] [CrossRef]
- Das, P.K.; Baaj, h.; Tighe, S.; Kringos, N. Atomic force microscopy to investigate asphalt binders: A state-of-the-art review. Road Mater. Pavement Des. 2016, 17, 693–718. [Google Scholar] [CrossRef]
- Król, J.; Radziszewski, P.; Kowalski, K.J. Influence of microstructural behavior on multiple stress creep recovery (MSCR) in modified bitumen. Procedia Eng. 2015, 111, 478–484. [Google Scholar] [CrossRef]
- ASTM E1131-20; Standard Test Method for Compositional Analysis by Thermogravimetry. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D4124-09; Standard Test Method for Separation of Asphalt into Four Fractions. ASTM International: West Conshohocken, PA, USA, 2018.
- Ragab, M. Enhancing the Performance of Crumb Rubber Modified Asphalts Through Controlling the Internal Network Structure Developed. Ph.D. Thesis, NDSU, Fargo, ND, USA, 2016. [Google Scholar]
- ASTM D2872-22; Test Method for Effect of Heat and Air on a Moving Film of Asphalt (Rolling Thin-Film Oven Test). ASTM International: West Conshohocken, PA, USA, 2022.
- Williams, P.T.; Besler, S. Pyrolysis-thermogravimetric analysis of tyres and tyre components. Fuel 1995, 74, 1277–1283. [Google Scholar] [CrossRef]
- Senneca, O.; Salatino, P.; Chirone, R. A fast heating-rate thermogravimetric study of the pyrolysis of scrap tyres. Fuel 1999, 78, 1575–1581. [Google Scholar] [CrossRef]
- Seidelt, S.; Müller-Hagedorn, M.; Bockhorn, H. Description of tire pyrolysis by thermal degradation behaviour of main components. J. Anal. Appl. Pyrolysis 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, W.-K.; Cho, S.-G.; Kim, I.; Ha, C.-S. Quantitative analysis of unknown compositions in ternary polymer blends: A model study on NR/SBR/BR system. J. Anal. Appl. Pyrolysis 2007, 78, 85–94. [Google Scholar] [CrossRef]
- Chen, F.; Qian, J. Studies of the thermal degradation of waste rubber. Waste Manag. 2003, 23, 463–467. [Google Scholar] [CrossRef]
- Prime, R.B.; Bair, H.E.; Vyazovkin, S.; Gallagher, P.K.; Riga, A. Thermogravimetric analysis (TGA). In Thermal Analysis of Polymers, Fundamentals and Applications, 1st ed.; Menczel, J.D., Prime, R.B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 241–317. [Google Scholar]
- Hatakeyama, T.; Zhenhai, L. Handbook of Thermal Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Ghavibazoo, A.; Abdelrahman, M. Composition analysis of crumb rubber during interaction with asphalt and effect on properties of binder. Int. J. Pavement Eng. 2013, 14, 517–530. [Google Scholar] [CrossRef]
- Zhong, Q.; Inniss, D.; Kjoller, K.; Elings, V. Fractured polymer/silica fiber surface studied by tapping mode atomic force microscopy. Surf. Sci. Lett. 1993, 290, L688–L692. [Google Scholar]
- Loeber, L.; Sutton, O.; Morel, J.; Valleton, J.M.; Muller, G. New direct observations of asphalts and asphalt binders by scanning electron microscopy and atomic force microscopy. J. Microsc. 1996, 182, 32–39. [Google Scholar] [CrossRef]
- Stark, M.; Guckenberger, R. Fast low-cost phase detection setup for tapping-mode atomic force microscopy. Rev. Sci. Instrum. 1999, 70, 3614–3619. [Google Scholar] [CrossRef]
- Anczykowski, B.; Krüger, D.; Fuchs, H. Cantilever dynamics in quasinoncontact force microscopy: Spectroscopic aspects. Phys. Rev. B 1996, 53, 15485–15488. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Spatz, J.; Sheiko, S.; Möller, M.; Reineker, P.; Marti, O. Imaging material properties by resonant tapping-force microscopy: A model investigation. Phys. Rev. B 1996, 54, 8908–8912. [Google Scholar] [CrossRef] [PubMed]
- Peralta, J.; Silva, H.M.R.D.; Machado, A.V.; Pais, J.; Pereira, P.A.A.; Sousa, J.B. Changes in rubber due to its interaction with bitumen when producing asphalt rubber. Road Mater. Pavement Des. 2010, 11, 1009–1031. [Google Scholar] [CrossRef]
- Bouldin, M.G.; Collins, J.H.; Berker, A. Rheology and microstructures of polymer/asphalt blends. Rubber Chem. Technol. 1991, 64, 577–600. [Google Scholar] [CrossRef]
- Masson, J.F.; Leblond, V.; Margeson, J. Bitumen morphologies by phase-detection atomic force microscopy. J. Microsc. 2006, 221, 17–29. [Google Scholar] [CrossRef]
- Sengoz, B.; Isikyakar, G. Evaluation of the properties and microstructure of SBS and EVA polymer modified bitumen. Constr. Build. Mater. 2008, 22, 1897–1905. [Google Scholar] [CrossRef]
- Wu, S.-P.; Pang, L.; Mo, L.-T.; Chen, Y.-C.; Zhu, G.-J. Influence of aging on the evolution of structure, morphology and rheology of base and SBS modified bitumen. Constr. Build. Mater. 2009, 23, 1005–1010. [Google Scholar] [CrossRef]
- Pauli, A.; Grimes, R.; Beemer, A.; Turner, T.; Branthaver, J. Morphology of asphalts, asphalt fractions and model wax-doped asphalts studied by atomic force microscopy. Int. J. Pavement Eng. 2011, 12, 291–309. [Google Scholar] [CrossRef]
- Nagao, E.; Dvorak, J.A. Phase imaging by atomic force microscopy: Analysis of living homoiothermic vertebrate cells. Biophys. J. 1999, 76, 3289–3297. [Google Scholar] [CrossRef]
- Marrese, M.; Guarino, V.; Ambrosio, L. Atomic force microscopy: A powerful tool to address scaffold design in tissue engineering. J. Funct. Biomater. 2017, 8, 7. [Google Scholar] [CrossRef]


| Binder Properties | |||
| Property | B1 | B2 | |
| Asphalt Fractions (ASTM D 4124-09 [26]) | Saturates (%) | 16.23 | 11.85 |
| Aromatics (%) | 40.46 | 37.11 | |
| Resins (%) | 29.64 | 35.37 | |
| Asphaltenes (%) | 13.67 | 15.67 | |
| Rubber Properties | |||
| Type | Cryogenic | ||
| Source | A mixed source of scrap tires | ||
| Rubber Components (ASTM E1131-20 [25]) | Oily Components (%) | 7.00 | |
| Natural Rubber (%) | 28.00 | ||
| Synthetic Rubber (%) | 21.00 | ||
| Filler (%) | 44.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deef-Allah, E.; Ragab, M.; Attia, M.; Abdelrahman, M. Microstructural Evolution and Rheological Enhancement of Asphalt–Rubber Binders: Unveiling the Role of Morphology in Performance. Buildings 2025, 15, 1963. https://doi.org/10.3390/buildings15121963
Deef-Allah E, Ragab M, Attia M, Abdelrahman M. Microstructural Evolution and Rheological Enhancement of Asphalt–Rubber Binders: Unveiling the Role of Morphology in Performance. Buildings. 2025; 15(12):1963. https://doi.org/10.3390/buildings15121963
Chicago/Turabian StyleDeef-Allah, Eslam, Mohyeldin Ragab, Mohamed Attia, and Magdy Abdelrahman. 2025. "Microstructural Evolution and Rheological Enhancement of Asphalt–Rubber Binders: Unveiling the Role of Morphology in Performance" Buildings 15, no. 12: 1963. https://doi.org/10.3390/buildings15121963
APA StyleDeef-Allah, E., Ragab, M., Attia, M., & Abdelrahman, M. (2025). Microstructural Evolution and Rheological Enhancement of Asphalt–Rubber Binders: Unveiling the Role of Morphology in Performance. Buildings, 15(12), 1963. https://doi.org/10.3390/buildings15121963






