Effect of Silica Fume Concentration and Water–Cement Ratio on the Compressive Strength of Cement-Based Mortars
Abstract
1. Introduction
2. Materials and Methods
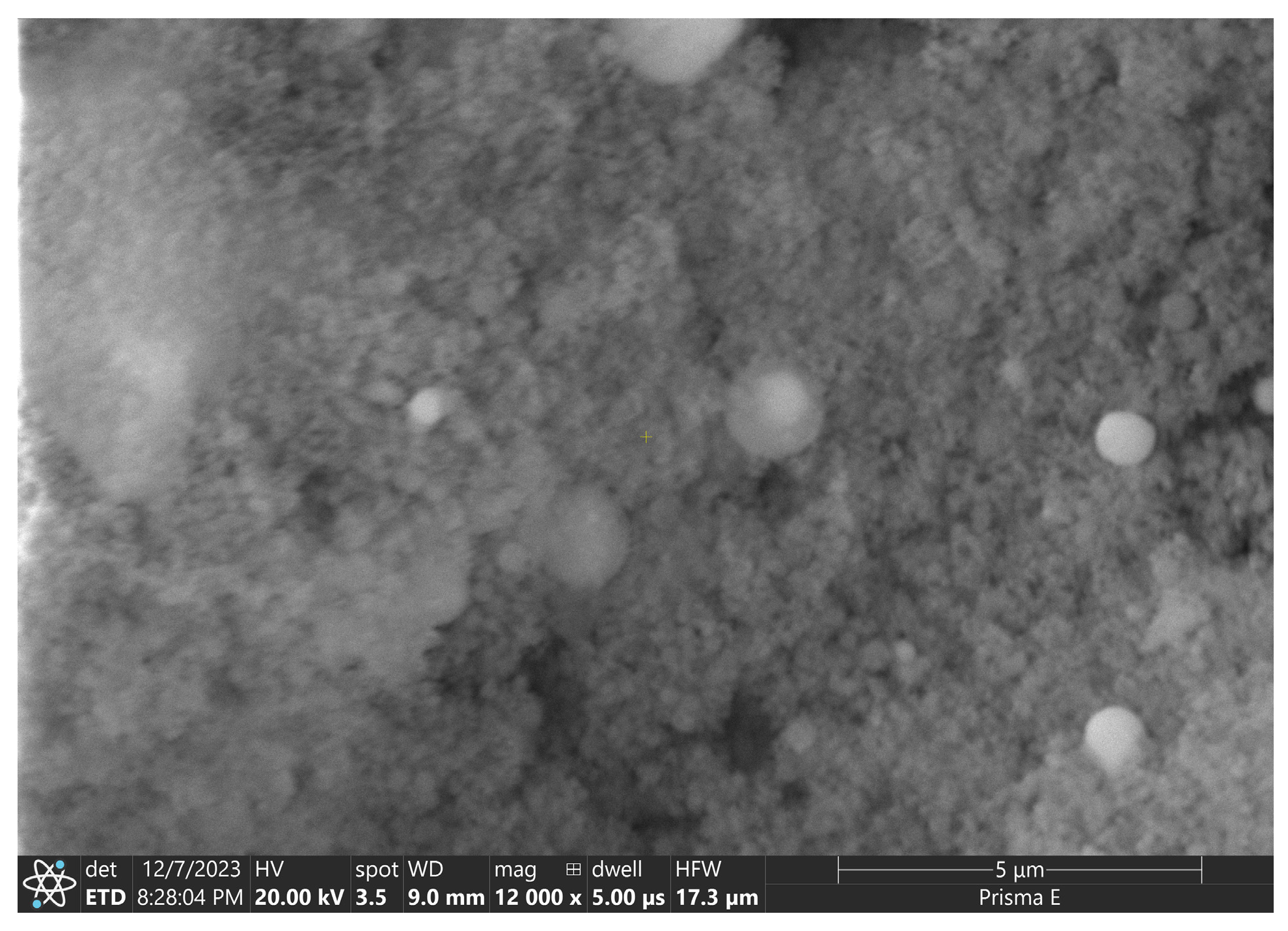
2.1. Materials
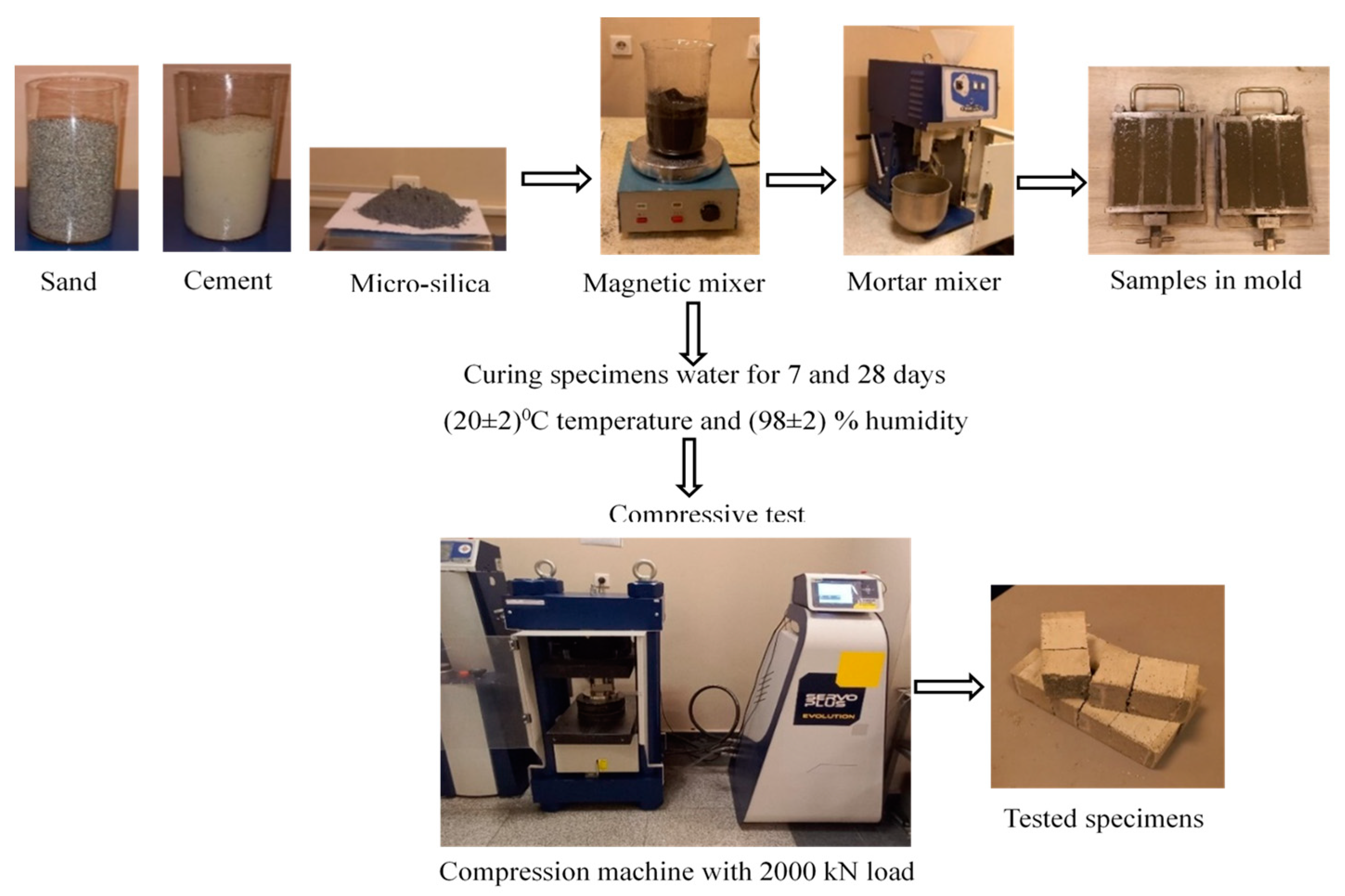
2.2. Mixing and Sample Preparation
2.3. Compressive Strength Testing
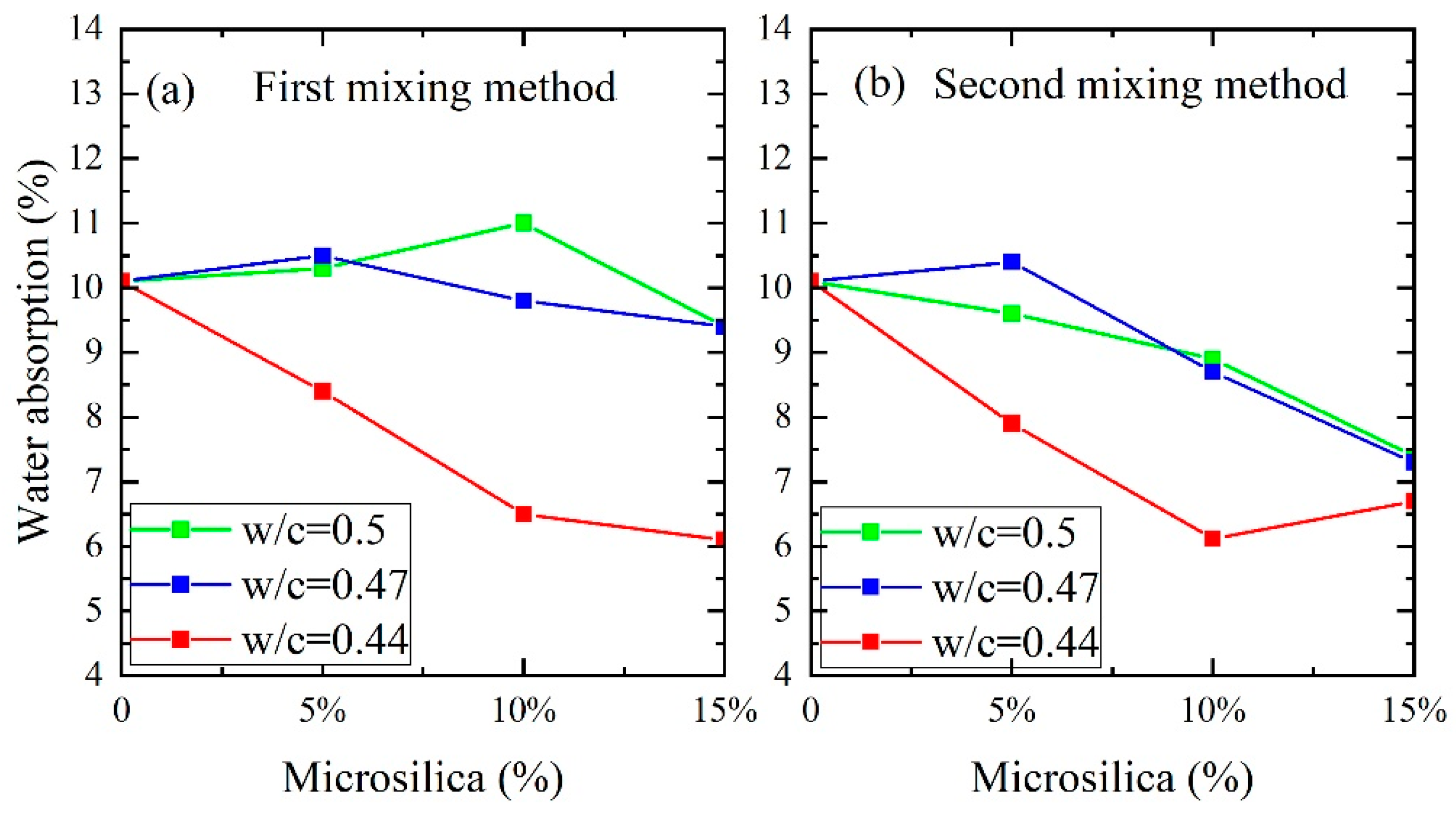
2.4. Water Absorption Calculation
3. Results and Discussion
4. Conclusions
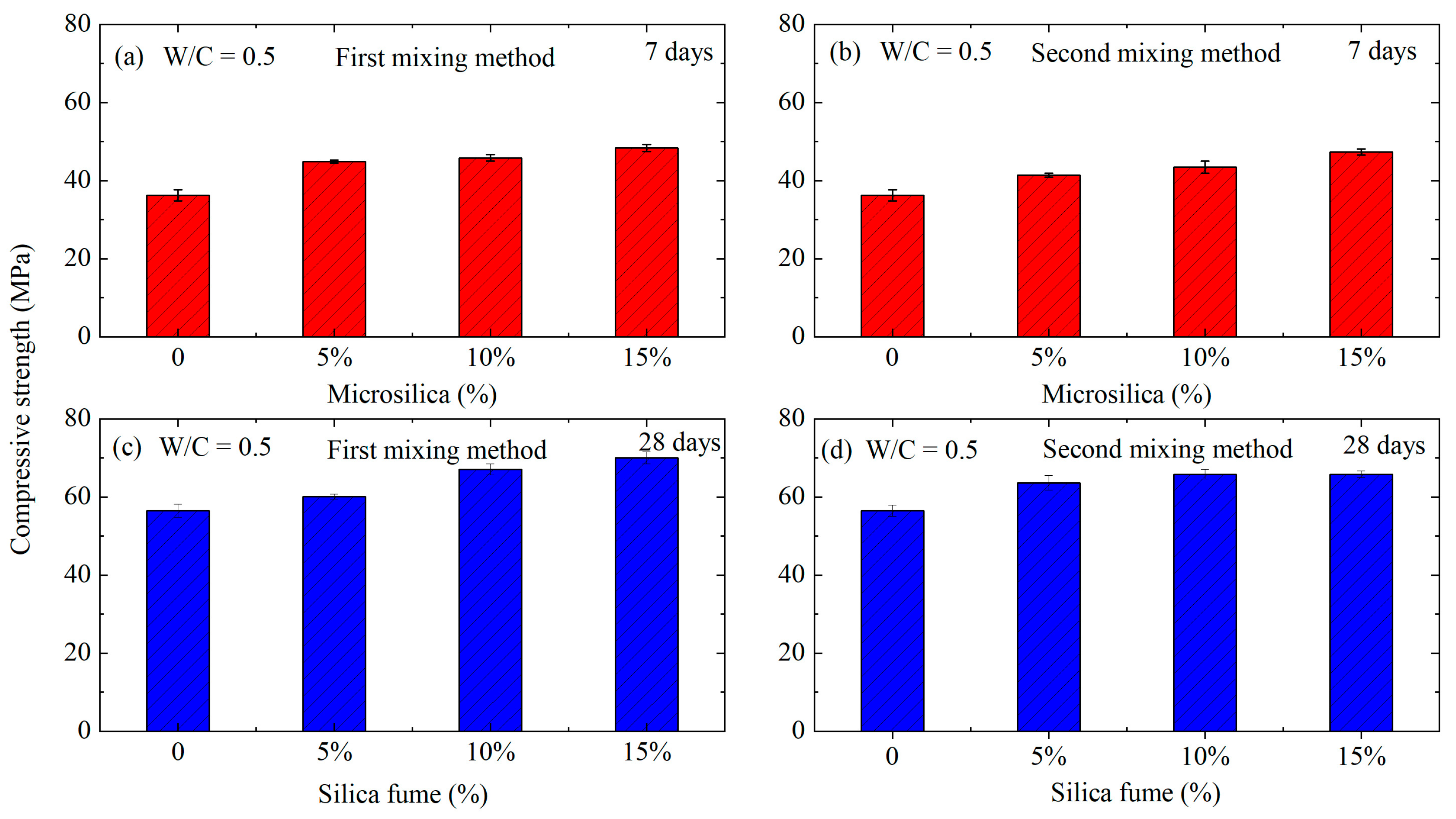
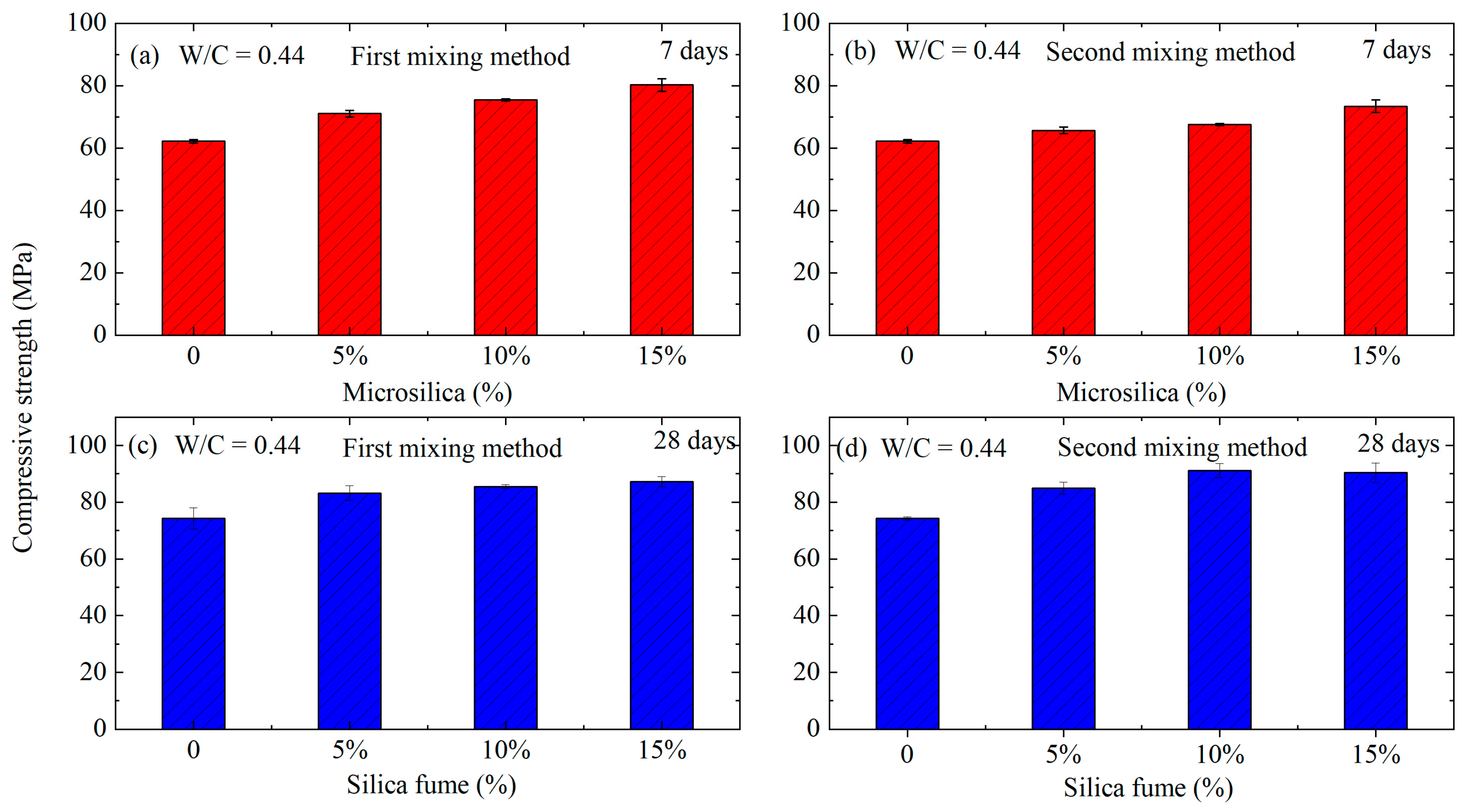
- In accordance with the second technological method, it was observed that samples prepared with water–cement ratios (W/C) of 0.47 and 0.44 exhibited higher compressive strengths compared to those prepared using the first method. However, this trend did not hold for the W/C ratio of 0.5. This phenomenon can be attributed to the inherent tendency of all mineral additives to aggregate, leading to a reduction in specific surface area. To address this, these additives are typically mixed either in a dry state with other components or in water. Stirring silica fume with water, for instance, facilitates the separation of aggregated particles through wedging with water and collision impacts during the mixing process. However, it was noted that within 5 min, lumps of silica fume persisted in the suspension, diminishing its effectiveness.
- Notably, at the lowest water–cement ratio (W/C = 0.44), the components of the mixture experience more constrained conditions. This contributes to the formation of a matrix with a denser structure, increasing the strength of the conglomerate. However, it is crucial to consider that at an increased silica fume content of 15%, the concrete mixture became rigid, making sample compaction challenging and leading to a slight decrease in strength (by 0.7 MPa). To mitigate this undesirable effect, a comprehensive modification approach is necessary, such as the addition of a plasticizer into silica.
- Based on the aforementioned data, it can be concluded that the optimal composition is achieved with a W/C ratio of 0.44 and a silica fume content of 10% by the weight of cement, as per the second scheme. This formulation exhibited a 22.5% increase in strength compared to the base sample. Furthermore, it maintained a high pH environment, making this concrete suitable for reinforced concrete structures. This comprehensive analysis underscores the significance of the intricate interplay between water–cement ratio and silica fume content, highlighting the need for a nuanced approach to achieve optimal concrete properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scarfato, P.; Maio, L.D.; Fariello, M.L. Preparation and evaluation of polymer/clay nanocomposite surface treatments for concrete durability enhancement. Cem. Concr. Compos. 2012, 34, 297–305. [Google Scholar] [CrossRef]
- Fuat, K.; Fatih, A.; Ilhami, Y.; Yus, S. Combined effect of silica fume and steel fiber on the mechanical properties of high strength concretes. Constr. Build. Mater. 2008, 22, 1874–1880. [Google Scholar] [CrossRef]
- Khayat Kamal, H.; Mitchell, D. Transportation research board, self-consolidating concrete for precast, prestressed concrete bridge elements. Technol. Eng. 2009, 91. Available online: https://www.semanticscholar.org/paper/Self-Consolidating-Concrete-for-Precast%2C-Concrete-Khayat-Mitchell/2d75c789b391893c5574b183d66ffc6e2fc11007 (accessed on 15 January 2024).
- ACI Committee 234. Guide for the use of silica fume in concrete (ACI 234R). ACI Mater. J. 1995, 92, 437–440. [Google Scholar]
- Malhotra, V.M. Fly ash, slag, silica fume, and rice-husk ash in concrete: A review. Concr. Int. 1993, 15, 23–28. [Google Scholar]
- Almusallam, A.A.; Beshr, H.; Maslehuddin, M.; Al-Amoudi, O.S.B. Effect of silica fume on the mechanical properties of low quality coarse aggregate concrete. Cem. Concr. Compos. 2004, 26, 891–900. [Google Scholar] [CrossRef]
- Phan, L.T.; Carino, N.J. Effects of test conditions and mixture proportions on behavior of high-strength concrete exposed to high temperatures. ACI Mater. J. 2002, 99, 54–66. Available online: https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=860335 (accessed on 18 January 2024).
- Sarshar, R.; Khoury, G.A. Material and environmental factors influencing the compressive strength of unsealed cement paste and concrete at high temperatures. Mag. Concr. Res. 1993, 45, 51–61. [Google Scholar] [CrossRef]
- Poon, C.; Azhar, S.; Anson, M.; Wong, Y. Comparison of the strength and durability performance of normal and high-strength pozzolanic concretes at elevated temperatures. Cem. Concr. Res. 2001, 31, 1291–1300. [Google Scholar] [CrossRef]
- Behnood, A.; Ziari, H. Effects of silica fume addition and water to cement ratio on the properties of high-strength concrete after exposure to high temperatures. Cem. Concr. Compos. 2008, 30, 106–112. [Google Scholar] [CrossRef]
- Felicetti, R.; Gambarova, P.G. Effects of high temperature on the residual compressive strength of high-strength siliceous concretes. ACI Mater. J. 1998, 95, 395–406. [Google Scholar]
- Chan, S.Y.N.; Peng, G.F.; Anson, M. Fire behavior of high-performance concrete made with silica fume at various moisture contents. ACI Mater. J. 1999, 96, 405–411. [Google Scholar]
- Parrott, L.J. Production and properties of high-strength concrete. Concrete 1969, 3, 443–448. [Google Scholar]
- Hoff, G.; Bilodeau, A.; Malhotra, V.M. Elevated temperature effects on HSC residual strength. Concr. Int. 2000, 22, 41–47. [Google Scholar]
- Janotka, I.; Bagel, L. Pore structures, perm abilities, and compressive strength of concrete at temperatures up to 800 °C. ACI Mater. J. 2002, 99, 196–200. [Google Scholar]
- Bentur, A.; Goldman, A. Curing effects, strength and physical properties of high strength silica fume concretes. J. Mater. Civ. Eng. 1989, 1, 46–58. [Google Scholar] [CrossRef]
- Hooton, R.D. Influence of silica fume replacement of cement on physical properties and resistance to sulfate attack freezing and thawing, and alkali–silica reactivity. ACI Mater. J. 1993, 90, 143–152. [Google Scholar]
- Castillo, C.; Durrani, A.J. Effect of transient high temperature on high strength concrete. ACI Mater. J. 1990, 87, 47–53. [Google Scholar]
- Sanjayan, G.; Stocks, L.J. Spalling of high-strength silica fume concrete in fire. ACI Mater. J. 1993, 90, 170–173. [Google Scholar]
- Khayat, K.H.; Aitcin, P.C. Silica Fume: A Unique Supplementary Cementations Material. In Mineral Admixtures in Cement and Concrete; Ghosh, S.N., Ed.; ABI Books Private Limited: Delhi, India, 1993; Volume 4, pp. 227–265. [Google Scholar]
- Silica Fume Association. Silica Fume Manual; Silica Fume Association: Lovettsville, VA, USA, 2005. [Google Scholar]
- Hsu, L.S.; Hsu, C.T. Complete stress–strain behavior of high-strength concrete under compression. Mag. Concr. Res. 1994, 46, 301–312. [Google Scholar] [CrossRef]
- Bhanja, S.; Sengupta, B. Influence of silica fume on the tensile strength of concrete. Cem. Concr. Res. 2005, 35, 743–747. [Google Scholar] [CrossRef]
- Igarashi, S.I.; Watanabe, A.; Kawamura, M. Evaluation of capillary pore size characteristics in high-strength concrete at early ages. Cem. Concr. Res. 2005, 35, 513–519. [Google Scholar] [CrossRef]
- Mazloom, M.; Ramezanianpour, A.A.; Brooks, J.J. Effect of silica fume on mechanical properties of high-strength concrete. Cem. Concr. Compos. 2004, 26, 347–357. [Google Scholar] [CrossRef]
- Brooks, J.J.; Cabrera, J.G.; Megat, J.M.A. Factors Affecting the Autogenous Shrinkage of Silica Fume High-Strength Concrete. In Proceedings of the International Workshop on Autogenous Shrinkage of Concrete; Japan Concrete Institute: Hiroshima, Japan, 1998; pp. 185–192. [Google Scholar]
- Tao, Z.; Weizu, Q. Tensile creep due to restraining stresses in high-strength concrete at early ages. Cem. Concr. Res. 2006, 36, 584–591. [Google Scholar] [CrossRef]
- Tazawa, E.; Yonekura, A.; Tanaka, S. Rate of Hydration and Drying Shrinkage of Condensed Silica Fume Mortar Prepared by Double Mixing. In Proceedings of the 3rd CANMET/ACI International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete, Trondheim, Norway, 19–26 June 1989; Alsali, M., Ed.; American Concrete Institute: Detroit, MI, USA, 1989; pp. 350–364. [Google Scholar]
- Tazawa, E.; Miyazawa, S. Autogenous Shrinkage of Concrete and Its Importance in Concrete Technology. In Proceedings of the 5th International RILEM Symposium on Creep and Shrinkage of Concrete, Barcelona, Spain, 6–9 September 1993; E & FN Spon: London, UK, 1993; pp. 159–168. [Google Scholar]
- Uddin, M.A.; Bashir, M.T.; Khan, A.M.; Alsharari, F.; Farid, F.; Alrowais, R. Effect of Silica Fume on Compressive Strength and Water Absorption of the Portland Cement–Silica Fume Blended Mortar. Arab. J. Sci. Eng. 2023, 1–9. [Google Scholar] [CrossRef]
- Su, Y.; Li, J.; Wu, C.; Wu, P.; Li, Z.-X. Influences of nano-particles on dynamic strength of ultra-high performance concrete. Compos. B Eng. 2016, 91, 595–609. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Zhu, J.; Kwan, A.; Chen, H. Synergistic effects of micro-silica and nano-silica on strength and microstructure of mortar. Constr. Build. Mater. 2017, 140, 229–238. [Google Scholar] [CrossRef]
- Kargari, A.; Eskandari-Naddaf, H.; Kazemi, R. Effect of cement strength class on the generalization of Abrams’ law. Struct. Concr. 2019, 20, 493–505. [Google Scholar] [CrossRef]
- Elaqra, H.; Godin, N.; Peix, G.; R’Mili, M.; Fantozzi, G. Damage evolution analysis in mortar, during compressive loading using acoustic emission and X-ray tomography: Effects of the sand/cement ratio. Cem. Concr. Res. 2007, 37, 703–713. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of superplasticizer and shrinkage-reducing admixtures on alkali-activated slag pastes and mortars. Cem. Concr. Res. 2005, 35, 1358–1367. [Google Scholar] [CrossRef]
- Haach, V.G.; Vasconcelos, G.; Lourenço, P.B. Influence of aggregates grading and water/cement ratio in workability and hardened properties of mortars. Constr. Build. Mater. 2011, 25, 2980–2987. [Google Scholar] [CrossRef]
- De Vargas, A.S.; Dal Molin, D.C.; Vilela, A.C.; Da Silva, F.J.; Pavao, B.; Veit, H. The effects of Na2O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cem. Concr. Compos. 2011, 3, 653–660. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; Rong, H.; Liang, J.; Zhang, G.; Huang, Y. The leaching-deterioration properties and leaching mechanism of cement mortar under dry-wet cycles. Constr. Build. Mater. 2023, 400, 132672. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sousa, R.; Coelho, L.; Azenha, M.; de Almeida, J.M.; Jorge, P.A.S.; Silva, C.J.R. Alkali-silica reaction in concrete: Mechanisms, mitigation and test methods. Constr. Build. Mater. 2019, 222, 903–931. [Google Scholar] [CrossRef]
- Mehta, P. History and Status of Performance Tests for Evaluation of Soundness of Cements. In Cement Standards-Evolution and Trends; ASTM STP663-EB; American Society for Testing and Materials: Philadelphia, PA, USA, 1977; pp. 35–60. [Google Scholar]
- Kabir, H.; Hooton, R.D.; Popoff, N.J. Evaluation of cement soundness using the ASTM C151 autoclave expansion test. Cem. Concr. Res. 2020, 136, 106159. [Google Scholar] [CrossRef]
- Arzumanyan, A.A.; Tadevosyan, V.G.; Muradya, N.G.; Navasardyan, H.V. Study of “Saralsk” Deposit for Practical Applications in Construction. J. Arch. Eng. Res. 2021, 1, 3–6. [Google Scholar] [CrossRef]
- GOST EN 196-1-2002; Methods of Testing Cement. Part 1. Determination of Strength. Available online: https://www.armstandard.am/en/standart/5556 (accessed on 31 July 2023).
- GOST EN 196-2-2002; Methods of Testing Cement. Part 2. Chemical Analysis of Cement. Available online: https://www.armstandard.am/en/standart/5557 (accessed on 31 July 2023).
- GOST EN 196-3-2002; Methods of Testing Cement. Part 3. Determination of Setting Time and Soundness. Available online: https://www.armstandard.am/en/standart/5558 (accessed on 31 July 2023).
- GOST 12730.3-2020; Concretes. Method of Determination of Water Absorption. Available online: https://www.armstandard.am/en/standart/5508 (accessed on 31 July 2023).


| Characteristics | Days | Results Obtained | ||||||
|---|---|---|---|---|---|---|---|---|
| Standard consistency (%) | - | 28 | ||||||
| Specific gravity (g/cm3) | - | 3.1 | ||||||
| Blaine fineness (cm2/g) | - | 4552 | ||||||
| Compressive strength (MPa) | 3 days | 21 | ||||||
| 7 days | 38 | |||||||
| 28 days | 52 | |||||||
| Setting time (min) | Initial | 55 | ||||||
| Final | 325 | |||||||
| Chemical composition of cement (wt.%) | ||||||||
| Al2O3 | SiO2 | Fe2O3 | CaO | MgO | SO3 | Loss on ignition | Insol. Residue | Free CaO |
| 4.5 | 21.9 | 2.17 | 61.6 | 1.1 | 2.1 | 3.2 | 1.9 | 1.5 |
| Fineness Modulus | Specific Gravity | Zone | Bulk Density in Compact State (kg/m3) | Bulk Density in Loose State (kg/m3) |
|---|---|---|---|---|
| 2.35 | 2.50 | II | 1641 | 1470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalyan, M.M.; Muradyan, N.G.; Shainova, R.S.; Arzumanyan, A.A.; Kalantaryan, M.A.; Sukiasyan, R.R.; Yeranosyan, M.; Laroze, D.; Vardanyan, Y.V.; Barseghyan, M.G. Effect of Silica Fume Concentration and Water–Cement Ratio on the Compressive Strength of Cement-Based Mortars. Buildings 2024, 14, 757. https://doi.org/10.3390/buildings14030757
Badalyan MM, Muradyan NG, Shainova RS, Arzumanyan AA, Kalantaryan MA, Sukiasyan RR, Yeranosyan M, Laroze D, Vardanyan YV, Barseghyan MG. Effect of Silica Fume Concentration and Water–Cement Ratio on the Compressive Strength of Cement-Based Mortars. Buildings. 2024; 14(3):757. https://doi.org/10.3390/buildings14030757
Chicago/Turabian StyleBadalyan, Maria M., Nelli G. Muradyan, Roza S. Shainova, Avetik A. Arzumanyan, Marine A. Kalantaryan, Rafayel R. Sukiasyan, Mkrtich Yeranosyan, David Laroze, Yeghiazar V. Vardanyan, and Manuk G. Barseghyan. 2024. "Effect of Silica Fume Concentration and Water–Cement Ratio on the Compressive Strength of Cement-Based Mortars" Buildings 14, no. 3: 757. https://doi.org/10.3390/buildings14030757
APA StyleBadalyan, M. M., Muradyan, N. G., Shainova, R. S., Arzumanyan, A. A., Kalantaryan, M. A., Sukiasyan, R. R., Yeranosyan, M., Laroze, D., Vardanyan, Y. V., & Barseghyan, M. G. (2024). Effect of Silica Fume Concentration and Water–Cement Ratio on the Compressive Strength of Cement-Based Mortars. Buildings, 14(3), 757. https://doi.org/10.3390/buildings14030757








