Experimental Study on the Properties and Hydration Mechanism of Gypsum-Based Composite Cementitious Materials
Abstract
1. Introduction
2. Materials and Experiments
2.1. Raw Materials
2.2. Test Programme
2.2.1. Mixing Ratio Design
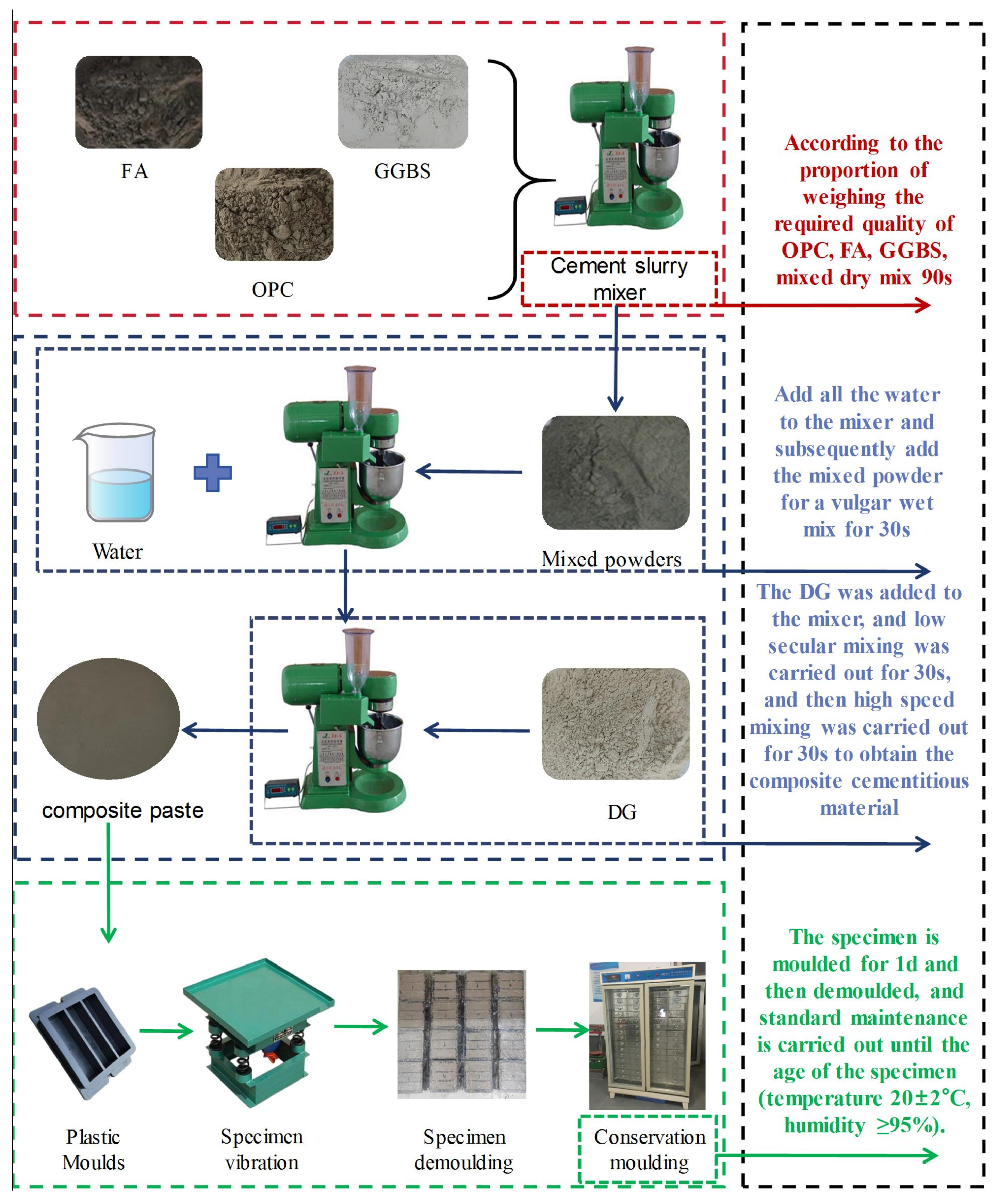
2.2.2. Sample Preparation
2.3. Test Methods
2.3.1. Mechanical Performance
2.3.2. Dry Density
2.3.3. Water Absorption
2.3.4. Softening Factor
2.3.5. X-ray Diffraction Test (XRD)
2.3.6. Scanning Electron Microscopy Test (SEM)
2.3.7. Mercury Impression Test (MIP)
3. Results and Discussions
3.1. Visual Analyses
3.2. Analysis of Variance
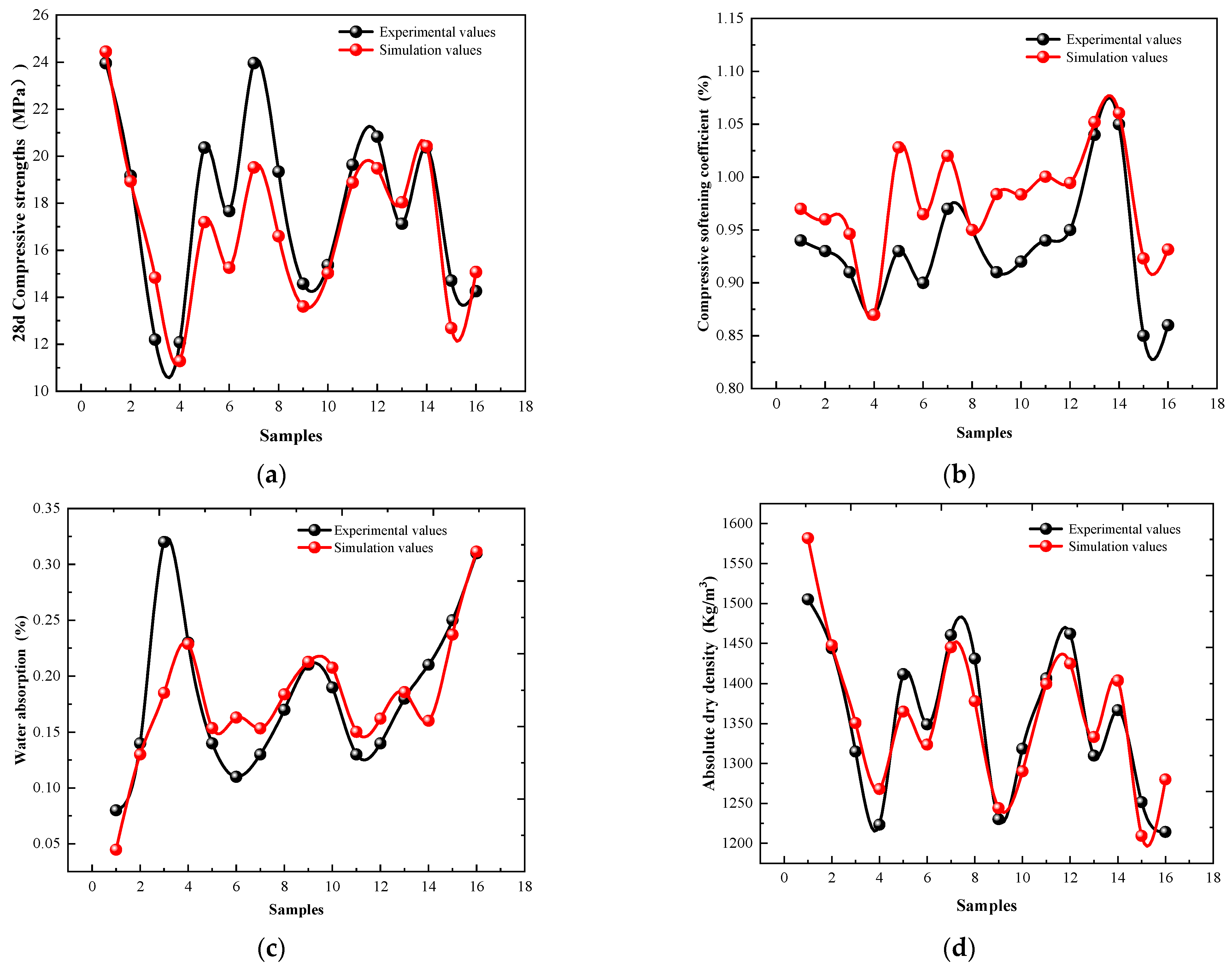
3.3. Multiple Linear Regression Model Analysis
3.4. X-ray Diffraction Test (XRD)
3.5. Scanning Electron Microscopy Test (SEM)
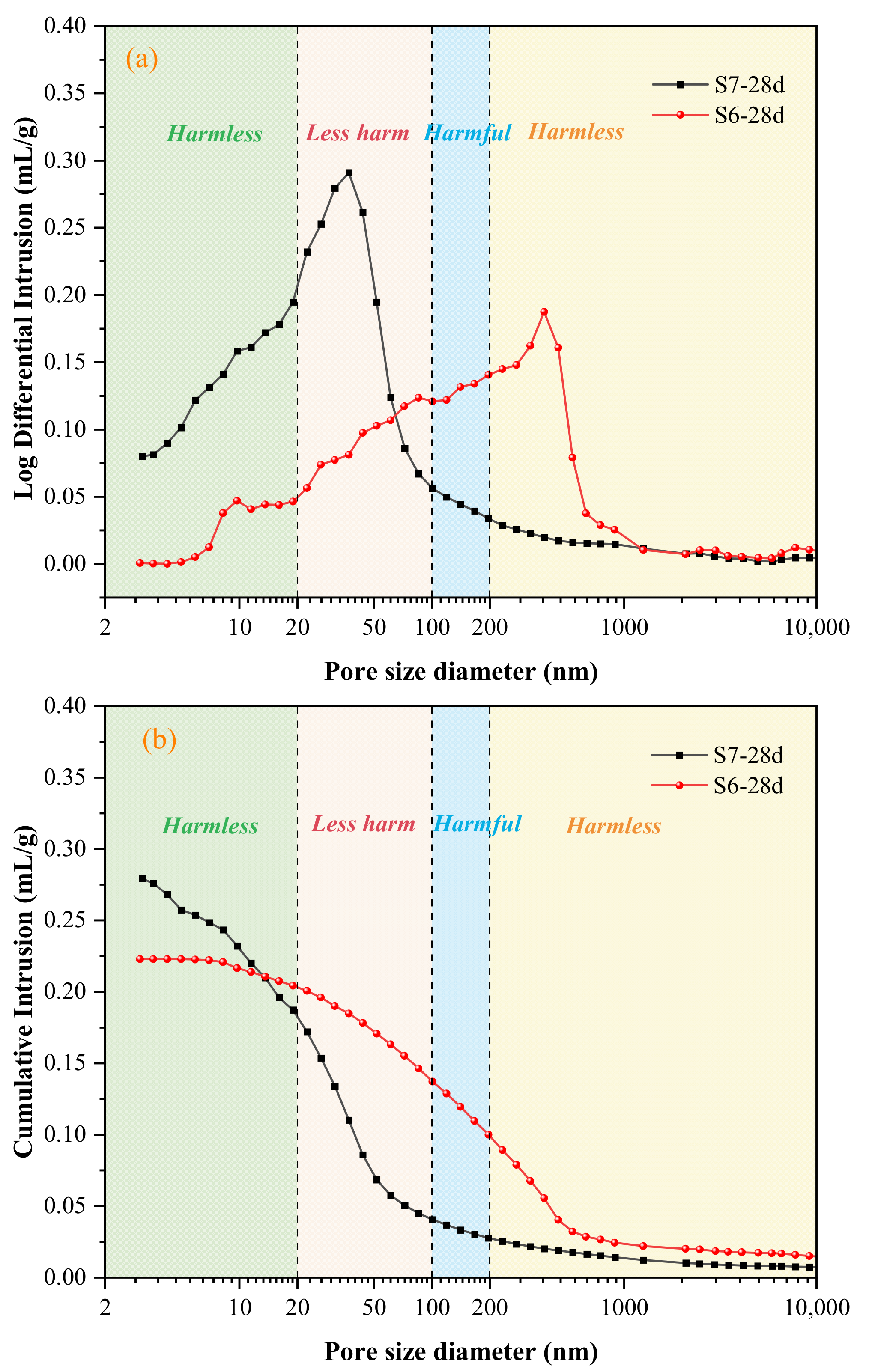
3.6. Mercury Impression Test (MIP)
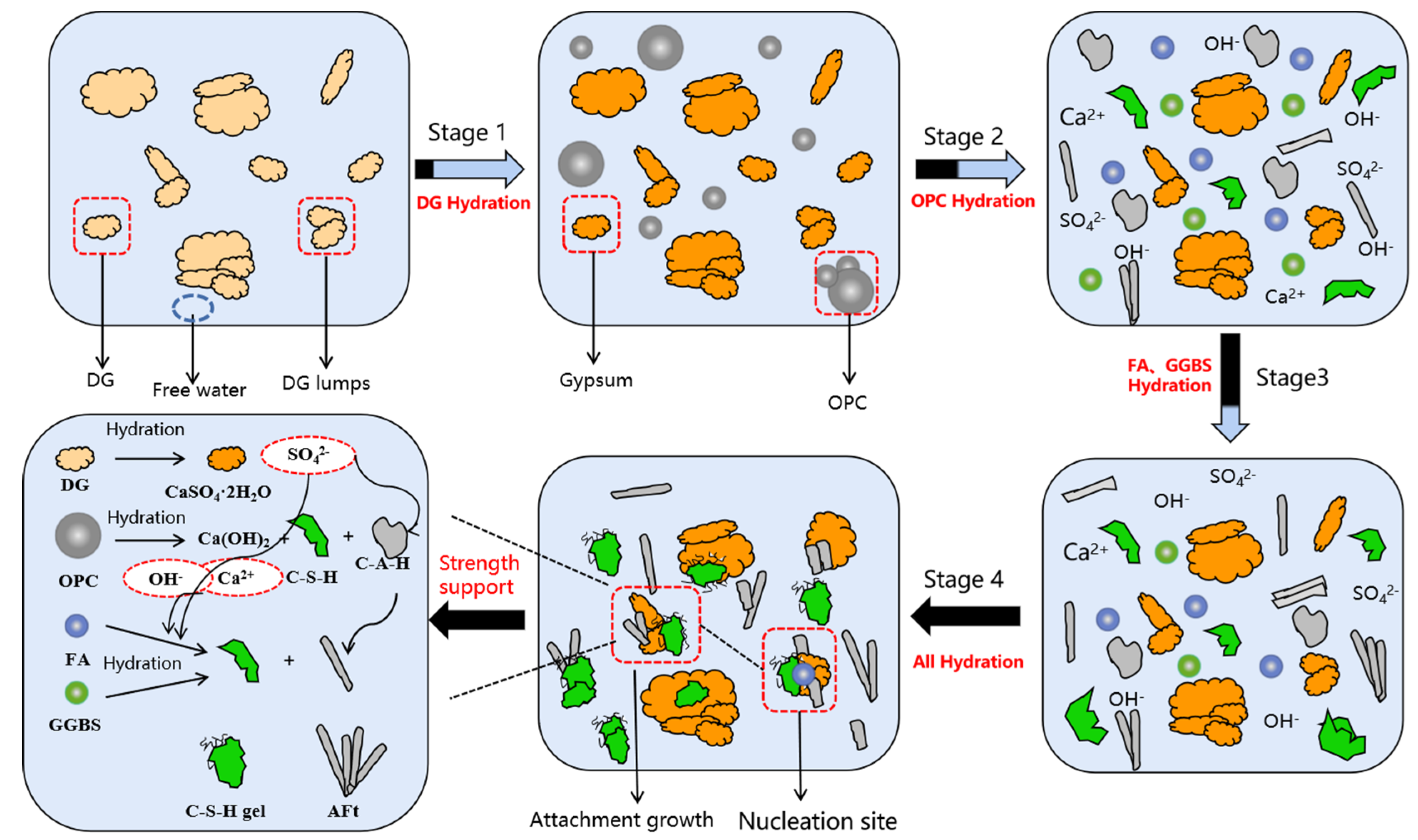
3.7. Hydration Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, S.W.; Cai, R.J.; He, Z.; Cai, X.H.; Shao, H.Y.; Li, Z.J.; Yang, H.M.; Chen, E. Continuous Microstructural Correlation of Slag/Superplasticizer Cement Pastes by Heat and Impedance Methods Via Fractal Analysis. Fractals 2017, 25, 1740003. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Huang, M.; Yin, H.; Jiang, K.; Xiao, K.; Tang, S. Influence of Different Alkali Sulfates on the Shrinkage, Hydration, Pore Structure, Fractal Dimension and Microstructure of Low-Heat Portland Cement, Medium-Heat Portland Cement and Ordinary Portland Cement. Fractal Fract. 2021, 5, 79. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, H.; Cai, X.; Chen, L.; Liu, M.; Lin, H.; Wang, X. Characteristics of CO2 and atmospheric pollutant emissions from China’s cement industry: A life-cycle perspective. J. Clean. Prod. 2021, 282, 124533. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K. Empirical assessing cement CO2 emissions based on China’s economic and social development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, H.; Yi, Z.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 2: The residues after iron recovery from iron ore tailings to prepare cementitious material. J. Hazard. Mater. 2010, 174, 78–83. [Google Scholar] [CrossRef]
- Li, D.; Song, X.; Gong, C.; Pan, Z. Research on cementitious behavior and mechanism of pozzolanic cement with coal gangue. Cem. Concr. Res. 2006, 36, 1752–1759. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Zhao, F.; Huo, T.; Chen, E.; Tang, S. Comparison between the Influence of Finely Ground Phosphorous Slag and Fly Ash on Frost Resistance, Pore Structures and Fractal Features of Hydraulic Concrete. Fractal Fract. 2022, 6, 598. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, S.; Shi, Y.; Huang, Y.; Zhao, F.; Huo, T.; Tang, S. The Influence of Fly Ash Dosages on the Permeability, Pore Structure and Fractal Features of Face Slab Concrete. Fractal Fract. 2022, 6, 476. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Liu, W.; Lin, L.; Wang, S.; Peng, X.; Wu, B.; Sun, K.; Zeng, L. Setting and Hardening Behaviour of Alkali-Activated Landfilled Fly Ash-Slag Binder at Room Temperature. Materials 2020, 13, 3130. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; He, X.; Su, Y.; Zeng, J.; Xiong, L.; Zeng, L.; Yu, X.; Tan, H. Low-carbon wet-ground fly ash geopolymer activated by single calcium carbide slag. Constr. Build. Mater. 2022, 353, 129084. [Google Scholar] [CrossRef]
- Magalhães, L.F.d.; França, S.; Oliveira, M.d.S.; Peixoto, R.A.F.; Bessa, S.A.L.; Bezerra, A.C.d.S. Iron ore tailings as a supplementary cementitious material in the production of pigmented cements. J. Clean. Prod. 2020, 274, 123260. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Hou, G. Utilization of fly ash microsphere powder as a mineral admixture of cement: Effects on early hydration and microstructure at different curing temperatures. Powder Technol. 2020, 375, 262–270. [Google Scholar] [CrossRef]
- Yong, C.L.; Mo, K.H.; Koting, S. Phosphorus slag in supplementary cementitious and alkali activated materials: A review on activation methods. Constr. Build. Mater. 2022, 352, 129028. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, S.; Zhou, Y.; Zhang, C.; Meng, T.; Jiang, S.; Liu, L.; Hu, G. Effect of incorporation of rice husk ash and iron ore tailings on properties of concrete. Constr. Build. Mater. 2022, 338, 127584. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, J.; Deng, F.; Li, H.; Wang, K.; Tang, S. Hydration behavior and strength development of supersulfated cement prepared by calcined phosphogypsum and slaked lime. J. Build. Eng. 2023, 80, 108075. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, S.; Wang, K.; Qunaynah, S.A.; Wan, S.; Yuan, Z.; Xu, P.; Tang, S. A study on the hydration of calcium aluminate cement pastes containing silica fume using non-contact electrical resistivity measurement. J. Mater. Res. Technol. 2023, 24, 8135–8149. [Google Scholar] [CrossRef]
- Kakasor Ismael Jaf, D.; Ismael Abdulrahman, P.; Salih Mohammed, A.; Kurda, R.; Qaidi, S.M.A.; Asteris, P.G. Machine learning techniques and multi-scale models to evaluate the impact of silicon dioxide (SiO2) and calcium oxide (CaO) in fly ash on the compressive strength of green concrete. Constr. Build. Mater. 2023, 400, 132604. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Yao, G.; Liu, Q.; Wang, J.; Wu, P.; Lyu, X. Effect of mechanical grinding on pozzolanic activity and hydration properties of siliceous gold ore tailings. J. Clean. Prod. 2019, 217, 12–21. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.; Liu, J.; Zhu, Z.; Wang, S.; Xu, X.; Meng, J. Enhancement mechanism of micro-iron ore tailings on mechanical properties and hydration characteristics of cement-steel slag system. J. Build. Eng. 2023, 79, 107882. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Zhang, G.; Ding, Q.; Sun, X. The Preparation of Ground Blast Furnace Slag-Steel Slag Pavement Concrete Using Different Activators and Its Performance Investigation. Buildings 2023, 13, 1590. [Google Scholar] [CrossRef]
- Dai, X.; Aydın, S.; Yardımcı, M.Y.; Lesage, K.; Schutter, G.D. Effect of Ca(OH)2 Addition on the Engineering Properties of Sodium Sulfate Activated Slag. Materials 2021, 14, 4266. [Google Scholar] [CrossRef]
- Kim, T.; Jun, Y. Mechanical Properties of Na2CO3-Activated High-Volume GGBFS Cement Paste. Adv. Civ. Eng. 2018, 2018, 8905194. [Google Scholar] [CrossRef]
- Gu, X.; Ge, X.; Liu, J.; Song, G.; Wang, S.; Hu, Z.; Wang, H. Study on the synergistic effect of calcium carbide residue -fly ash enhanced desulphurisation gypsum under high temperature maintenance condition. Constr. Build. Mater. 2024, 412, 134706. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yucel Yardimci, M.; De Schutter, G. Fresh and hardened state properties, reaction kinetics and microstructure of sodium sulfate/sodium hydroxide—Activated slag mixtures. Constr. Build. Mater. 2023, 401, 132943. [Google Scholar] [CrossRef]
- Wansom, S.; Chintasongkro, P.; Srijampan, W. Water resistant blended cements containing flue-gas desulfurization gypsum, Portland cement and fly ash for structural applications. Cem. Concr. Compos. 2019, 103, 134–148. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Liu, C.; Zhao, Y. Effect of neutralization on the setting and hardening characters of hemihydrate phosphogypsum plaster. Constr. Build. Mater. 2018, 190, 53–64. [Google Scholar] [CrossRef]
- Duan, P.X.; Zhang, Y.; Miao, Y.C.; Li, Y. A Study of the Influence of FGD Gypsum Used as Cement Retarder on the Properties of Concrete. Adv. Mater. Res. 2011, 374–377, 1311–1319. [Google Scholar] [CrossRef]
- Wang, J.H.; Luan, Y.C.; Ma, T.; Zhang, W.G.; Xu, G.J. Experimental investigation on the mechanical performance and microscopic characterization of desulfurization gypsum-reinforced ternary geopolymer. Constr. Build. Mater. 2023, 392, 131855. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, H. Characterization and optimization of eco-friendly cementitious materials based on titanium gypsum, fly ash, and calcium carbide residue. Constr. Build. Mater. 2022, 349, 128635. [Google Scholar] [CrossRef]
- GB/T 14506.28-2010; Methods for Chemical Analysis of Silicate Rocks-Part 28: Determination of 16 Major and Minor Elements Content. Standardization Administration of the People’s Republic of China: Beijing, China, 2010.
- GB/T 19077-2016; Particle Size Analysis-Laser Diffraction Methods. Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- JY/T 0587-2020; General Rules for X-Ray Polycrystalline Diffractometry. Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- JY/T 0584-2020; General Analysis Rules for Transmission Electron Microscope. Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- Wang, S.; Gu, X.; Liu, J.; Zhu, Z.; Wang, H.; Ge, X.; Xu, X.; Nehdi, M.L. Modulation of the workability and Ca/Si/Al ratio of cement-metakaolin cementitious material system by using fly ash: Synergistic effect and hydration products. Constr. Build. Mater. 2023, 404, 133300. [Google Scholar] [CrossRef]
- Moghaddam, F.; Sirivivatnanon, V.; Vessalas, K. The effect of fly ash fineness on heat of hydration, microstructure, flow and compressive strength of blended cement pastes. Case Stud. Constr. Mater. 2019, 10, e00218. [Google Scholar] [CrossRef]
- Jin, H.; Li, F.; Li, X. Research on the Micro-Pore Structures of AAFAM. Arab. J. Sci. Eng. 2021, 46, 10885–10900. [Google Scholar] [CrossRef]
- Mathapati, M.; Amate, K.; Durga Prasad, C.; Jayavardhana, M.L.; Hemanth Raju, T. A review on fly ash utilization. Mater. Today: Proc. 2022, 50, 1535–1540. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- GB/T 23451-2009; Light Weight Panels for Partition Wall Used in Buildings. Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- Han, X.; Feng, J.; Wang, B. Relationship between fractal feature and compressive strength of fly ash-cement composite cementitious materials. Cem. Concr. Compos. 2023, 139, 105052. [Google Scholar] [CrossRef]
- Sarbapalli, D.; Dhabalia, Y.; Sarkar, K.; Bhattacharjee, B. Application of SAP and PEG as curing agents for ordinary cement-based systems: Impact on the early age properties of paste and mortar with water-to-cement ratio of 0.4 and above. Eur. J. Environ. Civ. Eng. 2016, 21, 1237–1252. [Google Scholar] [CrossRef]
- Şimşek, B.; İç, Y.T.; Şimşek, E.H. A RSM-Based Multi-Response Optimization Application for Determining Optimal Mix Proportions of Standard Ready-Mixed Concrete. Arab. J. Sci. Eng. 2015, 41, 1435–1450. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S. Influence of water-to-cement ratio and curing period on pore structure of cement mortar. Constr. Build. Mater. 2013, 38, 804–812. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Tian, Y.; Wu, P.; Guo, Z.; Qiu, J.; Xing, J.; Xiaowei, G. Modification of high-volume fly ash cement with metakaolin for its utilization in cemented paste backfill: The effects of metakaolin content and particle size. Powder Technol. 2021, 393, 539–549. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, C.; Sun, W. Fly ash effects. Cem. Concr. Res. 2004, 34, 2061–2066. [Google Scholar] [CrossRef]
- Liu, J.; Ge, X.; Liu, P.; Song, G.; Hu, Z. Experimental study on the preparation of cementitious materials from iron ore tailings by activation. Constr. Build. Mater. 2023, 385, 131409. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Kong, D.; Peng, D.; Zheng, T. Research on optimizing performance of desulfurization-gypsum-based composite cementitious materials based on response surface method. Constr. Build. Mater. 2022, 341, 127874. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, H.; Yu, T.; Yuan, P.; Deng, L.; Zhang, B. Utilization of Calcium Carbide Residue as Solid Alkali for Preparing Fly Ash-Based Geopolymers: Dependence of Compressive Strength and Microstructure on Calcium Carbide Residue, Water Content and Curing Temperature. Materials 2022, 15, 973. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, X.; Liu, B.; Li, Z.; Zhang, W.; Liu, J.; Nehdi, M.L. Evaluation of waste powder from open pit mines as supplementary cementitious material: Crystal structure and hydration characteristics. J. Build. Eng. 2023, 71, 106514. [Google Scholar] [CrossRef]
- Zhong, S.; Ni, K.; Li, J. Properties of mortars made by uncalcined FGD gypsum-fly ash-ground granulated blast furnace slag composite binder. Waste Manag. 2012, 32, 1468–1472. [Google Scholar] [CrossRef]
- Gu, X.; Wang, H.; Zhu, Z.; Liu, J.; Xu, X.; Wang, Q. Synergistic effect and mechanism of lithium slag on mechanical properties and microstructure of steel slag-cement system. Constr. Build. Mater. 2023, 396, 131768. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on microstructure of blended cement paste. Constr. Build. Mater. 2007, 21, 1534–1541. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Yang, H.; Lv, X.; Guo, F.; Shi, Y.; Hanif, A. Investigation and Application of Fractal Theory in Cement-Based Materials: A Review. Fractal Fract. 2021, 5, 247. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Xu, X.; Zhang, Z. Using calcium carbide residue to prepare ecological alkali activated slag composites: Effect of anion type. Ceram. Int. 2023, 49, 25092–25104. [Google Scholar] [CrossRef]
- Tang, S.W.; Wang, L.; Cai, R.J.; Cai, X.H.; He, Z.; Chen, E. The Evaluation of Electrical Impedance of Three-Dimensional Fractal Networks Embedded in a Cube. Fractals 2017, 25, 1740005. [Google Scholar] [CrossRef]
- Tang, S.W.; A, H.B.; Yu, P.; Wang, L.; Cai, R.J.; Chen, E. The Analysis of Thermal Resistance of Three-Dimensional Fractal Networks Embedded in a Sphere. Fractals 2018, 26, 1850036. [Google Scholar] [CrossRef]
- Han, X.; Wang, B.; Feng, J. Relationship between fractal feature and compressive strength of concrete based on MIP. Constr. Build. Mater. 2022, 322, 126504. [Google Scholar] [CrossRef]
- Zeng, H.; Qu, S.; Qin, Y. Microstructure and transport properties of cement-based material enhanced by graphene oxide. Mag. Concr. Res. 2021, 73, 1011–1024. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Geng, Z.; Xu, X.; Yu, W.; A, H.; Chen, J. Structure, Fractality, Mechanics and Durability of Calcium Silicate Hydrates. Fractal Fract. 2021, 5, 47. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Zhou, S.; Tang, S.; Lu, X. Investigation of microstructure of C-S-H and micro-mechanics of cement pastes under NH4NO3 dissolution by 29Si MAS NMR and microhardness. Measurement 2021, 185, 110019. [Google Scholar] [CrossRef]
- Lootens, D.; Bentz, D.P. On the Relation of Setting and Early-Age Strength Development to Porosity and Hydration in Cement-Based Materials. Cem. Concr. Compos. 2016, 68, 9–14. [Google Scholar] [CrossRef]
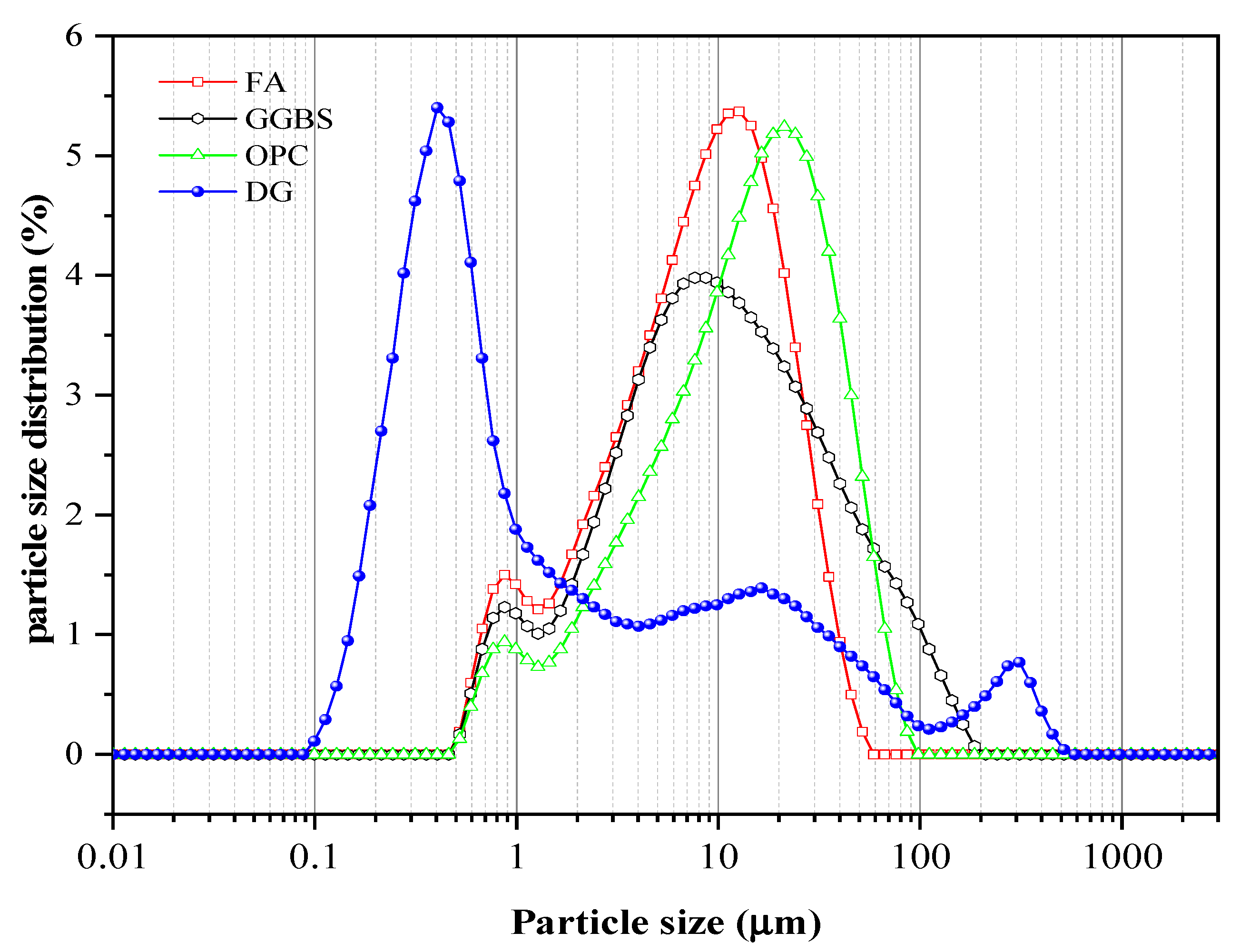
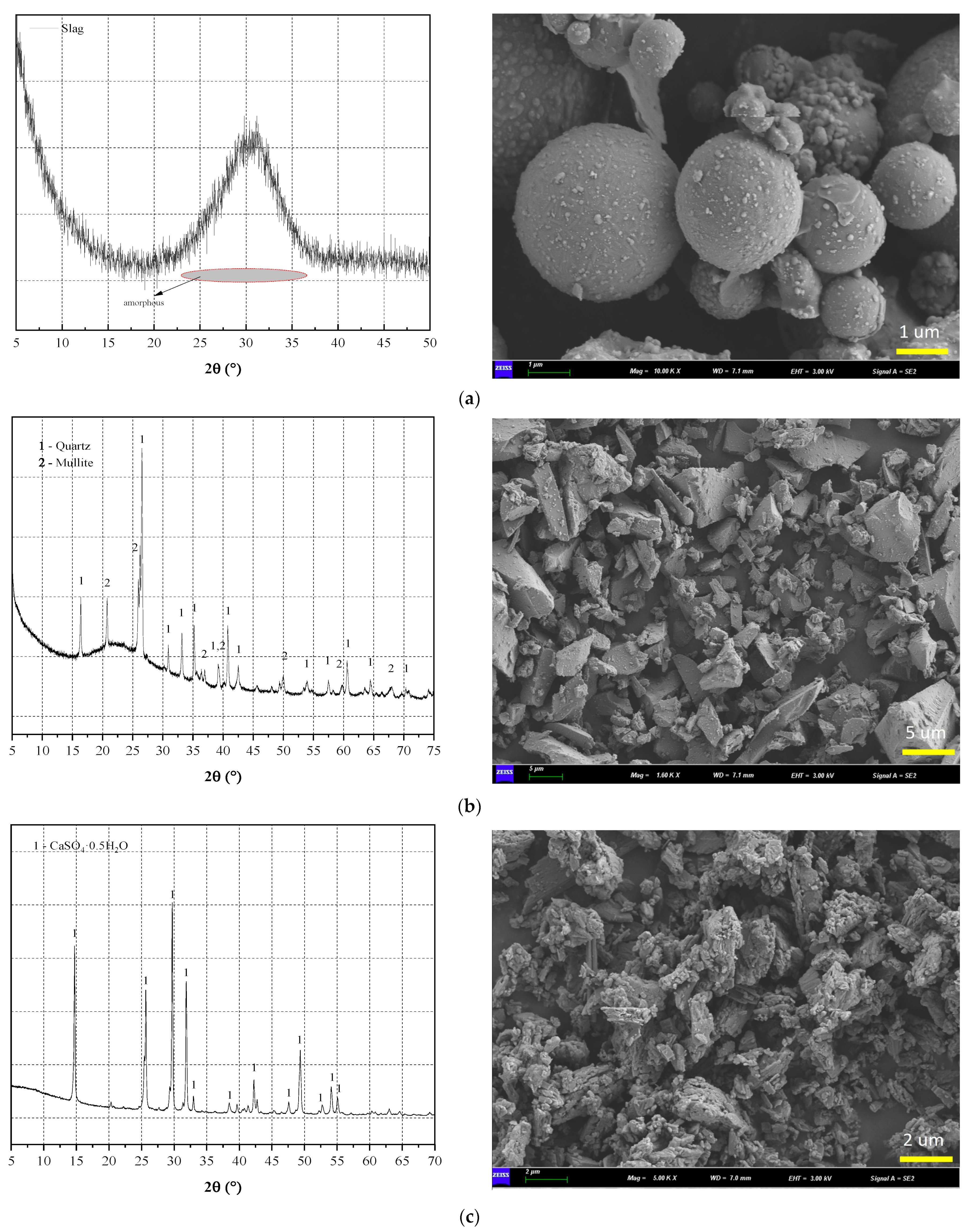




| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | K2O | Na2O | SO3 | TiO2 | |
|---|---|---|---|---|---|---|---|---|---|
| OPC | 25.53 | 9.01 | 54.01 | 3.29 | 3.36 | ||||
| DG | 2.24 | 0.55 | 48.476 | 0.71 | 0.462 | 46.481 | 0.033 | ||
| FA | 56.2 | 18.67 | 9.89 | 2.12 | 6.08 | 1.23 | 1.15 | 2.95 | |
| GGBS | 33.98 | 15.22 | 36.91 | 9.27 | 0.62 | 0.41 | 0.39 | 1.81 |
| Factors | A | B | C | D | |
|---|---|---|---|---|---|
| Levels | OPC/(FGDG + FA + GGBS) | FGDG/(FA + GGBS) | FA/GGBS | W/B | |
| 1 | 15:85 | 50:50 | 1:1 | 0.55 | |
| 2 | 20:80 | 60:40 | 2:1 | 0.6 | |
| 3 | 25:75 | 70:30 | 3:1 | 0.65 | |
| 4 | 30:70 | 80:20 | 4:1 | 0.7 | |
| Number | OPC (%) | FGDG (%) | FA (%) | GGBS (%) | W/B |
|---|---|---|---|---|---|
| S1 | 15 | 42.5 | 21.3 | 21.2 | 0.55 |
| S2 | 15 | 51 | 22.7 | 11.3 | 0.6 |
| S3 | 15 | 59.5 | 19.1 | 6.34 | 0.65 |
| S4 | 15 | 68 | 13.6 | 3.4 | 0.7 |
| S5 | 20 | 40 | 26.7 | 13.3 | 0.65 |
| S6 | 20 | 48 | 16 | 16 | 0.7 |
| S7 | 20 | 56 | 19.2 | 4.8 | 0.55 |
| S8 | 20 | 64 | 12 | 4 | 0.6 |
| S9 | 25 | 37.5 | 28.1 | 9.4 | 0.7 |
| S10 | 25 | 45 | 24 | 6 | 0.65 |
| S11 | 25 | 52.5 | 11.3 | 11.2 | 0.6 |
| S12 | 25 | 60 | 10 | 5 | 0.55 |
| S13 | 30 | 35 | 28 | 7 | 0.6 |
| S14 | 30 | 42 | 21 | 7 | 0.55 |
| S15 | 30 | 49 | 14 | 7 | 0.7 |
| S16 | 30 | 56 | 7 | 7 | 0.65 |
| Number | Flexural Strengths (MPa) | Compressive Strengths (MPa) | Softening Coefficient (%) | Water Absorption (%) | Dry Density (kg/m3) | |||
|---|---|---|---|---|---|---|---|---|
| 7d | 28d | 7d | 28d | Flexural | Compressive | |||
| S1 | 2.75 | 5.16 | 13.54 | 23.96 | 0.95 | 0.94 | 8.26 | 1505.2 |
| S2 | 2.01 | 4.64 | 9.02 | 19.17 | 0.94 | 0.93 | 13.98 | 1443.9 |
| S3 | 1.31 | 3.71 | 4.68 | 12.20 | 0.92 | 0.91 | 31.65 | 1315.0 |
| S4 | 1.03 | 3.47 | 4.43 | 12.08 | 0.89 | 0.87 | 23.04 | 1223.2 |
| S5 | 1.64 | 4.97 | 8.93 | 20.36 | 0.93 | 0.93 | 13.71 | 1411.5 |
| S6 | 1.63 | 3.41 | 8.29 | 17.67 | 0.91 | 0.90 | 11.16 | 1348.9 |
| S7 | 2.89 | 5.35 | 9.66 | 23.96 | 0.96 | 0.97 | 12.80 | 1460.5 |
| S8 | 2.11 | 4.71 | 7.81 | 19.34 | 0.95 | 0.95 | 16.81 | 1430.8 |
| S9 | 1.31 | 3.68 | 6.18 | 14.57 | 0.92 | 0.91 | 21.34 | 1230.1 |
| S10 | 1.27 | 3.86 | 5.62 | 15.37 | 0.92 | 0.92 | 18.97 | 1318.5 |
| S11 | 2.11 | 4.55 | 8.25 | 19.64 | 0.94 | 0.94 | 12.69 | 1406.3 |
| S12 | 2.05 | 4.50 | 9.15 | 20.83 | 0.97 | 0.95 | 13.62 | 1462.2 |
| S13 | 1.97 | 4.23 | 8.32 | 17.13 | 0.92 | 0.93 | 17.91 | 1309.7 |
| S14 | 2.44 | 4.86 | 9.86 | 20.38 | 0.93 | 1.05 | 21.31 | 1366.8 |
| S15 | 1.05 | 3.65 | 5.45 | 14.71 | 0.90 | 0.85 | 24.96 | 1251.6 |
| S16 | 1.43 | 3.50 | 5.96 | 14.26 | 0.92 | 0.86 | 30.98 | 1214.2 |
| Factors | k1 | k2 | k3 | k4 | R | |
|---|---|---|---|---|---|---|
| 28 d Compressive strengths (MPa) | A | 16.85 | 20.33 | 17.6 | 16.62 | 3.72 |
| B | 19.0 | 18.15 | 17.62 | 16.63 | 2.38 | |
| C | 18.88 | 18.77 | 16.62 | 17.13 | 2.26 | |
| D | 22.28 | 18.82 | 15.55 | 14.76 | 7.52 | |
| Compressive softening coefficient (%) | A | 0.91 | 0.94 | 0.93 | 0.95 | 0.04 |
| B | 0.96 | 0.95 | 0.92 | 0.91 | 0.05 | |
| C | 0.91 | 0.91 | 0.96 | 0.95 | 0.05 | |
| D | 0.98 | 0.96 | 0.9 | 0.88 | 0.1 | |
| Water absorption (%) | A | 0.19 | 0.14 | 0.17 | 0.24 | 0.1 |
| B | 0.15 | 0.16 | 0.21 | 0.21 | 0.06 | |
| C | 0.16 | 0.17 | 0.23 | 0.18 | 0.07 | |
| D | 0.14 | 0.15 | 0.24 | 0.2 | 0.1 | |
| Absolute dry density (kg/m3) | A | 1384.4 | 1412.9 | 1354.3 | 1285.6 | 127.35 |
| B | 1376.6 | 1369.6 | 1358.3 | 1332.6 | 43.99 | |
| C | 1381.2 | 1392.3 | 1335.7 | 1327.9 | 64.35 | |
| D | 1461.2 | 1397.7 | 1314.8 | 1263.5 | 197.7 |
| Factors | Square Sum | Mean Square | F Value | Significance | |
|---|---|---|---|---|---|
| 28 d Compressive strengths (MPa) | A | 34.928 | 11.643 | 7.718 | 0.064 |
| B | 15.703 | 5.234 | 3.47 | 0.167 | |
| C | 11.866 | 3.955 | 2.622 | 0.225 | |
| D | 141.812 | 47.271 | 31.336 | 0.009 | |
| Inaccuracy | 4.256 | 1.509 | / | / | |
| Aggregate | 5307.88 | / | / | / | |
| Compressive softening coefficient (%) | A | 0.003 | 0.001 | 2.694 | 0.114 |
| B | 0.007 | 0.002 | 1.565 | 0.361 | |
| C | 0.007 | 0.002 | 1.529 | 0.368 | |
| D | 0.025 | 0.008 | 5.965 | 0.088 | |
| Inaccuracy | 0.004 | 0.001 | / | / | |
| Aggregate | 13.959 | / | / | / | |
| Water absorption (%) | A | 0.024 | 0.008 | 16.374 | 0.023 |
| B | 0.01 | 0.003 | 6.862 | 0.074 | |
| C | 0.012 | 0.004 | 7.891 | 0.062 | |
| D | 0.022 | 0.007 | 14.902 | 0.026 | |
| Inaccuracy | 0.001 | 0 | / | / | |
| Aggregate | 0.607 | / | / | / | |
| Absolute dry density (kg/m3) | A | 35857.8 | 11952.62 | 6.272 | 0.083 |
| B | 4471.3 | 1490.432 | 0.782 | 0.578 | |
| C | 12434.9 | 4144.984 | 2.175 | 0.27 | |
| D | 92055 | 30685.04 | 16.101 | 0.024 | |
| Inaccuracy | 5717.5 | 1905.82 | / | / | |
| Aggregate | 29712538.7 | / | / | / |
| Model | Model Coefficients (t Value) | ||||||
|---|---|---|---|---|---|---|---|
| R Value | F Value | Significance | |||||
| 28 d Compressive strengths (MPa) | 0.881 | 9.534 | 0.001 | −0.024 | 0.007 | 0.366 | −0.783 |
| Compressive softening coefficient (%) | 0.889 | 10.335 | 0.001 | −1.54 | 1.059 | −1.973 | −5.314 |
| Water absorption (%) | 0.812 | 2.825 | 0.078 | −0.623 | 0.667 | −2.337 | 2.044 |
| Absolute dry density (kg/m3) | 0.93 | 17.624 | 0.001 | 2.735 | 1.844 | 4.487 | −6.884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Song, G.; Ge, X.; Liu, B.; Liu, K.; Tian, Y.; Wang, X.; Hu, Z. Experimental Study on the Properties and Hydration Mechanism of Gypsum-Based Composite Cementitious Materials. Buildings 2024, 14, 314. https://doi.org/10.3390/buildings14020314
Liu J, Song G, Ge X, Liu B, Liu K, Tian Y, Wang X, Hu Z. Experimental Study on the Properties and Hydration Mechanism of Gypsum-Based Composite Cementitious Materials. Buildings. 2024; 14(2):314. https://doi.org/10.3390/buildings14020314
Chicago/Turabian StyleLiu, Jianping, Ge Song, Xiaowei Ge, Bing Liu, Kaixin Liu, Yulin Tian, Xu Wang, and Zhihang Hu. 2024. "Experimental Study on the Properties and Hydration Mechanism of Gypsum-Based Composite Cementitious Materials" Buildings 14, no. 2: 314. https://doi.org/10.3390/buildings14020314
APA StyleLiu, J., Song, G., Ge, X., Liu, B., Liu, K., Tian, Y., Wang, X., & Hu, Z. (2024). Experimental Study on the Properties and Hydration Mechanism of Gypsum-Based Composite Cementitious Materials. Buildings, 14(2), 314. https://doi.org/10.3390/buildings14020314





