Abstract
Curing early age concrete (hereinafter referred to as EAC) with CO2 as a new method for capturing and storing CO2 can not only result in energy savings and emission reductions, but can also improve the performance of early age concrete and shorten the curing time, which leads to various application prospects. In this paper, we collect the existing research results at home and abroad to explain the reaction mechanisms of early age CO2-cured concrete (hereinafter referred to as EACC); summarize the effects of external factors such as carbonation time, CO2 pressure and concentration, and intrinsic factors (such as the active admixture, the water/cement ratio, and the water content) on the carbonation effect of early age CO2; detail the existing theoretical and numerical models of EACC; investigate the technology of EACC in four fields, i.e., precast concrete, cast-in-place concrete, recycled concrete, and fibre-reinforced concrete; and summarize the problems faced by existing research in application.
1. Introduction
In 2020, CO2 emissions from the cement industry accounted for about 14.3% of the total national carbon emissions [1]. According to the plan, China requires CO2 emissions to reach their peak by 2030 and is striving to achieve the “double carbon” goal of carbon neutrality by 2060; thus, the traditional cement industry is facing severe pressure to reduce its carbon emissions [2]. In order to achieve this goal of carbon emission reduction, scholars have been developing technologies and materials from various aspects, including (1) ultra-high-performance concrete, which involves promoting the application of ultra-high-strength concrete to reduce the amount of cement used in large projects [3]; (2) new cementitious materials, which involves replacing traditional calcium cement with geopolymer cement, composite cement, magnesium oxide cement, etc., thus reducing the overall carbon emissions of the cement industry [4,5]; (3) steel–concrete combination structures, which involves using steel and concrete to form a combination structure to reduce the amount of concrete used [6,7,8,9,10,11]; (4) composite concrete reinforcement technology, in which concrete is reinforced, for example, by steel fibers, to improve its durability [12,13,14]; and (5) early age carbon dioxide curing technology, where the concrete is cured by the carbonation curing method in the early stages after the concrete is poured to capture and sequester CO2.
Current ultra-high-strength concrete has the characteristic of a high thermal conductivity, which tends to relatively increase the energy consumption of the building in later years [15]. The promotion and application of new cementitious materials are subject to various constraints such as cost and raw material supply, and their industrial application faces challenges. For steel–concrete composite structures, steel refining also requires a large amount of energy, which produces carbon emissions, and the material cost is high. For composite concrete reinforcement technology, adding steel fibers to concrete increases the cost of the project. In recent years, the technology of capturing and sequestering CO2 using EACC has attracted a lot of attention from many scholars [16,17]. For a long time, industry and academia categorized concrete carbonation in the field of structural durability [18,19,20], mainly because concrete carbonation decreases the pH value inside the concrete, which destroys the passivation film on the surface of reinforcing steel and induces its rusting and swelling, which in turn leads to concrete cracking and hinders the overall performance of the structure. However, for plain concrete, relevant studies have shown that the use of active carbonation techniques for curing concrete as it is being formed not only positively affect its mechanical properties, but also reduce the conventional curing time required for concrete [21,22,23,24]. In addition, EAC has a reduced porosity after carbonation curing [25,26], which can improve the durability of concrete structures [27,28,29]. What is more noteworthy is that in the production process of assembled precast concrete, the use of early age carbon dioxide curing technology has huge advantages over traditional curing methods in terms of energy consumption and environmental protection [30]. For the carbon sequestration capacity of cement-based materials, Zhan et al. calculated the theoretical maximum carbon sequestration capacity of cement mortar to be about 50% of the mass of cement clinker by analyzing the reactive composition of cement clinker [31]. In summary, early age carbon dioxide curing technology is of great significance in reducing carbon emissions, protecting the environment, and developing the building materials industry, and it is also recognized as one of the most effective technical paths to achieve the “double carbon” goal in the cement industry.
The purpose of this paper is to summarize the research progress of early age carbon dioxide curing technology in recent years, to explain the reaction mechanisms and influencing factors of early age carbon-dioxide-cured concrete, to detail the existing theoretical and numerical models of early age carbon-dioxide-cured concrete, to analyze the application prospects of early age carbon dioxide curing technology in the fields of precast concrete, cast-in-place concrete, recycled concrete, and fiber-reinforced concrete, and finally to summarize the challenges faced by the existing research in the application and promotion of this technology and to give corresponding suggestions.
2. Early Age Carbon Dioxide Curing Technology and Its Reaction Mechanism
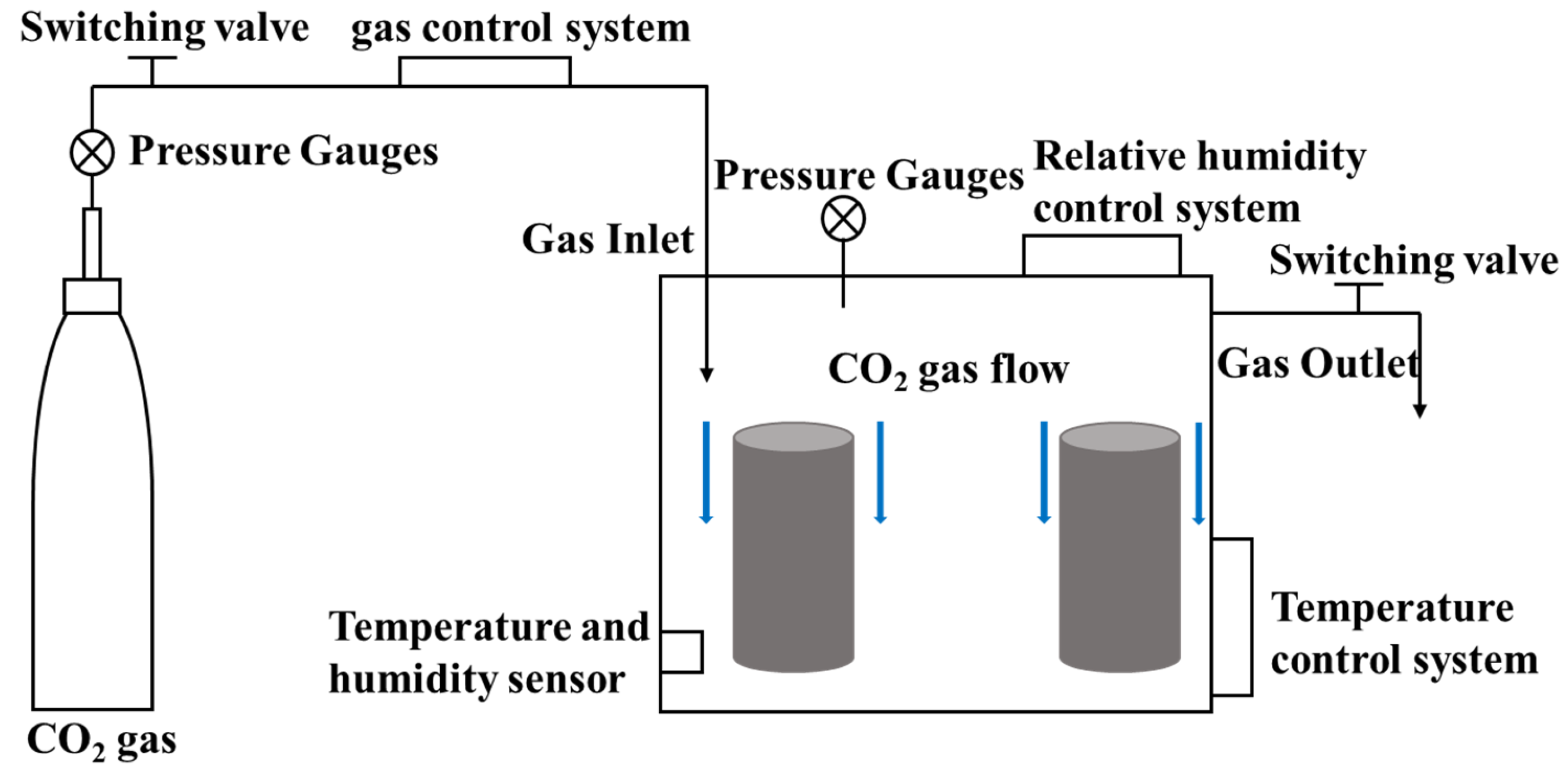
Early age CO2 curing technology refers to CO2 curing of fresh concrete using carbonation curing devices after forming and demolding, where the curing devices are shown in Figure 1. Berger et al. [32] at the University of Illinois first proposed carbonation-cured concrete technology in the 1970s to study the effect of CO2 on calcium silicate hydration, giving the following reaction equation during the carbonation reaction:
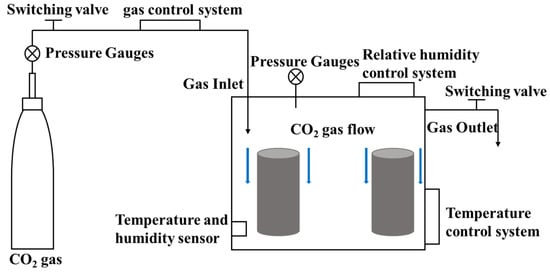
Figure 1.
CO2 curing device.
All the above reactions are exothermic and the x and y in the equation are related to the degree of reaction. The results of many studies so far indicate that the CxSHy generated by the carbonation reaction is a polymerized silica gel [33], and its morphology is more similar to that of the C–S–H gel generated in the hydration reaction in cement-based materials.
Klemm and Berger [34,35] performed early age carbonation curing on a slurry prepared from C3S and C2S, and found that the strength of the slurry was significantly improved after 5 min of curing, reaching a compressive strength of 20.68 MPa. The early strength improvement was due to the rapid accumulation of silica gel in the polymerized state. Additionally, in the subsequent carbonation reaction process of cementitious materials, CxSHy will be further decalcified and carbonized, and the final products generated will be calcium carbonate and SiO2-mH2O (silica gel), with the equation is shown below.
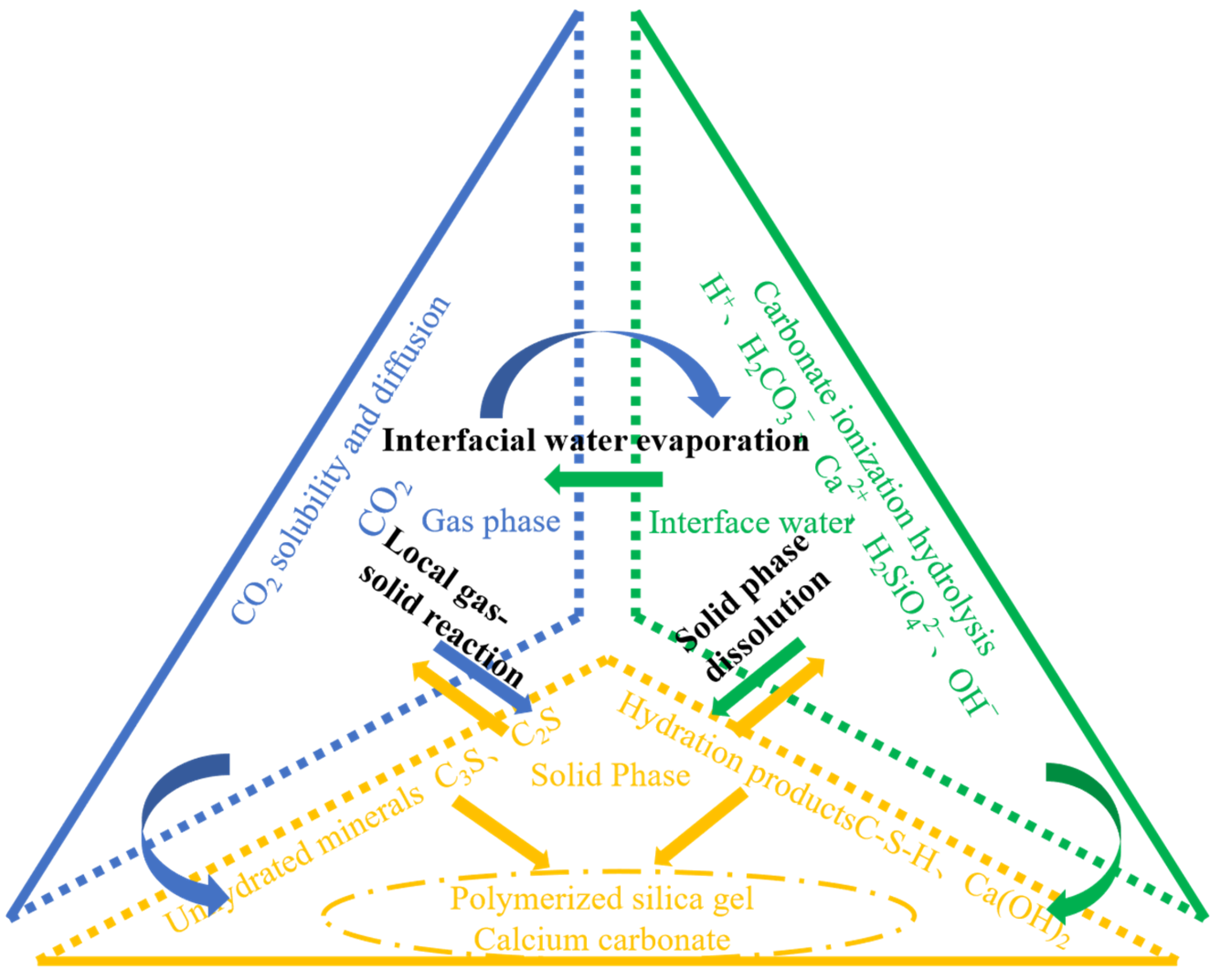
The carbonation process of CO2 with concrete is essentially a diffusion-controlled process. The reaction process of CO2 with cementitious materials is shown in Figure 2. Chen et al. [36] summarized a series of physicochemical reaction processes of concrete during carbonation: (1) dissolution and diffusion of CO2, (2) dissolution of Ca(OH)2, (3) hydration of cementitious materials, and (4) carbonation of hydration products and hydrated cementitious materials with CO2. The mechanism of action and kinetics of these carbonation reactants above are different, and Ca(OH)2 and C–S–H gels can directly chemically react with dissolved CO2·C3S. Its hydration product Ca(OH)2 is the key reactant in the early carbonation reaction process, and the carbonation reaction of C3S and Ca(OH)2 is preceded by C2S and C–S–H. In the next step, C2S and C–S–H gel will undergo carbonation reactions with CO2, but this reaction has little effect on the overall carbonation process. The carbonation process of concrete is accelerated by the carbonation reaction of unhydrated C3S and C2S with CO2, and this process is mainly controlled by the reaction rate of C3S and C2S with CO2 [37]. The C3A present in the cement composition basically does not react chemically with CO2, but the calcium alumina generated in the hydration reaction of C3A is easily carbonized with CO2 and decomposed into a variety of compounds. The presence of a large amount of calcium carbonate in concrete can cause concrete expansion and cracking, while the consumption of calcium carbonate in the carbonation curing reaction can have the benefit of improving the performance of concrete [38]. The reaction of C4AF with CO2 mainly produces calcite, C3AH6, C4ACH11, trace amounts of sphalerite, aragonite, and alumina trihydrate [39], but the extent of these chemical reactions is very limited and has a small impact on the total carbonation reaction process.
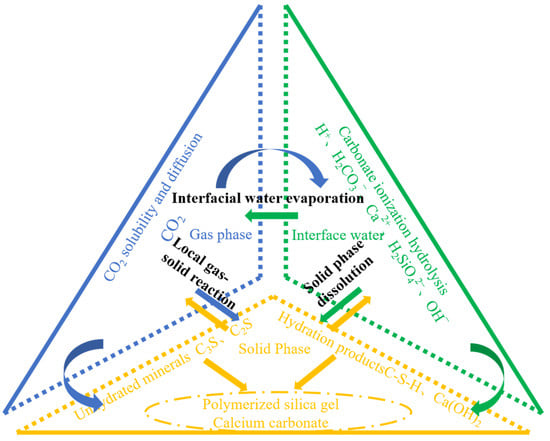
Figure 2.
Reaction kinetics of CO2-cured concrete.
3. Factors Influencing the Carbonation Effect of Early Age Carbon-Dioxide-Cured Concrete
The current research shows that there are two main factors affecting carbonation. One is the external factors, including carbonation time, CO2 pressure, CO2 concentration, relative humidity, and temperature, and the other is the internal factors, mainly dopants, water/cement ratio, and water content. All, from the acceleration of CO2 transport to the reaction rate, affect the carbonation effect.
3.1. Carbonation Time
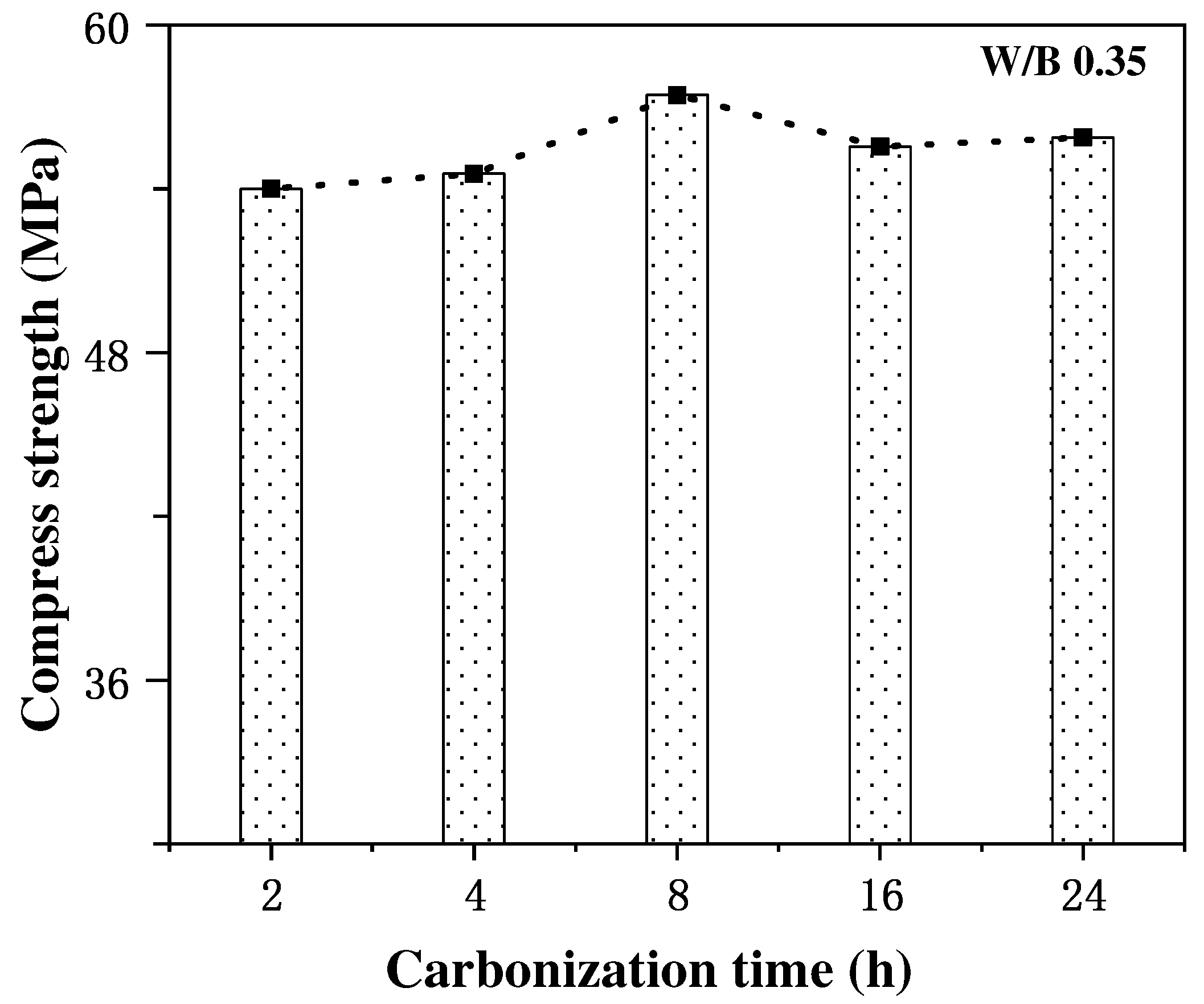
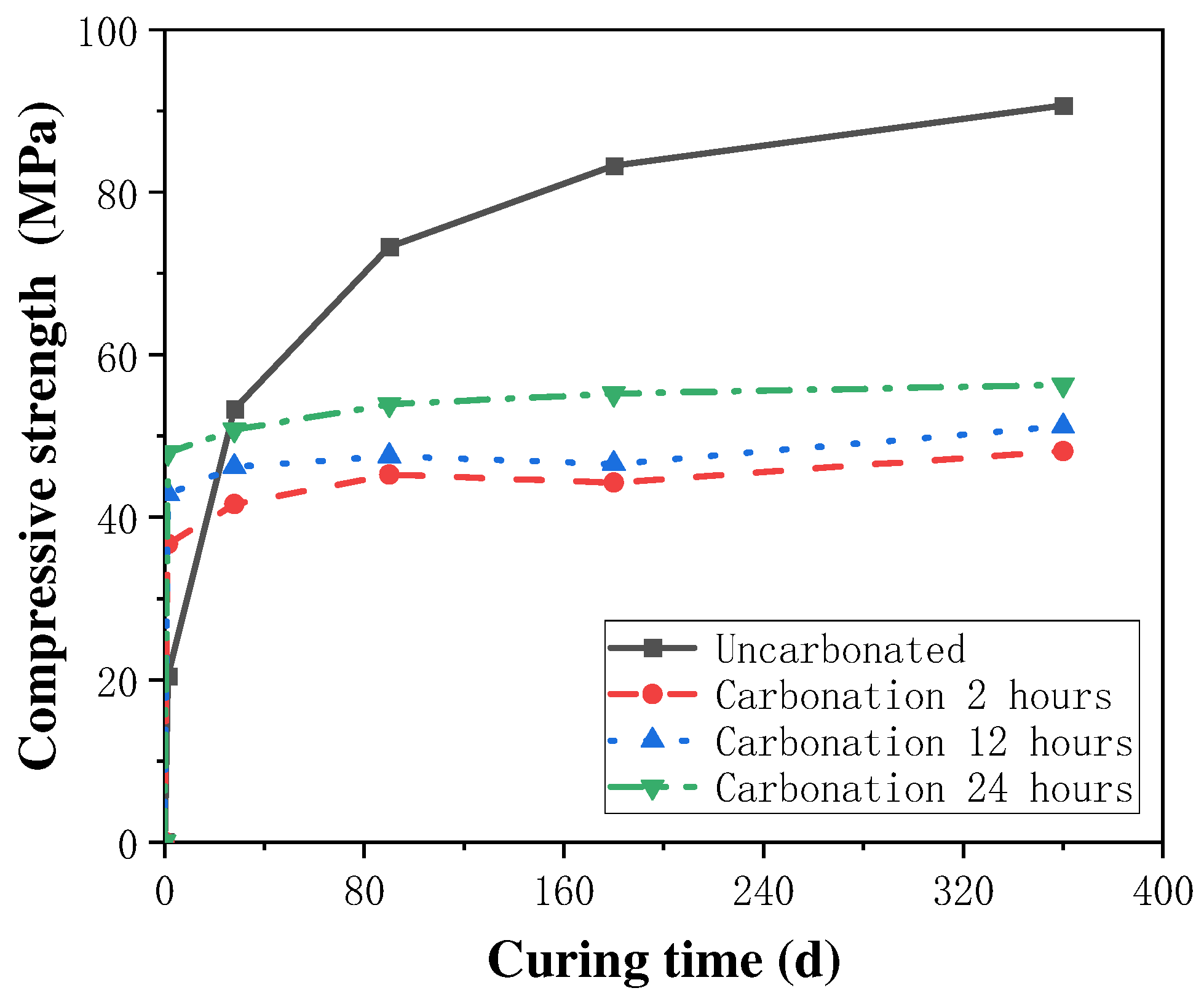
The effect of carbonation curing time on the strength enhancement effect of concrete specimens and the degree of carbonation reaction is more complex, and an appropriate extension of carbonation time can promote the rapid development of the strength of cement mortar, while an excessive extension of carbonation curing time may not continue to improve the compressive strength of concrete and may even have a negative impact on the enhancement of the mechanical properties of concrete. Thus, the selection of an optimum carbonation time in the process of concrete carbonation strengthening is very critical for concrete performance improvement. In the study of the effect of carbonation curing on the strength of steel slag concrete, Feng Zhang et al. [40,41] found that the compressive strength of steel slag concrete could be increased to 20 MPa after 1 d of carbonation curing treatment, and with the extension of the curing time to 14 d, the compressive strength could reach 40 MPa, which was 4.2–6.3 times stronger than the specimens which did not undergo carbonation curing treatment. Additionally, Wang et al. [25] studied the effect of different carbonation curing times on the enhancing the strength of cement mortar, and found that the rate of strength increase of cement mortar gradually decreased with the increase in carbonation curing time. The rate of increase in the compressive strength was at a maximum at the curing time of 8 h, which tended to be stable at any time, as shown in Figure 3, which led to the authors concluding that the optimal carbonation curing time of cement mortar was 6~12 h, after analyzing the characteristics of thermogravimetric curves and XRD patterns. Similar conclusions were obtained by Junior et al. [42] through experimental results, where the excessive prolongation of the carbonation time adversely affected the concrete strength development. Some scholars [43] believe that this is mainly due to that when the carbonation time is too long, the external area of the cementitious material carbonation is too high, the separation degree of the inner and outer side of the cementitious material increases, and the hydration products degrade, resulting in the phenomenon of the concrete compressive strength increasing and then decreasing with the carbonation time.
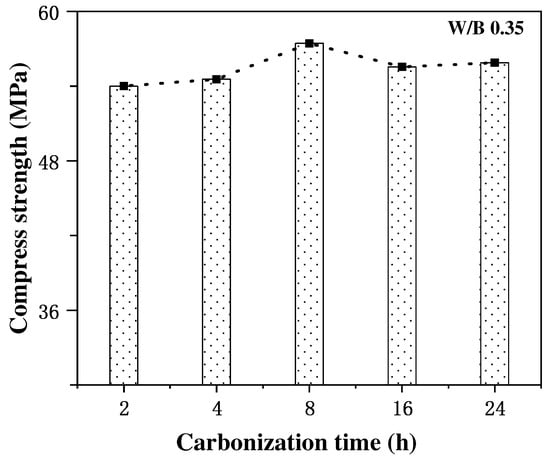
Figure 3.
Variation in compressive strength with carbonation curing time [43].
3.2. CO2 Pressure
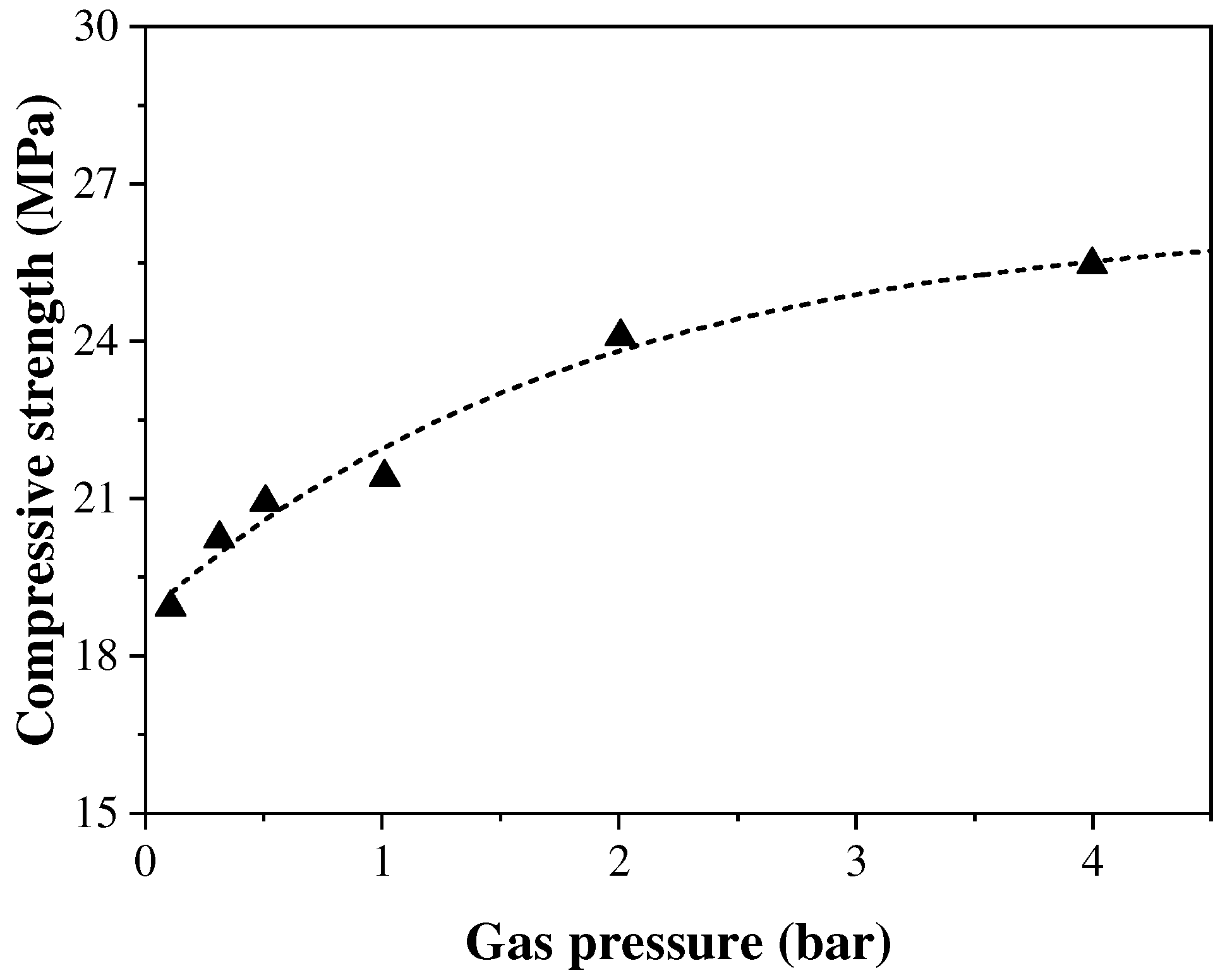
The CO2 pressure value during the carbonation curing process will directly affect the CO2 diffusion rate and thus the carbonation curing of concrete specimens. In general, increasing the gas pressure can enhance the diffusion and dissolution rate of CO2 into the concrete. However, when the gas pressure reaches a certain value, a better carbonation curing effect is not obtained. Zhan et al. [44] found that when the pressure was increased from 0.01 MPa to 0.05 MPa, the carbonation of concrete was increased from 23.8% to 28.2%, but when the pressure was increased from 0.05 MPa to 0.4 MPa, the carbonation was only increased to 34.3% and the fitting results showed that there was not a linear relationship between the compressive strength of concrete and the pressure, as shown in Figure 4. Ahmad et al. [45] reached the same conclusion by adjusting the magnitude of the gas pressure value to study the carbonation curing effect; increasing the pressure value from 10 psi (≈0.07 MPa) to 60 psi (≈0.41 MPa) did not have a significant effect on the strength gain of the concrete.
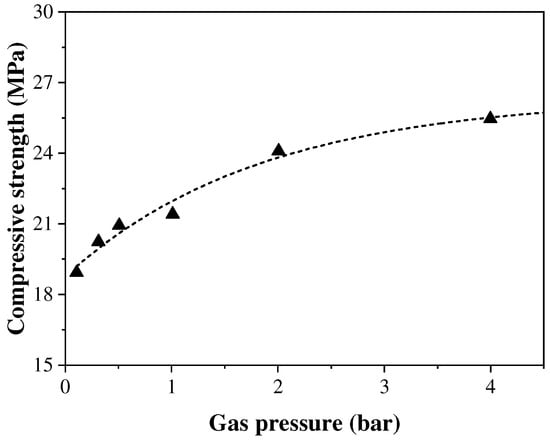
Figure 4.
Influence of CO2 pressure and concentration on carbonation curing [44].
3.3. CO2 Concentration
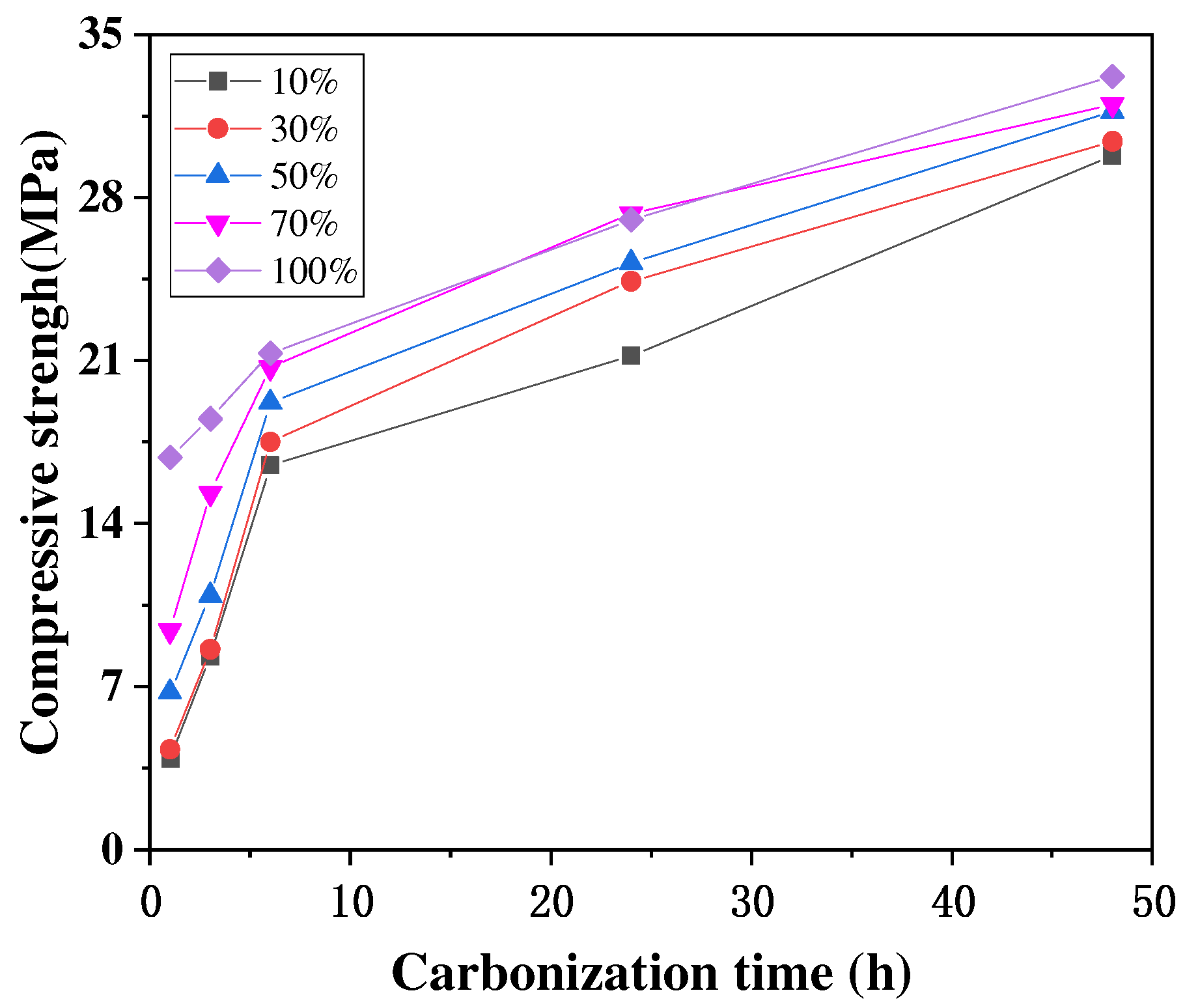
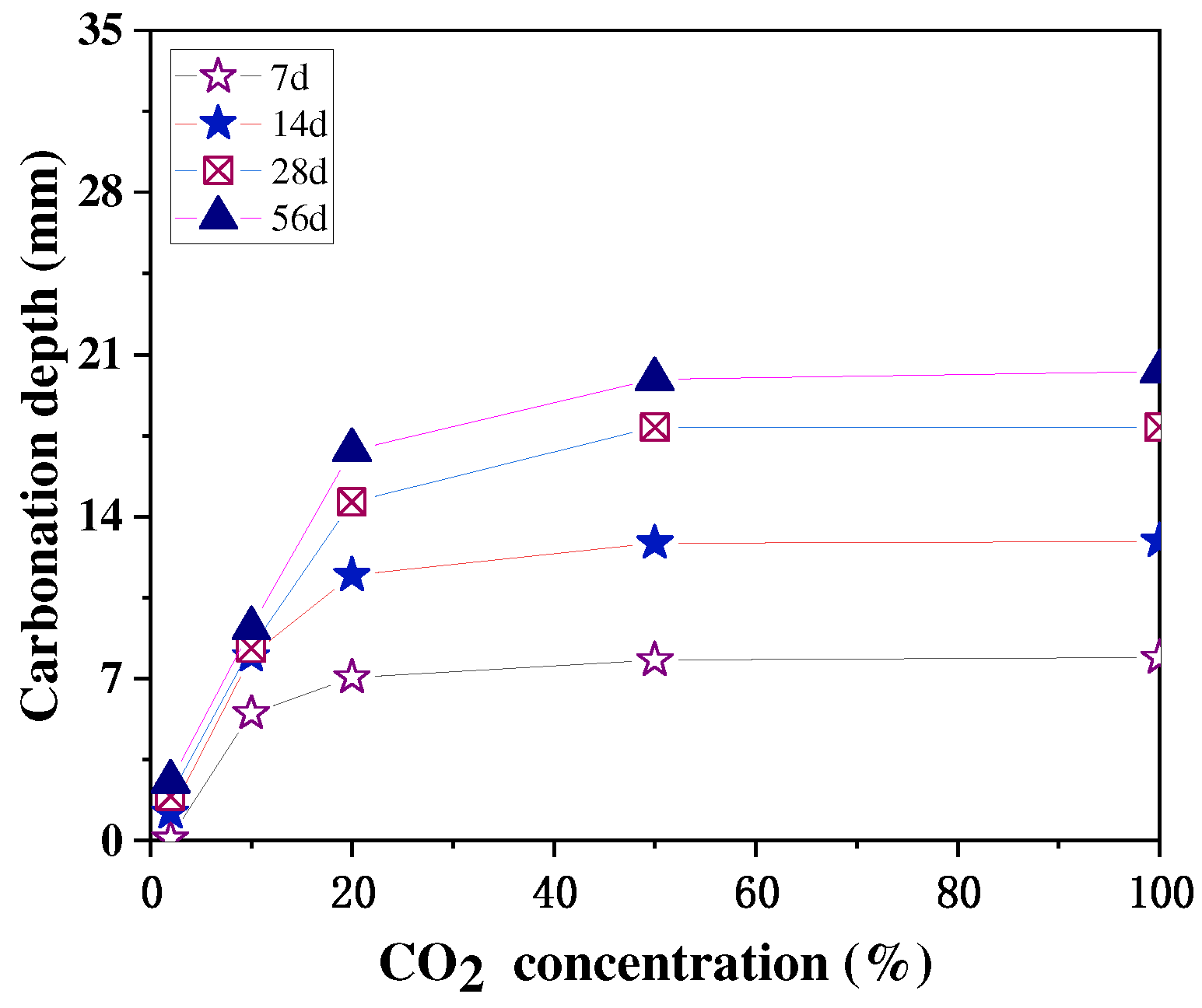
A high CO2 concentration environment is beneficial to accelerate the carbonation of concrete, but when the CO2 concentration is too high, it has little effect on the final carbonation of concrete. The main reason for this is that after a certain CO2 concentration, it is difficult for the CO2 to pass through the already carbonated concrete and carbonate the concrete even further. The study by Xuan et al. [46] showed that a higher CO2 concentration led to a faster strength development of the concrete only in the initial stages, that the strength growth rate at different concentrations tended to be the same in the later stage, and that the final strength after 48 h was relatively similar, as shown in Figure 5. Cui et al. [47] obtained similar experimental results, finding that at the same age of carbonation, the concrete had a faster growth rate of carbonation depth at low CO2 concentrations (2–20%) and a lower growth rate of carbonation depth at high CO2 concentrations (50–100%), and the depths of carbonation measured at concentrations of 50% to 100% at 28 and 56 d were almost the same, as shown in Figure 6.
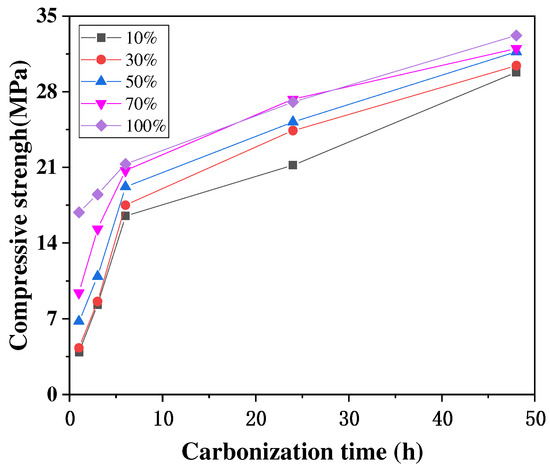
Figure 5.
Relationship between carbonization time and compressive strength [46].
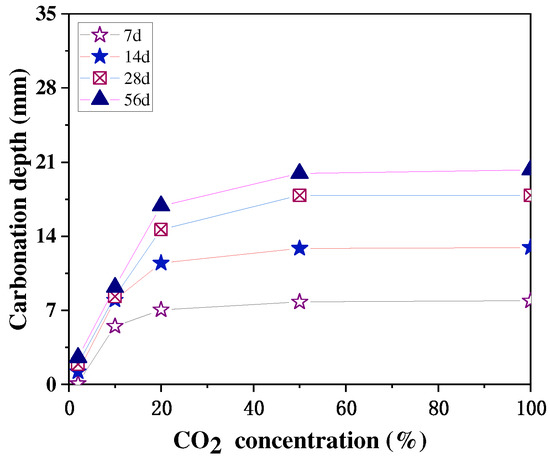
Figure 6.
Relationship between CO2 concentration and carbonation depth [47].
3.4. Relative Humidity
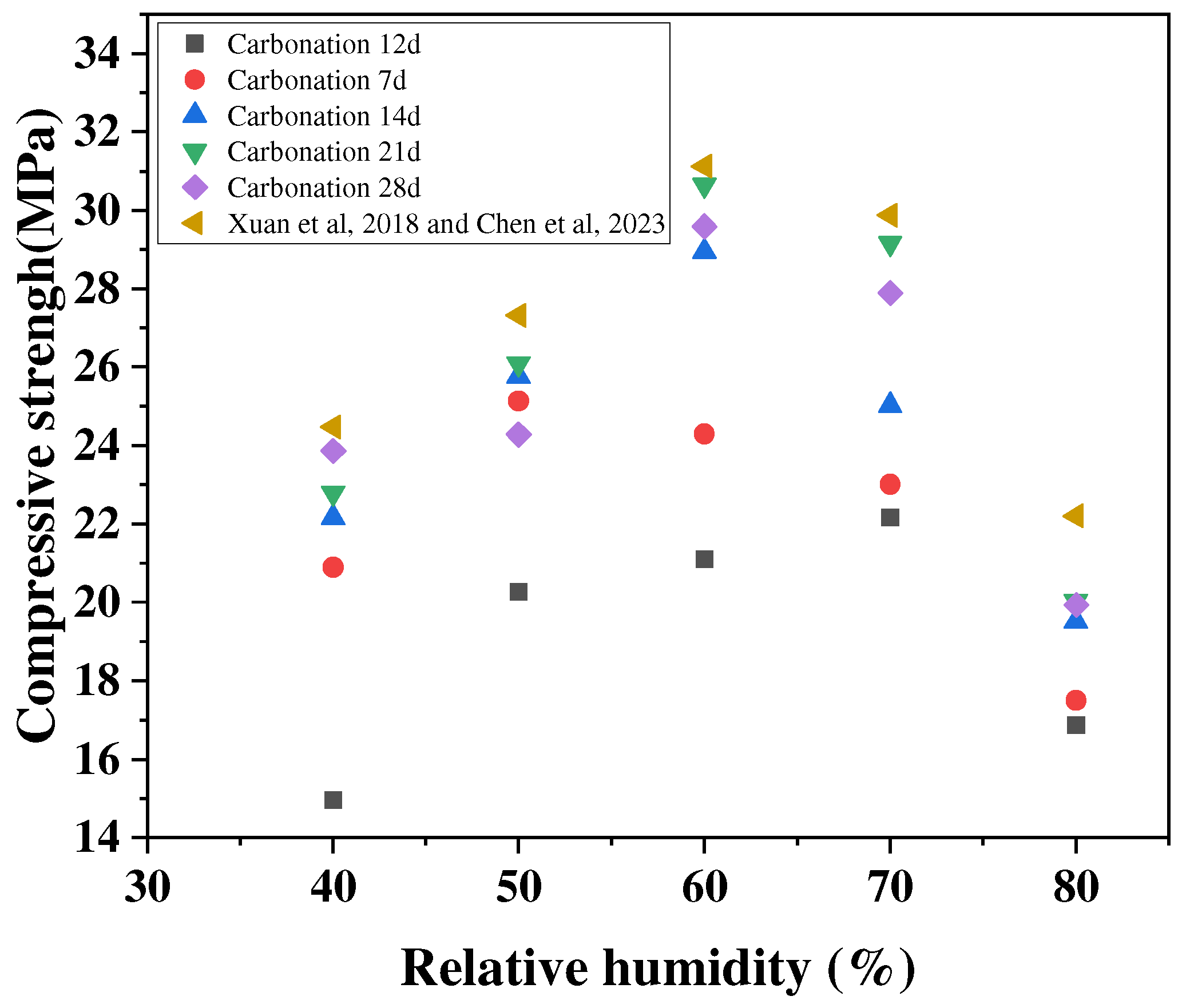
The relative humidity affects the reaction rate mainly by influencing the diffusion and dissolution processes of CO2 within the cementitious material [48,49]. Xuan et al. [46] studied the compressive strength of concrete after 48 h of carbonation curing, finding that the strength enhancement of concrete at a relative humidity of 50% was higher than that at an ambient humidity of 80%, as shown in Figure 7. When the humidity is too high, water molecules fill the micro-pores, which may block the diffusion path of CO2 and slow down the diffusion rate of CO2. Additionally, when the humidity is low, the concrete is in a drier state, and although the CO2 diffusion rate will be accelerated, the concrete surface cannot easily gather the amount of water molecules needed for reaction and the dissolution process of Ca(OH)2 on the surface of the adsorbed water is limited. This step is the key to controlling the carbonation rate, and thus the reaction is slowed down or even prevented from proceeding [50,51]. Therefore, choosing a suitable relative humidity in the carbonation curing process has a certain promoting effect on the enhancement of the mechanical properties of concrete. The current experimental data show that the rate of the carbonation reaction of concrete is faster when the relative humidity is between 50% and 70% [52].
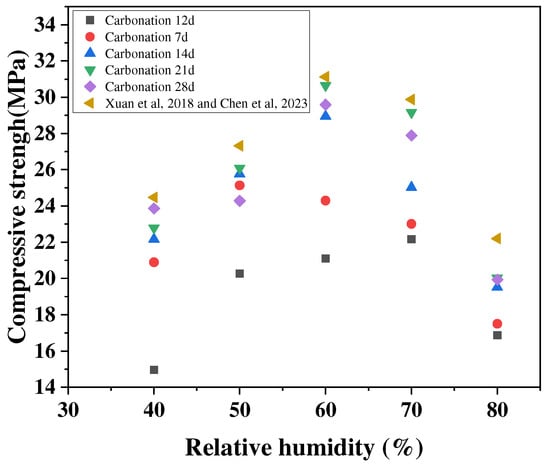
Figure 7.
Effect of relative humidity on conservation with carbonation [46,53].
3.5. Temperature
Studies have shown that controlling the ambient temperature within 20~100 °C is beneficial to the carbonation reaction and effectively improves the CO2 sequestration rate; however, an increase in temperature will reduce the efficiency and inhibit the progress of the carbonation reaction. The reason for this is that the essence of a chemical reaction is the effective collision of reactant molecules, and the increase in temperature can effectively increase the energy of reactant molecules and promote the thermal movement of molecules, thus promoting the diffusion of CO2 and increasing the reaction rate. However, too high a temperature will reduce the solubility of CO2 and calcium ions in the water in the pores and affect the carbonation. Therefore, a suitable temperature should be sought to balance the advantages and disadvantages. Zhan et al. [44] found that the carbonation of concrete could be increased from 52.6% to 55.0% by increasing the temperature from 20 °C to 80 °C. Wang et al. also studied the effect of temperature on the degree of carbonation of concrete, and the test results showed that the degree of carbonation of the specimens at different temperatures varied greatly, with the carbonation rate the largest at 100 °C, reaching 16.4%. However, as the temperature continued to increase, the degree of carbonation began decrease, and only 5% carbonation was recorded at 300 °C [54].
3.6. Active Dopants
The addition of fly ash, limestone powder, and other admixtures facilitates the diffusion of carbon dioxide into the interior of the cement paste, promotes the absorption of carbon dioxide, and increases the depth of carbonation [55]. Qin et al. studied the effect of limestone powder, fly ash, and ground granulated blast furnace slag on the carbonation curing of cement paste, and the test results showed that the addition of mineral admixtures reduced the early compressive strength of concrete, but enhanced the effect of carbonation curing on the improvement in compressive strength [56]. Monkman et al. investigated the effect of CO2 curing on the early strength of slag concrete and showed that the the compressive strength reached 70–80% after 2 h CO2 curing compared to that of 24 h standard curing [57]. Mo et al. investigated the effect of different pressures and carbonation times on the compressive strength of fly ash–MgO–Portland cement blends, and the results showed that the compressive strength of 90% dosed fly ash and MgO specimens increased by 76.2% after 3 h of carbonation curing compared to the conventional curing method, and even increased by 195% after 14 days of carbonation curing [58]. However, it has also been shown that cement specimens mixed with excessive amounts of volcanic ash reactive substances, such as fly ash, negatively affect the compressive strength of concrete at a later stage after carbonation curing, as shown in Figure 8 [59]. Although the addition of fly ash drives the diffusion of CO2 into the cement paste, Ca(OH)2 is consumed in the reaction with volcanic ash [60,61]. Wang et al. inferred that the higher the degree of early carbonation, the more products, such as calcium carbonate and silica gel, are generated, which cover the surface of unreacted clinker, hindering the subsequent hydration and inhibiting the development of the compressive strength of concrete [62].
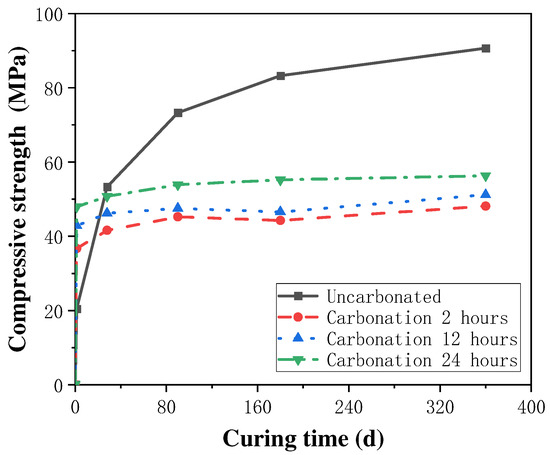
Figure 8.
Variation in compressive strength with age growth of concrete with 50% fly ash content after carbonation curing [59].
3.7. Water-to-Glue Ratio and Water Content
The water/cement ratio of concrete materials is directly related to their porosity, which is an important factor affecting the strengthening effect of carbonation on concrete [63]. For concrete, when the water/cement ratio is high, the compressive strength decreases before carbonation, but the high porosity at high water/cement ratios promotes the effective diffusion of CO2, which increases the carbonation degree. It has been shown that the effective diffusion coefficient of CO2 in concrete exhibits an exponential increase with the increase in porosity [64,65]. Wang et al. demonstrated the effect of slurry porosity on carbonation and the evolution of the slurry microstructure by setting different water/collagen ratios (w/c), and the experimental results showed that the carbonation depth and carbonation rate increased with the increase in the w/c ratio [66]. Siddique et al. [37] similarly investigated the effect of different water/gel ratios on carbonation and came to the same conclusion. For the specimen moisture content, when the internal moisture content is too low, the dissolution of CO2 and Ca2+ is restricted, which affects the carbonation of the specimen, while when the moisture is too high, the diffusion migration of CO2 is also restricted [67].
Shi Caijun et al. [68,69] tested the water content of specimens after different curing times and found that there exists an optimal residual water/cement ratio that maximizes the carbonation and compressive strength of concrete under carbonation curing conditions. Additionally, once this optimum moisture content is exceeded, the carbonation effect may be drastically reduced [70]. Zeng Haima et al. [71] tested the compressive strength of concrete specimens before and after carbonation at different water loss rates, and the results showed that when the water loss rate was 80%, the strength was reduced by nearly 20 MPa compared to at a 74.4% water loss rate. Therefore, in order to achieve the highest degree of concrete carbonation, the optimum moisture content needs to be confirmed before carbonation curing. However, it should also be noted that during the drying and water loss processes, the evaporation rate of water needs to be reasonably controlled, as rapid water evaporation may lead to microcracks due to dry shrinkage of the sample, thus reducing the strength of the specimen after carbonation curing.
4. Effect of CO2 Curing on the Performance of Concrete
4.1. Volumetric Stability
Many studies have found that EAC after carbonation curing can significantly improve the volumetric stability and effectively reduce the drying shrinkage and microstrain values of concrete compared to steam and water curing [24,40]. In fully hydrated cementitious materials, the main components are C–S–H gel, Ca(OH)2, AFt, and AFm, of which the percentage of C–S–H gel is about 70%. C–S–H gel is porous and has a large specific surface area, which can release and adsorb water molecules in dry and moist environments, thus leading to shrinkage and swelling of cementitious materials. However, after carbonation curing of concrete, the cementitious products are mainly calcium carbonate and silica gel formed by C–S–H decalcification, which reduces the specific surface area of C–S–H gel. Although silica gel is equally capable of adsorbing and releasing water molecules during the wetting and drying processes [24], it has a weaker effect on the shrinkage of cementitious materials in a dry environment because the percentage of silica gel is much lower than that of calcium carbonate. Calcium carbonate, on the other hand, has now been shown to have a good volumetric stability and chemical inertness in the cement matrix [72]; thus, the cementitious composites can exhibit better volumetric stability under dry shrinkage conditions after carbonation curing.
4.2. Compressive Strength
Early age concrete generates C–S–H and calcium carbonate products during the carbonation curing process, of which C–S–H plays a major role in promoting the growth in concrete strength, while calcium carbonate can effectively fill concrete pores and improve compactness, prompting the rapid development of concrete strength in early stages [44]. However, the long-term performance improvement of concrete by carbonation curing technology may be limited. Zhan et al. [16] showed that the compressive strength of cement slurry specimens after 1 d of carbonation curing was 48.4 MPa, which was twice as high as that of standard-cured 1 d specimens and close to that of standard-cured 28 d specimens (52.4 MPa). The compressive strength of the carbonized specimens at 3 d was about 63.9% higher than that of the standard-cured specimens for specimens subjected to carbonized curing for 7 h at early age, as determined by Liu et al. [26]. The difference in strength between the two at 28 d was not significant. From the above results, it is clear that carbonation curing of concrete at early ages can induce the rapid development of early strength, but for long-term strength, the difference in compressive strength between carbonation-cured and standard-cured specimens is small. It has been inferred that this is mainly due to the fact that after the carbonation reaction, calcium carbide covers the surface layer of unhydrated C3S and C2S, which slows down the hydration reaction of cement clinkers at a later stage, and also because the CO2 surface treatment only affects the surface layer of concrete [73].
4.3. Durable Performance
The durability of concrete structures is mainly due to the long-term exposure to some harsh environments, such as chloride salts and the freeze–thaw process, which leads to a degradation in concrete performance. The current research results show that in carbonation-cured concrete, CO2 precipitates as crystalline calcium carbonate through chemical action and interlaces with C–S–H gel to form a compact structure with low permeability, and thus the durability performance of the concrete is improved [25], such as its freeze–thaw resistance [74], chloride ion resistance [29], and permeability resistance [75], as shown in Table 1.

Table 1.
Effect of carbonation curing on the durability of concrete.
However, there is a decrease in the pH value of concrete after carbonation curing due to the consumption of Ca(OH)2. Under natural conditions, once the pH of the porous solution inside the carbonated concrete decreases to 8.3~9, the passivation film around the reinforcement enters an unstable state, which puts the reinforcement at risk of rusting during the chloride salt attack. However, it has been pointed out that, unlike natural carbonation, carbonation treatment of early age concrete does not increase the risk of reinforcement corrosion. There are several reasons for this: (1) after carbonation curing, the concrete porosity is reduced, which can effectively stop the erosion process by salt solutions, thus improving the durability of concrete [25]; (2) a reduction in the internal pH of concrete due to early carbonation curing is improved after subsequent hydration; and (3) the carbonation depth of concrete after carbonation curing is much lower than the thickness of protective layer in reinforced concrete [45,77].
Although subsequent hydration can restore the concrete to a higher pH and mitigate the adverse effects of early CO2 curing, the durability of reinforced concrete after carbonation curing currently requires further in-depth assessments, especially the effect of secondary carbonation on the durability of reinforced concrete structures during service.
5. Theoretical and Numerical Modeling of Early Age Carbon-Dioxide-Cured Concrete Technology
The establishment of theoretical and numerical models is one of the key research areas in the study of carbonation of cementitious materials. The establishment of a carbonation model enables the simulation and prediction of key parameters such as the carbonation depth and post-carbonation strength of cementitious materials; in addition, some important mechanistic aspects of the carbonation reaction process can be revealed.
For concrete carbonation under natural conditions, scholars have established a variety of concrete carbonation models, which can better simulate the carbonation process of concrete in the service process. Papadakis [78] established a theoretical model based on Fick’s first law that could predict the carbonation rate and carbonation depth of concrete at maturity more accurately. Subsequently, the scholar further refined this carbonation model by establishing a mathematical model for natural carbonation of concrete in the range of ambient relative humidity [79]. Peter et al. [80] extended this carbonation model based on Papadakis’ study by considering the carbonation and hydration reactions of hydrated calcium silicate and unhydrated C2S and C3S mineral components, and investigated the relationship between the competing mechanisms of hydration reactions of unhydrated minerals and carbonation reactions in the carbonation process of concrete at maturity. The numerical simulation results showed that the effect of carbonation of unhydrated C2S and C3S on the overall carbonation process of concrete could be neglected in the carbonation model of concrete at maturity. Saetta et al. [81] considered the effects on CO2 transport coefficients under multifactorial action (mainly including ambient temperature, ambient humidity, hydration of cement hydration minerals, concrete porosity variation, and loading action) and developed a multifactorial carbonation model for concrete at maturity. Ishida et al. [82] proposed a model of carbonation based on thermo-hygro-physics by introducing C–S–H gel reactions and calcium hydroxide reactions into the existing model, and considered the volume change and the increase in surface area of hydration products, which was combined with water balance/transport to provide a reasonable and unified prediction of carbonation processes at low and high CO2 concentrations. In addition, many other scholars have also considered the development of cracks during carbonation, aggregate content, pore saturation, and other factors to establish a relevant concrete carbonation model [83,84].
However, the active carbonation of early age concrete differs from the passive carbonation of mature concrete in the natural environment. Mechanistically, the carbonation enhancement process of early age concrete is more complex, mainly due to the fact that the hydration reaction generally proceeds simultaneously with the carbonation reaction. In addition, during the carbonation reaction of early age concrete, the unhydrated hydrated minerals in cement minerals will also undergo carbonation reactions with CO2 to produce calcium carbonate and C–S–H gel or silica gel with lower Ca/Si [36]. From the analysis of external conditions, carbonation of early age concrete generally requires high pressure and high temperature environments, with the main purpose of these being the acceleration of the diffusion of CO2 to the interior and the acceleration of the reaction rate. There are also relevant studies considering the special properties of supercritical CO2 (temperature and pressure exceeding 304.12 K and 7.38 MPa, respectively), i.e., its low viscosity and low surface tension, and their effect on further accelerating the carbonation reaction.
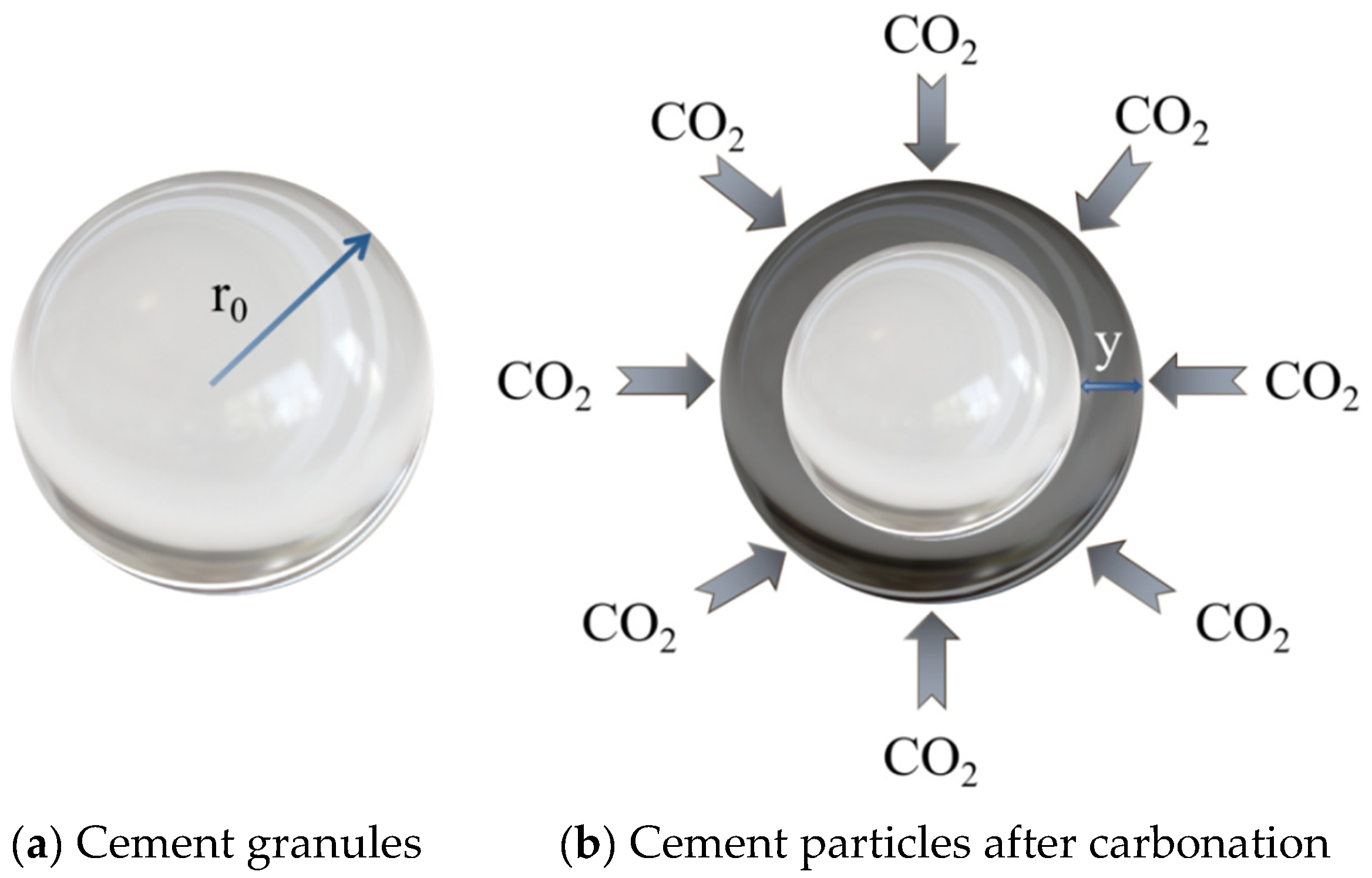
For this reason, many scholars have carried out further theoretical and numerical modeling studies on carbonation-enhanced early age concrete based on the existing carbonation theory. Caijun Shi et al. [85] derived a kinetic theory model for CO2-cured early age concrete based on the solid phase reaction model, using cement particles as the research object, as shown in Figure 9.
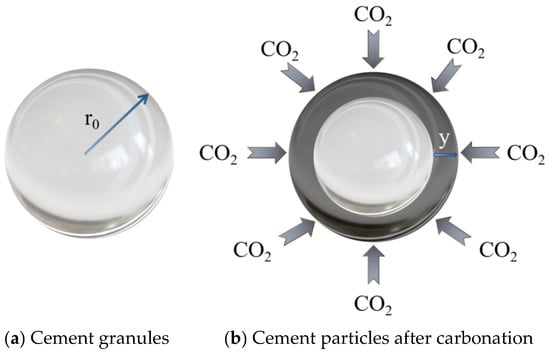
Figure 9.
Comparison of cement particles before and after carbonation [85].
Phung et al. [86] developed a model for accelerated carbonation to predict the microstructure and transport properties of molded cement paste considering diffusion and convection effects. Kashef-Haghighi [87] developed a theoretical model for CO2 uptake and distribution in concrete during carbonation of EAC and numerically described the partial differential equations for CO2 transport, water dissolution in concrete pores, and hydration and carbonation reactions with cementitious material compounds. Zha et al. [88] considered the chemical reaction rate, the mass conservation of gas–liquid two-phase flow, the diffusion and dispersion of CO2 in water, the energy conservation of porous media, and the solubility of CO2 in concrete pore fluid and established a theoretical model to simulate supercritical carbonation in cementitious materials. Tie-Feng Chen et al. [89] established a theoretical model of one-dimensional carbonation for the carbonation conservation of cement slurry, simulating and predicting the key parameters, such as carbonation efficiency, carbonation depth, and porosity, during the carbonation of cement slurry under the combined effect of gas concentration and flow rate. The authors pointed out through this theoretical model, the carbonation depth and rate of early age concrete under carbonation conservation are mainly affected by the content of C3S and CH, and the carbonation of C3S and CH is more preferential compared to C2S and C–S–H, which are the main determinants of the early carbonation of cement paste. However, the model assumes uniform and constant ambient temperature and humidity and ignores the exchange of temperature and humidity with the outside world. Xuan et al. [46] investigated the effects of CO2 curing conditions on the strength development of different concrete block mixtures, studying the effects of the CO2 concentration, the gas flow rate, the relative humidity, and the carbonation duration. Based on the experimental results and maturity theory, the strength development of carbonated concrete blocks was established. Thiery et al. [90] developed a semi-analytical model to study the carbonation mechanism of recycled concrete aggregates at a concentration of 10% CO2, linking the diffusion of CO2 to the carbonation of the cement matrix and pointing out the influence of the characteristics of mortar attached to the recycled aggregates on the CO2-reinforced recycled aggregates. Xiaopeng Hu et al. [91,92] established a calculation model to determine the early carbonation depth of mature concrete, considering factors such as mineral admixture type, admixture amount, curing time, carbonation time, carbonation site, and water/cement ratio.
As can be seen from the above, the current research on concrete carbonation theory and numerical models has considered the effects of environmental humidity, gas concentration, gas flow rate, carbonation time, and other factors, and scholars have established increasingly complete prediction models and tools. However, there is still a lack of consideration of temperature; the above model studies are based on room temperature or constant temperature conditions and do not consider the influence of temperature changes. However, temperature has a large impact on the physical properties of carbonation reactants and chemical reaction processes and the ambient temperature is also a key parameter in the optimization of the early age concrete carbonation strengthening process. Consequently, the materialization process will also affect the local temperature changes, so in theoretical research, it is necessary to consider the effect of temperature and establish a relevant early age concrete carbonation strengthening process. It is necessary to consider the effect of temperature in theoretical studies and to establish the corresponding multiphysics model for early age concrete carbonation curing.
6. Prospects for CO2 in Cementitious Materials
6.1. Precast Concrete
Precast concrete components such as blocks, fiber cement wall panels, concrete pipes, concrete piles, and hollow core panels are usually cured by atmospheric or high-pressure steam during the production process to achieve a higher early strength. However, the steam curing of concrete consumes more energy, resulting in higher production costs. In addition, some studies have shown that steam curing can adversely affect the internal pore structure of concrete [93], thus affecting the concrete strength, durability, watertightness, abrasion resistance, volume stability, and resistance to the freeze–thaw process and chloride ion attack. In addition, as concrete brittleness increases, the concrete surface micro-cracks, the poor distribution of hydration products, etc., increase. These above-mentioned defects lead to different degrees of deterioration in the later life stages of concrete. In contrast, recent studies have shown that the use of carbonation curing on precast concrete elements can reduce the time required for curing [30] and improve the durability of concrete relative to traditional steam curing [27], as shown in Table 2. Shi et al. [30] performed carbonation curing and steam curing on lightweight concrete blocks separately and found that the strength of the blocks after 4–8 h of carbonation curing was comparable to that of steam curing for 18–24 h, which greatly reduced the curing time, improved the production efficiency, and reduced energy consumption.

Table 2.
Comparison of the effects of carbonation curing and steam curing [27].
Carbonation curing can not only shorten the curing time, but can also overcome the downfalls of a high energy consumption, a high production cost, and the adverse effects on the durability of concrete caused by steam curing [93]. The utilization rate of CO2 is also improved, so carbonation curing has good application prospects in the production of precast concrete elements. However, there are still some limitations in the research of carbonation curing, i.e., the current application tests of CO2-cured concrete focus on small-sized specimens such as blocks, and there are a lack of tests on large-sized specimens, especially for the research on carbonation curing processes of large-sized precast concrete.
6.2. Cast-in-Place Concrete
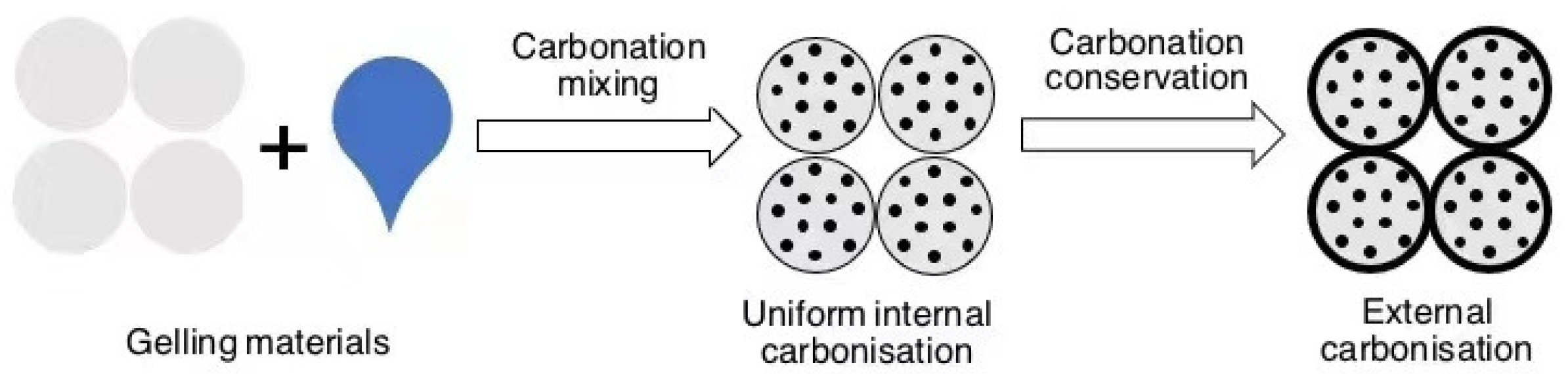
The carbonation reaction of concrete specimens proceeds from the outside to the inside, and the higher the strength of the specimen, the greater its compactness and the more difficult it is for CO2 to diffuse to the inside, making carbonation eventually achieve only a superficial treatment effect, which in turn leads to a difference in the properties of the concrete between the inside and the outside. This may not be effective in improving the overall mechanical performance and durability of large components, and the carbon sequestration potential of cementitious materials cannot be fully reached, which is the core problem to be solved before the application of carbonation-cured concrete technology. A feasible technical path is to introduce CO2 into the mixing process of concrete, so that the cement slurry is evenly contacted and carbonated with CO2 during the mixing process, evenly distributing the reaction products inside the material, as shown in Figure 10, which can also further improve the carbon sequestration of concrete.
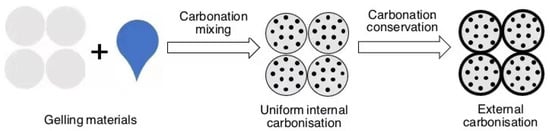
Figure 10.
Stirred carbonation process.
Many scholars have conducted studies on the introduction of CO2 application tests in the concrete placement process. Many scholars have carried out the study of mixing carbonation with internal CO2 particles instead of cement mass. He et al. [94] studied the effect of internal CO2 mixing on the CO2 absorption and strength gain of concrete, and the results showed that carbonation mixing can significantly increase CO2 absorption compared to carbonation curing, but it also reduced the strength of concrete. Liu Yixiang et al. [95] studied the effect of a dry ice mixture on the performance of recycled normal concrete and recycled normal steel pipe concrete, and found that the mixing with dry ice can indeed carbonate the Ca(OH)2 in concrete, but the dry ice easily evaporates during the reaction of hydrated cement and the discharged gas forms pores inside the concrete, which reduces its strength. Jang et al. [96] studied the effect of mixing with NaHCO3 internally. The test results of this study showed that the addition of NaHCO3 can induce a uniform carbonation reaction inside the slurry, and the strength can be improved when the added NaHCO3 is less than 5% of the weight of the cementitious material, but the strength of the concrete may be adversely affected when the weight exceeds 5%.
In addition to the mixing method with internal CO2 particles, Monkman and MacDonaldl [97] carried out mixing carbonation by introducing CO2 gas during the mixing and forming of concrete, and the results of the study showed that the introduction of CO2 had some negative effects on the compaction, density, and strength of the concrete slurry. However, the authors then improved the process by adding water to the mixing process to counteract the negative effects. Lili Liu [98] used ultrasonic mixing to further optimize the process of concrete mixing carbonation, and the results showed that the ultrasonic mixing process can not only substantially increase the ultimate absorption of CO2 by the cement paste, but also a large number of carbonation products form crystalline nuclei, improving and enhancing the microscopic pore structure of the cement paste and improving the compressive strength and durability of the concrete.
Mixing carbonation for fresh concrete is not only limited to the improvement in cementitious materials after forming, but also can be used in the production of both precast and cast-in-place concrete. Although some research results have been presented on mixing carbonation, the carbonation reaction processes and mechanisms are more complicated compared to CO2-cured early age concrete, especially for mixing with internal particles. At the same time, the excessive amount of admixture will have a certain negative impact on the performance of concrete, so it is necessary to further optimize the carbonation mixing process of cement-based materials to reduce the negative effects of the internal CO2 admixture on the concrete.
6.3. Recycled Concrete
Compared with natural aggregates, recycled aggregates have the unwanted properties of high water absorption, high porosity, and a high crushing index. In addition, recycled aggregate particles are not easily formed and the surface adheres to the old mortar, so that there is a transition area between the old and new mortar in the recycled concrete [99], deteriorating its performance as the amount of recycled aggregate increases [100]. A large number of studies have now shown that carbonation enhancement of recycled aggregates helps to improve their basic properties and microstructure, as shown in Table 3.

Table 3.
Effect of carbonation on basic properties of recycled aggregates.
Carbonation strengthening of recycled aggregates is mainly based on the fact that chemicals such as CaO, Ca(OH)2, and CaSiO3 in the adhered mortar can react with CO2 during carbonation, which can effectively reduce the water absorption rate to one similar to that of natural aggregates [106], thus improving the fluidity of the recycled concrete slurry, improving the dry shrinkage properties of recycled concrete, and increasing the compressive strength as well as the modulus of elasticity. A study has shown that the fluidity of the treated recycled aggregate mortar increased by about 35.4% [101] and the dry shrinkage of recycled concrete was reduced by about 10% to 15% [108].
In addition to the improved physical and mechanical properties, the durability of recycled concrete after carbonation strengthening is improved to some extent. Gao Yueqing et al. showed that the chloride ion diffusion coefficient of recycled aggregates could be reduced by about 46.0% to 67.7% after carbonation strengthening, and also pointed out that the poorer the quality of recycled aggregates, the more significant the improvement in their resistance to chloride ion penetration. However, it is worth noting that the enhancement effect of carbonation strengthening of recycled aggregates can be affected by many factors. Zhan et al. [109] studied the effect of the strength of virgin concrete on the enhancement effect of carbonation strengthening of recycled aggregates using recycled aggregates with different strengths of recycled concrete as variables. The test results showed that the higher the strength of the virgin concrete, the more obvious the enhancement effect in the recycled aggregates. Liang et al. [110] argued that this is mainly because the higher the strength of the virgin concrete, the less pores and fine pores exist inside its recycled aggregate and the lower the initial water absorption, leading to a more significant enhancement effect. In addition, smaller pores are more easily filled by the products generated by carbonation reactions. In addition, the carbonation time also affects the recycled aggregate strengthening. In the early stage of the reaction, the carbonation degree of the recycled aggregate is low and the carbonation degree becomes higher and higher with time, and when the carbonation reaction enters the later stages, the carbonation degree only slightly increases or even remains roughly the same [111]. It has been suggested that this is mainly attributed to the fact that the encapsulation of the recycled aggregates by the carbonation reaction products prevents the penetration of CO2 into the interior of the matrix [112].
The application of carbonation technology can effectively solve the problem of the performance deterioration of recycled concrete due to recycled aggregate defects; improve the workability, mechanical properties, and durability of recycled concrete; and further reduce the performance gap between recycled concrete and virgin concrete, which has a very positive significance for the promotion of recycled concrete. However, some influencing factors, such as the effect of temperature and carbonation time on the rate and efficiency of carbonation strengthening of recycled aggregates, need to be further studied in order to determine the optimal conditions for carbonation strengthening of recycled aggregates. In addition, the structural and mechanical properties of the CO2-reinforced recycled concrete members also need to be further investigated.
6.4. Fiber-Reinforced Concrete
Fiber-reinforced resin matrix composites have the advantages of a light weight, a high strength, and a high resistance to chloride salt erosion [113]. However, the results of many scholars at home and abroad show that the highly alkaline environment of concrete can lead to a deterioration in the performance of fiber-reinforced composites, and as the pH of the solution in the pore increases, the more obvious the corrosive effect of the environment on the resin and fibers [114,115]. Related studies have shown that carbonation curing can improve the mechanical properties and durability of composite fibers [116]. After carbonation curing, the pH value inside the concrete is reduced, and at the same time, the impermeability of the concrete surface layer is increased, thus serving to protect the resin matrix of the cured fiber [117]. On the other hand, after carbonation curing of fiber-reinforced concrete, calcium carbonate precipitation promotes pore densification, thus reducing the dry shrinkage and pore volume of concrete and increasing the excess interfacial area around the cementitious material and fibers [118].
In summary, carbonation-cured fiber-reinforced concrete technology has the potential to improve the mechanical properties and slow down the aging of various fiber-reinforced composites. In addition, compared to conventional reinforced concrete structures, fiber-reinforced concrete structures can reduce corrosion and reinforcement due to the reduction in pH after the carbonation of concrete. However, there are some limitations of carbonation conservation technology applied to fiber-reinforced concrete, as the current research progress shows that the carbonation depth of concrete reinforced by carbonation is generally low, which affects the improvement in the durability of fiber-reinforced concrete structures.
7. Conclusions and Outlook
As a new technology for capturing and sequestering CO2, early age CO2 curing technology can provide the long-term and stable sequestration of CO2, and also improve the mechanical properties and durability of concrete, giving it a wide range of application prospects. This paper analyzes the research progress and application prospects of early age CO2 curing technology for precast concrete, cast-in-place concrete, recycled concrete, and fiber-reinforced concrete materials, obtaining the following conclusions: carbonation curing of early age concrete can promote the rapid development of early strength and improve the durability of concrete; direct internal mixing of CO2 can induce uniform carbonation inside concrete; the application of carbonation-enhanced recycled aggregate technology can effectively improve the deterioration in performance of recycled concrete caused by the defects in recycled aggregates and further narrows the performance gap between recycled concrete and virgin concrete; and the factors affecting carbonation-cured concrete include curing time, CO2 concentration, CO2 pressure, relative humidity, and temperature. Among all the influencing factors, the curing time and temperature may adversely affect the curing if they exceed certain values.
Although EACC application technology has achieved positive results so far, the following issues still need to be studied in depth to promote the application and use of CO2-enhanced concrete in engineering. In the experimental studies of CO2-enhanced concrete/recycled aggregates, not enough tests have been conducted on large-sized specimens, especially in the study of the carbonation curing process for large precast elements. It is necessary to investigate the effect of coupling CO2 concentration and pressure and ambient temperature and humidity on the carbonation curing effect and compare it with the study of single influencing factors so as to find the optimal combination of curing conditions. The incorporation of excessive amounts of volcanic ash reactive substances, such as fly ash, will affect the compressive strength of concrete in the later stages after carbonation curing, but further research is needed to assess the mechanisms of action of Ca(OH)2 and low pH values of the pore solution on the long-term reactions of the volcanic ash and mineral admixture after carbonation curing. These studies would determine the influence of mineral admixture incorporation on the improvement in carbonation curing. In theoretical studies, the influence of temperature variation, humidity variation, CO2 concentration, and other influencing factors on the relevant physical and chemical parameters should be considered to establish theoretical and numerical models which consider multifactorial coupling effects of EAC carbonation to carry out a more in-depth analysis of the key process parameters.
Author Contributions
Methodology, L.Z.; Formal Analysis, W.L.; Data Curation, J.N.; Writing-Original Draft, L.Z.; Writing—Review & Editing, L.Z.; Visualization, J.N.; Supervision, X.Z.; Project Administration, X.Z.; Funding Acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (no 52178129).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ding, M.R. Analysis of the current situation of carbon emissions in the cement industry and exploration of key paths for emission reduction. China Cem. 2021, 7, 46–49. [Google Scholar]
- Martín, D.; Flores-Alés, V.; Aparicio, P. Proposed methodology to evaluate CO2 capture using construction and demolition waste. Minerals 2019, 9, 612. [Google Scholar] [CrossRef]
- Habert, G.; Arribe, D.; Dehove, T.; Espinasse, L.; Roy, R.L. Reducing environmental impact by increasing the strength of concrete: Quantification of the improvement to concrete bridges. J. Clean. Prod. 2012, 35, 250–262. [Google Scholar] [CrossRef]
- Shen, W.; Cao, L.; Li, Q.; Wen, Z.; Wang, J.; Liu, Y.; Dong, R.; Tan, Y.; Chen, R. Is magnesia cement low carbon? Life cycle carbon footprint comparing with Portland cement. J. Clean. Prod. 2016, 131, 20–27. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting factors and properties of limestone calcined clay cements (LC3). Green Mater. 2019, 07, 3–14. [Google Scholar] [CrossRef]
- Long, Y.L.; Zeng, L. A refined model for local buckling of rectangular CFST columns with binding bars. Thin-Walled Struct. 2018, 132, 431–441. [Google Scholar] [CrossRef]
- Long, Y.L.; Zeng, L.; Gardner, L.; Wadee, M.A. A new model for calculating the elastic local buckling stress of steel plates in square CFST columns. Thin-Walled Struct. 2022, 171, 108756. [Google Scholar] [CrossRef]
- Liao, J.; Zeng, J.J.; Long, Y.L.; Cai, J.; Ouyang, Y. Behavior of square and rectangular concrete-filled steel tube (CFST) columns with horizontal reinforcing bars under eccentric compression. Eng. Struct. 2022, 271, 114899. [Google Scholar] [CrossRef]
- Long, Y.L.; Wan, J.; Cai, J. Theoretical study on local buckling of rectangular CFT columns under eccentric compression. J. Constr. Steel Res. 2016, 120, 70–80. [Google Scholar] [CrossRef]
- Cai, J.; Long, Y. Local buckling of steel plates in rectangular CFT columns with binding bars. J. Constr. Steel Res. 2009, 65, 965–972. [Google Scholar] [CrossRef]
- Zeng, J.J.; Zheng, Y.Z.; Long, Y.L. Axial compressive behavior of FRP-concrete-steel double skin tubular columns with a rib-stiffened Q690 steel tube and ultra-high strength concrete. Compos. Struct. 2021, 268, 113912. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, X.; Niu, Q.; Zhang, Y.; Zhao, B. Durability degradation simulation of RC structure based on gamma process considering two-dimensional chloride diffusion and life probabilistic prediction. Structures 2022, 48, 159–171. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, Q.; Liu, X.; Zhang, Y.; Zhao, T.; Liu, M. Durability life prediction of reinforced concrete structure corroded by chlorine based on the Gamma process. ASCE-ASME J. Risk Uncertain. Eng. Syst. Part A Civ. Eng. 2021, 7, 04021061. [Google Scholar] [CrossRef]
- Zhang, Z.; Paul, C.P.; Panda, B.; Huang, Y.; Garg, A.; Zhang, Y.; Garg, A.; Zhang, W. Assessment of flexural and splitting strength of steel fiber reinforced concrete using automated neural network search. Adv. Concr. Constr. 2020, 10, 81–92. [Google Scholar]
- Nicholas, L.; Ling, T.-C.; Pan, S.-Y. Towards carbon-neutral construction materials: Carbonation of cement-based materials and the future perspective. J. Build. Eng. 2020, 28, 101062. [Google Scholar]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Mechanism for rapid hardening of cement pastes under coupled CO2-water curing regime. Cem. Concr. Compos. 2019, 97, 78–88. [Google Scholar] [CrossRef]
- Xin, Q.; Wang, J.; Yi, F.; Liang, W. Carbon dioxide as an admixture for better performance of OPC-based concrete. J. CO2 Util. 2018, 25, 31–38. [Google Scholar]
- Wei, L.; Zhang, L.; Lu, Z. Effect of carbonation and cracking of concrete on reinforcement corrosion and structural durability. J. Xiangtan Min. Inst. 1992, 7, 164–172. [Google Scholar]
- Jin, Z.Q.; Sun, W.; Zhang, Y.S.; Liu, Z.Y. Study on Carbonati on of Concrete under Loading. J. Build. Mater. 2005, 8, 179–183. [Google Scholar]
- Jiang, J.Y.; Sun, W.; Jin, Z.Q.; Wang, C.H. Service Life Prediction of Structural Concrete under Coupled Interactions of Fatigue Loading and Carbonation Factor. J. Build. Mater. 2010, 13, 304–309. [Google Scholar]
- Pu, Q.; Yao, Y.; Wang, L.; Liu, Y.X.; Shi, X.X. An investigation of the pH variation in carbonated concrete under different depth. New Build. Mater. 2017, 44, 1–4. [Google Scholar]
- Pu, Q.; Xue, W.Y.; Jiang, L.H.; Xi, F.D.; Zhang, C.Z.; Dai, D.D.; Xie, X.R. Research on fracture performance of concrete under the action of carbonization and freeze-thaw. Concrete 2020, 9, 1–6. [Google Scholar]
- Huang, H.; Guo, R.; Wang, T.; Hu, X.; Garcia, S.; Fang, M.; Luo, Z.; Maroto-Valer, M.M. Carbonation curing for wollastonite-Portland cementitious materials: CO2 sequestration potential and feasibility assessment. J. Clean. Prod. 2019, 211, 830–841. [Google Scholar] [CrossRef]
- Shi, C.; Wang, D.; He, F.; Liu, M. Weathering properties of CO2-cured concrete blocks. Resour. Conserv. Recycl. 2012, 65, 11–17. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.; Cai, X.; Deng, H.; Li, B. Mechanical and Microstructural Characteristics of Calcium Sulfoaluminate Cement Exposed to Early-Age Carbonation Curing. Materials 2021, 14, 3515. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Qi, L. Effects of Temperature and Carbonation Curing on the Mechanical Properties of Steel Slag-Cement Binding Materials. Constr. Build. Mater. 2016, 124, 999–1006. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation Curing versus Steam Curing for Precast Concrete Production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Kosior-Kazberuk, M.; Berkowski, P. Surface scaling resistance of concrete subjected to freeze-thaw cycles and sustained load. Procedia Eng. 2017, 172, 513–520. [Google Scholar] [CrossRef]
- Pan, X.; Shi, C.; Farzadnia, N.; Hu, X.; Zheng, J. Properties and microstructure of CO2 surface treated cement mortars with subsequent lime-saturated water curing. Cem. Concr. Compos. 2019, 99, 89–99. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Wu, Y. Effect of pre-conditioning on CO2 curing of lightweight concrete blocks mixtures. Constr. Build. Mater. 2012, 26, 257–267. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.; Shi, C. CO2 curing for improving the properties of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of Hydration of Calcium Silicates by Carbon Dioxide Treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Shtepenko, O.; Hills, C.; Brough, A.; Thomas, M. The effect of carbon dioxide on β-dicalcium silicate and Portland cement. Chem. Eng. J. 2006, 118, 107–118. [Google Scholar] [CrossRef]
- Klemm, W.A.; Berger, R.L. Accelerated curing of cementitious systems by carbon dioxide: Part I. Portland cement. Cem. Concr. Res. 1972, 2, 567–576. [Google Scholar] [CrossRef]
- Berger, R.L.; Klemm, W.A. Accelerated curing of cementitious systems by carbon dioxide: Part II. Hydraulic calcium silicates and aluminates. Cem. Concr. Res. 1972, 2, 647–652. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X.; Qin, L. Mathematical modeling of accelerated carbonation curing of Portland cement paste at early age. Cem. Concr. Res. 2019, 120, 187–197. [Google Scholar] [CrossRef]
- Siddique, S.; Naqi, A.; Jang, J.G. Influence of water to cement ratio on CO2 uptake capacity of belite-rich cement upon exposure to carbonation curing. Cem. Concr. Compos. 2020, 111, 103616. [Google Scholar] [CrossRef]
- Ma, H.; Li, Z. Ettringite Formation in Concrete. Build. Sci. 2007, 23, 105–110. [Google Scholar]
- Wang, D.; Chang, J. Comparison on accelerated carbonation of β-C2S, Ca(OH)2, and C4AF: Reaction degree, multi-properties, and products. Constr. Build. Mater. 2019, 224, 336–347. [Google Scholar] [CrossRef]
- Zhang, F.; Mo, L.; Deng, M. Effect of Carbonation Curing on Mechanical Strength and Volume Stability of Steel Slag Concrete. J. Chin. Ceram. Soc. 2016, 44, 640–646. [Google Scholar]
- GB/T 11970-1997; Test Methods for Bulk Density, Moisture and Water Absorption of Aerated Concrete. The State Bureau of Quality and Technical Supervision: Beijing, China, 1997.
- Junior, A.N.; Fairbairn, E.d.M.R.; Dweck, J. A study of the carbonation profile of cement pastes by thermogravimetry and its effect on the compressive strength. J. Therm. Anal. Calorim. 2014, 116, 69–76. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X. Effect of carbonation curing regime on strength and microstructure of Portland cement paste. J. CO2 Util. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Effect of curing parameters on CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 71, 122–130. [Google Scholar] [CrossRef]
- Ahmad, S.; Assaggaf, R.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Adekunle, S.K.; Ali, S.I. Effects of carbonation pressure and duration on strength evolution of concrete subjected to accelerated carbonation curing. Constr. Build. Mater. 2017, 136, 565–573. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. A maturity approach to estimate compressive strength development of CO2-cured concrete blocks. Cem. Concr. Compos. 2018, 85, 153–160. [Google Scholar] [CrossRef]
- Cui, H.; Tang, W.; Liu, W.; Dong, Z.; Xing, F. Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Constr. Build. Mater. 2015, 93, 522–527. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, B.; Cai, Y. A model for predicting carbonation of high-volume fly ash concrete. Cem. Concr. Res. 2000, 30, 699–702. [Google Scholar] [CrossRef]
- Steiner, S.; Lothenbach, B.; Proske, T.; Borgschulte, A.; Winnefeld, F. Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite. Cem. Concr. Res. 2020, 135, 106116. [Google Scholar] [CrossRef]
- Dheilly, R.M.; Tudo, J.; Sebaïbi, Y.; Quéneudec, M. Influence of storage conditions on the carbonation of powdered Ca(OH)2. Constr. Build. Mater. 2002, 16, 155–161. [Google Scholar] [CrossRef]
- Shih, S.M.; Ho, C.U.S.; Song, Y.S.; Lin, J.P. Kinetics of the reaction of Ca(OH)2 with CO2 at low temperature. Ind. Eng. Chem. Res. 1999, 38, 1316–1322. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H.; Xi, P.; Yu, T.; Zhang, B. Influence of carbonization humidity on mechanics and durability of carbonization block. J. Jilin Univ. (Eng. Technol. Ed.) 2023, 1–9. [Google Scholar] [CrossRef]
- Wang, D.; Noguchi, T.; Nozaki, T.; Higo, Y. Investigation of the carbonation performance of cement-based materials under high temperatures. Constr. Build. Mater. 2020, 272, 121634. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Huang, J.; Yu, K.; Zhong, G.; Chen, F.; Chen, X.; Yang, W.; Wang, Y. Effects of temperature, humidity and CO2 concentration on carbonation of cement-based materials: A review. Constr. Build. Mater. 2022, 346, 128399. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- Monkman, S.; Shao, Y. Carbonation curing of slag-cement concrete for binding CO2 and improving performance. J. Mater. Civ. Eng. 2010, 22, 296–304. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Effects of carbonation treatment on the properties of hydrated fly ash-MgO-Portland cement blends. Constr. Build. Mater. 2015, 96, 147–154. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y.; Cai, X. Carbonation Curing of Precast Fly Ash Concrete. J. Mater. Civ. Eng. 2016, 28, 04016127. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Qian, J.; Zhong, M.; Zhao, S. Behavior and Mechanism of Pozzolanic Reaction Heat of Fly Ash and Ground Granulated Blastfurnace Slag at Early Age. J. Chin. Ceram. Soc. 2012, 40, 1050–1058. [Google Scholar]
- Qiao, H.; Sun, B.; Wang, F.; Wang, J.; Ding, Z.; Yang, T. Age Performance of Leshan Giant Buddha Restoration Material by Metakaolin Modified. Bull. Chin. Ceram. Soc. 2020, 39, 543–551. [Google Scholar]
- Wang, Y.; Lu, B.; Hu, X.; Liu, J.; Zhang, Z.; Pan, X.; Xie, Z.; Chang, J.; Zhang, T.; Nehdi, M.L.; et al. Effect of CO2 surface treatment on penetrability and microstructure of cement-fly ash–slag ternary concrete. Cem. Concr. Compos. 2021, 123, 104194. [Google Scholar] [CrossRef]
- Bai, J.; Lin, N.; Wang, J.; Liu, J. Effect of ash dosage on microstructure of carbonized cement paste. J. Water Resour. Water Eng. 2022, 33, 7. [Google Scholar]
- Li, J.; Wu, Z.; Shi, C.; Yuan, Q.; Zhang, Z. Durability of ultra-high performance concrete–A review. Constr. Build. Mater. 2020, 255, 119296. [Google Scholar] [CrossRef]
- Houst, Y.F.; Wittmann, F.H.J.C. Influence of porosity and water content on the diffusivity of CO2 and O2 through hydrated cement paste. Cem. Concr. Res. 1994, 24, 1165–1176. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zhou, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influence of porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]
- Gu, H.; Wu, Q.; Wu, Y.; Min, Z. Strength and Microstructure of Carbonated Cement-Steel Slag Composite Gelled Material. J. Mater. Sci. Eng. 2019, 37, 35–39. [Google Scholar]
- Shi, C.; He, P.; Tu, Z.; Cao, Z. Effect of Pre-conditioning on Process and Microstructure of Carbon Dioxide Cured Concrete. J. Chin. Ceram. Soc. 2014, 42, 996–1004. [Google Scholar]
- GB/T 50081-2002; Standard for Test Method of Mechanical Properties on Ordinary Concrete. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Ministry of Development of the People’s Republic of China: Beijing, China, 2002.
- Mehdipour, I.; Falzone, G.; Plante, E.C.L.; Simonetti, D.A. How microstructure and pore moisture affect strength gain in portlandite-enriched composites that mineralize CO2. ACS Sustain. Chem. 2019, 7, 13053–13061. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, Z.; Wang, F. Effect of Accelerated Carbonation Curing on Mechanical Property and Microstructure of High Volume Steel Slag Mortar. J. Chin. Ceram. Soc. 2020, 48, 1801–1807. [Google Scholar]
- Shi, C.; Tu, Z.; Guo, M.; Wang, D. Accelerated carbonation as a fast curing technology for concrete blocks. Sustain. Nonconv. Constr. Mater. Using Inorg. Bond. Fiber Compos. 2017, 313–341. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Early age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 2016, 113, 134–143. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Surface scaling of CO2-cured concrete exposed to freeze-thaw cycles. J. CO2 Util. 2018, 27, 137–144. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2022, 56, 101861. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Shao, Y. Weathering carbonation behavior of concrete subject to early-age carbonation curing. J. Mater. Civ. Eng. 2020, 32, 04020038. [Google Scholar] [CrossRef]
- Papadakis, V.G. Physical and chemical characteristics affecting the durability of concrete. ACI Mater. J. 1991, 8, 186–196. [Google Scholar]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Peter, M.A.; Muntean, A.; Meier, S.A.; Böhm, M. Competition of several carbonation reactions in concrete: A parametric study. Cem. Concr. Res. 2008, 38, 1385–1393. [Google Scholar] [CrossRef]
- Saetta, A.V.; Vitaliani, R.V. Experimental investigation and numerical modeling of carbonation process in reinforced concrete structures: Part II. Pract. Applications. Cem. Concr. Res. 2005, 35, 958–967. [Google Scholar] [CrossRef]
- Ishida, T.; Li, C.H. Modeling of carbonation based on thermo-hygro physics with strong coupling of mass transport and equilibrium in micro-pore structure of concrete. J. Adv. Concr. Technol. 2008, 6, 303–316. [Google Scholar] [CrossRef]
- Kwon, S.J.; Song, H.W. Analysis of carbonation behavior in concrete using neural network algorithm and carbonation modeling. Cem. Concr. Res. 2010, 40, 119–127. [Google Scholar] [CrossRef]
- Khunthongkeaw, J.; Tangtermsirikul, S.; Leelawat, T. A study on carbonation depth prediction for fly ash concrete. Constr. Build. Mater. 2006, 20, 744–753. [Google Scholar] [CrossRef]
- Shi, C.; Zou, Q.; He, F. Study on CO2 curing kinetics of concrete. J. Chin. Ceram. Soc. 2010, 38, 1179–1184. [Google Scholar]
- Phung, Q.T.; Maes, N.; Jacques, D.; Schutter, G.D.; Ye, G.; Perko, J. Modelling the carbonation of cement pastes under a CO2 pressure gradient considering both diffusive and convective transport. Constr. Build. Mater. 2016, 114, 333–351. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Zha, X.; Yu, M.; Ye, J.; Feng, G. Numerical modeling of supercritical carbonation process in cement-based materials. Cem. Concr. Res. 2015, 72, 10–20. [Google Scholar] [CrossRef]
- Chen, T. Carbonation Curing Mechanism of Cement-Based Materials and Its Improvement on Pervious Concrete; Harbin Institute of Technology: Harbin, China, 2020. [Google Scholar]
- Thiery, M.; Dangla, P.; Belin, P.; Habert, G.; Roussel, N. Carbonation kinetics of a bed of recycled concrete aggregates: A laboratory study on model materials. Cem. Concr. Res. 2013, 46, 50–65. [Google Scholar] [CrossRef]
- Hu, X.; Wu, X.; Peng, G. Calculation Model of Early Carbonation Depth of Mineral Admixture Concrete. Ind. Constr. 2020, 50, 106–111. [Google Scholar]
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Ministry of Housing and Urban-Rural Development of the People’s Republic of China, General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2009.
- Geng, J.; Peng, B.; Sun, J. Effect of Steam Curing System on Pore Structure of Cement Paste. J. Build. Mater. 2011, 14, 116–118+123. [Google Scholar]
- He, Z.; Li, Z.; Shao, Y. Effect of Carbonation Mixing on CO2 Uptake and Strength Gain in Concrete. J. Mater. Civ. Eng. 2017, 29, 04017176. [Google Scholar] [CrossRef]
- Liu, X.; Zha, X. The Experimental Study on Modified Property of Recycled Aggregate Concrete and lts Application in Concrete Filled Steel Tube. Prog. Steel Build. Struct. 2011, 13, 7. [Google Scholar]
- Jang, J.G.; Kim, H.J.; Park, S.M.; Lee, H.K. The influence of sodium hydrogen carbonate on the hydration of cement. Constr. Build. Mater. 2015, 94, 746–749. [Google Scholar] [CrossRef]
- Monkman, S.; Mac Donald, M. Carbon dioxide upcycling into industrially produced concrete blocks. Constr. Build. Mater. 2016, 124, 127–132. [Google Scholar] [CrossRef]
- Liu, L. Research on CO2 Absorption Ability and the Reverse Action Mechanism of Fresh Cement Paste; China University of Mining and Technology: Beijing, China, 2021. [Google Scholar]
- Shi, C.; Cao, Z.; Xie, Z. Research Progress in the Mechanical Properties of Recycled Aggregate Concrete. Mater. Rep. 2016, 30, 96–103. [Google Scholar]
- Liu, J.; Wu, C. Development status and performance research of recycled concrete. Concrete 2018, 344, 148–150. [Google Scholar]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.; Xie, Z. Influence of carbonated recycled concrete aggregate on properties of cement mortar. Constr. Build. Mater. 2015, 98, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, X.; Zhu, P.; Chen, C.; Wang, X.; Yang, W. Carbonation treatment to repair the damage of repeatedly recycled coarse aggregate from recycled concrete suffering from coupling action of high stress and freeze-thaw cycles. Constr. Build. Mater. 2022, 349, 128688. [Google Scholar] [CrossRef]
- Lu, Z.; Tan, Q.; Lin, J.; Wang, D. Properties investigation of recycled aggregates and concrete modified by accelerated carbonation through increased temperature. Constr. Build. Mater. 2022, 341, 127813. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Shi, X.; Wang, Q.; Abomohra, A. Improving recycled concrete aggregates using flue gas based on multicyclic accelerated carbonation: Performance and mechanism. Constr. Build. Mater. 2022, 361, 129623. [Google Scholar] [CrossRef]
- Wu, K.; Luo, S.; Zheng, J.; Yan, J.; Xiao, J. Influence of carbonation treatment on the properties of multiple interface transition zones and recycled aggregate concrete. Cem. Concr. Compos. 2022, 127, 104402. [Google Scholar] [CrossRef]
- Huang, L.; Huang, J.; Tian, J.; Ying, J. CO2 strengthening of recycled aggregate with different properties and its effect on compressive strength of concrete. Concrete 2021, 392, 94–97+102. [Google Scholar]
- Ying, J.; Meng, Q.; Xiao, J. Effect of CO2-Modified Recycled Aggregate on Compressive Strength of Concrete. J. Build. Mater. 2017, 20, 277–282. [Google Scholar]
- Kou, S.C.; Zhan, B.; Poon, C.S. Use of a CO2 curing step to improve the properties of concrete prepared with recycled aggregates. Cem. Concr. Compos. 2014, 45, 22–28. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, Q.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2014, 67, 3–7. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2019, 105, 103446. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Development of a new generation of eco-friendly concrete blocks by accelerated mineral carbonation. J. Clean. Prod. 2016, 133, 1235–1241. [Google Scholar] [CrossRef]
- Pu, H.; Shi, X.; Wang, Q.; Li, W.; Fu, L. Review on recycled concrete aggregates cured by CO2. Concrete 2020, 368, 65–69. [Google Scholar]
- Dong, Z.; Wu, G.; Zhao, X.L.; Zhu, H.; Lian, J.L. Durability test on the flexural performance of seawater sea-sand concrete beams completely reinforced with FRP bars. Constr. Build. Mater. 2018, 19, 671–682. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Xie, J. Study on the degradation of bfrp bars wrapped in seawater sea sand concrete. Ind. Constr. 2019, 49, 18–21. [Google Scholar]
- Xian, G.; Li, H.; Su, X. Water absorption and hygrothermal ageing of ultraviolet cured glass-fiber reinforced acrylate composites. Polym. Compos. 2012, 33, 1120–1128. [Google Scholar] [CrossRef]
- Santos, S.F.; Schmidt, R.; Almeida, A.E.F.S.; Tonoli, G.H.D.; Savastano, H., Jr. Supercritical carbonation treatment on extruded fibre–cement reinforced with vegetable fibres. Cem. Concr. Compos. 2015, 56, 84–94. [Google Scholar] [CrossRef]
- Pizzol, V.D.; Mendes, L.M.; Savastano, H., Jr.; Frías, M.; Davila, F.J.; Cincotto, M.A.; John, V.M.; Tonoli, G.H.D. Mineralogical and microstructural changes promoted by accelerated carbonation and ageing cycles of hybrid fiber–cement composites. Constr. Build. Mater. 2014, 68, 750–756. [Google Scholar] [CrossRef]
- Tonoli, G.H.D.; Pizzol, V.D.; Urrea, G.; Santos, S.F.; Mendes, L.M.; Santos, V.; John, V.M.; Frías, M.; Savastano, H., Jr. Rationalizing the impact of aging on fiber–matrix interface and stability of cement-based composites submitted to carbonation at early ages. J. Mater. Sci. 2016, 51, 7929–7943. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).