Reuse of Abandoned Shield Residues Stabilized by a Sustainable Binder: Assessment of Strength, Durability, and Environmental Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
| Properties | Standard | Value |
|---|---|---|
| Moisture content, w (%) | 28.07 | |
| Density, ρd (g/cm3) | 2.15 | |
| Dry density, ρd (g/cm3) | 1.72 | |
| Specific gravity, Gs | 2.63 | |
| Liquid limit, wL (%) | ASTM D4318 [32] | 24.04 |
| Plastic limit, wP (%) | ASTM D4318 [32] | 15.86 |
| Liquidity index, IL | ASTM D4318 [32] | 0.63 |
| Plasticity index, IP | ASTM D4318 [32] | 8.18 |
| Soil classification | ASTM D2487 [30] | Lean Clay |
| pH | ASTM D4927 [29] | 10.13 |
| Electrical conductibility (EC, μs/cm) | 325 |
| Oxide Chemistry | Soil (%) | GGBS (%) | MgO (%) |
|---|---|---|---|
| CaO | 1.3 | 34.0 | 0.23 |
| SiO2 | 67.9 | 34.3 | 0.28 |
| Al2O3 | 14.1 | 17.9 | 0.28 |
| Fe2O3 | 5.0 | 1.02 | 0 |
| MgO | 2.5 | 6.02 | 86.3 |
| Loss of ignition | 5.20 | 1.42 | 2.14 |
| Material | Properties | Value |
|---|---|---|
| Basalt fiber | Length (mm) | 6, 9, 12 |
| Diameter (μm) | 18–20 | |
| Breaking strength (MPa) | 1240 | |
| Elasticity modulus (MPa) | 63,000 | |
| Breaking elongation (%) | 2.64 | |
| GGBS | Alkalinity | 1.627 |
| Specific surface areas (m2/g) | 0.2863 | |
| pH | 10.92 | |
| Electrical conductibility (EC, μs/cm) | 431.5 | |
| MgO | MgO content (%) | 86.3% |
| Reactivity (s) | 85 | |
| Specific surface areas (m2/g) | 28.791 | |
| pH | 11.25 | |
| Electrical conductibility (EC, μs/cm) | 316.5 |
2.2. Specimen Preparation Method
2.3. Experimental Procedure
2.3.1. Unconfined Compressive Strength Test
2.3.2. Direct Shear Test
2.3.3. PH Test
2.3.4. Modified Dry and Soaking Cycle Test
2.3.5. Binder Material Ratio Design
3. Results and Discussion
3.1. Fiber-Stabilized Shield Residues Ratio Test
3.2. MgO-Activated GGBS-Fiber-Stabilized Shield Residues Ratio Test
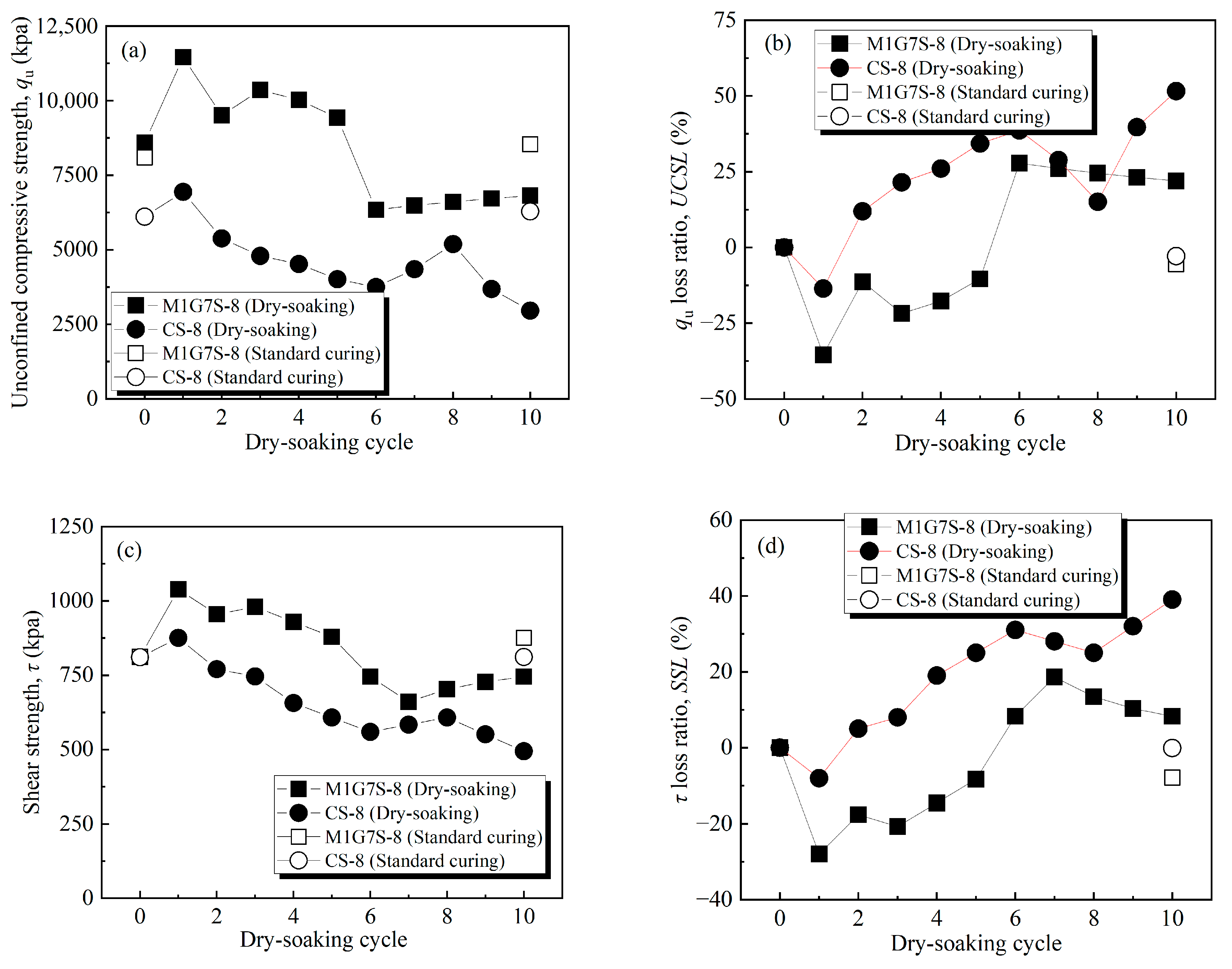
3.3. Modified Dry and Soaking Cycle Test
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, K.R. Development and Thinking of Tunnels and Underground Engineering in China in Recent 2 Years (From 2017 to 2018). Tunn. Constr. 2019, 39, 710–723. [Google Scholar]
- Xiao, M.Q. Representative projects and development trend of underwater shield tunnels in china. Tunn. Constr. 2018, 38, 360–367. [Google Scholar]
- China Association of Metros. Statistical and Analytical Report of China Urban Rail Transit in 2017; China Association of Metros: Beijing, China, 2018; pp. 1–32. (In Chinese) [Google Scholar]
- Ooishi, Y.; Murakawa, S.; Hatakoshi, A.; Mori, T. Development of discharged soil measuring device for shield tunneling machines. J. Terramechanics 1992, 29, 465–475. [Google Scholar] [CrossRef]
- Oggeri, C.; Fenoglio, T.M.; Vinai, R. Tunnel spoil classification and applicability of lime addition in weak formations for muck reuse. Tunn. Undergr. Space Technol. 2014, 44, 97–107. [Google Scholar] [CrossRef]
- Grohs, H. Cost-efficient regeneration of bore slurry for driving of the weser tunnel. Aufbereitungstechnik 2002, 43, 30–37. [Google Scholar]
- Zhou, S.; Li, X.; Ji, C.; Xiao, J. Back-fill grout experimental test for discharged soils reuse of the large-dayiameter size slurry shield tunnel. KSCE J. Civ. Eng. 2017, 21, 725–733. [Google Scholar] [CrossRef]
- Blengini, G.A.; Garbarino, E.; Solar, S.; Shields, D.J.; Hámor, T.; Vinai, R.; Agioutantis, Z. Life cycle assessment guidelines for the sustainable production and recycling of aggregates: The Sustainable Aggregates Resource Management Project (SARMa). J. Clean. Prod. 2012, 27, 177–181. [Google Scholar] [CrossRef]
- Bellopede, R.; Marini, P. Aggregates from tunnel muck treatments. Properties and uses. Physicochem. Probl. Miner. 2011, 47, 259–266. [Google Scholar]
- Riviera, P.P.; Bellopede, R.; Marini, P.; Bassani, M. Performance-based re-use of tunnel muck as granular material for subgrade and sub-base formation in road construction. Tunn. Undergr. Space Technol. 2014, 40, 160–173. [Google Scholar] [CrossRef]
- Guo, W.S.; Wang, B.Q.; Li, Y.Z.; Mo, S. Status quo and prospect of harmless disposal and reclamation of shield muck in China. Tunn. Constr. 2020, 40, 1101. [Google Scholar]
- Du, L.; Feng, Y.; Lu, W.; Kong, L.; Yang, Z. Evolutionary game analysis of stakeholders’ decision-making behaviours in construction and demolition waste management. Environ. Impact Assess. 2020, 84, 106408. [Google Scholar] [CrossRef]
- Zhu, W.; Qian, Y.J.; Wang, L.; Wei, B.; Lu, K.J.; Fang, Z.Q.; Meng, L.F. Classification, treatment, and utilization techniques of shield tunnel abandoned soil and related issues. Tunn. Constr. 2021, 41, 1–13. [Google Scholar]
- Tanner, S.; Katra, I.; Argaman, E.; Ben-Hur, M. Erodibility of waste (Loess) soils from construction sites under water and wind erosional forces. Sci. Total Environ. 2018, 616–617, 1524. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, B.; Wang, W.; Zhan, L.; Xue, Q.; Gao, Y.; Zhang, N.; Chen, H.; Liu, T.; Li, A. Mechanism of the December 2015 Catastrophic Landslide at the Shenzhen Landfill and Controlling Geotechnical Risks of Urbanization. Engineering 2016, 2, 230–249. [Google Scholar] [CrossRef]
- Entacher, M.; Resch, D.; Reichel, P.; Galler, R. Recycling of tunnel spoil—Laws affecting waste from mining and tunnelling. J. Geomech. Tunnelbau. 2011, 4, 692–701. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Liu, T.; Zhang, Y.; Su, X.T.; Zheng, J.G. The Curing and Strength Properties of Highly Moist Waste Mud from Slurry Shield Tunnel Construction. Appl. Sci. 2022, 12, 3762. [Google Scholar] [CrossRef]
- Shi, Q.T.; Wu, W.Q.; Lu, Y. Mixing Proportion and Working Mechanism of Inorganic Binder-Waste Slurry Composite Cementing Materials. Tunn. Constr. 2020, 40, 629–635. [Google Scholar]
- Yang, A.W.; Wang, T.; Xu, Z.L. Experimental study on lime and its additional agent to cure tianjin marine soft soil. Eng. Geol. 2015, 23, 996–1004. [Google Scholar]
- Boz, A.; Sezer, A.; Özdemir, T.; Hızal, G.E.; Azdeniz Dolmacı, Ö. Mechanical properties of lime-treated clay reinforced with different types of randomly distributed fibers. Arab. J. Geosci. 2018, 11, 122. [Google Scholar] [CrossRef]
- Higgins, D.D. Soil Stabilisation with Ground Granulated Blastfurnace Slag; Cementitious Slag Makers Association (CSMA): London, UK, 2005; Volume 1, p. 15. [Google Scholar]
- Swamy, R.N. Cement Replacement Materials; Surrey University Press: Surrey, UK, 1986. [Google Scholar]
- Cheng, F.A.; Wei, R.L.; Li, H. Status of recycling technology of blast furnace slag. J. Xi’an Univ. Archit. Technol. (Nat. Sci.). 2010, 42, 446–450. (In Chinese) [Google Scholar]
- Wang, X.J.; Lu, W.X.; Shao, X. Progress in granulated blast furnace slay used as admixture for high performance concrete. J. Shanghai Univ. (Nat. Sci.) 2004, 10, 170–175. [Google Scholar]
- Li, W.T.; Li, R.X.; Chen, Y.; Xiao, H.L. Comparison of Two Sulfate-Bearing Soils Stabilized with Reactive Magnesia-Activated Ground Granulated Blast Furnace Slag: Swelling, Strength, and Mechanism. Buildings 2023, 13, 230. [Google Scholar] [CrossRef]
- Zhang, M.T.; Zhao, M.; Tan, K.F. Experimental study on the new non-clinker slag cement. Bull. Chin. Ceram. Soc. 2011, 30, 920–924. [Google Scholar]
- Haha, M.B.; Lothenbach, B.; Saout, G.L. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Evaluation of novel reactive MgO activated slag binder for the immobilisation of lead and zinc. Chemosphere 2014, 117, 285–294. [Google Scholar] [CrossRef]
- ASTM D4972; Standard Test Methods for pH of Soils. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM D2487; Standard Test Methods for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM: West Conshohocken, PA, USA, 2017.
- GB/T 18046; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. Standardization Administration of the People’s Republic of China Press: Beijing, China, 2017.
- ASTM D4318; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D4219; Standard Test Method for Short-Term Unconfined Compressive Strength Index of Chemically Grouted Soils. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D3080/D3090M-11; Standard Test Method for Direct Shear Test of Soils Under Consolidated Drained Conditions (Withdrawn 2020). ASTM: West Conshohocken, PA, USA, 2011.
- Kamon, M.; Nontananandh, S.; Katsumi, T. Utilization of stainless-steel slag by cement hardening. Soils Found. 1993, 33, 118–129. [Google Scholar] [CrossRef]
- Darve, F.; Vardoulakis, I. Degradations and Instabilities in Geomaterials; Springer: New York, NY, USA, 2014. [Google Scholar]
- Shihata, S.; Baghdadi, Z. Long-term strength and durability of soil cement. J. Mater. Civ. Eng. 2001, 13, 161–165. [Google Scholar] [CrossRef]
- ASTM D4843-88; Standard Test Method for Wetting and Drying Test of Solid Wastes. ASTM: West Conshohocken, PA, USA, 2016.
- Kamon, M.; Ying, C.Y.; Katsumi, T. Effect of acid rain on lime and cement stabilized soils. J. Soils Found. 1996, 36, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Boz, A.; Sezer, A. Influence of fibre type and content on freeze-thaw resistance of fibre stabilized lime stabilized clay. Cold Reg. Sci. Technol. 2018, 151, 359–366. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y. Strength characteristics of cemented sand-bentonite mixtures with fibre and metakaolin additions. Mar. Georesour. Geotechnol. Taylor Francis 2017, 35, 414–425. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.S.; Wang, D.Y.; Pei, X.J.; Shi, B. Effect of discrete fibre reinforcement on soil tensile strength. J. Rock Mech. Geotech. 2014, 6, 133–137. [Google Scholar] [CrossRef]
- Tang, C.; Shi, B.; Gao, W. Strength and mechanical behavior of short polypropylene fibre stabilized and cement stabilized clayey soil. Geotext. Geomembr. 2007, 25, 194–202. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F. Hydration mechanisms of super sulphated slag cement. Cem. Concr. Res. 2008, 38, 983–992. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Al-Tabbaa, A. Initial Investigation into the Use of GGBS-MgO in Soil Stabilisation. In Grouting and Deep Mixing; American Society of Civil Engineers: New Orleans, LA, USA, 2012; pp. 444–453. [Google Scholar]
- Yi, Y.; Liska, M.; Al-Tabbaa, A. Properties of two model soils stabilized with different blends and contents of GGBS, MgO, lime, and PC. J. Mater. Civ. Eng. 2014, 26, 267–274. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-activated ground-granulated blast furnace slag for stabilization of marine soft clay. J. Mater. Civ. Eng. 2015, 27, 04014146. [Google Scholar] [CrossRef]
- Yi, Y.; Zheng, X.; Liu, S.Y.; Al-Tabbaa, A. Comparison of reactive magnesia-and carbide slag-activated ground granulated blastfurnace slag and Portland cement for stabilisation of a natural soil. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Larsson, S.; Benzerzour, M.; Maherzi, W.; Amar, M. Effect of basalt fiber inclusion on the mechanical properties and microstructure of cement-solidified kaolinite. Constr. Build. Mater. 2020, 241, 118085. [Google Scholar] [CrossRef]
- Zheng, J.R.; Sun, X.X.; Guo, L.J.; Zhang, S.M.; C, J.Y. Strength and hydration products of cemented paste backfill from sulphide-rich tailings using reactive MgO-activated slag as a binder. Constr. Build. Mater. 2019, 203, 111–119. [Google Scholar] [CrossRef]
- Estabragh, A.R.; Jahani, A.; Javadi, A.A.; Babalar, M. Assessment of different agents for stabilisation of a clay soil. Int. J. Pavement. Eng. 2022, 23, 160–170. [Google Scholar] [CrossRef]
- Sun, X.P.; Ding, Z.H.; Bi, Y.Z.; Wang, X.Y. Water-Holding Properties of Clinoptilolite/Sodium Polyacrylate-Modified Compacted Clay Cover of Tailing Pond. Int. J. Environ. Res. Public Health 2022, 19, 15554. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Li, J.L. Application of cement soil pile composite foundation. J. Highw. 2012, 1, 86–88. (In Chinese) [Google Scholar]
- Inthurraide, G.J.B.; Oliver, J. High performance concrete for French Nuclear Reactor Containment Vessels. J. Util. High Strength Concr. 1993, 1, 20–24. [Google Scholar]
- Fu, X.Q.; Feng, J.D.; Xie, Y.J. Mechanical behavior of soil cement under ambient with sulfate conditions. Rock Soil Mech. 2008, 29 (Suppl. S1), 659–662. (In Chinese) [Google Scholar]
- Rollings, R.; Burkes, J.; Rollings, M. Sulfate attack on cement-stabilized sand. J. Geotech. Geoenviron. 1999, 125, 364–372. [Google Scholar] [CrossRef]
- Wild, S.; Kinuthia, J.M.; Jones, G.I. Effects of partial substitution of lime with ground granulated blast furnace slag (GGBS) on the strength properties of lime-stabilised sulphate-bearing clay soils. Eng. Geol. 1998, 51, 37–53. [Google Scholar] [CrossRef]
- Wild, S.; Kinuthia, J.M.; Jones, G.I. Suppression of swelling associated with ettringite formation in lime stabilized sulphate bearing clay soils by partial substitution of lime with ground granulated blastfurnace slag (GGBS). Eng. Geol. 1999, 51, 257–277. [Google Scholar] [CrossRef]

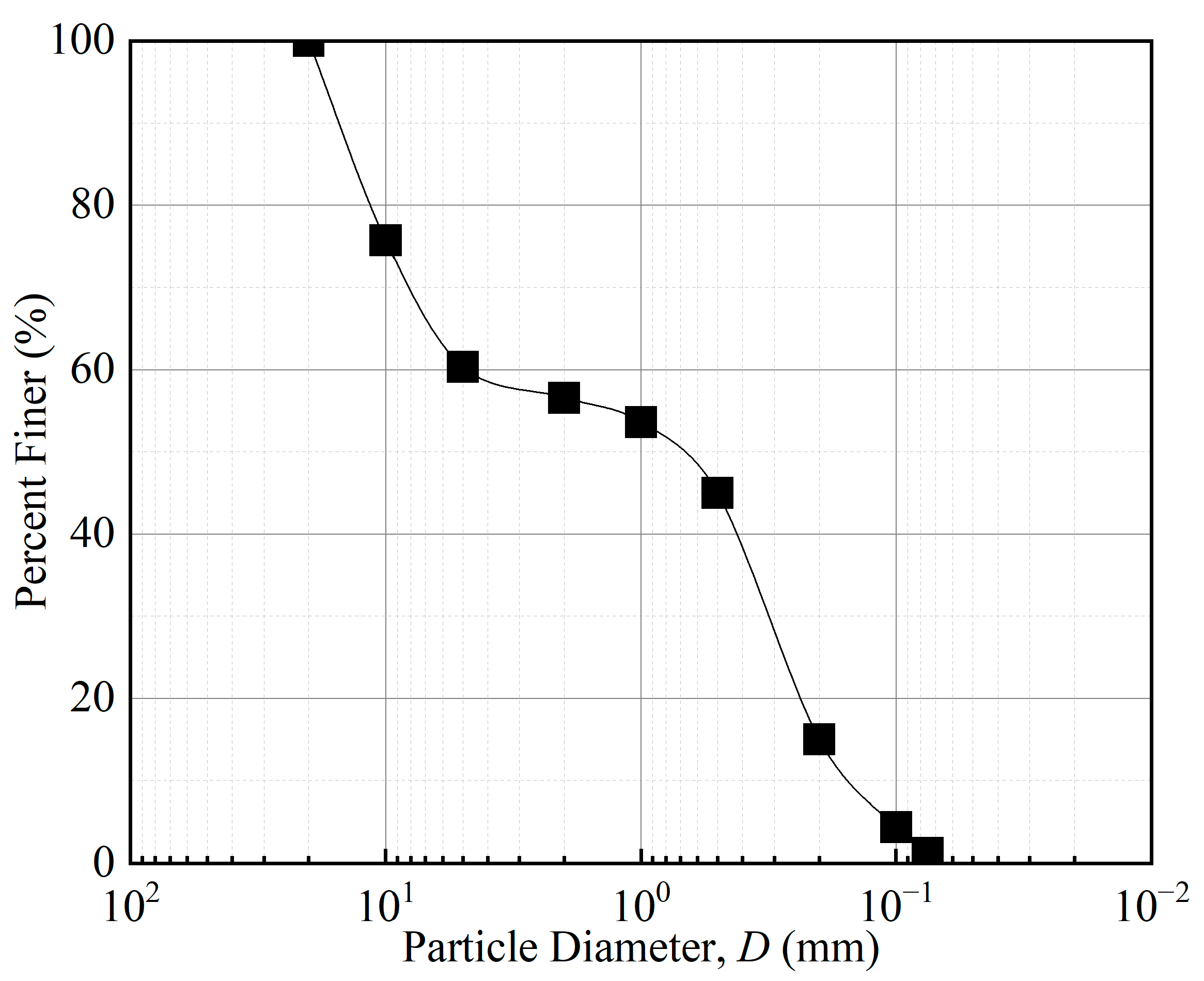
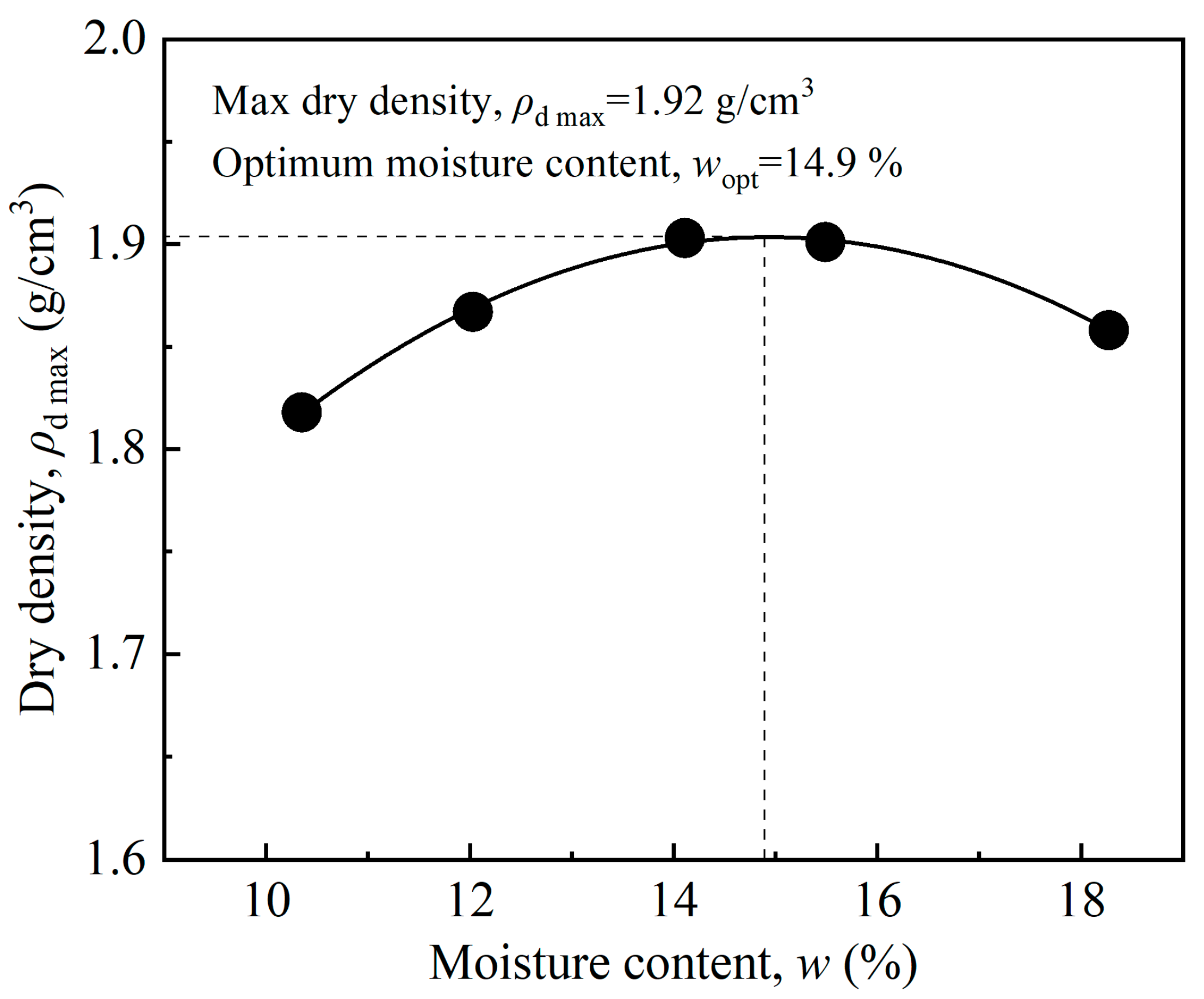

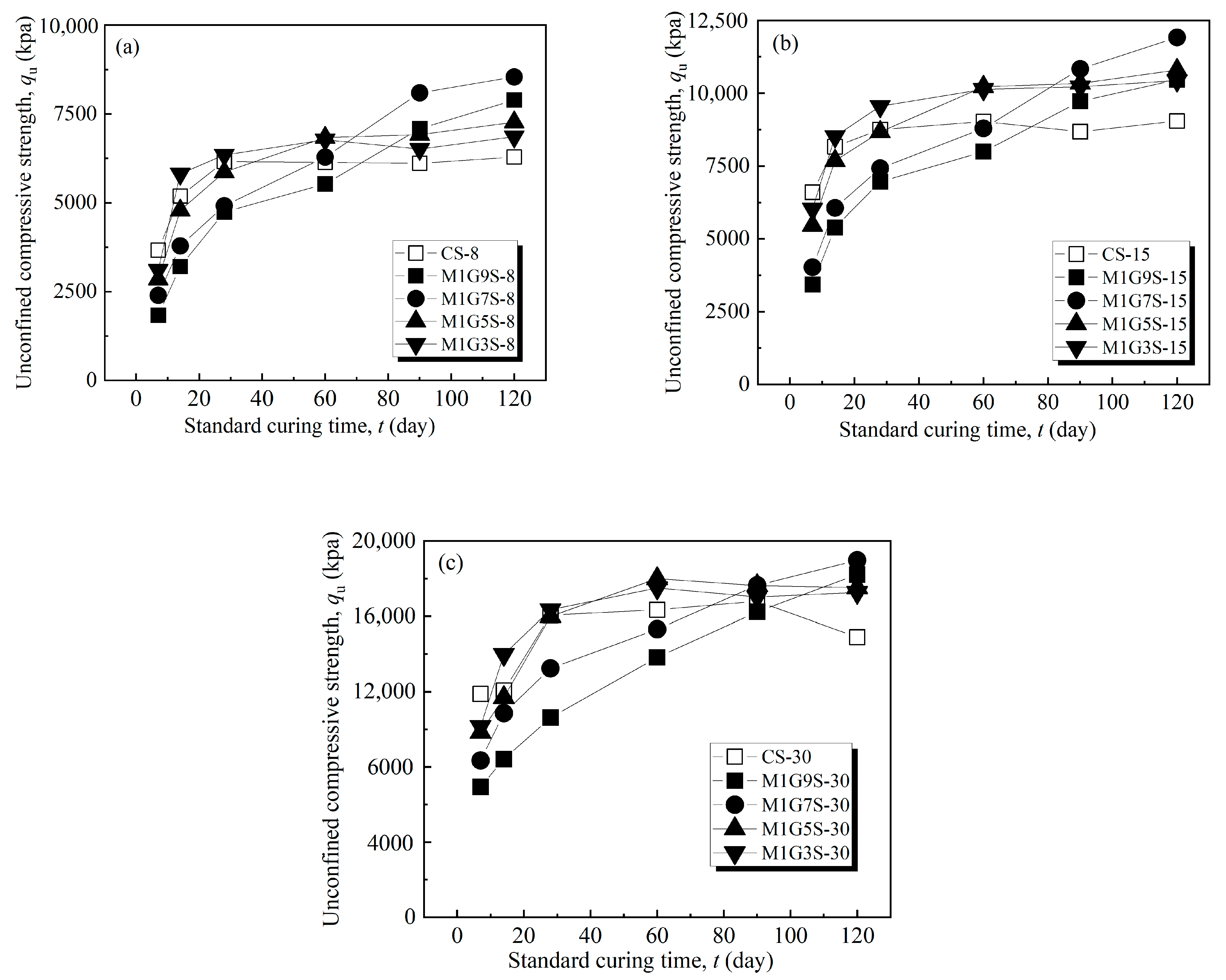
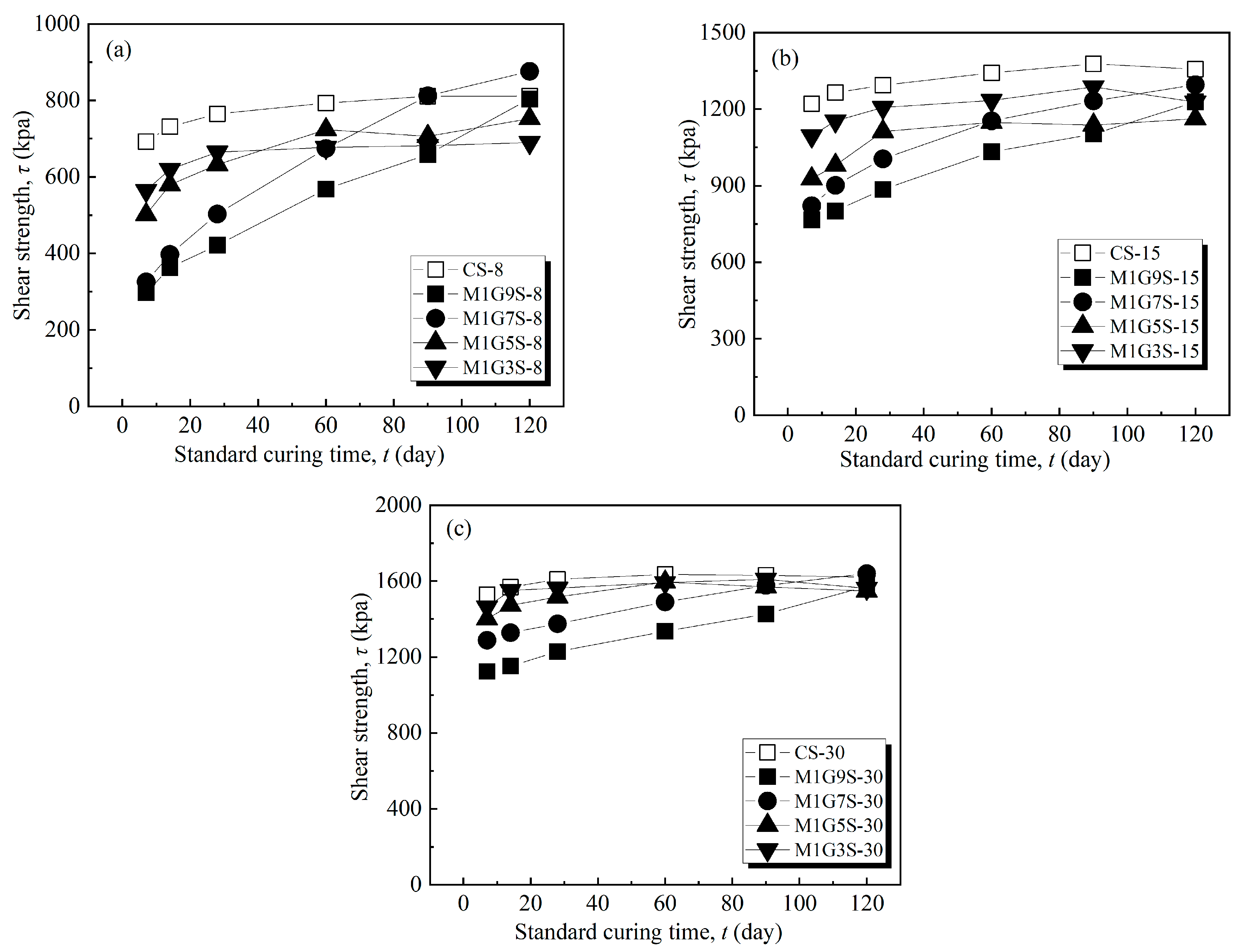



| Fiber Length (mm) | Fiber Content (%) | Dry Density (%) | Moisture Content (%) |
|---|---|---|---|
| 6 | 0.1 | 1.9 | 15 |
| 6 | 0.2 | 1.9 | 15 |
| 6 | 0.3 | 1.9 | 15 |
| 6 | 0.4 | 1.9 | 15 |
| 9 | 0.1 | 1.9 | 15 |
| 9 | 0.2 | 1.9 | 15 |
| 9 | 0.3 | 1.9 | 15 |
| 9 | 0.4 | 1.9 | 15 |
| 12 | 0.1 | 1.9 | 15 |
| 12 | 0.2 | 1.9 | 15 |
| 12 | 0.3 | 1.9 | 15 |
| 12 | 0.4 | 1.9 | 15 |
| Mix Code | Binder Content (%) | MgO to GGBS | Fiber Content (%) | Fiber Length (mm) | Dry Density (%) | Moisture Content (%) |
|---|---|---|---|---|---|---|
| M1G9S-8 | 8 | 1:9 | 0.1 | 12 | 1.9 | 15 |
| M1G7S-8 | 8 | 1:7 | 0.1 | 12 | 1.9 | 15 |
| M1G5S-8 | 8 | 1:5 | 0.1 | 12 | 1.9 | 15 |
| M1G3S-8 | 8 | 1:3 | 0.1 | 12 | 1.9 | 15 |
| M1G9S-15 | 15 | 1:9 | 0.1 | 12 | 1.9 | 15 |
| M1G7S-15 | 15 | 1:7 | 0.1 | 12 | 1.9 | 15 |
| M1G5S-15 | 15 | 1:5 | 0.1 | 12 | 1.9 | 15 |
| M1G3S-15 | 15 | 1:3 | 0.1 | 12 | 1.9 | 15 |
| M1G9S-30 | 30 | 1:9 | 0.1 | 12 | 1.9 | 15 |
| M1G7S-30 | 30 | 1:7 | 0.1 | 12 | 1.9 | 15 |
| M1G5S-30 | 30 | 1:5 | 0.1 | 12 | 1.9 | 15 |
| M1G3S-30 | 30 | 1:3 | 0.1 | 12 | 1.9 | 15 |
| CS-8 | 8 | 0 | 0 | 0 | 1.9 | 15 |
| CS-15 | 15 | 0 | 0 | 0 | 1.9 | 15 |
| CS-30 | 30 | 0 | 0 | 0 | 1.9 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Zhao, J.; Jiang, Z.-Y.; Li, Y.-Z.; Che, C. Reuse of Abandoned Shield Residues Stabilized by a Sustainable Binder: Assessment of Strength, Durability, and Environmental Properties. Buildings 2023, 13, 738. https://doi.org/10.3390/buildings13030738
Cao W, Zhao J, Jiang Z-Y, Li Y-Z, Che C. Reuse of Abandoned Shield Residues Stabilized by a Sustainable Binder: Assessment of Strength, Durability, and Environmental Properties. Buildings. 2023; 13(3):738. https://doi.org/10.3390/buildings13030738
Chicago/Turabian StyleCao, Wei, Jun Zhao, Zhe-Yuan Jiang, Ying-Zhen Li, and Chi Che. 2023. "Reuse of Abandoned Shield Residues Stabilized by a Sustainable Binder: Assessment of Strength, Durability, and Environmental Properties" Buildings 13, no. 3: 738. https://doi.org/10.3390/buildings13030738
APA StyleCao, W., Zhao, J., Jiang, Z.-Y., Li, Y.-Z., & Che, C. (2023). Reuse of Abandoned Shield Residues Stabilized by a Sustainable Binder: Assessment of Strength, Durability, and Environmental Properties. Buildings, 13(3), 738. https://doi.org/10.3390/buildings13030738





