Abstract
This study provides an overview of how phase change materials (PCMs) can improve the resistance of concrete pavement to freeze–thaw cycles and mitigate the urban heat island (UHI) effect. The investigation covers different types of PCMs and methods for integrating them into concrete pavement, as well as the mechanical properties and compressive strength of concrete pavement when employing various PCMs. Prior studies have identified porous aggregates, microencapsulation, and pipelines containing liquid PCM as common approaches for PCM integration. Researchers have observed that the utilization of PCMs in concrete pavement yields favorable thermal properties, suggesting the potential for anti-freezing and UHI mitigation applications. However, the choice of PCM materials should be informed by local climate conditions.
1. Introduction
Pavements are a vital part of our modern life, and their coverage is up to 45% in some cities [1,2,3]. The pavement can be applied to roads, driveways, parking lots, sidewalks, commercial plazas, playgrounds, and airstrips, which play an essential role in our everyday lives [4]. They are categorized into three groups: (1) flexible (full-depth asphalt pavement, conventional layered flexible pavement, and rock-contained asphalt), (2) rigid pavements (conventional concrete pavements, pre-stressed and precast concrete, roller compacted concrete, and pervious or porous concrete), and (3) composite pavements (semi-rigid pavement structures, premium composite pavements, long-life pavements, flexible composite pavements, and maintenance-free pavements) [5,6,7,8,9].
Prior literature attempted to develop pavements technically in terms of physical, thermal, and mechanical properties. However, the freezing and urban heat island (UHI) phenomena are the two main critical issues interacting between pavement and the surroundings during cold and hot seasons.
Due to freeze–thaw, internal frost damage and surface scaling are the most common types of frost damage on pavements [10]. Depending on the type of exposure (water, deicer), the rapid freezing of water inside the concrete causes hydraulic pressure or cryosuction and/or glue-spall stress due to the deicer solution on the surface, causing scaling damage [11,12,13] and decreasing pavement life. Also, frost and snow are critical factors in traffic jams and accidents during cold seasons. Therefore, it is necessary to thaw ice and snow through different methods [11] of snow removal and/or melting, etc. The most used techniques for removing ice and snow from the concrete pavement surface are deicing salts and snowplowing in urban areas, including transport to dumping sites, which are costly, time consuming, and labor intensive. It should be noted that using deicing salts also negatively affects the environment [14,15,16]. Therefore, considering a more sustainable (less energy consumption, less emissions, less consumption of primary raw materials) technique for removing ice and snow on pavements appears to be necessary [17].
Regarding the UHI, some prior studies showed that the ambient temperature of 450 cities has increased [18,19] due to the UHI phenomenon [20,21,22,23], which can negatively affect energy consumption, the environment, and people’s health. Forecasts of future cooling energy consumption of residential and commercial buildings reveal that by 2050, it may increase up to 750% and 275% due to local and global climate change, tremendous population growth, and the expected rise in cooling system employment worldwide [24]. The building and pavement materials have an essential role in UHI mitigation. Akbari and Rose [25] found that the average urban surface of four different metropolitan areas was 29–41% vegetation, 19–25% roofs, and 29–39% paved surfaces. This demonstrates that hard, artificial, heat-absorbent surfaces can cover over 60% of an urban surface. Therefore, the impact of pavements on UHI development is significant. Reflecting materials such as light-color materials can reflect most solar radiation to reduce the surface and ambient temperature. However, they may be dangerous for the thermal comfort of people on the street [26]. Based on the available literature, many researchers developed new materials and technologies to reduce the surface temperature of pavements as an essential method to mitigate UHI [27,28,29,30]. The high temperature on the pavement’s surface is one of the reasons for the UHI phenomena [31,32]. Thus, reducing the surface temperature of pavements through the deduction of solar gains, enhancing heat transfer, and increasing the heat capacity of pavement are the most reported strategies to mitigate UHI [20].
As an innovative method, the phase change materials (PCMs) have been incorporated into the concrete pavement to increase the anti-freezing effect and mitigate the UHI by enhancing latent heat capacity. PCMs have the potential to be deployed to save energy during the phase change in terms of solidification (exothermic process) and melting (endothermic process) [33,34]. Thus, the current study aims to evaluate the current knowledge of PCMs in concrete pavements. We searched the keywords on online databases and found that considerably more recent studies incorporated PCMs into asphalt pavement [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] compared to concrete pavement (Figure 1). However, the scope of this study is limited to the effect of PCMs on freezing and UHI mitigation of concrete pavements. For this reason, the following sections are related to the prior literature on using PCMs in concrete pavement for anti-freezing and UHI mitigation applications.
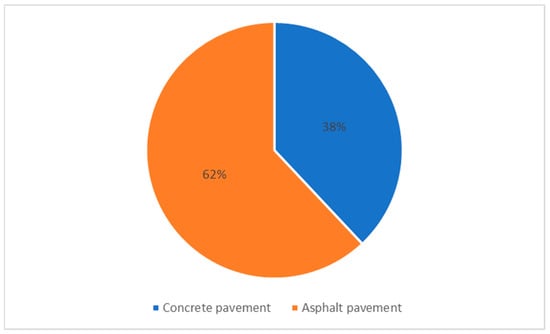
Figure 1.
The number of publications on the PCM asphalt pavement vs. PCM concrete pavement during 2016–2022.
2. Phase Change Materials (PCMs) in Concrete Pavements
One of the most promising methods for increasing the thermal energy storage of pavement is incorporating phase change materials (PCMs). Incorporating PCMs into the concrete can increase heat storage capacity by minimizing temperature fluctuation despite heat transfer to/from materials [72,73]. By heating a PCM, the temperature rises before solidus temperature (Ts) and after liquidus temperature (Tl). It should be noted that the temperature of a PCM does not change by heating/cooling of the PCM between Ts and Tl, and only the phase of material changes (Figure 2). The solid and liquid phases are available during this process, and their amount changes over time. The fraction of liquid phase (fl) and solid phase (fs) as a function of temperature can be calculated by the following equations [67]:
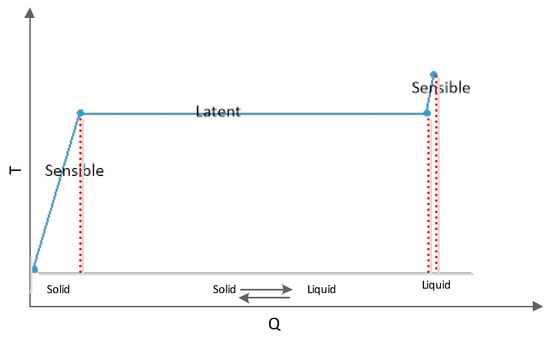
Figure 2.
Temperature vs. heating or cooling during sensible and latent heat storage capacity.
Thus, the energy equation for the PCM based on the enthalpy can be written as follows:
where is , Tm is the melting temperature (Tm = Ts + ɛ, Tm = Tl − ɛ, ɛ > 0), and lf is the latent heat of fusion.
It should be noted that the PCM is incorporated into the concrete pavement in different percentages and methods, which are discussed in Section 2.2. Thus, the pavement temperature is not constant during the phase changing of PCMs in actual conditions; however, it can fluctuate less than the pavement without PCMs. Athukorallage et al. [67] suggested the following model for the heat transfer in a pavement layer containing different amounts of PCMs:
where is the volume fraction of PCM in the matrix, is the heat capacity of the pavement, and is the thermal conductivity as a function of kpa, kpcm, Ø.
2.1. Type of PCMs in Concrete Pavements
As demonstrated in Equations (2) and (3), selecting the proper PCM for different applications is related to the thermodynamic properties such as entropy (S), latent heat of fusion (lf) (i.e., enthalpy (H)), and melting point (Tm) of PCMs. Entropy measurement during the changing phase is a challenging issue. However, the enthalpy of phase change and melting temperature can be measured using a differential scanning calorimeter (DSC). Figure 3 shows different PCMs categorized based on the enthalpy of fusion and phase-changing temperature [74]. Generally, organic, inorganic (MnH2O), and eutectic are the three main types of PCMs used in cement-based materials. Organic PCMs are paraffin-based (CnH2n+2) and non-paraffin-based CH3 (CH2)2nCOOH [75]. Salt hydrates and metal are the two main inorganic PCMs. Eutectics can be organic–organic, inorganic–organic, and inorganic–inorganic [76]. Recently, another classification was illustrated based on the state of PCMs as solid–liquid, liquid–gas, and solid–solid [77].
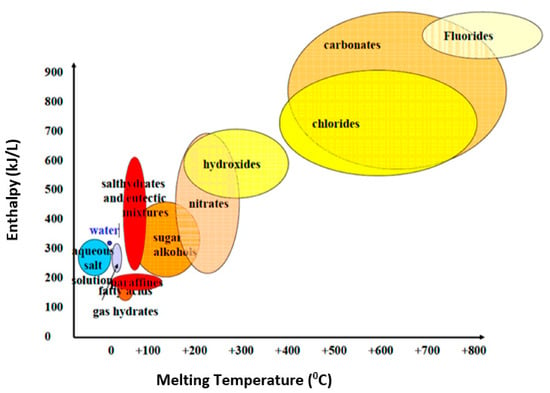
Figure 3.
Melting point vs. heat storage capacity of various PCMs reprinted/adapted from Refs. [74,76].
As mentioned previously, the phase change temperature of PCMs should be appropriate based on their application [30]. Prior literature showed that the latent heat of PCMs used in the concrete pavement for anti-freezing and UHI mitigation applications is 122 to 240.5 J/g. Moreover, the phase change temperature of PCMs used in the concrete pavement for melting ice and anti-freezing purposes is slightly above zero (in the range of −0.5 to 5.7 °C). However, PCMs with higher phase change temperatures (temperature range of 28 to 51 °C) were applied for other applications, such as reducing the surface temperature of concrete pavement.
2.2. Methods of Incorporation
Generally, the PCMs are incorporated into the materials by immersion, impregnation, and encapsulation (macro- and microencapsulation) (Figure 4) [78,79,80,81,82]. Regarding the cement-based materials, the previous literature revealed that the incorporation of PCMs directly into the binder (cementitious system) might have significantly reduced the strength of concrete due to the displacement of some of the cementitious materials by the PCMs. This displacement minimizes the amount of cement paste to bind the aggregate together. Moreover, PCMs may interfere with the chemical reactions that occur during the hydration process of cement, which is critical for developing concrete strength [83,84,85].
The following references [75,86,87,88,89,90] explain the methods of incorporating PCMs into the concrete. Impregnating PCMs in different carrier agents such as lightweight aggregates (LWA) or encapsulation is common to integrate PCMs into cement-based materials [91,92,93,94]. Moreover, the available literature revealed other methods to incorporate PCMs into the concrete pavement, such as immersion or pipes filled with PCMs. However, a few studies also used direct incorporation and immersion in concrete pavement. Table 1 summarizes the type, physical properties, and incorporation method of PCMs.
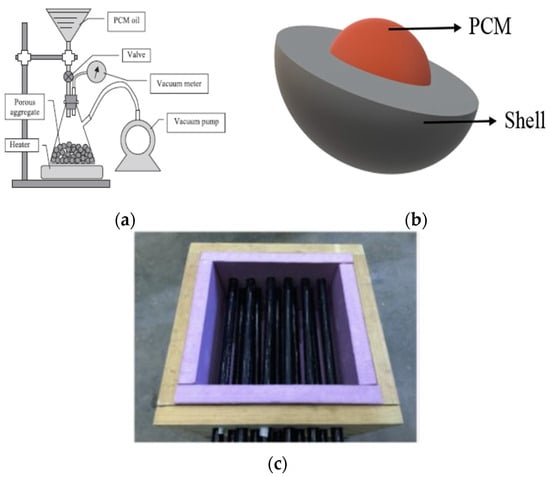
Figure 4.
Methods of PCM incorporation: (a) impregnation adapted with permission from Ref. [78], (b) microencapsulated PCM adapted with permission from Ref. [30], and (c) using PCM in the concrete pavement through pipes adopted from adapted with permission from Ref. [95].

Table 1.
Physical properties and method of incorporation of PCMs into the concrete pavement.
Table 1.
Physical properties and method of incorporation of PCMs into the concrete pavement.
| Aim of Using PCM in Concrete Pavement | PCM | Density (g/cm3) | Latent Heat (J/g) | Phase Change Temperature (°C) | Method of Incorporation | Ref. |
|---|---|---|---|---|---|---|
| Anti-freezing | Polyol | 0.82 | 240.5 | 4.53 | Pipe | [11] |
| Paraffin | 0.77 | 129.4 | 2.9 | Using LWA and embedded tube | [96] | |
| Methyl laurate | 0.87 | 160.4 | 1.9 | |||
| Paraffin oil (C14-C16) | 0.77 | 157.8 | 5.7 | Impregnation | [95] | |
| Pipe | ||||||
| Paraffin (C14-H13) | 0.75 | 224.5 | 4.5 | Microencapsulate | [97] | |
| Paraffin | 0.88 (solid) 0.77 (liquid) | 200 | 1–3 | Impregnation | [60] | |
| Paraffin | - | 200–225 | 4.5 | Microencapsulate | [98] | |
| Methyl laurate | 0.87 | - | 5.2 | Impregnation | [99] | |
| Paraffin (OP2E) | 0.77 | 205 | 1–3 | |||
| Paraffin (OP3E) | 0.77 | 250 | 3–5 | |||
| Paraffin | 0.75 | 193 | 4.5 | Impregnation | [100] | |
| Paraffin | 0.77 | 122 | 2–2.5 | |||
| Paraffin | 0.78 | 171 | −0.5 | |||
| Reduce surface temperature | Paraffin | - | 150 | 28 | Impregnation | [101] |
| Paraffin | 0.86 | 180 | 45 | Microencapsulate | [102] | |
| Paraffin | 0.96 | 172 | 48–51 | Direct mixing | [103] | |
| Paraffin | 0.96 (solid) 0.87 (liquid) | 171 | 34–35 | Impregnation | [104] | |
| Paraffin | 0.90 (solid) 0.86 (liquid) | 199 | 43–44 |
In the impregnation method, the porous carrier agent (LWA is the most familiar carrier agent because of its high porosity and absorption capacity) is impregnated by liquid PCMs [60,95,96,99,100,101,105]. The porosity of LWA can be filled with liquid PCMs through the vacuum saturation technique (Figure 4a). Evaluation of the previous studies indicated that the water absorption capacity and size of LWAs (expanded shale, expanded clay, and expanded perlite) used as a PCM carrier are 9–250% and 0–8 mm, respectively. However, the water absorption capacity and size of normal coarse and fine aggregate depend strongly on the type of material. Still, the examples are in the ranges of 0.4–0.57%, 5–20 mm, and 1–1.52%, 0–5 mm, respectively (Table 2).

Table 2.
Examples of physical properties of aggregate used in PCM concrete pavement.
The main disadvantage of impregnation in concrete pavement is the leakage of PCMs while changing phases. Therefore, encapsulating PCMs into a polymer shell before incorporating them into the mixture can reduce leakage and incompatibility problems [90]. The microencapsulation method encapsulates the micro-sized PCMs within the polymeric shells in different sizes from 1 to 300 mm [106,107,108]. However, the typical core size (paraffin) and the shell were reported to be around 40 mm and 2 mm, respectively [109]. The shell can protect the PCMs from environmental conditions and leakage (Figure 4b), and prior literature revealed several advantages and disadvantages of microencapsulated PCMS [110,111]. One of the main advantages of this method is optimizing heat transfer because of the high surface area-to-volume ratio [89]. However, the low durability, low stiffness, and chemical and thermal stability of the polymer shell can be mentioned as the main drawbacks of this method [110].
It is expected to use water pipes to cool massive concrete [112]. Recently, the closed pipe system was applied to increase the heat capacity of concrete using liquid PCMS instead of water inside the pipeline [11,95,96]. The main advantages of this method are reducing the leakage and limiting the chemical and physical damage of PCMs if the pipes do not corrode, which may occur in the other methods. This method provided frost-resistant concrete pavement simply by avoiding concrete freezing. However, the practicality of this method is in doubt due to its cost and applicability in extremely cold countries like Norway, where temperatures remain below freezing for several months.
3. The Effect of PCMs on the Mechanical Properties of Concrete Pavement
Incorporating PCMs into concrete pavements may affect the mechanical properties differently. Incorporating PCMs into concrete pavements can impede the chemical reactions that happen during the hydration process of cement, which is essential for the formation of CSH and concrete’s strength development. This hindrance can slow down or lessen the rate of hydration, leading to a reduction in the amount of CSH produced and weaker concrete. Also, using LWA as a PCM carrier agent decreases the strength of cementitious materials due to its pore structure compared to natural aggregate [83,93,113]. In a study, 10% LWA as sand replacement on a volumetric basis decreased the compressive strength by 2%. However, incorporating LWA and rice husk ash (RHA) presoaked in PCM decreased the compressive strength of cementitious materials by 10% and more than 35%, respectively [105]. Due to its high absorption capacity, RHA is investigated as a PCM carrier agent in cementitious materials. It is reported that using RHA decreased compressive strength by about 28%. However, incorporating PCMs into cementitious materials with the RHA decreased the compressive strength by 36%.
Decreasing the compressive strength with the use of PCMs filled in the LWA in concrete is reported by other studies, which is due to the chemical interaction of leaked PCMs with hydration products, which cause expansion and cracking and decrease the connection at the interfacial transition zone (ITZ) [113,114]. ITZ is a aggregate particles and the bulk cement paste [115]. However, it is reported that coating the LWA as a PCM carrier agent with a capillary crystalline waterproofing material and Portland cement can prevent PCM leakage. The investigation showed that cement as a coating material might be beneficial for providing a proper ITZ and preventing leakage of PCM [99].
When PCMs are directly added to cementitious materials, the compressive strength decreases significantly, which could be attributed to its destructive effect on the hydration reaction and reduction in ITZ connection [83,93,105]. It is reported that the compressive and flexural strengths of cementitious materials containing raw PCM are lower than specimens containing PCM filled in LWA [100]. The results indicated that incorporating 10% and 15% PCMs (by mass of cement) filled in LWA and raw PCM decreased the compressive strengths by 13%, 18%, 27%, and 36%, respectively. Also, the flexural strengths for specimens with 10% and 15% PCMs (by mass of cement) filled in LWA decreased by 5.5% and 7%, and for samples with raw PCMs by 8% and 14%, respectively [100]. However, the investigation showed that the PCMs could be directly mixed in the concrete pavement in both solid and liquid states. The results showed that the specimens in the dry state had higher compressive strength values than those in the liquid state. According to the IRC 58:2015 standard [116] for concrete pavement, which limits the 28 day compressive strength to more than 40 MP, the PCMs by 6% and 8% at liquid and dry states can be used in concrete pavement, respectively [103].
According to another study, cementitious materials’ compressive and flexural strengths were negatively impacted by the microencapsulation of PCMs with a melamine-formaldehyde resin. Incorporating 10% and 20% microencapsulated PCMs (by cement mass) decreased the compressive strengths by 27.7% and 46.9%, and the flexural strengths reduced by 17.5% and 22.3%, respectively [97]. Microencapsulated PCMs make voids in the matrix and cracks in cementitious materials, leading to decreased compressive and flexural strengths. Also, the structure of microencapsulated PCMs inside the cementitious materials is weaker than cement hydration products, which could be considered a weak point for failure [113,117,118,119].
Thus, incorporating PCMs into the concrete pavement (i.e., LWAs, directly and by microencapsulation) could decrease its mechanical properties by about 46%. However, considering the required costs and advanced technology for manufacturing microcapsules and high-strength decrease for microencapsulation and direct methods, using LWA as a PCM carrier agent could be recommended. Notably, using cement or any other coating material might be beneficial for providing a proper ITZ and preventing leakage of PCM. According to previous studies, Figure 5 shows the percentages of compressive strength reduction with the incorporation of PCMs filled in LWA, raw PCMs, and microencapsulated PCMs compared to the control mixture (without any PCM) [87,98,103]. All the percentages of PCMs in this figure are by mass of cement.
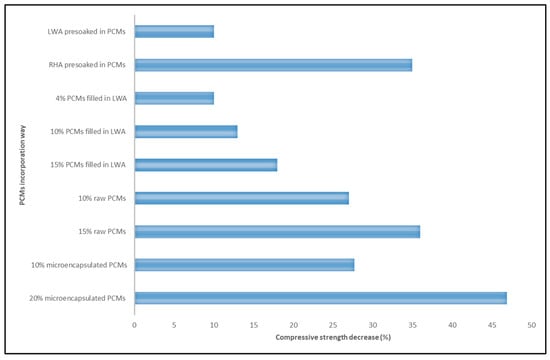
Figure 5.
Percentages of compressive strength reduction with the incorporation of PCMs in cementitious materials.
4. The Effect of PCMs on the Heat of Hydration of Concrete
The heat released during hydration can typically be divided into four stages: initial, dormant, acceleration, and retardation. The maximum heat of hydration is usually released during the acceleration and the post-acceleration phases, which typically take place between two and ten hours [120]. Some countries, such as China, Japan, and Korea, have been developing a new technology that can reduce the heat of hydration by encapsulating PCMs [121]. Generally, there is an inverse relationship between the amount of heat released during the hydration process and the mechanical properties of concrete. Also, a higher amount of heat of hydration contributes to the micro-cracking of massive concrete elements, leading to lower mechanical performance during service life [122]. Therefore, incorporating materials with latent heat storage capacity, i.e., PCMs, into cementitious materials is receiving increasing interest among researchers worldwide.
Fernandes et al. [123] used paraffin-based microencapsulated PCMs in cement mortars and reported a reduction in peak temperature during cement hydration. In another study [122], a small amount of two paraffin-based microencapsulated PCMs (up to 1% in weight of cement) with different melting temperatures of 18 and 25 °C were used in cement-based cubes. The results showed a decrease in the internal temperature of the cement paste by 5 °C. Mihashi et al. [124] applied a retarder containing PCMs in a paraffin microcapsule to control the heat of hydration. They reported that the maximum temperatures in semi-adiabatic curing could be reduced in large concrete specimens. Choi et al. [125] investigated different types of inorganic PCMs in conditions similar to concrete materials. They found a strontium-based PCM to be the most appropriate to decrease the heat of hydration in mass concrete. Yun et al. [88] evaluated the feasibility of adding strontium-based powder (Sr(OH)2-8H2O) PCMs into concrete to mitigate the hydration heat of mass concrete. The mixed PCMs in concrete were 3% of the weight of the binder. The results showed a delay in initial and final setting time and about a 15–21% decrease in the temperature rise of concrete.
Therefore, incorporating PCMs into the concrete pavement could considerably decrease the heat of hydration. In addition, the thermal cracking could be reduced by adding PCMs into the concrete mix. However, very few investigations exist in the literature on the relationship between the uses of PCMs and thermal cracking in concrete. Thus, future studies on these issues can explore more critical findings.
5. Frost-Resistant Concrete Pavement with PCMs
PCMs can meet various working temperatures (see Figure 3). Researchers invested in using phase change materials (PCMs) in concrete pavements as an anti-freezing and snow-melting method. They evaluated the impact of PCMs on the thermal response of concrete pavements in cold winter regions through experimental and numerical simulation frameworks utilizing finite element analysis [11,94,95,96,97,99,126,127].
The results of studies indicated that utilizing PCMs can prevent ice formation and decrease the number of freeze–thaw cycles in concrete due to the release of heat during phase changing. Moreover, some of these studies reported that PCMs could increase the service life of pavements due to the prevention of sudden temperature drops. Also, the results showed that macro encapsulation of PCMs via LWA had desirable performance in terms of maintaining a temperature of concrete pavement above 0 °C.
In summary, limited studies consider the effect of PCMs on concrete pavement freezing compared to other applications like UHI. As mentioned, most reported that incorporating PCMs can effectively increase freeze–thaw resistance and ice melting. However, the effectiveness of a PCM can be related to the amount of utilization; latent heat of fusion; and, of course, the phase-changing temperature. Table 1 demonstrates that the literature’s phase-changing temperature of applied PCMs varies from −0.5 °C to 5.7 °C. The results seem acceptable for regions with mild snow and moderate winter. However, the efficiency of utilizing PCMs in areas with severe cold winters is a controversial issue. For example, the air temperature in many Nordic countries like Norway remains below 0 for several months. Further research is essential to comprehensively assess the efficacy of phase change materials (PCM) in cold regions characterized by varying air temperatures. An illustrative example of this necessity is evident in the provided Appendix A, where a simulation delves into the impact of PCM volume and phase-changing points on the surface temperature of concrete pavement. This simulation not only underscores the need for additional studies but also highlights the intricate interplay between PCM properties and environmental conditions, emphasizing the importance of a more nuanced understanding for effective implementation in diverse climates. Consequently, a more extensive exploration of PCM behavior in cold environments is warranted to refine our understanding and optimize its practical applications.
6. The Effect of PCMs on the Surface Temperature and UHI Phenomena
Generally, the UHI effect of pavement should be controlled by reducing its surface temperature. Pavement can significantly reflect a considerable amount of solar radiation to decrease the surface temperature. Using titanium dioxide (TiO2), lime (calcium hydroxide), and/or colored coating are the usual methods to increase the reflection of concrete pavements [128,129]. Thermal properties of concrete pavements, such as thermal conductivity (k) and specific heat capacity (Cp), are also influential factors in their surface temperature [75,130,131,132].
Pavements are heated by the sun and increase the surrounding air due to poor thermal conductivity and heat capacity [133,134]. Thermal conductivity is a material property that demonstrates its capability in heat transfer through conduction [135,136]. It has been reported that the PCMs with higher thermal conductivity must change their phase faster. Also, a concrete pavement with higher thermal conductivity is desirable to reduce surface temperature and melt ice and snow [102]. The heat capacity of a material is its ability in heat storage capability. Concrete pavements with higher heat capacity are valuable for improving temperature stability against changing temperatures. Incorporating the PCMs increases cement-based materials’ thermal performance [83,137]. Therefore, most researchers correctly hypothesized that incorporating the PCMs with concrete pavement can increase the heat capacity of pavement. However, incorporating the PCMs with the reflective coating is a proper method to enhance the cooling surface efficiency [138].
On the other hand, the surface temperature of pavement increases due to the absorption of solar radiation. The absorbed heat transfers to the lower layers through conduction. Also, the pavement’s surface transfers heat to the surroundings through convection and radiation. The amount of heat transferred to the environment and the human body is related to the heat transfer coefficient and surface temperature. Thus, it is an excellent strategy to reduce pavement surface temperature for UHI mitigation by using PCMs.
Prior literature indicated that incorporating PCMs into the concrete pavement reduces the peak temperature and increases the time lag to reach the peak temperature. For example, the peak temperatures of mortars containing 1, 3, and 5% PCM were reduced by 9, 12, and 15%, and the times of reaching the peak temperature were 15, 18, and 22% longer, respectively [84]. Another study predicted a 6–10 °C reduction in the peak temperature of concrete pavement when 5% of cement or aggregate is replaced by PCM [16].
In summary, this section presents the calculated or simulated PCM concrete pavement surface temperature in the available literature. Sharifi and Mahboub [139] evaluated the surface temperature of samples in two different scenarios. In one design, the PCMs were incorporated into the entire samples; in another, the PCMs were incorporated into the top layer of concrete samples. The results revealed that the differences between the surface temperature of PCMs and non-PCM samples were not meaningful in the first scenario. However, they reported that increasing the thermal inertia just in the top layer of concrete (applying PCM in this layer) can significantly reduce the surface temperature of concrete pavement.
7. The Chemical Reaction of PCMs Inside Concrete
Most organic PCMs are chemically stable, safe, recyclable, non-reactive, and compatible with conventional construction materials [126]. Also, organic PCMs do not suffer phase segregation and crystallize with little or no supercooling. However, most inorganic PCMs are corrosive to metals and undergo supercooling and phase decomposition. There are limited data on the properties of many combinations of eutectic PCMs.
In most cases, chemical reactions between the PCMs and cementitious matrix, regardless of the incorporation, could result in fundamental problems inside the concrete. It has been observed that some PCMs leaking out of LWAs may chemically interact with hydration products, causing expansion and cracking. An example of such a problem is given in the study by Wei et al. [119], who reported a 25% reduction in enthalpy of the phase change of PCM microcapsules due to a chemical reaction of the melamine formaldehyde shell with sulfate ions, causing the release of the core material and its reaction with the pore solution. The proposed mechanism is schematically described in Figure 6.
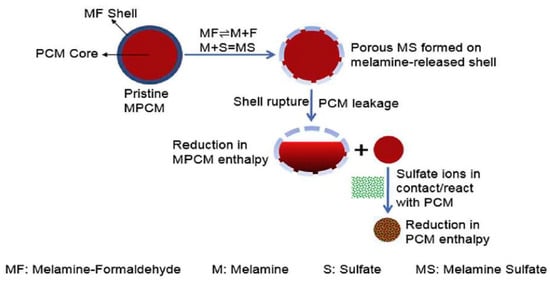
Figure 6.
The proposed chemical interaction pathway results in enthalpy reduction of the PCMs following exposure to caustic solutions containing sulfate ions adapted with permission from Ref. [119].
8. Discussion
The extensive coverage of pavements in urban areas underscores their significant impact on the local environment [29,101]. In cold regions, the predominant challenge faced by pavements is freezing, leading to various forms of cracking [97]. Paradoxically, many urban areas grapple with the urban heat island (UHI) effect. The surface temperature of concrete pavement emerges as a pivotal factor influencing both freezing and UHI phenomena. Typically, surface temperatures exhibit diurnal variations, being higher during the day due to solar radiation and lower than the ambient temperature at night owing to radiation from the surface to the sky [140].
Addressing the freezing predicament has prompted the exploration of diverse solutions. These include entraining air voids [141], employing high-strength concrete to obviate the need for air entrainment, integrating anti-freezing additives [142], incorporating fly ash or slag as supplementary cementitious materials [143], utilizing super absorbent polymer [144], and introducing phase change materials (PCMs) [97].
Regarding the UHI mitigation, Santamouris [145] suggested the following ways to reduce the surface temperature of pavements: (i) enhancing the albedo of pavements by using lightweight aggregates, involving resin-based pavements, applying colorless reflective binders, adding fly ash, replacing gray cement by white cement, applying infrared-reflective, etc.; (ii) enhancing the evaporation process by increasing the porosity, permeability, etc.; (iii) decreasing the solar absorption by using shading; (iv) dissipating the excess heat using water tubes as the cooling systems; and (v) increasing the heat capacity of pavement using PCMs.
Incorporating PCMs into concrete pavement emerges as a promising solution to mitigate UHI effects by lowering surface temperatures during the day and preventing freezing in cold regions by increasing temperatures at night, owing to the high latent heat of fusion [146,147,148]. Studies have demonstrated that PCM inclusion results in lower maximum surface temperatures and higher minimum surface temperatures compared to control samples, attributed to the heat released during phase change [149], aligning with the findings in [103].
However, challenges accompany the utilization of PCM concrete pavement, including PCM leakage; the low thermal conductivity of PCMs; and pavement strength reduction due to insufficient PCM strength, which delays hydration and increases porosity [150,151,152,153,154]. Despite an increasing trend in publications, significant knowledge gaps persist, particularly regarding the impact of PCMs on preventing freezing in icy regions, as highlighted in Section 5.
To address these gaps, future research directions are proposed:
- A.
- Thermal conductivity:
- 1.
- Consider the thermal conductivity of PCM concrete pavement, as it plays a crucial role in heat transfer speed.
- 2.
- Investigate the implications of higher thermal conductivity on surface temperature fluctuation in different climate conditions.
- 3.
- Examine the effect of PCM application in different layers to manage heat transfer and energy consumption.
- B.
- PCMs:
- 1.
- Develop innovative methods to address PCM leakage in encapsulation.
- 2.
- Evaluate PCM stability under loaded conditions to withstand the rigors of pavement fatigue.
- 3.
- Assess the impact of solidification’s latent heat on PCM concrete pavement temperature to limit the UHI effect during warm nights.
- C.
- Energy and Environment:
- 1.
- Design studies to evaluate the impact of PCM concrete pavement on city temperature and annual energy consumption.
- 2.
- Conduct life cycle assessment (LCA) studies to understand the environmental impacts of PCM concrete pavement in various regions.
In summary, while PCM concrete pavement holds promise for addressing freezing and UHI issues, addressing these recommendations in future research will contribute to a more comprehensive understanding of its potential benefits and challenges.
9. Conclusions
In conclusion, this review underscores the significance of integrating phase change materials (PCMs) into concrete pavement for enhanced anti-freezing capabilities and urban heat island (UHI) mitigation. The reviewed literature establishes that PCMs, particularly those based on paraffin, exhibit potential in elevating surface temperatures for anti-freezing purposes and concurrently reducing maximum surface temperatures for cooling applications. Researchers have explored a range of PCMs with phase change temperatures spanning −0.5 °C to 51 °C, accompanied by varying latent heats from 122 to 240.5 J/g. Notably, PCMs with phase transition temperatures near freezing points show promise in diminishing freezing events in concrete, yet their applicability in regions characterized by prolonged snowfall and sub-zero temperatures, such as Nordic countries, remains uncertain.
On the front of UHI mitigation, incorporating PCMs into concrete pavement exhibits the capacity to reduce maximum surface temperatures by up to 15%. However, the deployment of PCMs in concrete pavements is not without its challenges. The findings highlight a reduction in mechanical properties, such as compressive strength, upon the inclusion of PCMs. Additionally, concerns regarding the leakage of microencapsulation add to the list of drawbacks.
Considering these drawbacks, we propose avenues for future research. These include exploring alternative PCM types beyond paraffin, developing multilayer concrete pavement configurations with and without PCM incorporation, and conducting comprehensive assessments of the environmental impact and energy efficiency associated with PCM–concrete pavement. This holistic approach aims to address current limitations and propel the effective and sustainable integration of PCMs into concrete pavement technology.
Funding
This research received no external funding and Open Access Funding was funded by the University for Continuing Education Krems.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author Mehdi Maghfouri was employed by the company Wisma Hume. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A. Simulation of the Effect of the Volume and Phase Changing Point of PCM on the Surface Temperature of Concrete Pavement
Utilizing information gleaned from the literature, we opted for phase change materials that undergo a transformation within the temperature range spanning the minimum and maximum air temperatures. These materials were employed to emulate the surface temperature of concrete pavements, both with and without PCM, across diverse locations, namely, Trondheim (Norway), Beijing (China), Zanjan (Iran), Berlin (Germany), and New York (USA). Historical air temperature data for 15 January spanning the years 2010 to 2023 were sourced from World Weather Online https://www.worldweatheronline.com (accessed on 1 April 2023). The maximum surface temperature increment (Ts with PCM—Ts without PCM) resulting from the application of 5% to 20% PCM is presented in Table A1. It is essential to acknowledge that the phase change temperature is assumed to be equivalent to the temperature at which the maximum differences manifest.

Table A1.
Surface temperature with and without PCM.
Table A1.
Surface temperature with and without PCM.
| Location | Average Air Temperature at the 15th of January (°C) | Surface Temperature Difference (°C) Maximum (Ts with PCM − Ts without PCM) | |||
|---|---|---|---|---|---|
| Min | Max | ||||
| Trondheim | −8.2 | −4.2 | f = 0.05 | Tm = −3 | 1.9 |
| f = 0.1 | 2.5 | ||||
| f = 0.15 | 2.6 | ||||
| f = 0.2 | 2.8 | ||||
| Beijing | −7.0 | 0.2 | f = 0.05 | Tm = −2 | 2.8 |
| f = 0.1 | 3.3 | ||||
| f = 0.15 | 3.5 | ||||
| f = 0.2 | 3.6 | ||||
| Zanjan | −5.5 | 2.5 | f = 0.05 | Tm = −0.5 | 2.9 |
| f = 0.1 | 3.3 | ||||
| f = 0.15 | 3.5 | ||||
| f = 0.2 | 3.6 | ||||
| Berlin | −0.1 | 4.0 | f = 0.05 | Tm = 2 | 0.9 |
| f = 0.1 | 1.0 | ||||
| f = 0.15 | 1.0 | ||||
| f = 0.2 | 1.0 | ||||
| New York | −2.5 | 3.5 | f = 0.05 | Tm = 0 | 1.7 |
| f = 0.1 | 1.9 | ||||
| f = 0.15 | 2.0 | ||||
| f = 0.2 | 2.0 | ||||
The selection of phase change materials (PCMs) to prevent freezing is contingent upon the specific weather conditions of the location. For instance, in Zanjan and New York, it appears possible that PCM application can elevate the surface temperature above freezing, whereas, in Trondheim, this outcome seems less probable. Figure A1 illustrates the maximum disparity in surface temperature for concrete pavement across various regions when 20% PCM, with a phase-changing temperature corresponding to the average air temperature, is incorporated into the concrete (refer to Table A1 for details). The simulation in Figure A1 indicates that with 20% PCM (melting temperature assumed to be equal to the average air temperature), only in Berlin and New York is the 20% PCM effective in maintaining the surface temperature above freezing. It is important to note that this simulation simplifies heat transfer in a single direction to determine the peak difference. In real-world conditions, a multitude of factors influence pavement surface temperature when PCMs are applied. For a more realistic depiction of surface temperature fluctuations with and without PCM, refer to the findings of Nayak et al. [155], Yeon [100], and Somani and Gaur [103].
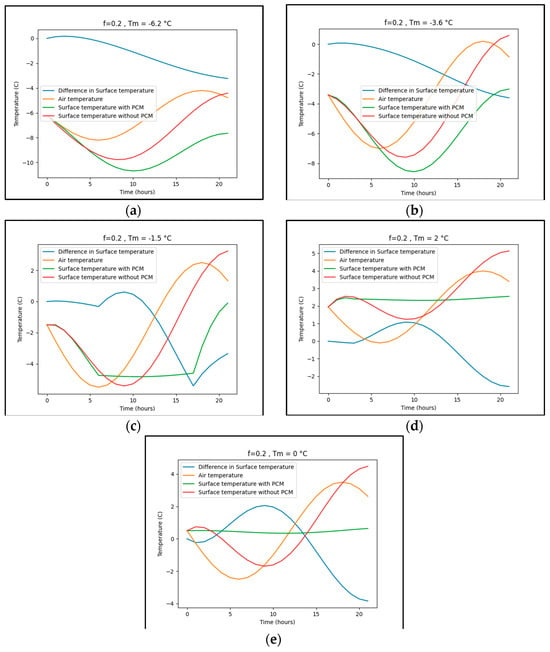
Figure A1.
The effect of PCMs on the surface temperature of the pavement. (a) Trondheim. (b) Beijing. (c) Zanjan. (d) Berlin. (e) NY.
Furthermore, it is crucial to note that the surface temperature difference presented in Table A1 reflects the maximum disparity between the surface temperatures of concrete with and without phase change material (PCM). Nevertheless, there are instances during the day when the surface temperature of concrete with PCM is lower than that of concrete without PCM, as depicted in Figure A2. Table A1 indicates that PCM exhibits ineffectiveness in mitigating peak surface temperatures at certain specific melting points. Figure A2 visually illustrates the impact of various phase-changing temperatures on the peak surface temperature of the pavement.
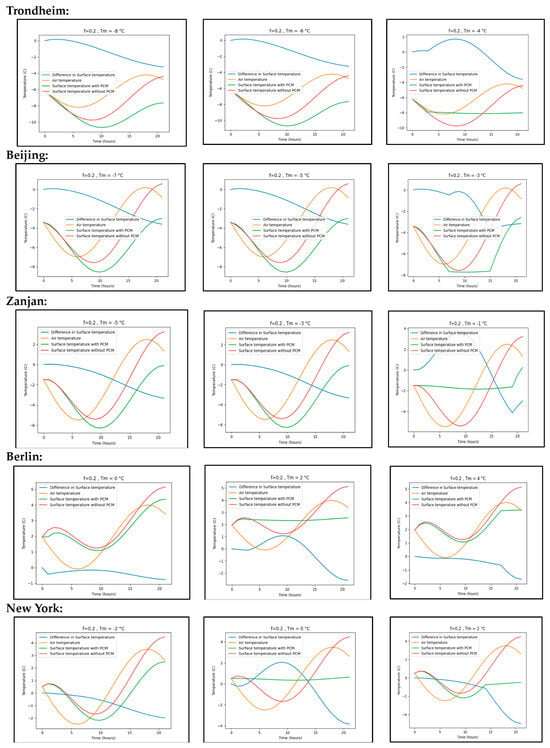
Figure A2.
Difference of surface temperatures for various phase changing temperatures.
References
- Madhumathi, A.; Subhashini, S.; VishnuPriya, J. The Urban Heat Island Effect its Causes and Mitigation with Reference to the Thermal Properties of Roof Coverings. In Proceedings of the International Conference on Urban Sustainability: Emerging Trends, Themes, Concepts & Practices (ICUS), Jaipur, India, 16 March–25 February 2018. [Google Scholar]
- Akbari, H.; Menon, S.; Rosenfeld, A. Global cooling: Increasing world-wide urban albedos to offset CO2. Clim. Change 2009, 94, 275–286. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Y.; Li, F. A review on thermoelectric energy harvesting from asphalt pavement: Configuration, performance and future. Constr. Build. Mater. 2019, 228, 116818. [Google Scholar] [CrossRef]
- Mallick, R.B.; El-Korchi, T. Pavement Engineering: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Delatte, N.J. Concrete Pavement Design, Construction, and Performance; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mohod, M.V.; Kadam, K. A comparative study on rigid and flexible pavement: A review. IOSR J. Mech. Civ. Eng. 2016, 13, 84–88. [Google Scholar]
- Núñez, O. Composite Pavements: A Technical and Economic Analysis during the Pavement Type Selection Process; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2007. [Google Scholar]
- Hashemi, M.; Shafigh, P.; Asadi, I.; Mahpour, A.; Samadian, A. The effect of superplasticizer admixture on the engineering characteristics of roller-compacted concrete pavement. Int. J. Pavement Eng. 2022, 23, 2432–2447. [Google Scholar] [CrossRef]
- Shafigh, P.; Hashemi, M.; Hyun Nam, B.; Asadi, I. Laboratory comparison of roller-compacted concrete and ordinary vibrated concrete for pavement structures. Građevinar 2020, 72, 127–137. [Google Scholar]
- Asadi, I.; Kanstad, T.; Skjølsvold, O.; Jacobsen, S. Frost-Salt Testing Non-Air Entrained High-Performance Fly-Ash Concrete: Relations Liquid Uptake-Internal Cracking–Scaling. Available online: https://ssrn.com/abstract=4447555 (accessed on 13 May 2023).
- Gao, Y.; Huang, L.; Zhang, H. Study on anti-freezing functional design of phase change and temperature control composite bridge decks. Constr. Build. Mater. 2016, 122, 714–720. [Google Scholar] [CrossRef]
- Liu, Z.; Hansen, W. Effect of hydrophobic surface treatment on freeze-thaw durability of concrete. Cem. Concr. Compos. 2016, 69, 49–60. [Google Scholar] [CrossRef]
- Valenza, J.J., II; Scherer, G.W. A review of salt scaling: II. Mechanisms. Cem. Concr. Res. 2007, 37, 1022–1034. [Google Scholar] [CrossRef]
- Farnam, Y.; Dick, S.; Wiese, A.; Davis, J.; Bentz, D.; Weiss, J. The influence of calcium chloride deicing salt on phase changes and damage development in cementitious materials. Cem. Concr. Compos. 2015, 64, 1–15. [Google Scholar] [CrossRef]
- Farnam, Y.; Bentz, D.; Hampton, A.; Weiss, W.J. Acoustic emission and low-temperature calorimetry study of freeze and thaw behavior in cementitious materials exposed to sodium chloride salt. Transp. Res. Rec. 2014, 2441, 81–90. [Google Scholar] [CrossRef]
- Arora, A.; Sant, G.; Neithalath, N. Numerical simulations to quantify the influence of phase change materials (PCMs) on the early-and later-age thermal response of concrete pavements. Cem. Concr. Compos. 2017, 81, 11–24. [Google Scholar] [CrossRef]
- Nilimaa, J.; Zhaka, V. An Overview of Smart Materials and Technologies for Concrete Construction in Cold Weather. Eng 2023, 4, 1550–1580. [Google Scholar] [CrossRef]
- Santamouris, M. Analyzing the heat island magnitude and characteristics in one hundred Asian and Australian cities and regions. Sci. Total Environ. 2015, 512, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Santamouris, M. Innovating to zero the building sector in Europe: Minimising the energy consumption, eradication of the energy poverty and mitigating the local climate change. SoEn 2016, 128, 61–94. [Google Scholar] [CrossRef]
- Santamouris, M.; Yun, G.Y. Recent development and research priorities on cool and super cool materials to mitigate urban heat island. Renew. Energy 2020, 161, 792–807. [Google Scholar] [CrossRef]
- Shirani-Bidabadi, N.; Nasrabadi, T.; Faryadi, S.; Larijani, A.; Roodposhti, M.S. Evaluating the spatial distribution and the intensity of urban heat island using remote sensing, case study of Isfahan city in Iran. Sustain. Cities Soc. 2019, 45, 686–692. [Google Scholar] [CrossRef]
- Taleghani, M. Outdoor thermal comfort by different heat mitigation strategies—A review. Renew. Sustain. Energy Rev. 2018, 81, 2011–2018. [Google Scholar] [CrossRef]
- Akbari, H.; Cartalis, C.; Kolokotsa, D.; Muscio, A.; Pisello, A.L.; Rossi, F.; Santamouris, M.; Synnefa, A.; Wong, N.H.; Zinzi, M. Local climate change and urban heat island mitigation techniques–the state of the art. J. Civ. Eng. Manag. 2016, 22, 1–16. [Google Scholar] [CrossRef]
- Kyriakodis, G.; Santamouris, M. Using reflective pavements to mitigate urban heat island in warm climates-Results from a large scale urban mitigation project. Urban. Clim. 2018, 24, 326–339. [Google Scholar] [CrossRef]
- Akbari, H.; Rose, L.S. Urban surfaces and heat island mitigation potentials. J. Hum-Environ. Syst. 2008, 11, 85–101. [Google Scholar] [CrossRef]
- Santamouris, M.; Ding, L.; Fiorito, F.; Oldfield, P.; Osmond, P.; Paolini, R.; Prasad, D.; Synnefa, A. Passive and active cooling for the outdoor built environment–Analysis and assessment of the cooling potential of mitigation technologies using performance data from 220 large scale projects. SoEn 2017, 154, 14–33. [Google Scholar] [CrossRef]
- Al-Humairi, S.; Alias, A.; Haron, N.; Hassim, S.; Jakarni, F.M. Sustainable pavement: A review on the usage of pavement as a mitigation strategy for UHI. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1075, 012010. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Yang, Y.; Bai, S. Characterizing thermal impacts of pavement materials on urban heat island (UHI) effect. DEStech Trans. Eng. Technol. Res. 2016. [Google Scholar] [CrossRef]
- Nwakaire, C.M.; Onn, C.C.; Yap, S.P.; Yuen, C.W.; Onodagu, P.D. Urban Heat Island Studies with Emphasis on Urban Pavements; a review. Sustain. Cities Soc. 2020, 63, 102476. [Google Scholar] [CrossRef]
- Anupam, B.; Sahoo, U.C.; Rath, P. Phase change materials for pavement applications: A review. Constr. Build. Mater. 2020, 247, 118553. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.-H.; Kaloush, K.E.; Dylla, H. Effect of pavement thermal properties on mitigating urban heat islands: A multi-scale modeling case study in Phoenix. Build. Environ. 2016, 108, 110–121. [Google Scholar] [CrossRef]
- Fahed, J.; Kinab, E.; Ginestet, S.; Adolphe, L. Impact of urban heat island mitigation measures on microclimate and pedestrian comfort in a dense urban district of Lebanon. Sustain. Cities Soc. 2020, 61, 102375. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.d.; De Gracia, A.; Fernández, A. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Ma, B.; Shi, H.; Xu, J.; Wei, K.; Wang, X.; Xiao, Y. Thermal-Insulation Effect and Evaluation Indices of Asphalt Mixture Mixed with Phase-Change Materials. Materials 2020, 13, 3738. [Google Scholar] [CrossRef]
- Kakar, M.R.; Refaa, Z.; Bueno, M.; Worlitschek, J.; Stamatiou, A.; Partl, M.N. Investigating bitumen’s direct interaction with Tetradecane as potential phase change material for low temperature applications. Road Mater. Pavement Des. 2020, 21, 2356–2363. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Li, X.; Chen, M.; Wu, Y.; Sun, C.; Wang, X. Antiaging Property and Mechanism of Phase-Change Asphalt with PEG as an Additive. Adv. Mater. Sci. Eng. 2020, 2020, 7598049. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Jia, J.; Sun, H.; Wang, H.; Qiao, H. Preparation and characterization of temperature-adjusting asphalt with diatomite-supported PEG as an additive. J. Mater. Civ. Eng. 2020, 32, 04020019. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wu, Y.; Li, X. Preparation and Characterization of Polyurethane Shell Microencapsulated Phase Change Materials by Interfacial Polymerization. In Proceedings of the 2020 International Conference on Artificial Intelligence and Electromechanical Automation (AIEA), Tianjin, China, 26–28 June 2020; pp. 711–714. [Google Scholar]
- Ma, B.; Wei, K.; Huang, X.; Shi, W.; Chen, S.; Hu, Y.; Shi, H. Preparation and Investigation of NiTi Alloy Phase-Change Heat Storage Asphalt Mixture. J. Mater. Civ. Eng. 2020, 32, 04020250. [Google Scholar] [CrossRef]
- Tahami, A.; Gholikhani, M.; Dessouky, S. A Novel Thermoelectric Approach to Energy Harvesting from Road Pavement. Proceedings of International Conference on Transportation and Development, Seattle, WA, USA, 26–29 May 2020; American Society of Civil Engineers: Seattle, WA, USA, 2020; pp. 174–181. [Google Scholar]
- Jia, M.; Sha, A.; Jiang, W.; Wang, W.; Li, J.; Dai, J.; Lu, Z. Laboratory evaluation of poly (ethylene glycol) for cooling of asphalt pavements. Constr. Build. Mater. 2021, 273, 121774. [Google Scholar] [CrossRef]
- Ma, B.; Hu, Y.; Si, W.; Wei, K.; Chang, X. Study on the temperature control effects of an epoxy resin composite thermoregulation agent on asphalt mixtures. Constr. Build. Mater. 2020, 257, 119580. [Google Scholar] [CrossRef]
- Yinfei, D.; Pusheng, L.; Jiacheng, W.; Hancheng, D.; Hao, W.; Yingtao, L. Effect of lightweight aggregate gradation on latent heat storage capacity of asphalt mixture for cooling asphalt pavement. Constr. Build. Mater. 2020, 250, 118849. [Google Scholar] [CrossRef]
- Si, W.; Ma, B.; Ren, J.; Hu, Y.; Zhou, X.; Tian, Y.; Li, Y. Temperature responses of asphalt pavement structure constructed with phase change material by applying finite element method. Constr. Build. Mater. 2020, 244, 118088. [Google Scholar] [CrossRef]
- Ma, B.; Chen, S.-s.; Ren, Y.-z.; Zhou, X.-y. The thermoregulation effect of microencapsulated phase-change materials in an asphalt mixture. Constr. Build. Mater. 2020, 231, 117186. [Google Scholar] [CrossRef]
- She, Z.; Wei, Z.; Young, B.A.; Falzone, G.; Neithalath, N.; Sant, G.; Pilon, L. Examining the effects of microencapsulated phase change materials on early-age temperature evolutions in realistic pavement geometries. Cem. Concr. Compos. 2019, 103, 149–159. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Wang, H.; Huang, W.; Sun, W.; Xu, T. Preparation and effectiveness of composite phase change material for performance improvement of Open Graded Friction Course. J. Clean. Prod. 2019, 214, 259–269. [Google Scholar] [CrossRef]
- Ma, B.; Chen, S.-s.; Wei, K.; Liu, F.-w.; Zhou, X.-y. Analysis of thermoregulation indices on microencapsulated phase change materials for asphalt pavement. Constr. Build. Mater. 2019, 208, 402–412. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, M.; Wu, S.; Riara, M.; Wan, J.; Li, Y. Thermal and rheological performance of asphalt binders modified with expanded graphite/polyethylene glycol composite phase change material (EP-CPCM). Constr. Build. Mater. 2019, 194, 83–91. [Google Scholar] [CrossRef]
- Tahami, S.A.; Gholikhani, M.; Nasouri, R.; Dessouky, S.; Papagiannakis, A. Developing a new thermoelectric approach for energy harvesting from asphalt pavements. Appl. Energy 2019, 238, 786–795. [Google Scholar] [CrossRef]
- Bueno, M.; Kakar, M.R.; Refaa, Z.; Worlitschek, J.; Stamatiou, A.; Partl, M.N. Modification of asphalt mixtures for cold regions using microencapsulated phase change materials. Sci. Rep. 2019, 9, 20342. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, P.; Wang, J.; Wang, H.; Hu, S.; Tian, J.; Li, Y. Laboratory investigation of phase change effect of polyethylene glycolon on asphalt binder and mixture performance. Constr. Build. Mater. 2019, 212, 1–9. [Google Scholar] [CrossRef]
- Kakar, M.R.; Refaa, Z.; Worlitschek, J.; Stamatiou, A.; Partl, M.N.; Bueno, M. Thermal and rheological characterization of bitumen modified with microencapsulated phase change materials. Constr. Build. Mater. 2019, 215, 171–179. [Google Scholar] [CrossRef]
- Tian, Y.-x.; Ma, B.; Liu, F.-w.; Li, N.; Zhou, X.-y. Thermoregulation effect analysis of microencapsulated phase change thermoregulation agent for asphalt pavement. Constr. Build. Mater. 2019, 221, 139–150. [Google Scholar] [CrossRef]
- Jin, J.; Liu, L.; Liu, R.; Wei, H.; Qian, G.; Zheng, J.; Xie, W.; Lin, F.; Xie, J. Preparation and thermal performance of binary fatty acid with diatomite as form-stable composite phase change material for cooling asphalt pavements. Constr. Build. Mater. 2019, 226, 616–624. [Google Scholar] [CrossRef]
- Wei, K.; Ma, B.; Huang, X.; Xiao, Y.; Liu, H. Influence of NiTi alloy phase change heat-storage particles on thermophysical parameters, phase change heat-storage thermoregulation effect, and pavement performance of asphalt mixture. Renew. Energy 2019, 141, 431–443. [Google Scholar] [CrossRef]
- Kakar, M.R.; Refaa, Z.; Worlitschek, J.; Stamatiou, A.; Partl, M.N.; Bueno, M. Effects of aging on asphalt binders modified with microencapsulated phase change material. Compos. Part. B Eng. 2019, 173, 107007. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.; Ma, B.; Shi, W.; Duan, S.; Liu, F. Study on rheological properties and phase-change temperature control of asphalt modified by polyurethane solid–solid phase change material. Sol. Energy 2019, 194, 893–902. [Google Scholar] [CrossRef]
- Zhou, X.; Kastiukas, G.; Lantieri, C.; Tataranni, P.; Vaiana, R.; Sangiorgi, C. Mechanical and thermal performance of macro-encapsulated phase change materials for pavement application. Materials 2018, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, S.; Chen, S.; Yang, J.; Zhu, X.; Du, Y.; Yang, Z. Low-temperature organic phase change material microcapsules for asphalt pavement: Preparation, characterisation and application. J. Microencapsul. 2018, 35, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, S.; Chen, S.; Yang, Z.; Yang, J.; Zhu, X.; Du, Y.; Zhou, P.; Cheng, Z. Preparation of low-temperature phase change materials microcapsules and its application to asphalt pavement. J. Mater. Civ. Eng. 2018, 30, 04018303. [Google Scholar] [CrossRef]
- Refaa, Z.; Kakar, M.R.; Stamatiou, A.; Worlitschek, J.; Partl, M.N.; Bueno, M. Numerical study on the effect of phase change materials on heat transfer in asphalt concrete. Int. J. Therm. Sci. 2018, 133, 140–150. [Google Scholar] [CrossRef]
- Jin, J.; Xiao, T.; Zheng, J.; Liu, R.; Qian, G.; Xie, J.; Wei, H.; Zhang, J.; Liu, H. Preparation and thermal properties of encapsulated ceramsite-supported phase change materials used in asphalt pavements. Constr. Build. Mater. 2018, 190, 235–245. [Google Scholar] [CrossRef]
- Si, W.; Ma, B.; Zhou, X.-y.; Ren, J.-p.; Tian, Y.-x.; Li, Y. Temperature responses of asphalt mixture physical and finite element models constructed with phase change material. Constr. Build. Mater. 2018, 178, 529–541. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, M.; Wu, S.; Liu, Q.; Wan, J. Preparation of expanded graphite/polyethylene glycol composite phase change material for thermoregulation of asphalt binder. Constr. Build. Mater. 2018, 169, 513–521. [Google Scholar] [CrossRef]
- Athukorallage, B.; Dissanayaka, T.; Senadheera, S.; James, D. Performance analysis of incorporating phase change materials in asphalt concrete pavements. Constr. Build. Mater. 2018, 164, 419–432. [Google Scholar] [CrossRef]
- Bai, G.; Fan, Q.; Song, X.-M. Preparation and characterization of pavement materials with phase-change temperature modulation. J. Therm. Anal. Calorim. 2019, 136, 2327–2331. [Google Scholar] [CrossRef]
- Ryms, M.; Denda, H.; Jaskuła, P. Thermal stabilization and permanent deformation resistance of LWA/PCM-modified asphalt road surfaces. Constr. Build. Mater. 2017, 142, 328–341. [Google Scholar] [CrossRef]
- Jin, J.; Lin, F.; Liu, R.; Xiao, T.; Zheng, J.; Qian, G.; Liu, H.; Wen, P. Preparation and thermal properties of mineral-supported polyethylene glycol as form-stable composite phase change materials (CPCMs) used in asphalt pavements. Sci. Rep. 2017, 7, 16998. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, X.-y.; Liu, J.; You, Z.; Wei, K.; Huang, X.-f. Determination of specific heat capacity on composite shape-stabilized phase change materials and asphalt mixtures by heat exchange system. Materials 2016, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Asadi, I.; Baghban, M.H.; Hashemi, M.; Izadyar, N.; Sajadi, B. Phase change materials incorporated into geopolymer concrete for enhancing energy efficiency and sustainability of buildings: A review. Case Stud. Constr. Mater. 2022, 17, e01162. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Ghajar, A.J.; Ma, H. Heat and Mass Transfer: Fundamentals & Applications, 4th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Lilley, D.; Menon, A.K.; Kaur, S.; Lubner, S.; Prasher, R.S. Phase change materials for thermal energy storage: A perspective on linking phonon physics to performance. J. Appl. Phys. 2021, 130, 220903. [Google Scholar] [CrossRef]
- Shafigh, P.; Asadi, I.; Mahyuddin, N.B. Concrete as a thermal mass material for building applications-A review. J. Build. Eng. 2018, 19, 14–25. [Google Scholar] [CrossRef]
- Mabrouk, R.; Naji, H.; Benim, A.C.; Dhahri, H. A state of the art review on sensible and latent heat thermal energy storage processes in porous media: Mesoscopic Simulation. Appl. Sci. 2022, 12, 6995. [Google Scholar] [CrossRef]
- Liu, L.; Hammami, N.; Trovalet, L.; Bigot, D.; Habas, J.-P.; Malet-Damour, B. Description of phase change materials (PCMs) used in buildings under various climates: A review. J. Energy Storage 2022, 56, 105760. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Zhou, J.; Wu, K. Development of thermal energy storage concrete. Cem. Concr. Res. 2004, 34, 927–934. [Google Scholar] [CrossRef]
- Lee, T. Latent and Sensible Heat Storage in Concrete Blocks; Concordia University: Montreal, QC, Canada, 1998. [Google Scholar]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. ApEn 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Entrop, A.; Brouwers, H.; Reinders, A. Experimental research on the use of micro-encapsulated phase change materials to store solar energy in concrete floors and to save energy in Dutch houses. SoEn 2011, 85, 1007–1020. [Google Scholar] [CrossRef]
- Sá, A.V.; Azenha, M.; de Sousa, H.; Samagaio, A. Thermal enhancement of plastering mortars with phase change materials: Experimental and numerical approach. Energy Build. 2012, 49, 16–27. [Google Scholar] [CrossRef]
- Sharifi, N.P.; Sakulich, A. Application of phase change materials to improve the thermal performance of cementitious material. Energy Build. 2015, 103, 83–95. [Google Scholar] [CrossRef]
- Eddhahak, A.; Drissi, S.; Colin, J.; Caré, S.; Neji, J. Effect of phase change materials on the hydration reaction and kinetic of PCM-mortars. J. Therm. Anal. Calorim. 2014, 117, 537–545. [Google Scholar] [CrossRef]
- Hajilar, S.; Shafei, B. Nano-scale investigation of elastic properties of hydrated cement paste constituents using molecular dynamics simulations. Comput. Mater. Sci. 2015, 101, 216–226. [Google Scholar] [CrossRef]
- Adesina, A.; Awoyera, P.; Sivakrishna, A.; Kumar, K.R.; Gobinath, R. Phase change materials in concrete: An overview of properties. Mater. Today Proc. 2020, 27, 391–395. [Google Scholar] [CrossRef]
- Adesina, A. Use of phase change materials in concrete: Current challenges. Renew. Energy Environ. Sustain. 2019, 4, 9. [Google Scholar] [CrossRef]
- Yun, H.-D.; Ahn, K.-L.; Jang, S.-J.; Khil, B.-S.; Park, W.-S.; Kim, S.-W. Thermal and mechanical behaviors of concrete with incorporation of strontium-based phase change material (PCM). Int. J. Concr. Struct. Mater. 2019, 13, 18. [Google Scholar] [CrossRef]
- Šavija, B. Smart crack control in concrete through use of phase change materials (PCMs): A review. Materials 2018, 11, 654. [Google Scholar] [CrossRef]
- Sharma, B. Incorporation of Phase Change Materials into Cementitious Systems; Arizona State University: Phoenix, AZ, USA, 2013. [Google Scholar]
- Tyagi, V.; Pandey, A.; Kothari, R.; Tyagi, S. Thermodynamics and performance evaluation of encapsulated PCM-based energy storage systems for heating application in building. JTAC 2014, 115, 915–924. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Zheng, S.; Park, Y.; Frost, R.L. Preparation and thermal energy storage properties of paraffin/calcined diatomite composites as form-stable phase change materials. Thermochim. Acta 2013, 558, 16–21. [Google Scholar] [CrossRef]
- Sakulich, A.R.; Bentz, D.P. Incorporation of phase change materials in cementitious systems via fine lightweight aggregate. Constr. Build. Mater. 2012, 35, 483–490. [Google Scholar] [CrossRef]
- Bentz, D.P.; Turpin, R. Potential applications of phase change materials in concrete technology. Cem. Concr. Compos. 2007, 29, 527–532. [Google Scholar] [CrossRef]
- Farnam, Y.; Esmaeeli, H.S.; Zavattieri, P.D.; Haddock, J.; Weiss, J. Incorporating phase change materials in concrete pavement to melt snow and ice. Cem. Concr. Compos. 2017, 84, 134–145. [Google Scholar] [CrossRef]
- Farnam, Y.; Krafcik, M.; Liston, L.; Washington, T.; Erk, K.; Tao, B.; Weiss, J. Evaluating the use of phase change materials in concrete pavement to melt ice and snow. J. Mater. Civ. Eng. 2016, 28, 04015161. [Google Scholar] [CrossRef]
- Yeon, J.H.; Kim, K.-K. Potential applications of phase change materials to mitigate freeze-thaw deteriorations in concrete pavement. Constr. Build. Mater. 2018, 177, 202–209. [Google Scholar] [CrossRef]
- Urgessa, G.; Yun, K.-K.; Yeon, J.; Yeon, J.H. Thermal responses of concrete slabs containing microencapsulated low-transition temperature phase change materials exposed to realistic climate conditions. Cem. Concr. Compos. 2019, 104, 103391. [Google Scholar] [CrossRef]
- Li, W.; Ling, C.; Jiang, Z.; Yu, Q.-q. Evaluation of the potential use of form-stable phase change materials to improve the freeze-thaw resistance of concrete. Constr. Build. Mater. 2019, 203, 621–632. [Google Scholar] [CrossRef]
- Yeon, J.H. Thermal behavior of cement mortar embedded with low-phase transition temperature PCM. Constr. Build. Mater. 2020, 252, 119168. [Google Scholar] [CrossRef]
- Veeraragavan, R.K.; Sakulich, A.; Mallick, R.B. An Evaluation of Cool Pavement Strategies on Concrete Pavements. In Airfield and Highway Pavements 2017; ASCE Library: Reston, VA, USA, 2017; pp. 20–32. [Google Scholar]
- Young, B.A.; Falzone, G.; She, Z.; Thiele, A.M.; Wei, Z.; Neithalath, N.; Sant, G.; Pilon, L. Early-age temperature evolutions in concrete pavements containing microencapsulated phase change materials. Constr. Build. Mater. 2017, 147, 466–477. [Google Scholar] [CrossRef]
- Somani, P.; Gaur, A. Evaluation and reduction of temperature stresses in concrete pavement by using phase changing material. Mater. Today Proc. 2020, 32, 856–864. [Google Scholar] [CrossRef]
- BR, A.; Sahoo, U.C.; Rath, P. Thermal and mechanical performance of phase change material incorporated concrete pavements. Road Mater. Pavement Des. 2022, 23, 1287–1304. [Google Scholar]
- Sharifi, N.P.; Jafferji, H.; Reynolds, S.E.; Blanchard, M.G.; Sakulich, A.R. Application of lightweight aggregate and rice husk ash to incorporate phase change materials into cementitious materials. J. Sustain. Cem. Based Mater. 2016, 5, 349–369. [Google Scholar] [CrossRef]
- Tyagi, V.; Kaushik, S.; Tyagi, S.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K.; Gupta, N.K. Potential of microencapsulated PCM for energy savings in buildings: A critical review. Sustain. Cities Soc. 2020, 53, 101884. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, K.H.; Yang, J.K. Thermal analysis of hydration heat in concrete structures with pipe-cooling system. Comput. Struct. 2001, 79, 163–171. [Google Scholar] [CrossRef]
- Sakulich, A.R.; Bentz, D.P. Increasing the service life of bridge decks by incorporating phase-change materials to reduce freeze-thaw cycles. J. Mater. Civ. Eng. 2012, 24, 1034–1042. [Google Scholar] [CrossRef]
- Meshgin, P.; Xi, Y. Effect of Phase-Change Materials on Properties of Concrete. ACI Mater. J. 2012, 109, 71–80. [Google Scholar]
- Asadi, I.; Shafigh, P.; Hashemi, M.; Akhiani, A.R.; Maghfouri, M.; Sajadi, B.; Mahyuddin, N.; Esfandiari, M.; Talebi, H.R.; Metselaar, H.S.C. Thermophysical properties of sustainable cement mortar containing oil palm boiler clinker (OPBC) as a fine aggregate. Constr. Build. Mater. 2021, 268, 121091. [Google Scholar] [CrossRef]
- Congress, I.R. Guidelines for the design of plain jointed rigid pavements for highways (IRC 58). In Proceedings of the Indian Roads Congress, New Delhi, India, 25 June 2015. [Google Scholar]
- Hunger, M.; Entrop, A.; Mandilaras, I.; Brouwers, H.; Founti, M. The behavior of self-compacting concrete containing micro-encapsulated phase change materials. Cem. Concr. Compos. 2009, 31, 731–743. [Google Scholar] [CrossRef]
- Norvell, C.; Sailor, D.J.; Dusicka, P. The effect of microencapsulated phase-change material on the compressive strength of structural concrete. J. Green. Build. 2013, 8, 116–124. [Google Scholar] [CrossRef]
- Wei, Z.; Falzone, G.; Wang, B.; Thiele, A.; Puerta-Falla, G.; Pilon, L.; Neithalath, N.; Sant, G. The durability of cementitious composites containing microencapsulated phase change materials. Cem. Concr. Compos. 2017, 81, 66–76. [Google Scholar] [CrossRef]
- Abbas, Z.H.; Majdi, H.S. Study of heat of hydration of Portland cement used in Iraq. Case Stud. Constr. Mater. 2017, 7, 154–162. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, S.J.; Kim, Y.G.; Choi, Y.C.; Kim, J.H.; Lee, J.G. Development of building materials by using micro-encapsulated phase change material. Korean J. Chem. Eng. 2007, 24, 332–335. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; D’Alessandro, A.; Ubertini, F.; Cabeza, L.F.; Cotana, F. Effect of PCM on the hydration process of Cement-Based mixtures: A novel Thermo-Mechanical investigation. Materials 2018, 11, 871. [Google Scholar] [CrossRef]
- Fernandes, F.; Manari, S.; Aguayo, M.; Santos, K.; Oey, T.; Wei, Z.; Falzone, G.; Neithalath, N.; Sant, G. On the feasibility of using phase change materials (PCMs) to mitigate thermal cracking in cementitious materials. Cem. Concr. Compos. 2014, 51, 14–26. [Google Scholar] [CrossRef]
- Mihashi, H.; Nishiyama, N.; Kobayashi, T.; Hanada, M. Development of a smart material to mitigate thermal stress in early age concrete. Control Crack. Early Age Concr. 2002, 385–392. [Google Scholar]
- Choi, W.-C.; Khil, B.-S.; Chae, Y.-S.; Liang, Q.-B.; Yun, H.-D. Feasibility of using phase change materials to control the heat of hydration in massive concrete structures. Sci. World J. 2014, 2014, 781393. [Google Scholar] [CrossRef]
- Ling, T.-C.; Poon, C.-S. Use of phase change materials for thermal energy storage in concrete: An overview. Constr. Build. Mater. 2013, 46, 55–62. [Google Scholar] [CrossRef]
- Stoll, F.; Drake, M.L.; Salyer, I.O. Use of Phase Change Materials to Prevent Overnight Freezing of Bridge Decks; Transportation Research Board: Washington, DC, USA, 1996. [Google Scholar]
- Gartland, L.M. Heat Islands: Understanding and Mitigating Heat in Urban Areas; Routledge: Abingdon, UK, 2012. [Google Scholar]
- Santamouris, M.; Synnefa, A.; Kolokotsa, D.; Dimitriou, V.; Apostolakis, K. Passive cooling of the built environment–use of innovative reflective materials to fight heat islands and decrease cooling needs. Int. J. Low-Carbon. Technol. 2008, 3, 71–82. [Google Scholar] [CrossRef]
- Demirboğa, R.; Gül, R. The effects of expanded perlite aggregate, silica fume and fly ash on the thermal conductivity of lightweight concrete. Cem. Concr. Res. 2003, 33, 723–727. [Google Scholar] [CrossRef]
- Shafigh, P.; Asadi, I.; Akhiani, A.; Mahyuddin, N.; Hashemi, M. Thermal properties of cement mortar with different mix proportions. Mater. Construcción 2020, 70, 224. [Google Scholar] [CrossRef]
- Talebi, H.R.; Kayan, B.A.; Asadi, I.; Hassan, Z.F.B.A. Investigation of Thermal Properties of Normal Weight Concrete for Different Strength Classes. J. Environ. Treat. Tech. 2020, 8, 908–914. [Google Scholar]
- Ferguson, B.; Fisher, K.; Golden, J.; Hair, L.; Haselbach, L.; Hitchcock, D.; Kaloush, K.; Pomerantz, M.; Tran, N.; Waye, D. Reducing Urban Heat Islands: Compendium of Strategies-Cool Pavements. 2008. Available online: https://trid.trb.org/view/920168 (accessed on 15 May 2023).
- Qin, Y. A review on the development of cool pavements to mitigate urban heat island effect. Renew. Sustain. Energy Rev. 2015, 52, 445–459. [Google Scholar] [CrossRef]
- Tong, X.C. Characterization Methodologies of Thermal Management Materials. In Advanced Materials for Thermal Management of Electronic Packaging; Springer: New York, NY, USA, 2011; pp. 59–129. [Google Scholar]
- Zhang, W.; Min, H.; Gu, X.; Xi, Y.; Xing, Y. Mesoscale model for thermal conductivity of concrete. Constr. Build. Mater. 2015, 98, 8–16. [Google Scholar] [CrossRef]
- Sharifi, N.P.; Freeman, G.E.; Sakulich, A.R. Using COMSOL modeling to investigate the efficiency of PCMs at modifying temperature changes in cementitious materials–case study. Constr. Build. Mater. 2015, 101, 965–974. [Google Scholar] [CrossRef]
- Karlessi, T.; Santamouris, M.; Synnefa, A.; Assimakopoulos, D.; Didaskalopoulos, P.; Apostolakis, K. Development and testing of PCM doped cool colored coatings to mitigate urban heat island and cool buildings. Build. Environ. 2011, 46, 570–576. [Google Scholar] [CrossRef]
- Sharifi, N.P.; Mahboub, K.C. Application of a PCM-rich concrete overlay to control thermal induced curling stresses in concrete pavements. Constr. Build. Mater. 2018, 183, 502–512. [Google Scholar] [CrossRef]
- Bentz, D.P. A Computer Model to Predict the Surface Temperature and Time-of-Wetness of Concrete Pavements and Bridge Decks; NIST Publications: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Mayercsik, N.P.; Vandamme, M.; Kurtis, K.E. Assessing the efficiency of entrained air voids for freeze-thaw durability through modeling. Cem. Concr. Res. 2016, 88, 43–59. [Google Scholar] [CrossRef]
- Polat, R. The effect of antifreeze additives on fresh concrete subjected to freezing and thawing cycles. Cold Reg. Sci. Technol. 2016, 127, 10–17. [Google Scholar] [CrossRef]
- Cai, L.; Wang, H.; Fu, Y. Freeze–thaw resistance of alkali—Slag concrete based on response surface methodology. Constr. Build. Mater. 2013, 49, 70–76. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Schröfl, C.; Wyrzykowski, M.; Gorges, M.; Lura, P.; Cusson, D.; Margeson, J.; De Belie, N.; Snoeck, D.; Ichimiya, K. Effect of superabsorbent polymers (SAP) on the freeze–thaw resistance of concrete: Results of a RILEM interlaboratory study. Mater. Struct. 2017, 50, 14. [Google Scholar] [CrossRef]
- Santamouris, M. Using cool pavements as a mitigation strategy to fight urban heat island—A review of the actual developments. Renew. Sustain. Energy Rev. 2013, 26, 224–240. [Google Scholar] [CrossRef]
- Ma, B.; Si, W.; Ren, J.; Wang, H.-n.; Liu, F.-w.; Li, J. Exploration of road temperature-adjustment material in asphalt mixture. Road Mater. Pavement Des. 2014, 15, 659–673. [Google Scholar] [CrossRef]
- Liston, L.; Krafcik, M.; Farnam, Y.; Tao, B.; Erk, K.; Weiss, J. Toward the Use of Phase Change Materials (PCM) in Concrete Pavements: Evaluation of Thermal Properties of PCM; Leah Liston Purdue University: Washington, DC, USA, 2014. [Google Scholar]
- Yuan, X.; Wang, B.; Chen, P.; Luo, T. Study on the Frost Resistance of Concrete Modified with Steel Balls Containing Phase Change Material (PCM). Materials 2021, 14, 4497. [Google Scholar] [CrossRef]
- Nayak, S.; Krishnan, N.A.; Das, S. Microstructure-guided numerical simulation to evaluate the influence of phase change materials (PCMs) on the freeze-thaw response of concrete pavements. Constr. Build. Mater. 2019, 201, 246–256. [Google Scholar] [CrossRef]
- Haurie, L.; Serrano, S.; Bosch, M.; Fernandez, A.I.; Cabeza, L.F. Single layer mortars with microencapsulated PCM: Study of physical and thermal properties, and fire behaviour. Energy Build. 2016, 111, 393–400. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Das, M.K.; Rath, P. Application of TCE-PCM based heat sinks for cooling of electronic components: A review. Renew. Sustain. Energy Rev. 2016, 59, 550–582. [Google Scholar] [CrossRef]
- Figueiredo, A.; Lapa, J.; Vicente, R.; Cardoso, C. Mechanical and thermal characterization of concrete with incorporation of microencapsulated PCM for applications in thermally activated slabs. Constr. Build. Mater. 2016, 112, 639–647. [Google Scholar] [CrossRef]
- Eddhahak-Ouni, A.; Drissi, S.; Colin, J.; Neji, J.; Care, S. Experimental and multi-scale analysis of the thermal properties of Portland cement concretes embedded with microencapsulated Phase Change Materials (PCMs). Appl. Therm. Eng. 2014, 64, 32–39. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Qian, X. Integrating phase change materials into concrete through microencapsulation using cenospheres. Cem. Concr. Compos. 2017, 80, 317–325. [Google Scholar] [CrossRef]
- Nayak, S.; Lyngdoh, G.A.; Das, S. Influence of microencapsulated phase change materials (PCMs) on the chloride ion diffusivity of concretes exposed to Freeze-thaw cycles: Insights from multiscale numerical simulations. Constr. Build. Mater. 2019, 212, 317–328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).