Properties of Fine Graded Perlite-Based Lightweight Cement Mortars Subjected to Elevated Temperatures
Abstract
:1. Introduction
| Hydrate/Compound | Occurrence in Cement Type | Temperature [°C] |
|---|---|---|
| Ettringite | OPC, CSAC | 120–130 [55]; 160–173 [56]; 80 [57]; <180 [58]; 100–110 [59] |
| C-S-H phase | OPC, CSAC | 690 [57]; <660 [58] |
| C-S-H gel water | OPC, CSAC | <150 [55]; <180 [58] |
| CH | OPC, CSAC | 420–550 [55]; 480–490 [56]; 430 [57] |
| CaC03 | OPC, CSAC | 650–800 [55]; 660–740 [58]; 710–730 [59] |
| AFm | OPC, CSAC | 200–210 [59] |
| AH3 | CSAC, CAC | 276–282 [56]; 250 [57]; 180–280 [58]; 240 [59]; 280–310 [60]; 266–310 [61]; 210–300 [46] |
| AH3 gel water | CSAC, CAC | <150 [60]; <120 [62]; 100 [46] |
| CAH10 | CAC | <150 [60]; 110–120 [61]; 120 [46] |
| C2AH8 | CAC | <150 [60]; 170–230 [61]; 170–195 [46] |
| C2ASH8 | CAC, CSAC | <150 [60]; 220–240 [61] |
| C3AH6 | CAC | 280–310 [60]; 290–350 [61]; 240–370 [46] |
| C4A3H3 | CAC | 440 [61] |
| C4AH19 | CAC | 280–310 [60] |
| C4AH | CAC | 183 [60] |
Motivation for and Novelty of the Present Research
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fresh Mixes Properties
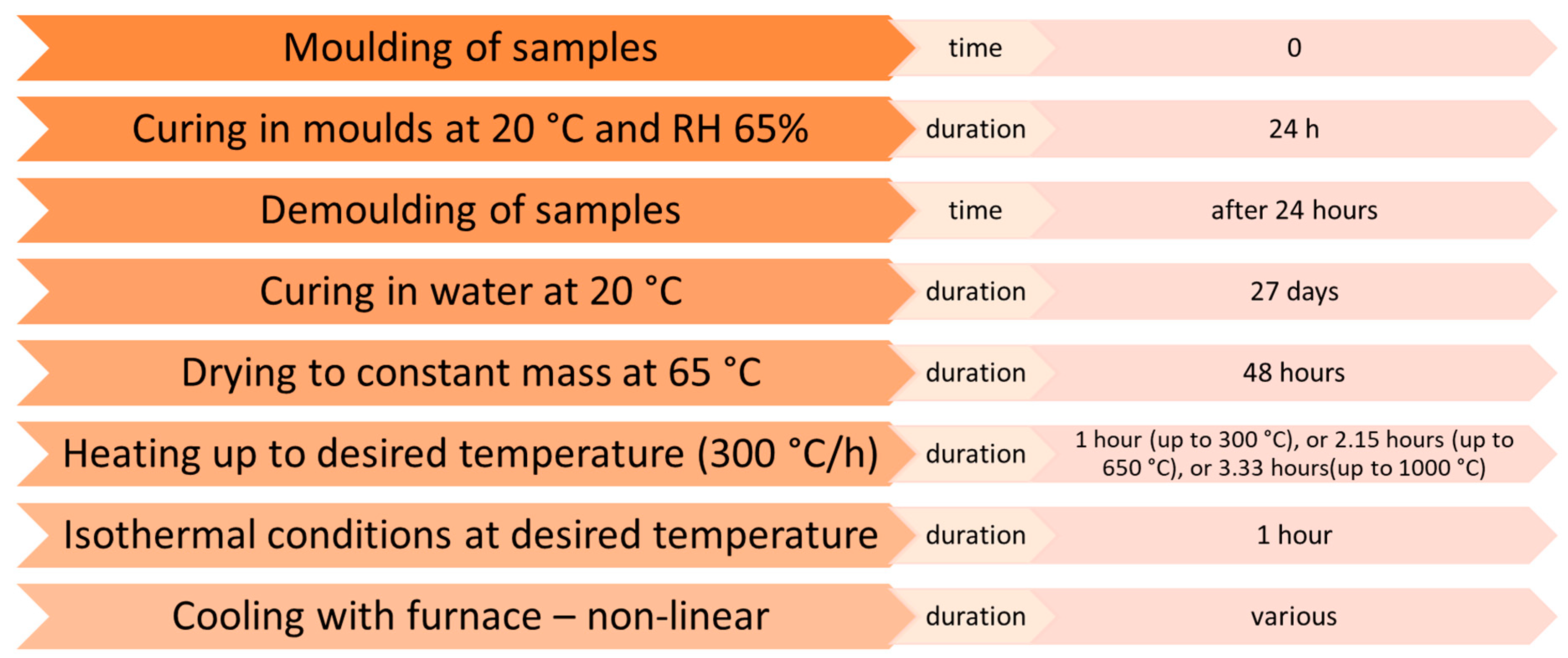
2.2.2. Hardened Mortar Preparation
- 300 °C—above or at the end of AH3 and most hydration products of CAC decomposition, above AFm, ettringite decomposition
- 650 °C—above CH, above or at the end of C-S-H decomposition
- 1000 °C—above CaCO3 decomposition, in range of softening of typical perlite (871–1093 °C) but below melting point (~1260–1343 °C) [68].
2.2.3. Hardened Mortar Properties
3. Results and Discussion
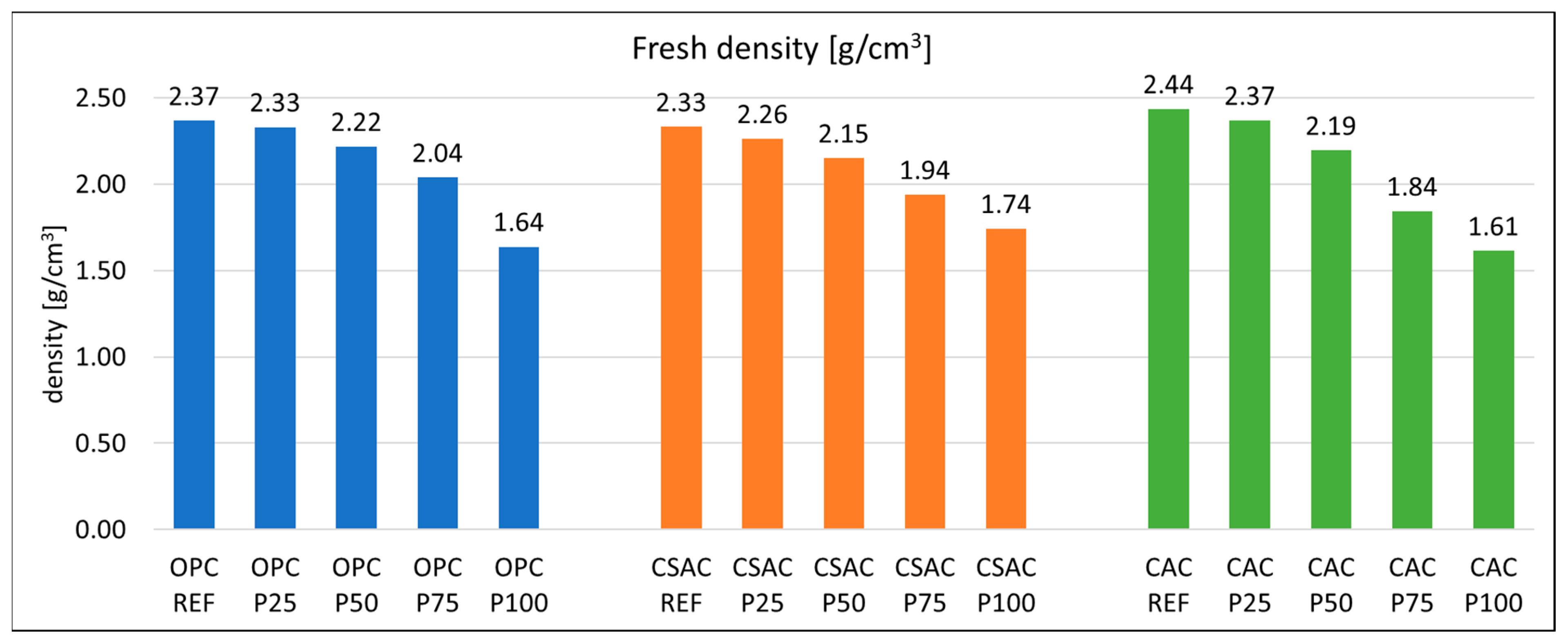
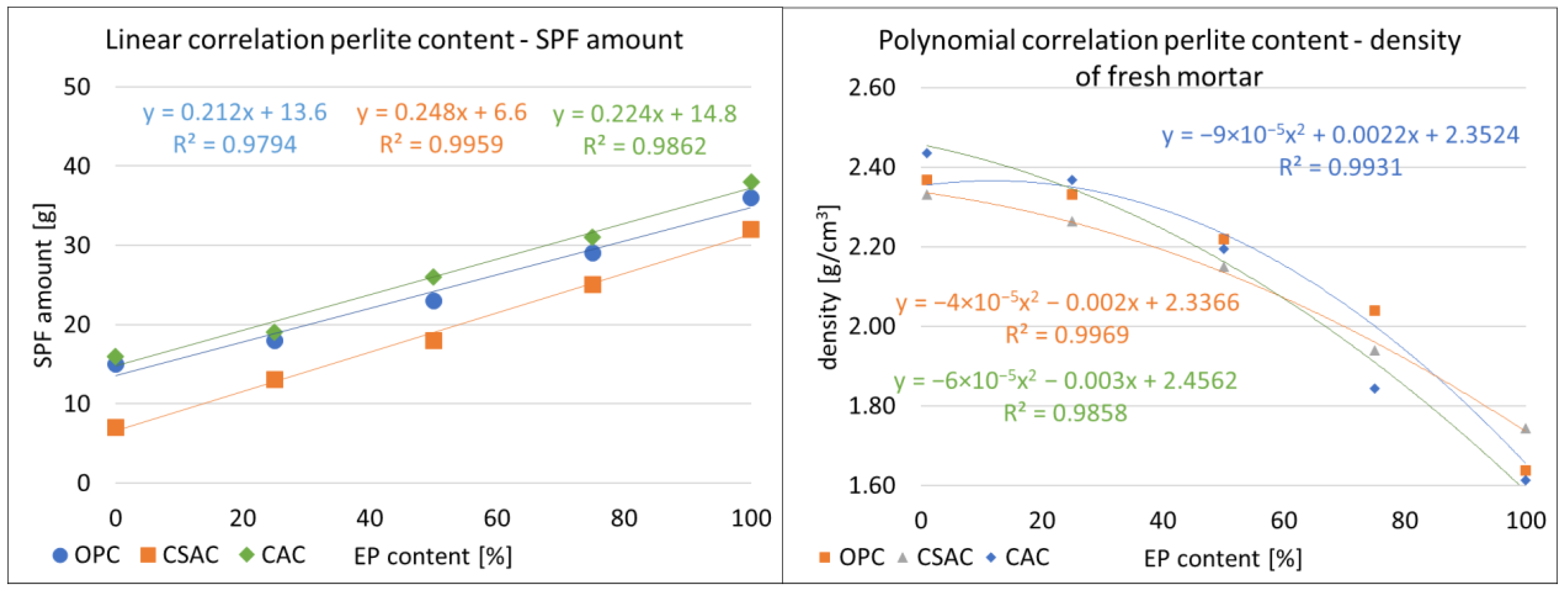
3.1. Properties of the Fresh Mixes
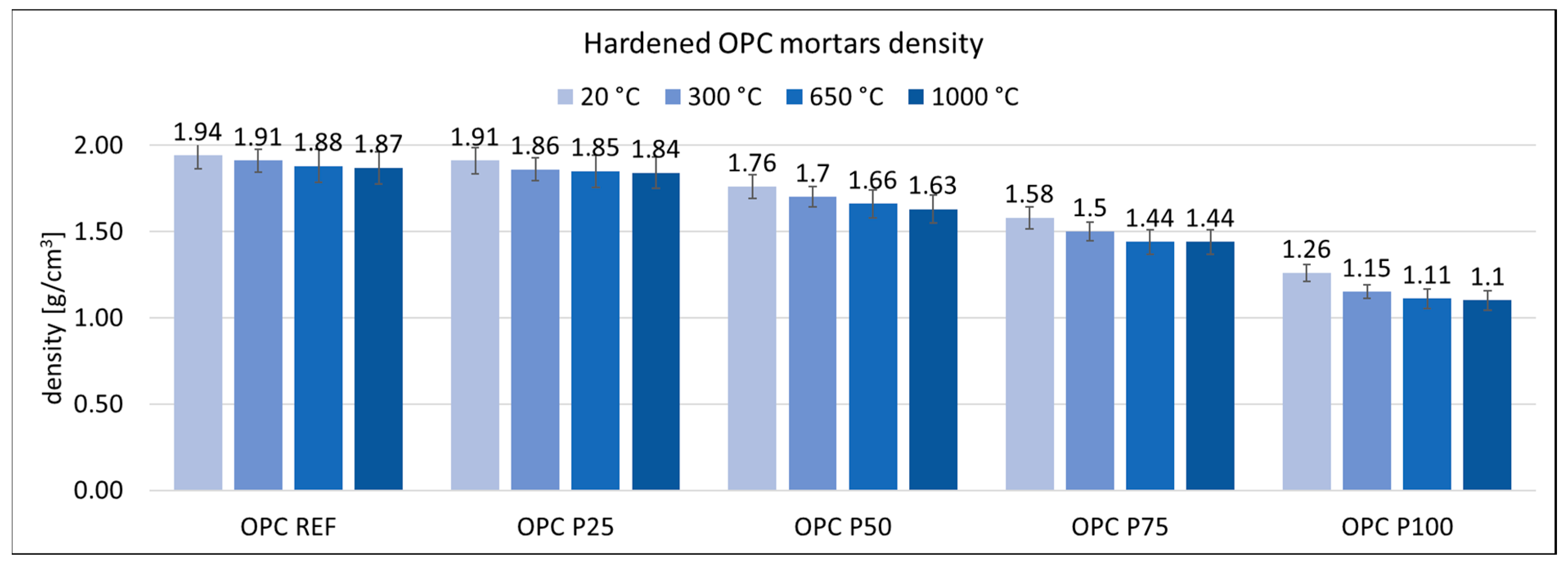
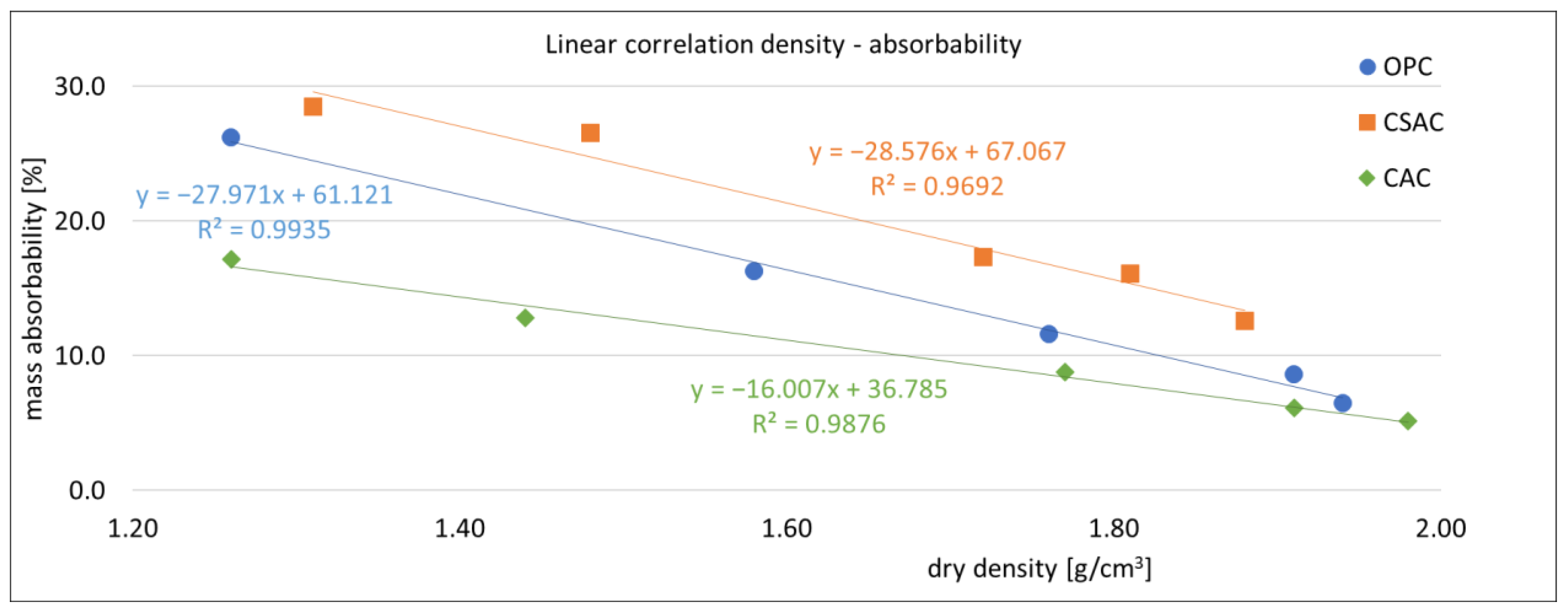
3.2. Physical Properties of Mortars
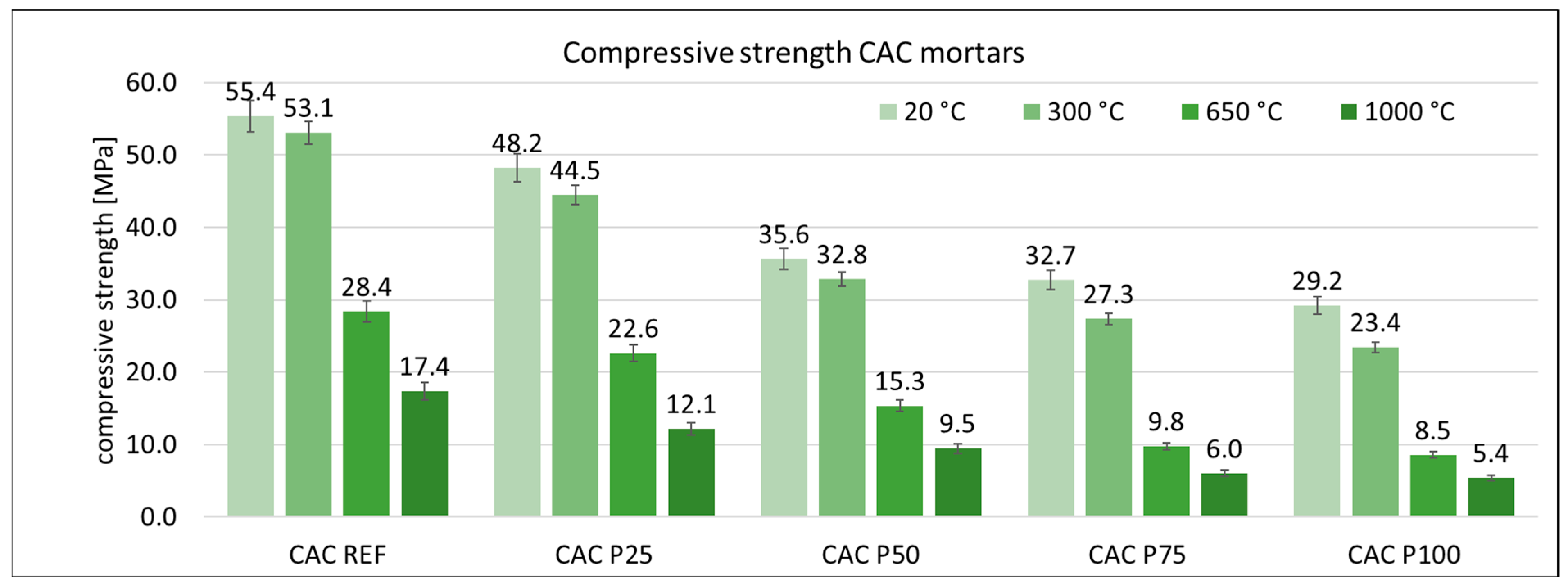
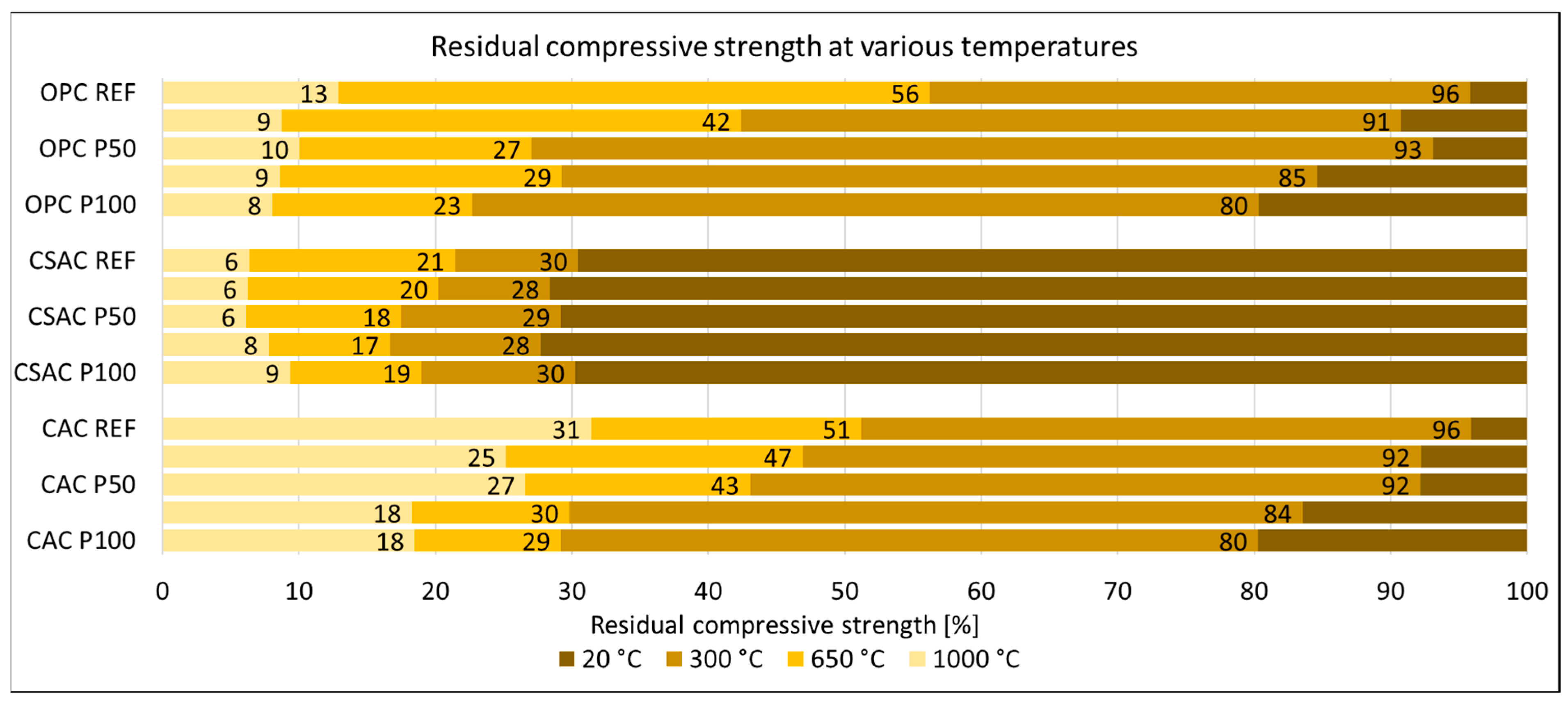
3.3. Mechanical Properties of Mortars
3.4. Photography of Surfaces of Mortars after Heating
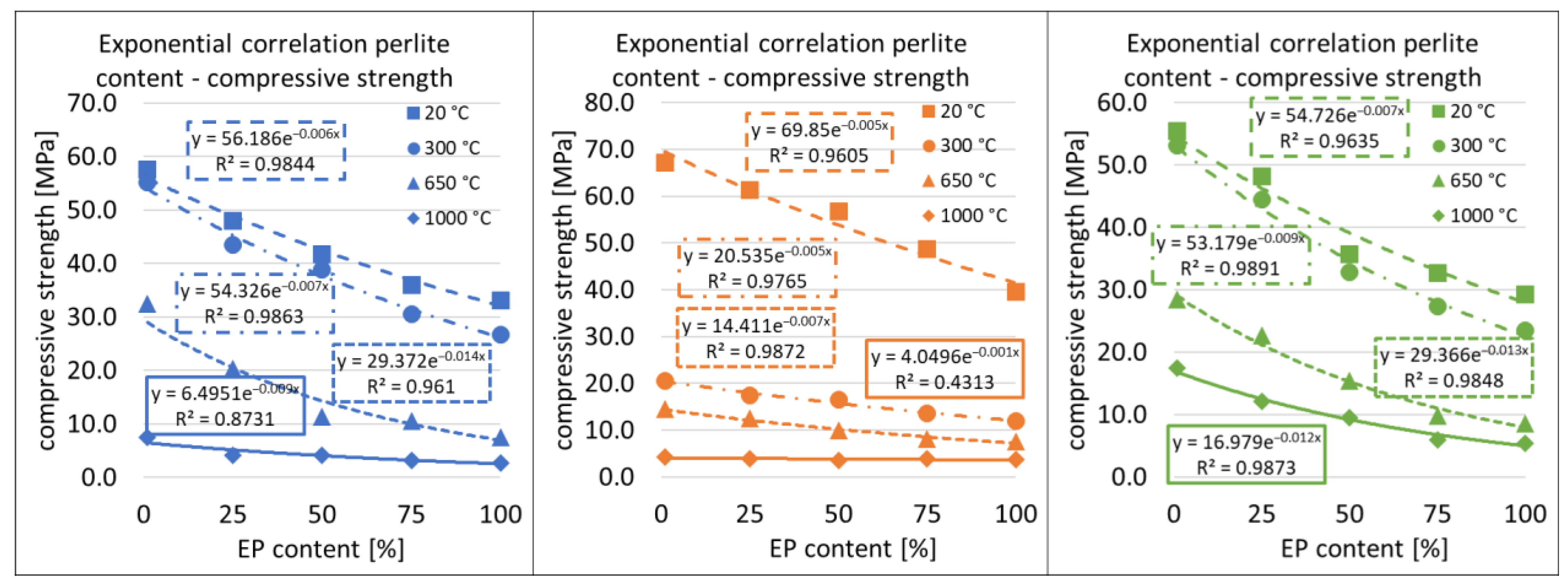
3.5. Correlations
4. Summary of Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Fabrication of Nanostructures Using Natural Synthesis: Optical Materials Using Silk; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9781845698027. [Google Scholar]
- Pacheco-Torgal, F. Introduction to the Environmental Impact of Construction and Building Materials. In Eco-Efficient Construction and Building Materials: Life Cycle Assessment (LCA), Eco-Labelling and Case Studies; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–10. [Google Scholar] [CrossRef]
- Poranek, N.; Łaźniewska-Piekarczyk, B.; Czajkowski, A.; Pikoń, K. Circular Economy for Municipal Solid Waste Incineration Bottom Ash (MSWIBA) Management in Mortars with CSA and CEM I, MSWIBA Glassy Phase, and DTG. Energies 2021, 15, 135. [Google Scholar] [CrossRef]
- Poranek, N.; Łaźniewska-Piekarczyk, B.; Lombardi, L.; Czajkowski, A.; Bogacka, M.; Pikoń, K. Green Deal and Circular Economy of Bottom Ash Waste Management in Building Industry—Alkali (NaOH) Pre-Treatment. Materials 2022, 15, 3487. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yao, J.; Deng, F.; Li, H.; Wang, K.; Tang, S. Hydration Behavior and Strength Development of Supersulfated Cement Prepared by Calcined Phosphogypsum and Slaked Lime. J. Build. Eng. 2023, 2023, 108075. [Google Scholar] [CrossRef]
- Krupnova, T.G.; Rakova, O.V.; Shefer, E.A.; Semenenko, D.P.; Saifullin, A.F. Domestic Energy-Saving Behavior Index as Sustainability Indicator: Are Russians Ready for Sacrifices to Protect the Environment? Environ. Sustain. Indic. 2022, 16, 100209. [Google Scholar] [CrossRef]
- Li, D.; Huang, C. Thermal Insulation Performances of Carbonized Sawdust Packed Bed for Energy Saving in Buildings. Energy Build 2022, 254, 111625. [Google Scholar] [CrossRef]
- Bao, J.; Zheng, R.; Zhang, P.; Cui, Y.; Xue, S.; Song, Q.; Ma, Y. Thermal Resistance, Water Absorption and Microstructure of High-Strength Self-Compacting Lightweight Aggregate Concrete (HSSC-LWAC) after Exposure to Elevated Temperatures. Constr. Build. Mater. 2023, 365, 130071. [Google Scholar] [CrossRef]
- Tanhadoust, A.; Yang, T.Y.; Dabbaghi, F.; Chai, H.K.; Mohseni, M.; Emadi, S.B.; Nasrollahpour, S. Predicting Stress-Strain Behavior of Normal Weight and Lightweight Aggregate Concrete Exposed to High Temperature Using LSTM Recurrent Neural Network. Constr. Build. Mater. 2023, 362, 129703. [Google Scholar] [CrossRef]
- Karakaş, H.; İlkentapar, S.; Durak, U.; Örklemez, E.; Özuzun, S.; Karahan, O.; Atiş, C.D. Properties of Fly Ash-Based Lightweight-Geopolymer Mortars Containing Perlite Aggregates: Mechanical, Microstructure, and Thermal Conductivity Coefficient. Constr. Build. Mater. 2023, 362, 129717. [Google Scholar] [CrossRef]
- Koksal, F.; Coşar, K.; Dener, M.; Benli, A.; Gencel, O. Insulating and Fire-Resistance Performance of Calcium Aluminate Cement Based Lightweight Mortars. Constr. Build. Mater. 2023, 362, 129759. [Google Scholar] [CrossRef]
- Gou, M.; Hou, W.; Zhou, L.; Zhao, J.; Zhao, M. Preparation and Properties of Calcium Aluminate Cement with Bayer Red Mud. Constr. Build. Mater. 2023, 373, 130827. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; da Costa, M.L.; Pöllmann, H. Waste Clay from Bauxite Beneficiation to Produce Calcium Sulphoaluminate Eco-Cements. Constr. Build. Mater. 2022, 340, 127703. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Zhou, W.; Lu, Y.; Liu, X.; Chang, X. A Fast-Setting and Eco-Friendly Superhydrophobic High Belite Sulphoaluminate Cement Mortar. J. Mater. Res. Technol. 2023, 23, 2690–2702. [Google Scholar] [CrossRef]
- Yousefi, A.; Tang, W.; Khavarian, M.; Fang, C.; Wang, S. Thermal and Mechanical Properties of Cement Mortar Composite Containing Recycled Expanded Glass Aggregate and Nano Titanium Dioxide. Appl. Sci. 2020, 10, 2246. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.E.; Álvarez Rabanal, F.P.; Lázaro, M.; Alonso-Martínez, M.; Alvear, D.; Del Coz-Díaz, J.J. Assessment of Lightweight Concrete Thermal Properties at Elevated Temperatures. Appl. Sci. 2021, 11, 10023. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Zhang, S.; Hou, J. Performance Study of Lightweight Insulating Mortar Reinforced with Straw Fiber. Materials 2023, 16, 2266. [Google Scholar] [CrossRef]
- Ulusu, İ.; Kurnuç Seyhan, A. Effect of Expanded Perlite Aggregate Plaster on the Behavior of High-Temperature Reinforced Concrete Structures. Buildings 2023, 13, 384. [Google Scholar] [CrossRef]
- Hao, D.L.C.; Razak, R.A.; Kheimi, M.; Yahya, Z.; Abdullah, M.M.A.B.; Nergis, D.D.B.; Fansuri, H.; Ediati, R.; Mohamed, R.; Abdullah, A. Artificial Lightweight Aggregates Made from Pozzolanic Material: A Review on the Method, Physical and Mechanical Properties, Thermal and Microstructure. Materials 2022, 15, 3929. [Google Scholar] [CrossRef]
- Neto, A.; Expanded, A.; Yan, J.-B.; Ji, J.; Wang, Y.; Campos de Assis Neto, P.; Priscilla Bonfim Sales, L.; Karolayne Santos Oliveira, P.; Costa da Silva, I.; Maria da Silva Barros, I.; et al. Expanded Vermiculite: A Short Review about Its Production, Characteristics, and Effects on the Properties of Lightweight Mortars. Buildings 2023, 13, 823. [Google Scholar] [CrossRef]
- Šeputyt E-Jucik, J.; Ejelis, S.; Kizinievič, V.; Kairyt, A.E.; Vaitkus, S. The Effect of Expanded Glass and Crushed Expanded Polystyrene on the Performance Characteristics of Lightweight Concrete. Appl. Sci. 2023, 13, 4188. [Google Scholar] [CrossRef]
- Rashad, A.M. A Synopsis about Perlite as Building Material—A Best Practice Guide for Civil Engineer. Constr. Build. Mater. 2016, 121, 338–353. [Google Scholar] [CrossRef]
- Xu, F.; Lin, X.; Zhou, A. Performance of Internal Curing Materials in High-Performance Concrete: A Review. Constr. Build. Mater. 2021, 311, 125250. [Google Scholar] [CrossRef]
- Hammam, A.; Ismail, M.; Roushdy, M.; El Ghonemy, M.; Abdel Raouf, M.; El Maghraby, Y.; Abou-Zeid, M.N. Incorporation of Perlite and Recycled Aggregates for Internal Concrete Curing. International Conference on Transportation and Development 2021: Transportation Planning and Development; American Society of Civil Engineers: Reston, VA, USA, 2021; pp. 225–234. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, D.-S.; Lim, C.-S.; Lee, S.-P.; Yang, J.-E.; Chung, D.-Y.; Lee, K.-S.; Lee, D.-S.; Lim, C.-S.; Lee, S.-P.; et al. Water Retention Characteristics of Various Sizes of Expanded Perlite Produced from Two Different Types of Rocks. Horticulturae 2022, 8, 805. [Google Scholar] [CrossRef]
- Grzeszczyk, S.; Janus, G. Lightweight Reactive Powder Concrete Containing Expanded Perlite. Materials 2021, 14, 3341. [Google Scholar] [CrossRef] [PubMed]
- Leyton-Vergara, M.; Pérez-Fargallo, A.; Pulido-Arcas, J.; Cárdenas-Triviño, G.; Piggot-Navarrete, J. Influence of Granulometry on Thermal and Mechanical Properties of Cement Mortars Containing Expanded Perlite as a Lightweight Aggregate. Materials 2019, 12, 4013. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, M.; Barnat-Hunek, D.; Grzegorczyk-Frańczak, M.; Trochonowicz, M. Surface Modification of Lightweight Mortars by Nanopolymers to Improve Their Water-Repellency and Durability. Materials 2020, 13, 1350. [Google Scholar] [CrossRef] [PubMed]
- Ariyaratne, I.E.; Ariyanayagam, A.; Mahendran, M. Bushfire-Resistant Lightweight Masonry Blocks with Expanded Perlite Aggregate. Fire 2022, 5, 132. [Google Scholar] [CrossRef]
- Alhamad, A.; Yehia, S.; Lublóy, É.; Elchalakani, M. Performance of Different Concrete Types Exposed to Elevated Temperatures: A Review. Materials 2022, 15, 5032. [Google Scholar] [CrossRef]
- Fehérvári, S. The Effect of Elevated Temperature on Ordinary Portland Cements. Period. Polytech. Civ. Eng. 2023, 67, 224–231. [Google Scholar] [CrossRef]
- Xu, C.; Wang, C.; Li, W.; Niu, H.; Sun, K.; Qin, S.; Wang, L. Preparation and Characterization of Multi-Component Fire-Resistant Cement-Based Materials. Constr. Build. Mater. 2022, 346, 128429. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Peng, Y.; Tang, S.; Wang, L.; Shi, Y.; Li, Y.; Wang, Y.; Geng, Z.; Wu, K. Hydration and Fractal Analysis on Low-Heat Portland Cement Pastes Using Thermodynamics-Based Methods. Fractal Fract. 2023, 7, 606. [Google Scholar] [CrossRef]
- Xu, C.; Gong, Y.; Yan, G. Research on Cement Demand Forecast and Low Carbon Development Strategy in Shandong Province. Atmosphere 2023, 14, 267. [Google Scholar] [CrossRef]
- Plaza, M.G.; Martínez, S.; Rubiera, F. CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations. Energies 2020, 13, 5692. [Google Scholar] [CrossRef]
- Cement—Analysis—IEA. Available online: https://www.iea.org/reports/cement (accessed on 5 April 2023).
- Durastanti, C.; Moretti, L. Environmental Impacts of Cement Production: A Statistical Analysis. Appl. Sci. 2020, 10, 8212. [Google Scholar] [CrossRef]
- Xie, J.; Yang, F.; Tan, N.; Wang, W.; Wang, W.; Wang, Z. Calcium Sulphoaluminate Cement from Solid Waste with Nano-TiO2 Addition for High-Efficiency CO2 Capture. Constr. Build. Mater. 2023, 367, 130267. [Google Scholar] [CrossRef]
- Sodol, K.A.; Kaczmarek, Ł.; Szer, J.; Miszczak, S.; Stegliński, M. Impact of Elevated Temperatures on Strength Properties and Microstructure of Calcium Sulfoaluminate Paste. Materials 2021, 14, 6751. [Google Scholar] [CrossRef] [PubMed]
- Kodur, V.; Khaliq, W. Effect of Temperature on Thermal Properties of Different Types of High-Strength Concrete. J. Mater. Civ. Eng. 2010, 23, 793–801. [Google Scholar] [CrossRef]
- Hager, I.; Tracz, T.; Choińska, M.; Mróz, K. Effect of Cement Type on the Mechanical Behavior and Permeability of Concrete Subjected to High Temperatures. Materials 2019, 12, 3021. [Google Scholar] [CrossRef]
- Gawin, D.; Pesavento, F.; Schrefler, B.A. What Physical Phenomena Can Be Neglected When Modelling Concrete at High Temperature? A Comparative Study. Part 2: Comparison between Models. Int. J. Solids Struct. 2011, 48, 1945–1961. [Google Scholar] [CrossRef]
- Hager, I. Behaviour of Cement Concrete at High Temperature. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 145–154. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Tantawy, M.A. Effect of High Temperatures on the Microstructure of Cement Paste. J. Mater. Sci. Chem. Eng. 2017, 5, 33–48. [Google Scholar] [CrossRef]
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete; Hewlett, P., Ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 1999. [Google Scholar]
- Cardoso, F.A.; Innocentini, M.D.M.; Akiyoshi, M.M.; Pandolfelli, V.C. Effect of Curing Time on the Properties of CAC Bonded Refractory Castables. J. Eur. Ceram Soc. 2004, 24, 2073–2078. [Google Scholar] [CrossRef]
- Amer, A.I.H.; El-Maddah, A.A.; Abuelela, A.M.; Ahmed, Y.M.Z.; Hassan, A.M.; El-Amir, A.A.M. Maximize the Use of Municipal Waste Generated by the Hydrogen Peroxide Industry in the Production of High-Quality Refractory CAC. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bareiro, W.G.; de Andrade Silva, F.; Sotelino, E.D.; da Gomes, O.F.M. The Influence of Alumina Content on the Chemical and Mechanical Behavior of Refractory Concretes Fired at Different Temperatures. Constr. Build. Mater. 2018, 187, 1214–1223. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, Z.; Zheng, K.; Ma, C. Inorganic Cementing Materials. Civ. Eng. Mater. 2021, 17–57. [Google Scholar] [CrossRef]
- Lee, J.M.; Yang, H.J.; Jang, I.Y.; Kim, S.K.; Jung, D.H. Comparison of Calcium Aluminate Cements on Hydration and Strength Development at Different Initial Curing Regimes. Case Stud. Constr. Mater. 2022, 17, e01596. [Google Scholar] [CrossRef]
- Lee, N.K.; Koh, K.T.; Park, S.H.; Ryu, G.S. Microstructural Investigation of Calcium Aluminate Cement-Based Ultra-High Performance Concrete (UHPC) Exposed to High Temperatures. Cem. Concr. Res. 2017, 102, 109–118. [Google Scholar] [CrossRef]
- Podwórny, J.; Dudek, K.; Psiuk, B.; Krause, O.; Holleyn, F.; Brochen, E.; Simmat, R. High-temperature phase transformations in the matrix of high-alumina monolithics. In Proceedings of the 61st International Colloquium on Refractories, UROGRESS, Aachen, Germany, 26–27 September 2018. [Google Scholar]
- Kim, G.M.; Park, S.M.; Park, S.W. Chloride Removal of Calcium Aluminate Cements: Reaction and Physicochemical Characteristics. Case Stud. Constr. Mater. 2023, 18, e01975. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E.; Kalmykov, S.N. An Introduction to Nuclear Waste Immobilisation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–512. [Google Scholar] [CrossRef]
- Ogirigbo, O.R. Influence of Slag Composition and Temperature on the Hydration and Performance of Slag Blends in Chloride Environments; University of Leeds: Leeds, UK, 2016. [Google Scholar]
- Gastaldi, D.; Canonico, F.; Capelli, L.; Bianchi, M.; Pace, M.; Telesca, A.; Valenti, G. Hydraulic Behaviour of Calcium Sulfoaluminate Cement Alone and in Mixture with Portland Cement. In Proceedings of the 13th International Congress on the Chemistry of Cement, Madrid, Spain, 3–8 July 2011. [Google Scholar]
- Zhang, J.; Ke, G.; Liu, Y. Early Hydration Heat of Calcium Sulfoaluminate Cement with Influences of Supplementary Cementitious Materials and Water to Binder Ratio. Materials 2021, 14, 642. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Z.; Meng, Y.; Tang, J.; Yang, L. Effect of Flue Gas Desulfurization Gypsum on the Properties of Calcium Sulfoaluminate Cement Blended with Ground Granulated Blast Furnace Slag. Materials 2021, 14, 382. [Google Scholar] [CrossRef]
- Gao, D.; Meng, Y.; Yang, L.; Tang, J.; Lv, M. Effect of Ground Granulated Blast Furnace Slag on the Properties of Calcium Sulfoaluminate Cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Madej, D.; Kruk, A. Tracing the Early and Long-Term Hydration of Fast Setting Cementitious Material (Ca7ZrAl6O18) and Calcium Aluminate Cement (CAC) Pastes by Means of Electrochemical Impedance Spectroscopy and Other Methods. Constr. Build. Mater. 2018, 164, 94–102. [Google Scholar] [CrossRef]
- Valix, M. Test Method 2B.5 Analysis of Calcium Aluminate Cement Hydrates by Thermal Gravimetric Analysis; University of Sydney: Sydney, Australia, 2020. [Google Scholar]
- Luz, A.P.; Pandolfelli, V.C. Halting the Calcium Aluminate Cement Hydration Process. Ceram. Int. 2011, 37, 3789–3793. [Google Scholar] [CrossRef]
- Guo, C.; Wang, R. Using Sulphoaluminate Cement and Calcium Sulfate to Modify the Physical–Chemical Properties of Portland Cement Mortar for Mechanized Construction. Constr. Build. Mater. 2023, 367, 130252. [Google Scholar] [CrossRef]
- Sodol, K.A.; Kaczmarek, Ł.; Szer, J. Fire-Temperature Influence on Portland and Calcium Sulfoaluminate Blend Composites. Materials 2020, 13, 5230. [Google Scholar] [CrossRef]
- EN 1015-3:2000/A2:2007; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). European Committee for Standardization: Brussels, Belgium, 2007.
- EN 1015-6:1999/A1:2007; Methods of Test for Mortar for Masonry—Part 6: Determination of Bulk Density of Fresh Mortar. European Committee for Standardization: Brussels, Belgium, 2007.
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2018.
- Perlite as High Temperature Insulation. 2013. Available online: https://www.perlite.org/wp-content/uploads/2018/03/perlite-high-temp-applications.pdf (accessed on 16 November 2023).
- EN 1015-10:2000/A1:2007; Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density. European Committee for Standardization: Brussels, Belgium, 2007.
- EN 13755:2008; Natural Stone Test Methods—Determination of Water Absorption at Atmospheric Pressure. European Committee for Standardization: Brussels, Belgium, 2008.
- Tao, Y.; Rahul, A.V.; Mohan, M.K.; De Schutter, G.; Van Tittelboom, K. Recent Progress and Technical Challenges in Using Calcium Sulfoaluminate (CSA) Cement. Cem. Concr. Compos. 2023, 137, 104908. [Google Scholar] [CrossRef]
- Biolzi, L.; Cattaneo, S.; Guerrini, G.; Afroughsabet, V. Sustainable Concretes for Structural Applications. In Regeneration of the Built Environment from a Circular Economy Perspective; Springer: Berlin/Heidelberg, Germany, 2020; pp. 249–261. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Al-Radadi, N.S. Estimating the Impact of Nanophases on the Production of Green Cement with High Performance Properties. Materials 2020, 13, 4197. [Google Scholar] [CrossRef] [PubMed]
- Julphunthong, P.; Joyklad, P. Utilization of Several Industrial Wastes as Raw Material for Calcium Sulfoaluminate Cement. Materials 2019, 12, 3319. [Google Scholar] [CrossRef]
- Akhlaghi, O.; Menceloglu, Y.Z.; Akbulut, O. Poly(Carboxylate Ether)-Based Superplasticizer Achieves Workability Retention in Calcium Aluminate Cement. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Lewis, R.; Fidjestøl, P. Microsilica as an Addition. In Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Waltham, MA, USA, 2019; pp. 509–535. [Google Scholar] [CrossRef]
- Huang, G.; Pudasainee, D.; Gupta, R.; Liu, W.V. Thermal Properties of Calcium Sulfoaluminate Cement-Based Mortars Incorporated with Expanded Perlite Cured at Cold Temperatures. Constr. Build. Mater. 2021, 274, 122082. [Google Scholar] [CrossRef]
- Gluth, G.J.G.; Hillemeier, B. Pore Structure and Permeability of Hardened Calcium Aluminate Cement Pastes of Low w/c Ratio. Mater. Struct. Mater. Et Constr. 2013, 46, 1497–1506. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Huang, M.; Yin, H.; Jiang, K.; Xiao, K.; Tang, S. Influence of Different Alkali Sulfates on the Shrinkage, Hydration, Pore Structure, Fractal Dimension and Microstructure of Low-Heat Portland Cement, Medium-Heat Portland Cement and Ordinary Portland Cement. Fractal Fract. 2021, 5, 79. [Google Scholar] [CrossRef]
- Park, S.; Jeong, Y.; Moon, J.; Lee, N. Hydration Characteristics of Calcium Sulfoaluminate (CSA) Cement/Portland Cement Blended Pastes. J. Build. Eng. 2021, 34, 101880. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, J.; Cai, R.; Wei, X.; Zhao, C.; Cai, X.; He, Z.; Chen, E. Continuous Monitoring for Leaching of Calcium Sulfoaluminate Cement Pastes Incorporated with ZnCl2 under the Attacks of Chloride and Sulfate. Chemosphere 2019, 223, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Ge, P. Research on the Calculation Method of Concrete Compressive Strength Based on Volume Content of Recycled Coarse Aggregate and Fine Aggregate. J. Asian Archit. Build. Eng. 2023, 2023, 2238021. [Google Scholar] [CrossRef]
- Stepien, A. Recycling in Building Materials: Analysis of the Possibilities and Results of Using Recycled Glass Sand in Autoclaved Materials. Energies 2023, 16, 3529. [Google Scholar] [CrossRef]
- Ascensão, G.; Faleschini, F.; Marchi, M.; Segata, M.; Van De Sande, J.; Rahier, H.; Bernardo, E.; Pontikes, Y. High-Temperature Behavior of CaO-FeOx-Al2O3-SiO2-Rich Alkali Activated Materials. Appl. Sci. 2022, 12, 2572. [Google Scholar] [CrossRef]
- Miah, M.J.; Paul, S.C.; Babafemi, A.J.; Panda, B. Strength Properties of Sustainable Mortar Containing Waste Steel Slag and Waste Clay Brick: Effect of Temperature. Materials 2021, 14, 2113. [Google Scholar] [CrossRef] [PubMed]
- Koksal, F.; Gencel, O.; Kaya, M. Combined Effect of Silica Fume and Expanded Vermiculite on Properties of Lightweight Mortars at Ambient and Elevated Temperatures. Constr. Build. Mater. 2015, 88, 175–187. [Google Scholar] [CrossRef]
- Torres, M.L.; García-Ruiz, P.A. Lightweight Pozzolanic Materials Used in Mortars: Evaluation of Their Influence on Density, Mechanical Strength and Water Absorption. Cem. Concr. Compos. 2009, 31, 114–119. [Google Scholar] [CrossRef]
- Rajhans, P.; Panda, S.K.; Nayak, S. Sustainability on Durability of Self Compacting Concrete from C&D Waste by Improving Porosity and Hydrated Compounds: A Microstructural Investigation. Constr. Build. Mater. 2018, 174, 559–575. [Google Scholar] [CrossRef]
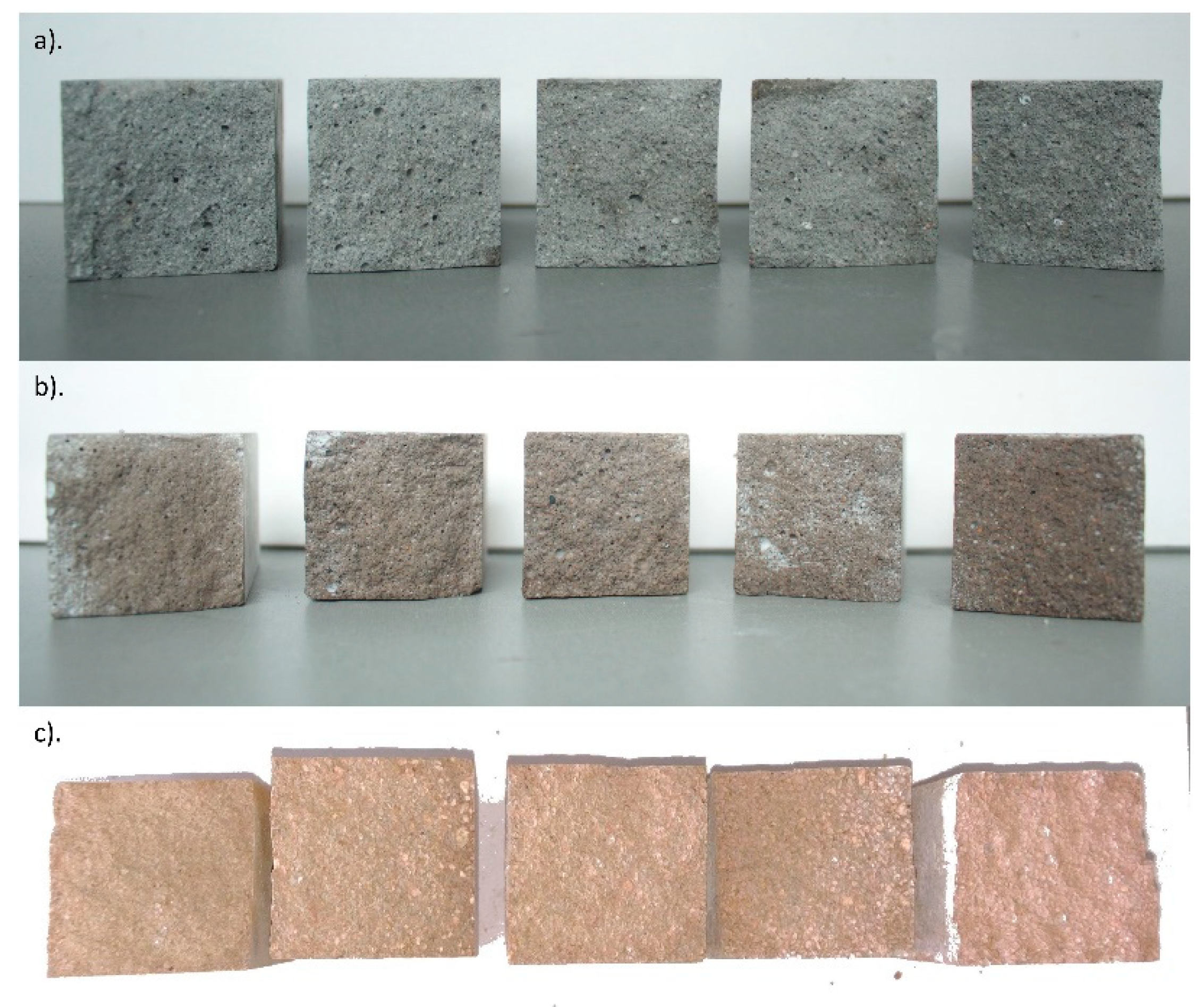



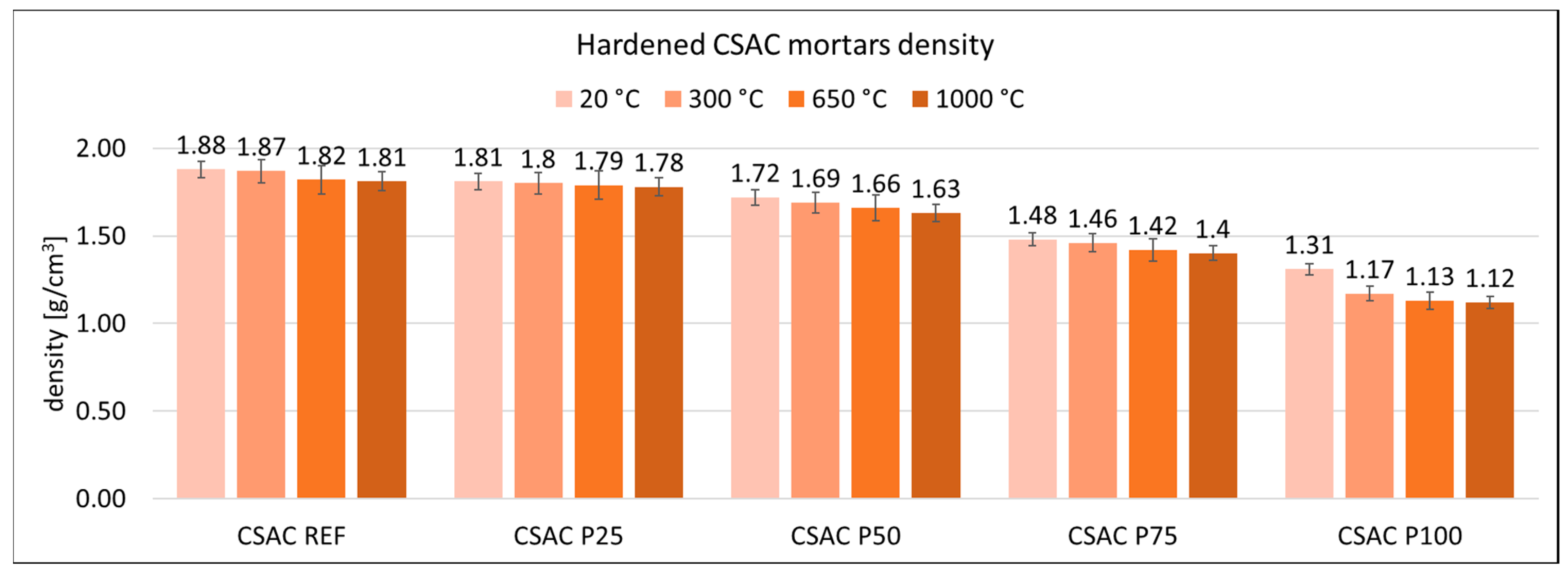

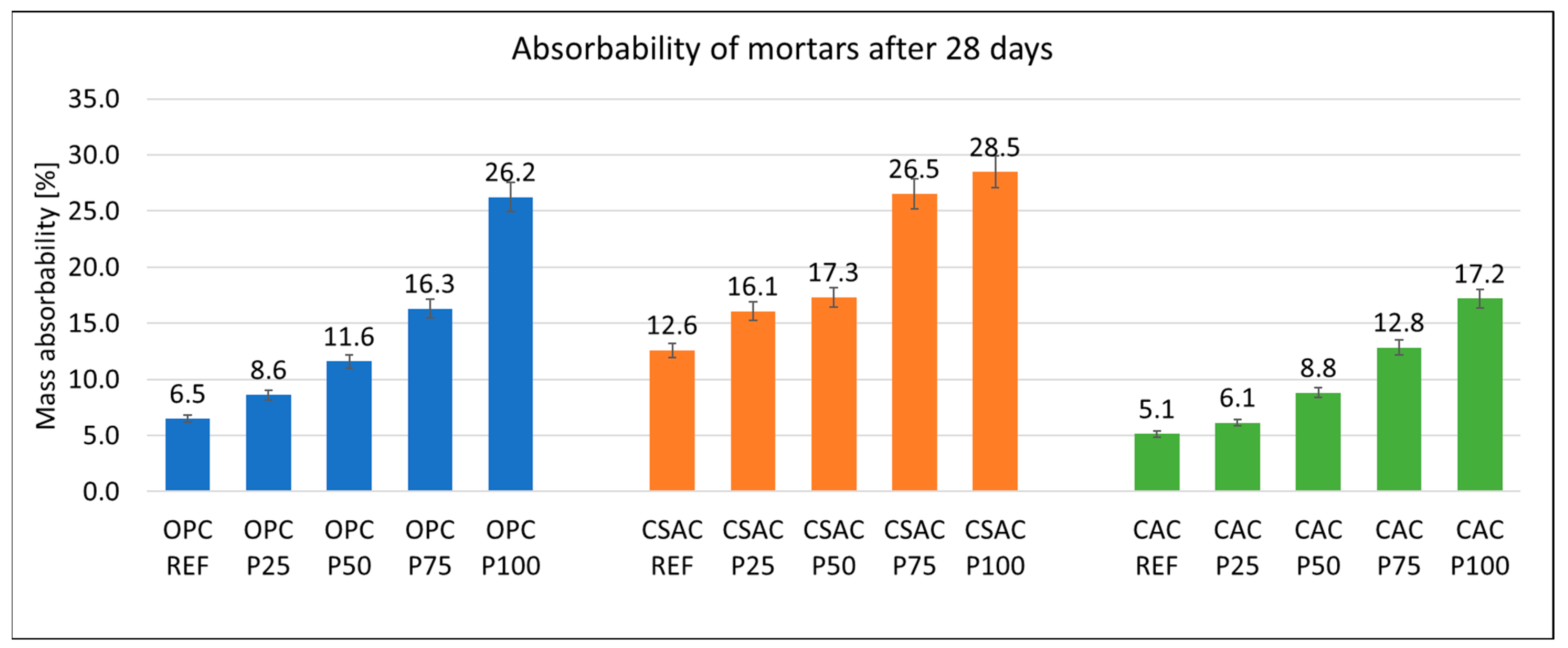
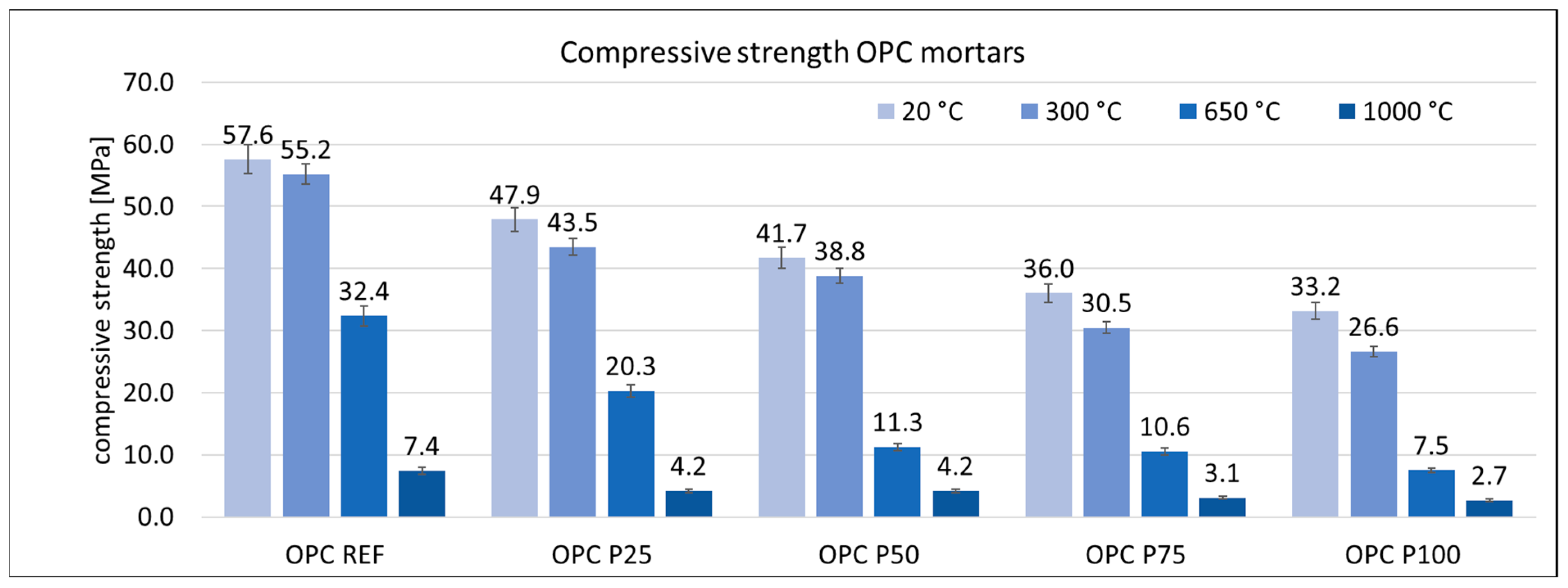
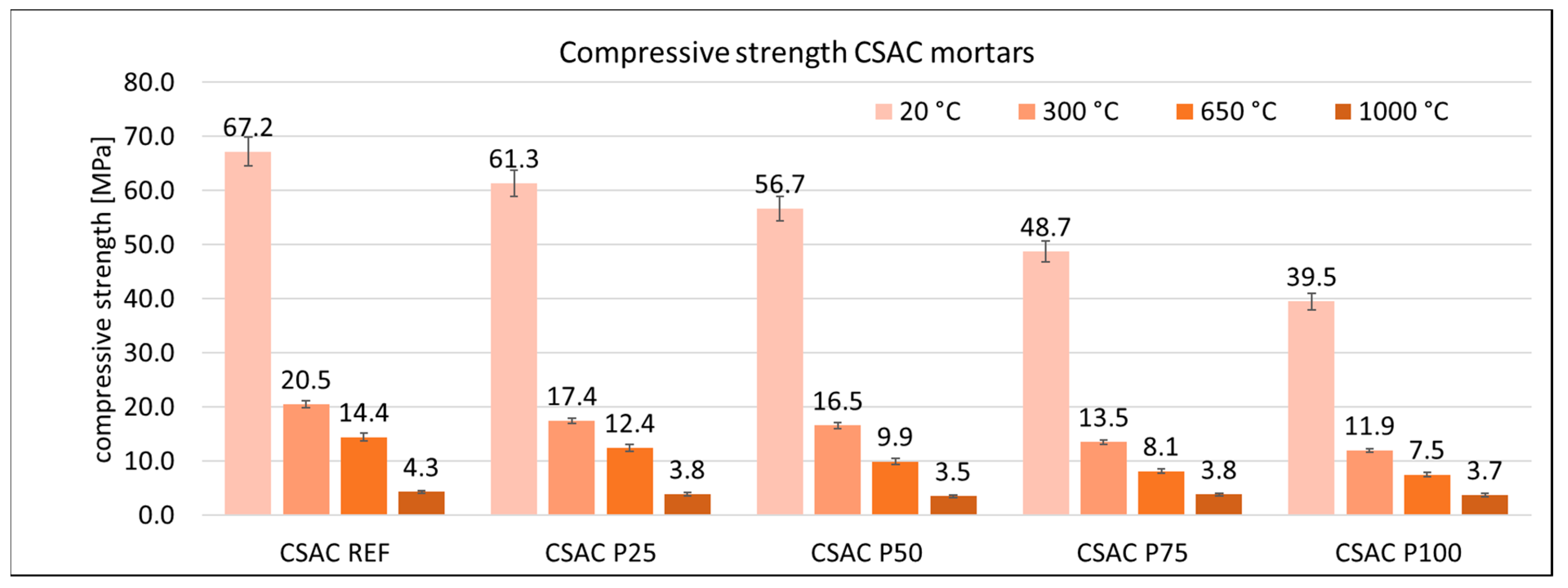


| Cement | C3S Alite | C2S Belite | C3A Celite | C4AF Brown Mille Rite | C4A3Ŝ Ye’elemite | CŜ Anhy Drite | C7MS4 Bredi Gite | MgO Peri Clase | C3MS2 Merwi Nite | CaCO3 Calcite | CA Krotite | C12A7 Mayenite | C2AS Gelhenite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 71.1 | 5.6 | 3.9 | 12.7 | 0.15 | 4.3 | |||||||
| CSAC | 4.8 | 2.6 | 43.5 | 16.4 | 11.9 | 3.7 | 5.9 | 2.7 | |||||
| CAC | 5.2 | 19.1 | 59.9 | 1.7 | 8.0 | ||||||||
| C–CaO, S–SiO2, A–Al2O3, F–FeO, Ŝ–SO3, M–MgO | |||||||||||||
| Bulk Density | Real Density | Fractions [mm] | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.0–0.063 | 0.063–0.125 | 0.125–0.25 | 0.25–0.5 | 0.5–1.0 | >1.0 | |||
| [g/dm3] | [%] | |||||||
| NS | 1651.2 | 2652.0 | 0.5 | 1.4 | 9.7 | 41.1 | 43.6 | 3.7 |
| EP | 90.2 | 2320.0 | 0.7 | 5.5 | 26.2 | 45.6 | 18.8 | 3.2 |
| SiO2 | Al2O3 | K2O | Na2O | MgO | CaO | Fe2O3 | |
|---|---|---|---|---|---|---|---|
| manufacturers information | 70–75 | 12–15 | 3–5 | 3–4 | 0.2–0.7 | 0.5–1.5 | 0.5–2.0 |
| Symbol | Binder | Water | Extruded Perlite | Sand | Superplasticizer | |||
|---|---|---|---|---|---|---|---|---|
| [mL] | [g] | [mL] | [g] | [g] | [% c.m.] | |||
| OPC REF | Ordinary Portland Cement (1620 g), silica fume (180 g) | 900 g (w/b ratio 0.5) | 0 | 0 | 4300 | 7100 | 15 | 0.83 |
| OPC P25 | 1075 | 97 | 3225 | 5325 | 18 | 1.00 | ||
| OPC P50 | 2150 | 194 | 2150 | 3550 | 23 | 1.28 | ||
| OPC P75 | 3225 | 291 | 1075 | 1775 | 29 | 1.61 | ||
| OPC P100 | 4300 | 388 | 0 | 0 | 36 | 2.00 | ||
| CSAC REF | Calcium Sulphoaluminate Cement (1620 g), silica fume (180 g) | 900 g (w/b ratio 0.5) | 0 | 0 | 4300 | 7100 | 7 | 0.39 |
| CSAC P25 | 1075 | 97 | 3225 | 5325 | 13 | 0.72 | ||
| CSAC P50 | 2150 | 194 | 2150 | 3550 | 18 | 1.00 | ||
| CSAC P75 | 3225 | 291 | 1075 | 1775 | 25 | 1.39 | ||
| CSAC P100 | 4300 | 388 | 0 | 0 | 32 | 1.78 | ||
| CAC REF | Calcium Aluminate Cement (1620 g), silica fume (180 g) | 900 g (w/b ratio 0.5) | 0 | 0 | 4300 | 7100 | 16 | 0.89 |
| CAC P25 | 1075 | 97 | 3225 | 5325 | 19 | 1.06 | ||
| CAC P50 | 2150 | 194 | 2150 | 3550 | 26 | 1.44 | ||
| CAC P75 | 3225 | 291 | 1075 | 1775 | 31 | 1.72 | ||
| CAC P100 | 4300 | 388 | 0 | 0 | 38 | 2.11 | ||
| OPC | CSAC | CAC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 300 °C | 650 °C | 1000 °C | 20 °C | 300 °C | 650 °C | 1000 °C | 20 °C | 300 °C | 650 °C | 1000 °C | |
| REF | 0.0 | 1.5 | 3.1 | 3.6 | 0.0 | 0.5 | 3.2 | 3.7 | 0.0 | 2.5 | 5.1 | 7.1 |
| P25 | 1.5 | 4.1 | 4.6 | 5.2 | 3.7 | 4.3 | 4.8 | 5.3 | 3.5 | 5.1 | 8.6 | 10.1 |
| P50 | 9.3 | 12.4 | 14.4 | 16.0 | 8.5 | 10.1 | 11.7 | 13.3 | 10.6 | 14.1 | 16.7 | 20.2 |
| P75 | 18.6 | 22.7 | 25.8 | 25.8 | 21.3 | 22.3 | 24.5 | 25.5 | 27.3 | 29.3 | 34.3 | 38.4 |
| P100 | 35.1 | 40.7 | 42.8 | 43.3 | 30.3 | 37.8 | 39.9 | 40.4 | 36.4 | 41.4 | 43.4 | 45.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizoń, J.; Konečný, P.; Mynarz, M.; Bílek, V. Properties of Fine Graded Perlite-Based Lightweight Cement Mortars Subjected to Elevated Temperatures. Buildings 2023, 13, 2969. https://doi.org/10.3390/buildings13122969
Pizoń J, Konečný P, Mynarz M, Bílek V. Properties of Fine Graded Perlite-Based Lightweight Cement Mortars Subjected to Elevated Temperatures. Buildings. 2023; 13(12):2969. https://doi.org/10.3390/buildings13122969
Chicago/Turabian StylePizoń, Jan, Petr Konečný, Miroslav Mynarz, and Vlastimil Bílek. 2023. "Properties of Fine Graded Perlite-Based Lightweight Cement Mortars Subjected to Elevated Temperatures" Buildings 13, no. 12: 2969. https://doi.org/10.3390/buildings13122969
APA StylePizoń, J., Konečný, P., Mynarz, M., & Bílek, V. (2023). Properties of Fine Graded Perlite-Based Lightweight Cement Mortars Subjected to Elevated Temperatures. Buildings, 13(12), 2969. https://doi.org/10.3390/buildings13122969








