A Study on the Effect of Ambient Air Plasma Treatment on the Properties of Methylammonium Lead Halide Perovskite Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Film Deposition
2.2. Plasma Processing
2.3. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ava, T.T.; Al Mamun, A.; Marsillac, S.; Namkoong, G. A Review: Thermal Stability of Methylammonium Lead Halide Based Perovskite Solar Cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhang, K.; Huang, P.; Li, D.; Yuan, L.; Cao, T.; Sun, Z.; Li, Z.; Chen, Q.; et al. 3,4-Dihydroxybenzhydrazide as an additive to improve the morphology of perovskite films for efficient and stable perovskite solar cells. Org. Electron. 2019, 66, 47–52. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.-K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 1808843, 1–25. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.K.; Bhattacharya, B.; Rhee, H.-W. Review of current progress in inorganic hole-transport materials for perovskite solar cells. Appl. Mater. Today 2019, 14, 175–200. [Google Scholar] [CrossRef]
- Huang, F.; Li, M.; Siffalovic, P. Environmental Science From scalable solution fabrication of perovskite films towards commercialization of solar cells. Energy Environ. Sci. 2019, 12, 518–549. [Google Scholar] [CrossRef]
- Adjokatse, S.; Kardula, J.; Fang, H.-H.; Shao, S.; Brink, G.H.T.; Loi, M.A. Effect of the Device Architecture on the Performance of FA0.85MA0.15PbBr0.45I2.55 Planar Perovskite Solar Cells. Adv. Mater. Interfaces 2019, 6, 1801667. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, M.; Game, O.S.; Wu, W.; Kwun, J.; Strauss, M.A.; Yan, Y.; Huang, J.; Zhu, K.; Padture, N.P. Manipulating Crystallization of Organolead Mixed-Halide Thin Films in Antisolvent Baths for Wide-Bandgap Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 2232–2237. [Google Scholar] [CrossRef]
- Uribe, J.I.; Ciro, J.; Montoya, J.F.; Osorio, J.; Jaramillo, F. Enhancement of Morphological and Optoelectronic Properties of Perovskite Films by CH3NH3Cl Treatment for Efficient Solar Minimodules. ACS Appl. Energy Mater. 2018, 1, 1047–1052. [Google Scholar] [CrossRef]
- Chu, Z.; Yang, M.; Schulz, P.; Wu, D.; Ma, X.; Seifert, E.; Sun, L.; Li, X.; Zhu, K.; Lai, K. Impact of grain boundaries on efficiency and stability of organic-inorganic trihalide perovskites. Nat. Commun. 2017, 8, 2230. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, S.; Lei, L.; Cao, Q.; Shao, J.; Zhang, S.; Liu, Y. Ultrasmooth Perovskite Film via Mixed Anti-Solvent Strategy with Improved Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-B.; Su, P.-Y.; Liu, J.-M.; Huang, J.-F.; Chen, Y.-F.; Qin, S.; Guo, J.; Xu, Y.-W.; Su, C.-Y. Interface engineering of perovskite solar cells with multifunctional polymer interlayer toward improved performance and stability. J. Power Sources 2018, 378, 483–490. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.-S.; Park, N.-G. Post-treatment of perovskite film with phenylalkylammonium iodide for hysteresis-less perovskite solar cells. Sol. Energy Mater. Sol. Cells 2018, 179, 57–65. [Google Scholar] [CrossRef]
- Dong, G.; Xia, D.; Yang, Y.; Sheng, L.; Su, T.; Fan, R.; Shi, Y.; Wang, J. Inverted thermal annealing of perovskite films: A method for enhancing photovoltaic device efficiency. RSC Adv. 2016, 6, 44034–44040. [Google Scholar] [CrossRef]
- Masood, M.T.; Weinberger, C.; Sarfraz, J.; Rosqvist, E.; Sandén, S.; Sandberg, O.J.; Vivo, P.; Hashmi, G.; Lund, P.D.; Österbacka, R.; et al. Impact of Film Thickness of Ultrathin Dip-Coated Compact TiO2 Layers on the Performance of Mesoscopic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 17906–17913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ling, H.; Lu, X.; Xia, J. The facile modification of PEDOT: PSS buffer layer by polyethyleneglycol and their effects on inverted perovskite solar cell. Sol. Energy 2019, 186, 398–403. [Google Scholar] [CrossRef]
- Cao, Q.; Yang, S.; Gao, Q.; Lei, L.; Yu, Y.; Shao, J.; Liu, Y. Fast and Controllable Crystallization of Perovskite Films by Microwave Irradiation Process. ACS Appl. Mater. Interfaces 2016, 8, 7854–7861. [Google Scholar] [CrossRef]
- Ren, Z.; Ng, A.; Shen, Q.; Gokkaya, H.C.; Wang, J.; Yang, L.; Yiu, W.-K.; Bai, G.; Djurišić, A.B.; Leung, W.W.-F.; et al. Thermal Assisted Oxygen Annealing for High Efficiency Planar CH3NH3PbI3 Perovskite Solar Cells. Sci. Rep. 2014, 4, 6752. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, S.; Li, M.; Xu, W.; Yin, G.; Wang, Z.; Sun, B.; Gao, X. Annealing Induced Re-crystallization in CH3NH3PbI3−xClx for High Performance Perovskite Solar Cells. Sci. Rep. 2017, 7, 46724. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Bao, C.; Fang, Y.; Dai, J.; Ecker, B.R.; Wang, C.; Lin, Y.; Tang, S.; Liu, Y.; Deng, Y.; et al. Argon Plasma Treatment to Tune Perovskite Surface Composition for High Efficiency Solar Cells and Fast Photodetectors. Adv. Mater. 2018, 30, 1705176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lin, Y.; Zhang, Y.; Yang, J.; Peng, Z.; Liu, A.; Zhang, F.; Hou, L. Dual Function of UV/Ozone Plasma-Treated Polymer in Polymer/Metal Hybrid Electrodes and Semitransparent Polymer Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 44656–44666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhao, W.; Liu, J.; Niu, J.; Liu, Y.; Ren, X.; Feng, J.; Liu, Z.; Sun, J.; Wang, D.; et al. CO2 Plasma-Treated TiO2 Film as an Effective Electron Transport Layer for High-Performance Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 33989–33996. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.T.; Weinberger, C.; Qudsia, S.; Rosqvist, E.; Sandberg, O.J.; Nyman, M.; Sandén, S.; Vivo, P.; Aitola, K.; Lund, P.D.; et al. Influence of titanium dioxide surface activation on the performance of mesoscopic perovskite solar cells. Thin Solid Films 2019, 686, 137418. [Google Scholar] [CrossRef]
- Černák, M.; Kováčik, D.; Ráhel’, J.; St’ahel, P.; Zahoranová, A.; Kubincová, J.; Tóth, A.; Černáková, L. Generation of a high-density highly non-equilibrium air plasma for high-speed large-area flat surface processing. Plasma Phys. Control. Fusion 2011, 53, 124031. [Google Scholar] [CrossRef]
- Medvecká, V.; Kováčik, D.; Zahoranová, A.; Černák, M. Atmospheric pressure plasma assisted calcination by the preparation of TiO2 fibers in submicron scale. Appl. Surf. Sci. 2018, 428, 609–615. [Google Scholar] [CrossRef]
- Mudra, E.; Streckova, M.; Pavlinak, D.; Medvecka, V.; Kovacik, D.; Kovalcikova, A.; Zubko, P.; Girman, V.; Dankova, Z.; Koval, V.; et al. Development of Al2O3 electrospun fibers prepared by conventional sintering method or plasma assisted surface calcination. Appl. Surf. Sci. 2017, 415, 90–98. [Google Scholar] [CrossRef]
- Kováčik, D.; Medvecká, V.; Tucekova, Z.; Zahoranova, A.; Cernak, M. Atmospheric pressure plasma assisted calcination of composite submicron fibers. Eur. Phys. J. Appl. Phys. 2016, 75, 24715. [Google Scholar]
- Medvecká, V.; Kováčik, D.; Zahoranová, A.; Stupavská, M.; Černák, M. Atmospheric pressure plasma assisted calcination of organometallic fibers. Mater. Lett. 2016, 162, 79–82. [Google Scholar] [CrossRef]
- Shekargoftar, M.; Dzik, P.; Stupavska, M.; Pavlinak, D.; Homola, T. Mineralization of flexible mesoporous TiO2 photoanodes using two low-temperature DBDs in ambient air. Contrib. Plasma Phys. 2018, 59, 102–110. [Google Scholar] [CrossRef]
- Homola, T.; Shekargoftar, M.; Dzik, P.; Krumpolec, R.; Ďurašová, Z.; Veselý, M.; Černák, M. Low-temperature (70 °C) ambient air plasma-fabrication of inkjet-printed mesoporous TiO2 flexible photoanodes. Flex. Print. Electron. 2017, 2, 35010. [Google Scholar] [CrossRef]
- Homola, T.; Pospišil, J.; Krumpolec, R.; Souček, P.; Dzik, P.; Weiter, M.; Černák, M. Atmospheric Dry Hydrogen Plasma Reduction of Inkjet-Printed Flexible Graphene Oxide Electrodes. ChemSusChem 2018, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chang, J.; Liu, Z.; Sun, X.; Lin, Z.; Chen, D.; Zhang, C.; Zhang, J.; Hao, Y. Enhanced planar perovskite solar cell efficiency and stability using a perovskite/PCBM heterojunction formed in one step. Nanoscale 2018, 10, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Adhikari, N.; Mabrouk, S.; Wu, F.; Chen, K.; Yang, S.; Qiao, Q. A strategic review on processing routes towards highly efficient perovskite solar cells. J. Mater. Chem. A 2018, 6, 2406–2431. [Google Scholar] [CrossRef]
- Lu, J.G.; Fujita, S.; Kawaharamura, T.; Nishinaka, H.; Kamada, Y.; Ohshima, T.; Ye, Z.Z.; Zeng, Y.J.; Zhang, Y.Z.; Zhu, L.P.; et al. Carrier concentration dependence of band gap shift in n-type ZnO:Al films. J. Appl. Phys. 2007, 101, 083705. [Google Scholar] [CrossRef]
- Shekargoftar, M.; Krumpolec, R.; Homola, T. Enhancement of electrical properties of flexible ITO/PET by atmospheric pressure roll-to-roll plasma. Mater. Sci. Semicond. Process. 2018, 75, 95–102. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Ma, Z.; Huang, J.; Ren, F. Low-cost synthesis, fluorescent properties, growth mechanism and structure of CH3NH3PbI3 with millimeter grains. Optik 2017, 142, 293–300. [Google Scholar] [CrossRef]
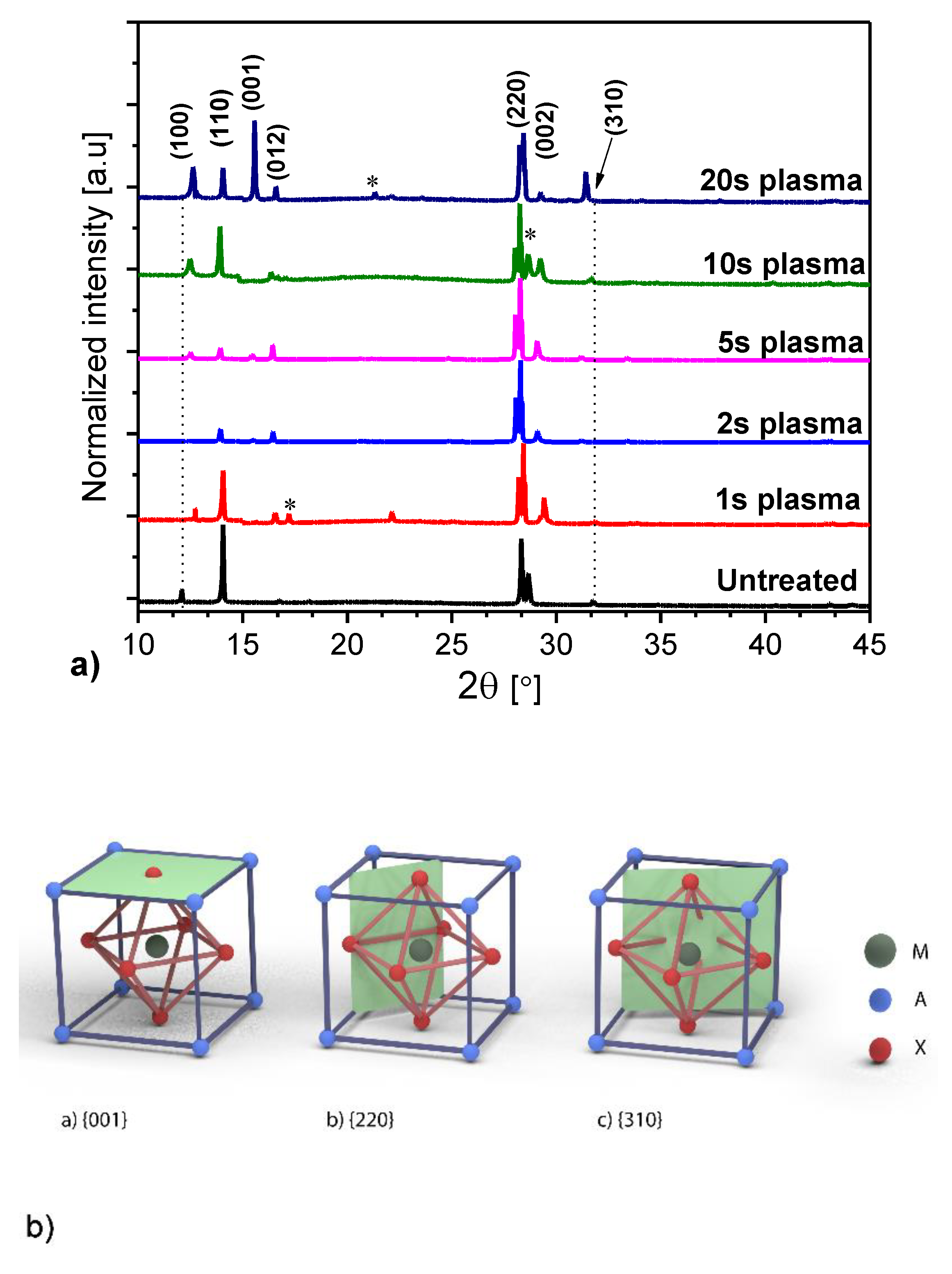
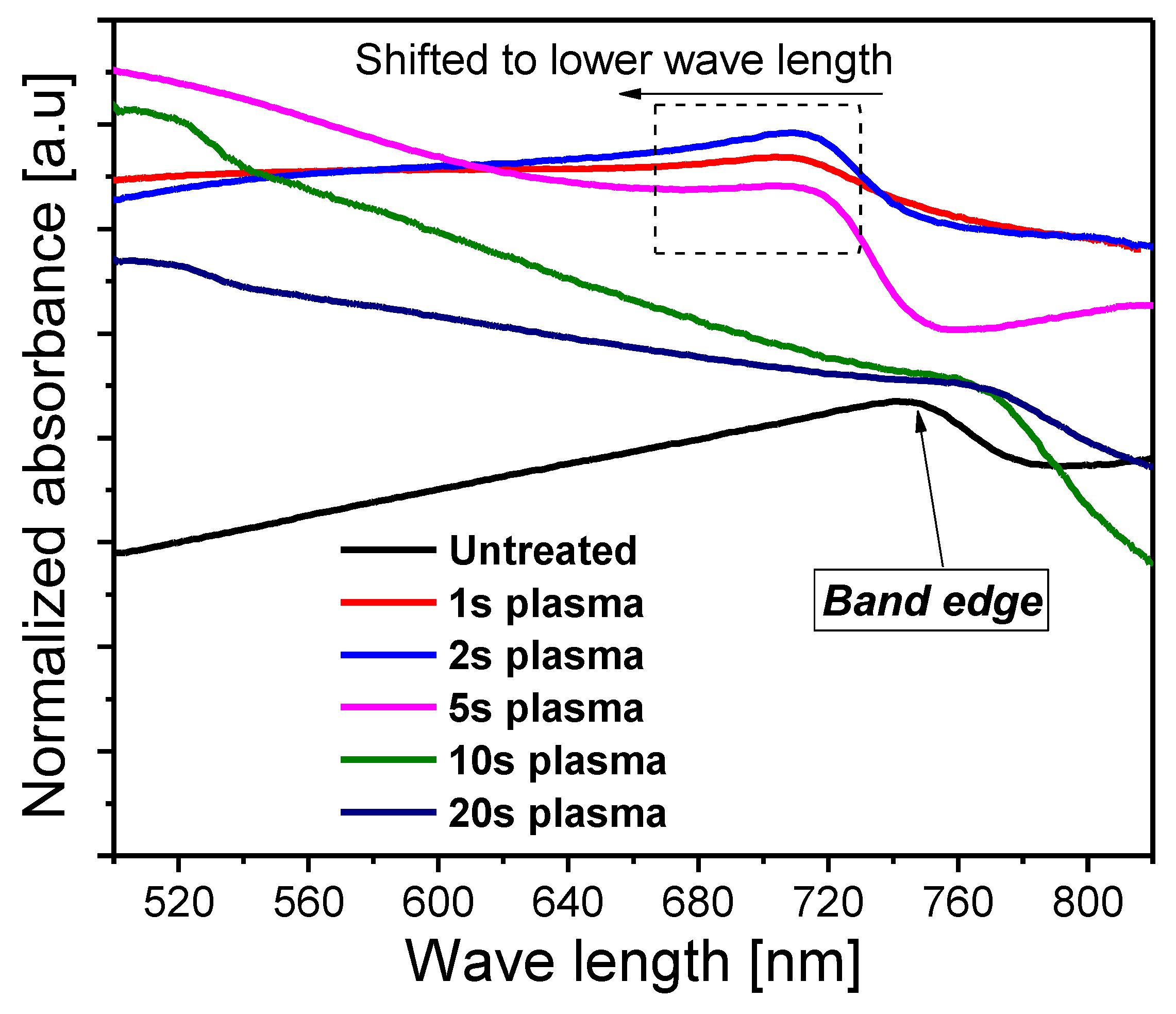
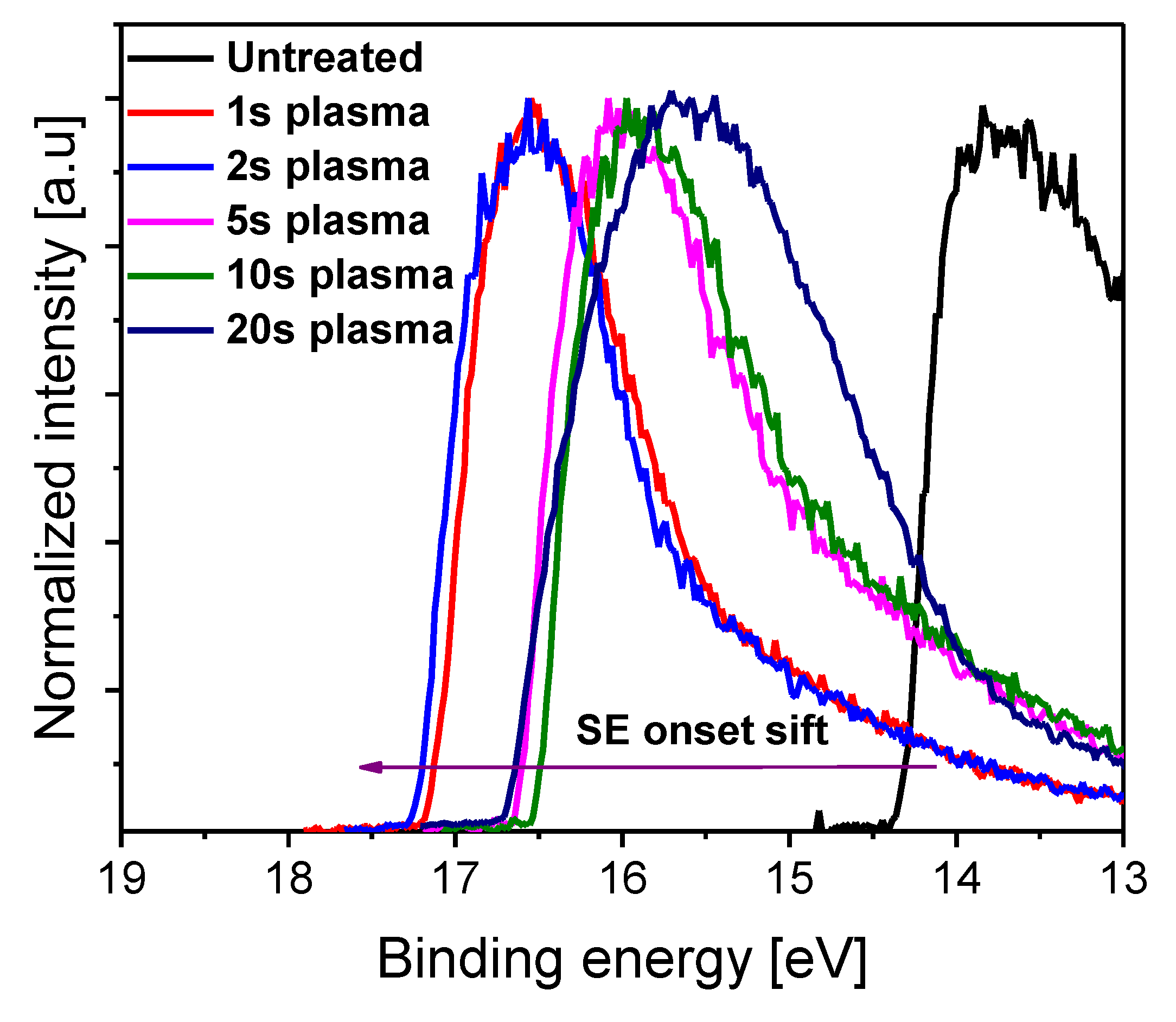


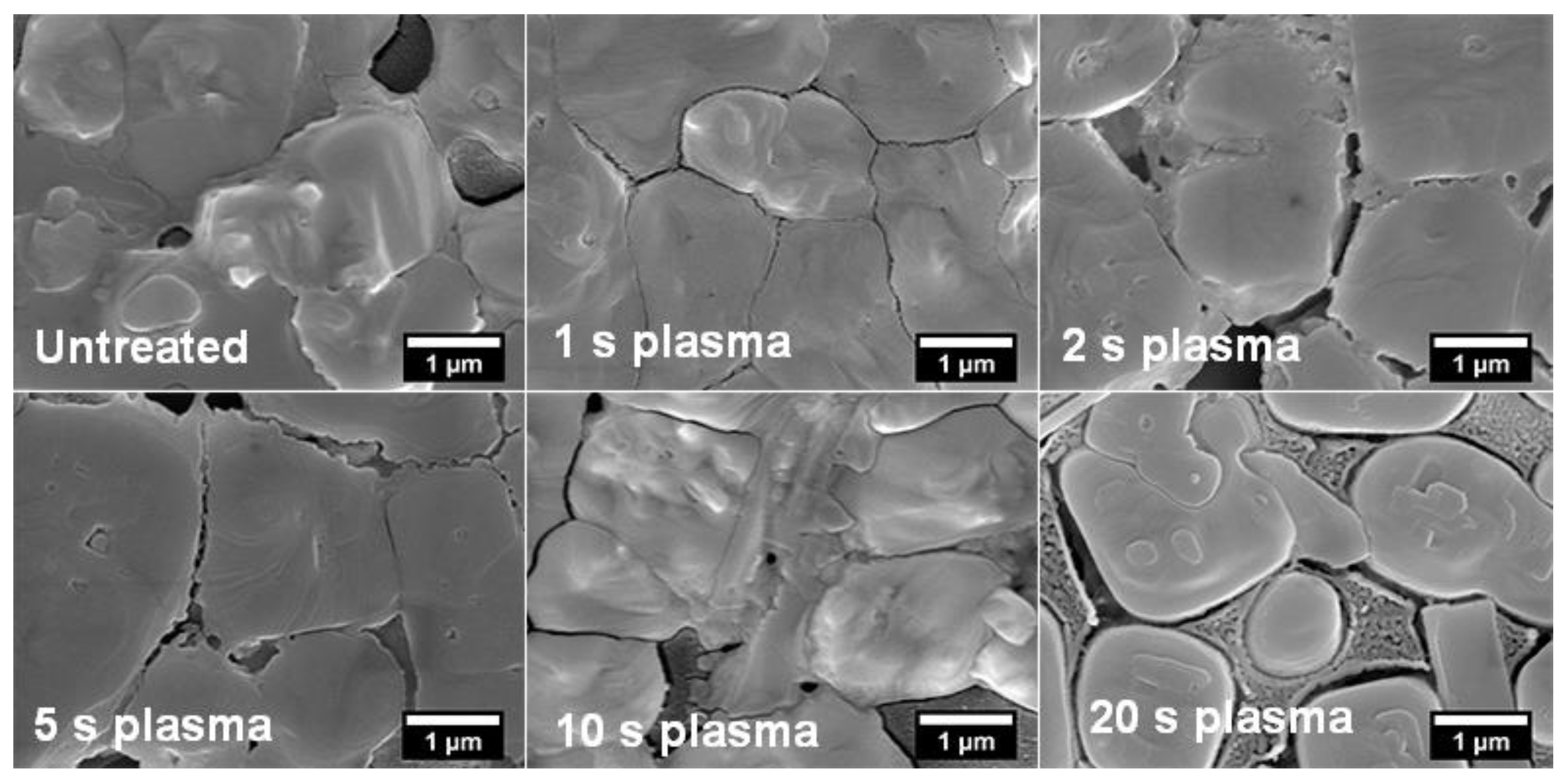
| Sample | Grain Size (µm) |
|---|---|
| Untreated | 0.92 |
| 1 s plasma | 1.36 |
| 2 s plasma | 1.81 |
| 5 s plasma | 1.93 |
| 10 s plasma | 0.78 |
| 20 s plasma | 0.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekargoftar, M.; Jurmanová, J.; Homola, T. A Study on the Effect of Ambient Air Plasma Treatment on the Properties of Methylammonium Lead Halide Perovskite Films. Metals 2019, 9, 991. https://doi.org/10.3390/met9090991
Shekargoftar M, Jurmanová J, Homola T. A Study on the Effect of Ambient Air Plasma Treatment on the Properties of Methylammonium Lead Halide Perovskite Films. Metals. 2019; 9(9):991. https://doi.org/10.3390/met9090991
Chicago/Turabian StyleShekargoftar, Masoud, Jana Jurmanová, and Tomáš Homola. 2019. "A Study on the Effect of Ambient Air Plasma Treatment on the Properties of Methylammonium Lead Halide Perovskite Films" Metals 9, no. 9: 991. https://doi.org/10.3390/met9090991





