Improvement in the Biological Properties of Titanium Surfaces with Low-Temperature Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium Disk Preparation
2.2. Low-Temperature Plasma Treatment
2.3. Scanning Electron Microscopic Analysis of Surfaces
2.4. Energy Dispersive Spectrometry
2.5. X-Ray Photoelectron Spectroscopy
2.6. Examination of Surface Wettability
2.7. Examination of Surface Roughness
2.8. Cell Viability
2.9. Porphyromonas gingivalis Culture
2.10. Quantification of Substrate and Products
2.11. Bacteria Counting Analysis
2.12. Statistical Analyses
3. Results
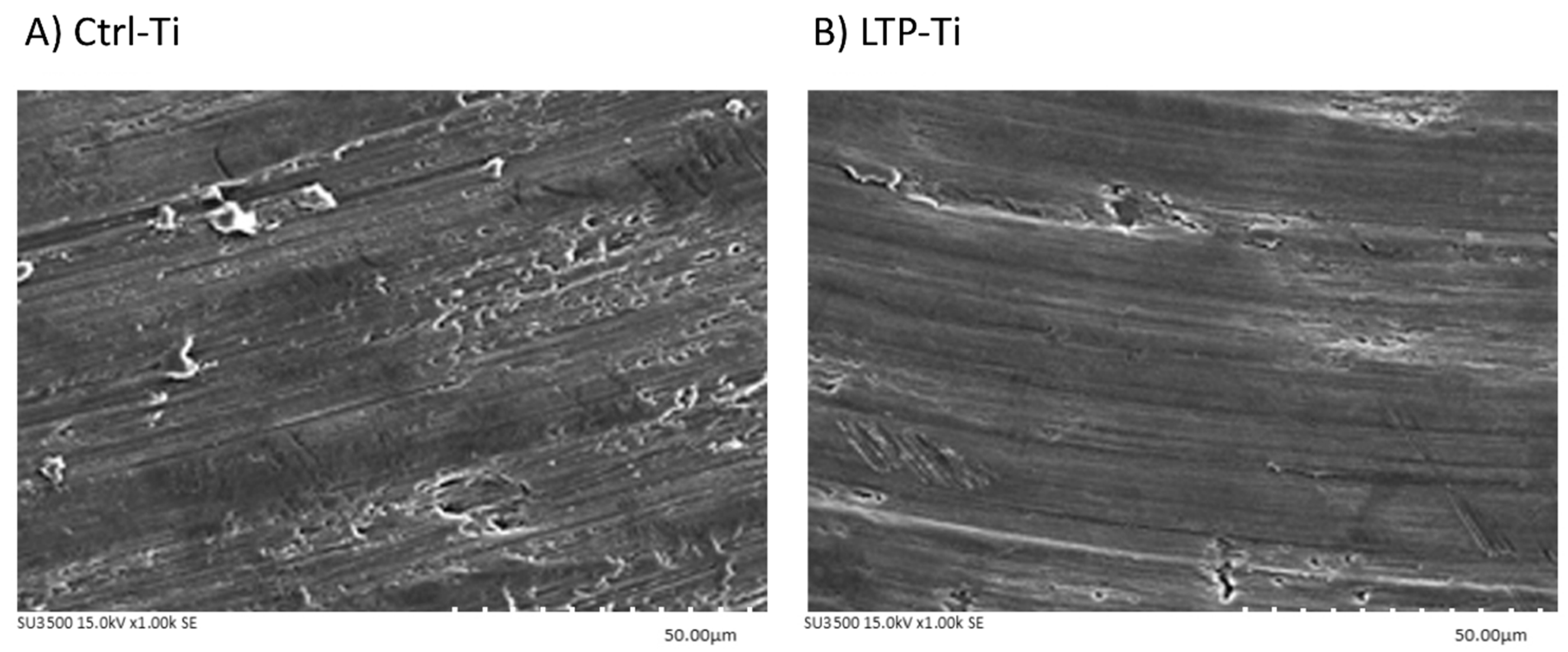
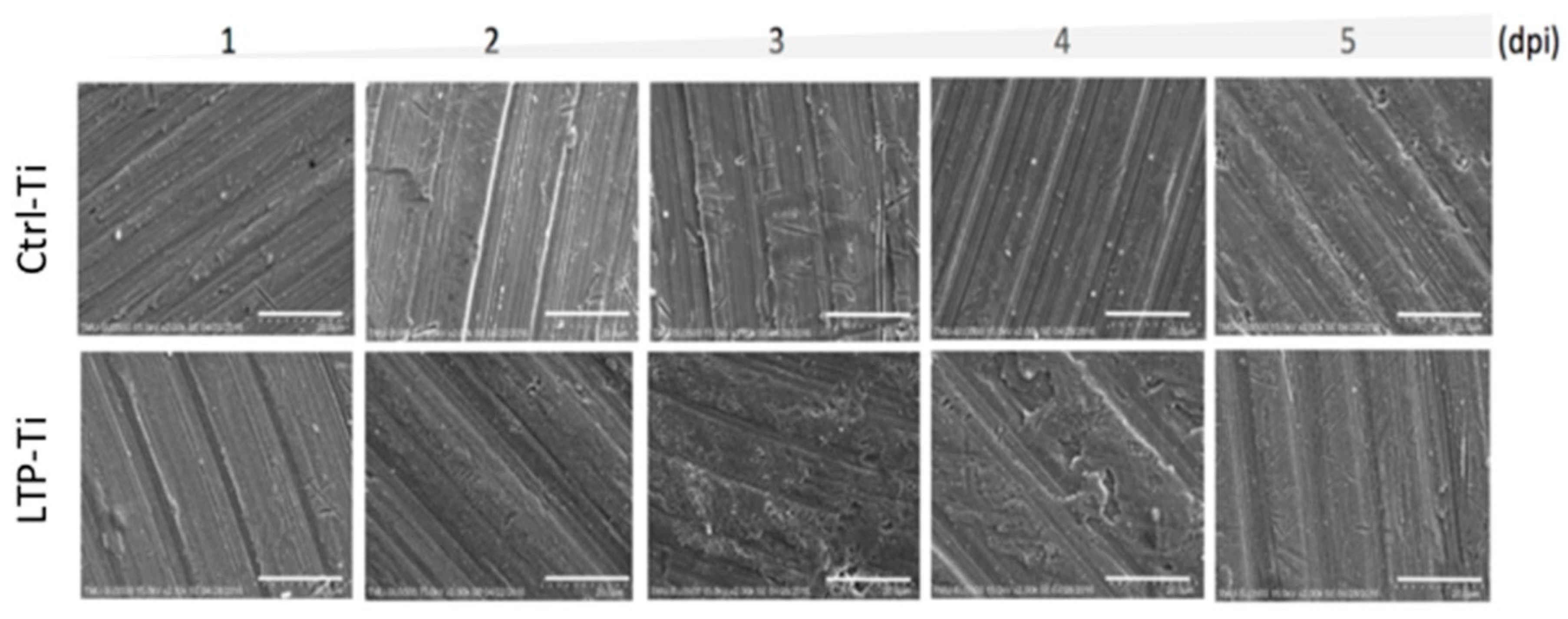
3.1. Surfaces Electron Microscopic Analysis
3.2. Energy Dispersive Spectrometry
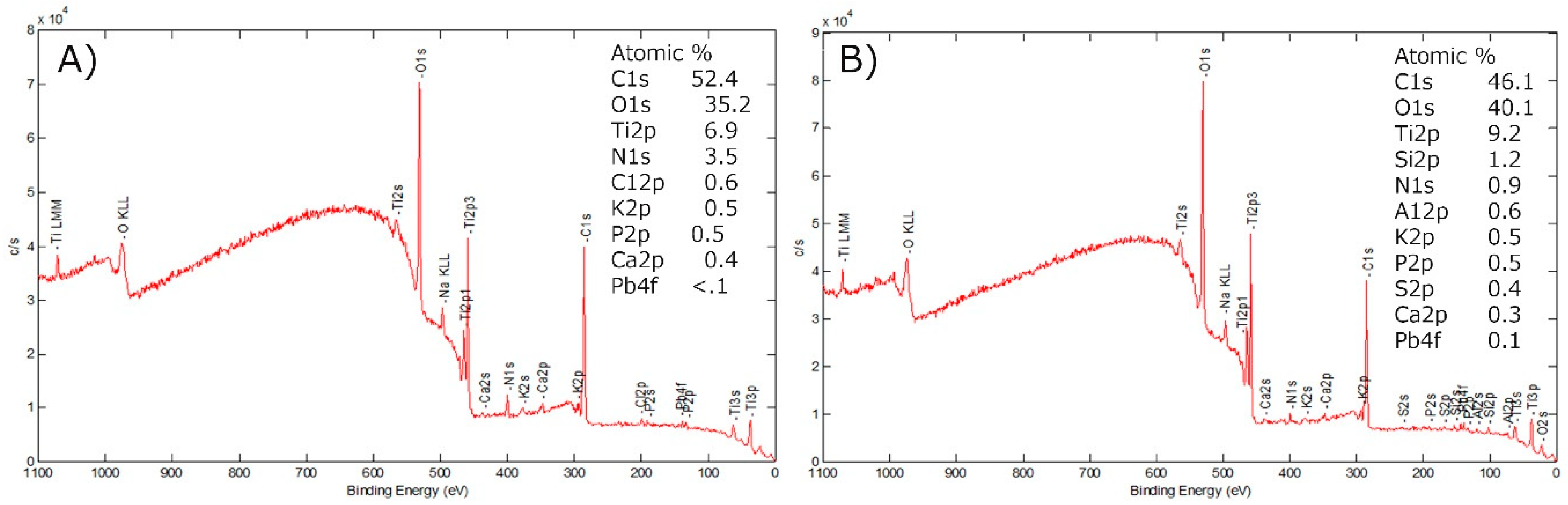
3.3. X-ray Photoelectron Spectroscopy
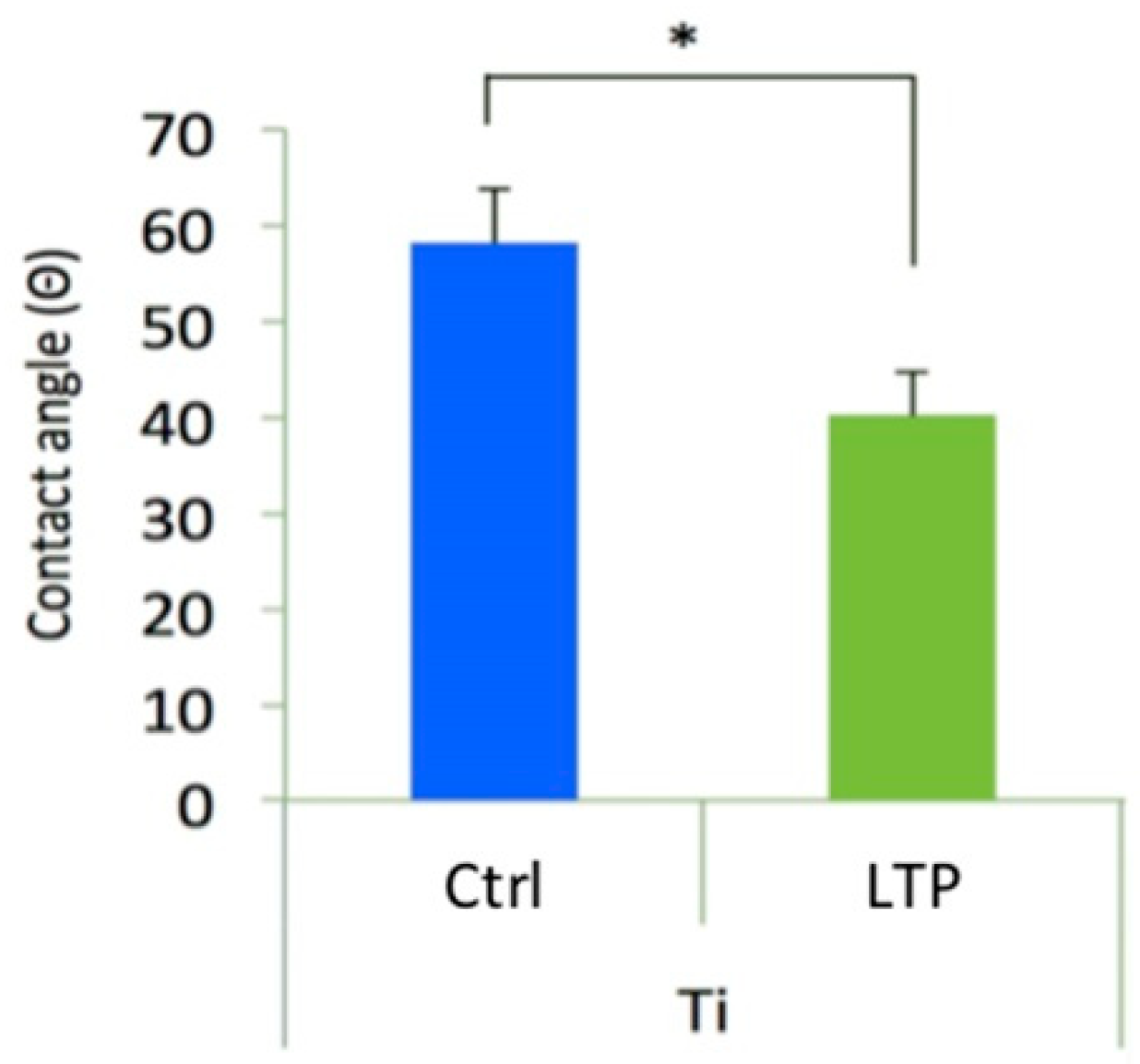
3.4. Examination of Surface Wettability
3.5. Surface Roughness Examination
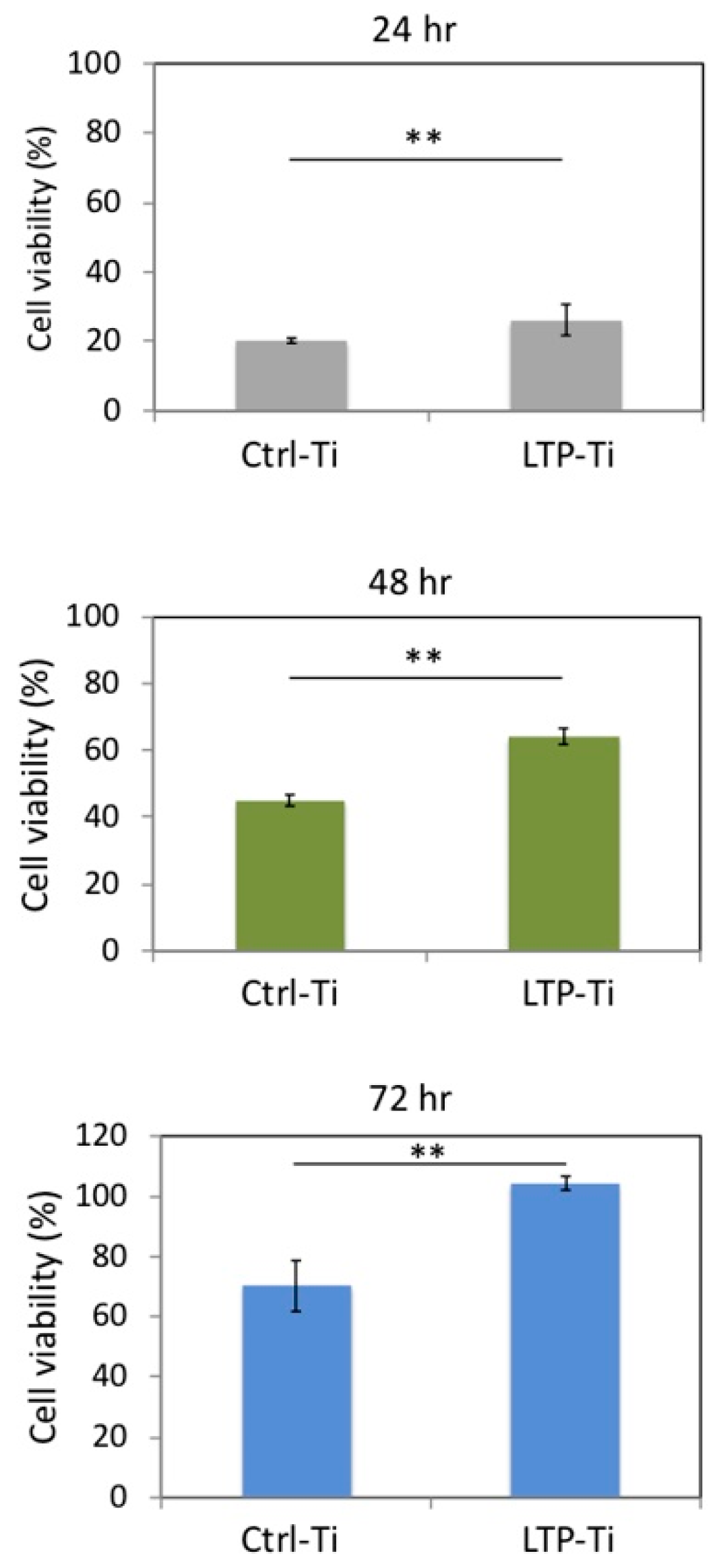
3.6. Cell Viability
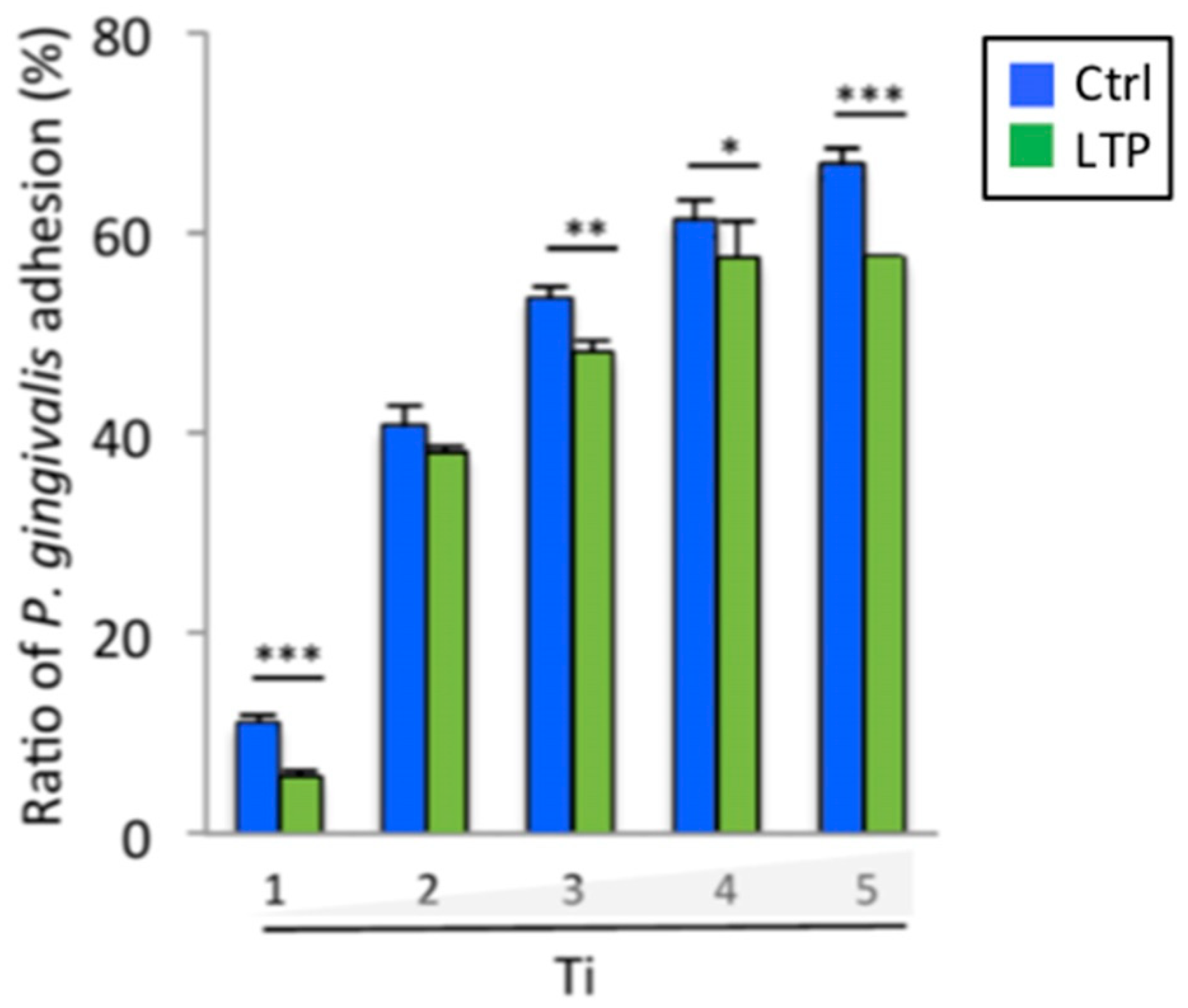
3.7. Porphyromonas Gingivalis Adhesion and Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adell, R.; Lekholm, U.; Rockler, B.; Branemark, P.-I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Mengel, R.; Behle, M.; Flores-De-Jacoby, L. Osseointegrated Implants in Subjects Treated for Generalized Aggressive Periodontitis: 10-Year Results of a Prospective, Long-Term Cohort Study. J. Periodontol. 2007, 78, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.; Lee, N.; Sivathasan, D.; Zhu, J.; Polster, A.; Darby, I.; Cheung, M.C.; Kao, P.; Vong, C.W. Interest in dental implantology and preferences for implant therapy: A survey of Victorian dentists. Aust. Dent. J. 2016, 61, 455–463. [Google Scholar]
- Branemark, P.-I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkvist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial Adhesion on Commercially Pure Titanium and Zirconium Oxide Disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Renvert, S.; Dahlén, G. Microbial findings at failing implants. Clin. Oral Implant. Res. 1999, 10, 339–345. [Google Scholar] [CrossRef]
- Hultin, M.; Gustafsson, A.; Hallström, H.; Johansson, L.-A.; Ekfeldt, A.; Klinge, B. Microbiological findings and host response in patients with peri-implantitis. Clin. Oral Implant. Res. 2002, 13, 349–358. [Google Scholar] [CrossRef]
- Mombelli, A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontology 2000 2000, 28, 177–189. [Google Scholar] [CrossRef]
- Tabanella, G.; Nowzari, H.; Slots, J. Clinical and Microbiological Determinants of Ailing Dental Implants. Clin. Implant. Dent. Relat. Res. 2009, 11, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Casado, P.L.; Otazu, I.B.; Balduino, A.; De Mello, W.; Barboza, E.P.; Duarte, M.E.L. Identification of Periodontal Pathogens in Healthy Periimplant Sites. Implant. Dent. 2011, 20, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of peri-implantitis. Periodontology 2000 1998, 17, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.M.; Knight, E.T.; Russell, A.A.; Tawse-Smith, A.; Leichter, J.W. Peri-implant disease: Current understanding and future direction. N. Z. Dent. J. 2013, 109, 55–62. [Google Scholar] [PubMed]
- Kotsakis, G.A.; Konstantinidis, I.; Karoussis, I.K.; Ma, X.; Chu, H. Systematic Review and Meta-Analysis of the Effect of Various Laser Wavelengths in the Treatment of Peri-Implantitis. J. Periodontol. 2014, 85, 1203–1213. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Zou, D.; Wang, G.; Kaigler, D. Clinical outcomes of dental implant therapy in alveolar cleft patients: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 1098–1105. [Google Scholar] [CrossRef]
- Romanos, G.E.; Javed, F.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L. Peri-implant diseases: A review of treatment interventions. Dent. Clin. N. Am. 2015, 59, 157–178. [Google Scholar] [CrossRef]
- Elemek, E.; Almas, K. Peri-implantitis: Etiology, diagnosis and treatment: An update. N. Y. State Dent. J. 2014, 80, 26–32. [Google Scholar]
- Malek, R.; Fisher, J.G.; Caleca, A.; Stinson, M.; Van Oss, C.J.; Lee, J.Y.; Cho, M.I.; Genco, R.J.; Evans, R.T.; Dyer, D.W. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J. Bacteriol. 1994, 176, 1052–1059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kato, I.; Vasquez, A.A.; Moyerbrailean, G.; Land, S.; Sun, J.; Lin, H.-S.; Ram, J.L. Oral microbiome and history of smoking and colorectal cancer. J. Epidemiol. Res. 2016, 2, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Tribble, G.D.; Kerr, J.E.; Wang, B.-Y. Genetic diversity in the oral pathogen Porphyromonas gingivalis: Molecular mechanisms and biological consequences. Futur. Microbiol. 2013, 8, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Martin, C.; Sanz, M. Biological complications and peri-implant clinical and radiographic changes at immediately placed dental implants. A prospective 5-year cohort study. Clin. Oral Implant. Res. 2012, 23, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological complications with dental implants: Their prevention, diagnosis and treatment. Clin. Oral Implant. Res. 2000, 11, 146–155. [Google Scholar] [CrossRef]
- Meschenmoser, A.; D’Hoedt, B.; Meyle, J.; Elßner, G.; Korn, D.; Hämmerle, H.; Schulte, W.; Meyle, J. Effects of Various Hygiene Procedures on the Surface Characteristics of Titanium Abutments. J. Periodontol. 1996, 67, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Placko, H.E.; Mishra, S.; Weimer, J.J.; Lucas, L.C. Surface characterization of titanium-based implant materials. Int. J. Oral Maxillofac. Implant. 2000, 15, 355–363. [Google Scholar]
- Webb, K.; Caldwell, K.; Tresco, P.A. Fibronectin Immobilized by a Novel Surface Treatment Regulates Fibroblast Attachment and Spreading. Crit. Rev. Biomed. Eng. 2000, 28, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Suggested guidelines for the topographic evaluation of implant surfaces. Int. J. Oral Maxillofac. Implant. 2000, 15, 331–344. [Google Scholar]
- Mustafa, K.; Wroblewski, J.; Lopez, B.S.; Wennerberg, A.; Hultenby, K.; Arvidson, K. Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin. Oral Implant. Res. 2001, 12, 515–525. [Google Scholar] [CrossRef]
- Kawashima, H.; Sato, S.; Kishida, M.; Yagi, H.; Matsumoto, K.; Ito, K. Treatment of Titanium Dental Implants with Three Piezoelectric Ultrasonic Scalers: An In Vivo Study. J. Periodontol. 2007, 78, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Lee, W.-F.; Feng, S.-W.; Huang, H.-M.; Lin, C.-T.; Teng, N.-C.; Chang, W.J. In Vitro Analysis of Fibronectin-Modified Titanium Surfaces. PLoS ONE 2016, 11, e0146219. [Google Scholar] [CrossRef] [PubMed]
- Bürgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, M.A.; Han, G.J.; Cho, B.H. Plasma in dentistry: A review of basic concepts and applications in dentistry. Acta Odontol. Scand. 2014, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hesby, R.M.; Haganman, C.R.; Stanford, C.M. Effects of radiofrequency glow discharge on impression material surface wettability. J. Prosthet. Dent. 1997, 77, 414–422. [Google Scholar] [CrossRef]
- Özden, N.; Akaltan, F.; Suzer, S.; Akovali, G.; Akovalı, G. Time-related wettability characteristic of acrylic resin surfaces treated by glow discharge. J. Prosthet. Dent. 1999, 82, 680–684. [Google Scholar] [CrossRef]
- Shibata, Y.; Hosaka, M.; Kawai, H.; Miyazaki, T. Glow discharge plasma treatment of titanium plates enhances adhesion of osteoblast-like cells to the plates through the integrin-mediated mechanism. Int. J. Oral Maxillofac. Implant. 2002, 17, 771–777. [Google Scholar]
- Kawai, H.; Shibata, Y.; Miyazaki, T. Glow discharge plasma pretreatment enhances osteoclast differentiation and survival on titanium plates. Biomaterials 2004, 25, 1805–1811. [Google Scholar] [CrossRef]
- Chang, Y.C.; Feng, S.W.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Lin, H.K.; Wang, P.D.; Chang, W.J. Surface analysis of titanium biological modification with glow discharge. Clin. Implant Dent. Relat. Res. 2015, 17, 469–475. [Google Scholar] [CrossRef]
- Chang, Y.C.; Ho, K.N.; Feng, S.W.; Huang, H.M.; Chang, C.H.; Lin, C.T.; Teng, N.C.; Pan, Y.H.; Chang, W.J. Fibronectin-Grafted Titanium Dental Implants: An In Vivo Study. BioMed Res. Int. 2016, 2016, 1–11. [Google Scholar]
- Annunziata, M.; Canullo, L.; Donnarumma, G.; Caputo, P.; Nastri, L.; Guida, L. Bacterial inactivation/sterilization by argon plasma treatment on contaminated titanium implant surfaces: In vitro study. Medicina Oral Patología Oral y Cirugia Bucal 2016, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Wang, H.-L.; Carossa, S.; Mussano, F. Plasma of Argon Increases Cell Attachment and Bacterial Decontamination on Different Implant Surfaces. Int. J. Oral Maxillofac. Implant. 2017, 32, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Shon, W.J.; Chung, S.H.; Kim, H.K.; Han, G.J.; Cho, B.H.; Park, Y.S. Peri-implant bone formation of non-thermal atmospheric pressure plasma–treated zirconia implants with different surface roughness in rabbit tibiae. Clin. Oral Implant. Res. 2014, 25, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R.F.A. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. Part A 2012, 100, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. Part A 2013, 101, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Shard, A.G. Quantitative Analysis of Adsorbed Proteins by X-ray Photoelectron Spectroscopy. Anal. Chem. 2011, 83, 8659–8666. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Pan, Y.-H.; Tsai, A.I.; Lin, P.-Y.; Lin, C.-K.; Huang, H.-M.; Teng, N.-C.; Wang, P.D.; Chang, W.-J. Enhancement of Osteoblastic-Like Cell Activity by Glow Discharge Plasma Surface Modified Hydroxyapatite/β-Tricalcium Phosphate Bone Substitute. Materials 2017, 10, 1347. [Google Scholar] [CrossRef]
- Batsukh, N.; Feng, S.W.; Lee, W.F.; Leu, S.-J.; Tsai, P.-Y.; Ho, K.-N.; Lin, C.T.; Su, C.-H.; Chang, W.-J. Effects of Porphyromonas gingivalis on Titanium Surface by Different Clinical Treatment. J. Med. Biol. Eng. 2017, 37, 35–44. [Google Scholar] [CrossRef]
- Erbektas, A.R.; Isgor, O.B.; Weiss, W.J. An accelerated testing protocol for assessing microbially induced concrete deterioration during the bacterial attachment phase. Cem. Concr. Compos. 2019, 104, 103339. [Google Scholar] [CrossRef]
- Koch, A.L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal. Biochem. 1970, 38, 252–259. [Google Scholar] [CrossRef]
- Aronsson, B.; Lausmaa, J.; Kasemo, B. Glow discharge plasma treatment for surface cleaning and modification of metallic biomaterials. J. Biomed. Mater. Res. 1997, 35, 49–73. [Google Scholar] [CrossRef]
- Socransky, S.; Haffajee, A.; Cugini, M.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-M.; Hsieh, S.-C.; Teng, N.-C.; Feng, S.-W.; Ou, K.-L.; Chang, W.-J. Biological surface modification of titanium surfaces using glow discharge plasma. Med. Biol. Eng. 2011, 49, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Cheng, H.-C.; Huang, C.-F.; Lin, C.-T.; Lee, S.-Y.; Chen, C.-S.; Ou, K.-L. Enhancement of Biocompatibility on Bioactive Titanium Surface by Low-Temperature Plasma Treatment. Jpn. J. Appl. Phys. 2005, 44, 8590–8598. [Google Scholar] [CrossRef]
- Matos, A.O.; Ricomini-Filho, A.P.; Beline, T.; Ogawa, E.S.; Oliveira, B.E.C.; De Almeida, A.B.; Junior, F.H.N.; Rangel, E.C.; Da Cruz, N.C.; Sukotjo, C.; et al. Three-species biofilm model onto plasma-treated titanium implant surface. Colloids Surf. B 2017, 152, 354–366. [Google Scholar] [CrossRef]

| Treatment | Component | ||
|---|---|---|---|
| Ti | O | C | |
| Crtl-Ti | 93.62 | 4.70 | 2.83 |
| LTP-Ti | 92.80 | 5.70 | 1.9 |
| p-value | 0.302 | 0.131 | * 0.038 |
| Treatment | Culturing Days after P. gingivalis Cultured | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Crtl-Ti | 63.10 cfu/cm2 | 1584.90 cfu/cm2 | 1,258,925.41 cfu/cm2 | 15,848,931.92 cfu/cm2 | 25,118,864.32 cfu/cm2 |
| LTP-Ti | 19.95 cfu/cm2 | 91.20 cfu/cm2 | 316,227.77 cfu/cm2 | 794,328.23 cfu/cm2 | 1,584,893.19 cfu/cm2 |
| p-value | *** p < 0.001 | *** p < 0.001 | * p < 0.05 | *** p < 0.001 | * p < 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.-H.; Yao, W.-L.; Lin, J.C.Y.; Salamanca, E.; Tsai, P.-Y.; Leu, S.-J.; Yang, K.-C.; Huang, H.-M.; Teng, N.C.; Chang, W.-J. Improvement in the Biological Properties of Titanium Surfaces with Low-Temperature Plasma. Metals 2019, 9, 943. https://doi.org/10.3390/met9090943
Pan Y-H, Yao W-L, Lin JCY, Salamanca E, Tsai P-Y, Leu S-J, Yang K-C, Huang H-M, Teng NC, Chang W-J. Improvement in the Biological Properties of Titanium Surfaces with Low-Temperature Plasma. Metals. 2019; 9(9):943. https://doi.org/10.3390/met9090943
Chicago/Turabian StylePan, Yu-Hwa, Wan-Ling Yao, Jerry Chin Yi Lin, Eisner Salamanca, Pei-Yo Tsai, Sy-Jye Leu, Kai-Chiang Yang, Haw-Ming Huang, Nai Chia Teng, and Wei-Jen Chang. 2019. "Improvement in the Biological Properties of Titanium Surfaces with Low-Temperature Plasma" Metals 9, no. 9: 943. https://doi.org/10.3390/met9090943
APA StylePan, Y.-H., Yao, W.-L., Lin, J. C. Y., Salamanca, E., Tsai, P.-Y., Leu, S.-J., Yang, K.-C., Huang, H.-M., Teng, N. C., & Chang, W.-J. (2019). Improvement in the Biological Properties of Titanium Surfaces with Low-Temperature Plasma. Metals, 9(9), 943. https://doi.org/10.3390/met9090943







