Continuous Cooling Transformation Diagram, Microstructures, and Properties of the Simulated Coarse-Grain Heat-Affected Zone in a Low-Carbon Bainite E550 Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
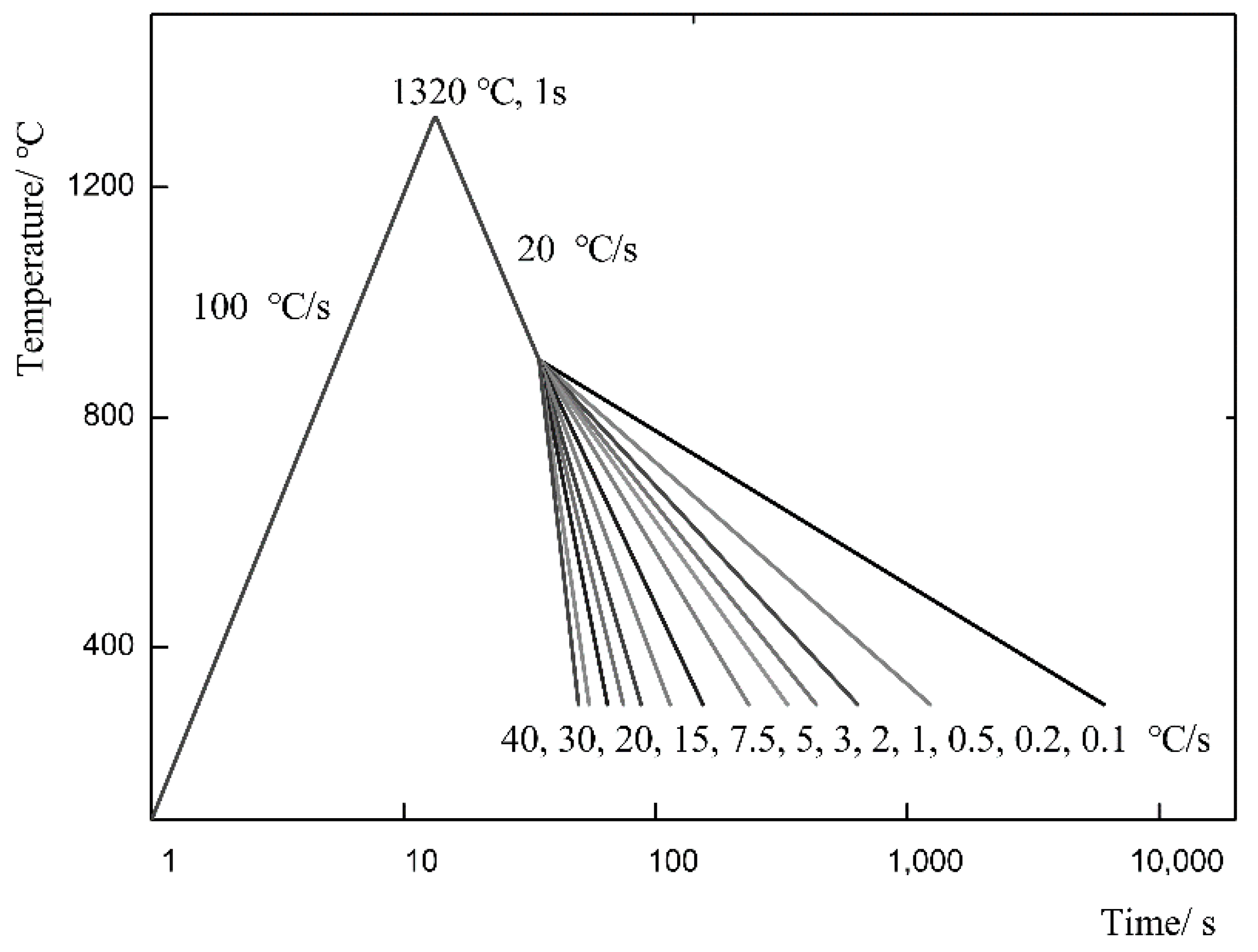
2.2. Experimental Method
3. Results
3.1. Comparison of Austenitic Transformation Temperature of Test Steel under Different Heating Rate
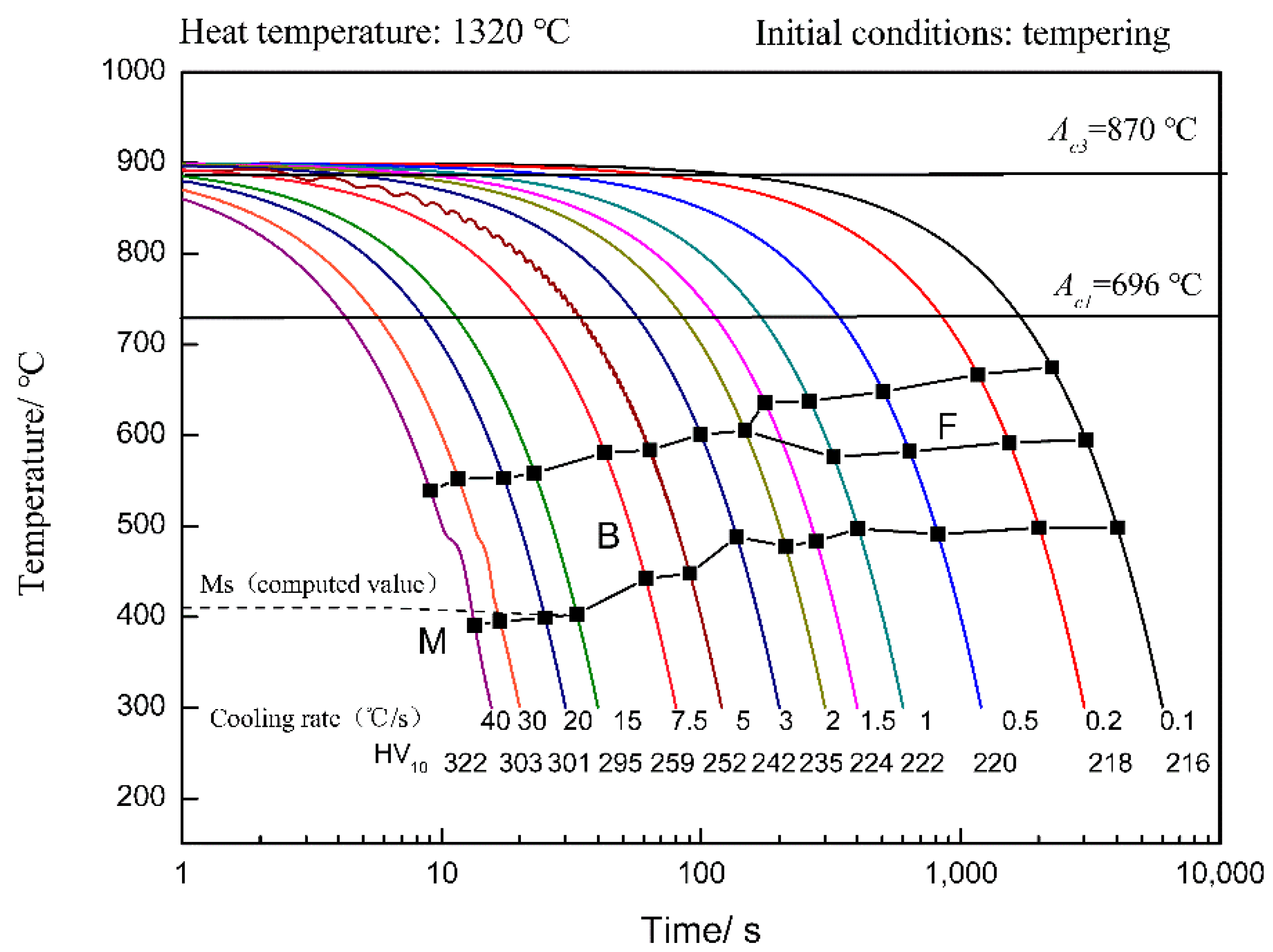
3.2. SH-CCT Diagram
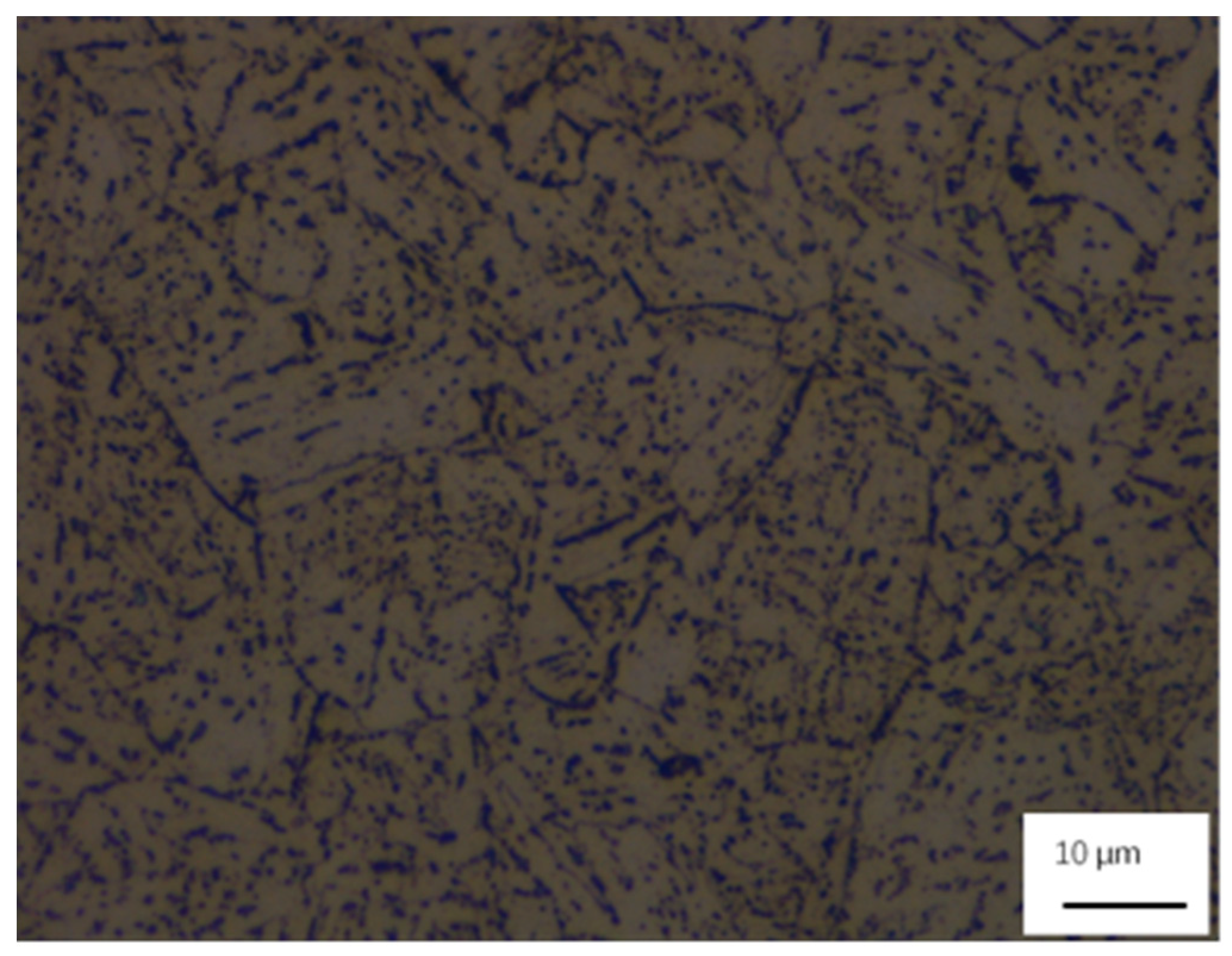
3.3. Microstructure in CGHAZ
3.4. Hardness and −40 °C Impact Toughness of CGHAZ
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.G.; Yang, C.F.; Shang, C.J. New Development of HSLA Steels in China. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015, Hang Zhou, China, 2015; The Chinese Society for Metals (CSM), Chinese Academy of Engineering (CAE), Eds.; Springer Nature: Basel, Switzerland, 2016; pp. 3–15. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Chen, J.; Liu, Z.Y. Corrosion Behavior of Rusted 550 MPa Grade Offshore Platform Steel. J. Iron Steel Res. Int. 2013, 20, 66–73. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Yang, S.W.; Shang, C.J.; Wang, X.M.; Liu, W.; He, X.L. The mechanical properties and corrosion behaviors of ultra-low carbon microalloying steel. Mater. Sci. Eng. A 2007, 454, 695–700. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, P.H.; Guan, Y.; Chen, Q.F.; Pu, S.K. The corrosion resistance of ultra-low carbon bainitic steel. Corros. Sci. 2009, 51, 954–961. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, L.B.; Qiu, C.J.; Wang, Z.C. Effects of Carbon and Boron on Structure and Properties of Austenitic Stainless Steel Coatings Fabricated by Laser Remanufacturing. Steel Res. Int. 2019, 90, 1800473. [Google Scholar] [CrossRef]
- Cizek, P.; Wynne, B.P.; Davies, C.H.J.; Hodgson, P.D. The Effect of Simulated Thermomechanical Processing on the Transformation Behavior and Microstructure of a Low-Carbon Mo-Nb Linepipe Steel. Metal. Mater. Trans. A 2015, 46, 407–425. [Google Scholar] [CrossRef]
- He, X.L.; Shang, C.J.; Yang, S.W. High Performance Low-Carbon Bainite Steel: Composition, Process, Organization, Performance and Application (Chinese); Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Chen, Y.L.; Chen, L.Q.; Zhou, X.J.; Zhao, Y.; Zha, X.W.; Zhu, F.C. Effect of Continuous Cooling Rate on Transformation Characteristic in Microalloyed Low Carbon Bainite Cryogenic Pressure Vessel Steel. Trans. Indian Inst. Met. 2016, 69, 817–821. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Misra, S.; Mondal, K. Corrosion Behavior of Annealed Steels with Different Carbon Contents (0.002, 0.17, 0.43 and 0.7% C) in Freely Aerated 3.5% NaCl Solution. J. Mater. Eng. Perform. 2019, 28, 4041–4052. [Google Scholar] [CrossRef]
- Sun, F.; Li, X.; Cheng, X. Effects of Carbon Content and Microstructure on Corrosion Property of New Developed Steels in Acidic Salt Solutions. Acta Met. Sin. (Engl. Lett.) 2014, 27, 115–123. [Google Scholar] [CrossRef]
- Nie, Y.; Shang, C.J.; Song, X.; You, Y.; Li, C.; He, X.L. Properties and homogeneity of 550 MPa grade TMCP steel for ship hull. Int. J. Min. Met. Mater. 2010, 17, 179–184. [Google Scholar] [CrossRef]
- Wang, B.; Song, Y.L.; Wen, X.M.; Wang, H.X.; Liu, Q.Y. Effect of Nb, Mo and Ti on the Microstructures and Mechanical Properties of 700MPa Weathering Steel. J. Iron Steel Res. Int. 2011, 18, 300–305. [Google Scholar] [CrossRef]
- He, X.L.; Shang, C.J. Microstructure Fining Theory of Low- carbon Bainitic Steel. In Ultra-Fine Grained Steels; Weng, Y.Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; p. 235. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, H.; Wang, C.; Zhao, J.; Ding, T. Work hardening behaviors of a low carbon Nb-microalloyed Si–Mn quenching–partitioning steel with different cooling styles after partitioning. Mater. Sci. Eng. A 2013, 585, 132–138. [Google Scholar] [CrossRef]
- Wang, B.X.; Dong, F.Z.; Wang, Z.D.; Wang, G.D. Microstructure and Property of Mn-Nb-B Low Carbon Bainite High Strength Steel Under Ultra-fast Cooling. J. Mater. Eng. 2016, 44, 26–33. [Google Scholar] [CrossRef]
- Lan, L.Y.; Qiu, C.L.; Zhao, D.W.; Gao, X.H.; Du, L.X. Effect of reheat temperature on continuous cooling bainite transformation behavior in low carbon microalloyed steel. J. Mater. Sci. 2013, 48, 4356–4364. [Google Scholar] [CrossRef]
- Barbaro, F.; Zhu, Z.X.; Kuzmikova, L.; Li, H.J.; Jian, H. Weld HAZ properties in modern high strength niobium pipeline steels. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015, Hang Zhou, China, 2015; The Chinese Society for Metals (CSM), Chinese Academy of Engineering (CAE), Eds.; Springer Nature: Basel, Switzerland, 2016; p. 453. [Google Scholar] [CrossRef]
- Di, X.J.; Ji, S.X.; Cheng, F.J.; Wang, D.P.; Cao, J. Effect of cooling rate on microstructure, inclusions and mechanical properties of weld metal in simulated local dry underwater welding. Mater. Des. 2015, 88, 505–513. [Google Scholar] [CrossRef]
- Shi, Y.W.; Han, Z.X. Effect of weld thermal cycle on microstructure and fracture toughness of simulated heat-affected zone for an 800 MPa grade high strength low alloy steel. J. Mater. Process. Technol. 2008, 207, 30–39. [Google Scholar] [CrossRef]
- Moeinifar, S.; Kokabi, A.H.; Madaah Hosseini, H.R. Influence of peak temperature during simulation and real thermal cycles on microstructure and fracture properties of the reheated zones. Mater. Des. 2010, 31, 2948–2955. [Google Scholar] [CrossRef]
- Shi, Z.R.; Wang, R.Z.; Wang, Q.F.; Su, H.; Chai, F.; Yang, C.F. Microstructures and continuous cooling transformation of CGHAZ in E36 class V-N-Ti, V-Ti and Nb-Ti shipbuilding steels. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015, Hang Zhou, China, 2015; The Chinese Society for Metals (CSM), Chinese Academy of Engineering (CAE), Eds.; Springer Nature: Basel, Switzerland, 2016; p. 517. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhang, H.Q.; Li, L.F.; Liu, W.M. Effect of Heat Input on Microstructure and toughness of coarse grain heat affected zone in Nb microalloyed HSLA steels. J. Iron Steel Res. Int. 2009, 16, 73–80. [Google Scholar] [CrossRef]
- Fonda, R.W.; Spanos, G. Microstructural evolution in ultra-low-carbon steel weldments—Part I: Controlled thermal cycling and continuous cooling transformation diagram of the weld metal. Met. Mater. Trans. A 2000, 31, 2145–2153. [Google Scholar] [CrossRef]
- Harrison, P.L.; Farrar, R.A. Application of continuous cooling transformation diagrams for welding of steels. Mater. Rev. 1989, 34, 35–51. [Google Scholar] [CrossRef]
- Wang, H.H.; Qin, Z.P.; Wang, X.L.; Wei, R.; Wu, K.M.; Misra, D. Continuous cooling transformation behavior and impact toughness in heat-affected zone of Nb-containing fire-resistant steel. Met. Mater. Int. 2017, 23, 848–854. [Google Scholar] [CrossRef]
- Li, H.; Liang, J.L.; Feng, Y.L.; Huo, D.X. Microstructure transformation of X70 pipeline steel welding heat-affected zone. Rare Met. 2014, 33, 493–498. [Google Scholar] [CrossRef]
- Singh, M.P.; Arora, K.S.; Shajan, N.; Pandu, S.R.; Shome, M.; Kumar, R.; Shukla, D. Comparative analysis of continuous cooling transformation behaviour in CGHAZ of API X-80 and X-65 line pipe steels. J. Therm. Anal. Calorim. 2019, 137, 1155–1167. [Google Scholar] [CrossRef]
- Poorhaydari, K.; Ivey, D.G. Heat-affected zone property diagrams for a grade 100 microalloyed steel. Weld. World 2018, 62, 551–564. [Google Scholar] [CrossRef]
- Hu, M.J.; Wang, P.; Lin, W.P.; Wang, X.Y.; Ji, L.K. SH-CCT of High-Strain Pipeline Steel X80. Adv. Mater. Res. 2012, 472, 1179–1182. [Google Scholar] [CrossRef]
- Liu, Z.C.; Ren, H.P.; Song, Y.Q. Solid Metal Phase Transition Tutorial (Chinese); Metallurgical Industry Press: Beijing, China, 2003; Chapter 2; pp. 46–47. [Google Scholar]
- Wang, G.D.; Shang, C.J.; Liu, Z.Y. Steel for Marine Applications; Chemical Industry Press (Chinese): Beijing, China, 2017; Chapter 3; p. 77. [Google Scholar]
- Lee, Y.K. Empirical formula of isothermal bainite start temperature of steels. J. Mater. Sci. Lett. 2002, 21, 1253–1255. [Google Scholar] [CrossRef]
- Gannon, L.; Yi, L.; Pegg, N.; Smith, M. Effect of welding sequence on residual stress and distortion in flat-bar stiffened plates. Mar. Struct. 2010, 23, 385–404. [Google Scholar] [CrossRef]




| Steel | C | Si | Mn | P | S | Nb | Ni | Cr | Mo | Ti | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E550 | 0.063 | 0.26 | 1.38 | 0.0081 | 0.0016 | 0.044 | 0.65 | 0.40 | 0.22 | 0.017 | 0.35 |
| Heating Rate (°C/S) | Tp1 (°C) | Holding Time (s) | T8/5 (s) |
|---|---|---|---|
| 100 | 1320 | 1 | 5, 10, 15, 30, 60, 100, 150 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, Y.; Liu, C.-M. Continuous Cooling Transformation Diagram, Microstructures, and Properties of the Simulated Coarse-Grain Heat-Affected Zone in a Low-Carbon Bainite E550 Steel. Metals 2019, 9, 939. https://doi.org/10.3390/met9090939
Zong Y, Liu C-M. Continuous Cooling Transformation Diagram, Microstructures, and Properties of the Simulated Coarse-Grain Heat-Affected Zone in a Low-Carbon Bainite E550 Steel. Metals. 2019; 9(9):939. https://doi.org/10.3390/met9090939
Chicago/Turabian StyleZong, Yun, and Chun-Ming Liu. 2019. "Continuous Cooling Transformation Diagram, Microstructures, and Properties of the Simulated Coarse-Grain Heat-Affected Zone in a Low-Carbon Bainite E550 Steel" Metals 9, no. 9: 939. https://doi.org/10.3390/met9090939
APA StyleZong, Y., & Liu, C.-M. (2019). Continuous Cooling Transformation Diagram, Microstructures, and Properties of the Simulated Coarse-Grain Heat-Affected Zone in a Low-Carbon Bainite E550 Steel. Metals, 9(9), 939. https://doi.org/10.3390/met9090939




