Influence of Microstructural Morphology on Hydrogen Embrittlement in a Medium-Mn Steel Fe-12Mn-3Al-0.05C
Abstract
1. Introduction
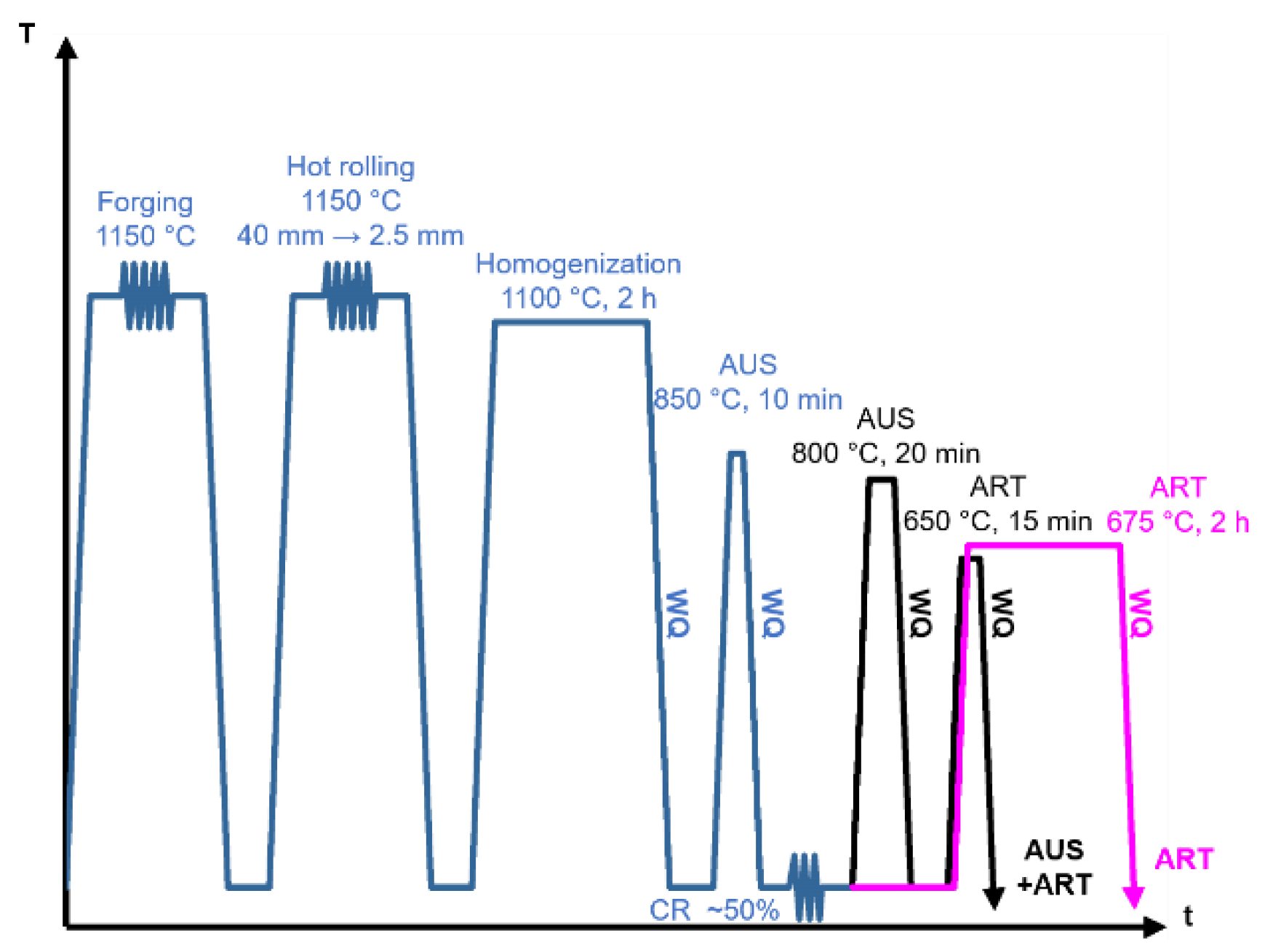
2. Materials and Methods
3. Results
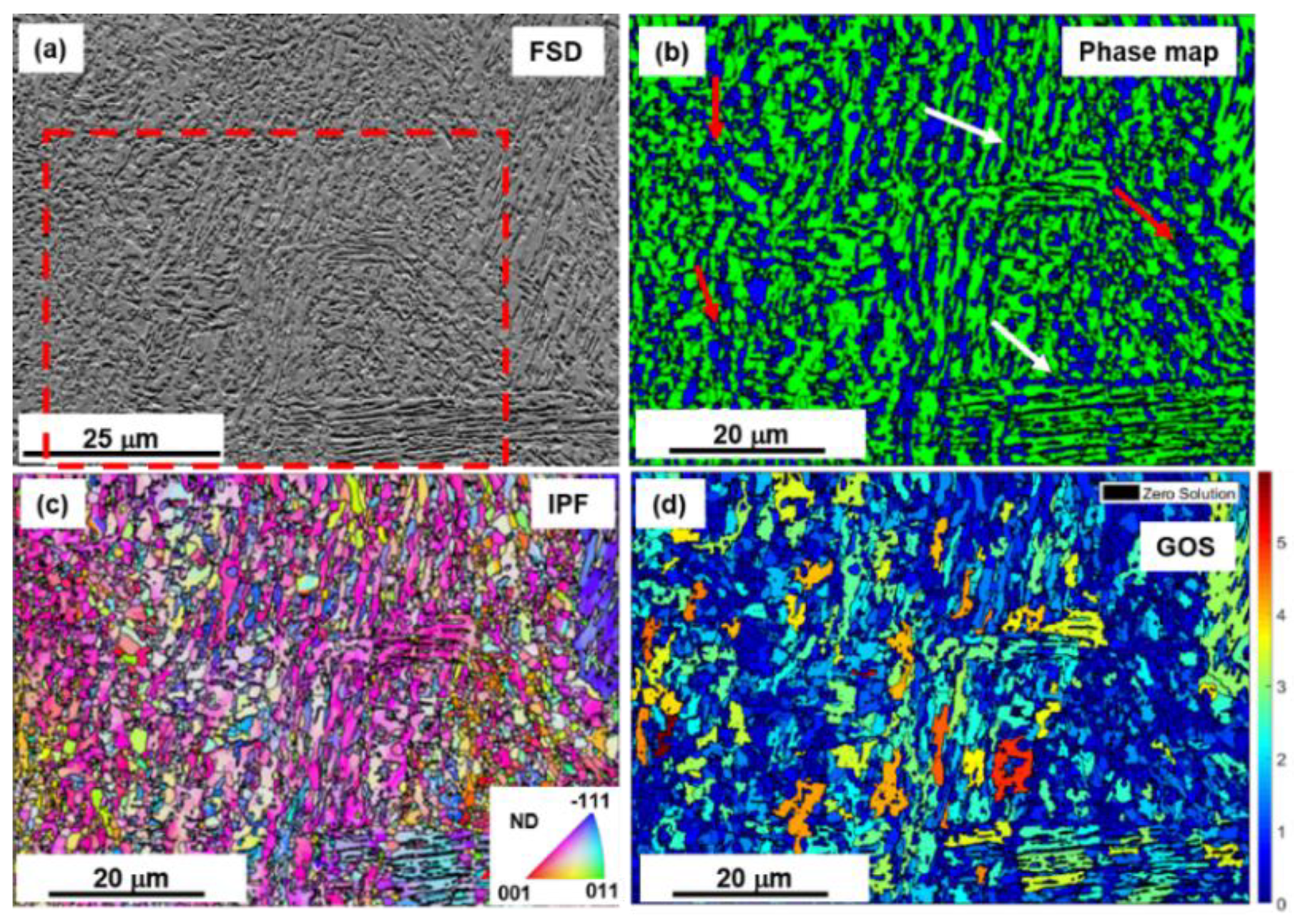
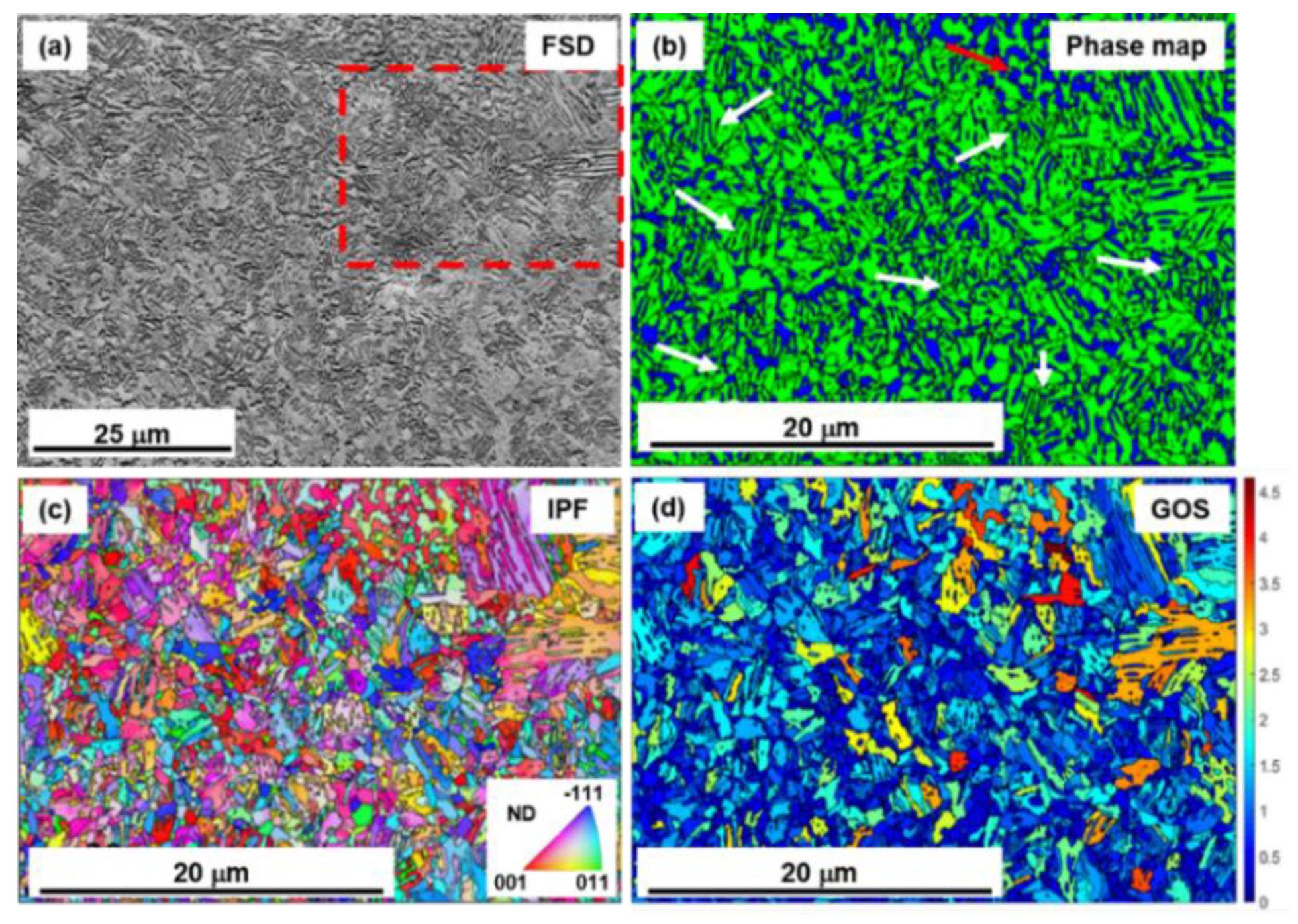
3.1. Microstructure
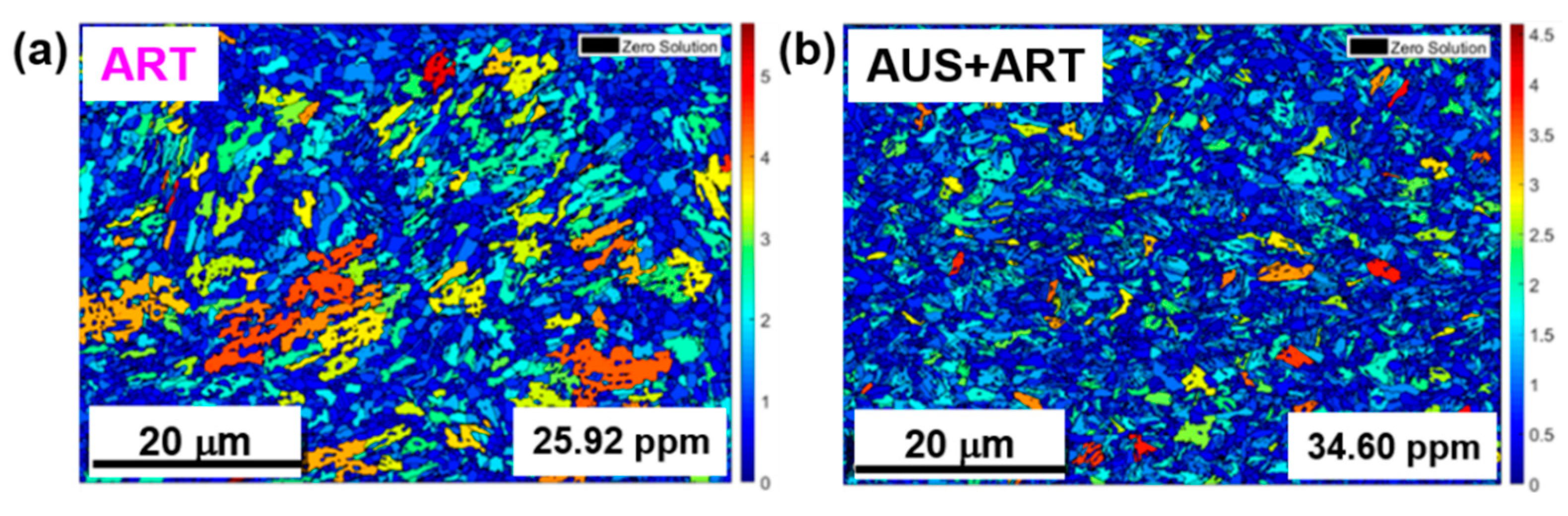
3.1.1. Electron Backscatter Diffraction (EBSD) Analysis of Microstructure
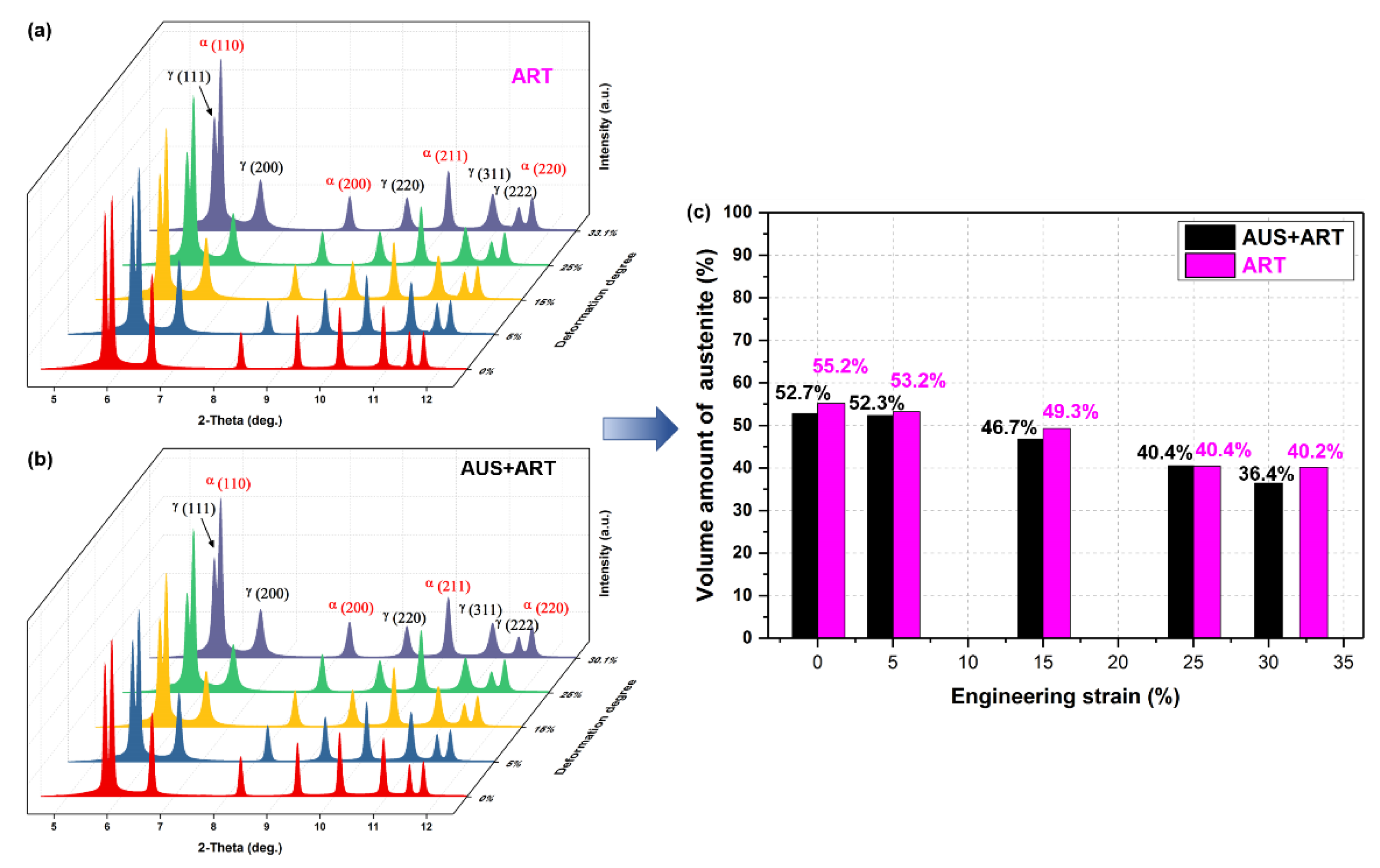
3.1.2. Quantitative Analysis of Microstructure by Synchrotron X-ray Diffraction (SYXRD)
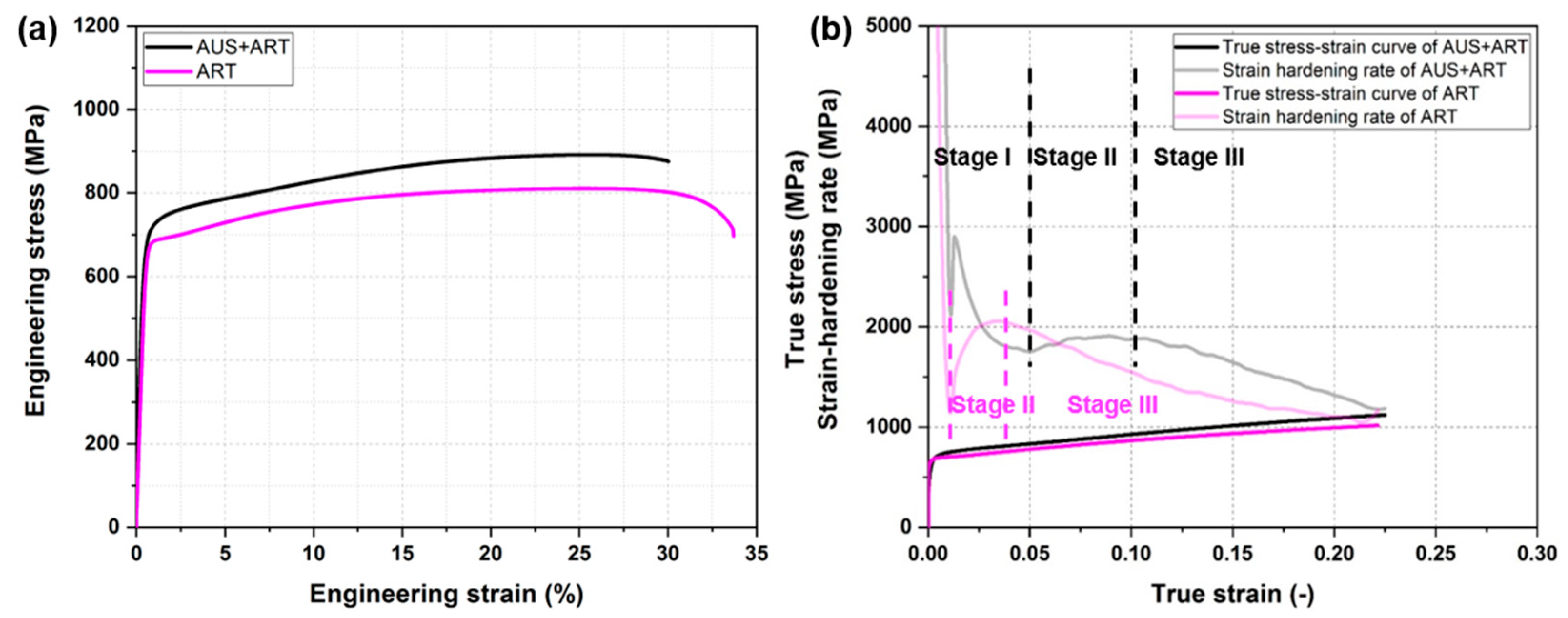
3.2. Mechanical Properties
3.2.1. Tensile Properties
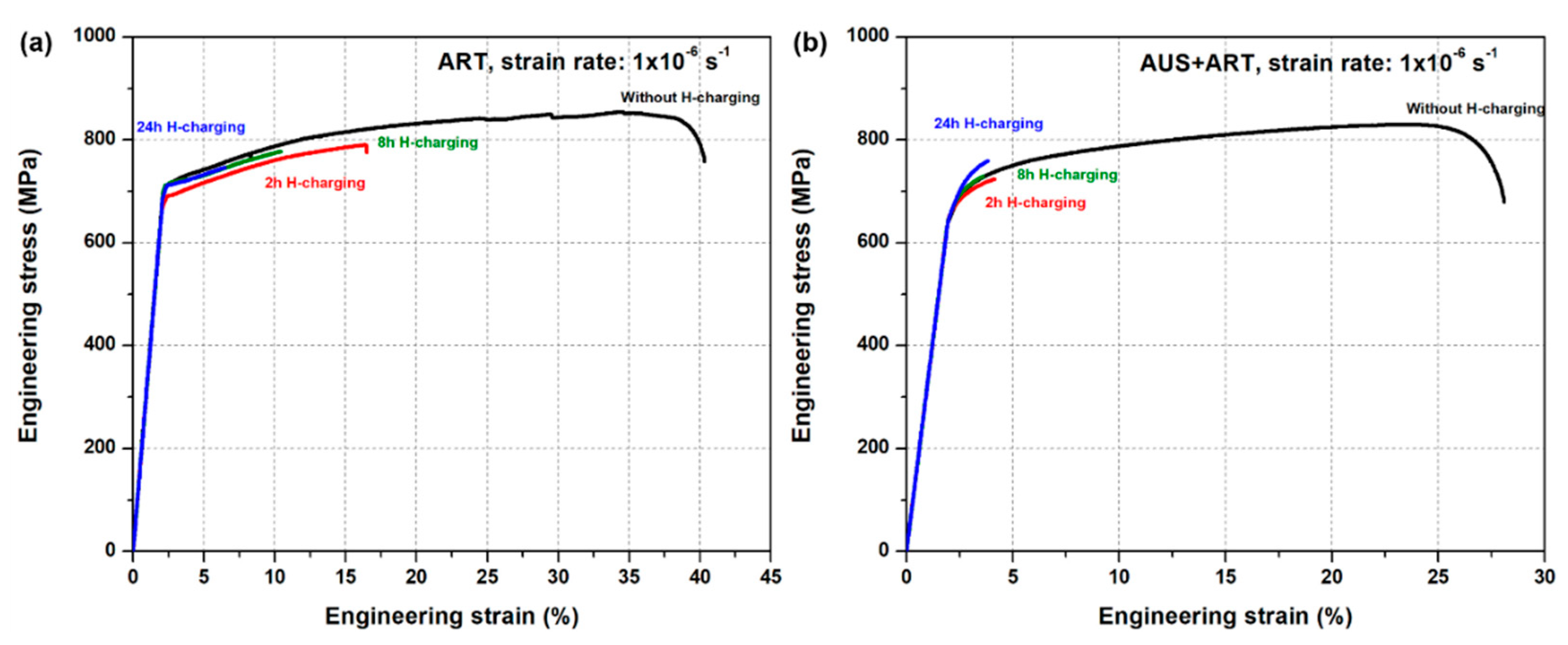
3.2.2. Mechanical Degradation Due to Hydrogen Ingression
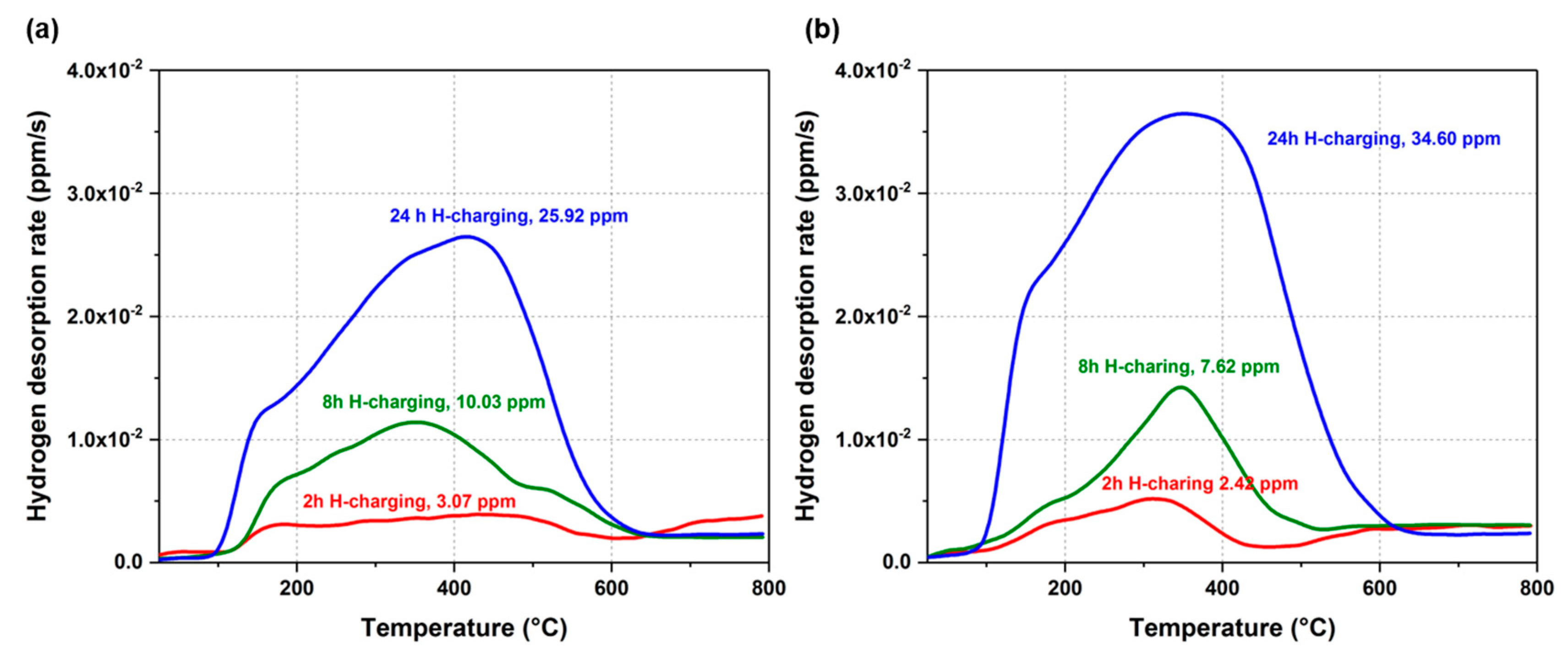
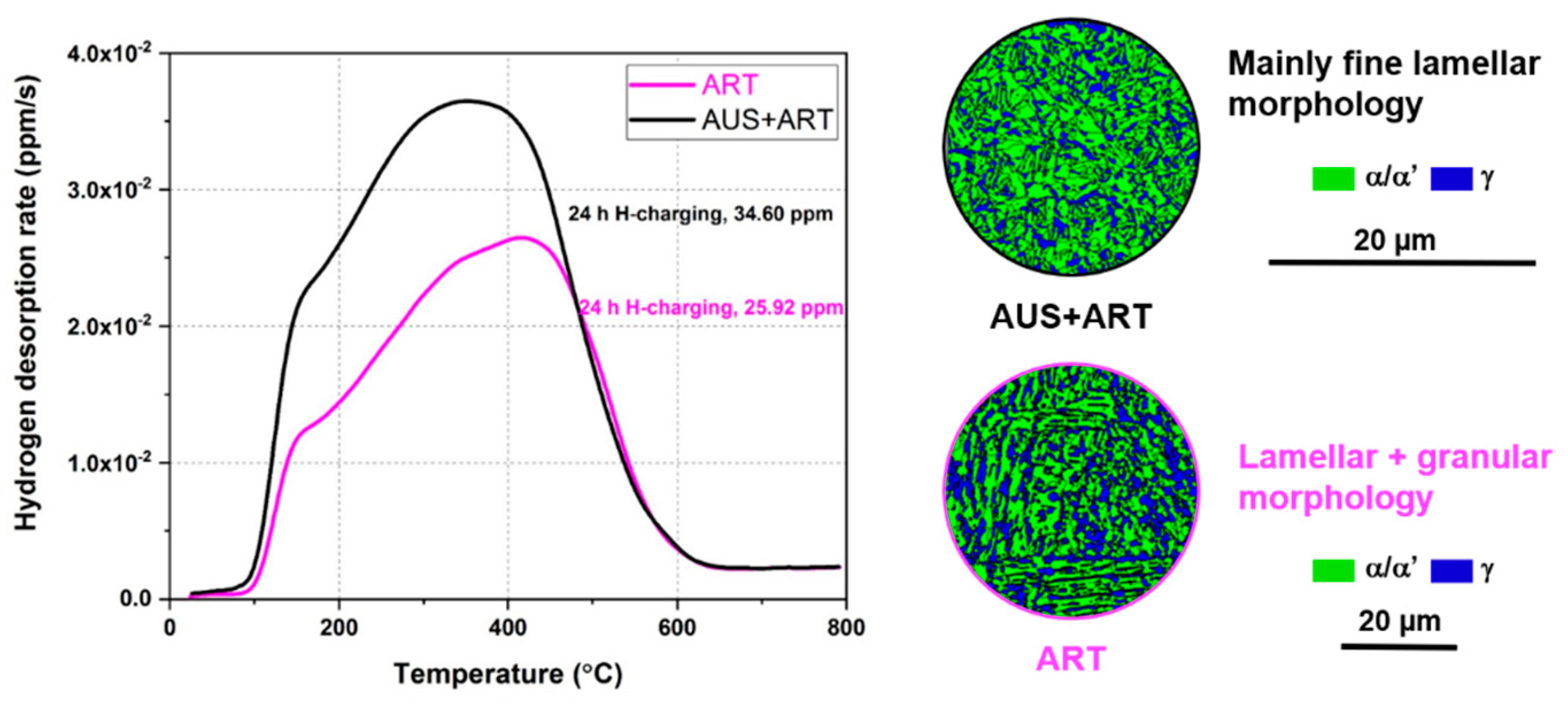
3.3. Hydrogen Uptake
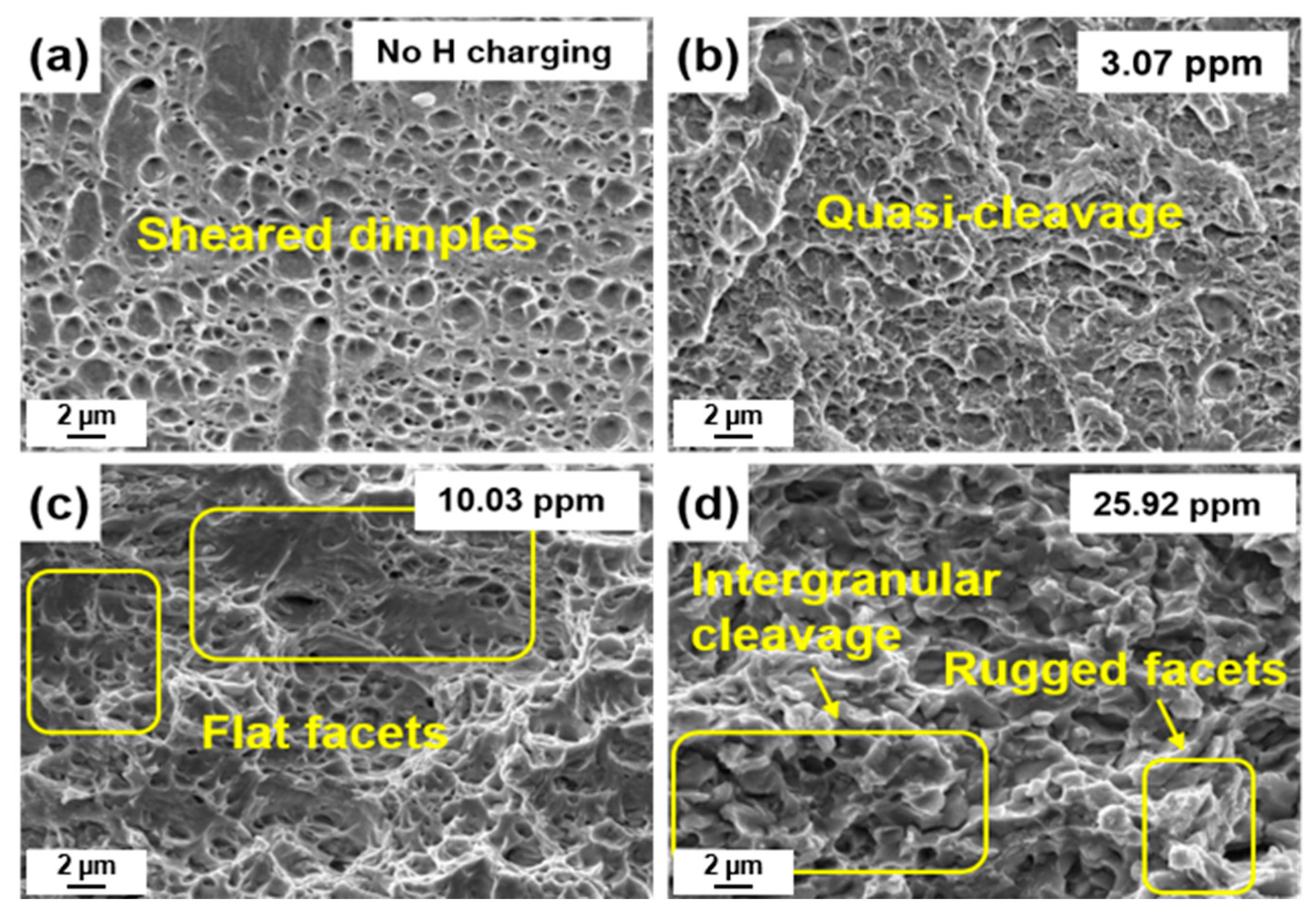
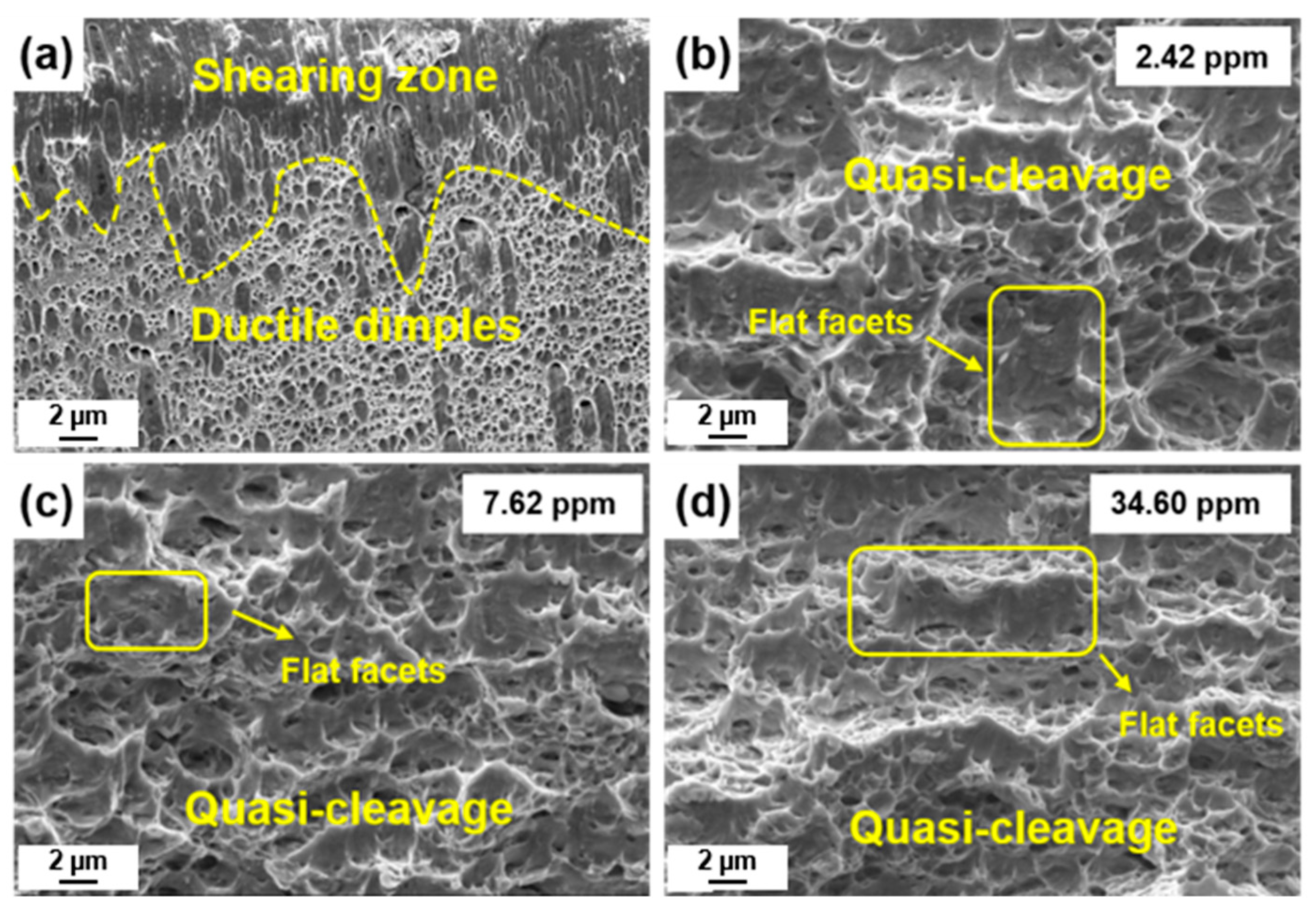
3.4. Fractography
4. Discussion
4.1. Microstructure–Mechanical Properties Correlation
4.2. Influences of Microstrucal Morphology on Hydrogen Embrittlement in Fe-12Mn-3Al-0.05C Steels
4.3. Thermodynamic Assessment
5. Conclusions
- (1)
- A combination of austenitization annealing (AUS) and austenite-reversed transformation (ART) produced comparable mechanical properties (UTS = 891 MPa, Y.S. = 701 MPa, total elongation = 30.1%) as that in a routine where the ART annealing was applied immediately after cold rolling.
- (2)
- The ultrafine-grained martensite colonies provided a large number of interfaces (prior austenite boundaries and lath boundaries) for hydrogen trapping, which increased the hydrogen ingression.
- (3)
- ART specimen revealed a clear ductile-brittle transition with increasing hydrogen concentration. Hydrogen embrittlement is considered to be predominated by concurrent contribution of HEDE and HELP mechanisms.
- (4)
- AUS + ART specimen exhibited extremely high hydrogen susceptibility of the ductility regardless of hydrogen concentration. The brittle failure in H-charged samples was attributed to the HEDE mechanism in the UFG microstructure with a large number of interfaces, and to possible contribution by the AIDE mechanism.
- (5)
- Consideration of thermodynamic factors suggest that the failure discrepancy in the UFG and non-UFG specimens was likely to be related to the facilitation of hydrogen-rich phase precipitation by interfacial defects.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, R.L. Ultrafine-grained microstructures and mechanical properties of alloy steels. Metall. Trans. 1972, 3, 905–912. [Google Scholar] [CrossRef]
- Han, J.; Lee, S.J.; Lee, C.Y.; Lee, S.; Jo, S.Y.; Lee, Y.K. The size effect of initial martensite constituents on the microstructure and tensile properties of intercritically annealed Fe–9Mn–0.05C steel. Mater. Sci. Eng. A 2015, 633, 9–16. [Google Scholar] [CrossRef]
- Lee, S.W.; De Cooman, B.C. Tensile behavior of intercritically annealed 10 pct Mn multi-phase steel. Metall. Mater. Trans. A. 2013, 45, 709–716. [Google Scholar] [CrossRef]
- Lee, S.W.; Woo, W.C.; De Cooman, B.C. Analysis of the tensile behavior of 12 pct Mn multi-phase (α + γ) TWIP + TRIP steel by neutron diffraction. Metall. Mater. Trans. A. 2016, 47A, 2125–2140. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.W.; De Cooman, B.C. Mn partitioning during the intercritical annealing of ultrafine-grained 6% Mn transformation-induced plasticity steel. Scripta Mater. 2011, 64, 649–652. [Google Scholar] [CrossRef]
- Luo, H.; Shi, J.; Wang, C.; Cao, W.; Sun, X.; Dong, H. Experimental and numerical analysis on formation of stable austenite during the intercritical annealing of 5Mn steel. Acta Mater. 2011, 59, 4002–4014. [Google Scholar] [CrossRef]
- Lee, Y.K.; Han, J. Current opinion in medium manganese steel. Mater. Sci. Tech. 2015, 31, 843–856. [Google Scholar] [CrossRef]
- Ma, Y. Medium-manganese steels processed by austenite-reverted-transformation annealing for automotive application. Mater. Sci. Tech. 2017, 33, 1713–1727. [Google Scholar] [CrossRef]
- Ma, Y.; Song, W.; Zhou, S.; Schwedt, A.; Bleck, W. Influence of intercritical annealing temperature on microstructure and mechanical properties of a cold-rolled medium-Mn steel. Metals 2018, 8, 357. [Google Scholar] [CrossRef]
- Haupt, M.; Dutta, A.; Ponge, D.; Sandlöbes, S.; Nellessen, M.; Hirt, G. Influence of intercritical annealing on microstructure and mechanical properties of a medium manganese steel. Procedia Engineer. 2017, 207, 1803–1808. [Google Scholar] [CrossRef]
- Edmonds, D.V.; He, K.; Rizzo, F.C.; De Cooman, B.C.; Matlock, D.K.; Speer, J.G. Quenching and partitioning martensite—A novel steel heat treatment. Mater. Sci. Eng. A. 2006, 438, 25–34. [Google Scholar] [CrossRef]
- Arlazarov, A.; Gouné, M.; Bouaziz, O.; Hazotte, A.; Kegel, F. Effect of intercritical annealing time on microstructure and mechanical behavior of advanced medium Mn steels. Mater. Sci. Forum. 2012, 706, 2693–2698. [Google Scholar] [CrossRef]
- Furukawa, T.; Huang, H.; Matsumura, O. Effects of carbon content on mechanical properties of 5%Mn steels exhibiting transformation induced plasticity. Mater. Sci. Tech. 1994, 10, 964–970. [Google Scholar] [CrossRef]
- De Moor, E.; Matlock, D.K.; Speer, J.G.; Merwin, M.J. Austenite stabilization through manganese enrichment. Scripta. Mater. 2011, 64, 185–188. [Google Scholar] [CrossRef]
- Furukawa, T. Dependence of strength–ductility characteristics on thermal history in low-carbon, 5 wt-%Mn steels. Mater. Sci. Tech. 1989, 5, 465–470. [Google Scholar] [CrossRef]
- Ryu, J.H.; Chun, Y.S.; Lee, C.S.; Bhadeshia, H.K.D.H.; Suh, D.W. Effect of deformation on hydrogen trapping and effusion in TRIP-assisted steel. Acta Mater. 2012, 60, 4085–4092. [Google Scholar] [CrossRef]
- Wang, M.; Tasan, C.C.; Koyama, M.; Ponge, D.; Raabe, D. Enhancing hydrogen embrittlement resistance of lath martensite by Introducing nano-films of interlath austenite. Metall. Mater. Trans. A. 2015, 46A, 3797–3802. [Google Scholar] [CrossRef]
- Han, J.; Nam, J.H.; Lee, Y.K. The mechanism of hydrogen embrittlement in intercritically annealed medium Mn TRIP steel. Acta Mater. 2016, 113, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, W.; Zhao, X.; Wang, C.; Cao, W.; Dong, H. Effect of reverted austenite fraction on hydrogen embrittlement of TRIP-aided medium Mn steel (0.1C-0.5Mn). Eng. Fail. Anal. 2019, 97, 605–616. [Google Scholar] [CrossRef]
- Jeong, I.; Ryu, K.M.; Lee, D.G.; Jung, Y.; Lee, K.; Lee, J.S.; Suh, D.W. Austenite morphology and resistance to hydrogen embrittlement in medium Mn transformation-induced plasticity steel. Scripta Mater. 2019, 169, 52–56. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen embrittlement understood. Metall. Mater. Trans. A 2015, 46A, 2323–2341. [Google Scholar] [CrossRef]
- Troiano, A.R. The role of hydrogen and other interstitials in the mechanical behavior of metals. Metallogr. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Oriani, R.A. A mechanistic theory of hydrogen embrittlement of steels. Berich. Bunsen. Gesell. 1972, 76, 848–857. [Google Scholar]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall. Mater. Trans. B. 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Robertson, I.M. The effect of hydrogen on dislocation dynamics. Eng. Fract. Mech. 2001, 68, 671–692. [Google Scholar] [CrossRef]
- Du, X.; Cao, W.; Wang, C.; Li, S.; Zhao, J.; Sun, Y. Effect of microstructures and inclusions on hydrogen-induced cracking and blistering of A537 steel. Mater. Sci. Eng. A. 2015, 642, 181–186. [Google Scholar] [CrossRef]
- Mohtadi-Bonad, M.A.; Ghesmati-kucheki, H. Important factors on the failure of pipeline steels with focus on hydrogen induced cracks and improvement of their resistance: Review paper. Met. Mater. Int. 2019, 1, 1–26. [Google Scholar]
- Mohtadi-Bonad, M.A.; Eskandari, M.; Szpunar, J.A. Effect of arisen dislocation density and texture components during cold rolling and annealing treatments on hydrogen induced cracking susceptibility in pipeline steel. J. Mater. Res. 2016, 31, 3390–3400. [Google Scholar] [CrossRef]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX—Free and Open Source Software Toolbox. SSP 2010, 160, 63–68. [Google Scholar] [CrossRef]
- Hielscher, R.; Schaeben, H. A novel pole figure inversion method: Specification of the MTEX algorithm. J. Appl. Crystallogr. 2008, 41, 1024–1037. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealized image or two-theta scan. High Pressure Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method, 4th ed.; Oxford University Press Inc.: New York, NY, USA, 2002. [Google Scholar]
- Field, D.P.; Bradford, L.T.; Nowell, M.M.; Lillo, T.M. The role of annealing twins during recrystallization of Cu. Acta Mater. 2007, 55, 4233–4241. [Google Scholar] [CrossRef]
- Henthorne, M. The slow strain rate stress corrosion cracking test—A 50 year retrospective. Corrosion 2016, 72, 1488–1518. [Google Scholar] [CrossRef]
- Park, Y.; Maroef, I.; Landau, A.; Olson, D. Retained austenite as a hydrogen trap in steel welds. Weld. J. 2002, 71, 27–35. [Google Scholar]
- Sevsek, S.; Haase, C.; Bleck, W. Strain-rate-dependent deformation behavior and mechanical properties of a multi-phase medium-manganese steel. Metals 2019, 9, 344. [Google Scholar] [CrossRef]
- Lynch, S.P. Metallographic contributions to understanding mechanisms of environmentally assisted cracking. Metallography 1989, 23, 147–171. [Google Scholar] [CrossRef]
- Spatschek, R.; Gobbi, G.; Hütter, C.; Chakrabarty, A.; Aydin, U.; Brinckmann, S.; Neugebauer, J. Scale bridging description of coherent phase equilibria in the presence of surfaces and interfaces. Phys. Rev. B 2016, 94, 134106. [Google Scholar] [CrossRef]
- Pezold, J.; Lymperakis, L. Neugebauer, Hydrogen-enhanced local plasticity at dilute bulk H concentrations: The role of H-H interactions and the formation of local hydrides. Acta Mater. 2011, 59, 2969–2980. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Tanaka, S.; Shiihara, Y.; Kohyama, M. Ab initio perspective of the (110) symmetrical tilt grain boundaries in bcc Fe: application of local energy and local stress. J. Mater. Sci. 2014, 49, 3980–3995. [Google Scholar] [CrossRef]
- Bai, Y.; Momotani, Y.; Chen, M.C.; Shibata, A.; Tsuji, N. Effect of grain refinement on hydrogen embrittlement behaviors of high-Mn TWIP steel. Mater. Sci. Eng. A 2016, 651, 935–944. [Google Scholar] [CrossRef]
| Element | C | Si | Mn | P | S | Al | Fe |
|---|---|---|---|---|---|---|---|
| wt.% | 0.064 | 0.2 | 11.7 | 0.006 | 0.003 | 2.9 | Bal. |
| Heat Treatment | UTS/MPa | A20/% | Effective Grain Size/μm | Austenite Fraction/% | |
|---|---|---|---|---|---|
| α’ Package | Globular Grains | ||||
| ART | 811 | 30.1 | 10–20 | ~1.5 | 55.2 |
| AUS + ART | 891 | 33.1 | 1–5 | ~1 | 53.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Song, W.; Sevsek, S.; Ma, Y.; Hüter, C.; Spatschek, R.; Bleck, W. Influence of Microstructural Morphology on Hydrogen Embrittlement in a Medium-Mn Steel Fe-12Mn-3Al-0.05C. Metals 2019, 9, 929. https://doi.org/10.3390/met9090929
Shen X, Song W, Sevsek S, Ma Y, Hüter C, Spatschek R, Bleck W. Influence of Microstructural Morphology on Hydrogen Embrittlement in a Medium-Mn Steel Fe-12Mn-3Al-0.05C. Metals. 2019; 9(9):929. https://doi.org/10.3390/met9090929
Chicago/Turabian StyleShen, Xiao, Wenwen Song, Simon Sevsek, Yan Ma, Claas Hüter, Robert Spatschek, and Wolfgang Bleck. 2019. "Influence of Microstructural Morphology on Hydrogen Embrittlement in a Medium-Mn Steel Fe-12Mn-3Al-0.05C" Metals 9, no. 9: 929. https://doi.org/10.3390/met9090929
APA StyleShen, X., Song, W., Sevsek, S., Ma, Y., Hüter, C., Spatschek, R., & Bleck, W. (2019). Influence of Microstructural Morphology on Hydrogen Embrittlement in a Medium-Mn Steel Fe-12Mn-3Al-0.05C. Metals, 9(9), 929. https://doi.org/10.3390/met9090929








