2. Materials and Methods
The composition of the steel used in this study was 0.0005C-7.19Mn-0.25Si wt% The as-received material was cast from a vacuum melt, reheated to 1230 °C, hot-rolled to a thickness of 2.87 mm and furnace-cooled from 650 °C, before being cold-rolled to a thickness of 1.42 mm (50 pct reduction). Three different conditions of the 7Mn steel were used in this study. The condition denoted from here on as ‘CR50′ is the material in its as-received, cold-rolled condition. Some of the CR50 material was further cold-rolled to a thickness of 0.97 mm (66 pct total reduction); these samples are denoted as ‘CR66′. Additionally, to provide a uniform initial microstructure for comparison to the cold-rolled conditions, samples were cut from the CR50 sheet, austenitized under vacuum at 850 °C for 300 s and quenched to 30 °C at 100 °C/s with Helium (He) gas before being placed in liquid nitrogen for 300 s; these samples are denoted as ‘AQ’ (as-quenched).
Rectangular samples measuring 4.0 mm × 10.0 mm of each material condition were machined with the long axis parallel to the rolling direction and processed under vacuum in a DIL 805A dilatometer (TA Instruments, New Castle, DE, USA). For in situ austenite volume fraction assessments during intercritical annealing, samples were heated to 650 °C at rates ranging from 0.05 to 96 °C/s and held for 1000 s. Subsequently, the samples were heated to 850 °C at a rate of 10 °C/s.
Samples for metallography were either heated to 650 °C and quenched or heated to 500 °C and held for 10,000 s and quenched. The
Ac1 temperature was determined via dilatometry by applying a linear offset corresponding to one volume pct austenite formation from the heating portion of the dilation data prior to the
Ac1 temperature. The
Ac1 temperature was determined to be the temperature where the experimental dilation curve intersected the linear offset. For samples with heating rates exceeding 3 °C/s, heating continued to 900 °C to ensure the
Ac1 temperature was realized upon heating.
Ac1 temperature measurements were taken on three samples for each condition and heating rate. The austenite volume fraction during intercritical annealing was assessed by application of a lever rule to dilation measurements [
6,
12].
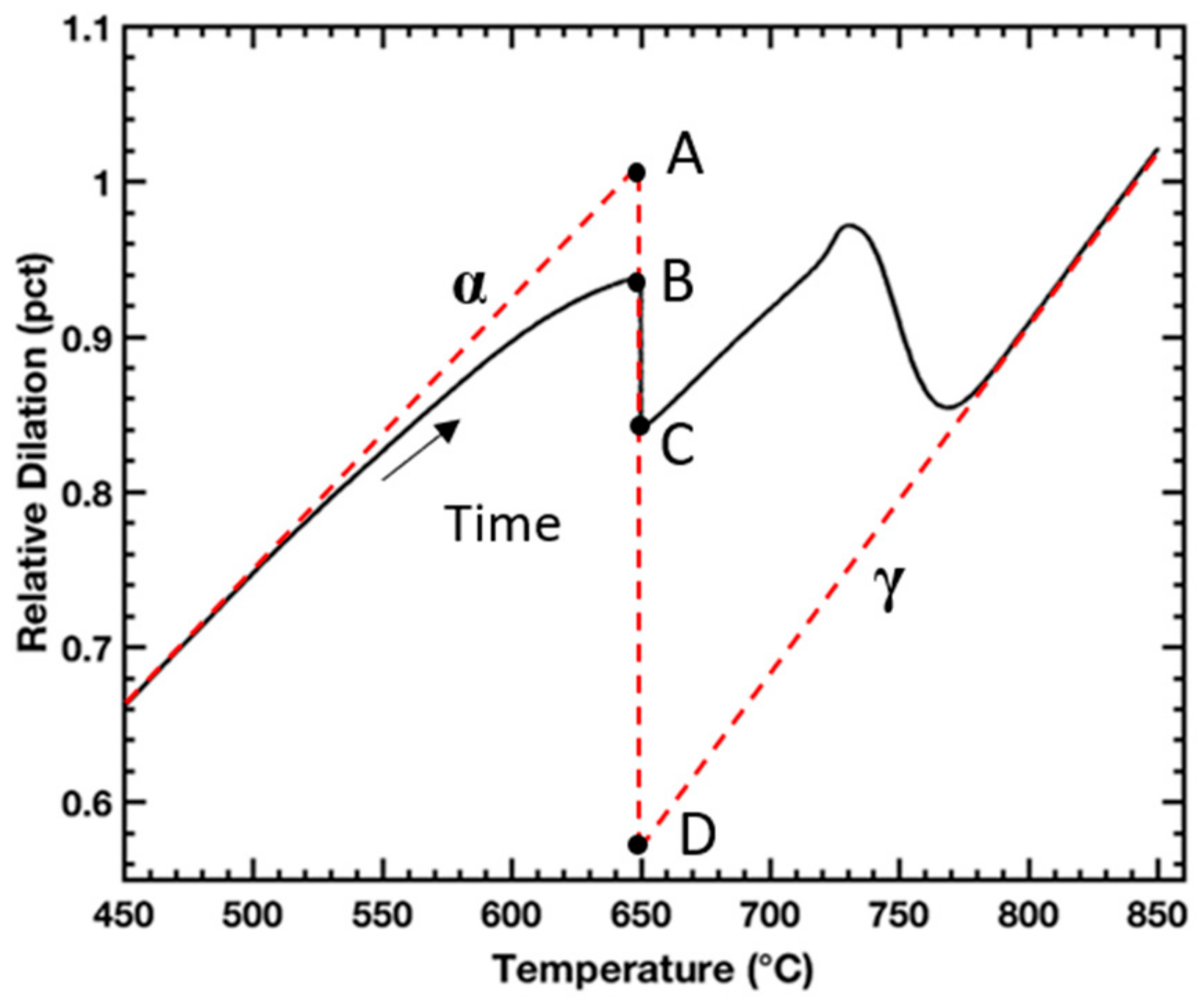
Figure 1 shows exemplary dilatometry data for a sample heated to 650 °C and held for 1000 s followed by heating to 850 °C. Overlaid on the data are dashed lines that extrapolate thermal expansion of the sample corresponding to regions when the sample is fully ferritic/tempered martensite and fully austenitic, labeled α and γ, respectively. Another overlaid line, vertical at 650 °C and bounded by points A and D, corresponds to the relative dilation of the sample corresponding to complete α→γ transformation. The length of line A-B divided by the length of line A-D corresponds to the austenite volume fraction formed upon heating, the length of line B-C divided by the length of line A-D corresponds to the austenite fraction formed during isothermal holding, the length of line A-C divided by the length of line A-D corresponds to total austenite fraction formed during intercritical annealing, and the length of line C-D divided by the length of line A-D corresponds to the fraction of additional austenite formed upon heating to 850 °C, after intercritical annealing.
For metallography, samples were sectioned with the cross-section normal perpendicular to the rolling direction. Samples were then mounted, polished, and etched with a one pct Nital solution and analyzed with a JOEL 7000 field-emission scanning electron microscope (FESEM) (JOEL USA, Peabody, MA, USA) using a 15 kV accelerating voltage and a working distance of 10 mm.
Simulations of microstructural evolution during intercritical annealing were conducted using the phase field simulation software MICRESS
® (version 7.117, ACCESS e.V., Aachen, Germany) utilizing the TQ module which allows MICRESS
® to interface with Thermo-Calc
® Software databases; the TCFE9 Steels/Fe-alloys database was used for all MICRESS
® simulations. All MICRESS
® simulations conducted were for intercritical annealing at 650 °C and a heating rate of 96 °C/s for a steel with a composition of 7.19Mn-0.25Si wt%. The 5 μm × 5 μm area simulated had an initial microstructure consisting of elongated ferrite which contained a specified amount of stored strain energy; this initial microstructure was constructed with the intent of representing cold-rolled ferrite. As time progressed in each simulation, the elongated ferrite was consumed concurrently by nucleation and growth of both strain-free ferrite and austenite. Strain-free ferrite nucleation was specified to nucleate within the initial elongated ferrite grains while austenite was specified to nucleate at ferrite grain boundaries, including grain boundaries between strain-free and deformed ferrite. The number of austenite nucleation sites was limited to a specified number of nucleation events. To reduce simulation time, all simulations began with heating from 400 °C.
Table 1 lists values used for the initial simulation relating to interfaces, austenite nucleation, Mn diffusivity in ferrite, and stored strain energy of ferrite. Interfacial energy values are identical to those used by Zhu and Militzer for a phase field simulation developed for predicting the microstructural evolution during intercritical annealing of dual-phase steels [
13]. The α-γ interface mobility pre-factor,
M0, and activation energy, ∆
G*, used by Zhu and Militzer were initially adopted for all interfaces in the simulation. The mobility pre-factor was adjusted to produce simulations that predicted stable austenite growth at 650 °C. The number of austenite nucleation sites was chosen to reflect qualitative metallography observations. The diffusivity pre-factor and activation energy for Mn in ferrite were adopted from De Cooman and Speer [
14]. The stored strain energy in ferrite was chosen to be greater than that use by Zhu and Militzer (2 J/cm
3) by approximately a factor of six and was specified as a range to reflect inhomogeneity in the amount of stored strain energy in cold-rolled ferrite.
Additional simulations were conducted using variations of select input parameters in an attempt to incorporate mechanisms that may cause accelerated α → γ transformation due to prior cold deformation; these parameter variations are listed in
Table 2. The number of austenite nucleation sites was varied from 10–40 to reflect a potential increase in ferrite grain boundary area, which serves as an austenite nucleation site. The diffusivity pre-factor for Mn in ferrite was varied from 0.756–7560 cm
2/s to reflect a potential increase in the effective bulk diffusivity of Mn through ferrite due to pipe diffusion in deformed ferrite. Initially, the stored strain energy in ferrite was also varied in order to reflect a potential increase in driving force due to stored strain energy in deformed ferrite, however, it was discovered that MICRESS
® does not consider the stored strain energy in ferrite to contribute to the driving force for the α → γ transformation. Stored strain energy is apparently only considered for like-phase grain boundary migration (e.g., recrystallization).
Author Contributions
Author contributions for this article are as follows: conceptualization, J.J.M., J.G.S., D.K.M., and E.D.M.; methodology, J.J.M.; formal analysis, J.J.M., J.G.S., D.K.M., and E.D.M.; investigation, J.J.M., J.G.S., D.K.M., and E.D.M.; writing—original draft preparation, J.J.M.; writing—review and editing, J.J.M., J.G.S., D.K.M., and E.D.M.; supervision, E.D.M.;
Funding
The authors gratefully acknowledge the support of Pacific Northwest National Laboratory, the sponsors of the Advanced Steel Processing and Products Research Center at the Colorado School of Mines, and the ASM Material Genome Toolkit. This work was funded by the Department of Energy’s Office of Vehicle Technologies under the Automotive Lightweighting Materials Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Merwin, M.J. Microstructure and Properties of Cold Rolled and Annealed Low-Carbon Manganese TRIP Steels. Iron Steel Technol. 2008, 5, 66–84. [Google Scholar]
- De Moor, E.; Matlock, D.K.; Speer, J.G.; Merwin, M.J. Austenite Stabilization Through Manganese Enrichment. Scr. Mater. 2011, 64, 185–188. [Google Scholar] [CrossRef]
- Gibbs, P.J.; De Moor, E.; Merwin, M.J.; Clausen, B.; Speer, J.G.; Matlock, D.K. Austenite stability effects on tensile behavior of manganese-enriched- austenite transformation-induced plasticity steel. Metall. Mater. Trans. A 2011, 42, 3691–3702. [Google Scholar] [CrossRef]
- Miller, R.L. Ultrafine-Grained Microstructures and Mechanical Properties of Alloy Steels. Metall. Trans. 1972, 3, 905–912. [Google Scholar] [CrossRef]
- Yang, D.Z.; Brown, E.L.; Matlock, D.K.; Krauss, G. Ferrite Recrystallization and Austenite Formation in Cold-Rolled Intercritically Annealed Steel. Metall. Trans. A 1985, 16, 1385–1392. [Google Scholar] [CrossRef]
- Huang, J.; Poole, W.J.; Militzer, M. Austenite Formation During Intercritical Annealing. Metall. Mater. Trans. A 2004, 35, 3363–3375. [Google Scholar] [CrossRef]
- Chbihi, A.; Barbier, D.; Germain, L.; Hazotte, A.; Gouné, M. Interactions Between Ferrite Recrystallization and Austenite Formation in High-Strength Steels. J. Mater. Sci. 2014, 49, 3608–3621. [Google Scholar] [CrossRef]
- Navara, E.; Bengtsson, B.; Easterling, K.E.; Easterling, K.E. Austenite Formation in Manganese-Partitioning Dual-Phase Steel. Mater. Sci. Technol. 1986, 2, 1196–1201. [Google Scholar] [CrossRef]
- Lee, S.; De Cooman, B.C. Influence of Carbide Precipitation and Dissolution on the Microstructure of ultra-fine-grained intercritically annealed medium manganese steel. Metall. Mater. Trans. A 2016, 47, 3263–3270. [Google Scholar] [CrossRef]
- Steinbach, I.; Pezzolla, F.; Nestler, B.; Seeßelberg, M.; Prieler, R.; Schmitz, G.J.; Rezende, J.L.L. A Phase Field Concept for Multiphase Systems. Phys. D 1996, 94, 135–147. [Google Scholar] [CrossRef]
- Militzer, M. Phase Field Modeling of Microstructure Evolution in Steels. Curr. Opin. Solid State Mater. Sci. 2011, 15, 106–115. [Google Scholar] [CrossRef]
- ASTM Int. Standard Practice for Quantitative Measurement and Reporting of Hypoeutectoid Carbon and Low-Alloy Steel Phase Transformations; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Zhu, B.; Militzer, M. Phase-Field Modeling for Intercritical Annealing of a Dual-Phase Steel. Metall. Mater. Trans. A 2014, 46, 1073–1084. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Speer, J.G. Fundamentals of Steel Product Physical Metallurgy; Association for Iron & Steel Technology: Materials Park, OH, USA, 2011; ISBN 978-1-935117-16-2. [Google Scholar]
- Azizi-Alizamini, H.; Militzer, M.; Poole, W.J. Austenite Formation in Plain Low-Carbon Steels. Mater. Sci. Eng. A 2011, 42, 1544–1557. [Google Scholar] [CrossRef]
- Mueller, J.J.; De Moor, E. Austenite growth and retention simulations in intercritically annealed medium manganese steels. In Proceedings of the Materials Science and Technology Conference and Exhibition 2017, Pittsburgh, PA, USA, 8–12 October 2017; Material Science and Technology: Warrendale, PA, USA, 2017. [Google Scholar]
- Dmitrieva, O.; Ponge, D.; Inden, G.; Millán, J.; Choi, P.; Sietsma, J.; Raabe, D. Chemical Gradients Across Phase Boundaries Between Martensite and Austenite in Steel Studied by Atom Probe Tomography and Simulation. Acta Mater. 2011, 59, 364–374. [Google Scholar] [CrossRef]
- Shewmon, P. Diffusion in Solids; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1989; ISBN 9780873391054. [Google Scholar]
- Balluffi, R.W.; Cahn, J.W. Mechanism for Diffusion Induced Grain Boundary Migration. Acta Metall. 1980, 29, 493–500. [Google Scholar] [CrossRef]
- Hillert, M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- De Moor, E.; Kang, S.; Speer, J.G.; Matlock, D.K. Manganese Diffusion in Third Generation Advanced High Strength Steels. In Proceedings of the International Conference on Mining, Materials and Metallurgical Engineering, Prague, Czech Republic, 11–12 August 2014. Keynote Lecture II. [Google Scholar]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
Figure 1.
Schematic depicting method for in situ austenite volume fraction assessments from dilatometry during intercritical annealing. Overlaid on the dilation data are dashed lines that extrapolate thermal expansion of the sample corresponding to regions when the sample is fully ferritic/tempered martensite and fully austenitic labeled α and γ, respectively. The line length fraction AC/AD corresponds to austenite volume fraction formed during heating and isothermal holding.
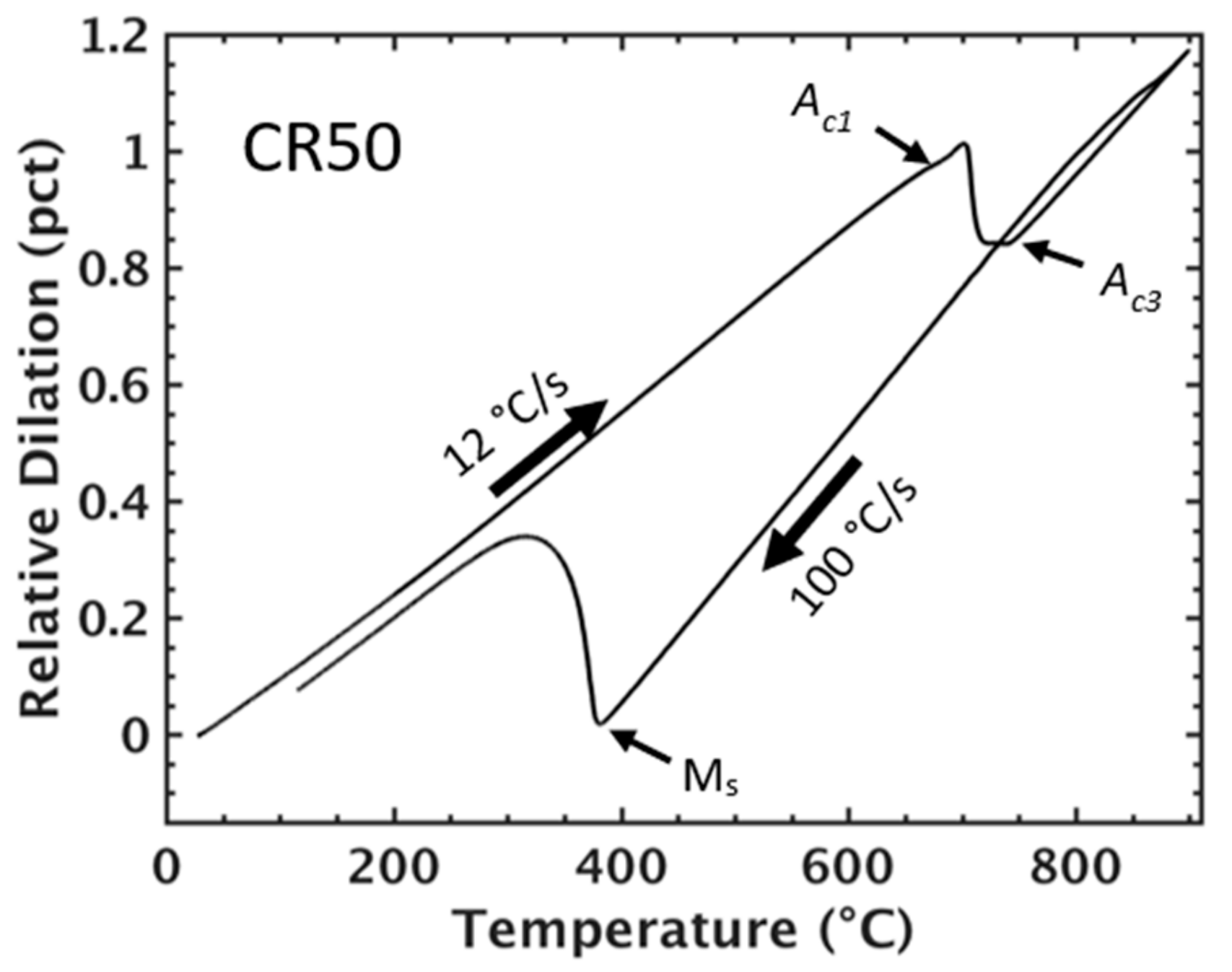
Figure 2.
Dilation response of the CR50 condition during heating to 900 °C followed by quenching to 30 °C at 100 °C/s.
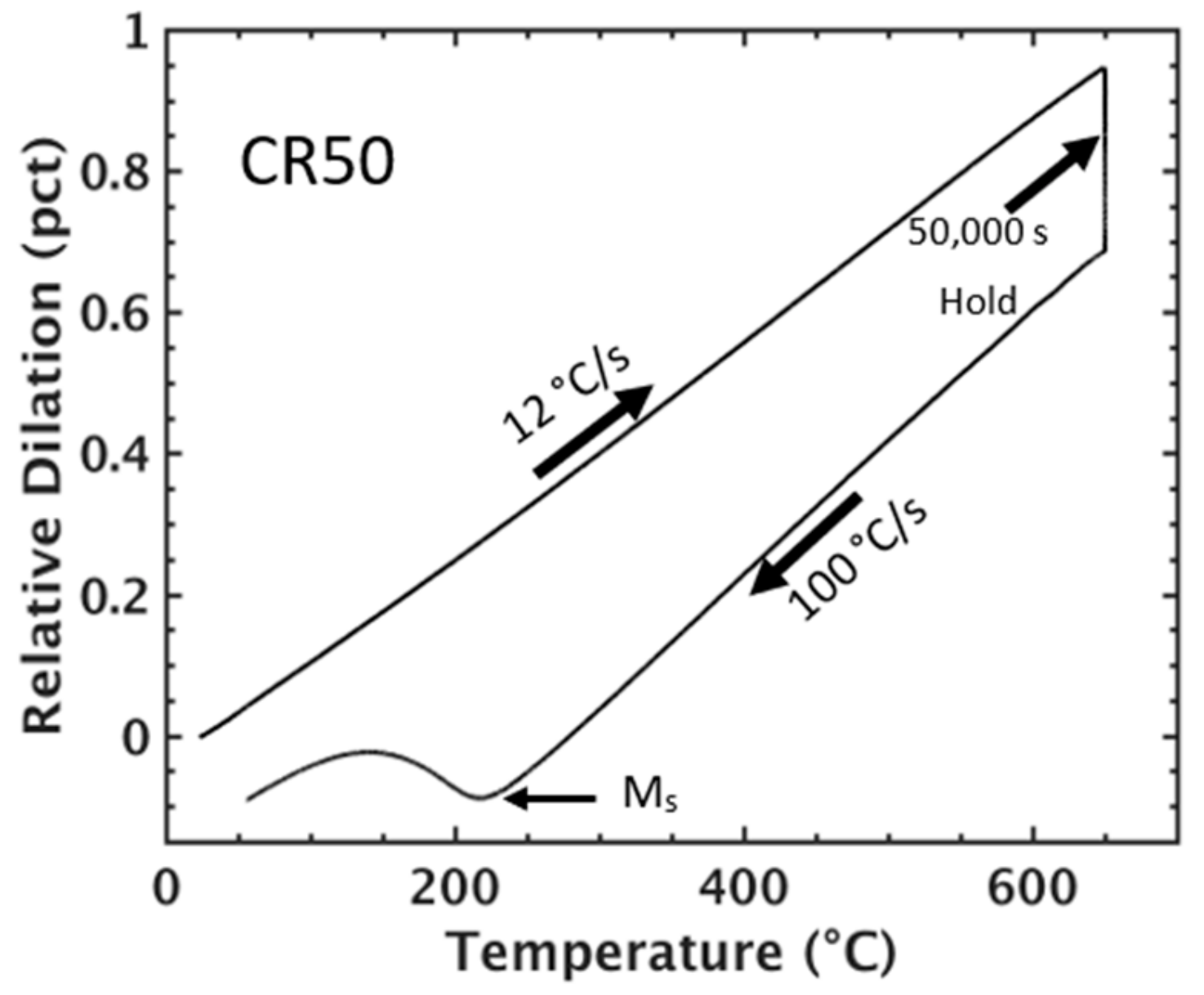
Figure 3.
Dilation response of the CR50 condition during heating and isothermal holding at 650 °C for 50,000 s followed by quenching to 30 °C at 100 °C/s.
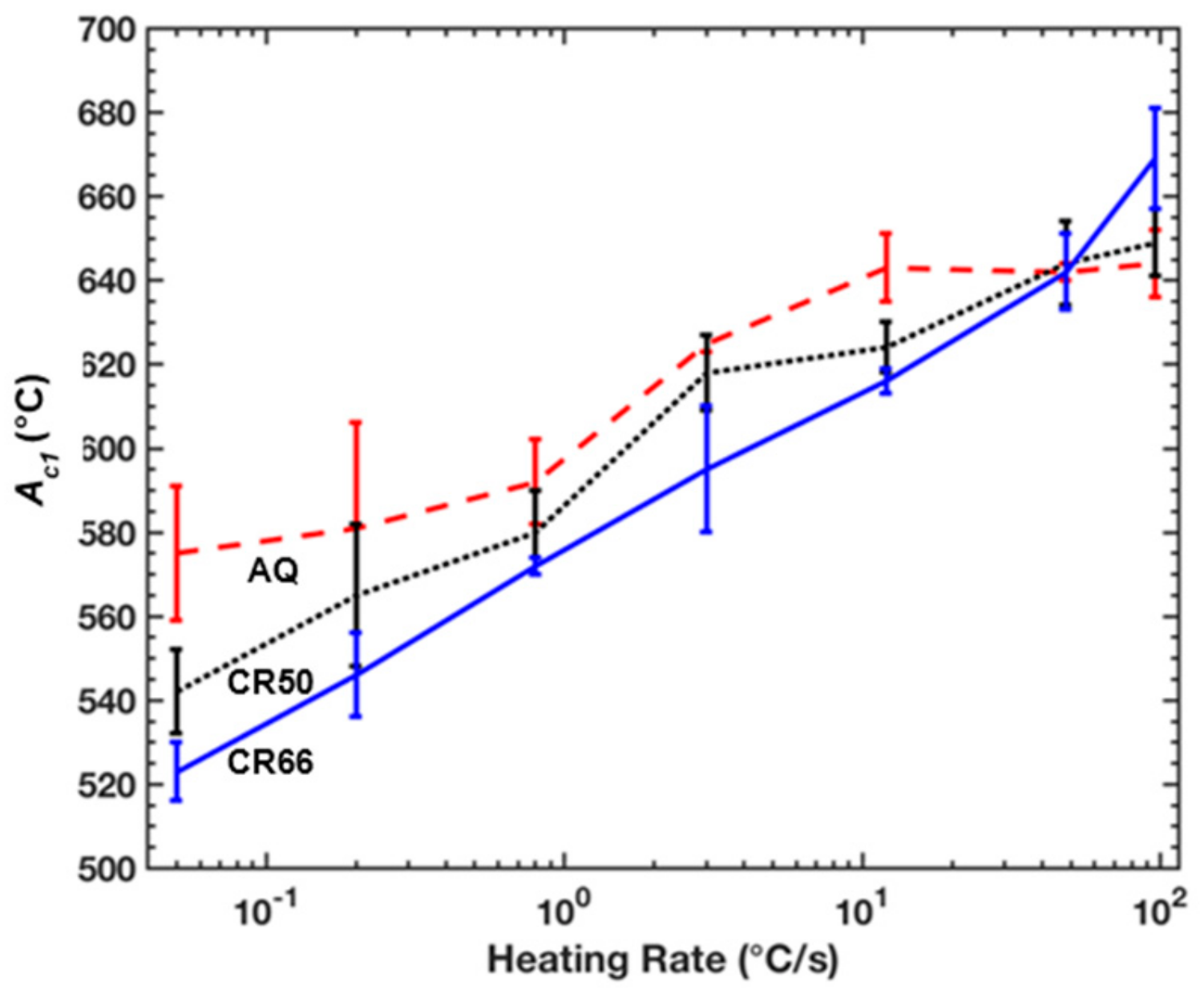
Figure 4.
Ac1 temperatures assessed via dilatometry for each condition and heating rate.
Figure 5.
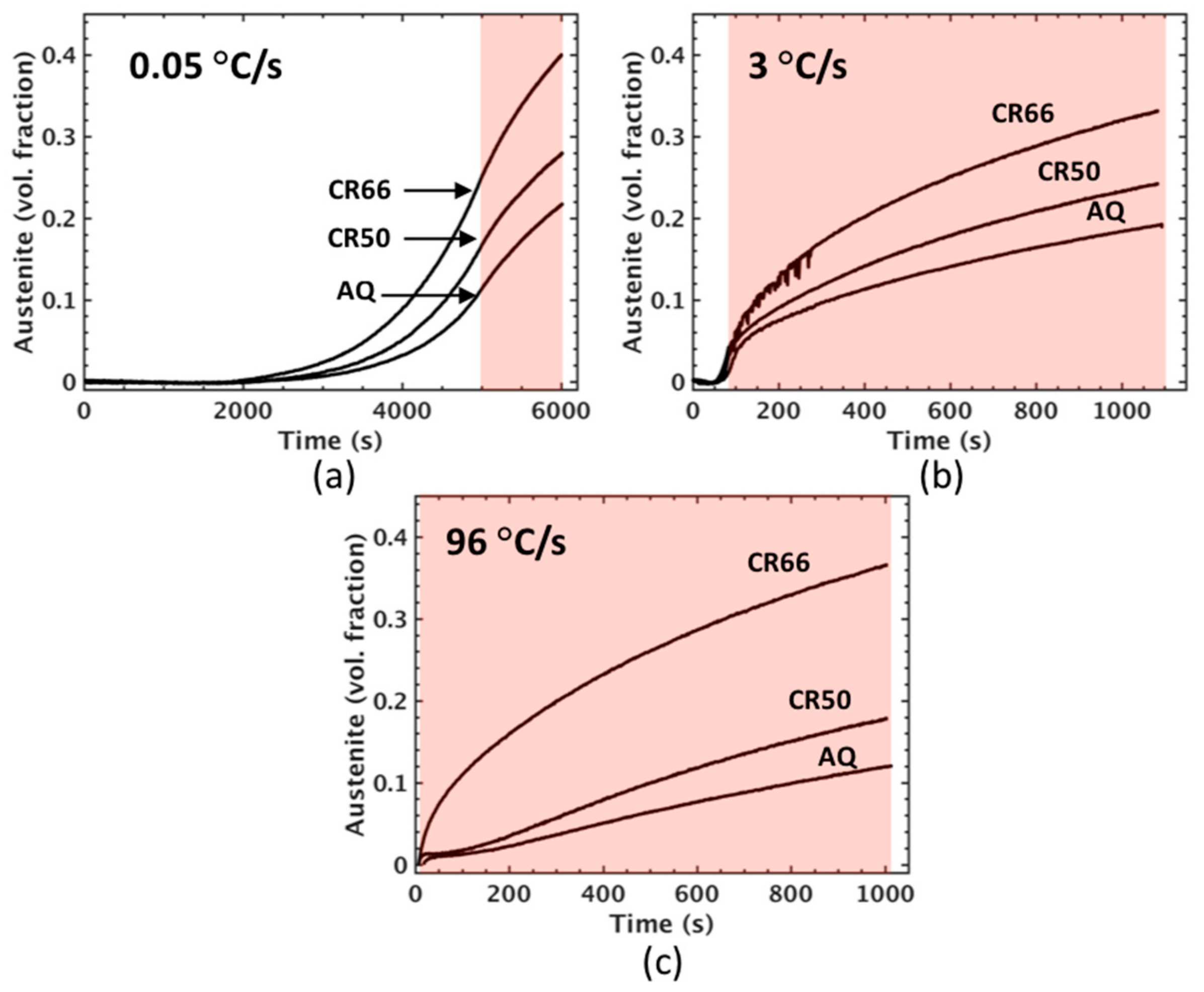
In situ austenite volume fraction assessments from dilatometry for intercritical annealing at 650 °C for each condition and heating rates of 0.05 °C/s (a), 3 °C/s (b), and 96 °C/s (c). Shaded areas indicate approximate duration of isothermal hold at 650 °C.
Figure 6.
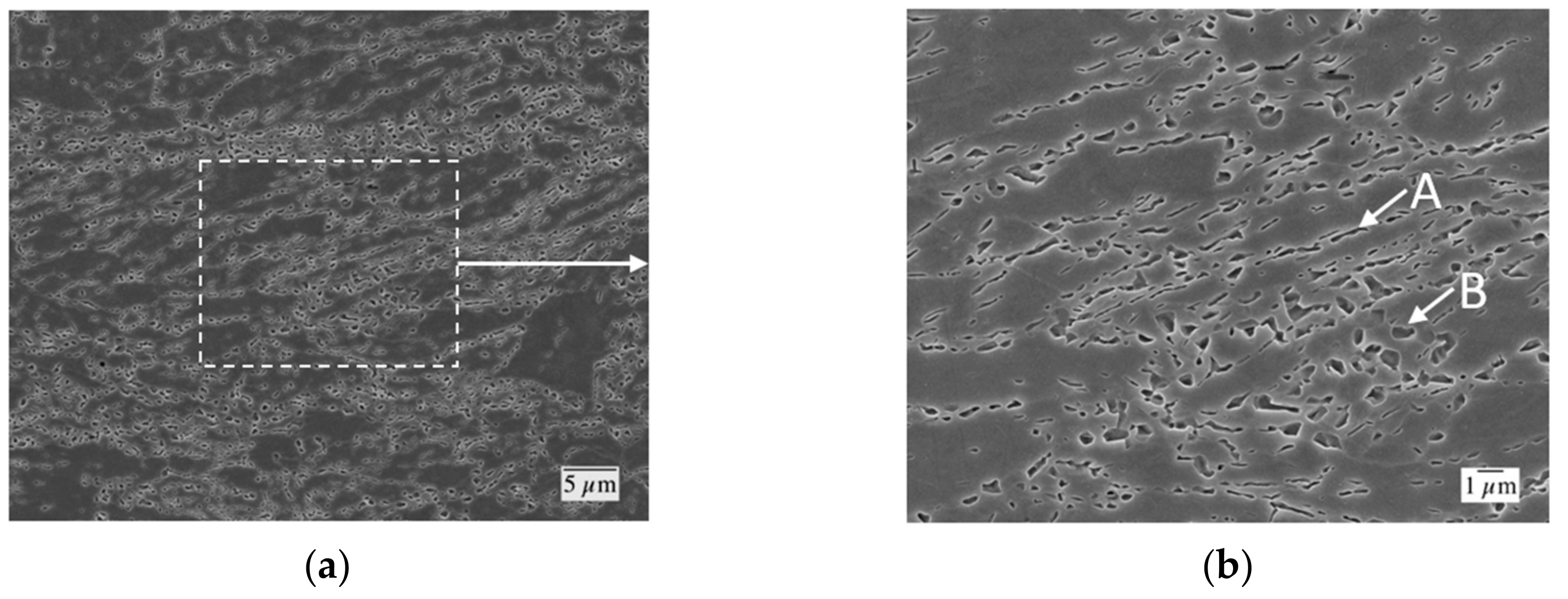
Field-emission scanning electron microscope (FESEM) images of the CR50 condition after heating to 650 °C at 0.05 °C/s followed by quenching to 30 °C at 100 °C/s. Higher magnification of the boxed region in (a) is shown in (b). Elongated regions of martensite (previously austenite) at deformed ferrite grain boundaries are labeled A. More equiaxed regions of martensite (previously austenite) are labeled B. One pct Nital Etch.
Figure 7.
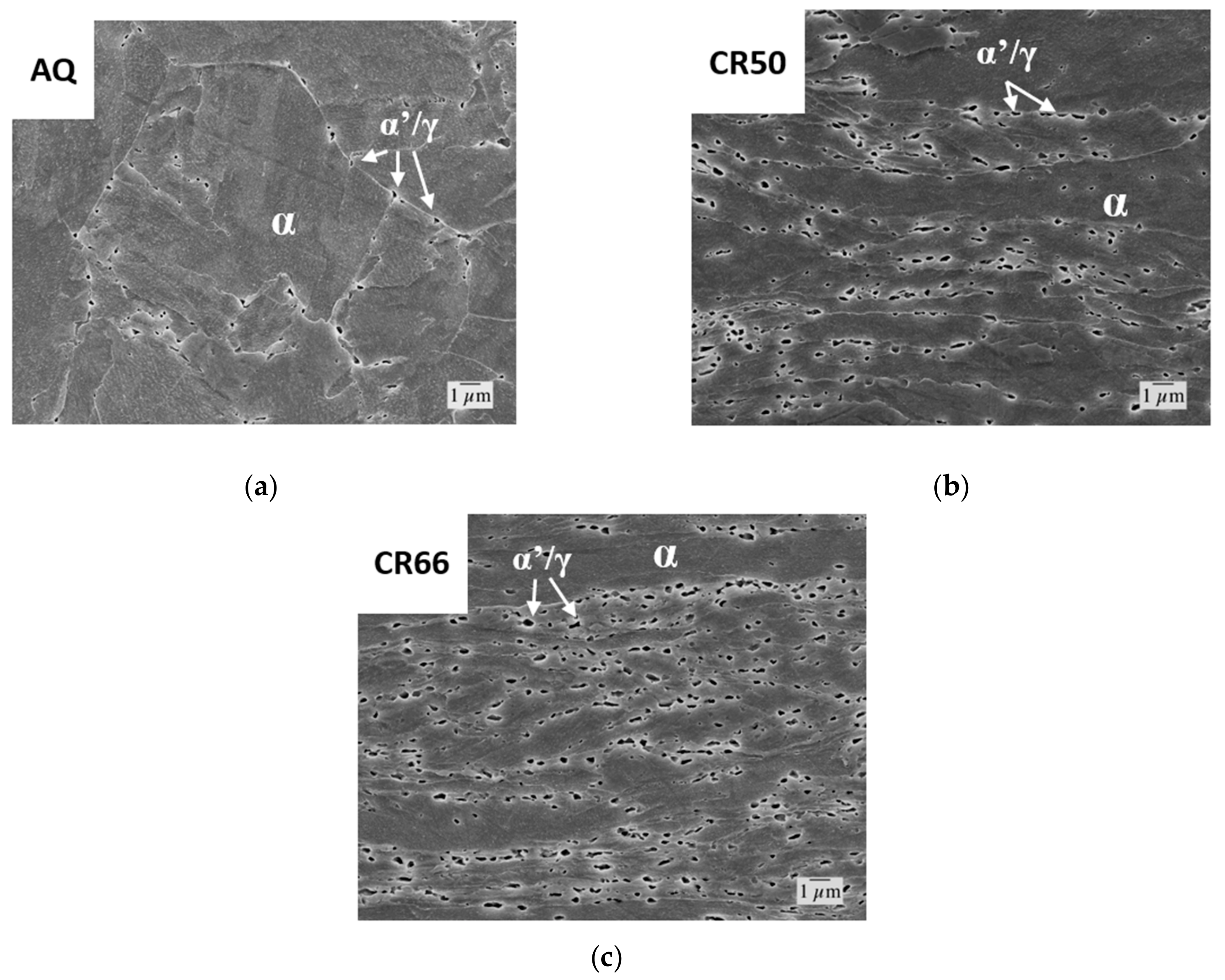
FESEM images of the (a) as-quenched (AQ), (b) CR50, and (c) CR66 conditions after heating to 500 °C at 12 °C/s and isothermal holding for 10,000 s followed by quenching to 30 °C at 100 °C/s. Regions of martensite (previously austenite) are labeled α’/γ. Regions of ferrite are labeled α. One pct Nital Etch.
Figure 8.
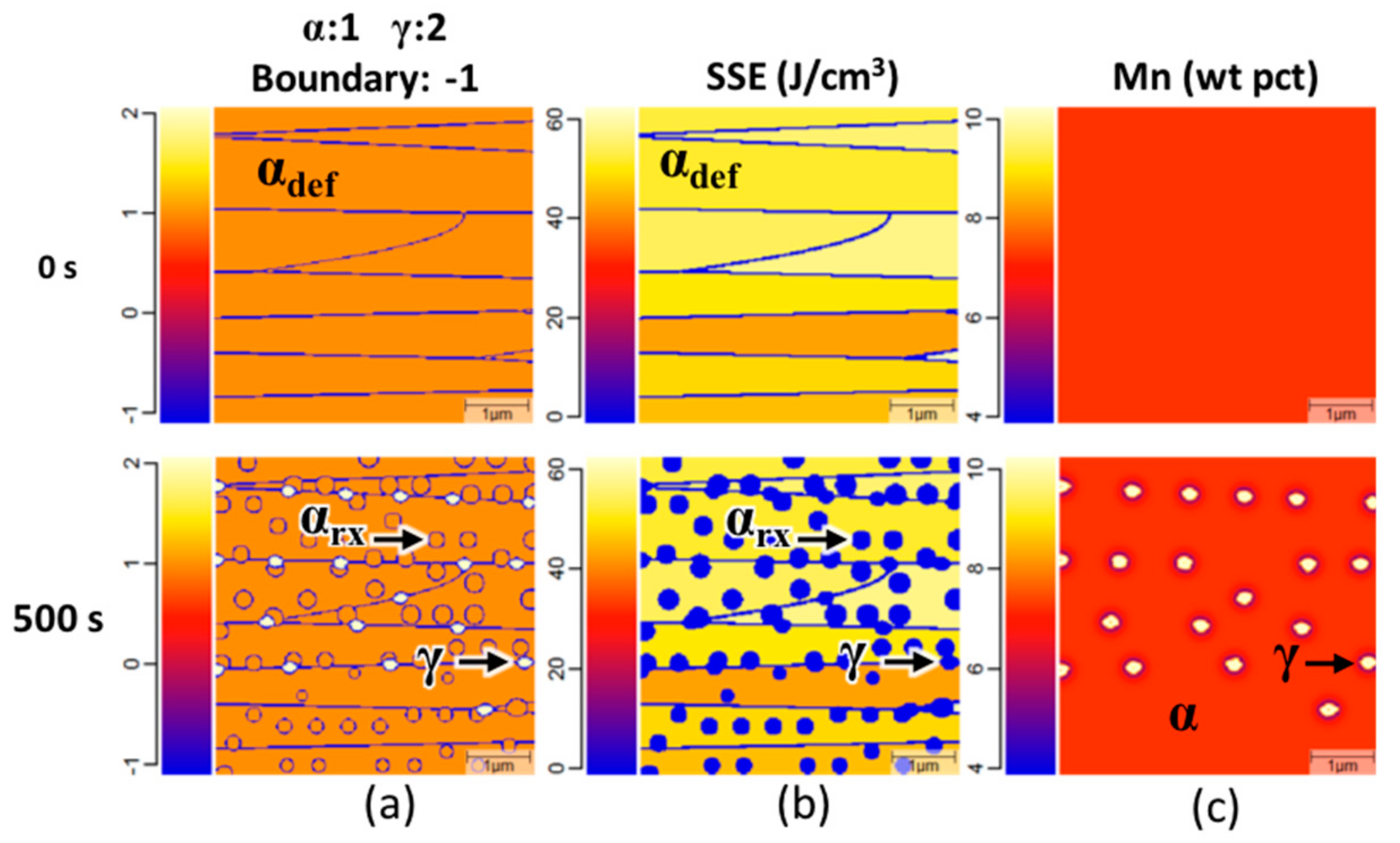
Exemplary MICRESS® simulation results of intercritical annealing with an isothermal holding temperature of 650 °C and a heating rate of 96 °C/s. Timesteps are shown at 0 and 500 s. In the phase maps: (a) The simulation is shown to initially contain only deformed ferrite; after 500 s the phase map shows the presence of homogenously nucleated recrystallized ferrite and ferrite grain boundary nucleated austenite. The stored strain energy maps (b) shows that the deformed ferrite has a distribution of stored strain energy and that recrystallized ferrite and austenite have no stored strain energy. The medium-manganese (Mn) distribution map (c) shows the simulation initially has a homogenous distribution of Mn and that austenite growth occurs with an enriched Mn concentration.
Figure 9.
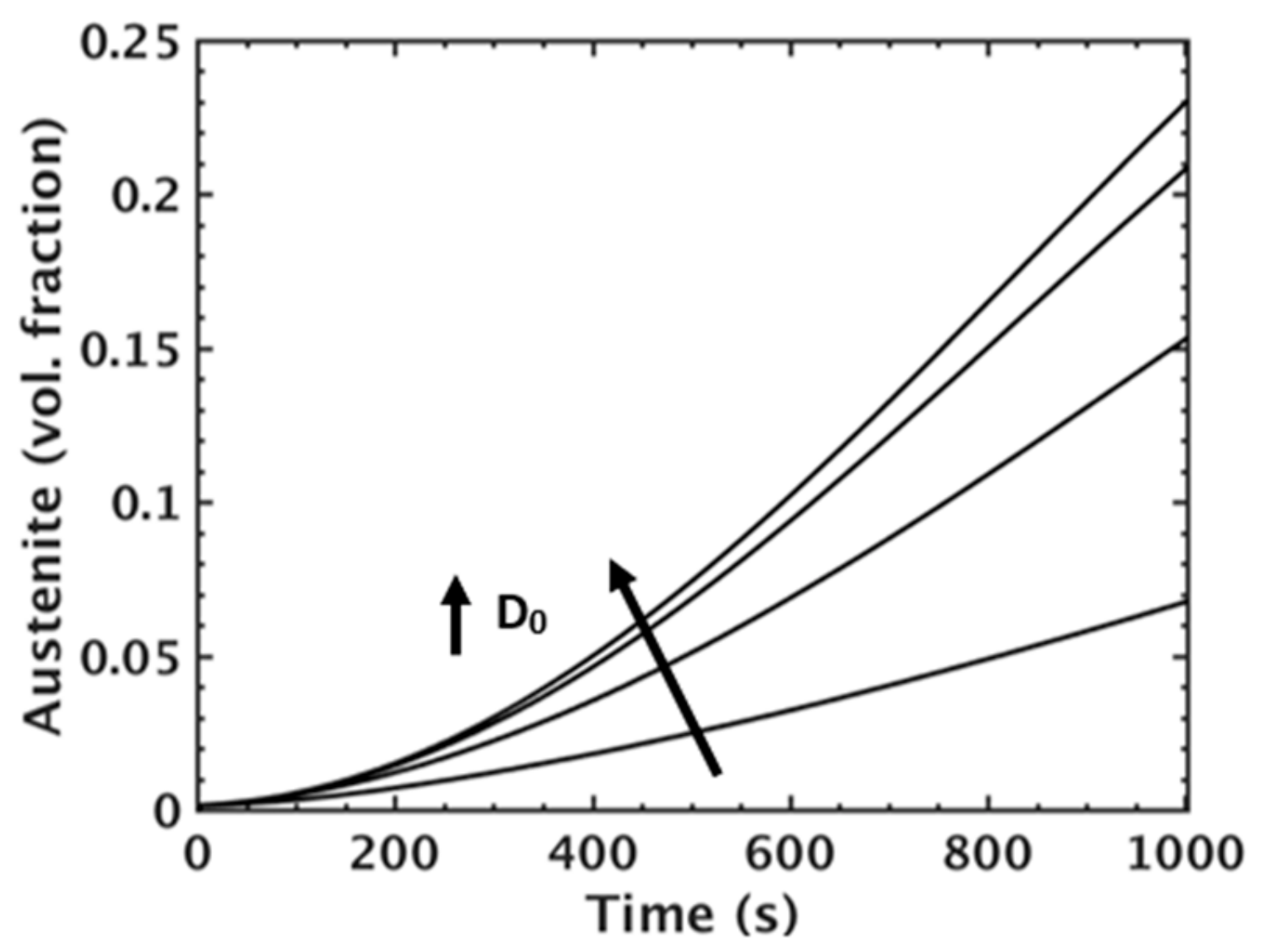
MICRESS® simulation results for austenite volume fraction during intercritical annealing for simulations incorporating different Mn diffusivity pre-factors for Mn diffusivity in ferrite (D0).
Figure 10.
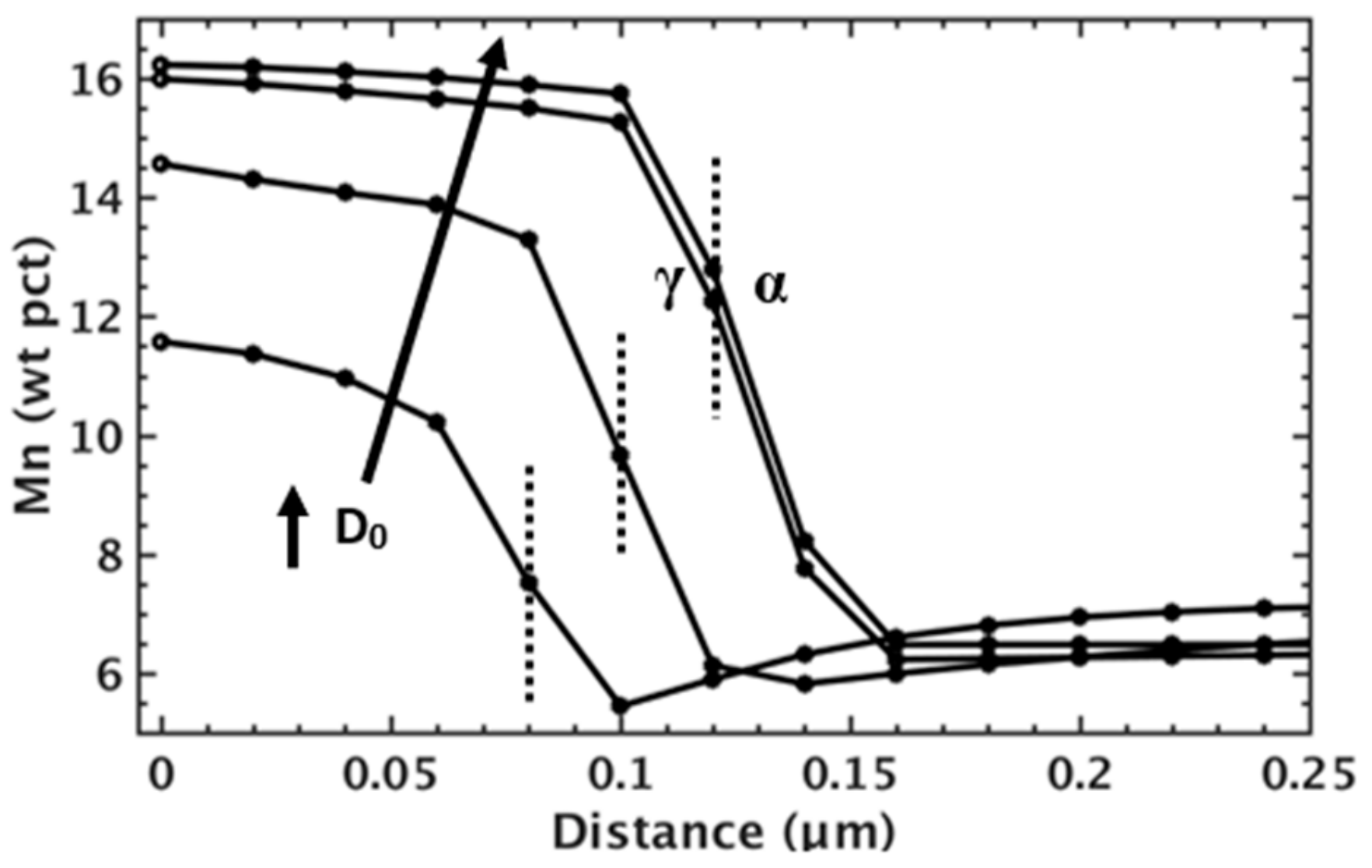
MICRESS® predictions for Mn distributions across the α-γ interface after a 500 s isothermal hold at 650 °C for simulations incorporating different Mn diffusivity pre-factors (D0) for Mn diffusivity in ferrite.
Figure 11.
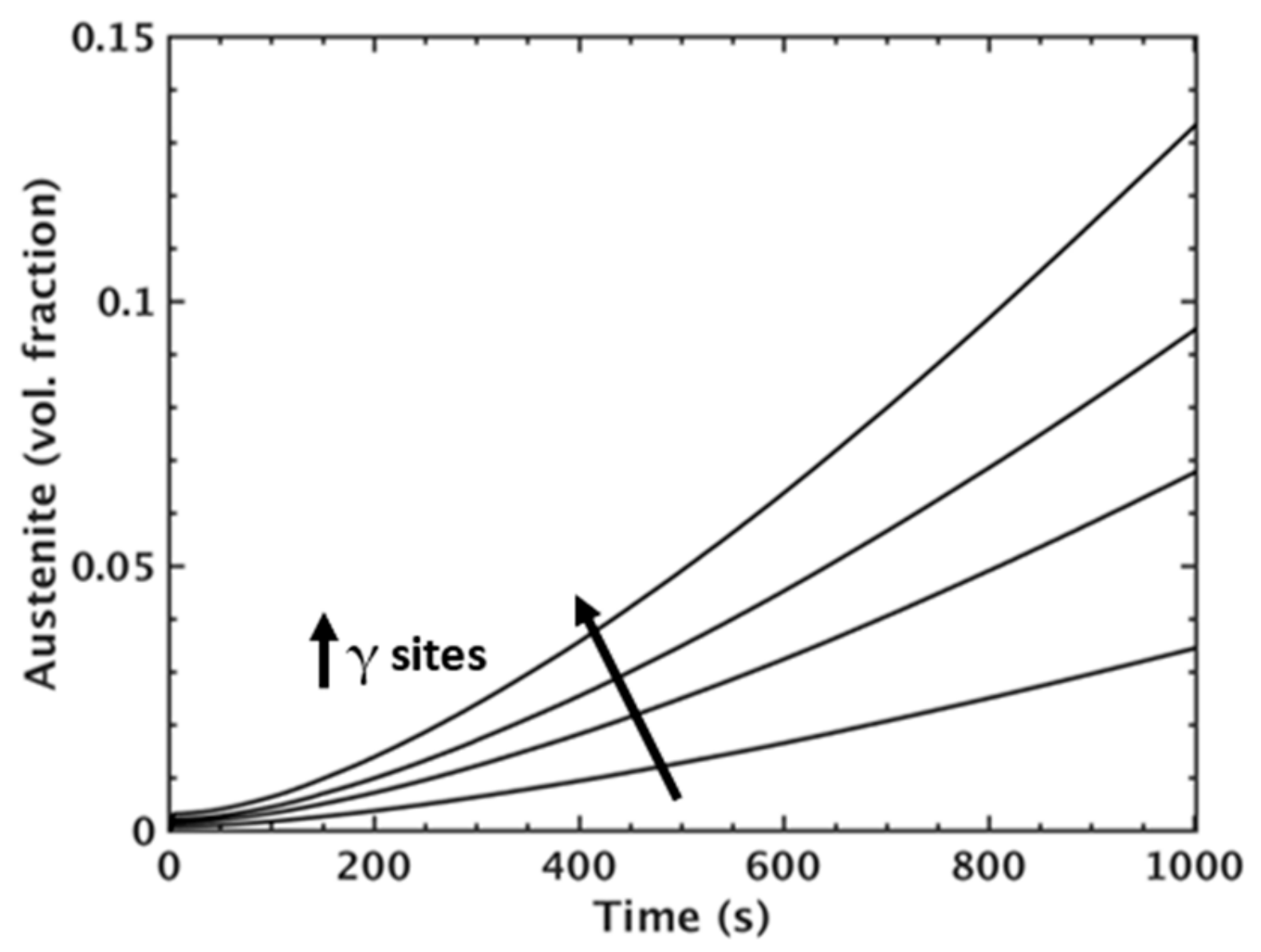
MICRESS® simulation results for austenite volume fraction during intercritical annealing for simulations incorporating different amounts of austenite nucleation sites (γ sites).
Figure 12.
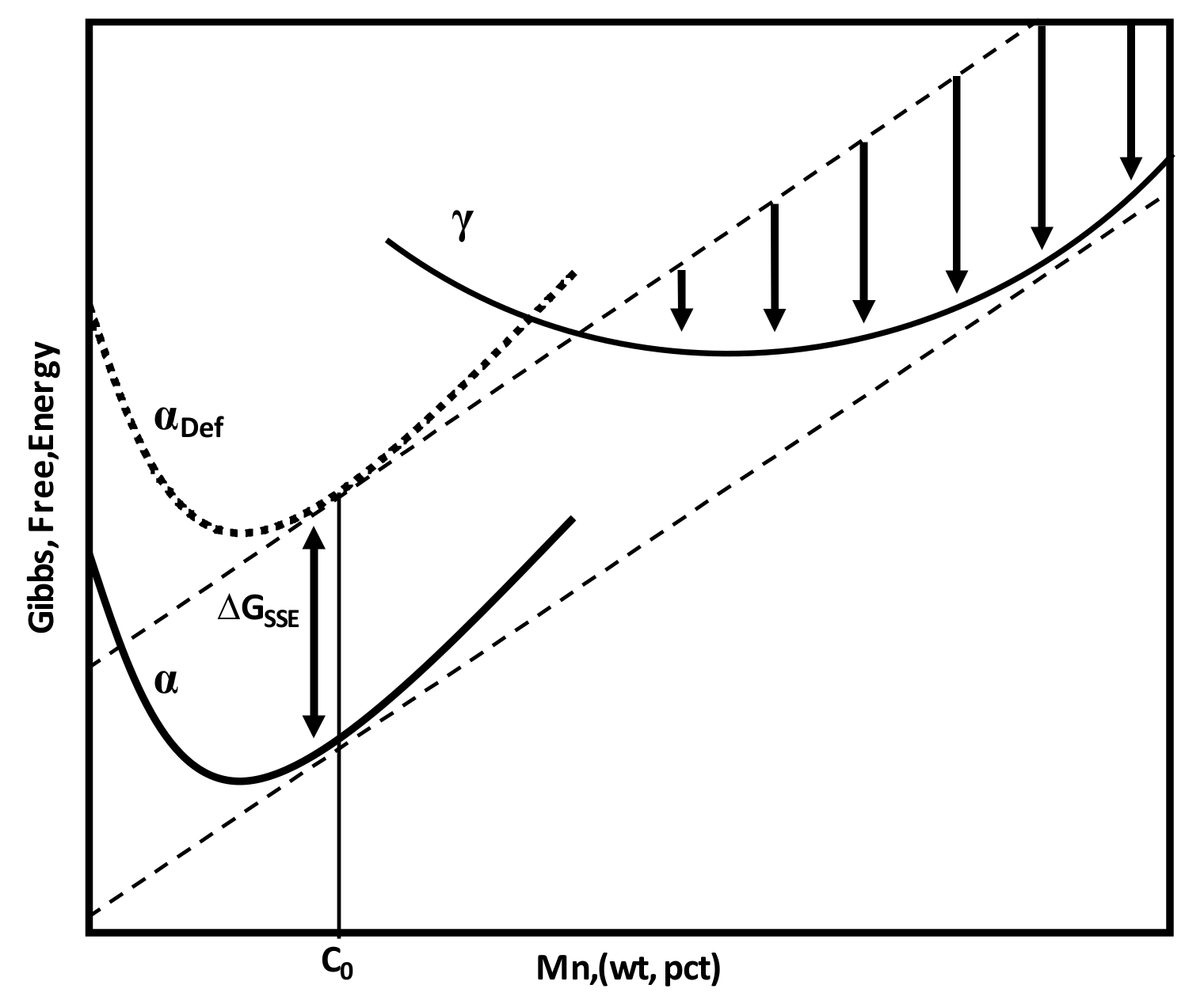
Schematic of Gibbs free energy curves for ferrite, deformed ferrite, and austenite showing driving force for austenite nucleation only from deformed ferrite.
Figure 13.
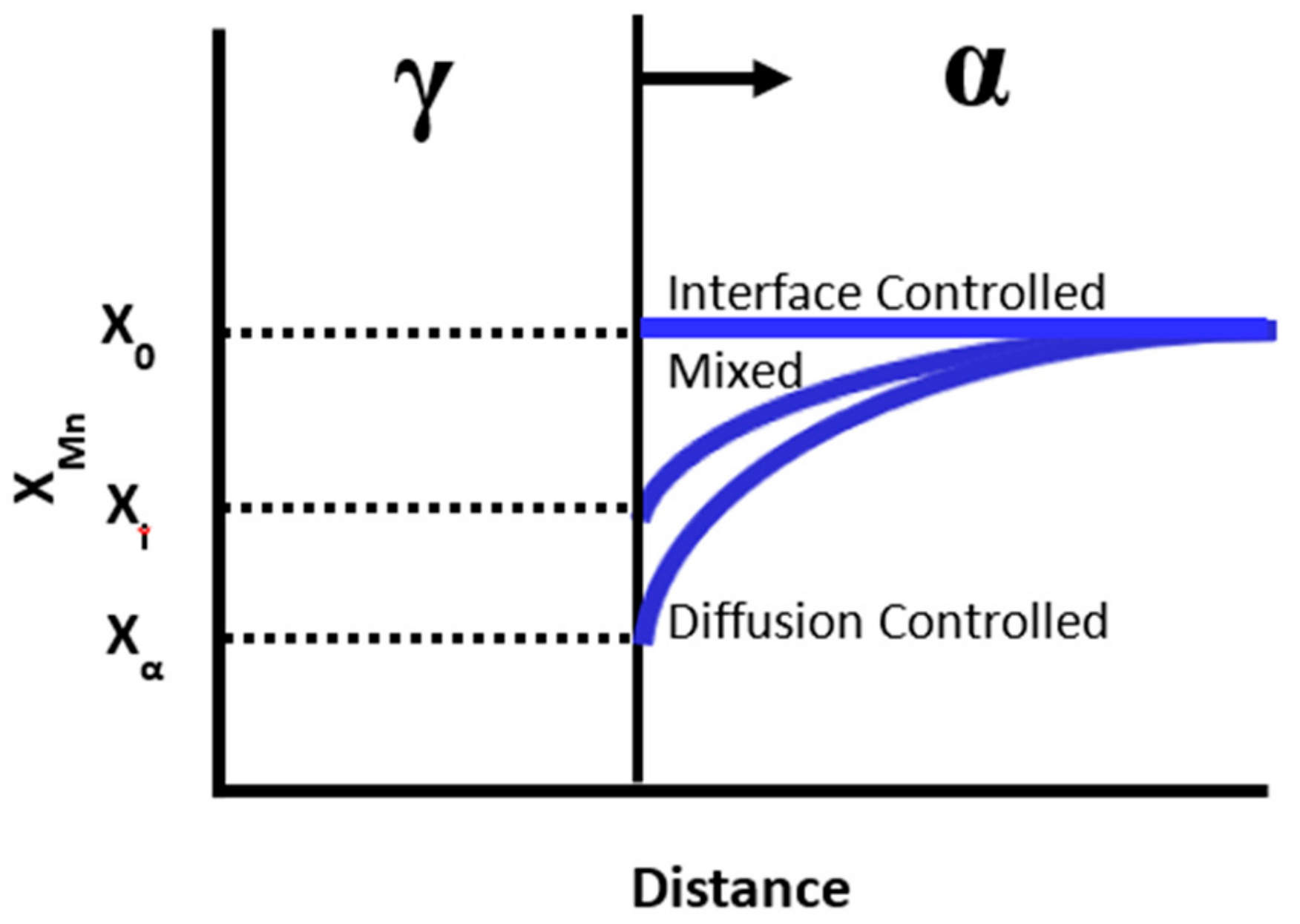
Schematic showing the Mn concentration in ferrite ahead of an advancing α-γ boundary.
Table 1.
Parameters for Initial MICRESS® Simulation.
| Parameter | Value |
|---|
| Interface Energy (J/cm2) | - |
| α-α | 8.0 × 10−5 |
| γ-γ | 5.0 × 10−5 |
| α-γ | 7.0 × 10−5 |
| Interface Mobility (α-α/γ-γ/α-γ) | - |
| M0 (cm4/J s) | 0.05 |
| ∆G* (J/mol) | 140,000 |
| Number of γ Nucleation Sites | 20 |
| Mn Diffusivity in α | - |
| D0 (cm2/s) | 0.756 |
| Q (J/mol) | 224,500 |
| Stored Strain Energy in α (J/cm3) | 10–15 |
Table 2.
Parameter Variations for MICRESS® Simulations.
| Parameter | Value |
|---|
| Number of γ Nucleation Sites | 10, 20, 30, 40 |
| Mn Diffusivity Pre-Factor in α-D0 (cm2/s) | 0.756, 7.56, 75.6, 7560 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).