Abstract
The sludge from a wet-off gas cleaning system of the iron blast furnace (BF) contains significant amounts of iron; however, they cannot be recycled due to their high content of zinc and alkalis. These compounds are detrimental to the optimal performance of iron and steelmaking furnaces. In this work, a comparative laboratory study to reduce zinc and alkali contained in the blast furnace sludge (BFS) is presented. The effect of leaching parameters such as oxidant (i.e., ferric ion, oxygen or ozone), aqueous solution media (i.e., 0.2 M NH4Cl, 0.2 M HCl and 0.1 M H2SO4) and temperature (i.e., 27 and 80 °C) on Zn and alkalis (Na2O and K2O) removal were studied by applying an experimental design. The results obtained show that Zn and K2O removal of 85% and 75% were achieved under the following conditions: Ozone as an oxidant agent and 0.1 M H2SO4 as an aqueous medium, temperature had no significant effect. The results are supported by thermodynamic diagrams and the possible chemical reactions are mentioned. Although the results also indicate that leaching under the above conditions dissolves up to 9% of iron, this loss is much less than leaching without the oxidizing conditions generated by the ozone. The BFS obtained from this treatment could be recirculated to the iron or steelmaking processes to recover iron values.
1. Introduction
The iron and steelmaking processes have been always related to the generation of dust/sludge, slag and other wastes or byproducts. Dust and sludge from the gas cleaning systems of the blast furnace (BF) and the basic oxygen furnace (BOF) contains significant amounts of iron, carbon and other elements. Such materials cannot be recycled because of its impurities (nonferrous metals, mainly zinc) and represent a loss of 2% of the iron and coal contained in the raw material. In 2018, the world mine production was of 2.5 billion tons of iron ore; then, dust and sludge can be representing a loss of 15 to 20 million tons of Fe units annually) [,]. The composition of the blast furnace and basic oxygen furnace dusts varies significantly depending on the process conditions. Blast furnace sludge (BFS) containing 0.77–5 wt.% Zn has been reported [,]. A high content of zinc in the raw material might damage the blast furnace refractory materials and, consequently, shorten its campaign life [,]. In the case of alkalis (Na and K), they increase coke consumption due to the transfer of heat to higher levels in the blast furnace, causing catastrophic swelling and disintegration of ores, pellets and coke. It also causes deterioration of the furnace lining. In brief, zinc and alkalis cause irregularities in the operation process and decrease the production, so only it is recycled up to 20% of the dust and sludge generated [,,].
As mentioned, not recycling of BFS represents an economical lost, due to the relatively high contents of iron (up to 50 wt.% Fe). This also represents an environmental issue, because this sludge contains leachable compounds of lead, cadmium and other elements that are classified as hazardous wastes []. This “waste” material is pile stocked on the steelmaking sites, which is an environmental hazard and occupies valuable land. To recycle BFS to the process, it is necessary to reduce their zinc and alkalis content to at least 0.03 wt.% and 0.12wt.%, respectively [,].
Pyrometallurgical and hydrometallurgical processes have been developed to recycle dust and/or to recover zinc from it. Pyrometallurgical routes are considered the primary choice because of its high potential metal recovery and among the most important processes are: Waelz, rotary heart furnace, PRIMUS, OXYCUP, coke-packed bed, Ausmelt, electric smelting reduction furnace, Plasamadust, plasma-arc, Elkem multi-purpose furnace, submerged plasma, etc. [,]. It is worth noting that in the metallurgical industry, the energy cost and pollution problems produced by pyrometallurgical processes (e.g., combustion gas emissions) have led to a more intensive search of hydrometallurgical alternatives [].
The hydrometallurgical processes developed for dust treatment might be classified as leaching in acid or alkaline media [,]. The efforts have been focused on conventional technology, based on H2SO4, as is shown in Table 1. As can be observed, almost all of studies have been realized for dust from the electric arc furnace steelmaking process (EAF). It is interesting to observe the relativity high H2SO4 concentration used (>1 M) and temperature (>50 °C). In the case of the treatment time, it varies from 15 min (microwave heating leaching assisted) to 1.5 h [,,].

Table 1.
Leaching studies for zinc removal base in H2SO4 media.
Table 1 shows that Zn removal is >70% but the iron loss has a great variability (4% to 60%), which could be due to the different composition between each material. Kukurugya et al. [] studied the behavior of various elements (i.e., zinc, iron and calcium) during the leaching of electric arc furnace dust in sulfuric acid solutions. The authors proposed that the rate limiting step for zinc and calcium was diffusion, while for iron it was the chemical reaction. This confirms the high solubility of some iron oxide compounds, but the iron loss will depend of the type of iron oxides contained in the material.
Other acid media has been studied to steelmaking dust. Shawabkeh [] studied the zinc extraction from a Jordanian electric arc furnace dust by testing different acids (i.e., nitric, hydrochloric and sulphuric) at different concentrations. The highest zinc extraction (i.e., 72%) was obtained with H2SO4 and 50 °C. Although 10% nitric acid concentration can dissolve approximately 33% of zinc [], this leaching agent must be discarded due to the large amount of iron dissolved (it is preferable that the iron remain in the dust to recycle it as raw material).
Novel leaching agents has been used for the treatment of EAF, BF and BOF dust as is shown in Table 2. Organic acid as butyric, citric or iminodiacetic have been studied to extract zinc from basic oxygen sludges or dust and electric arc furnace dust by using the coordination reaction between the organic ligand and zinc ions [,,,]. According to results shown in the table, in some case high Zn removal are obtained (up to 100%) with low Fe loss. The table also shows the alkaline leaching (hydroxide and carbonate solutions) that has been studied due to the insolubility of the ferric in these medium [,]. However, pre-treatment or high concentration of lixiviant is necessary to obtain a good Zn extraction (>70%).

Table 2.
Leaching studies for Zn removal using different aqueous medium.
It is worth mentioning that some processes based on chlorides were developed in the past by the industry. In the Hoogovens Ijmuiden and Delft process [,] the EAF dust is leached under oxidant conditions at approximately 140 °C with HCl to dissolve zinc and a small quantity of iron. This process only considers the Zn dissolution from zinc oxides, but not the presence of zinc ferrite. The limited amount of iron dissolved can be achieved controlling the pH of the solution to limit the attack of the ferric oxides and leaching with O2 to get enough oxidation conditions. A similar process was developed by Terra de Gaia, which is based on the dissolution of ZnO and ZnFe2O4 by FeCl3–HCl at 175 °C []. The dust is mixed with the solution of chloride, reacting with the dust of iron and producing gas chloride, then it is injected into the autoclave at 175 °C. The other one was the Ezinex process de Engite Impianti [] that uses ammonium chloride as aqueous media at 80 °C. The zinc oxide in the dust was dissolved but the zinc ferrite was not perceptibly attacked.
Most of the studies mentioned so far, have been focused on the removal and recovery of zinc, mainly in the electric arc furnace. Fewer studies have been done to treat basic oxygen furnace and blast furnace dust/sludge. For the case of the alkalis, the problem has been addressed since the operation conditions of the blast furnace perspective. Practically no study addresses the simultaneous removal and recovery of zinc and alkalis, respectively.
Considering the previous developments and the most recent studies, it could be concluded that the issue of the reduction of zinc and alkalis in iron and steelmaking dusts is still relevant for the industry due to the iron-containing values and the environment aspects. The aim of the present investigation was to evaluate the elimination of zinc and alkali in a BFS using H2SO4, HCl and NH4Cl solutions and employing different oxidizing agents (i.e., ferric chloride, ferric sulfate, oxygen and ozone) at 27 and 80 °C. Eh–pH diagrams were added to support the experimental results obtained.
2. Materials and Methods
2.1. Material
Samples of blast furnace sludge (BFS) were obtained from the wet-off gas cleaning system of an iron blast furnace (the collection system of the blast furnace dusts consists of three devices: Cyclone, baghouse and wet-off gas cleaning or wet scrubber; the samples were obtained from the latter). The sample was dried (110 °C by 24 h) and crumbled manually. The particle size was determined by wet sieving, using a Tyler sieve.
The BFS sample was analyzed by atomic absorption spectroscopy (AAS) using a Thermo Electron Solaar S4 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA); Ca, K and Na (reported as CaO, K2O and Na2O, respectively) (Sigma-Aldrich Inc., San Luis, MO, USA), a 0.2% w/v Cl3La and 0.1% w/v CsCl (Sigma-Aldrich Inc., San Luis, MO, USA)were added to prevent reduction of the signal by aluminum and as an ionization buffer, respectively. Mg and Al (reported as MgO and Al2O3) were measured under more oxidative conditions using N2O/acetylene mixed gases. SiO2 retained in the solid was determined by gravimetry (oxidation with HClO4). Determination of sulphur and total carbon was carried out using a LECO CS-244 analyzer (LECO, St. Joseph, MI, USA). Complementary chemical analyses were performed using X-Ray fluorescence (XRF) using a Brucker AXS S4 PIONEER spectrophotometer (Brucker, Billerica, MA, USA), following the Li-tetraborate fused bead technique. X-ray diffraction analysis was performance using a Brucker D8 diffractometer (Brucker, Billerica, MA, USA) and monochromatized CuKa radiation. Scanning electron microscopy analysis (Hitachi 5500 microscope; Hitachi, Tokyo, Japan) was realized using BFS powder dispersed in ethanol, sonicated and deposited on the carbon coated grid.
2.2. Experimental Procedure
Experimental tests were performed using a reactor (glass vessel) (Pyrex glass Vessel; Corning Inc., New York, NY, USA) with mechanic agitation. The BFS was mixed with a solution previously prepared, which could be a strong acid (0.1 M H2SO4 and 0.2 M HCl, pH = 0.9 and 1.1, respectively) or weak acid (0.2 M NH4Cl, pH = 5), prepared in deionized pure water (conductivity = 0.06 μS). The material and solution were poured in the reactor and were mixed using an agitation rate of 500 rpm. Solutions 0.1 M of ferric ion as ferric chloride (FeCl3 reagent grade, 97%, Sigma-Aldrich or ferric sulphate (Fe2(SO4)3, reagent grade 97%, Sigma-Aldrich), oxygen (industrial grade, 99.5%) and ozone (1% v/v O3 in O2, generated by a Pacific Ozone L22; Pacific Ozone Technology, Benicia, CA, USA) were used to have an oxidant condition in the aqueous media. Oxygen and ozone were injected to the solution through a bubbler positioned at the bottom of the reactor. The treatment duration was of 60 minutes, at room temperature (27 °C) and 80 °C.
Table 3 shows the experimental design used in this work. The variables studied were three different leaching media: (1) NH4Cl, (2) HCl and (3) H2SO4; three different oxidant agents: (1) Ferric ion (FeCl3 for NH4Cl and HCl, and Fe2(SO4)3 for H2SO4, respectively), (2) oxygen and (3) ozone; and two different temperature levels: (1) 27 °C and (2) 80 °C.

Table 3.
Experimental design implemented to study the different aqueous media, oxidant agents and temperature.
Samples of the solid material were withdrawn as well as samples of the aqueous solution. Chemical analyses were carried out in the collected samples to determine the final composition of solid residues and the barren solution.
3. Results
3.1. BFS Characterization
Table 4 shows the chemical composition and the main mineral species contained in the BFS. This reconstruction was based in the chemical analysis and the X-ray diffraction analysis (Figure 1) and elemental analysis by Scanning Electron Microscopy/Energy Dispersive X-Ray Spectroscopy (SEM/EDS) (Hitachi 5500 microscope, Hitachi, Tokyo, Japan), Figure 2.

Table 4.
Chemical analysis of the blast furnace sludge (BFS) and mineralogical reconstruction of the Fe compounds.
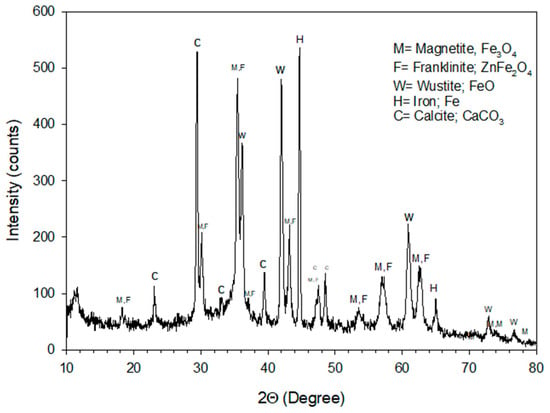
Figure 1.
X-Ray diffraction pattern obtained for the BFS.
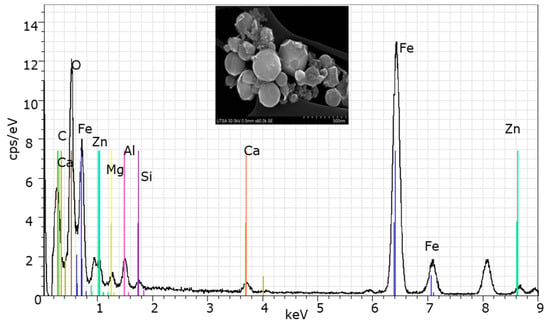
Figure 2.
SEM/EDS micrograph of the BFS.
Figure 1 shows the XRD pattern of the initial sample, indicating that the residue is formed by multiple compounds. We want to show only those of Fe to determine its phases and their possible applications. As can be seen, iron metallic, wustite (FeO) and magnetite (Fe3O4) the main phases observed.
Figure 2 presents the SEM image showing the size and morphology of BFS particles. These microstructures exhibit spherical-shaped particles and some side-striped eroded particles with octahedral shape, which result due to intense erosion that occurs during transportation through the dust collection system. On the other hand, can be observed that SEM shows a particle size below one micron.
Element mapping by scanning electron microscopy shown in Figure 3 indicate that zinc and alkalis are integrated to iron compounds (metallic and iron oxides). It is clear that BFS can have a homogeneous chemical composition, which suggests they are formed by multi-metallic oxides complex where iron, silicon, zinc, alkalis and minor elements could have joined by sintering due to high temperatures and atmospheric conditions prevailing during the extraction process. It is worth noting that liberated franklinite or sodium and potassium oxides were not observed.
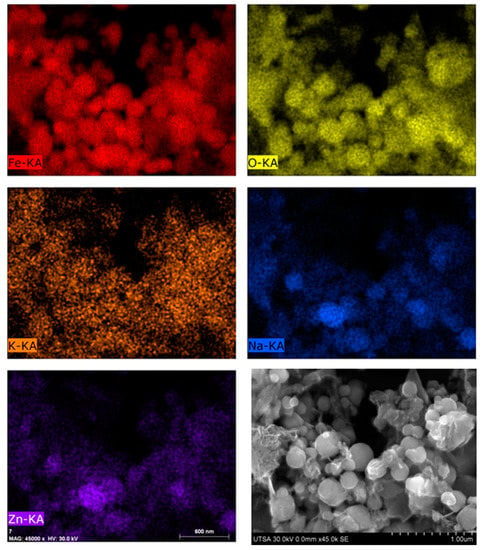
Figure 3.
SEM element mapping of BFS.
3.2. Removal of Zinc and Alkali from Blast Furnace Dust by Different Experimental Conditions
The following results show the removal of zinc and alkali obtained treating the steelmaking process BFS at different experimental conditions. Figure 4 shows the extraction of Zn and alkalis (as Na2O and K2O) for each of the tests carried out. The extraction was obtained from the balance of the chemical analysis of the aqueous solutions and solid residues sampling in the tests. The results showed in the figure indicate that there are great differences in the zinc and alkalis contents obtained with each experimental treatment.
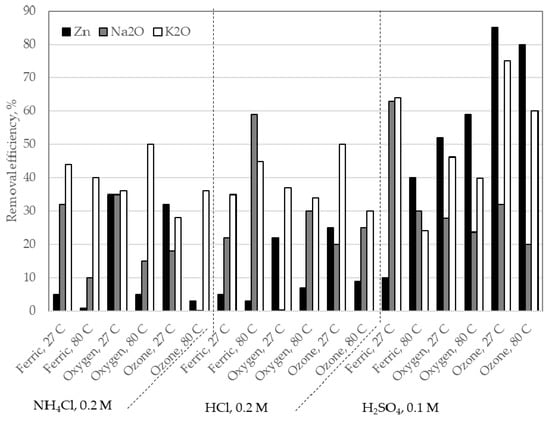
Figure 4.
Zinc and alkalis (Na2O and K2O) removal from BFS at different experimental tests.
Figure 4 also shows that the best results for zinc removal (>70%) were obtained when ozone and sulphuric acid were used. When other oxidants were used, it was not observed a significant decrease in the extraction of zinc. Exception case was obtained when NH4Cl was used, with a zinc extraction of 40%, due possibly to the dissolution of zinc oxides. It is interesting to note that the zinc removal was not observed when ferric ions were used. Possibly, the quantity of iron contained in the sample, mainly composed by hematite, under acid conditions have already conditions oxidant, but that are not enough for it to separate and to remove the zinc ferrites contained in the BFS sample. These results are in accordance with the conclusions made by other researchers [,], although the Zn/Fe ratio of the samples was different. It is important bear in mind that a smaller Zn/Fe ratio diminishes the effect of the ion ferric as an oxidant.
Figure 5 shows the remaining zinc and alkalis (Na2O + K2O) content after the BFS leaching tests. In this figure it can be appreciated that the removal of zinc varied greatly depending on the conditions of the aqueous media and the oxidant employed, as well as with the temperature. The results indicate that it was possible to obtain a zinc removal of 0.45% to less than 0.1%, when the sulfuric acid and the ozone were used. In these tests, a removal of alkalis of 75% and 60% for K2O and 30% and 20% for Na2O, respectively, were obtained.
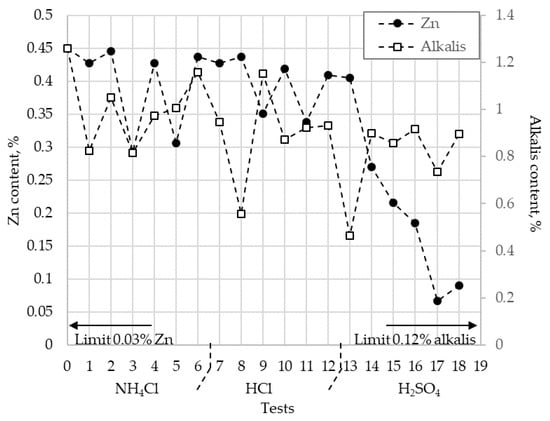
Figure 5.
Zn and alkalis content in the BFS at different experimental conditions.
It is interesting to note in Figure 5 that there were conditions allowing at the same time the removal of zinc and a significant decreasing of the alkali content. They were precisely those performed in an aqueous media that allowed dissolution of the sulphate-type compounds.
However, the results shown in Figure 6 indicate that there is a reduction in the iron containing in BFS, of 41.6 (original sample) to a minimum of 36%. However, this loss in iron is smaller than the one that would be presented if the oxidizing conditions were not present. These conditions allow the maintenance of the stability of the most acid soluble iron oxides species in the aqueous medium, also considering the low solubility of the metallic iron (the main element of the Fe compounds containing in the BFS in the low acid sulphuric concentration media). In any case, the recovery of iron of the solution is possibly increasing the pH of the aqueous solution, also under oxidant conditions, to obtain the precipitation of ferric oxide, previous separation of the zinc and alkalis dissolved in solution.
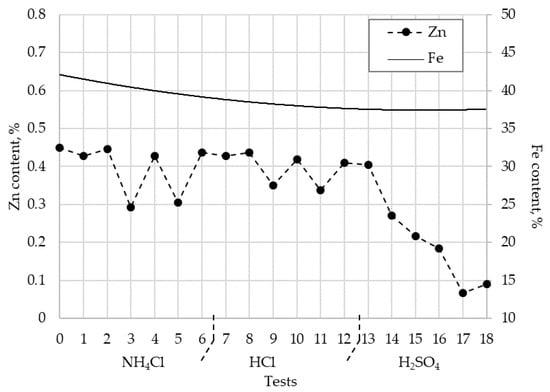
Figure 6.
Zn and Fe content in blast furnace sludge at a different experimental test.
3.3. 3× 3 × 2 Experimental Design and Analysis of Variance
The effect of the individual experimental variables on the Zn, Na2O and K2O removal was examined using the statistical analysis of data. Analysis of variance (ANOVA) of the experimental tests data at different conditions was used to evaluate the effect of each individual variable.
Table 5 shows the main effects, as determined by ANOVA, using NCSS software (NCSS 2000 software, NCSS, LLC, Kaysville, UT, USA) []. The table indicates the values of degree freedom (DF), sum of squares (SS), media of squares (MS), Fisher ratio (F), probability level (Prob Level) and the probability that a false null hypothesis can be rejected (Power) with a 95% level of confidence (α = 0.05). According to F, Prob Level and Power values, ANOVA shows that under the studied conditions, leaching media and the type of oxidant were the most important factors during the extraction of zinc. Results also indicated that within the analyzed range, the three variables studied had the lower effect in Na2O and K2O removal.

Table 5.
Analysis of variance (ANOVA) for Zn, Na2O and K2O removal from blast furnace sludge.
These results are graphically shown in Figure 7, which shows the box graph for the individual effects of the experimental parameters or variables. Each plot shows the box with the minor and major values, the average value and percentiles for each parameter. Furthermore, the comparison between different oxidants shows that for the case of zinc, the ozone could generate slightly more favorable conditions for increased extraction of zinc BFS. For alkalis, although the average removal of these compounds between the three oxidants was similar, under certain conditions the ferric ion allowed a better removal. These observations are discussed below.
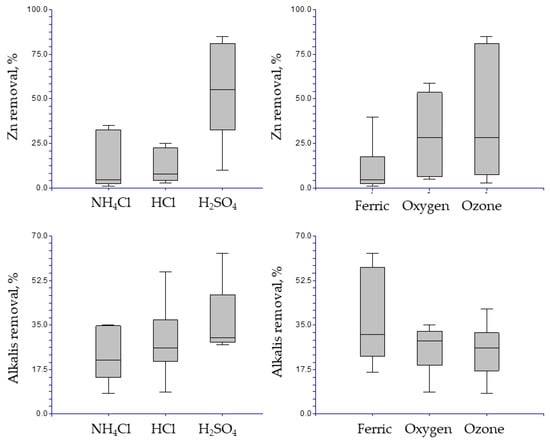
Figure 7.
Box graphs for the individual effects of leaching media and oxidant in Zn and alkalis removal.
Figure 8 shows the average response curves for the individual parameters to evaluate the effect of the ozone (considering that the ferric ion has the least effect in the Zn removal). The graphs were constructed with data from the tests that used the ozone and oxygen. The most significant comparative results were found for the oxidant (see Figure 8a), which Zn removal increased from 16% to 40% using oxygen and the ozone, respectively. However, alkalis removal was not affected by the change of oxidant. On the other hand, Fe loss increased from 4% to 9% when the ozone was employed. In the case of the type of aqueous solution, Figure 8b shows that the best result was obtained with H2SO4 where Zn removal increased from 20% and 18% to 80% using NH4Cl, HCl and H2SO4 respectively. Alkalis removal and Fe loss also increased when sulfuric acid was employed. In the case of temperature (see Figure 8c), Zn removal decreased by increasing temperature from 27 °C to 80 °C, while alkalis and Fe increased. Generally, in a leaching process, at higher temperature the velocity of dissolution is increased. However, an increment of temperature diminishes the absorption of ozone in the solution. In this case, it is possible that the effect of temperature in the reaction of dissolution were annulled, by which a change in the removal of zinc was observed.
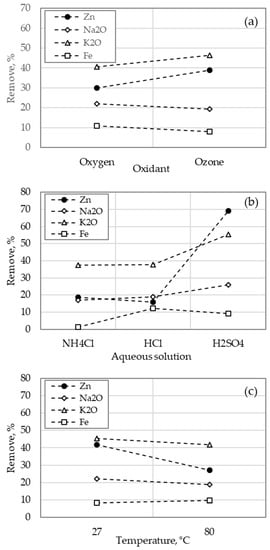
Figure 8.
Response curves of individual effects: (a) Oxidant, (b) type of aqueous solution and (c) temperature.
According to results, the best conditions to remove up to 70% of Zn and alkalis are using a solution of 0.1 M H2SO4 and ozone (1% v/v) as the oxidant, at 25 °C. Comparing with conditions shown in Table 1, the sulphuric acid concentration used in the present work was very low. Besides the additional advantage of using ambient temperature, it also has low loss of iron during leaching. Obtaining a zinc extraction, like those obtained in the studies cited in the table.
4. Discussion
The fundament of zinc and alkalis dissolution into strong or weak acid media using ozone or oxygen is that aqueous media react with the oxides of zinc and alkalis, incorporating them with the media as aqueous ions, just like it is shown by the average chemical analysis of the BFS with and without ozone treatment.
The presence of an oxidizing agent, like oxygen or ozone, allows the creation of severe oxidizing conditions in aqueous media, keeping stable and practically insoluble the ferric oxides. This oxidizing environment also creates the necessary conditions to transform oxides or ferrites of zinc and alkalis, as sodium and potassium oxides, to sulfates, chloride or chlorates, compounds with high solubility in an oxidizing aqueous media.
Eh–pH diagram [] for a Zn–O–S system (see Figure 9a), shows that zinc, which is in the form of zinc ferrite in the BFS, reacts in acid aqueous medium (pH < 5) under oxidant conditions (Eh > 0) to produce soluble species. According to the results discussed above, the aqueous medium appropriate is the sulfuric acid and ozone as an oxidizing agent. The following reactions can be possible:
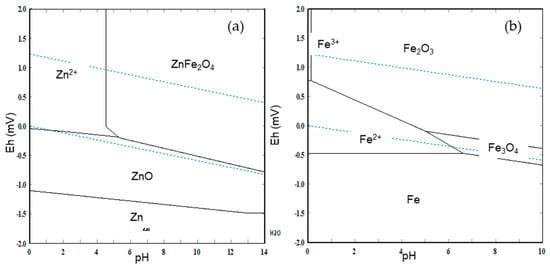
Figure 9.
Eh–pH diagrams. (a) Zn–O–S system and (b) Fe–O–S system; under standard conditions at 25 °C [].
In the absence of the ozone:
ZnFe2O4 + H2SO4 → Fe2O3 + ZnSO4(a) + H2O → ΔG°20 °C = −107 kJ.
In the presence of the ozone:
ZnFe2O4 + H2SO4 + 2/3O3(g) → Fe2O3 + ZnSO4(a) + H2O + O2(g) → ΔG°20 °C = −215 kJ.
ΔG°20 °C values were obtained from HSC Chemistry 6 [].
According to the Eh–pH diagram for a Fe system (Figure 9b), under very acid and oxidant conditions, iron oxides could be dissolved. Then, low concentrations of acid (pH > 5) decrease iron leaching. In the case of iron metallic or iron species as magnetite or wustite, they could dissolve to a pH between 1 and 5 (weak acid conditions). However, an oxidant acid media (Eh potential > 0.5 for pH > 2) could inhibit iron dissolution.
The alkalis contained in BFS are found as ferrites or sodium (potassium)-iron jarosites. These compounds could react in different aqueous medium to obtain soluble species. When an acid medium and oxidant agent is used, the alkalis can be dissolved and separated from the BFS. The possible reactions involved are:
In the absence of the ozone:
[K]Na2O*Fe2O3 + H2SO4 → [K]Na2SO4(a) + Fe2O3 + H2O → ΔG°20 °C = −277 kJ.
In the presence of the ozone:
[K]Na2O*Fe2O3 + H2SO4 + 2/3O3 → [K]Na2SO4(a) + Fe2O3 + H2O + O2 → ΔG°20 °C = −385 kJ.
According to the results, the removal of alkalis only was possible in the test where high oxidation conditions were employed, obtaining the best results when the ozone was employed as the oxidizing agent. The use of other oxidizing agents, as oxygen or ferric chloride, did not decrease the zinc and alkalis content in the blast furnace sludge.
The Zn/Fe and [K]Na/Fe ratios in the BFS allow zinc and alkalis to be as stable species (e.g., oxides/ferrites) under alkaline and acid non-oxidizing conditions, being necessary to increase the potential of oxidation of the aqueous medium to obtain the dissolution of these compounds. The use of the Fe3+ ion could inhibit the Zn dissolution. This is possible if some Fe dissolution from zinc ferrite can be necessary to zinc leaching. Then, an initial Fe concentration in solution could decrease the ferrite dissolution due to equilibrium between the Fe concentration in products. In the case of oxygen or, better, the ozone, the oxidant condition for zinc ferrite and alkalis removal is possible.
5. Conclusions
The high content of undesirable elements (alkalis and zinc) in iron blast furnace sludge (with respect to the 0.03% Zn and 0.12% alkalis required by iron and steelmaking industry) generated a loss of valuable units of iron, additionally to the potential environment hazards and the cost by handling and disposal/confinement. In accordance with the results presented, the BFS recycling by hydrometallurgical process is possible. The best conditions were obtained when the BFS was subjected to an acid leach process with sulfuric acid (0.1 M H2SO4), employing ozone (1% v/v O3) to obtain high oxidizing conditions. Only under these conditions, an important removal of zinc (up to 85%) and alkalis (up to 35% Na2O and 75% K2O) was possible to decrease the content of these compounds in the BFS. The fact that similar results were obtained under ambient temperature and at 80 °C indicates a compensation of two factors; the increment in the iron dissolution rate under high temperature and the diminishing of the oxidizing conditions due to the ozone decomposition. Although the zinc and alkalis concentration are still high according to the maximum values recommended, their lower content would allow it to increase the percentage of BFS added to the raw material of the blast furnace. On the other hand, the Fe loss (up to 13%) is still important, if blast furnace sludge volume is considered. Then, a Fe recovery treatment of the leaching solutions (e.g., precipitation) will be required.
Author Contributions
Data curation, F.R.C.P.; Formal analysis, F.R.C.P.; Funding acquisition, M.d.J.S.-A.; Investigation, M.d.J.S.-A., N.P.-R. and J.R.-C.; Methodology, F.R.C.P. and J.R.-C.; Project administration, M.d.J.S.-A.; Supervision, F.d.J.L.-S.; Validation, G.I.D.-P. and A.A.G.-I.; Visualization, F.d.J.L.-S.; Writing—original draft, F.R.C.P.; Writing—review & editing, G.I.D.-P. and A.A.G.-I.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank to International Center for Nanotechnology and Advanced Materials–Kleberg Advanced Microscopy Center–at the University of Texas at San Antonio (ICNAM-UTSA) for their technical assistance with the SEM characterization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trinkel, V.; Mallow, O.; Aschenbrenner, P.; Rechberger, H.; Fellner, J. Characterization of blast furnace sludge with respect to heavy metal distribution. Ind. Eng. Chem. Res. 2016, 55, 5590–5597. [Google Scholar] [CrossRef]
- Geological Survey, U.S. Mineral Commodity Summaries; United States Government Printing Office: Washington, DC, USA, 2019; p. 89.
- Halli, P.; Hamuyuni, J.; Leikola, M.; Lundström, M. Developing a sustainable solution for recycling electric arc furnace dust via organic acid leaching. Miner. Eng. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Sedlakova, Z.; Havlik, T. Appearance of non-ferrous metals in iron and steel making plant and their possible treatment. Acta Metall. Slovaca 2006, 12, 209–218. [Google Scholar]
- Kukurugya, F.; Vindt, T.; Havlík, T. Behavior of zinc, and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 20–32. [Google Scholar] [CrossRef]
- Dutra, A.J.B.; Paiva, P.R.P.; Tavares, L.M. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2006, 19, 478–485. [Google Scholar] [CrossRef]
- Williams, P.J.; Cloete, T.E. The production and use of cictric acid for the removal of potassium from the iron ore concentrate of the Sishen Iron Ore Mine, South Africa. S. Afr. J. Sci. 2010, 106, 1–5. [Google Scholar] [CrossRef][Green Version]
- Yusfin, Y.S.; Chernousov, P.I.; Garte, V.; Karpov, Y.A.; Petelin, A.L. The role of alkalis and conserving resources in blast-furnca smelting. Metallurgist 1999, 43, 54–58. [Google Scholar] [CrossRef]
- Aydin, S.; Dikec, F. The removal of alkalis from iron ores by chloride volatilization. Can. Metall. Q. 1990, 29, 213–216. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD): Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhang, Z.; Zhang, G. Removal of zinc from basic oxygen steelmaking filter cake by selective leaching with butyric acid. J. Cleaner Prod. 2019, 209, 1–9. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.M. Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using mononitrilotricetate anion and hexahydrated ferric chloride. J. Hazard. Mater. 2002, 91, 257–270. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.Y.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Cleaner Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- She, X.; Wang, J.; Wang, G.; Xue, Q.; Zhang, X. Removal mechanism of Zn, Pb and alcalis from metallurgical dusts in direct reduction process. J. Iron. Steel Res. Int. 2014, 21, 488–495. [Google Scholar] [CrossRef]
- Havlik, T.; Turzakova, M.; Stopic, S.; Friedrich, B. Atmospheric leaching of EAF dust with diluted sulphuric acid. Hydrometallurgy 2005, 77, 41–50. [Google Scholar] [CrossRef]
- Kul, M.; Oskay, K.O.; Simsir, M.; Subutay, H.; Kirgezen, H. Optimization of selective leaching of Zn from electric arc furnace steelmaking dust using response surface methodology. Trans. Nonferrous Met. Soc. China 2015, 25, 2753–2762. [Google Scholar] [CrossRef]
- Trung, Z.H.; Kukurugya, F.; Takacova, Z.; Orac, D.; Laubertova, M.; Miskufova, A.; Havlik, T. Acidic leaching both of zinc and iron from basic oxygen furnace sludge. J. Hazard. Mater. 2011, 192, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Veres, J.; Lovás, M.; Jakabský, S.; Sepelák, V.; Hredzák, S. Characterization of blast furnace slufge and removal of zinc by microwave assisted extraction. Hydrometallurgy 2012, 129–130, 67–73. [Google Scholar] [CrossRef]
- Shawabkeh, R.A. Hydrometallurgical extraction of zinc from Jordanian electric arc furnace dust. Hydrometallurgy 2010, 104, 61–65. [Google Scholar] [CrossRef]
- Ciba, J.; Zolotajkin, M.; Kluczka, J.; Loska, K.; Cebula, J. Comparison of methods for leaching heavy metals from composts. Waste Manage. 2003, 23, 897–905. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Yang, T.; Rao, S.; Hu, W.; Liu, W.; Chen, L. Selective leaching of zinc from blast furnace dust with mono-ligand and mixed-ligand complex leaching systems. Hydrometallurgy 2017, 169, 219–228. [Google Scholar] [CrossRef]
- Omran, M.; Fabrotius, T. Improved removal of zinc from blast furnace sludge by particle size separation and microwave heating. Miner. Eng. 2018, 127, 265–276. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Revitzer, H.; Lundström, M. Selection of leaching media for metal dissolution from electric arc furnace dust. J. Cleaner Prod. 2017, 164, 265–276. [Google Scholar] [CrossRef]
- Chaijraksa-Fujimoto, R.; Maruyama, K.; Miki, T.; Nagasaka, T. The selective alkaline leaching of zinc oxide from electric arc furace dust pre-treated with calcium oxide. Hydrometallurgy 2016, 159, 120–125. [Google Scholar] [CrossRef]
- Ruiz, O.; Clemente, C.; Alonso, M.; Alguacil, F.J. Recycling of an electric arc furnace flue dust to obtain high grade ZnO. J. Hazard. Mater. 2007, 141, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Genstskens, R. Pressure leaching of zinc-bearing blast furnace dust. In Proceedings of the Lead-Zinc ’90, Warrendale, PA, USA, 18–21 February 1990; Minerals, Metals & Materials Society: Warrendale, PA, USA, 1990; pp. 529–545. [Google Scholar]
- Van Weert, G.; Van Sandwijk, A.; Kat, W.; Honingh, S. The treatment of iron making blast furnace dust by chloride hydrometallurgy. In Proceedings of the Hydrometallurgy Fundamentals, Technology and Innovation, Littleton, CO, USA, 1–5 August 1993; Hiskey, J.B., Warren, G.W., Eds.; Society for Mining, Metallurgy and Exploration: Englewood, IL, USA, 1993; pp. 931–946. [Google Scholar]
- Mcelroy, R.O.; Murray, W. Developments in the Terra Gaia process for the treatment of EAF dust. In Proceedings of the Iron Control and Disposal, Montreal, QC, Canada, 20–23 October 1996; Dutrizac, J.E., Harris, G.B., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 1996; pp. 505–517. [Google Scholar]
- Olper, M. Zinc extraction from EAF dust with Ezinex Preocess. In Proceedings of the Recycling of Metals and Engineered Materials Minerals, Warrendale, PA, USA, 12–16 November 1995; Queneau, P.B., Peterson, R.D., Eds.; The Metals & Materials Society: Pittsburgh, PA, USA, 1995; pp. 563–578. [Google Scholar]
- Hintze, J. NCSS 2000. Available online: www.ncss.com (accessed on 19 April 2019).
- Roine, A. HSC Chemistry 6. Available online: www.outotec.com/hsc (accessed on 22 March 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).