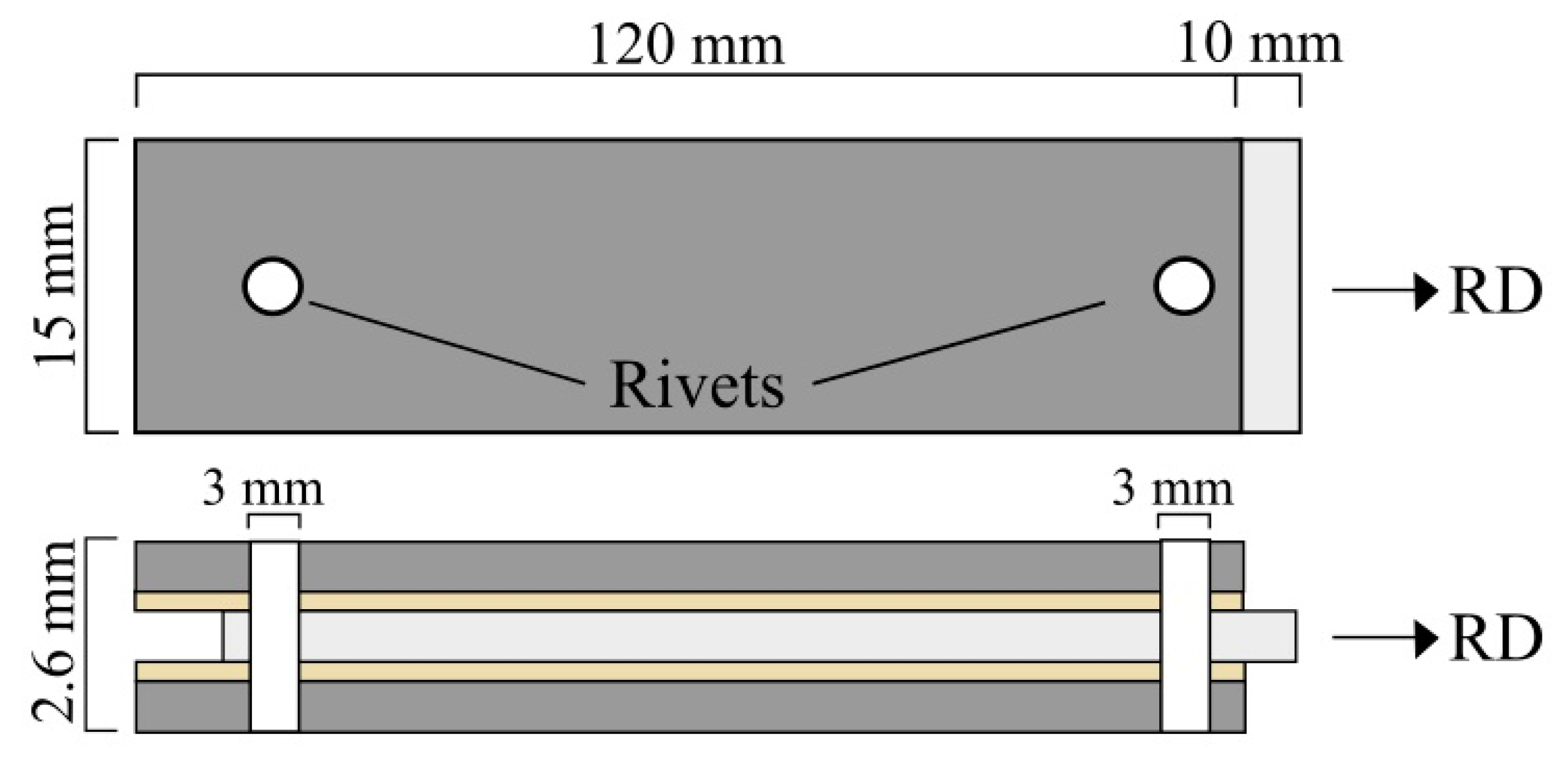
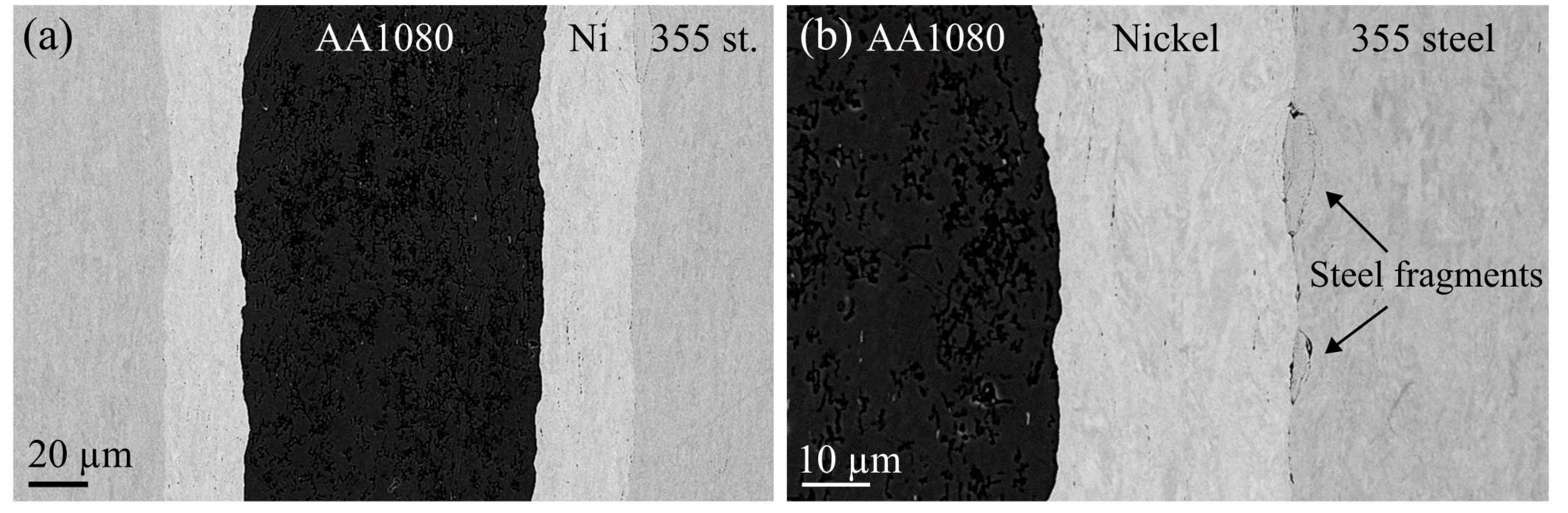
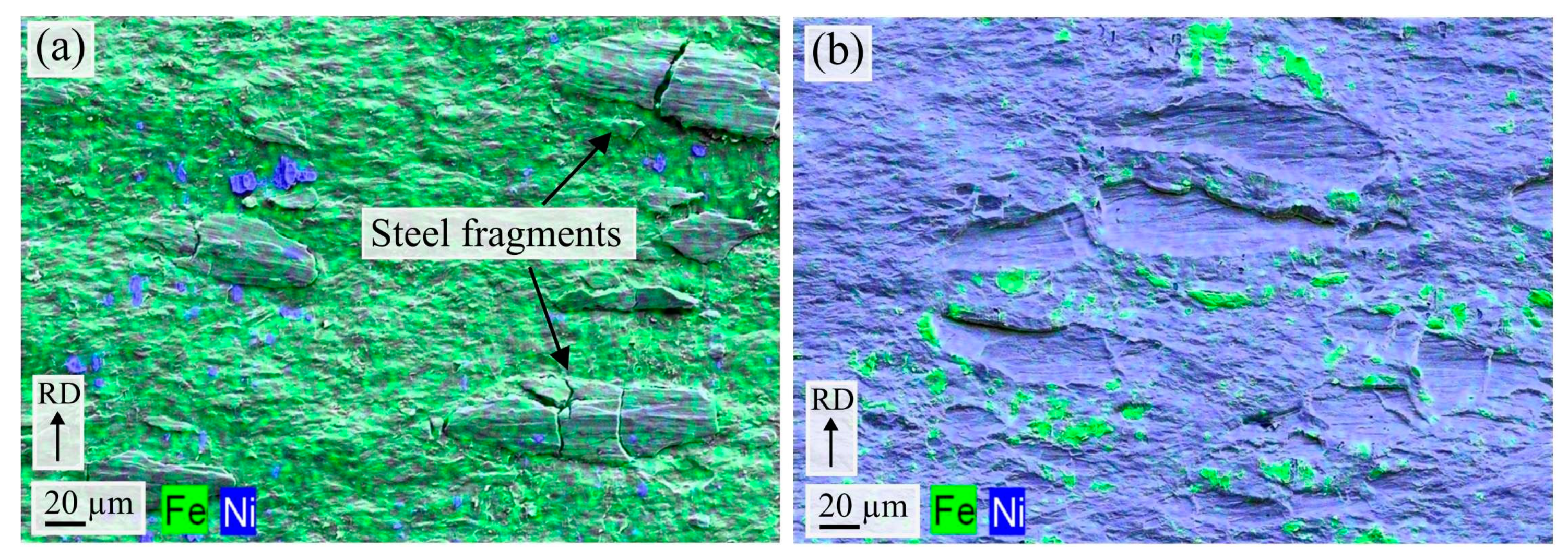
3.2. Characteritics of the Post-Rolling Heat Treated Composites
In order to investigate the interaction and IMP formation between the metals, post-rolling heat treatments were performed at 400 °C–550 °C for various times. Afterwards, cross-sections of the composites were studied in the SEM and TEM, in order to analyze the interface characteristics. In all the heat-treated composites, no IMP formation could be observed along the steel-nickel interface, consistent with findings in the literature [
15,
22]. The lack of IMPs between steel and nickel was expected based on the substantial solid solubility between iron and nickel, which can be observed from the binary Fe-Ni phase diagram [
33]. However, the phase, FeNi
3 could potentially have formed within the studied temperature range.
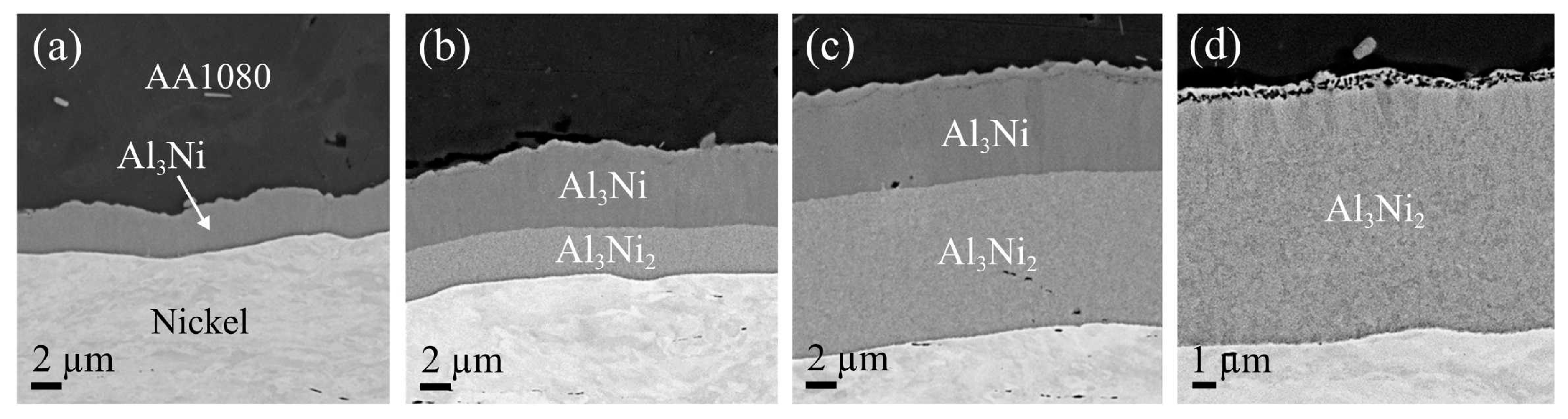
Along the aluminum-nickel interface, IMPs from the Al-Ni system were detected. SEM images of the aluminum-nickel interface are presented in
Figure 4a–d, showing the development of the formed Al-Ni IMP layer with increasing heat treatment temperature and time. In the composites heat treated at 400 °C and 450 °C, the IMP layer consisted mainly of one phase, shown in
Figure 4a. The results from the electron microprobe analyzer showed that the phase had a composition of 75 at% aluminum and 25 at% nickel, consistent with the Al
3Ni phase composition. The layer grew discontinuously along the interface, but was observed to become continuous with increasing heat treatment time, consistent with the observation made in other studies [
23,
24].
In the composites heat treated at 500 °C and 550 °C, two phases were observed along the aluminum-nickel interface, as shown in
Figure 4b,c. Both phases were observed even after short heat treatment times. The electron microprobe analysis showed that the second phase, growing between the Al
3Ni and the nickel layer, had a composition of 60 at% aluminum and 40 at% nickel, consistent with the phase Al
3Ni
2. The sequence across the interface was found to be: aluminum- Al
3Ni-Al
3Ni
2-nickel in all the heat-treated composites. Eventually, as shown in
Figure 4b–d, the Al
3Ni
2 phase became the dominating phase in the layer, growing at the expense of the Al
3Ni phase. In some areas along the interface, the Al
3Ni layer was found to be completely consumed by the Al
3Ni
2 phase, as shown in
Figure 4d. This was mainly the case in the composites subjected to heat treatment at 550 °C for long periods (two hours and more). This formation and growth sequence is consistent with findings in other studies focusing on the formation and growth of the Al-Ni IMP during heat treatment at 450 °C–620 °C [
24,
25,
26,
34].
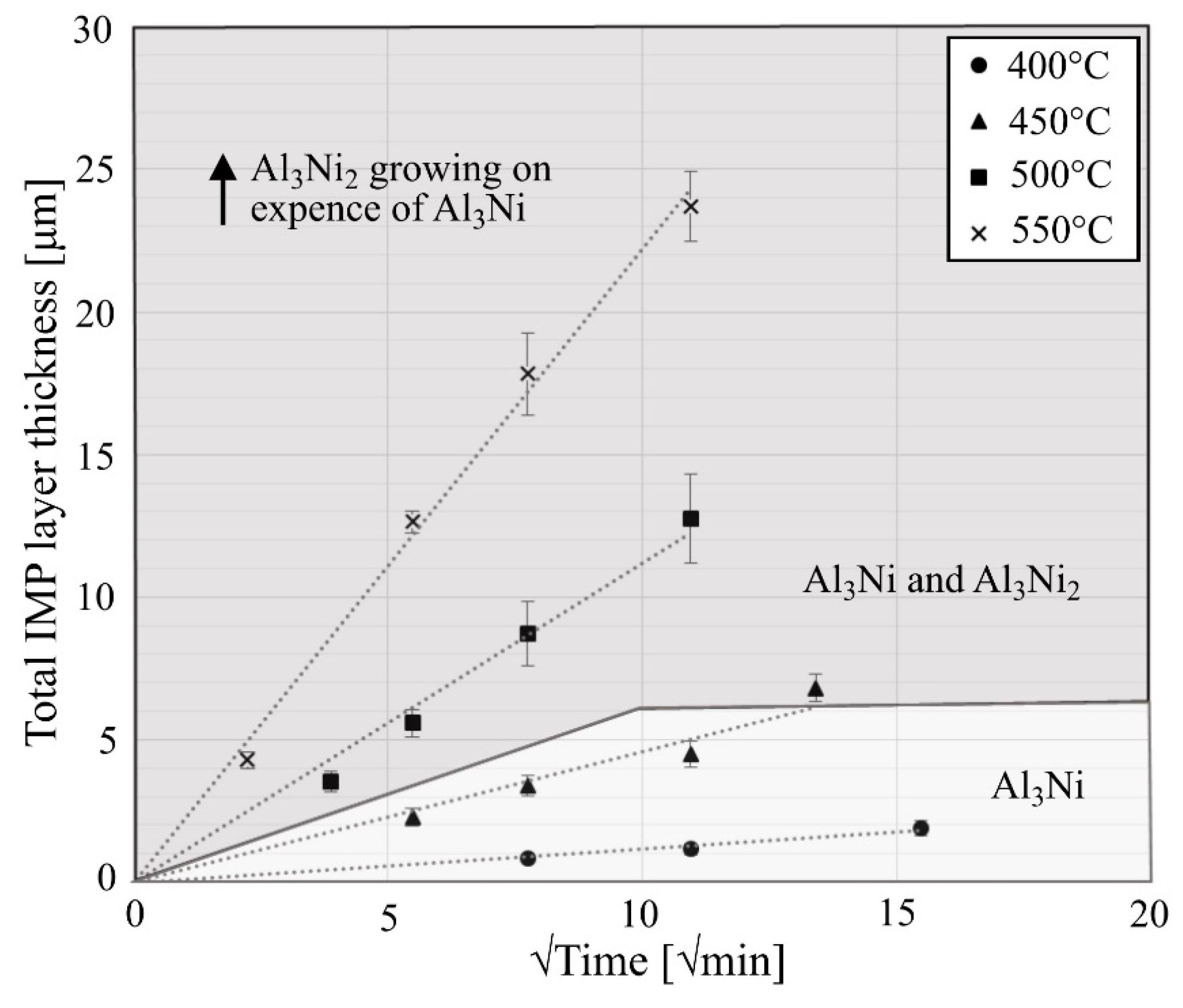
Figure 5 shows the average thickness of the total Al-Ni IMP layer as a function of the square root of heat treatment time for the different temperatures. The presented values are based on the average of twenty measurements (obtained at two different locations) from minimum two and maximum five different heat-treated composites.
Figure 5 is divided into two grey-scale colored areas, indicating which phases that were observed to grow at the different temperature-time combinations. Either only Al
3Ni (light grey) or both Al
3Ni and Al
3Ni
2 (dark grey) were observed to form. The thickness of the total Al-Ni IMP layer is observed to increase with increasing heat treatment temperature and time.
At 400 °C and 450 °C, only Al
3Ni was observed to grow along the interface, and it can be observed in
Figure 5 that the growth of Al
3Ni follows a linear relationship with the square root of time. At these temperatures, the growth of Al
3Ni
2 was negligible, and therefore, it can be concluded that below 500 °C, the growth rate of Al
3Ni is larger than the growth rate of Al
3Ni
2. During heat treatment above 500 °C, both Al
3Ni and Al
3Ni
2 was observed to grow along the interface, even for short heat treatment times, as indicated in
Figure 5. As already mentioned, at these temperatures the Al
3Ni layer was observed to be consumed by the growing Al
3Ni
2 phase. Thus, the increase in thickness of the IMP layer at these temperatures is assumed to be due to the growth of the Al
3Ni
2 phase. From
Figure 5 it can be observed that the growth of Al
3Ni
2 also follows a linear relationship with the square root of heat treatment time. Based on the observed growth characteristics at these temperatures it can be argued that above 500 °C, the growth rate of Al
3Ni
2 is larger than the growth rate of Al
3Ni.
Based on the observed growth rates from
Figure 5, the thickness (Δx) of the IMP layer can be expressed by the parabolic rate law, given in Equation (2):
where t is the heat treatment time, and k is the rate constant, which is temperature dependent. This means that the growth of the two phases, Al
3Ni and Al
3Ni
2 is volume diffusion controlled [
24,
25,
26,
34,
35].
3.2.1. Characteristics of the Al3Ni Layer
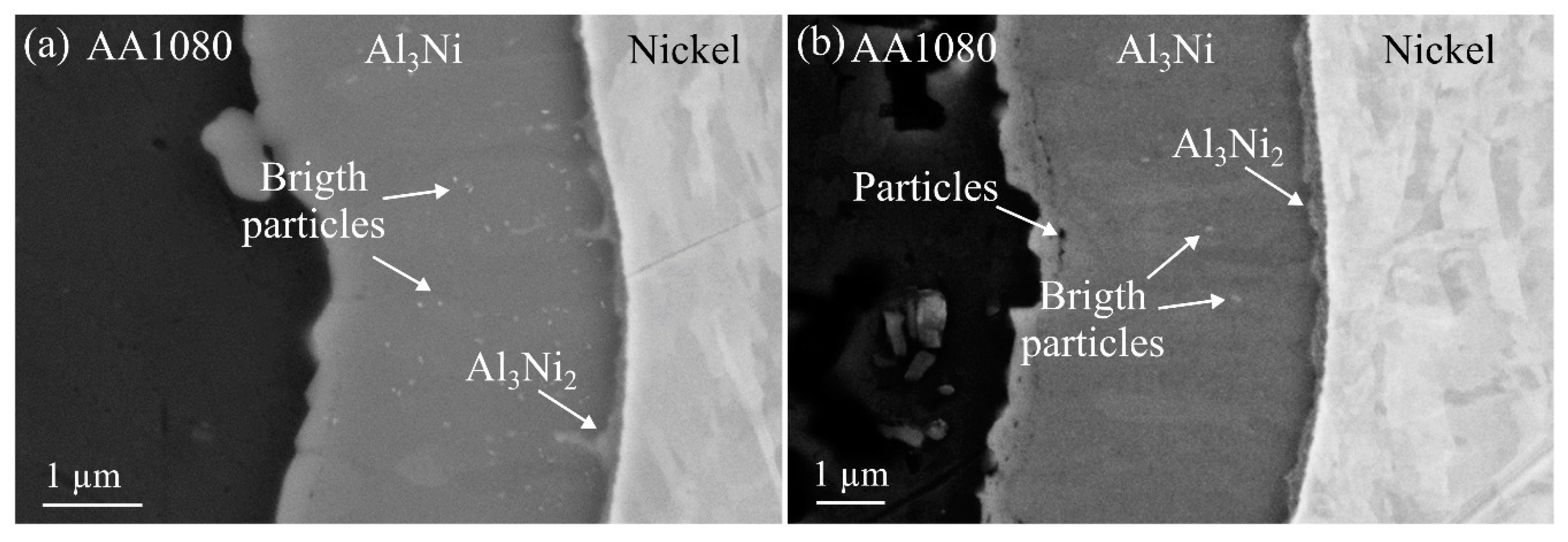
Figure 6a,b show the main observations made during SEM analysis of the Al
3Ni layer, which formed after heat treatment at 400 °C and 450 °C. In
Figure 6a, many small bright particles can be observed inside the Al
3Ni layer. However, as seen in
Figure 6b, with increasing heat treatment time, fewer particles could be observed inside the layer. Similar bright particles inside the Al
3Ni layer have not been reported in other studies where the Al
3Ni phase have been closely analyzed [
23,
24]. The bright particles were not subjected to any further analysis in the current study, since the work is focusing on the relationship between the Al-Ni IMP layers and the bond strength. The bright particles were assumed not to have any influence on the bond strength, as the fracture was observed not to occur inside the Al
3Ni layer where the particles were present.
In some areas, highlighted in
Figure 6a,b, the initial formation of the second phase, Al
3Ni
2 can be observed along the Al
3Ni-nickel interface. The images are taken from composites heat treated at 450 °C for one and two hours, respectively. The initial formation is already visible after one hour and the phase is observed to grow thicker as the heat treatment time increases. In the study by Adabi and Amadeh [
24], the formation of Al
3Ni
2 was first reported after heat treatment at 450 °C for 150 min, which is significantly longer than in the current study. However, their observation was reported after heat treatments performed between a nickel-coating and an AA6061 aluminum alloy. The AA6061 alloy contains silicon and magnesium, and silicon has been reported to influence and delay the Fe-Al IMP formation in steel-aluminum joints [
2,
36]. These alloying elements might be the reason for the slightly delayed formation of the Al
3Ni
2 phase reported in the study by Adabi and Amadeh [
24], compared to the observations made in the current study, where the commercially pure aluminum alloy AA1080 is used.
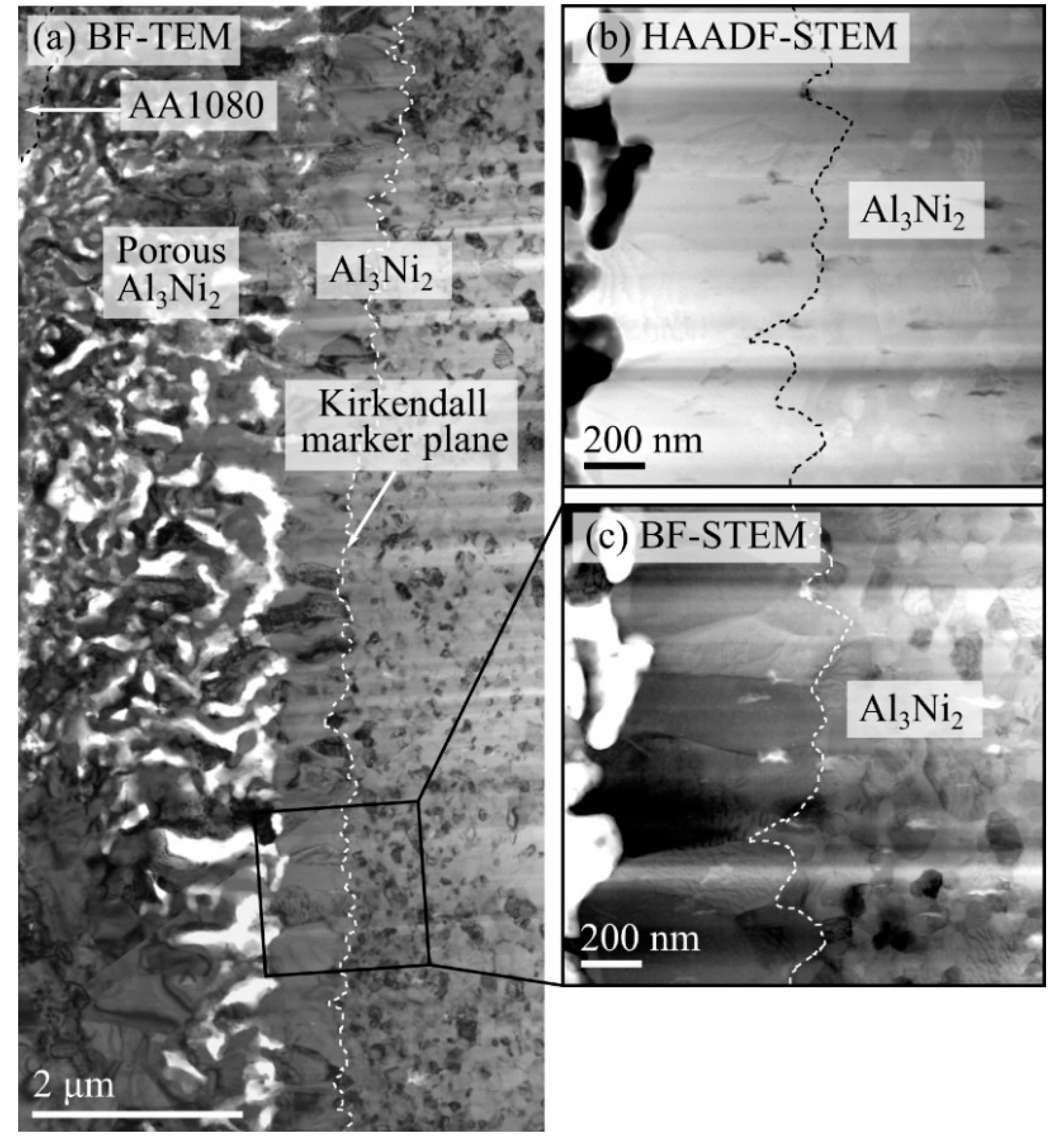
In
Figure 6b, a line, consisting of what appears to be many small particles can be observed inside the Al
3Ni layer, ~1 µm away from the interface towards the aluminum. Such particles were observed inside the Al
3Ni layer in all the studied composites, independent of heat treatment procedure. The particles were therefore further studied with TEM, which verified the SEM observations. The composite studied with TEM was heat treated at 550 °C for one hour and had an IMP layer consisting of both Al
3Ni and Al
3Ni
2.
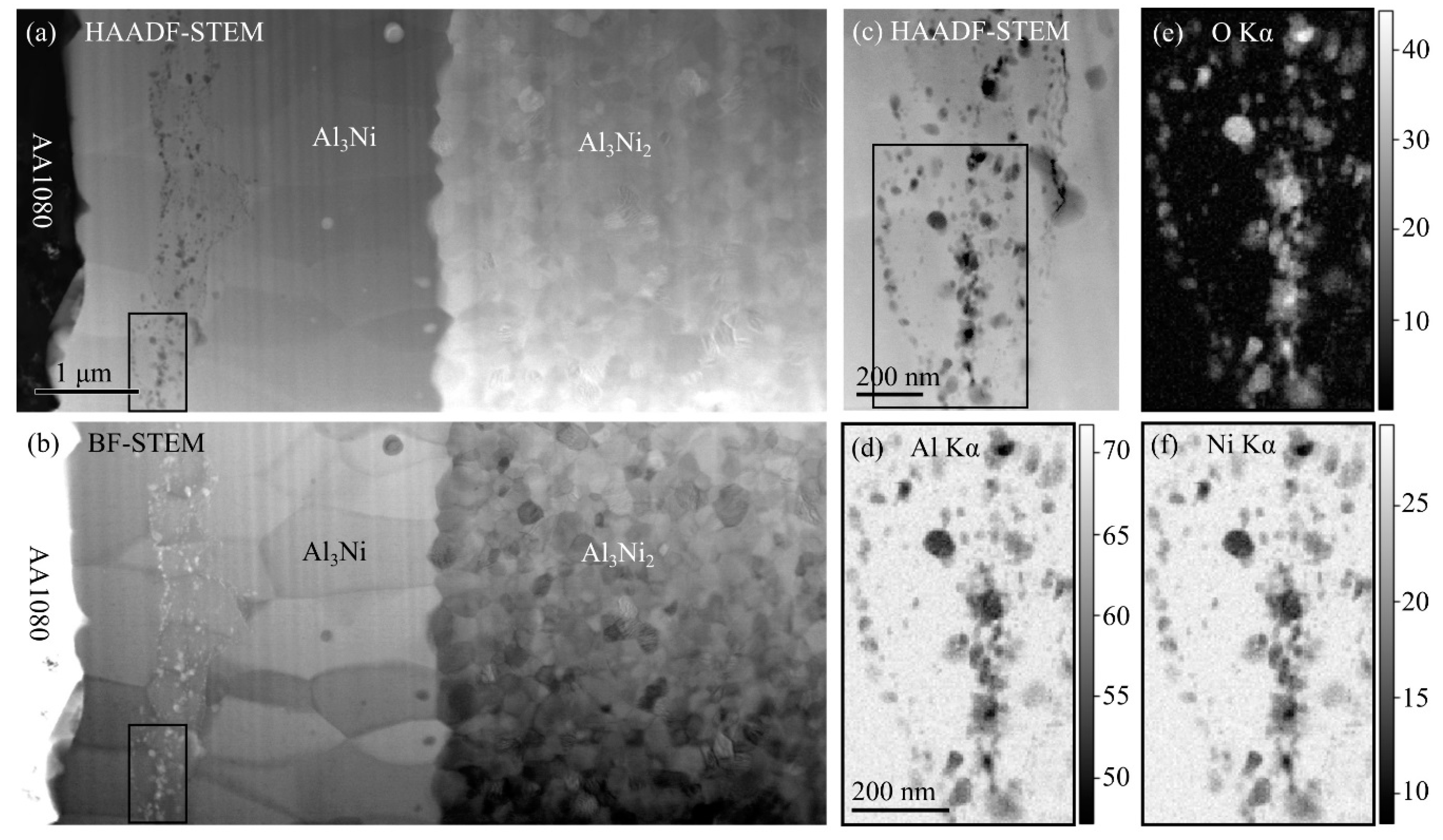
Figure 7a,b show an HAADF-STEM and a corresponding bright field (BF)-STEM image, while (c) shows a higher magnification HAADF-STEM image. HAADF-STEM images are often called Z-contrast images, where Z refers to atomic number, since the intensity scales as ~Z
2. Hence, phases with heavy elements appear bright and phases with light elements appear dark in typical HAADF-STEM images. Thus, Al
3Ni and Al
3Ni
2 are clearly distinguishable in (a), where the most nickel-rich phase, Al
3Ni
2, has the strongest contrast. In BF-STEM images, both diffraction contrast and mass-thickness contrast contribute, and the intensities of different grains depend on their crystal orientations. Thus, different grains are easier to distinguish in (b) than in (a).
In
Figure 7a,b, it can be observed that the Al
3Ni layer consists of several grains with a width in the order of ~1 µm, which are slightly elongated, growing perpendicular to the interface. Inside the Al
3Ni layer, the previously mentioned line of particles is again observed, and as can be observed in
Figure 7a,b, the line actually consists of many small particles, which lies on an approximate line. Denoised EDS maps for aluminum, oxygen and nickel are shown in
Figure 7d,e,f, respectively, obtained from the area highlighted in
Figure 7c. From the EDS maps, it is observed that the particles mainly consist of aluminum (~50–60 at%) and oxygen (~40–45 at%). Although the overlap of the particles with the Al
3Ni phase makes quantification challenging, Al
2O
3 is assumed to be the most likely phase. These particles probably originated from the Al
2O
3 layer that formed on the aluminum surface after brushing prior to the rolling process, as well as oxygen gas which was trapped between the layers due to mechanical interlocking during the rolling process.
The Al
2O
3 layer on the aluminum surface is assumed to break up together with the work-hardened surface layer during the rolling process, due to the large surface expansion. Thus, after rolling, the interface between aluminum and nickel is expected to consist of separated Al
2O
3 fragments, with gaps where fresh aluminum has come into contact with nickel (assuming that the film theory is the main bonding mechanism). If the Al
2O
3 particles were inert during the subsequent heat treatment process, they could have behaved like the inert markers used during the Kirkendall experiment [
11]. In the experiment, inert markers were placed along the interface between two dissimilar metals, and the movement of the markers during heat treatment was used to visualize the unequal diffusion of atoms and vacancies across the interface. The position of the inert markers was referred to as the Kirkendall marker plane. Based on the position of the Kirkendall marker plane and the growth mechanism of the IMPs between the two metals, the diffusivities of the atoms from each metal can be compared [
12,
13,
37]. Assuming that the Al
2O
3 particles behaved like similar inert markers, their apparent movement could be viewed as a result of the Kirkendall effect, and the location of the particles as a Kirkendall marker plane. If that were the case, the position of the oxide particles found here would indicate that aluminum diffuses faster than nickel through the Al
3Ni layer, based on the description found in [
37]. This assumption is in accordance with the suggestion made in other studies [
24,
25,
26]. However, Paul et al. [
13] stated that particles with a size less than 0.1 µm might be dragged by grain boundaries during the diffusion process, as their size is below typical inert marker size (1–5 µm, depending on the studied system), which is the case in the current study. Then, the position of the particles would not represent the position of a Kirkendall marker plane. However, it is outside the scope of the current work to study in detail if the observed Al
2O
3 particles behaved like stable inert markers or not. Further studies are required to understand the dynamical behavior of this system and the movement of these oxide fragments during the diffusion process.
3.2.2. Characteristics of the Al3Ni2 Layer
As previously introduced, in the composites heat treated at 500 °C and 550 °C (or for more than 2–3 h at 450 °C), the Al-Ni IMP layer consisted of both Al
3Ni and Al
3Ni
2. While the Al
3Ni layer consisted of slightly elongated grains, the Al
3Ni
2 layer was observed to consist of small equiaxed grains with sizes below ~0.5 µm (
Figure 7a,b). With increasing temperature and time, the Al
3Ni
2 layer was observed to become the dominating phase in the IMP layer, as previously shown in
Figure 4. Konieczny et al. [
34] also reported that the Al
3Ni
2 phase is the dominating phase after heat treatment of nickel-aluminum composites at 620 °C for one hour, fitting well with the observations in the current study and the reported findings in other studies [
24,
25].
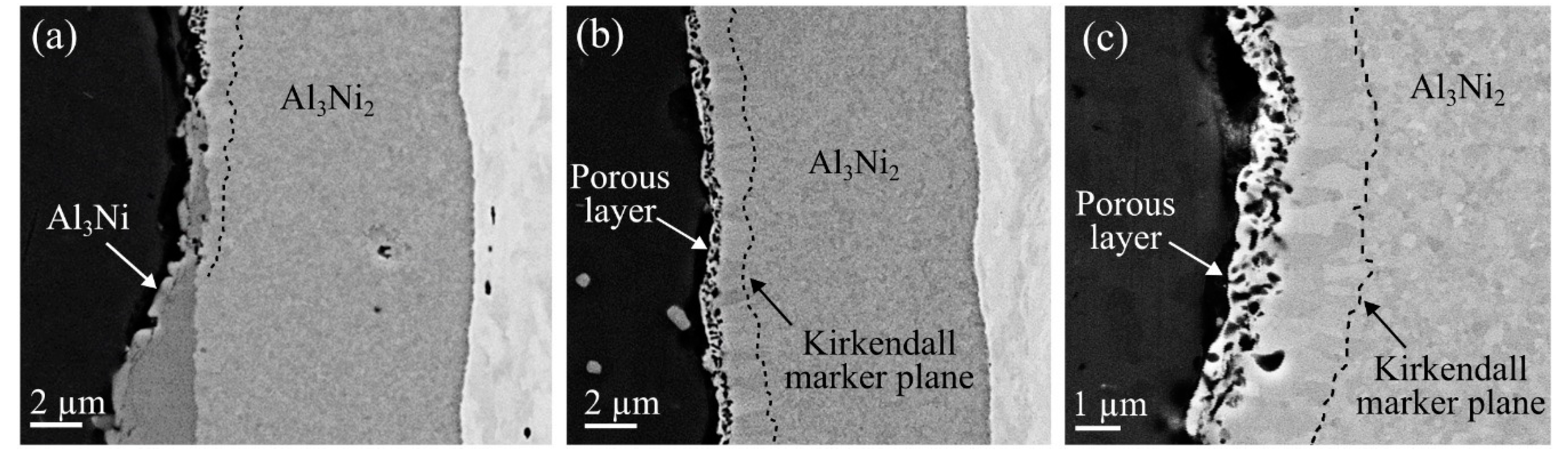
In the composites heat treated at 550 °C for two hours or more, areas, where only the Al
3Ni
2 phase was present along the interface between aluminum and nickel, were observed. This change in the interface characteristics is shown in
Figure 8a,b.
Figure 8a shows an area of the aluminum-nickel interface where the IMP layer goes from consisting of both Al
3Ni and Al
3Ni
2 to only consisting of a layer of Al
3Ni
2.
The extent of these areas, where only Al
3Ni
2 were present along the aluminum-nickel interface, increased with increasing heat treatment time. The growth rate of the Al
3Ni
2 layer is thus assumed to be much larger than for the Al
3Ni layer at 500 °C and 550 °C, as already discussed. Combined with the observations made by SEM and the thickness measurements of the two phases with increasing heat treatment time, it appears that the Al
3Ni
2 layer grows on behalf of the Al
3Ni layer, and that the Al
3Ni phase is transformed into Al
3Ni
2. As stated by Alimadadi et al. [
25], since the total thickness of the Al-Ni IMP layer still increases, the direct formation of Al
3Ni
2 along the interface is assumed to occur. Adabi and Amadeh [
24] observed the same trend and stated that the Al
3Ni
2 layer thickness increased due to the diffusion of Ni-atoms through the Al
3Ni
2 layer until the entire Al
3Ni layer was consumed. Where both phases were present along the interface, the Al
3Ni
2 layer consisted of small equiaxed grains. However, once the Al
3Ni layer was fully consumed by Al
3Ni
2, a duplex morphology was observed to develop inside the Al
3Ni
2 layer. As shown in
Figure 8b, and more clearly in
Figure 8c, the fine Al
3Ni
2 grains change into larger elongated grains, with the longest axes in the growth direction (perpendicular to the interface). The line where the change in morphology is observed lies approximately parallel to the Al
3Ni
2-aluminum interface. The duplex morphology was studied more closely by TEM. An overview BF-TEM and a higher magnification HAADF-STEM image with a corresponding BF-STEM image are shown in
Figure 9a–c, respectively.
The presence of a Kirkendall marker plane in the reaction zone during diffusion between two metals can not only be revealed by inert markers, but also by the presence of a duplex morphology [
13,
38]. The change in morphology then marks the location of the Kirkendall marker plane. Paul et al. [
38] stated that the presence of a Kirkendall plane indicates that the grains grow in two different ways from the two interfaces, due to different nucleation types. Alimadadi et al. [
25] also observed such a change in morphology inside the Al
3Ni
2 layer in their study. They stated that there is an increase in the availability of aluminum after the Al
3Ni layer is fully consumed. This changes the growth speed of the Al
3Ni
2 phase, resulting in the duplex morphology. As observed from
Figure 8c and
Figure 9, the Kirkendall marker plane is located closer to the aluminum layer than the nickel layer. Following the description found in the work by Paul [
37], the diffusivity of aluminum in Al
3Ni
2 is therefore assumed to be larger than the diffusivity of nickel. This assumption is in accordance with the discussion and observations made by Alimadadi et al. [
25] and Liu et al. [
26]. Adabi and Amadeh [
24] on the other hand argued that nickel is the dominating diffusing element during the growth of the Al
3Ni
2 layer.
In addition to the Kirkendall marker plane, a region inside the Al
3Ni
2 layer along the interface towards the aluminum became porous once the Al
3Ni layer was fully consumed, as shown in
Figure 8c and
Figure 9a. The formation of voids as a result of the Kirkendall effect is a known phenomenon occurring during diffusion [
12,
13], especially if there are large differences in the diffusivities of the different elements in a certain phase layer [
37]. Based on the location of the voids, the relative diffusivities of the elements in the phase can be compared, using the examples and descriptions found in [
13,
37]. Since the voids lie inside the Al
3Ni
2 layer, close to the aluminum, it indicates that aluminum diffuses faster than nickel in the Al
3Ni
2 layer. The voids are formed due to the necessary vacancy flux towards the aluminum side, to compensate for the slower diffusion of nickel. This corresponds well with the previous assumption regarding the diffusivity of the elements based on the observed position of the Kirkendall marker plane. Alimadadi et al. [
25] reported a porous layer inside the Al
3Ni
2 and found that small pores had sharp interfaces, while the rim of larger pores (>50 nm in diameter) contained a layer of Al
2O
3. Thus, they attributed that the pore formation was due to oxidation of Al
3Ni
2 layer. However, in the current study, the areas where the voids formed were not exposed to any source of oxygen during the void formation. Thus, the observed voids cannot be a result of oxidation and are assumed to be Kirkendall voids.
The heat treatments at 500 °C and 550 °C involve several different stages of growth, i.e., formation and growth of Al
3Ni, followed by the formation and growth of Al
3Ni
2, the transformation of Al
3Ni into Al
3Ni
2 and the development of Kirkendall voids along the Al
3Ni
2-aluminum interface. However, still, the thickness of the total IMP layer follows a linear relationship with the square root of time for all the conduced heat treatments, as shown in
Figure 5. This is similar to the findings reported in other studies [
24,
25,
26,
35]. Changes in the slope of the curve might be expected if the growth rate controlling mechanism changes during these different growth stages. However, from the measurements presented in
Figure 5, this cannot be concluded within the calculated error-bars. Further studies would be required in order to fully understand the kinetics regarding the formation and growth of the two Al-Ni IMPs and how the formation of the Kirkendall voids along the Al
3Ni
2-aluminum interface eventually influence the growth process.
3.3. Relationship between the Intermetallic Layer and Bond Strength
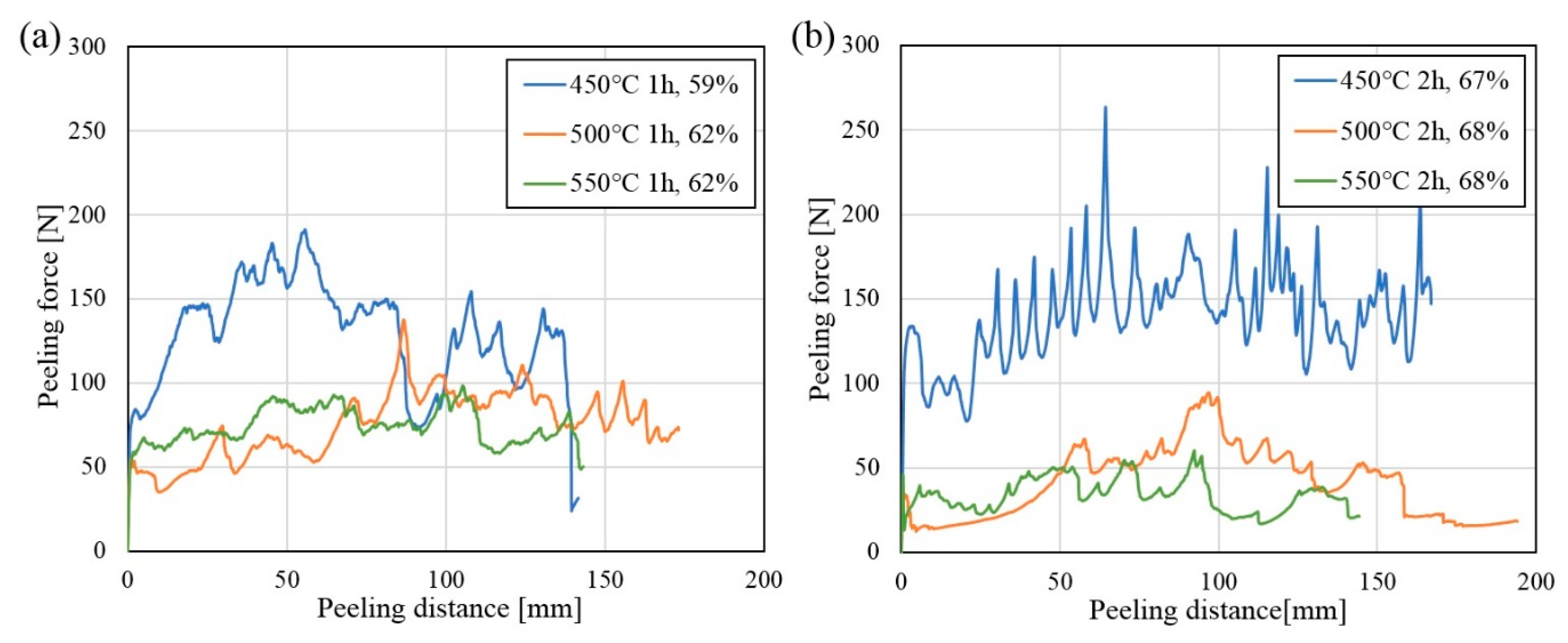
Selected composites with similar thickness reductions were chosen to be heat treated for the same period at 450 °C, 500 °C and 550 °C followed be peel-testing, in order to get an initial overview of the effect of the Al-Ni IMP layer on the bond strength. The results from the peel-tests are presented in
Figure 10a,b, after heat treatment at the three temperatures for one and two hours, respectively. The graphs show the force necessary to delaminate the peel-testing specimens (peeling force) as a function of specimen length (peeling distance). A high peeling force indicates a high bond strength between the layers that are delaminated, as given by Equation (1).
In
Figure 10a,b it is observed that a high peeling force is required in order to delaminate the composites. This means that the conducted post-rolling heat treatments have resulted in an increase in bond strength. The highest bond strengths are observed for the composites heat treated for one and two hours at 450 °C (blue curves,
Figure 10a,b). A decrease in bond strength with increasing heat treatment temperature and time is observed for the composites heat treated at 500 °C and 550 °C (orange and green curves,
Figure 10a,b).
In some of the presented peel-testing curves, large fluctuations can be observed. The produced composites were symmetrically stacked, with nickel on both sides of the aluminum in the middle and steel as the outer layers, as showed in
Figure 1. It was observed that the fracture changed location during the peel-test, occurring between the same metal layers, but alternating between the two similar locations in the composite, which resulted in these fluctuations.
After rolling and after the different heat treatments, hardness measurements were taken in the steel and aluminum layers. The nickel layers were too thin to obtain any valid hardness measurements. No differences in the hardness were observed in any of the layers after heat treatment for one and two hours, and therefore, the average hardness values presented in
Table 2 are based on measurements from both cases.
The measured hardness values were observed to be independent of the total thickness reduction. From
Table 2, it can be observed that the hardness of the aluminum is similar after heat treatment at the different temperatures, while the hardness of the steel decreases slightly with increasing heat treatment temperature. The decrease in hardness in the steel layers might influence the peel-testing results, as the peel-test requires the metal layers to be bent 90°, and less force is required to bend metals with a lower hardness. However, it is assumed that this effect has little influence on the measured bond strengths in the current study. When comparing the peel-testing curves for the composites heat treated at 450 °C and 500 °C in
Figure 10, a clear difference in the measured peeling force can be observed between the composites, even though the steel layers in the composites have a similar hardness after the two heat treatments.
The composites heat treated at 500 °C and 550 °C had the lowest bond strengths, which decreased with increasing heat treatment time, as shown in
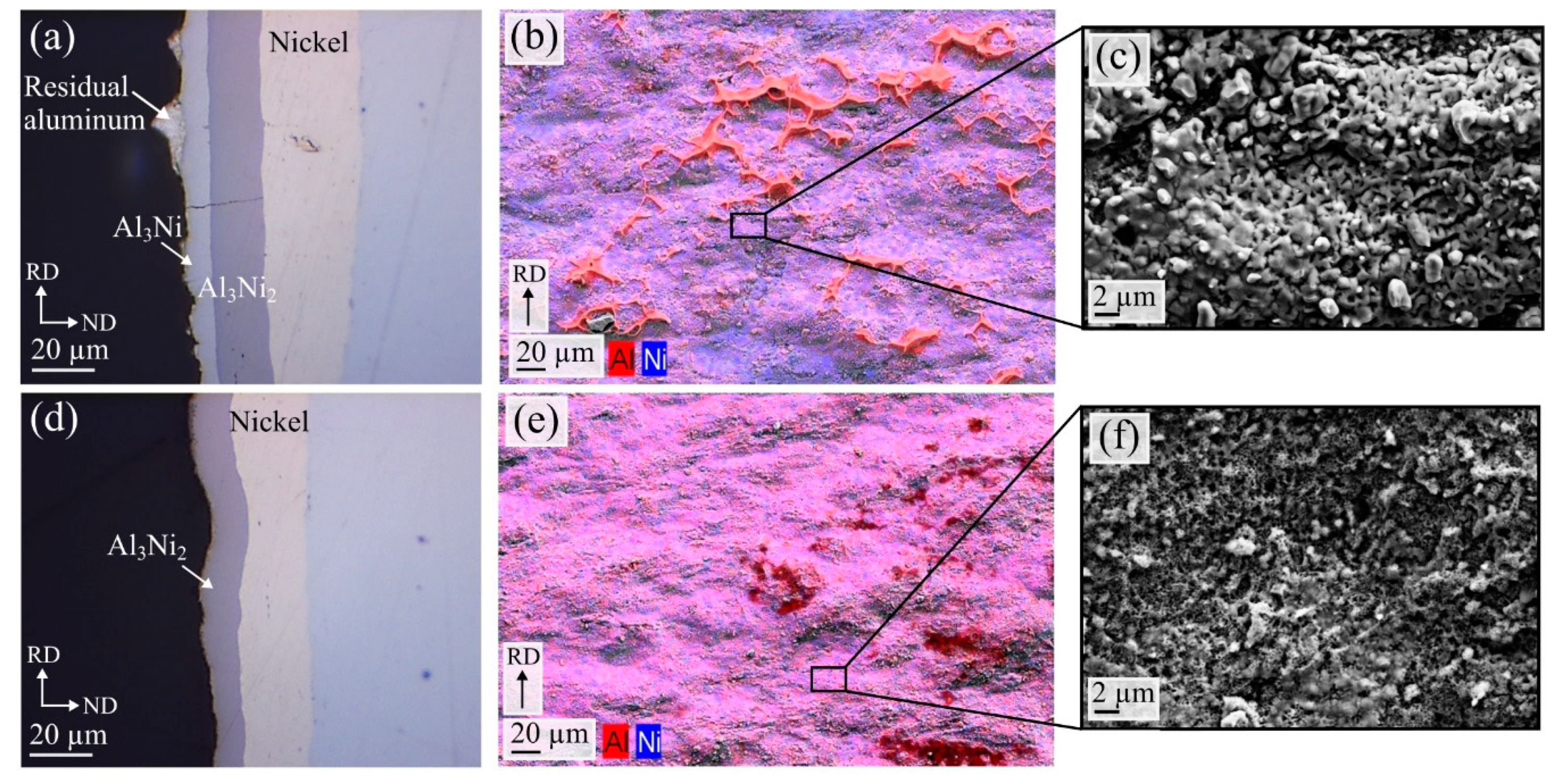
Figure 10a,b. Afterwards, the cross-section and the fracture surfaces were studied in order to investigate the fracture mechanism. The obtained images are shown in
Figure 11a–f, comparing the fracture mechanisms occurring after heat treatments at 500 °C and 550 °C. For the composites heat treated at 500 °C, the fracture occurred along the Al
3Ni-aluminum interface, as shown in
Figure 11a, showing the cross-section of the fracture surface.
Figure 11b shows an SEM image of the corresponding fracture surface combined with EDS color mapping. Residual aluminum on the Al
3Ni fracture surface can be observed in both the cross-section image,
Figure 11a, and on the fracture surface,
Figure 11b, where the detected aluminum is colored red. The residual aluminum has a dimpled characteristic, consistent with a ductile fracture. The rest of the fracture surface appears flat, indicating a brittle fracture. Thus, a thick Al-Ni IMP layer consisting of both Al
3Ni and Al
3Ni
2 was found to decrease the bond strength.
As previously shown for the composites heat treated at 550 °C, the Al
3Ni layer was in some areas completely consumed by the growing Al
3Ni
2 layer. The interface between the Al
3Ni
2 layer and the aluminum developed a porous structure (
Figure 8c), due to the Kirkendall effect. In the areas where only Al
3Ni
2 was present along the aluminum-nickel interface, the fracture characteristics shown in
Figure 11d–f were observed. From the cross-section image in
Figure 11d, a clear fracture between the aluminum and the Al
3Ni
2 layer can be observed. On the Al
3Ni
2 fracture surface,
Figure 11e, no dimpled residual aluminum can be seen, and the surface appears flat, consistent with a brittle fracture.
Figure 11c,f show high magnification images of the fracture surfaces with the two fracture mechanisms. The Kirkendall voids found along the Al
3Ni
2-aluminum interface resulted in the surface seen in
Figure 11f, with many small grooves on the fracture surface. This characteristic was only observed in the composites which obtained the lowest bond strength values. Due to the Kirkendall voids, the fracture has easily propagated along the Al
3Ni
2-aluminum interface [
12]. Thus, post-rolling heat treatments resulting in only an Al
3Ni
2 layer along the interface should be avoided, when a high bond strength is desired.
As shown by Shi et al. [
39] studying the Al-Ni IMP using density functional theory, the Al
3Ni phase was found to be ductile, while the Al
3Ni
2 phase was found to be brittle. In the current study, the fractures only occurred along the interfaces between the Al-Ni IMP layer and aluminum, and not inside the Al-Ni IMP layers themselves. As shown in
Figure 11a, the fracture runs along the Al
3Ni-aluminum interface, indicating that the Al
3Ni
2 layer bonds stronger to both Al
3Ni and nickel, than Al
3Ni bonds to aluminum. Hence, the fracture surfaces presented in
Figure 11 demonstrate that the Al
3Ni
2 phase is not what causes the fracture even though this phase was assumed to be the most brittle based on the work by Shi et al. [
39].
As observed in
Figure 10, the composites heat treated at 450 °C obtained the highest bond strength during the initial tests. In these composites, the IMP layer only consisted of a layer of Al
3Ni, as previously introduced, and the fracture characteristics observed in these composites are presented in
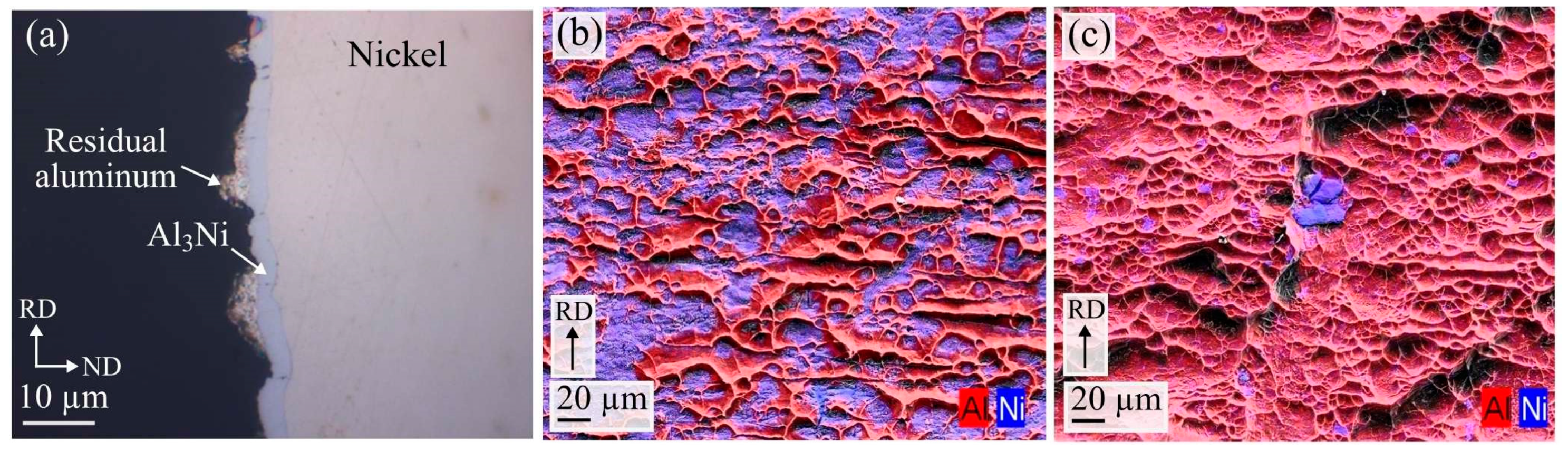
Figure 12a–c.
Figure 12a shows an optical microscopy image of the cross-section of the Al
3Ni fracture surface. A thin layer of Al
3Ni can be seen together with residual aluminum. No signs of fracture between the Al
3Ni layer and nickel is observed. Individual high magnification SEM images combined with EDS color mapping of the Al
3Ni and aluminum fracture surfaces are presented in
Figure 12b,c.
Large amounts of residual aluminum can be observed on the Al
3Ni fracture surface,
Figure 12b, which have a band like structure, lying perpendicular to the rolling direction. The residual aluminum has a clear dimple-like characteristic, indicating that a ductile fracture has occurred in the aluminum. The dimple-like characteristic is also clearly observed on the aluminum fracture surface,
Figure 12c. These images suggest that the main part of the fracture has occurred in the aluminum and also partially along the Al
3Ni-aluminum interface. Separation occurring between the Al
3Ni layer and the aluminum has been previously reported, similar to the current study [
24,
25]. However, in the study by Adabi and Amadeh [
24] the formation of Kirkendall voids along the Al
3Ni-aluminum interface was assumed to be the reason for the separation after heat treatment at 450 °C for 240 min. This was not observed in the current study.
When comparing the two Al
3Ni fracture surfaces in
Figure 11b and
Figure 12b, a large difference in the amount of residual aluminum can be observed. The observed decrease in the amount of residual aluminum on the Al
3Ni fracture surface in the composite heat treated at 500 °C can be related to the decrease in the measured bond strength for those composites.
Based on the initial peel-testing results (
Figure 10), the main focus was placed on the composites heat treated at 450 °C, which obtained the highest bond strengths, in order to better understand the relationship between the Al
3Ni layer thickness and the bond strength. Additional composites were heat treated, and composites with different thickness reductions were selected for heat treatment at one and two hours. The results from these peel-tests are shown in
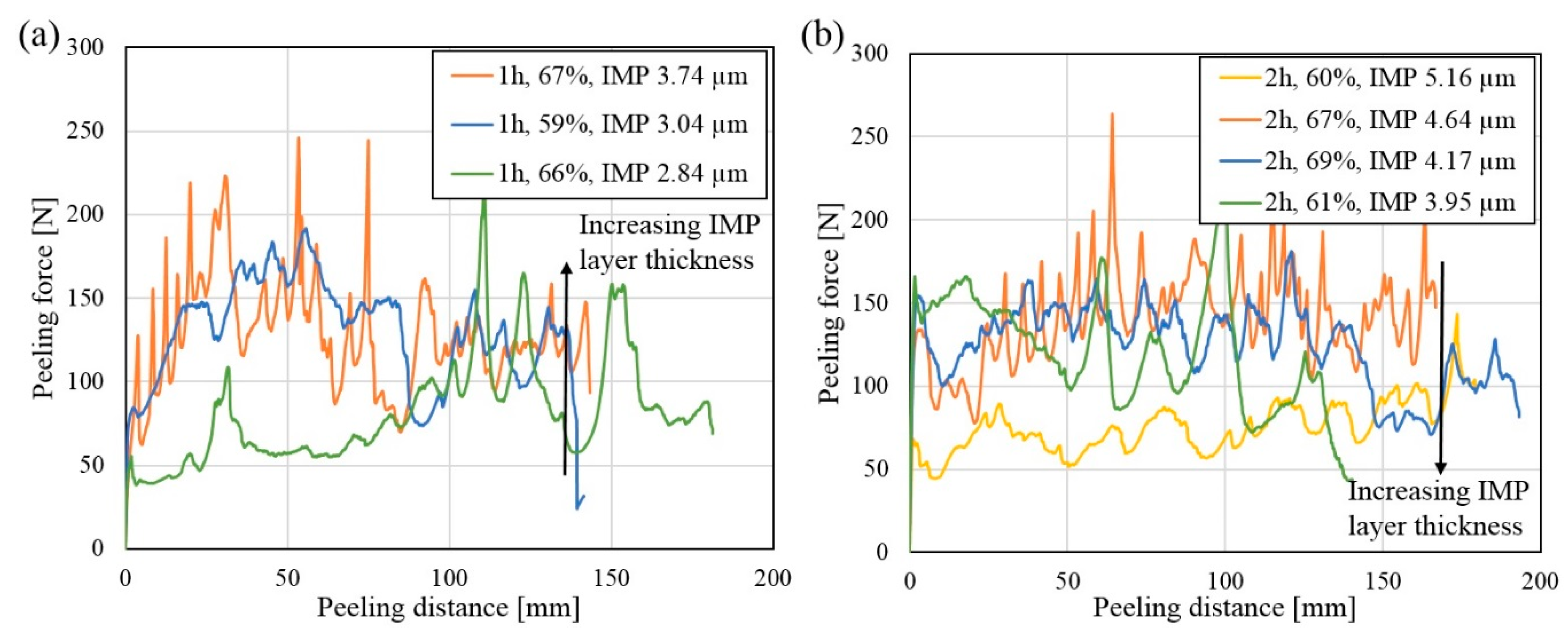
Figure 13a,b. The total thickness reduction and average Al
3Ni layer thickness are given in the legends in the two graphs. Again, as observed in the peel-testing curves in
Figure 10, there are large fluctuations in the measured values.
Figure 13a shows the peel-testing curves for the composites heat treated for one hour. The green curve is observed to lie slightly below the other two curves for the majority of the peeling distance. In these composites, the bond strength (given by the measured peeling force) is observed to increase with increasing Al
3Ni layer thickness, as indicated by the arrow.
Figure 13b shows the peel-testing curves for the composites heat treated for two hours. For the majority of these composites, the peeling force is observed to be similar, lying between ~100–160 N. However, for one composite, the peeling force is observed to be lower, lying around 60–70 N (yellow curve,
Figure 13b). Hence, this composite has a lower bond strength compared to the other composites. As can be seen in the legends in
Figure 13b, the composite that had the lowest measured peeling force is also the composite which has the thickest Al
3Ni layer. This shows that the bond strength decreases with increasing Al
3Ni layer thickness. For all the peel-tested composites, no clear influence of the total thickness reduction on the bond strength was observed.
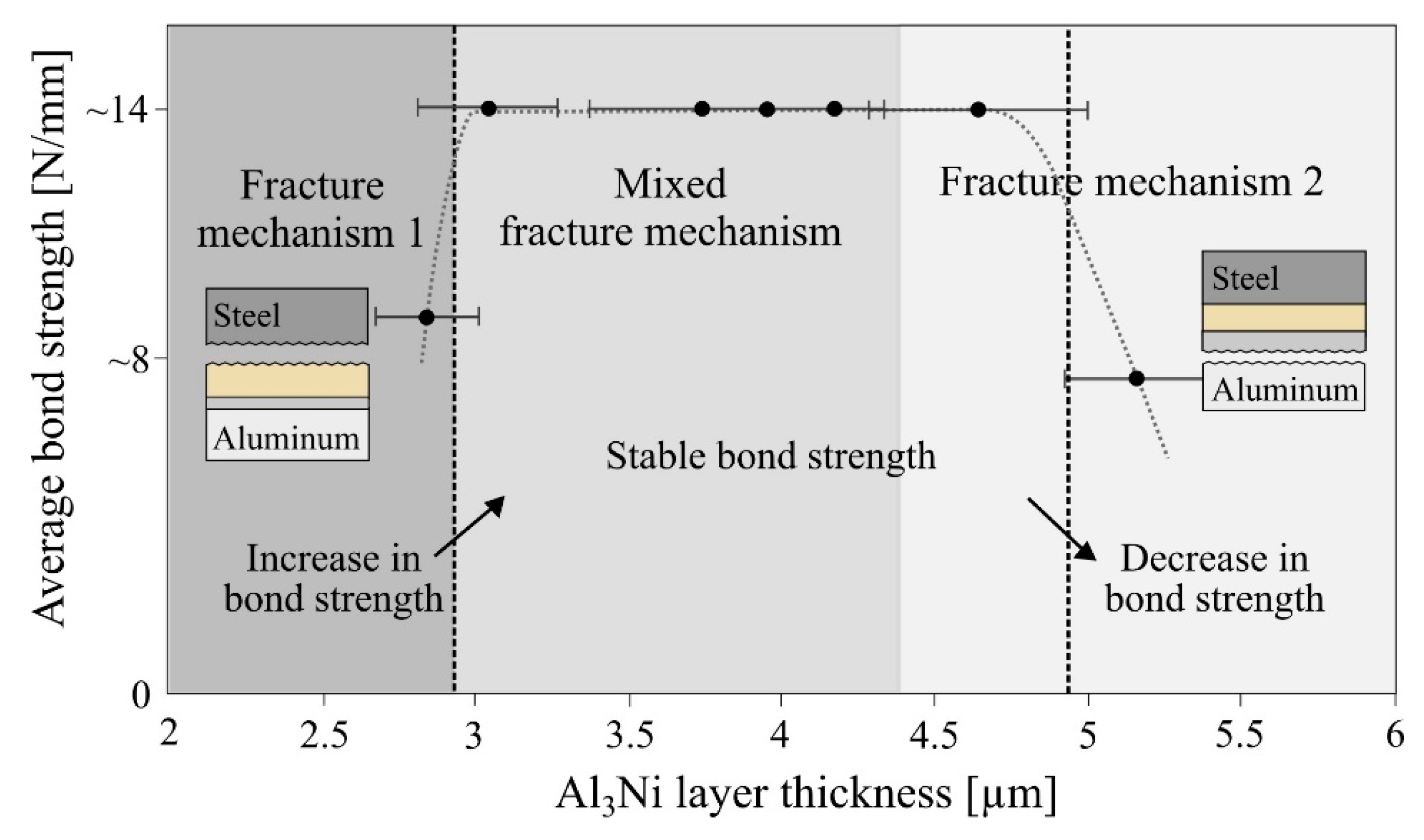
When comparing the Al
3Ni layer thickness and the obtained peel-testing curves for each of the composites heat treated at 450 °C, it is observed that bond strength is strongly influenced by the Al
3Ni layer thickness. The graphical illustration presented in
Figure 14 summarizes the relationship between the achieved average bond strength (calculated by Equation 1) and the Al
3Ni layer thickness. The different grey-scaled areas indicate the three different fracture mechanisms observed in the composites. The fracture in the peel-tested composites heat treated at 450 °C was observed to either occur between the steel and nickel layers (fracture mechanism 1), between the aluminum and the Al
3Ni layer (fracture mechanism 2) or a mix of both fracture mechanisms. Due to the large fluctuations in the peel-testing curves shown in
Figure 13a,b, it was difficult to calculate an accurate average bond strength for the composites, thus a rough estimate based on the peel-testing curves are used in
Figure 14.
In the composite which obtained the lowest bond strength after one hour heat treatment (green curve,
Figure 13a), the fracture occurred along the steel-nickel interface (fracture mechanism 1) and this composite was observed to have the lowest Al
3Ni layer thickness (<3 µm). For the rest of the composites, which had an Al
3Ni layer thickness between 3.0–4.5 µm, the fracture changed from mechanism 1 into the mixed fracture mechanism, and a clear increase in bond strength was observed. This indicates that for Al
3Ni layers between ~3–4.5 µm, neither the steel-nickel nor the Al
3Ni-aluminum interface is the weakest. With increasing heat treatment time and Al
3Ni layer thickness (>4.5 µm), the fracture changed into mechanism 2 and only occurred along the Al
3Ni-aluminum interface.
Typically, steel and nickel are diffusion bonded at temperatures between 800–950 °C [
15,
22]. From the observations made in the current study, it was found that a certain heat treatment time, at least one hour at 450 °C was necessary for the steel-nickel bonding to become sufficiently strong. Eventually, the Al
3Ni-aluminum interface became the weakest part of the joint. This indicates that a high bond strength is achieved by a combination of obtaining a sound joint between steel and nickel due to diffusion, and the growth of the Al
3Ni layer along the aluminum-nickel interface. Peel-tests of composites heat treated for shorter times at 450 °C were therefore not conducted.
An approximately similar bond strength could be observed in the composites which had an Al
3Ni layer thickness between ~3–5 µm, as shown in
Figure 14. This indicates that a certain Al
3Ni layer thickness promotes a high bond strength. Then, when the critical thickness is reached, the bond strength decreases. Based on the presented results in
Figure 14 it is suggested that an Al
3Ni layer with a thickness between ~3–5 µm is one of the main criteria for obtaining a high bond strength for the studied material combination. This observation corresponds well with the results reported in the study by Xiong et al. [
21] on diffusion bonded aluminum-copper composites with a nickel-interlayer. They reported that the thickness of the Al
3Ni layer was below 3.8 µm and that this resulted in a high bond strength.
From the IMP layer thickness measurements presented in
Figure 5, it can be observed that a total IMP layer thickness in the range of 3–5 μm can be obtained through heat treatments at all the investigated temperatures for very different heat treatment times. After heat treatment at 500 °C for 15 min, and 550 °C for 5 min, IMP layers had formed along the aluminum-nickel interfaces, which consisted of Al
3Ni and Al
3Ni
2 with a thickness of 3.9 μm and 4.2 μm, respectively. With such short heat treatment times, the IMP layers were observed to be slightly discontinuous along the interfaces, and the thickness was found to be very sensitive to small changes in heat treatment time. Thus, these temperature-time combinations were not subjected to any further studies, as they were argued to be unfit and non-applicable in industrial applications. During heat treatment at 400 °C, the IMP layer had only reached a thickness of 2.5 μm after four hours. Based on the obtained measurements, a heat treatment time of at least eight hours would have been necessary in order to reach a layer thickness in the optimal range of 3–5 μm. At 400 °C, the IMP layer consisted only of Al
3Ni, and since the relationship between the Al
3Ni layer thickness and the bond strength had already been thoroughly investigated through the conducted tests at 450 °C, additional experiments were not performed at 400 °C.