In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
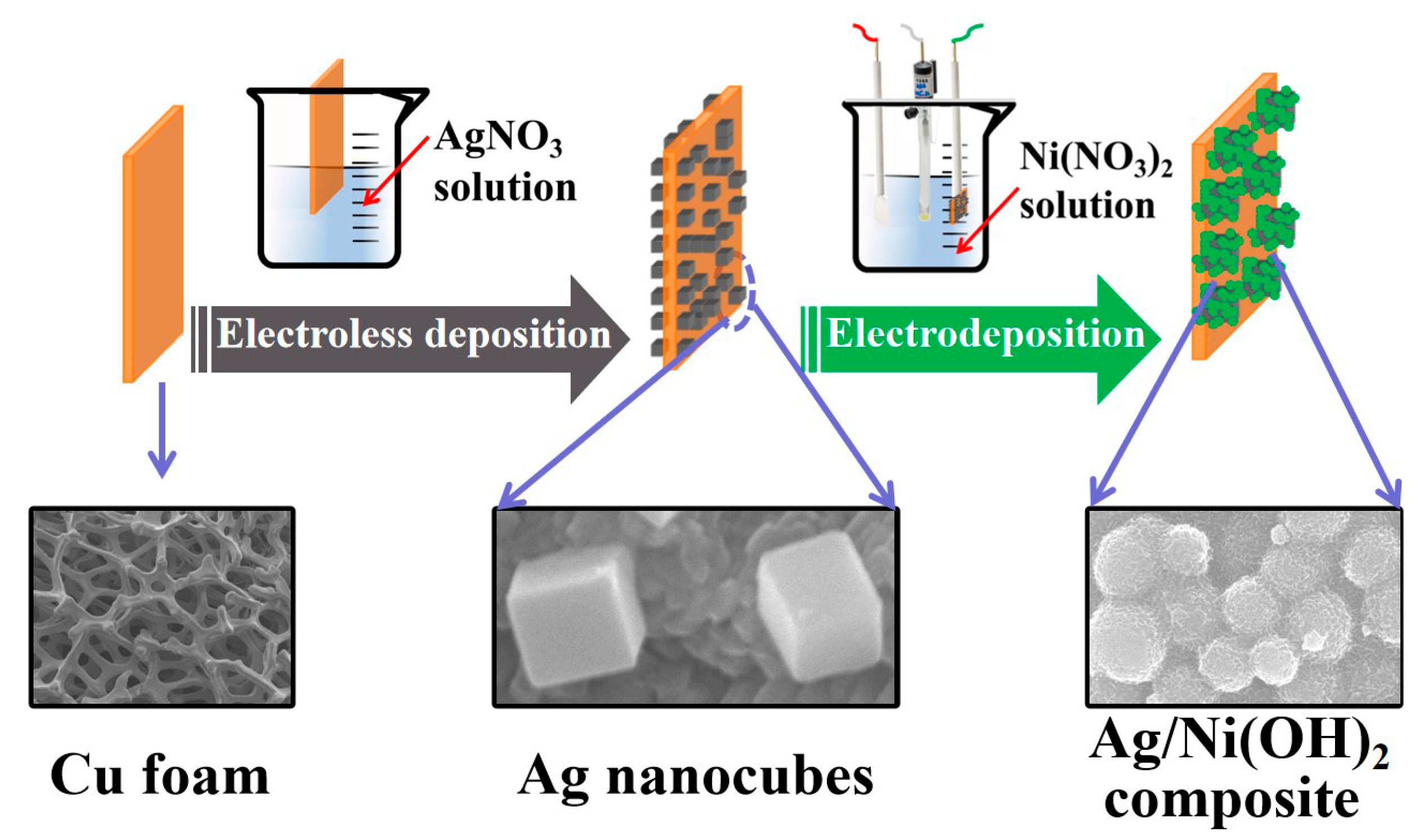
2.2. Synthesis of Ag/Ni(OH)2 Composite Electrode
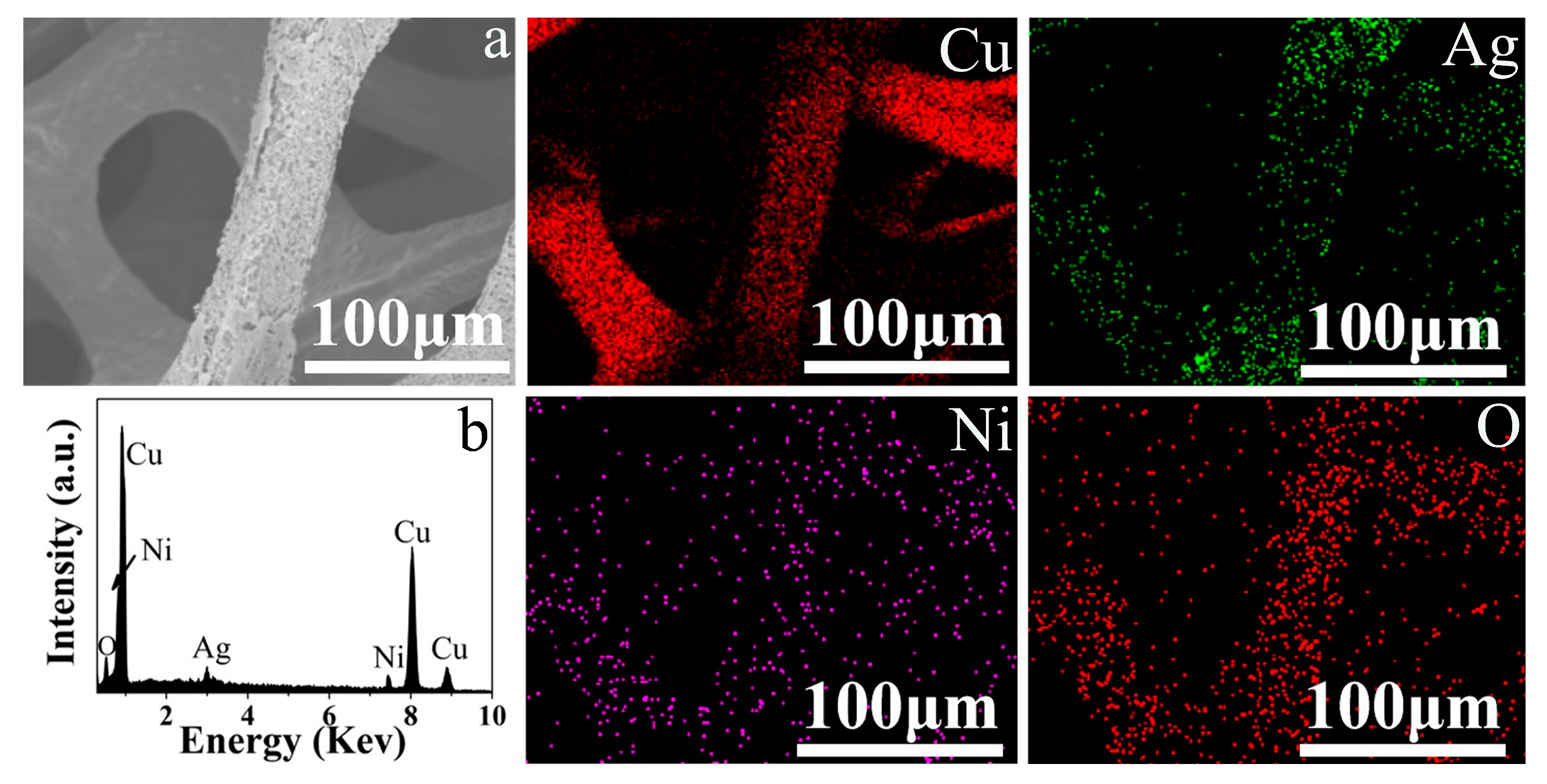
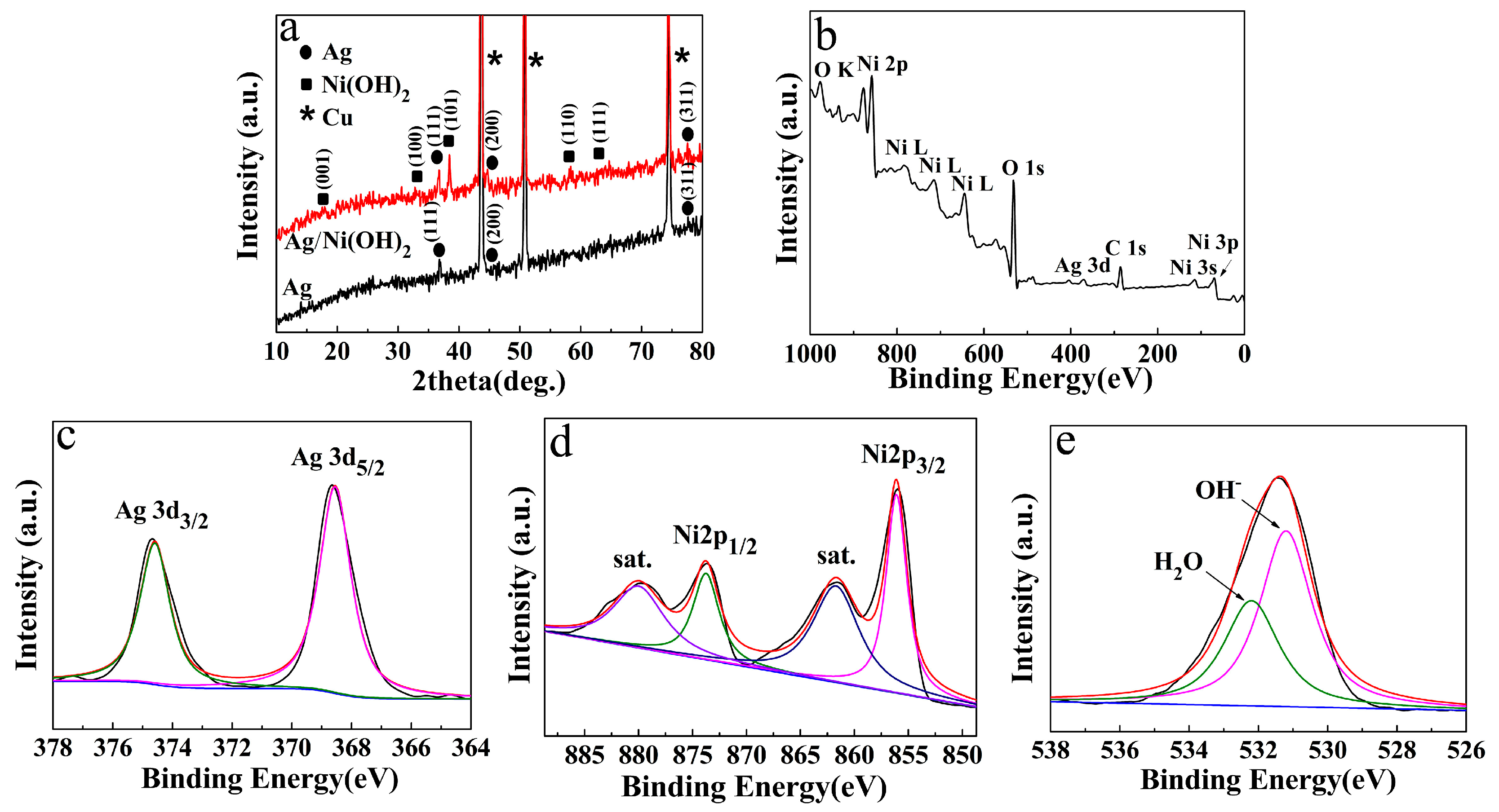
2.3. Materials Characterization
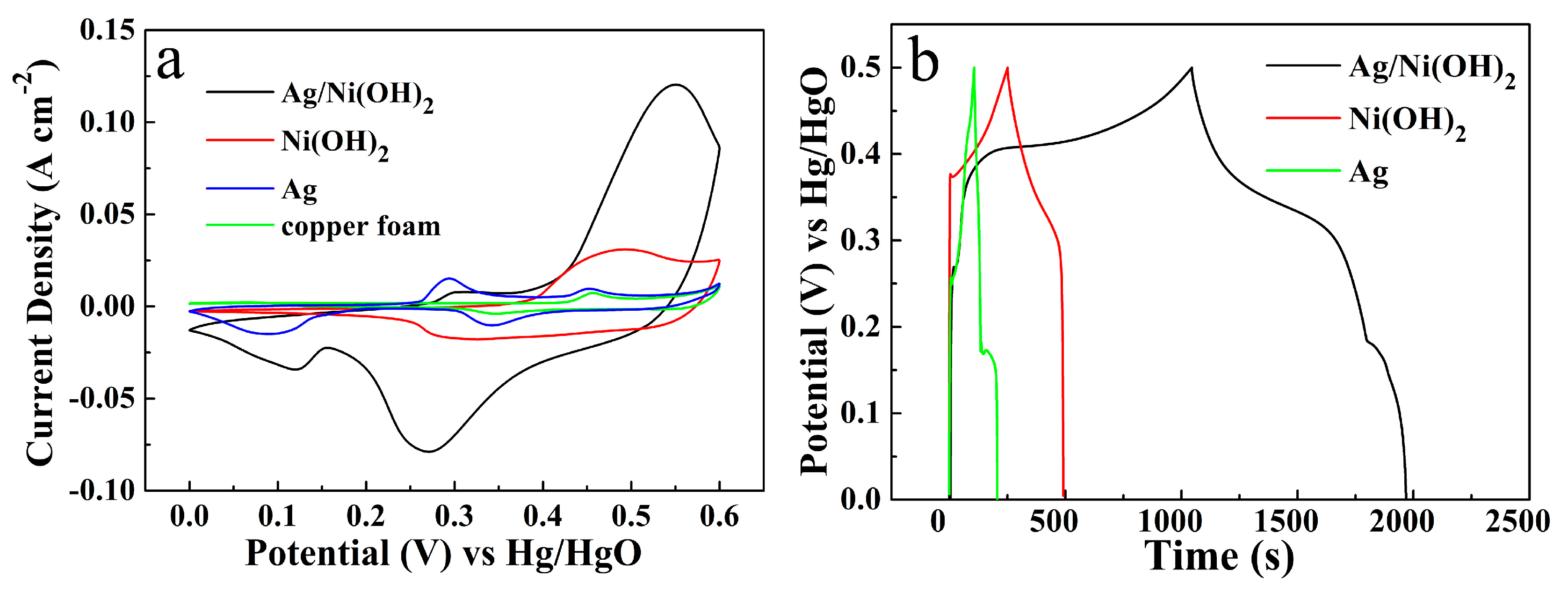
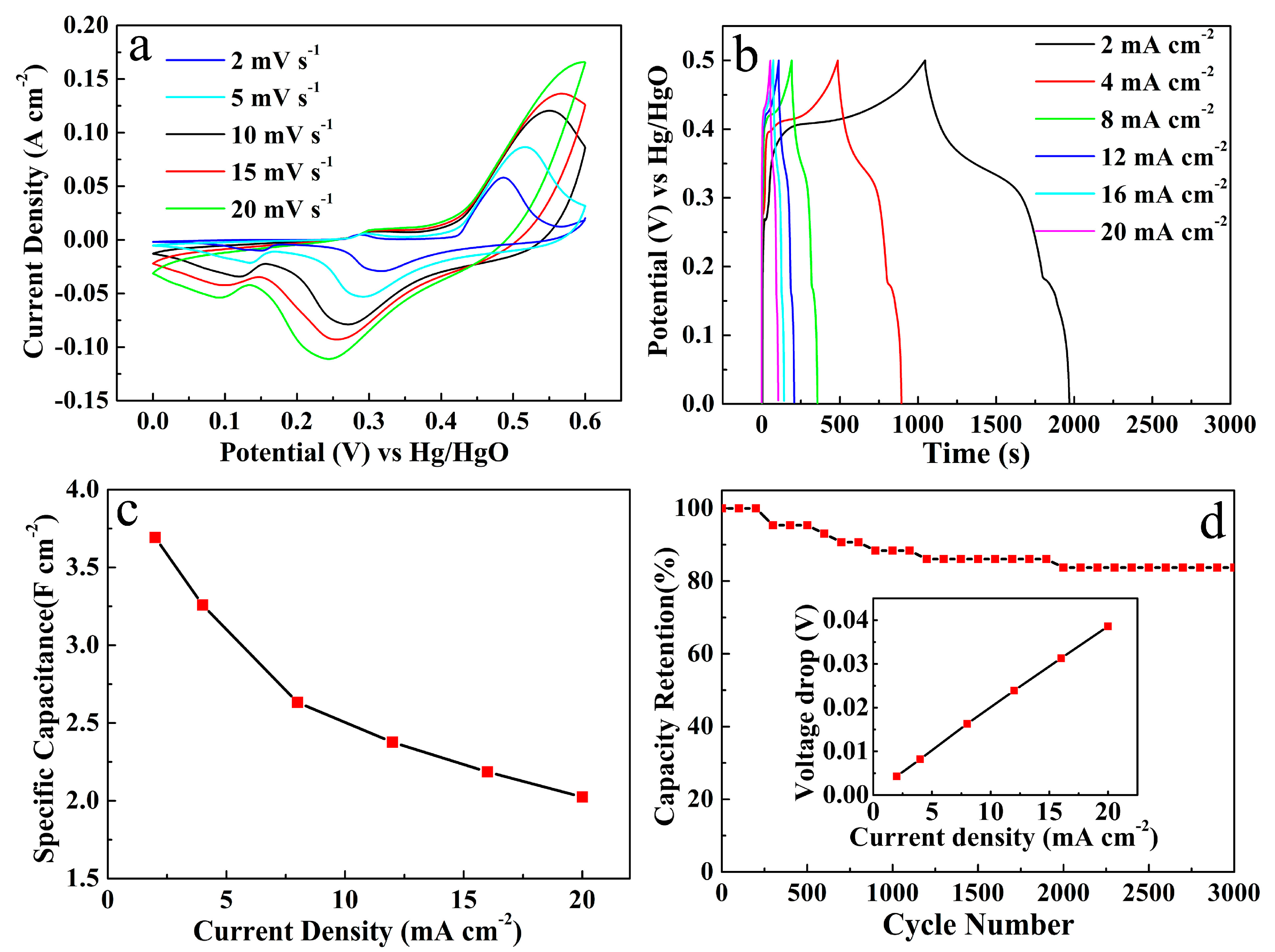
2.4. Electrochemical Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, J.; Wu, C.; Sun, X.; Hu, H.; Zhi, C.; Hou, L.; Yuan, C. Recent progresses in high-energy-density all pseudocapacitive-electrode-materials-based asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 9443–9464. [Google Scholar] [CrossRef]
- Hossain, A.; Bandyopadhyay, P.; Guin, P.S.; Roy, S. Recent developed different structural nanomaterials and their performance for supercapacitor application. Appl. Mater. Today 2017, 9, 300–313. [Google Scholar] [CrossRef]
- Saha, S.; Samanta, P.; Murmu, N.C.; Kuila, T. A review on the heterostructure nanomaterials for supercapacitor application. J. Energy Storage 2018, 17, 181–202. [Google Scholar] [CrossRef]
- Lokhande, P.; Chavan, U. Nanoflower-like Ni(OH)2 synthesis with chemical bath deposition method for high performance electrochemical applications. Mater. Lett. 2018, 218, 225–228. [Google Scholar] [CrossRef]
- Chiku, M.; Toda, M.; Higuchi, E.; Inoue, H. NiO layers grown on a Ni substrate by galvanostatic anodization as a positive electrode material for aqueous hybrid capacitors. J. Power Sources 2015, 286, 193–196. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, C.; Liu, T.; He, D.; Suo, H. Facile route to achieve book-like tricobalt tetraoxide microstructures on copper foam for high performance supercapacitor. Mater. Lett. 2018, 220, 78–81. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L.L. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21380–21423. [Google Scholar] [CrossRef]
- Zhang, G.D.; Liu, X.C.; Wang, Y.H.; Liu, C.Z.; Xing, S.X. Achieving MnO2 nanosheets via surface redox reaction on nickel nanochains for catalysis and energy storage. Chem. Eur. J. 2017, 23, 5557–5562. [Google Scholar] [CrossRef]
- Ruan, Y.J.; Wang, C.D.; Jiang, J.J. Nanostructured Ni compounds as electrode materials towards high-performance electrochemical capacitors. J. Mater. Chem. A 2016, 4, 14509–14538. [Google Scholar] [CrossRef]
- Zheng, M.; Li, B.; Xue, H.; Pang, H. High performance electrochemical capacitor materials focusing on nickel based materials. Inorg. Chem. Front. 2016, 3, 175–202. [Google Scholar]
- Hou, C.; Lang, X.Y.; Wen, Z.; Zhu, Y.F.; Zhao, M.; Li, J.C.; Zheng, W.T.; Lian, J.S.; Jiang, Q. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 23412–23419. [Google Scholar] [CrossRef]
- Aguilera, L.; Leyet, Y.; Peña-Garcia, R.; Hernández, E.P.; Passos, R.; Pocrifka, L. Cabbage-like α-Ni(OH)2 with a good long-term cycling stability and high electrochemical performances for supercapacitor applications. Chem. Phys. Lett. 2017, 677, 75–79. [Google Scholar] [CrossRef]
- Meng, X.; Deng, D. Bio-inspired synthesis of α-Ni(OH)2 nanobristles on various substrates and their applications. J. Mater. Chem. A 2016, 4, 6919–6925. [Google Scholar] [CrossRef]
- Lee, D.U.; Fu, J.; Park, M.G.; Liu, H.; Kashkooli, A.G.; Chen, Z. Self-Assembled NiO/Ni(OH)2 Nanoflakes as Active Material for High-Power and High-Energy Hybrid Rechargeable Battery. Nano Lett. 2016, 16, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Giri, S.; Mandal, A.; Das, C.K. Graphene decorated with Ni(OH)2 and Ag deposited Ni(OH)2 stacked nanoplate for supercapacitor application. Chem. Phys. Lett. 2013, 573, 41–47. [Google Scholar] [CrossRef]
- Kang, K.N.; Kim, I.H.; Ramadoss, A.; Kim, S.I.; Yoon, J.C.; Jang, J.H. Ultrathin nickel hydroxide on carbon coated 3D-porous copper structures for high performance supercapacitors. Phys. Chem. Chem. Phys. 2018, 20, 719–727. [Google Scholar] [CrossRef]
- Liao, G.; Fang, J.; Li, Q.; Li, S.; Xu, Z.; Fang, B. Ag-Based nanocomposites: Synthesis and applications in catalysis. Nanoscale 2019, 11, 7062–7096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Wang, Y.; Chen, G.; Xing, S. Dual Role of Polyaniline for Achieving Ag Dendrites and Enhancing Its Oxygen Reduction Reaction Catalytic Activity. ChemistrySelect 2017, 2, 10300–10303. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, L.; Yin, X.; Huang, T.J.; Gong, H. A one step processed advanced interwoven architecture of Ni(OH)2 and Cu nanosheets with ultrahigh supercapacitor performance. J. Mater. Chem. A 2016, 4, 12144–12151. [Google Scholar] [CrossRef]
- Liao, G.F.; Chen, J.; Zeng, W.G.; Yu, C.H.; Yi, C.F.; Xu, Z.S. Facile preparation of uniform nanocomposite spheres with loading silver nanoparticles on polystyrene-methyl acrylic acid spheres for catalytic reduction of 4-nitrophenol. J. Phys. Chem. C 2016, 120, 25935–25944. [Google Scholar] [CrossRef]
- Yuksel, R.; Coskun, S.; Kalay, Y.E.; Unalan, H.E. Flexible, silver nanowire network nickel hydroxide core-shell electrodes for supercapacitors. J. Power Sources 2016, 328, 167–173. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Nagaraju, G.; Yu, J.S. Conductive silver nanowires-fenced carbon cloth fibers-supported layered double hydroxide nanosheets as a flexible and binder-free electrode for high-performance asymmetric supercapacitors. Nano Energy 2017, 36, 58–67. [Google Scholar] [CrossRef]
- Salavagione, H.; Prieto, R.; Morallo´n, E.; Narciso, J. 3D Electrodes from aluminium foams prepared by replication process. J. Appl. Electrochem. 2010, 40, 241–246. [Google Scholar] [CrossRef]
- Wu, G.X.; Yang, S.J.; Liu, Q.M. Synthesis of silver nanostructures by simple redox under electrodeposited copper microcubes and the orient attachment growth of 2D silver. Appl. Surf. Sci. 2015, 357, 583–592. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Kiani, M.; Ghaemmaghami, M.; Kazemi, H. Nano-architectured MnO2 Electrodeposited on the Cu-decorated Nickel Foam substrate as Supercapacitor Electrode with Excellent Areal Capacitance. Electrochim. Acta 2016, 197, 107–116. [Google Scholar] [CrossRef]
- Usman, M.; Pan, L.; Sohail, A.; Mahmood, Z.; Cui, R. Fabrication of 3D vertically aligned silver nanoplates on nickel foam-graphene substrate by a novel electrodeposition with sonication for efficient supercapacitors. Chem. Eng. J. 2017, 311, 359–366. [Google Scholar] [CrossRef]
- Park, S.; Tan, A.W.M.; Wang, J.; Lee, P.S. Coaxial Ag–base metal nanowire networks with high electrochemical stability for transparent and stretchable asymmetric supercapacitors. Nanoscale Horiz. 2017, 2, 199–204. [Google Scholar] [CrossRef]
- Liu, P.; Liu, J.; Cheng, S.; Cai, W.; Yu, F.; Zhang, Y.; Wu, P.; Liu, M. A high-performance electrode for supercapacitors: Silver nanoparticles grown on a porous perovskite-type material La0.7Sr0.3CoO3−δ substrate. Chem. Eng. J. 2017, 328, 1–10. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; Jia, S.; Liu, X.; Wang, Y.; Xing, S. Facile route to achieve hierarchical hollow MnO2 nanostructures. Electrochim. Acta 2016, 203, 59–65. [Google Scholar] [CrossRef]
- He, D.; Wang, G.; Liu, G.; Suo, H.; Zhao, C. Construction of leaf-like CuO–Cu2O nanocomposites on copper foam for high-performance supercapacitors. Dalton Trans. 2017, 46, 3318–3324. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Xu, J.; Cheng, G.; Yang, W.; Kou, T.; Zhang, Z.; Ding, Y. 3D binder-free Cu2O@Cu nanoneedle arrays for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 18229–18235. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Yang, F.; Chu, X.; Wang, H.; Yang, J.; Chi, Y.; Yang, X. In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition. Metals 2019, 9, 826. https://doi.org/10.3390/met9080826
Lv S, Yang F, Chu X, Wang H, Yang J, Chi Y, Yang X. In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition. Metals. 2019; 9(8):826. https://doi.org/10.3390/met9080826
Chicago/Turabian StyleLv, Sa, Fan Yang, Xuefeng Chu, Huan Wang, Jia Yang, Yaodan Chi, and Xiaotian Yang. 2019. "In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition" Metals 9, no. 8: 826. https://doi.org/10.3390/met9080826
APA StyleLv, S., Yang, F., Chu, X., Wang, H., Yang, J., Chi, Y., & Yang, X. (2019). In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition. Metals, 9(8), 826. https://doi.org/10.3390/met9080826





