Effect of Hydrogen Content and Strain Rate on Hydrogen-induced Delay Cracking for Hot-stamped Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogen Measurements
2.3. Slow Strain Rate Tests
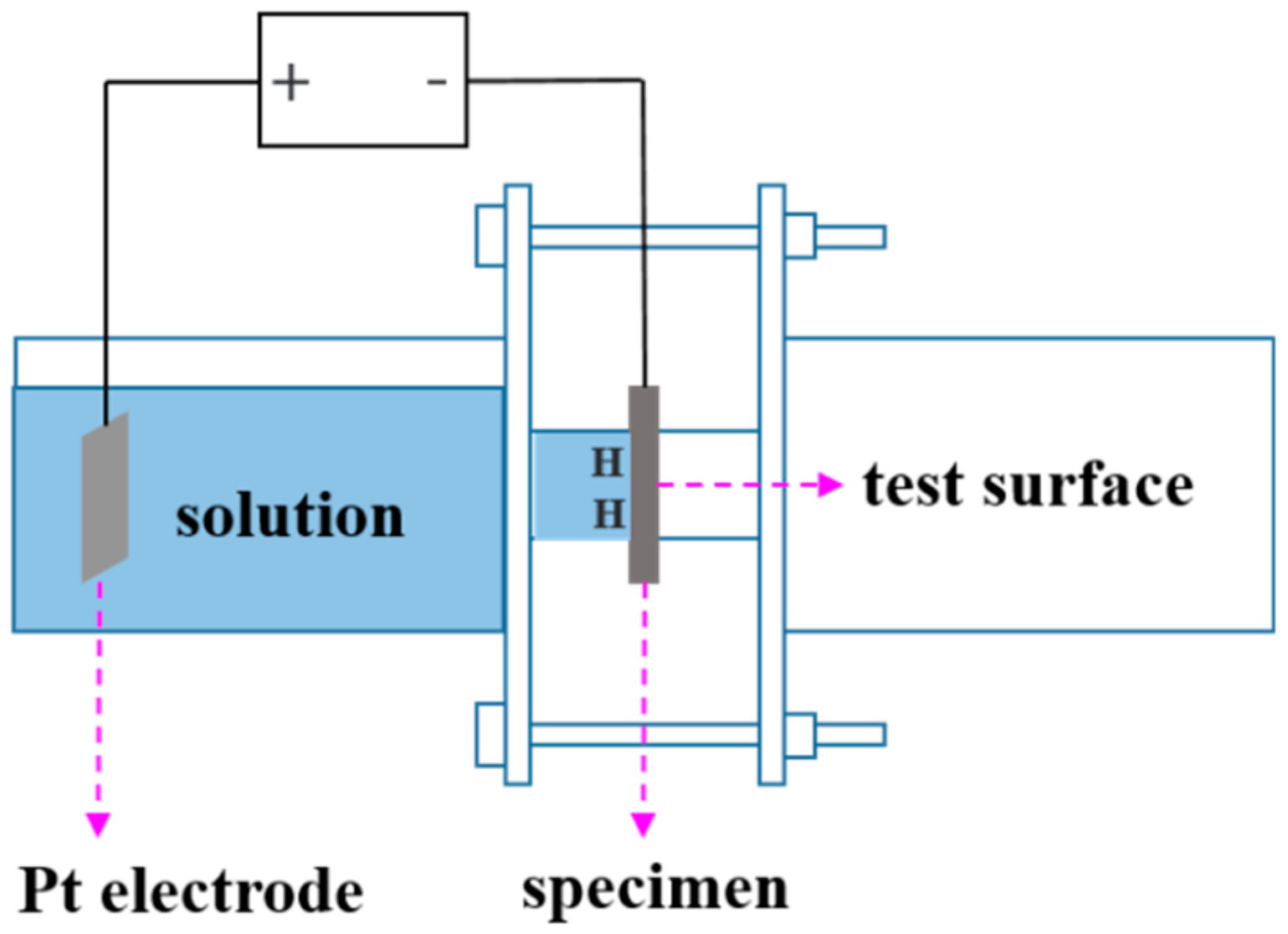
2.4. Scanning Kelvin Probe Force Microscope (SKPFM) Measurements
3. Results
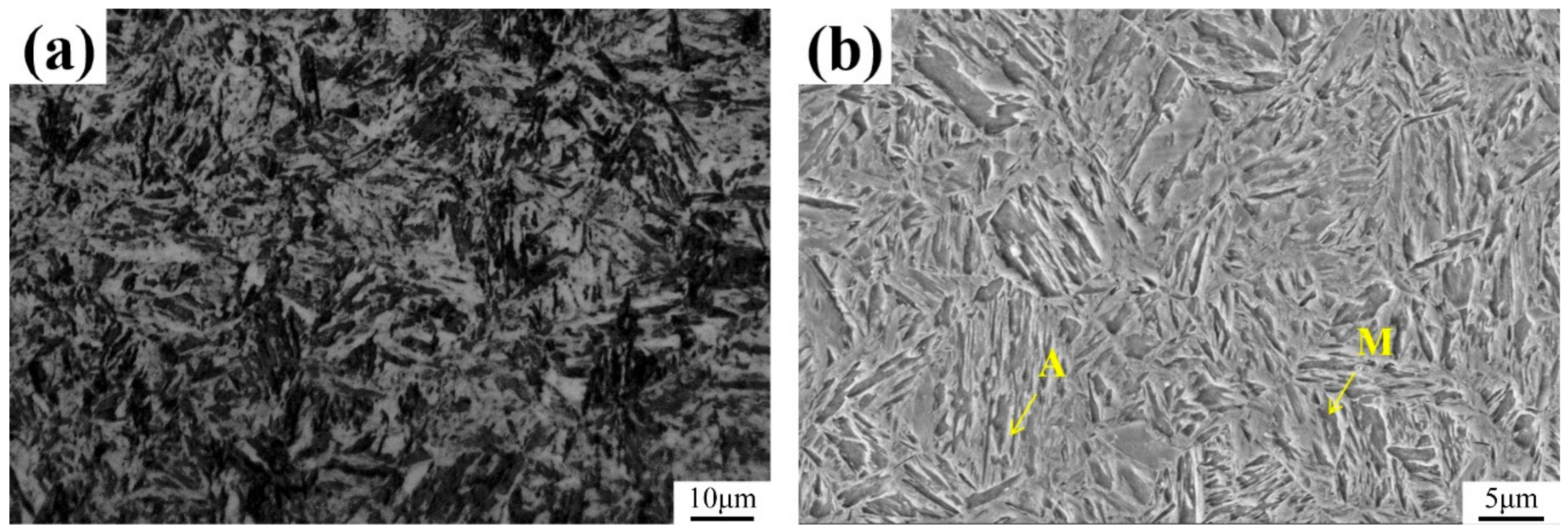
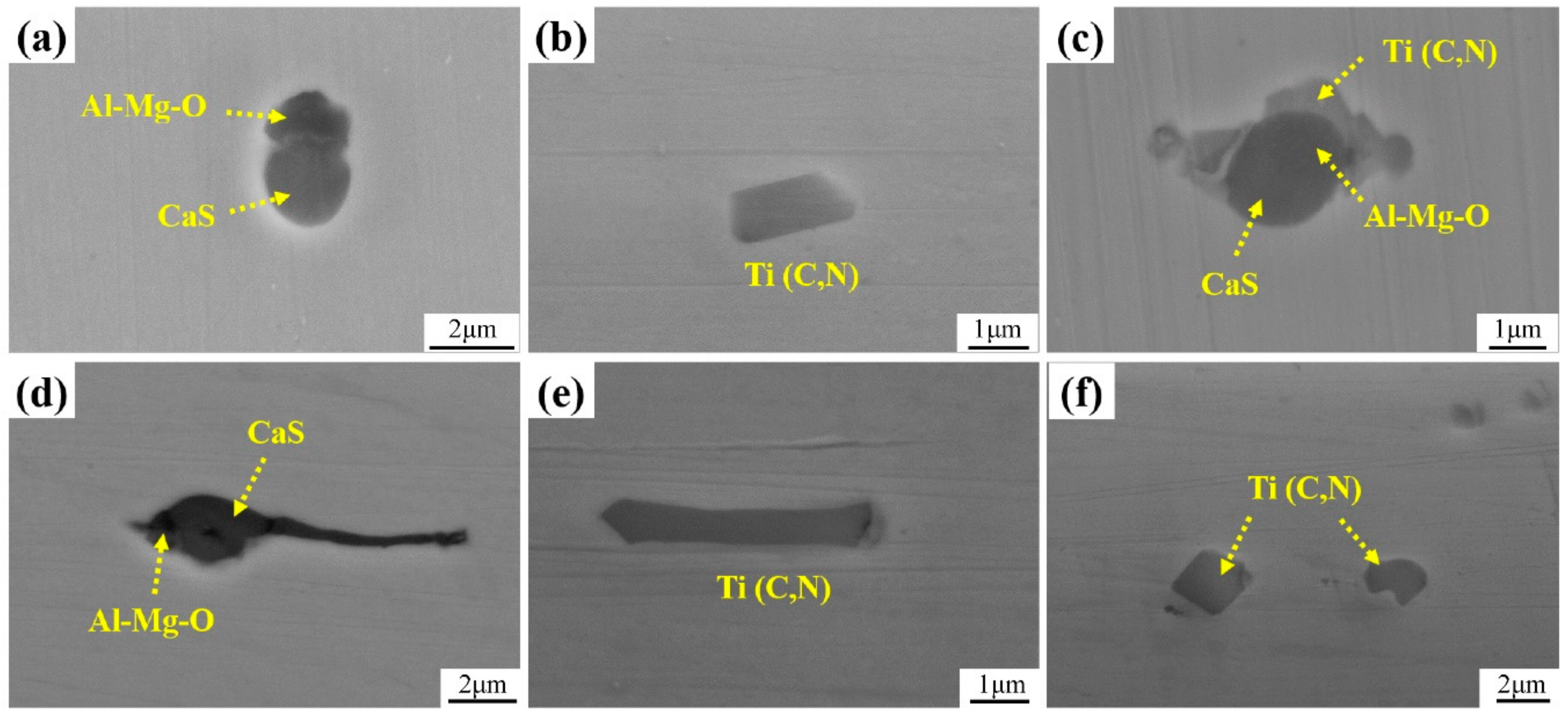
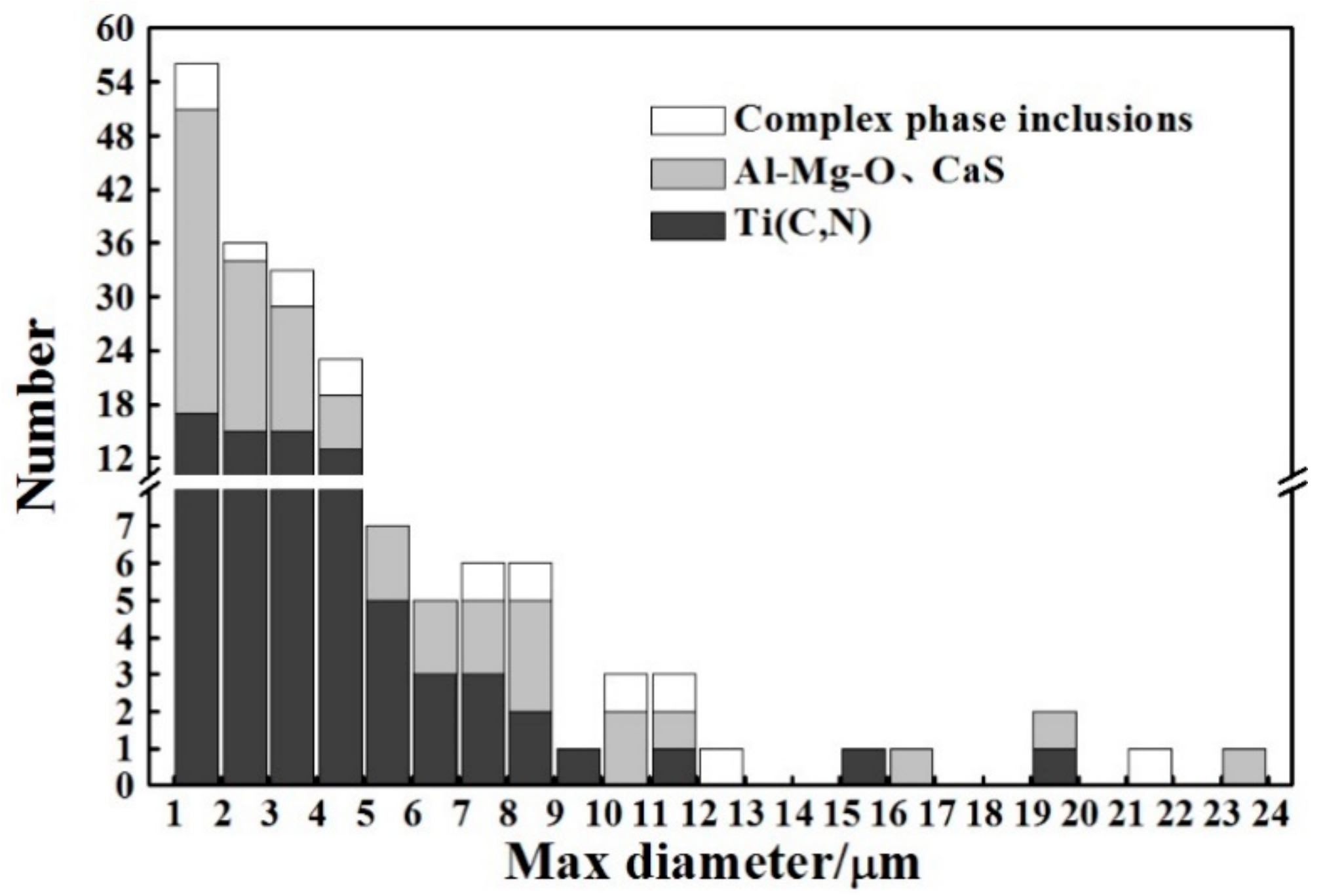
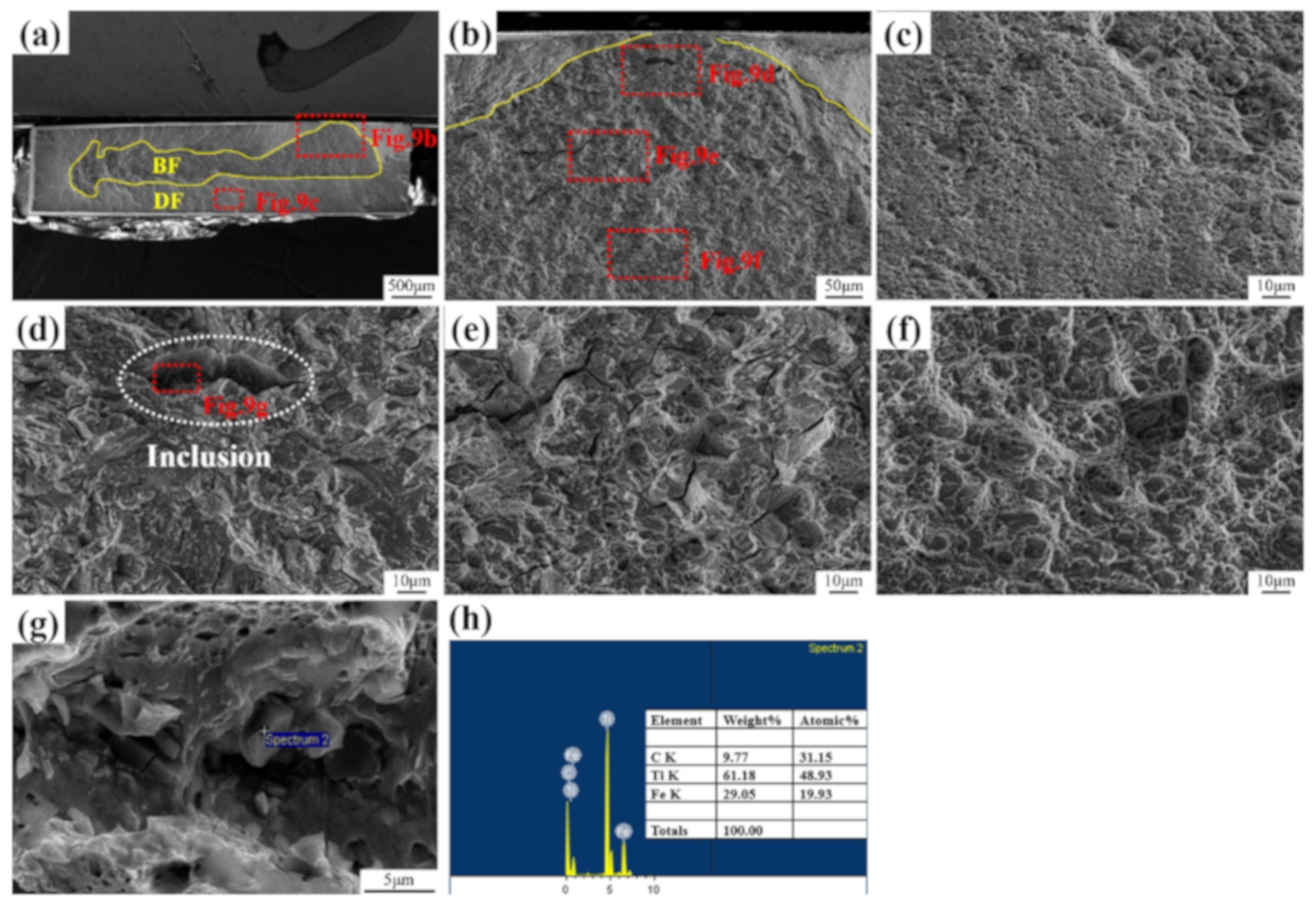
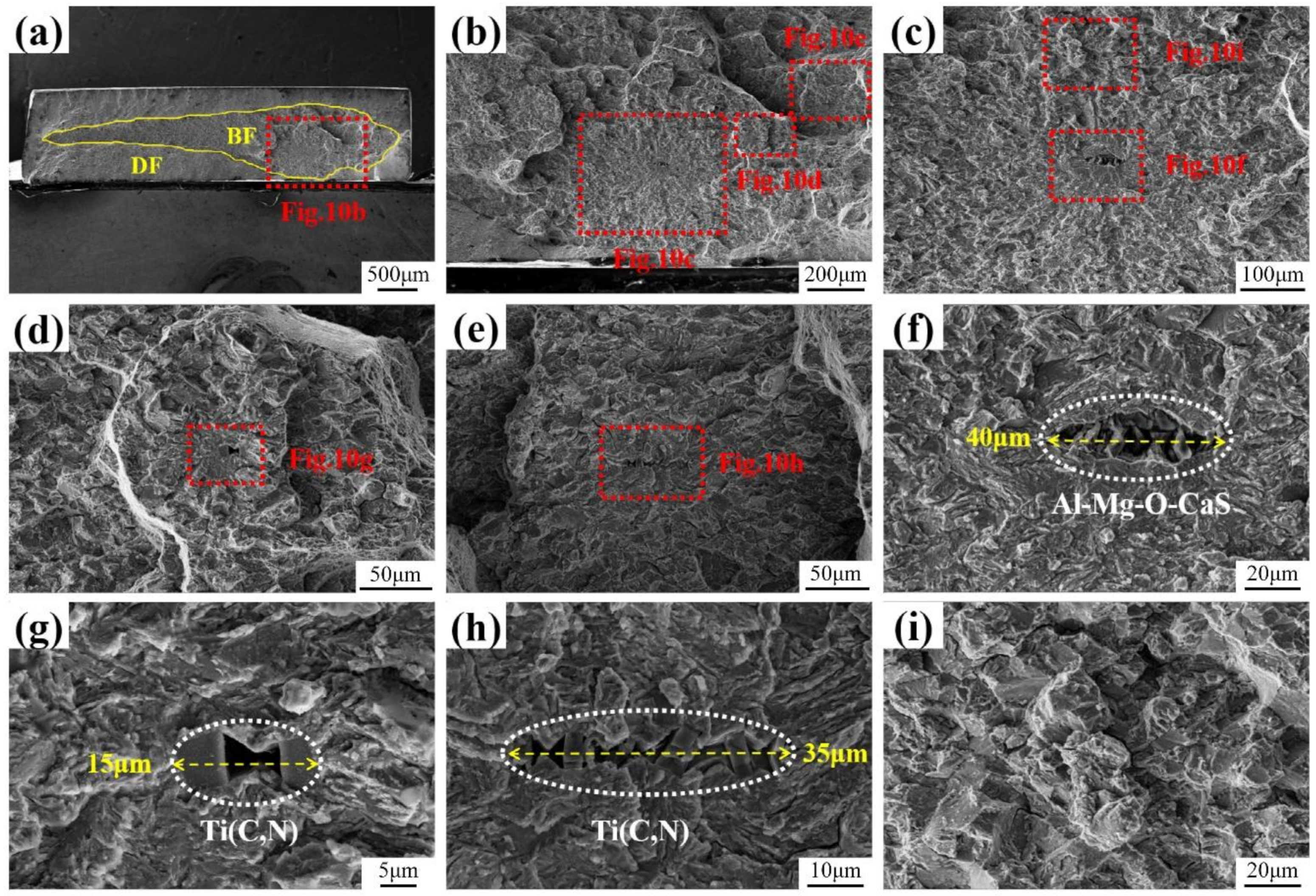
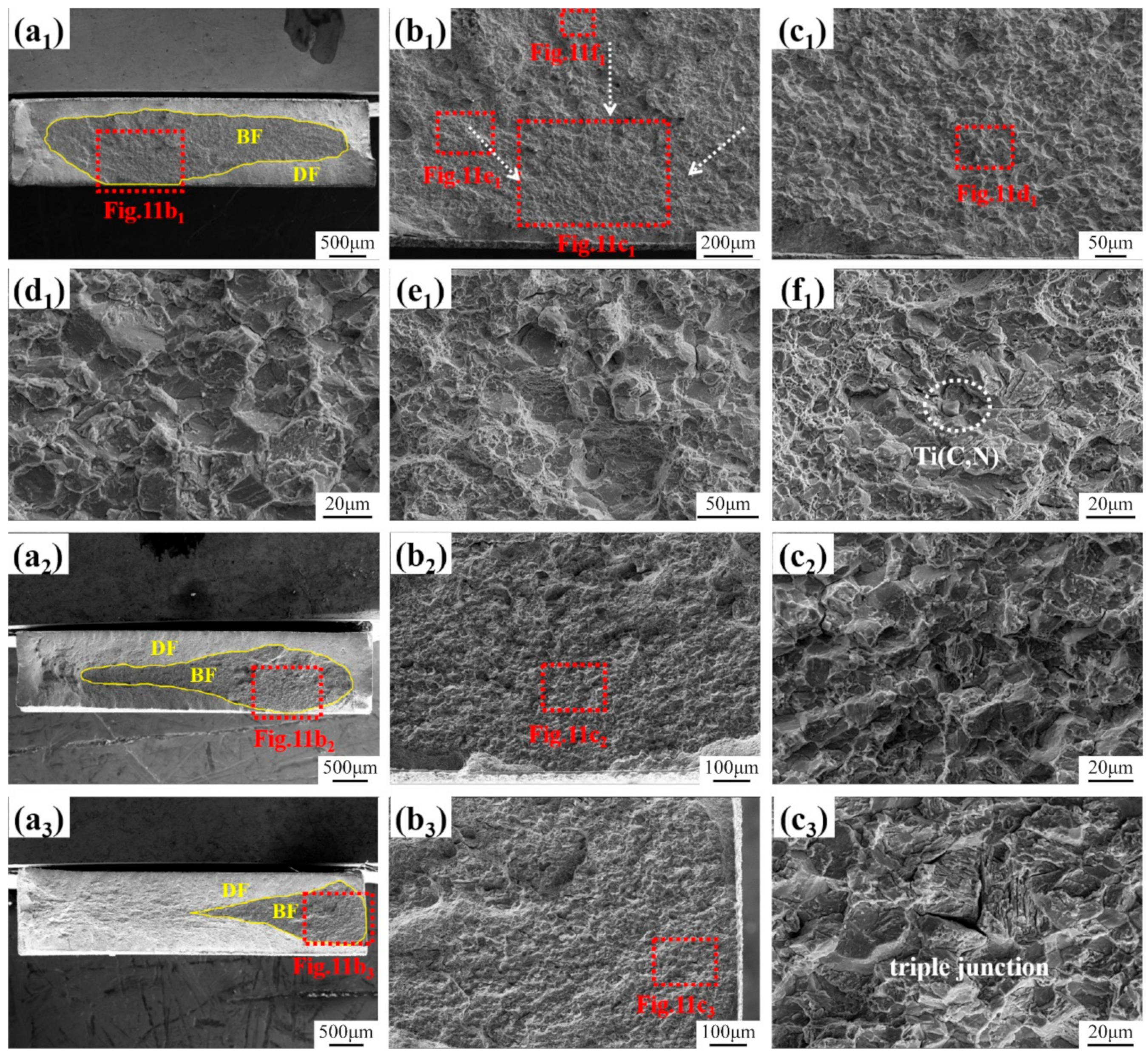
3.1. Microstructure and Inclusions
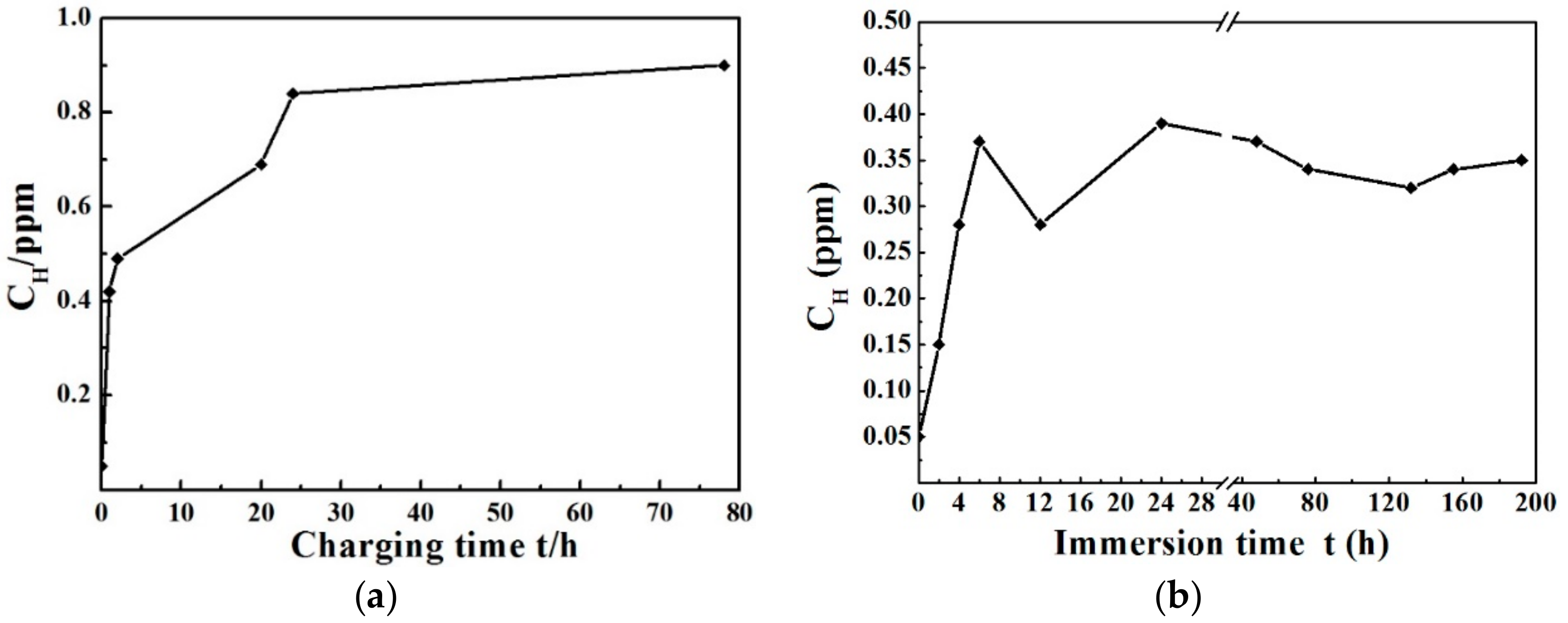
3.2. Hydrogen Measurements
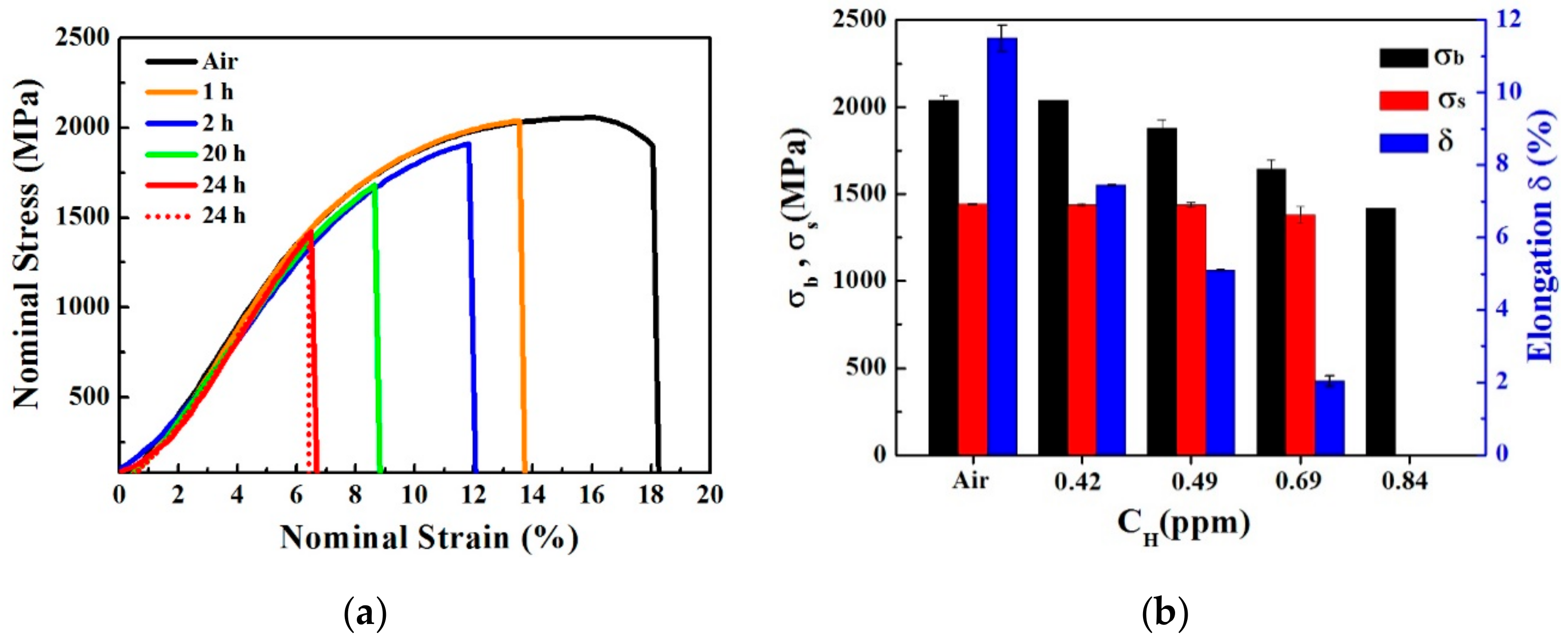
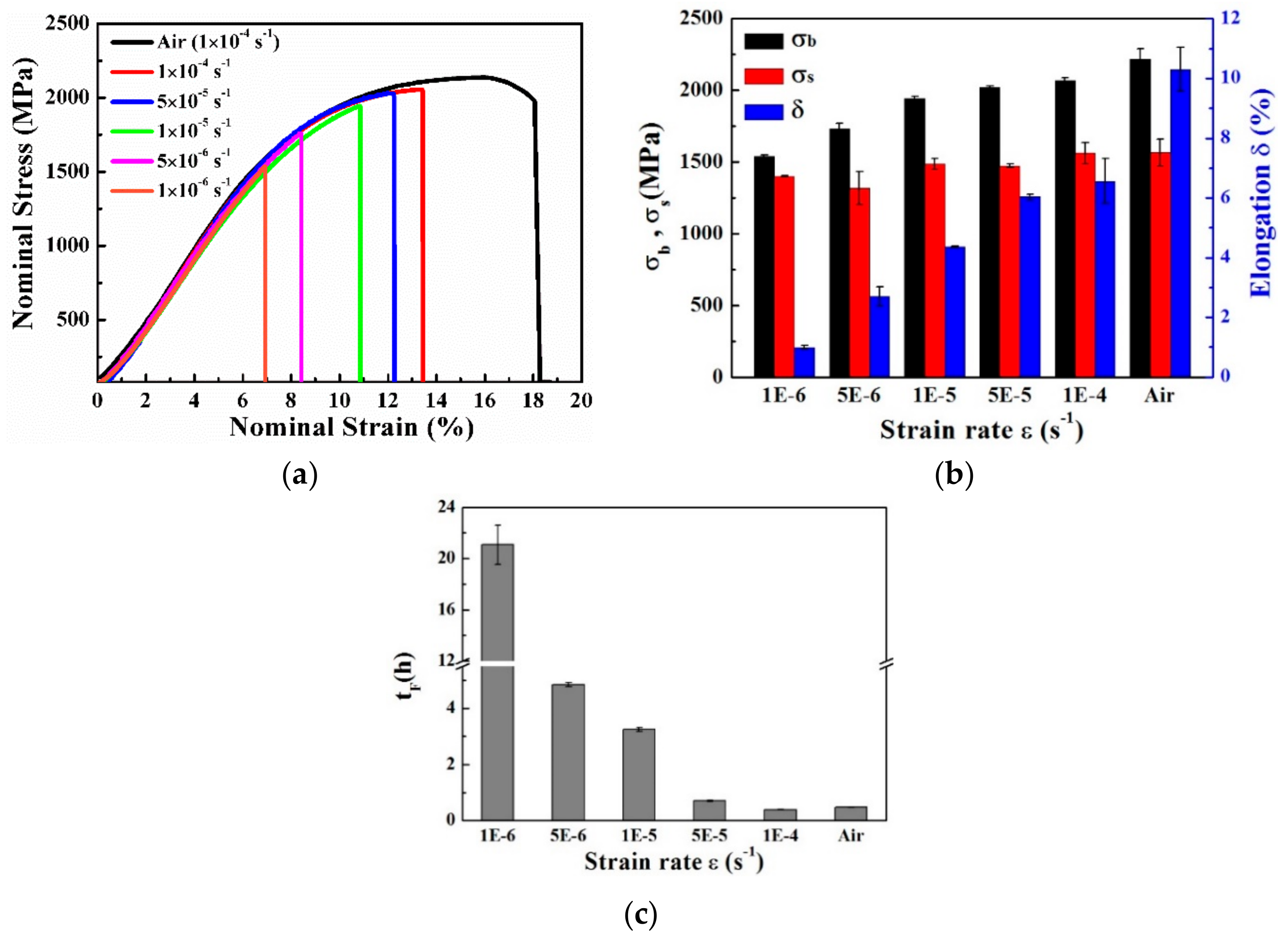
3.3. Mechanical Properties
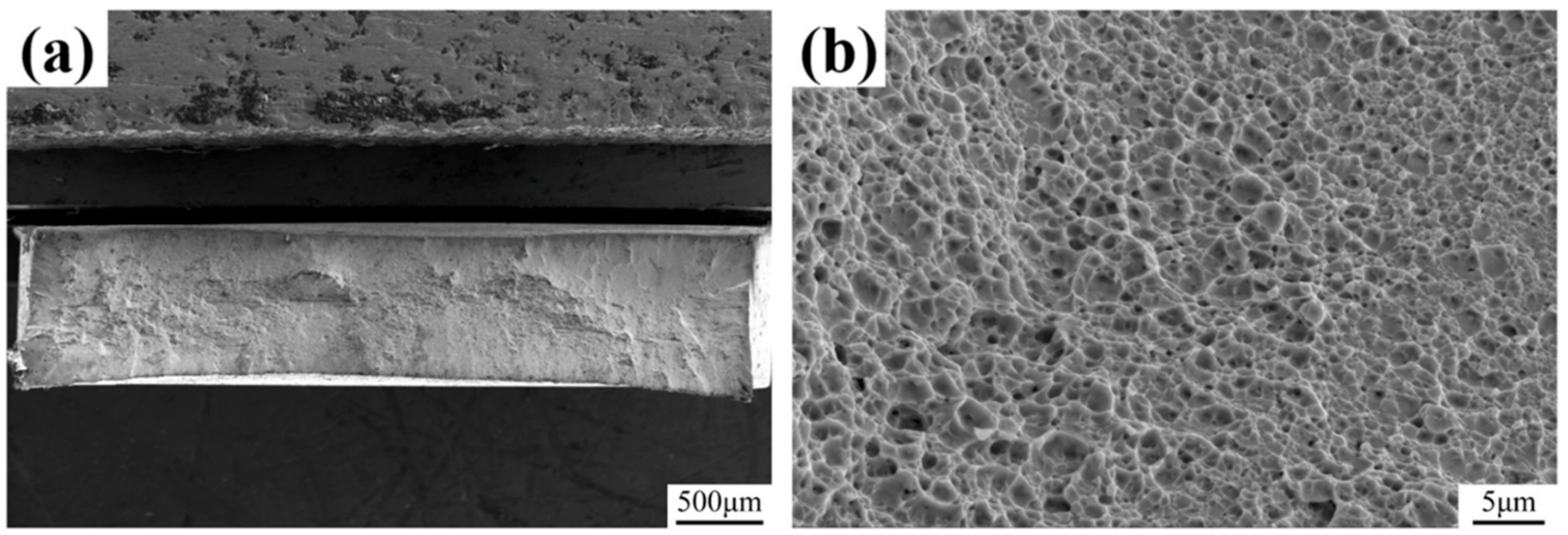
3.4. Fracture Surface Observation
3.5. Hydrogen Enrichment Measurement by in-situ SKPFM
4. Discussion
4.1. Role of Inclusions in Steels
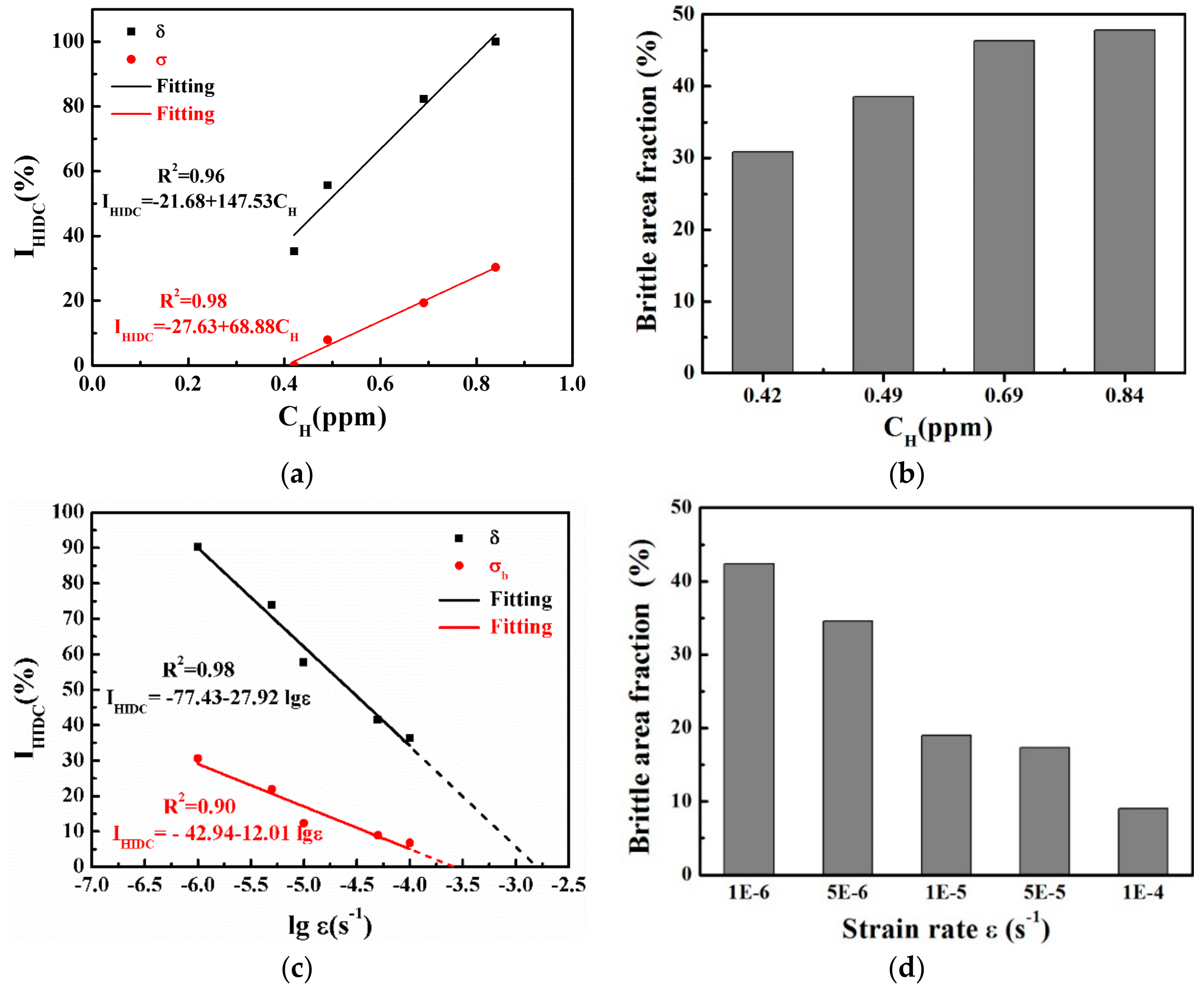
4.2. Effect of the Hydrogen Content on the HIDC
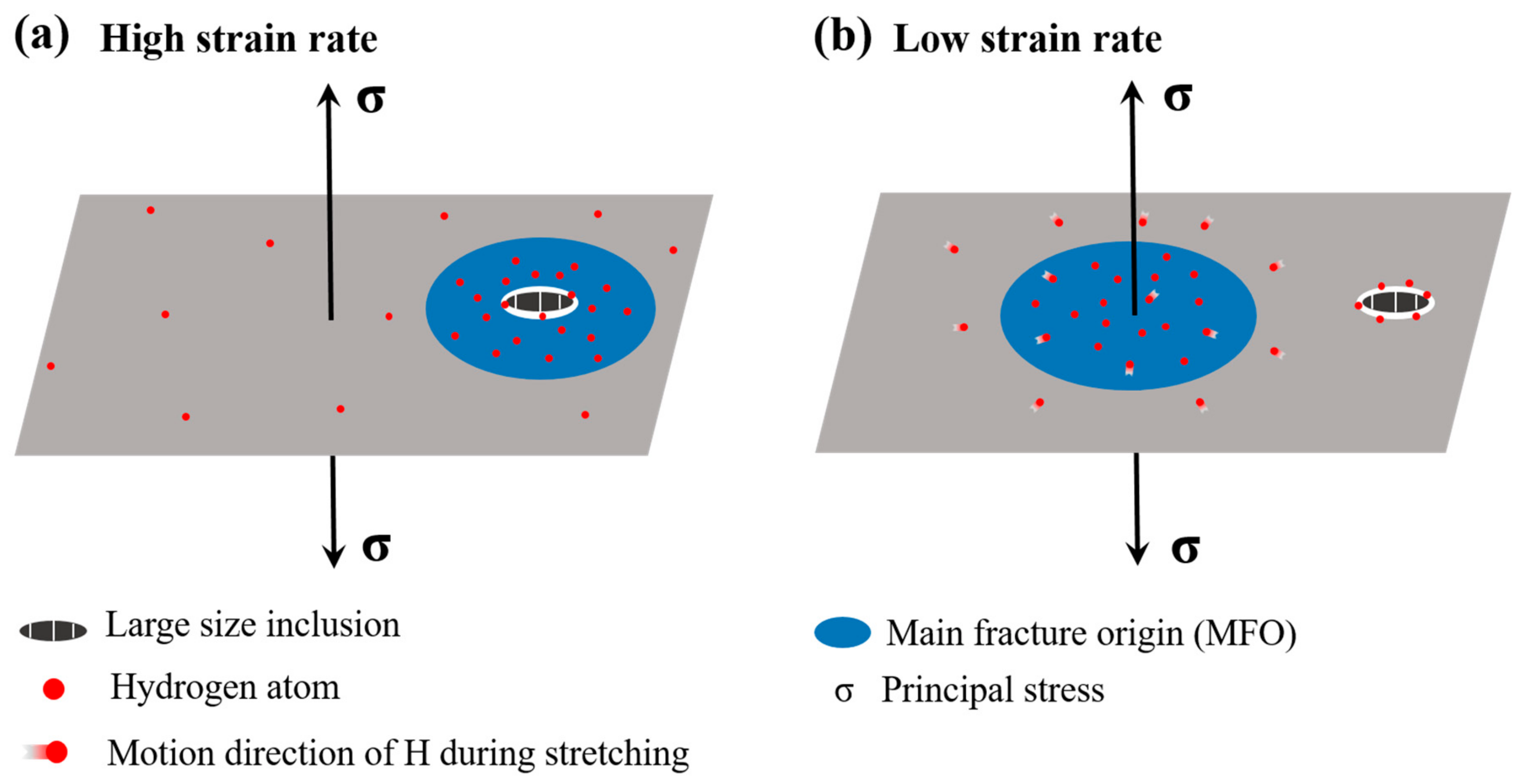
4.3. Effect of the Strain Rate on the HIDC
4.3.1. High hydrogen Content in Materials
4.3.2. Low Hydrogen Content in Materials
5. Conclusions
- (1)
- As the hydrogen content increased, the plasticity and strength decreased for the pre-charged hydrogen unsteady-state specimens. All of the fractures originated from large-sized inclusions and the brittle fracture area increased as the hydrogen content increased. The in-situ SKPFM results indicated that more hydrogen was accumulated around the inclusions after hydrogen charging.
- (2)
- For pre-charged hydrogen steady-state samples, the surroundings of large-sized inclusions induced the main fracture origin and fish-eyes were presented as quasi-cleavage features at a higher strain rate (of 1 × 10−3 s−1). At a lower strain rate (of 1 × 10−6 s−1), in contrast, the main fracture origin was not induced by the inclusions and appeared, instead, as a distinct inter-granular fracture. The above results mean that, when the sample was deformed at a relatively high strain rate, the micro-cracks originated from the vicinity of the inclusions, and the fracture surface showed more large-sized inclusions; therefore, the defect itself in the material had great influence on the tensile properties, in this case. The hydrogen in the material had enough time to diffuse and re-enrich when the sample was deformed at a slower strain rate; thus, the slower the strain rate, the greater the influence of hydrogen, so that the final fracture exhibited inter-granular characteristics.
- (3)
- For non-pre-charged specimens, on which in-situ continuous soaking SSRT was performed, as the strain rate decreased, the tensile strength and elongation gradually decreased. The fracture origin under all strain rates were corrosion pits at the edge of the samples, due to the presence of large inclusions near the sample surface, and the brittle fracture zone increased with a decrease of strain rate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Q.L.; Zhou, Q.J.; Venezuela, J.; Zhang, M.X.; Wang, J.Q.; Atrens, A. A review of the influence of hydrogen on the mechanical properties of DP, TRIP, and TWIP advanced high-strength steels for auto construction. Corros. Rev. 2016, 34, 127–152. [Google Scholar] [CrossRef]
- Laureys, A.; Depover, T.; Petrov, R.; Verbeken, K. Characterization of hydrogen induced cracking in TRIP-assisted steels. Int. J. Hydrogen Energy 2015, 40, 16901–16912. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sun, J.J.; Jiang, T.; Sun, Y.; Guo, S.W.; Liu, Y.N. A low-alloy high-carbon martensite steel with 2.6 GPa tensile strength and good ductility. Acta Mater. 2018, 158, 247–256. [Google Scholar] [CrossRef]
- Schaffner, T.; Hartmaier, A.; Kokotin, V.; Pohl, M. Analysis of hydrogen diffusion and trapping in ultra-high strength steel grades. J. Alloy Compd. 2018, 746, 557–566. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Miyashita, T.; Takai, K. Hydrogen behavior in high strength steels during various stress applications corresponding to different hydrogen embrittlement testing methods. Mater. Sci. Eng. A 2018, 735, 61–72. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, H.K.; Lee, C.W.; Yoo, B.G.; Jung, H.Y. Baking Effect on Desorption of Diffusible Hydrogen and Hydrogen Embrittlement on Hot-Stamped Boron Martensitic Steel. Metals 2019, 9, 636. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeon, S.H.; Yang, W.S.; Yoo, B.G.; Chung, Y.D.; Ha, H.Y.; Chung, H.Y. Effects of titanium content on hydrogen embrittlement susceptibility of hot-stamped boron steels. J. Alloy Compd. 2018, 735, 2067–2080. [Google Scholar] [CrossRef]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.D.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J.R.; et al. Understanding and mitigating hydrogen embrittlement of steels: A review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef]
- Koyama, M.; Tasan, C.C.; Akiyama, E.; Tsuzaki, K.; Raabe, D. Hydrogen-assisted decohesion and localized plasticity in dual-phase steel. Acta Mater. 2014, 70, 174–187. [Google Scholar] [CrossRef]
- Tiegel, M.C.; Martin, M.L.; Lehmberg, A.K.; Deutges, M.; Borchers, C.; Kirchheim, R. Crack and blister initiation and growth in purified iron due to hydrogen loading. Acta Mater. 2016, 115, 24–34. [Google Scholar] [CrossRef]
- Dong, C.F.; Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Effects of hydrogen-charging on the susceptibility of X100 pipeline steel to hydrogen-induced cracking. Int. J. Hydrogen Energy 2009, 34, 9879–9884. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization of corrosion of welded X70 pipeline steel in near-neutral pH solution. Corros. Sci. 2009, 51, 1714–1724. [Google Scholar] [CrossRef]
- Koyama, M.; Springer, H.; Merzlikin, S.V.; Tsuzaki, K.; Akiyama, E.; Raabe, D. Hydrogen embrittlement associated with strain localization in a precipitation-hardened Fe-Mn-Al-C light weight austenitic steel. Int. J. Hydrogen Energy 2014, 39, 4634–4646. [Google Scholar] [CrossRef]
- Fujita, S.; Murakami, Y. A New Nonmetallic Inclusion Rating Method by Positive Use of Hydrogen Embrittlement Phenomenon. Metall. Mater. Trans. A 2013, 44, 303–322. [Google Scholar] [CrossRef]
- Dunne, D.P.; Hejazi, D.; Saleh, A.A.; Haq, A.J.; Calka, A.; Pereloma, E.V. Investigation of the effect of electrolytic hydrogen charging of X70 steel: I. The effect of microstructure on hydrogen-induced cold cracking and blistering. Int. J. Hydrogen Energy 2016, 41, 12411–12423. [Google Scholar] [CrossRef]
- Ervasti, E.; Stahlberg, U. Void initiation close to a macro-inclusion during single pass reductions in the hot rolling of steel slabs: A numerical study. J. Mater. Process. Technol. 2005, 170, 142–150. [Google Scholar] [CrossRef]
- Liu, Q.L.; Zhou, Q.J.; Venezuela, J.; Zhang, M.X.; Atrens, A. The role of the microstructure on the influence of hydrogen on some advanced high-strength steels. Mat. Sci. Eng. A 2018, 715, 370–378. [Google Scholar] [CrossRef]
- Lovicu, G.; Bagliani, E.P.; De Sanctis, M.; Dimatteo, A.; Ishak, R.; Valentini, R. Hydrogen embrittlement of a medium carbon Q&P steel. Metall. Ital. 2013, 105, 3–10. [Google Scholar]
- Sojka, J.; Vodarek, V.; Schindler, I.; Ly, C.; Jerome, M.; Vanova, P.; Ruscassier, N.; Wenglorzova, A. Effect of hydrogen on the properties and fracture characteristics of TRIP 800 steels. Corros. Sci. 2011, 53, 2575–2581. [Google Scholar] [CrossRef]
- Madelen, O.; Todoshchenko, I.; Yagodzinskyy, Y.; Saukkonen, T.; Hanninen, H. Role of Nonmetallic Inclusions in Hydrogen Embrittlement of High-Strength Carbon Steels with Different Microalloying. Metall. Mater. Trans. A 2014, 45, 4742–4747. [Google Scholar] [CrossRef]
- Bal, B.; Koyama, M.; Gerstein, G.; Maier, H.J.; Tsuzaki, K. Effect of strain rate on hydrogen embrittlement susceptibility of twinning-induced plasticity steel pre-charged with high-pressure hydrogen gas. Int. J. Hydrogen Energy 2016, 41, 15362–15372. [Google Scholar] [CrossRef]
- Wu, X.Q.; Kim, I.S. Effects of strain rate and temperature on tensile behavior of hydrogen-charged SA508 Cl.3 pressure vessel steel. Mater. Sci. Eng. A 2003, 348, 309–318. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Effect of strain rate on hydrogen embrittlement in low-carbon martensitic steel. Int. J. Hydrogen Energy 2017, 42, 3371–3379. [Google Scholar] [CrossRef]
- Kan, B.; Yang, Z.X.; Wang, Z.; Li, J.X.; Zhou, Q.J.; Su, Y.J.; Qiao, L.J.; Volinsky, A.A. Hydrogen redistribution under stress-induced diffusion and corresponding fracture behaviour of a structural steel. Mater. Sci. Technol. 2017, 33, 1539–1547. [Google Scholar] [CrossRef]
- Ghosh, A.; Ray, A.; Chakrabarti, D.; Davis, C.L. Cleavage initiation in steel: Competition between large grains and large particles. Mater. Sci. Eng. A 2013, 561, 126–135. [Google Scholar] [CrossRef]
- Tervo, H.; Kaijalainen, A.; Pikkarainen, T.; Mehtonen, S.; Porter, D. Effect of impurity level and inclusions on the ductility and toughness of an ultra-high-strength steel. Mater. Sci. Eng. A 2017, 697, 184–193. [Google Scholar] [CrossRef]
- Senoz, C.; Evers, S.; Stratmann, M.; Rohwerder, M. Scanning Kelvin Probe as a highly sensitive tool for detecting hydrogen permeation with high local resolution. Electrochem. Commun. 2011, 13, 1542–1545. [Google Scholar] [CrossRef]
- Wang, G.; Yan, Y.; Yang, X.N.; Li, J.X.; Qiao, L.J. Investigation of hydrogen evolution and enrichment by scanning Kelvin probe force microscopy. Electrochem. Commun. 2013, 35, 100–103. [Google Scholar] [CrossRef]
- Kim, W.K.; Koh, S.U.; Yang, B.Y.; Kim, K.Y. Effect of environmental and metallurgical factors on hydrogen induced cracking of HSLA steels. Corros. Sci. 2008, 50, 3336–3342. [Google Scholar] [CrossRef]
- Qin, W.; Szpunar, J.A. A general model for hydrogen trapping at the inclusion-matrix interface and its relation to crack initiation. Philos. Mag. 2017, 97, 3296–3316. [Google Scholar] [CrossRef]
- Jin, T.Y.; Liu, Z.Y.; Cheng, Y.F. Effect of non-metallic inclusions on hydrogen-induced cracking of API5L X100 steel. Int. J. Hydrogen Energy 2010, 35, 8014–8021. [Google Scholar] [CrossRef]
- Oriani, R.A.; Hirth, J.P.; Smialowski, M. Hydrogen Degradation of Ferrous Alloys; Knovel Academic Civil Engineering & Construction Materials; Knovel Academic Metals & Metallurgy; Knovel Academic Oil & Gas Engineering; Noyes Publications: Park Ridge, NJ, USA, 1985; p. 886. [Google Scholar]
- Otsuka, T.; Tanabe, T. Hydrogen diffusion and trapping process around MnS precipitates in alpha Fe examined by tritium autoradiography. J. Alloy Compd. 2007, 446, 655–659. [Google Scholar] [CrossRef]
- Hanada, H.; Otsuka, T.; Nakashima, H.; Sasaki, S.; Hayakawa, M.; Sugisaki, M. Profiling of hydrogen accumulation in a tempered martensite microstructure by means of tritium autoradiography. Scr. Mater. 2005, 53, 1279–1284. [Google Scholar] [CrossRef]
- Liu, Q.L.; Zhou, Q.J.; Venezuela, J.; Zhang, M.X.; Atrens, A. Hydrogen influence on some advanced high-strength steels. Corros. Sci. 2017, 125, 114–138. [Google Scholar] [CrossRef]
- Liu, Q.; Irwanto, B.; Atrens, A. Influence of hydrogen on the mechanical properties of some medium-strength Ni-Cr-Mo steels. Mater. Sci. Eng. A 2014, 617, 200–210. [Google Scholar] [CrossRef]
- Gupta, A.; Goyal, S.; Padmanabhan, K.A.; Singh, A.K. Inclusions in steel: Micro-macro modelling approach to analyse the effects of inclusions on the properties of steel. Int. J. Adv. Manuf. Technol. 2015, 77, 565–572. [Google Scholar] [CrossRef]
- Laureys, A.; Depover, T.; Petrov, R.; Verbeken, K. Microstructural characterization of hydrogen induced cracking in TRIP-assisted steel by EBSD. Mater. Charact. 2016, 112, 169–179. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.S.; Choe, B.H. The Role of Hydrogen in Hydrogen Embrittlement of Metals: The Case of Stainless Steel. Metals 2019, 9, 406. [Google Scholar] [CrossRef]
- Bruck, S.; Schippl, V.; Schwarz, M.; Christ, H.J.; Fritzen, C.P.; Weihe, S. Hydrogen Embrittlement Mechanism in Fatigue Behavior of Austenitic and Martensitic Stainless Steels. Metals 2018, 8, 339. [Google Scholar] [CrossRef]
- Wang, L.W.; Xin, J.C.; Cheng, L.J.; Zhao, K.; Sun, B.Z.; Li, J.R.; Wang, X.; Cui, Z.Y. Influence of inclusions on initiation of pitting corrosion and stress corrosion cracking of X70 steel in near-neutral pH environment. Corros. Sci. 2019, 147, 108–127. [Google Scholar] [CrossRef]
- Zhu, L.K.; Qiao, L.J.; Li, X.Y.; Xu, B.Z.; Pan, W.; Wang, L.; Volinsky, A.A. Analysis of the tube-sheet cracking in slurry oil steam generators. Eng. Fail. Anal. 2013, 34, 379–386. [Google Scholar] [CrossRef]
- Zhu, L.K.K.; Yan, Y.; Qiao, L.J.J.; Volinsky, A.A. Stainless steel pitting and early-stage stress corrosion cracking under ultra-low elastic load. Corros. Sci. 2013, 77, 360–368. [Google Scholar] [CrossRef]
- Zhu, L.K.; Yan, Y.; Li, J.X.; Qiao, L.J.; Li, Z.C.; Volinsky, A.A. Stress corrosion cracking at low loads: Surface slip and crystallographic analysis. Corros. Sci. 2015, 100, 619–626. [Google Scholar] [CrossRef]
- Zhu, L.K.K.; Yan, Y.; Li, J.X.X.; Qiao, L.J.J.; Volinsky, A.A. Stress corrosion cracking under low stress: Continuous or discontinuous cracks? Corros. Sci. 2014, 80, 350–358. [Google Scholar] [CrossRef]
- Abbassi, F.; Mistou, S.; Zghal, A. Failure analysis based on microvoid growth for sheet metal during uniaxial and biaxial tensile tests. Mater. Des. 2013, 49, 638–646. [Google Scholar] [CrossRef]
- Bao, Y.B. Dependence of ductile crack formation in tensile tests on stress triaxiality, stress and strain ratios. Eng. Fract. Mech. 2005, 72, 505–522. [Google Scholar] [CrossRef]


| C | Si | Mn | S | P | Al | Ti | Cr | Ni | Nb | B |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.39 | 0.21 | 1.23 | 0.0022 | 0.014 | 0.033 | 0.03 | 0.12 | 0.015 | <0.01 | 0.0026 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Zhang, X.; Xu, J.; Sun, Y.; Li, J. Effect of Hydrogen Content and Strain Rate on Hydrogen-induced Delay Cracking for Hot-stamped Steel. Metals 2019, 9, 798. https://doi.org/10.3390/met9070798
Jia H, Zhang X, Xu J, Sun Y, Li J. Effect of Hydrogen Content and Strain Rate on Hydrogen-induced Delay Cracking for Hot-stamped Steel. Metals. 2019; 9(7):798. https://doi.org/10.3390/met9070798
Chicago/Turabian StyleJia, Hongxing, Xuewei Zhang, Juanping Xu, Yaping Sun, and Jinxu Li. 2019. "Effect of Hydrogen Content and Strain Rate on Hydrogen-induced Delay Cracking for Hot-stamped Steel" Metals 9, no. 7: 798. https://doi.org/10.3390/met9070798
APA StyleJia, H., Zhang, X., Xu, J., Sun, Y., & Li, J. (2019). Effect of Hydrogen Content and Strain Rate on Hydrogen-induced Delay Cracking for Hot-stamped Steel. Metals, 9(7), 798. https://doi.org/10.3390/met9070798




