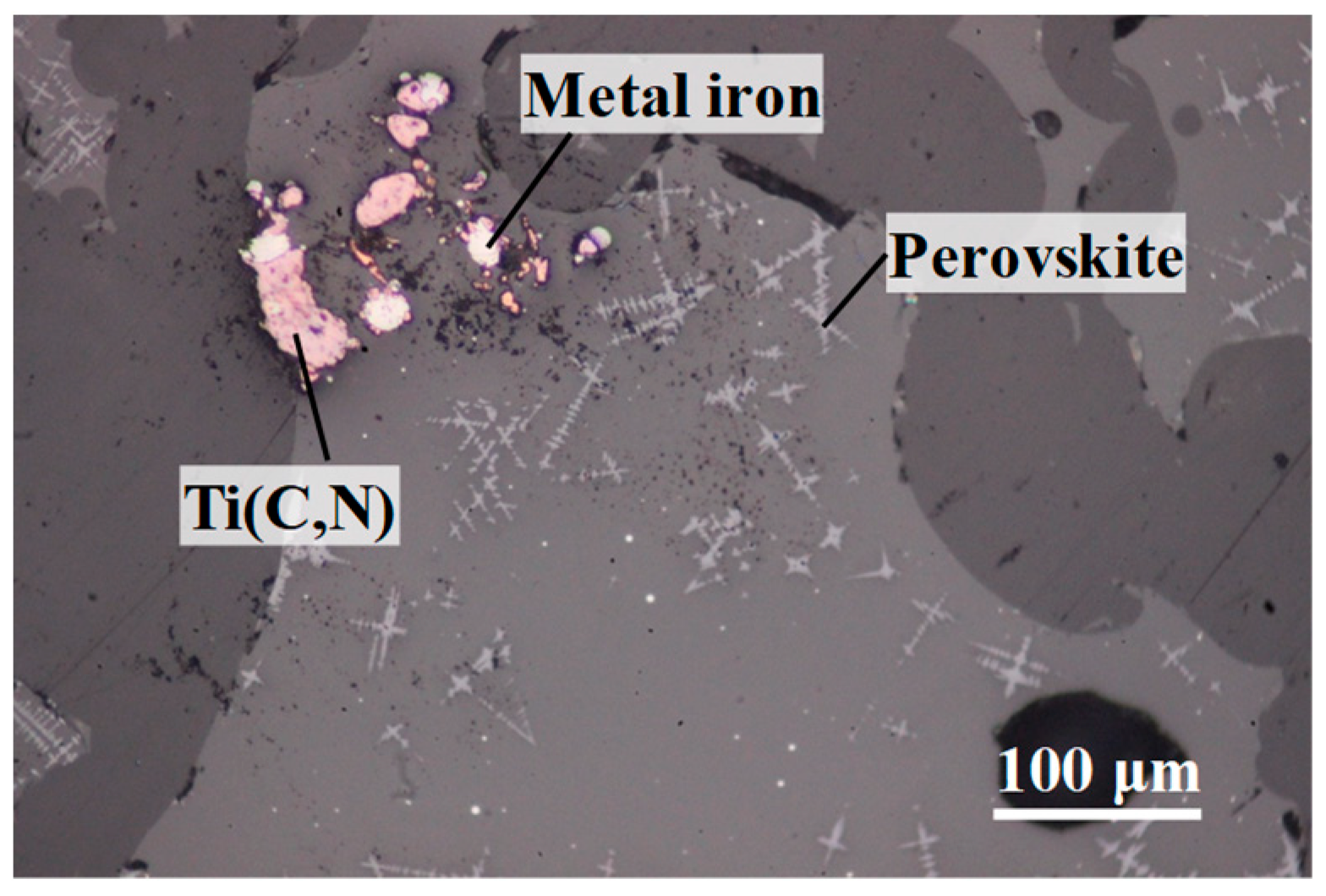
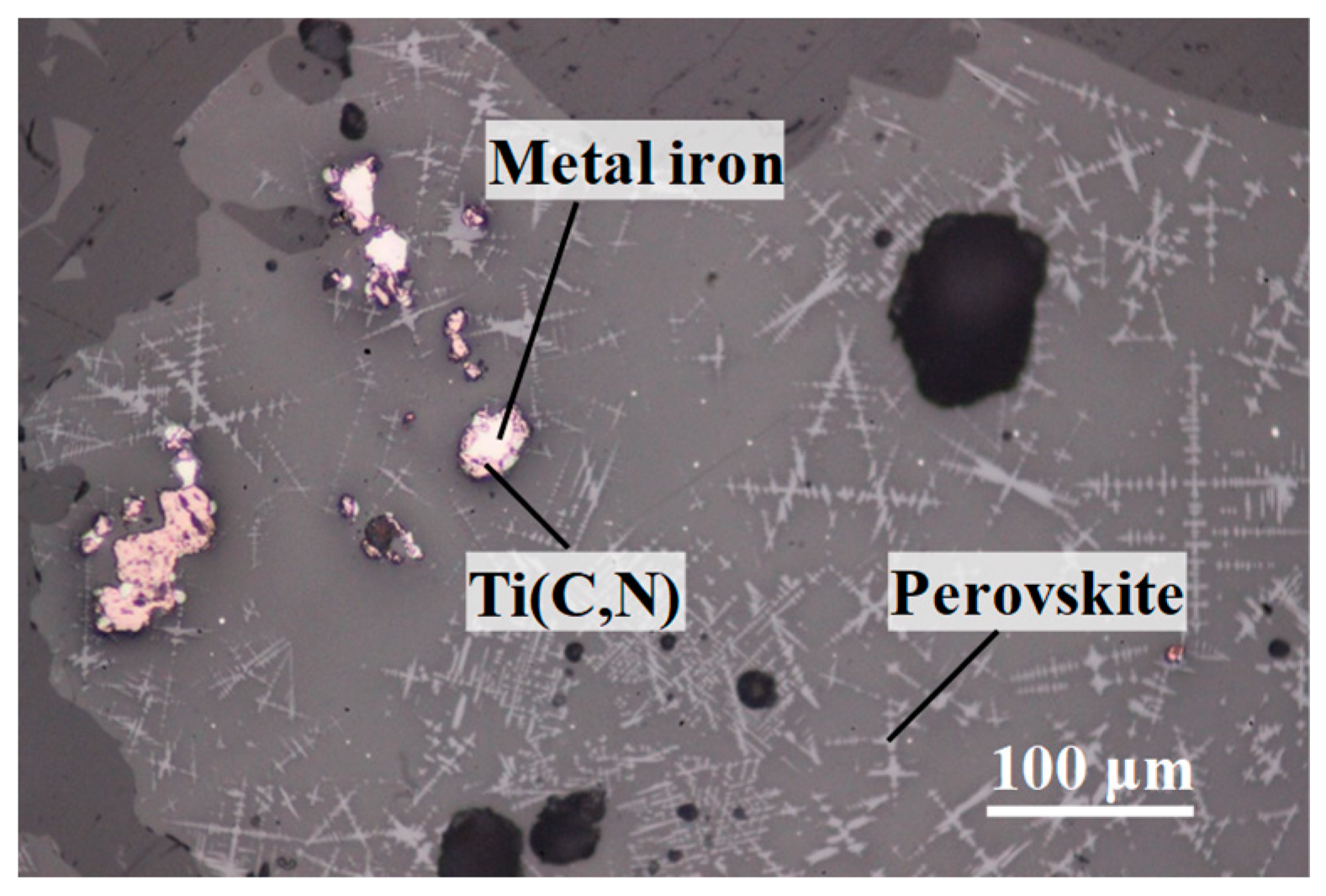
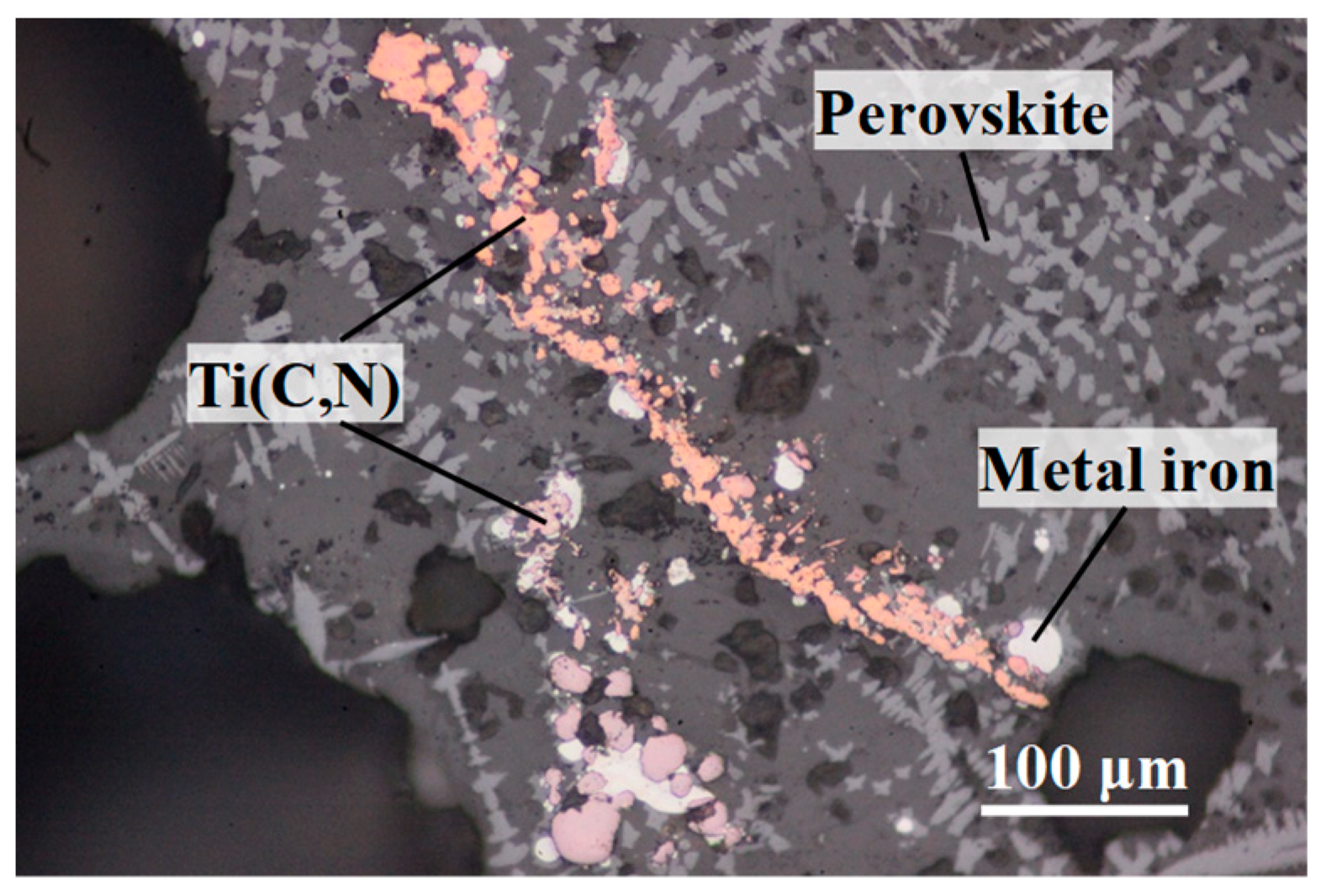
Viscosity of TiO
2-containing slags is an important factor influencing the processes of ironmaking, steelmaking and Ti-recycling industries [
1,
2,
3]. The content of titania (TiO
2) in high-titanium-type blast furnace slag is always more than 20 wt%. Therefore, it is inevitable that part of the TiO
2 in this slag was reduced to titanium carbonitride (Ti(C,N)) particles by coke during blast furnace production. The Ti(C,N) particle is harmful for the fluidity of the slag because it has a high melting point and can significantly increase the viscosity of the slag. According to Einstein’s theory [
4] about the relation between the volume fraction of solid particles and the viscosity of extremely dilute solutions, Roscoe presented a type of equation (Einstein–Roscoe type equation) to describe the viscosity of liquids containing high concentrations of solid suspensions [
5]:
where
η and
η0 are the viscosity of solid-containing and solid-free liquid, respectively;
f is the volume fraction of solid particles in the liquid;
a and
n are constants with regard to the volume fraction and geometrical shape of solid particles in liquid, and are 1.35 and 2.5 for spherical particles with a uniform size, respectively. This equation indicates that the viscosity of the melt should be related to the volume fraction and geometrical shape of solid particles in liquid. To explore this relationship in metallurgical slags, some research has been carried out by adding small amounts of solid particles to the slags. Wright et al. [
6] studied the viscosities of CaO-MgO-Al
2O
3-SiO
2 melts containing spinels with different sizes at 1646 K; Liu et al. [
7] studied the effect of Ti(C
0.3N
0.7) particles of 1.0 μm on the viscosities of CaO-MgO-Al
2O
3-SiO
2 blast furnace slag and Zhen et al. [
8] discussed the effect of TiC particles on the viscosity of CaO-MgO-Al
2O
3-SiO
2-TiO
2 slag. Their results suggested that the viscosity of the solid-containing melt increased with the addition of particles, and the Einstein–Roscoe type equation can well describe the viscosity variation behavior by allowing the parameters
a and
n to vary. To investigate the flow behavior of high-titanium-type slag, Jiang et al. [
9] studied the effect of TiC solid particles on the rheological behavior of blast furnace slags with 20 wt% of total TiO
2 and Yue et al. [
10] discussed the rheological behavior of Ti-bearing blast furnace slag with different TiN contents. Both of them pointed out that the slags will convert to non-Newtonian fluids if the volume fraction of the solid particles beyond certain values and the Einstein–Roscoe type equation could be not suitable at that condition.
In the last few decades, a large amount of research [
11,
12,
13,
14,
15,
16,
17,
18,
19] has attempted to establish an accurate description about the viscosity of the high-titanium-type slag and a lot of fruitful achievements have been obtained. However, the existing empirical and semi-empirical models still cannot describe the viscosity precisely. One of the possible reasons for this may be the improper method for preparing the experimental slag. For example, most of the previous studies prepared the high-titanium-type slag by adding the solid particles directly to the TiO
2-containing slags. However, this could not be enough to reflect the real characteristics of the on-site slag (slag in blast furnace). The morphology and distribution of TiC, TiN, and Ti(C,N) in on-site slag should be different from those directly added to the slag. Additionally, on-site slag is also different from the slag prepared by high purity reagents because there are some gas bubbles in on-site slag, which makes the structure of molten slag more complicated. Up to now, there is still a lack of accurate knowledge of viscosity properties for the high-titanium-type blast furnace slag. In order to control the iron-making process of titanium-vanadium-magnetite more efficiently, a further understanding of flow behaviors in high-titanium-type blast furnace slag should be necessary.
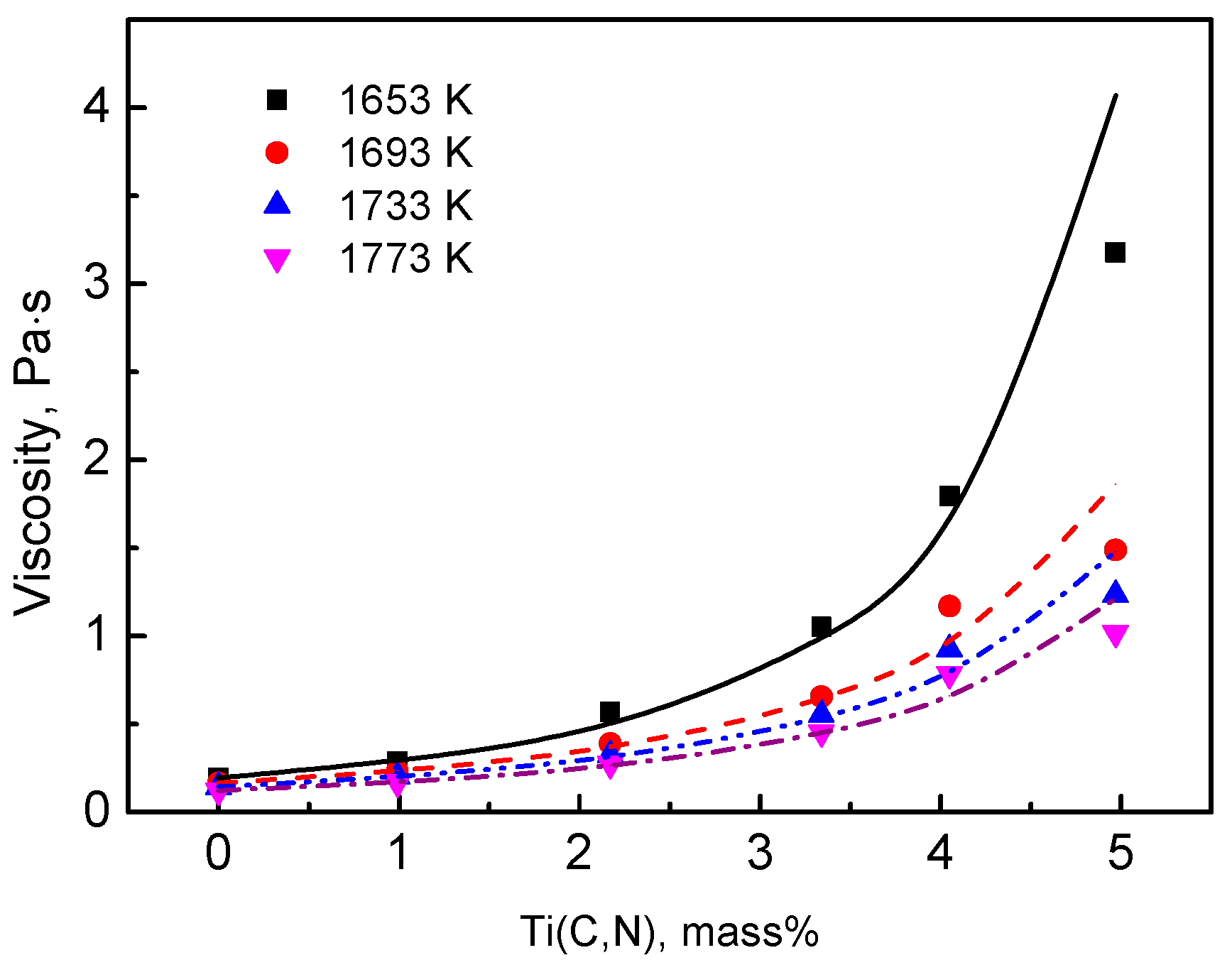
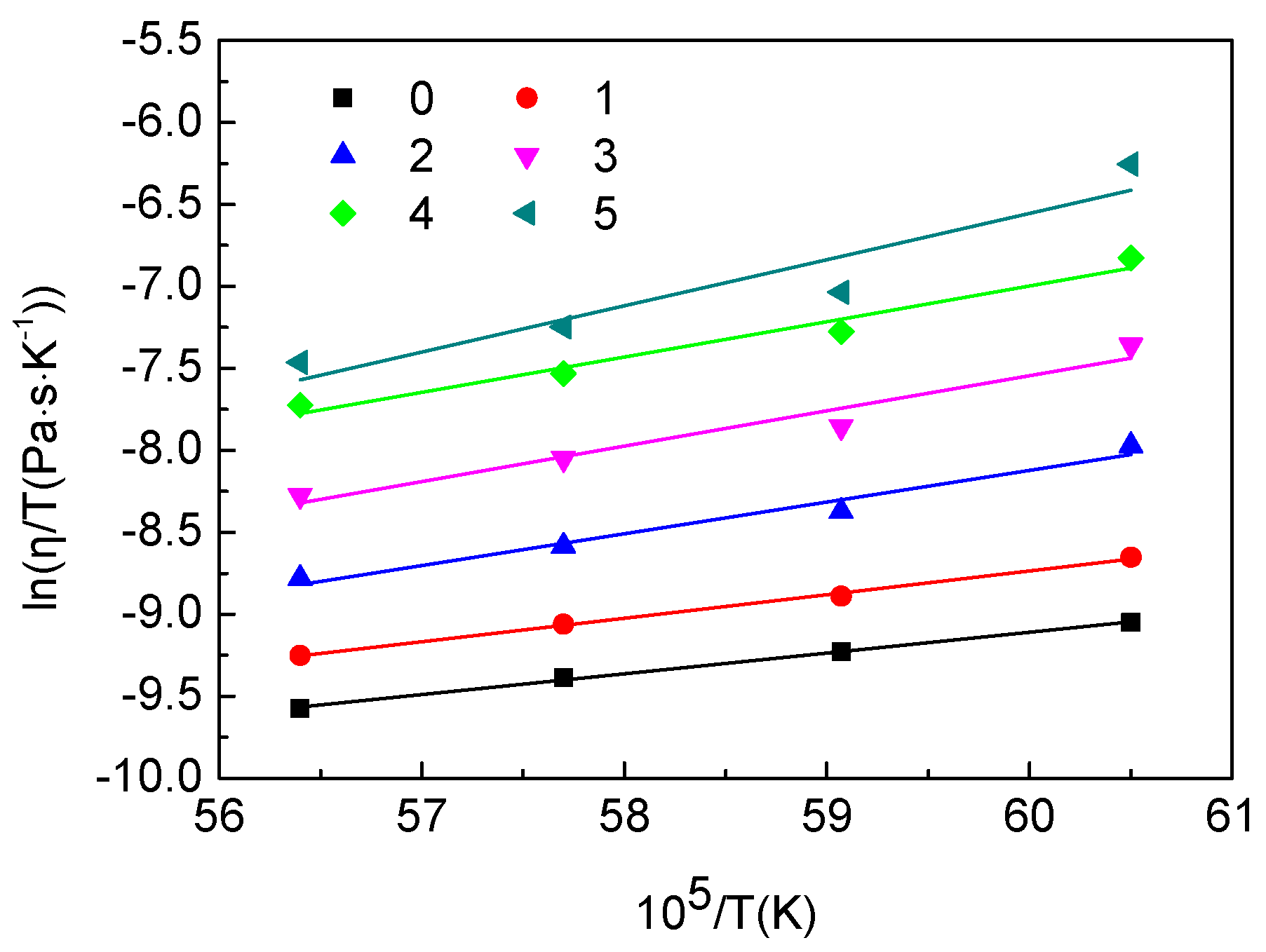
In the present study, to discuss the effect of titanium carbonitride on the viscosity of the high-titanium-type blast furnace slag, on-site slags with different contents of titanium carbonitride (reduced from TiO2) were prepared. Additionally, the viscosities of these slags were measured to clarify the relationships between slag fluidity and the contents of titanium carbonitride.