Oxidation of Thiosulfate with Oxygen Using Copper (II) as a Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure for Thiosulfate Transformation Using Copper Sulfate
3. Results and Discussion
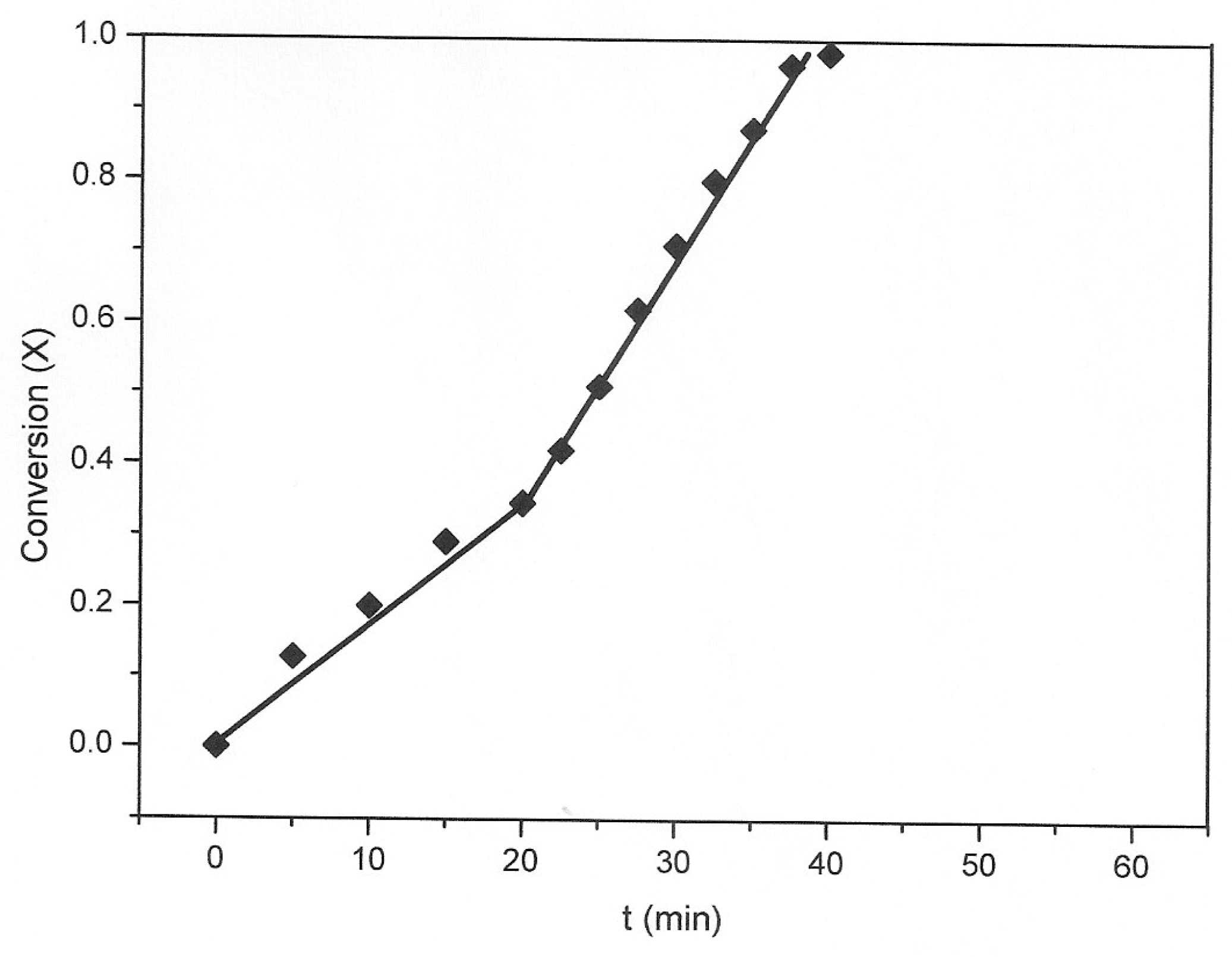
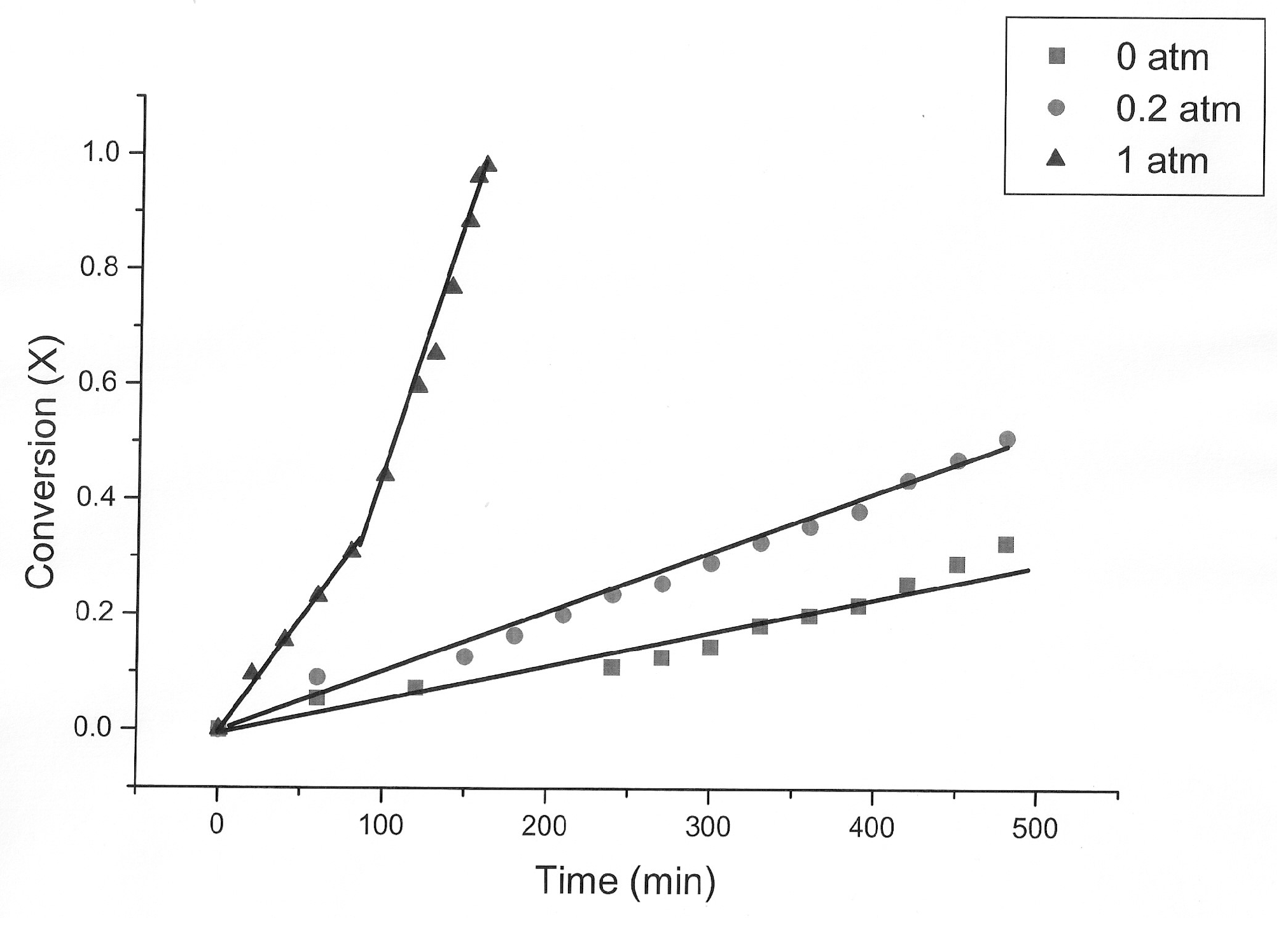
3.1. The Nature of the Thiosulfate Transformation by Reaction with Cu (II) Sulfate and Oxygen
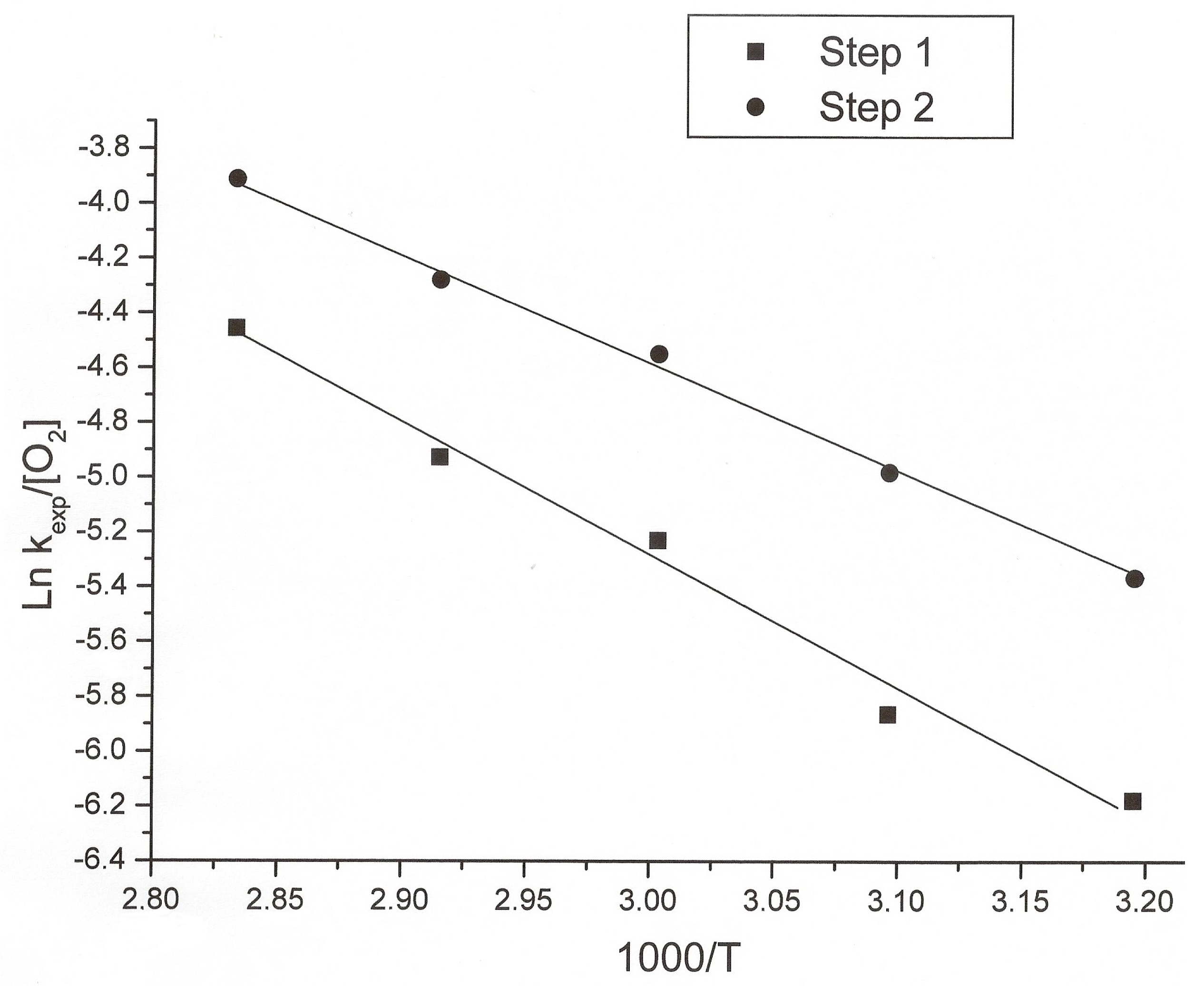
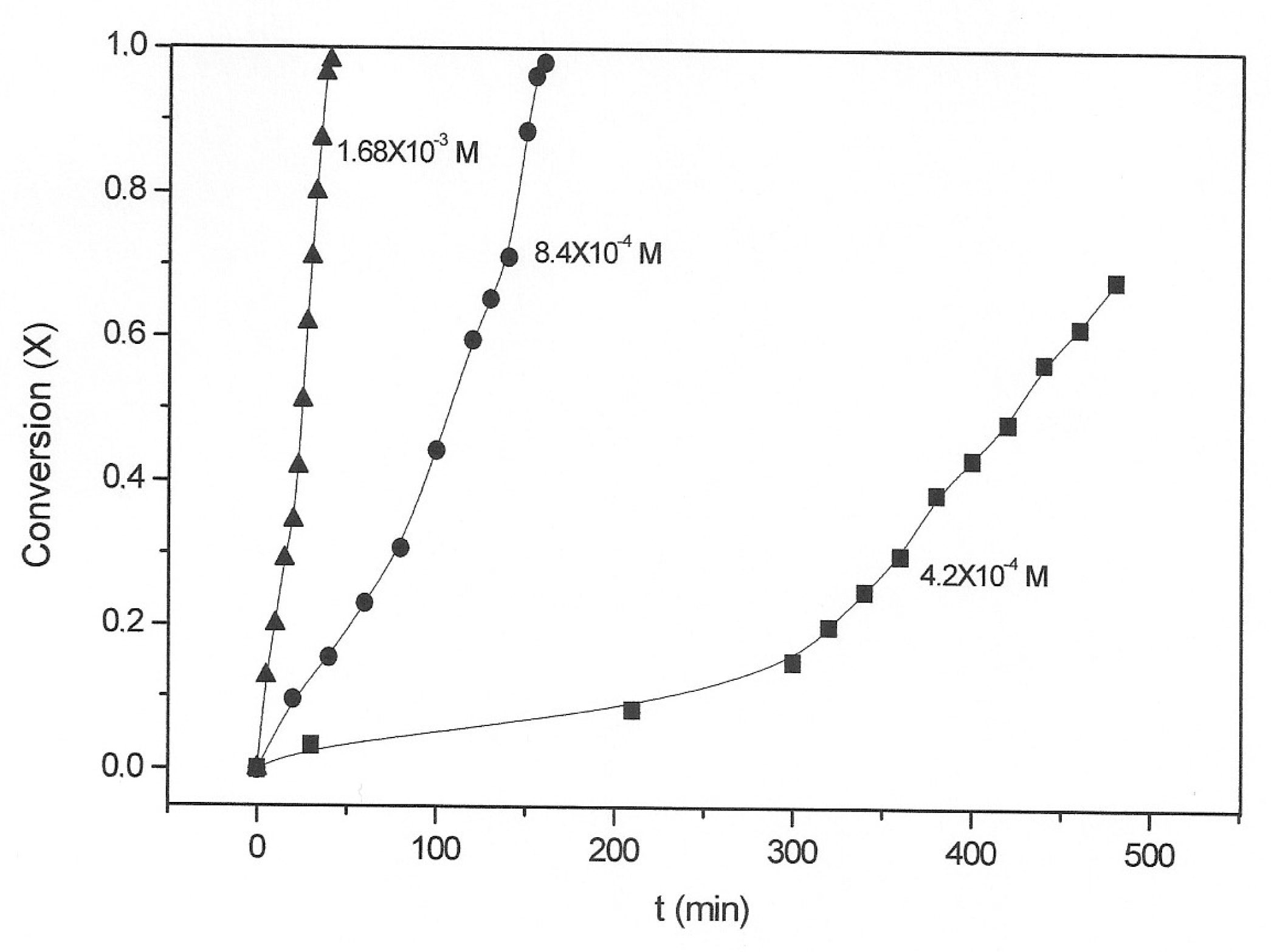
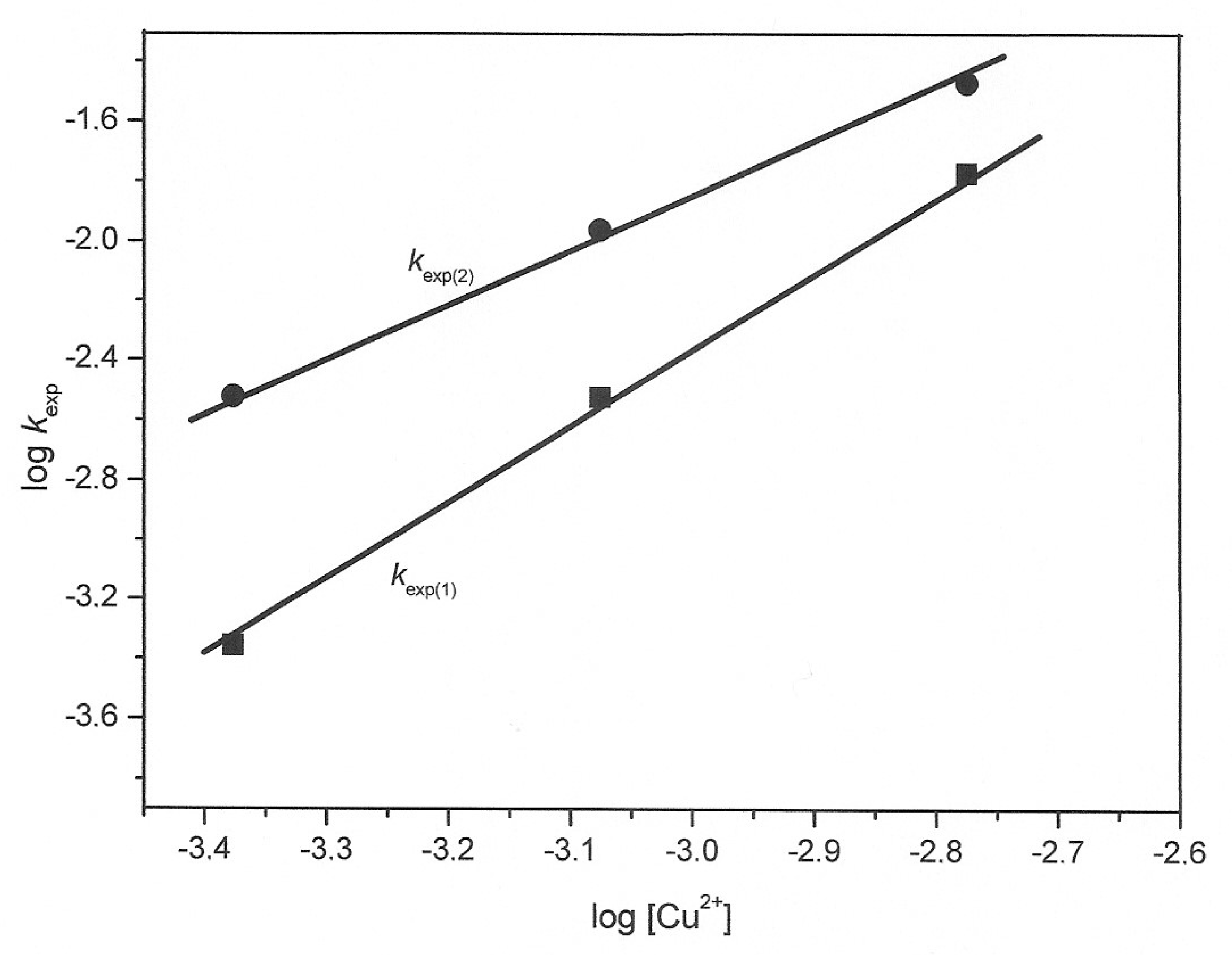
3.2. Kinetic Study of the Transformation of Thiosulfate by Reaction with Oxygen and Cu2+
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
List of Symbols
| Conversion | X (dimensionless) |
| kexp | reaction rate (min−1) |
| Activation energy | Ea (kJ·mol−1) |
References
- Breuer, P.L.; Jeffrey, M.I. Thiosulfate leaching kinetics of gold in the presence of copper and ammonia. Min. Eng. 2000, 13, 1071–1081. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D.; le Vier, M.; Wan, R.Y. The ammoniacal thiosulfate system for precious metal recovery. In Proceedings of the XIX International Mineral Processing Congress, Precious Metals Processing and Mineral Waste and the Environment, San Francisco, CA, USA, 22–27 October 1995; pp. 37–42, ISBN 0-87335-142-8. [Google Scholar]
- Senanayake, G. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulfate oxidation by ammoniacal copper (II) solutions. Hydrometallurgy 2004, 75, 55–75. [Google Scholar] [CrossRef]
- Zhang, S.; Nicol, J.M. An electrochemical study of the dissolution of gold in thiosulfate solutions. Part I. Alkaline solutions. J. Appl. Electrochem. 2003, 33, 767–775. [Google Scholar]
- Zhang, S.; Nicol, J.M. An electrochemical study of the dissolution of gold in thiosulfate solutions. Part II. Effect of copper. J. Appl. Electrochem. 2005, 35, 339–345. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources-A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A review of ammoniacal thiosulfate leaching of gold: An update useful for further research in Non-cyanide gold lixiviants. Miner. Process Extract. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Celep, O.; Altinkaya, P.; Yazici, E.Y.; Deveci, H. Thiosulphate leaching of silver from an arsenical refractory ore. Min. Eng. 2018, 122, 285–295. [Google Scholar] [CrossRef]
- Juárez, J.C.; Patiño, F.; Roca, A.; Teja, A.M.; Reyes, I.A.; Reyes, M.; Pérez, M.; Flores, M.U. Determination of dissolution rates of Ag contained in metallurgical and mining residues in the S2O3-O2-Cu2+system: Kinetic analysis. Minerals 2018, 8, 309. [Google Scholar] [CrossRef]
- Sitando, O.; Senanayake, G.; Dai, X.; Nikoloski, A.N.; Breuer, P. A review of factors affecting gold leaching in non-ammoniacal thiosulfate solutions including degradation and in-situ generation of thiosulfate. Hydrometallurgy 2018, 78, 151–175. [Google Scholar] [CrossRef]
- Deutsch, J.L.; Dreisinger, D.B. Silver sulfide leaching with thiosulfate inthe presence of additives. Part I: Ferric complexes and the application to silver sulfide ores. Hydrometallurgy 2013, 137, 165–172. [Google Scholar] [CrossRef]
- Puente-Siller, D.M.; Fuentes-Aceituno, J.C.; Nava-Alonso, F. A kinetic-thermodynamic study of silver leaching in thiosulfate-copper-ammonia-EDTA solutions. Hydrometallurgy 2013, 134–135, 124–131. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.; Huynh, T.H.; Jeong, J.; Pandey, B.D. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–129. [Google Scholar] [CrossRef]
- Camelino, S.; Rao, J.; Padilla, R.L.; Lucci, R. Initial studies about gold leching from printed circuit boardss (PCB’S) of waste cell phones. Procedia Mater. Sci. 2015, 9, 105–112. [Google Scholar] [CrossRef]
- Hagelüken, C.; Corti, C.W. Recycling of gold from electronics: Cost effective use through ‘Design for Recycling’. Gold Bull. 2010, 43, 209–220. [Google Scholar] [CrossRef]
- Kasper, A.C.; Veit, H.M. Leaching of Gold from Printed Circuit Boards Scrap of Mobile Phones. In Energy Technology; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-48602-4. [Google Scholar]
- Bas, A.D.; Yazici, E.Y.; Deveci, H. Recovery of silver from X-ray film processing effluents by hydrogen peroxide treatment. Hydrometallurgy 2012, 121–124, 22–27. [Google Scholar] [CrossRef]
- Mahdizadeh, F.; Eskandarian, M.; Zabarjadi, J.; Ehsani, A.; Afshar, A. Silver recovery from radiographic film processing effluents by hydrogen peroxide: Modeling and optimization using response surface methodology. Korean J. Chem. Eng. 2014, 31, 74–80. [Google Scholar] [CrossRef]
- Prado, P.F.A.; Ruotolo, L.A.M. Silver recovery from simulated photographic baths by electrochemical deposition avoiding Ag2S formation. J. Environ. Chem. Eng. 2016, 4, 3283–3292. [Google Scholar] [CrossRef]
- Labra, M.P.; Pérez, M.R.; Serrano, J.A.R.; Dávila, D.O.A.; Hernández, F.R.B.; Thangarasu, P. Silver cementation with zinc from residual X ray fixer, experimental and thermochemical study. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2016; pp. 605–613. [Google Scholar]
- Blair, A. Silver plating. Met. Finish. 1995, 93, 290–297. [Google Scholar] [CrossRef]
- Osaka, T.; Kodera, A.; Misato, T.; Homma, T.; Okinaka, Y. Electrodeposition of soft gold from thiosulfate-sulfite bath for electronics applications. J. Electrochem. Soc. 1997, 144, 3462–3469. [Google Scholar] [CrossRef]
- Ando, S.; Inoue, T.; Okudaira, H.; Takehara, Y.; Ushio, J.; Ohta, T.; Yamamoto, H. Super stable non-cyanide electroless gold plating bath which has been applied to advanced board manufacture. In Proceedings of the IEMT/IMC Symposium, 1st Joint International Electronic Manufacturing Symposium and the International Microelectronics Conference, Sonic City-Omiya, Tokyo, Japan, 16–18 April 1997; pp. 220–225. [Google Scholar]
- Berger, S.; Hoffacker, G. Novel and innovative non-cyanide silver process: A report on commercial plating experiences. Plat. Surf. Finish. 2005, 92, 46–51. [Google Scholar]
- Ren, F.-Z.; Yin, L.-T.; Wang, S.-S.; Volinsky, A.A.; Tian, B.-H. Cyanide-free silver electroplating process in thiosulfate bath and microstructure analysis of Ag coatings. Trans. Nonferr. Met. Soc. China 2013, 23, 3822–3828. [Google Scholar] [CrossRef]
- Sabir, S. Silver Recovery from Assorted Spent Sources: Toxicology of Silver Ions; World Scientific Publishing Europe Ltd.: London, UK, 2018; Chapter 1. [Google Scholar]
- Xu, Y.; Shoonen, M.A.M. Thiosulfate oxidation: Catalysis of synthetic sphalerite doped with transition metals. Geochim. Cosmochim. Acta 1996, 60, 4701–4710. [Google Scholar] [CrossRef]
- Chatterjee, D.; Shome, S.; Jaiswal, N.; Moi, S.C. Mechanism of the oxidation of thiosulfate with hydrogen peroxide catalyzed by aqua-ethylenediaminetetraacetatoruthenium (III). J. Mol. Catal. A Chem. 2014, 386, 1–4. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, F.; Khan, I.; Khan, A.D. Studies on the Oxidative Removal of Sodium Thiosulfate from Aqueous Solution. Arab. J. Sci. Eng. 2014, 40, 289–293. [Google Scholar] [CrossRef]
- González-Lara, J.M.; Roca, A.; Cruells, M.; Patiño, F. The oxidation of thiosulfates with copper sulfate. Application to an industrial fixing bath. Hydrometallurgy 2009, 95, 8–14. [Google Scholar] [CrossRef]
- Lourié, Y. Aide-Mémorie de Chimie Analytique; URSS: Moscou, Russia, 1975; pp. 114–115. [Google Scholar]
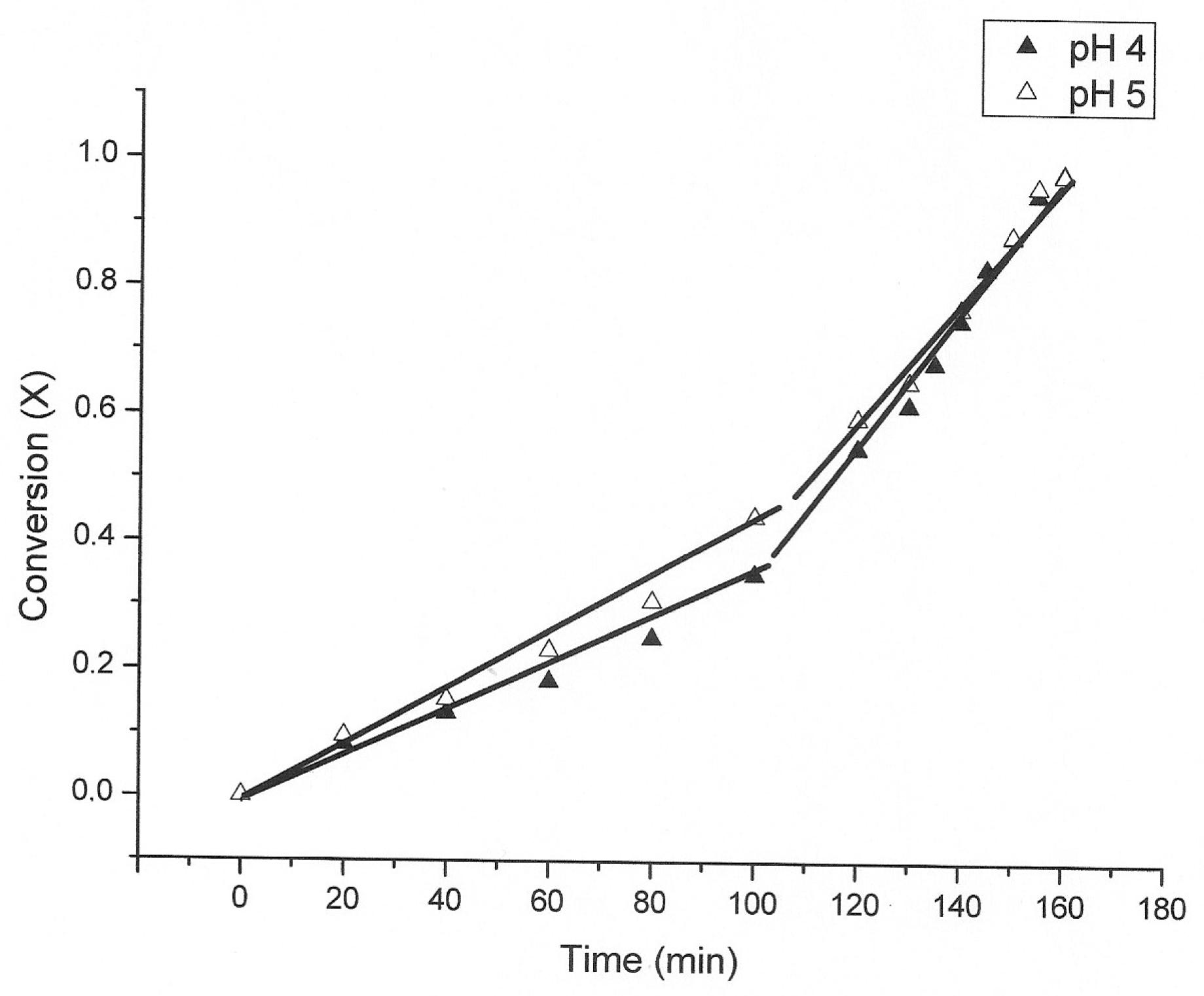



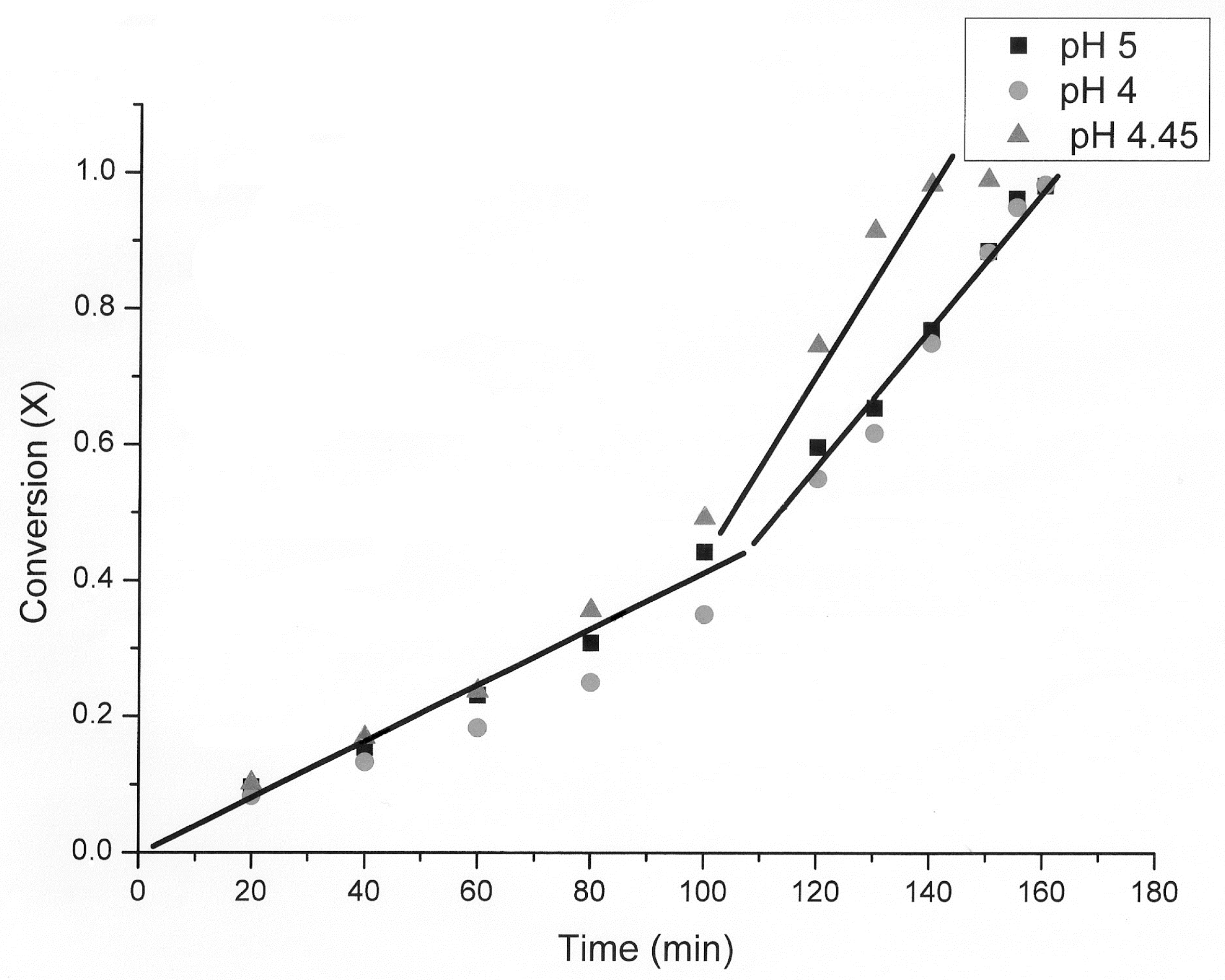

| pH | Time (min) First Step | Time (min) Second Step | kexp (min−1) First Step | kexp (min−1) Second Step |
|---|---|---|---|---|
| 5 | 0–80 | 80–160 | 0.0040 | 0.0070 |
| 4 | 0–100 | 100–160 | 0.0033 | 0.0110 |
| Temp. (oC) | 1000/T (K−1) | kexp(1) (min−1) | kexp(1)/[O2] (min−1/mol·m−3) | Ln(kexp(1)/[O2]) | kexp(2) (min−1) | kexp(2)/[O2] (min−1/mol·m−3) | Ln(kexp(2)/[O2]) |
|---|---|---|---|---|---|---|---|
| 40 | 3.195 | 0.0020 | 2.08 × 10−3 | −6.177 | 0.0045 | 4.67 × 10−3 | −5.366 |
| 50 | 3.096 | 0.0022 | 2.65 × 10−3 | −5.933 | 0.0057 | 6.87 × 10−3 | −4.981 |
| 60 | 3.003 | 0.0038 | 5.35 × 10−3 | −5.231 | 0.0075 | 0.0106 | −4.550 |
| 70 | 2.915 | 0.0042 | 7.24 × 10−3 | −4.928 | 0.0080 | 0.0138 | −4.280 |
| 80 | 2.833 | 0.0050 | 0.0116 | −4.457 | 0.0086 | 0.020 | −3.912 |
| [Cu2+] (mol·L−1) | kexp(1) (min−1) | kexp(2) (min−1) |
|---|---|---|
| 4.2 × 10−4 | 0.00044 | 0.0030 |
| 8.4 × 10−4 | 0.0030 | 0.011 |
| 1.68 × 10−3 | 0.017 | 0.034 |
| Line | [S2O32−] (mol·L−1) | log [S2O32−] (mol·L−1) | kexp (min−1) | log kexp (min−1) |
|---|---|---|---|---|
| 1 | 4.46 × 10−3 | −2.351 | 0.0410 | −1.387 |
| 2 | 6.70 × 10−3 | −2.174 | 0.0098 | −2.009 |
| 3 | 8.92 × 10−3 | −2.050 | 0.0060 | −2.222 |
| 4 | 8.92 × 10−3 | −2.050 | 0.0040 | −2.398 |
| 5 | 8.92 × 10−3 | −2.050 | 0.0070 | −2.155 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Lara, J.M.; Cardona, F.P.; Vallmajor, A.R.; Cadevall, M.C. Oxidation of Thiosulfate with Oxygen Using Copper (II) as a Catalyst. Metals 2019, 9, 387. https://doi.org/10.3390/met9040387
González Lara JM, Cardona FP, Vallmajor AR, Cadevall MC. Oxidation of Thiosulfate with Oxygen Using Copper (II) as a Catalyst. Metals. 2019; 9(4):387. https://doi.org/10.3390/met9040387
Chicago/Turabian StyleGonzález Lara, Juan Manuel, Francisco Patiño Cardona, Antonio Roca Vallmajor, and Montserrat Cruells Cadevall. 2019. "Oxidation of Thiosulfate with Oxygen Using Copper (II) as a Catalyst" Metals 9, no. 4: 387. https://doi.org/10.3390/met9040387
APA StyleGonzález Lara, J. M., Cardona, F. P., Vallmajor, A. R., & Cadevall, M. C. (2019). Oxidation of Thiosulfate with Oxygen Using Copper (II) as a Catalyst. Metals, 9(4), 387. https://doi.org/10.3390/met9040387




