High-Pressure Oxidative Leaching and Iodide Leaching Followed by Selective Precipitation for Recovery of Base and Precious Metals from Waste Printed Circuit Boards Ash
Abstract
:1. Introduction
2. Materials and Methods
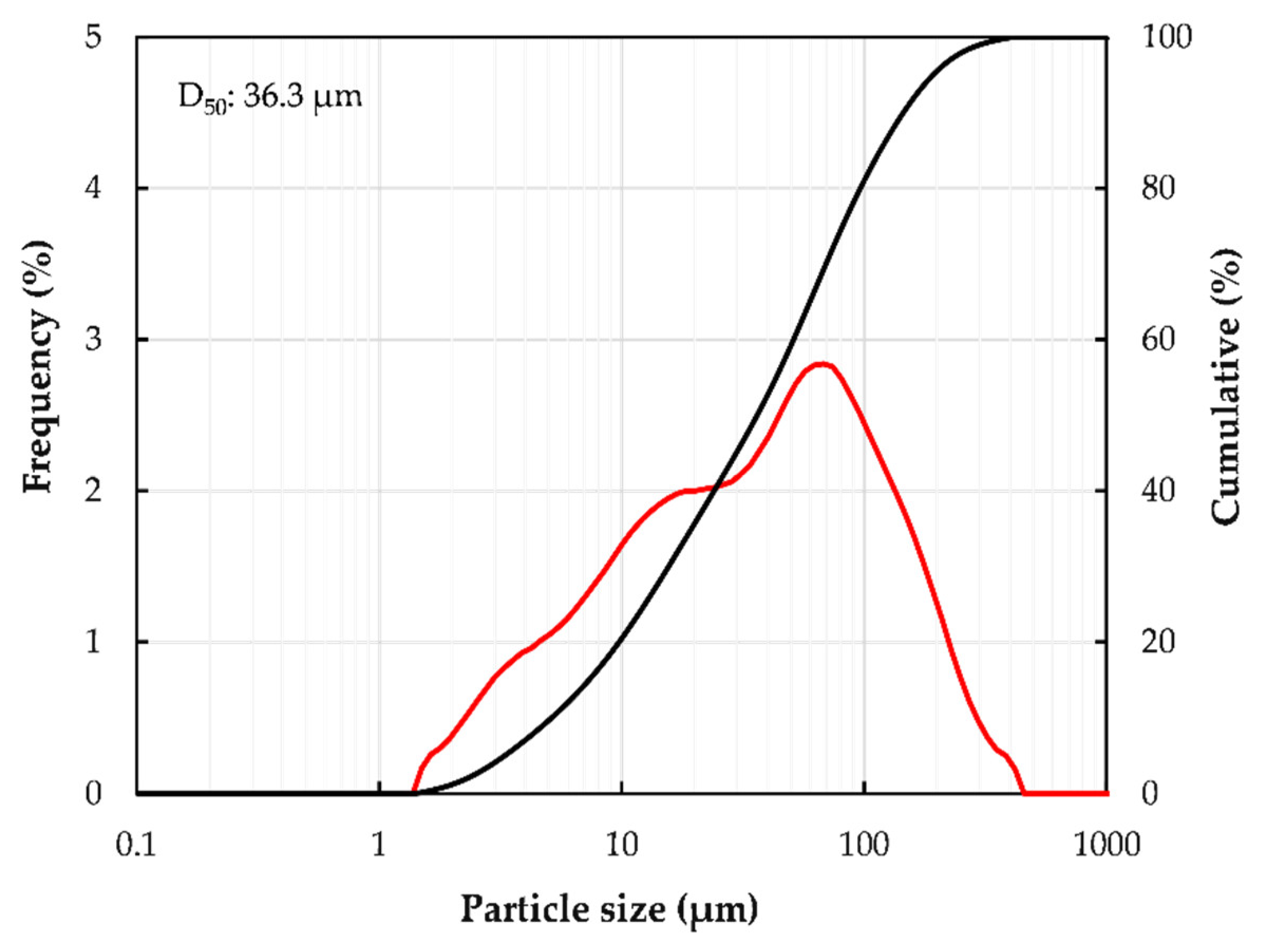
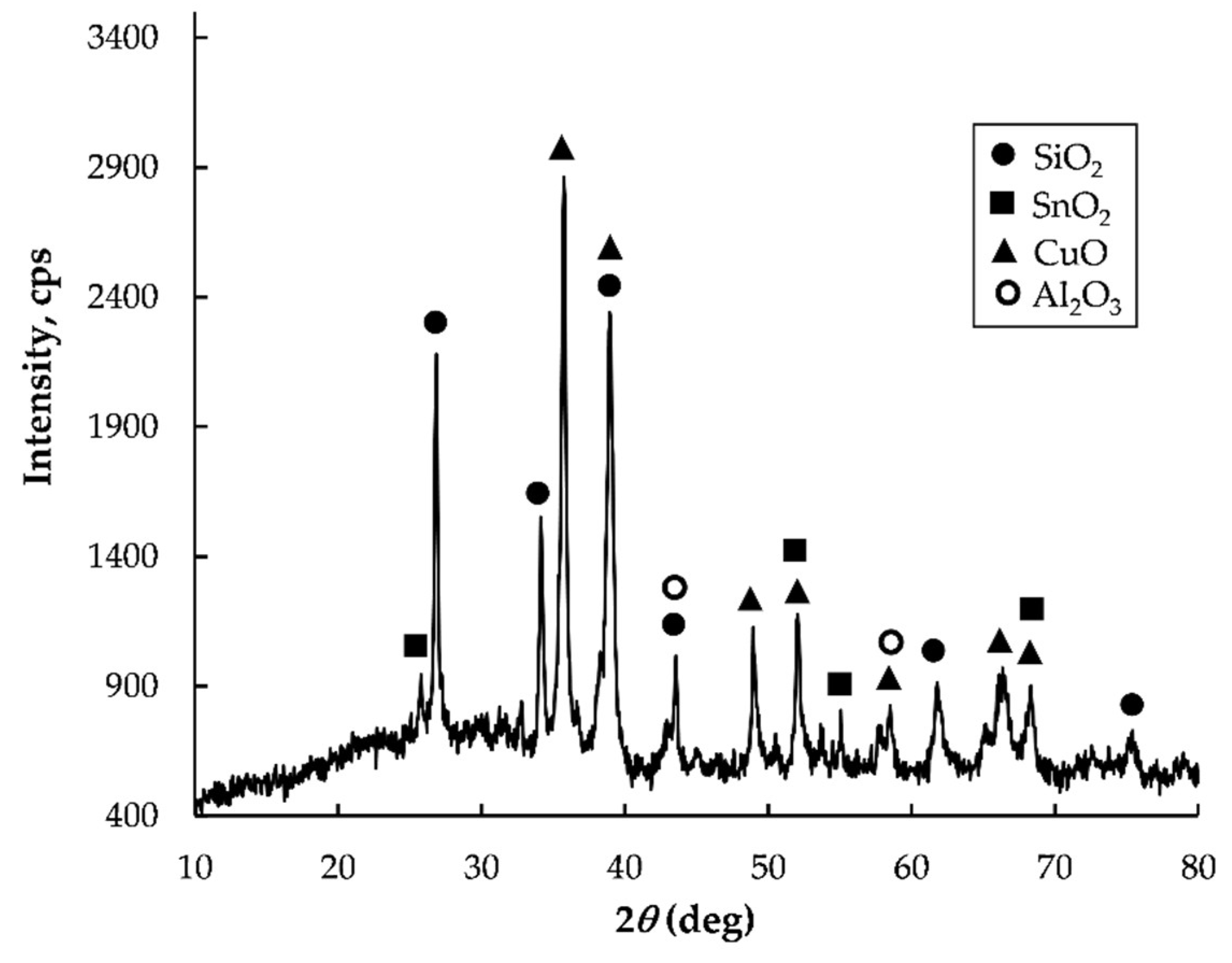
2.1. Materials
2.2. Leaching Experiments
2.2.1. Optimization of Iodide Leaching
2.2.2. High Pressure Oxidative Leaching
2.2.3. Iodide Leaching for Extraction of Gold from WPCBs Ash
2.3. Evaluation of Effectiveness of Iodide Leaching
2.4. Precipitation of Gold from Pregnant Leach Solution
3. Results and Discussion
3.1. Optimization of Iodide Leaching
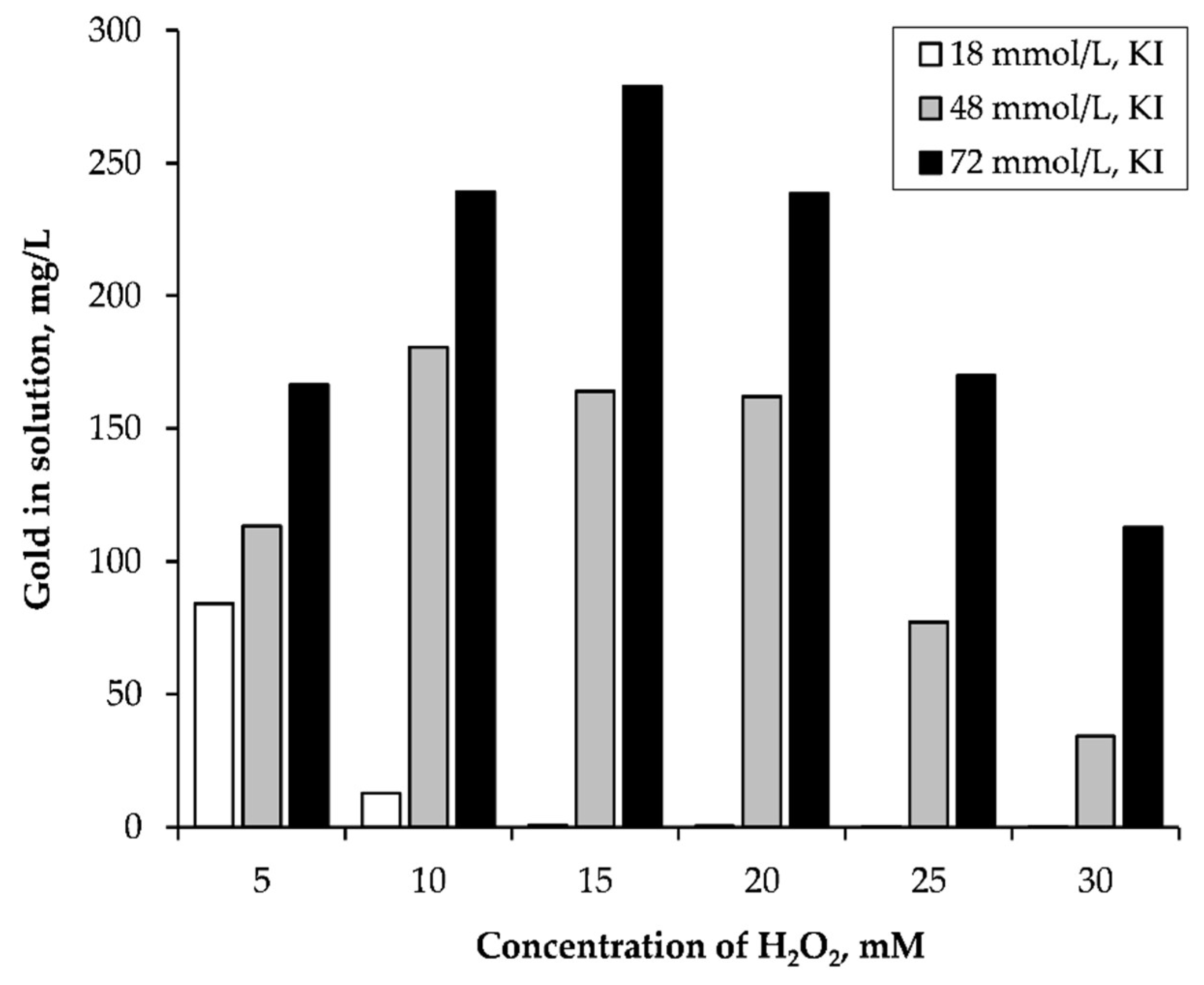
3.1.1. Effect of Potassium Iodide Concentration and Hydrogen Peroxide Concentration
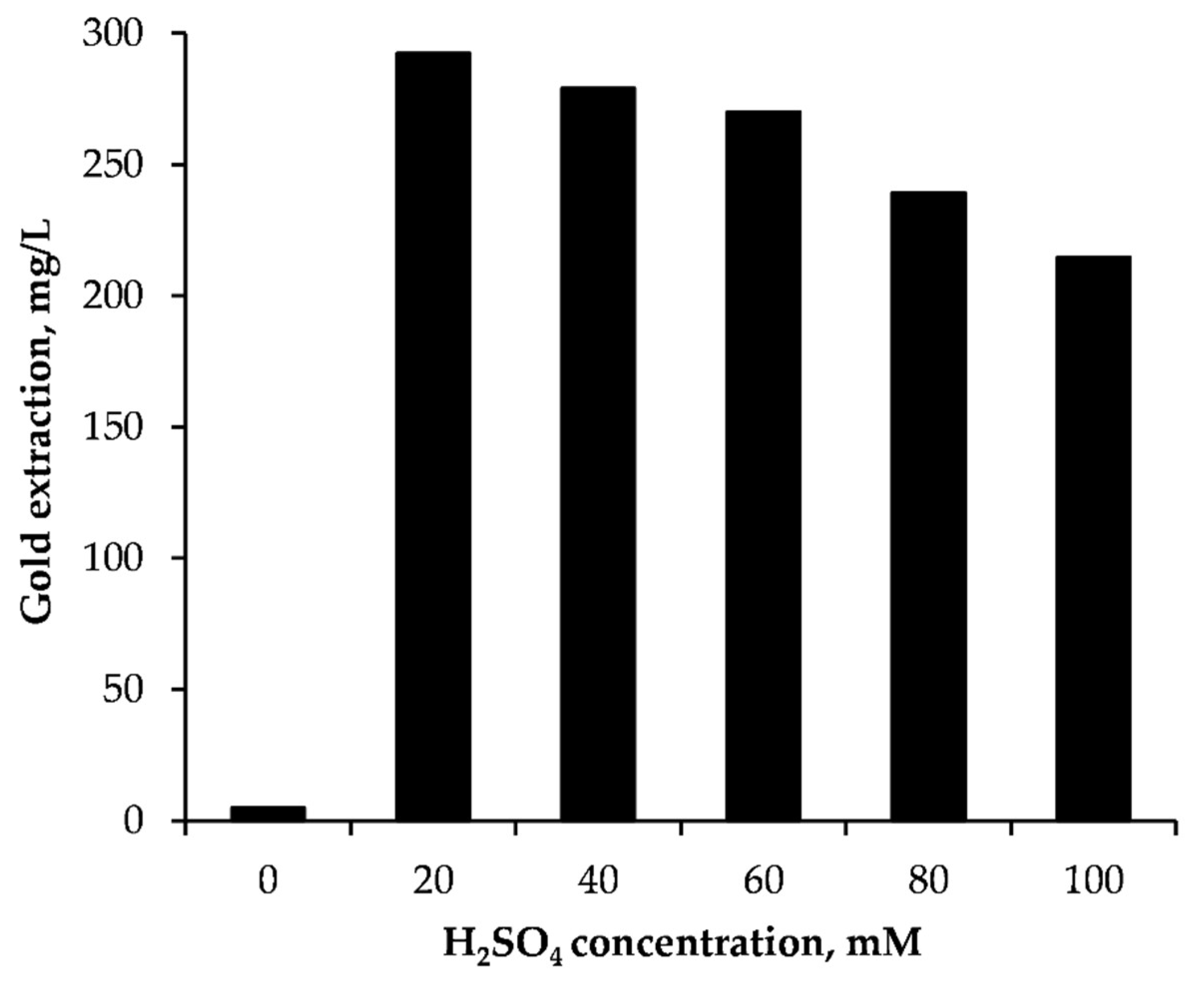
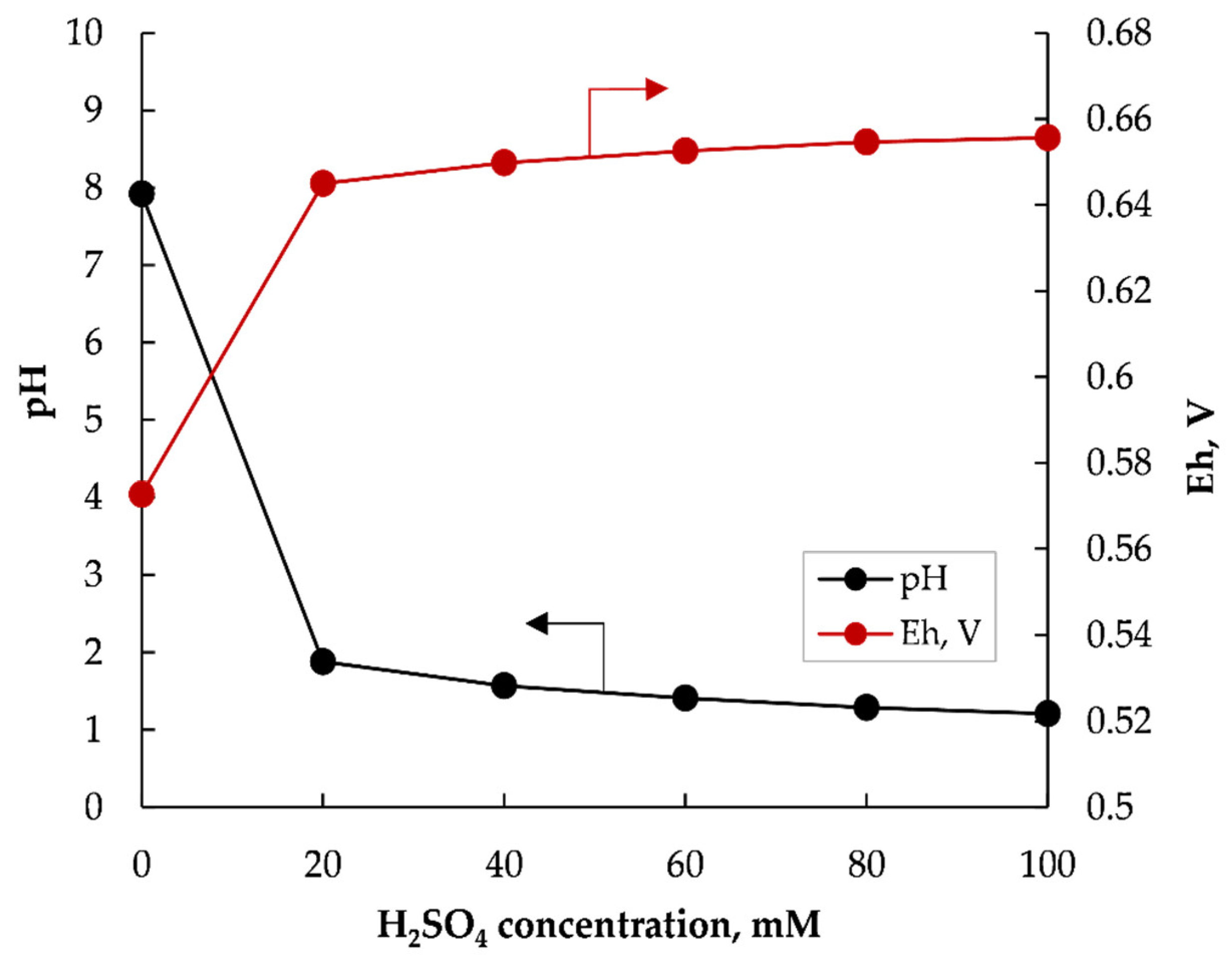
3.1.2. Effect of Sulfuric Acid Concentration
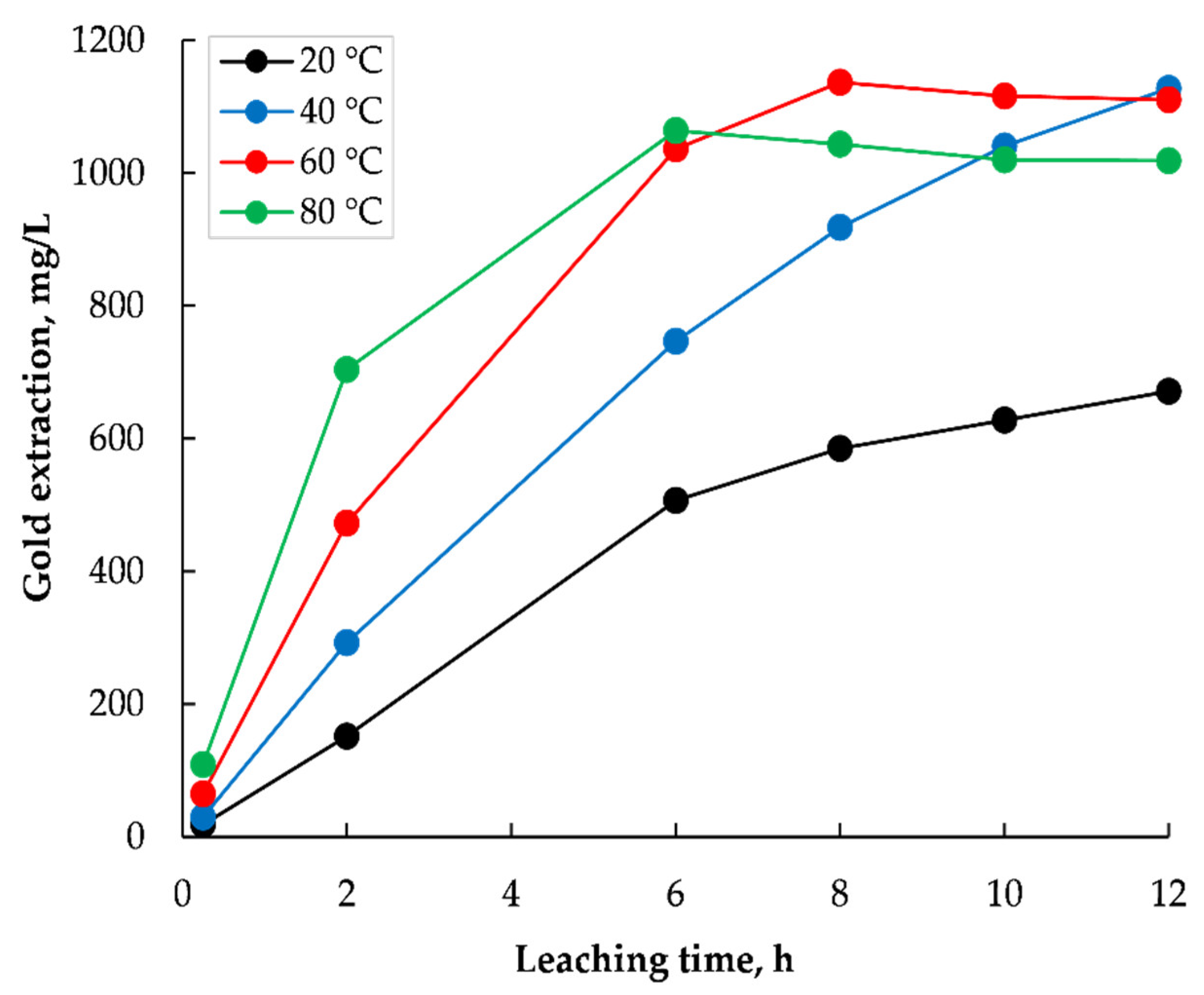
3.1.3. Effect of Leaching Temperature and Time
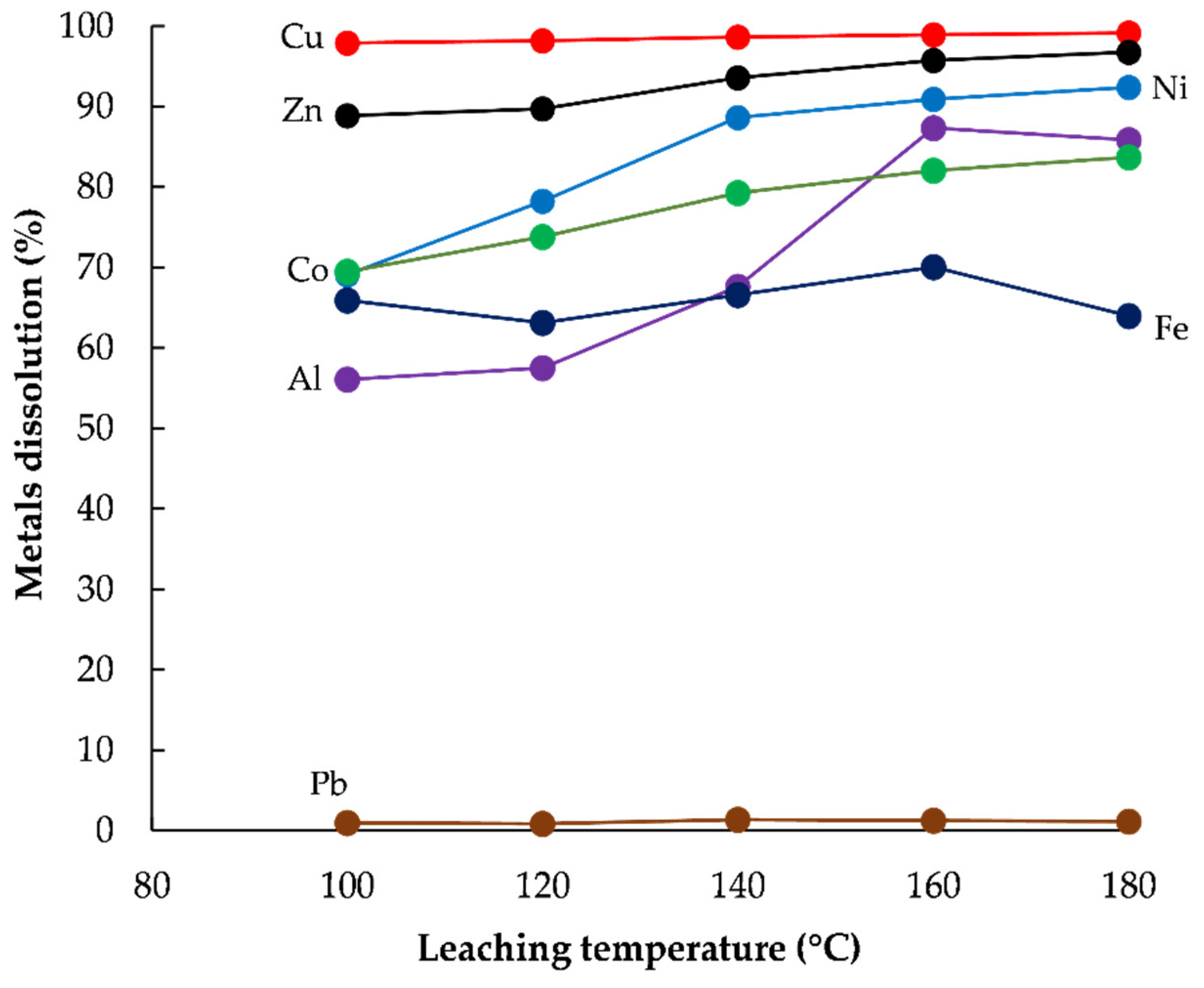
3.2. Extraction of Base Metals from WPCBs Ash by HPOL
Effect of Temperature
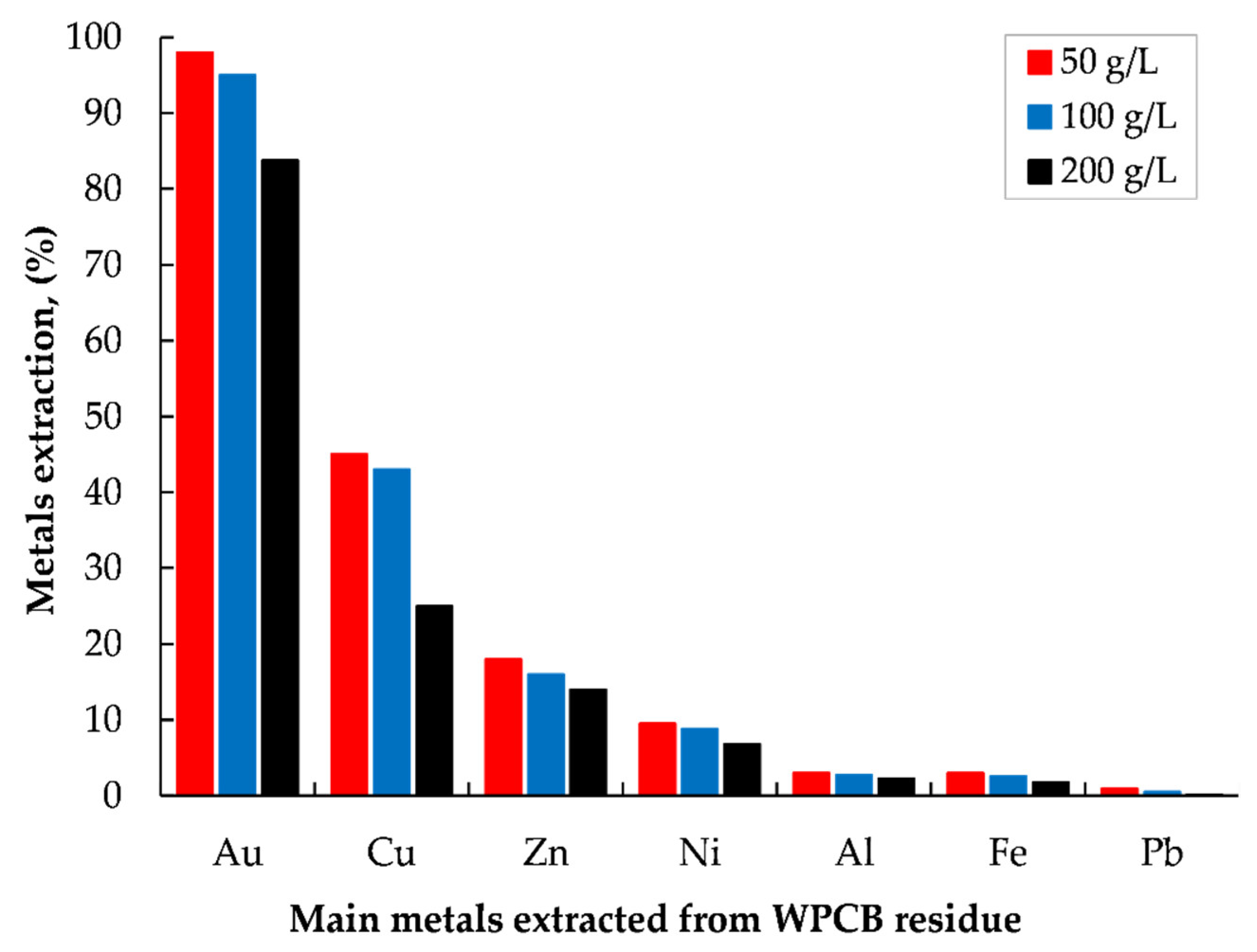
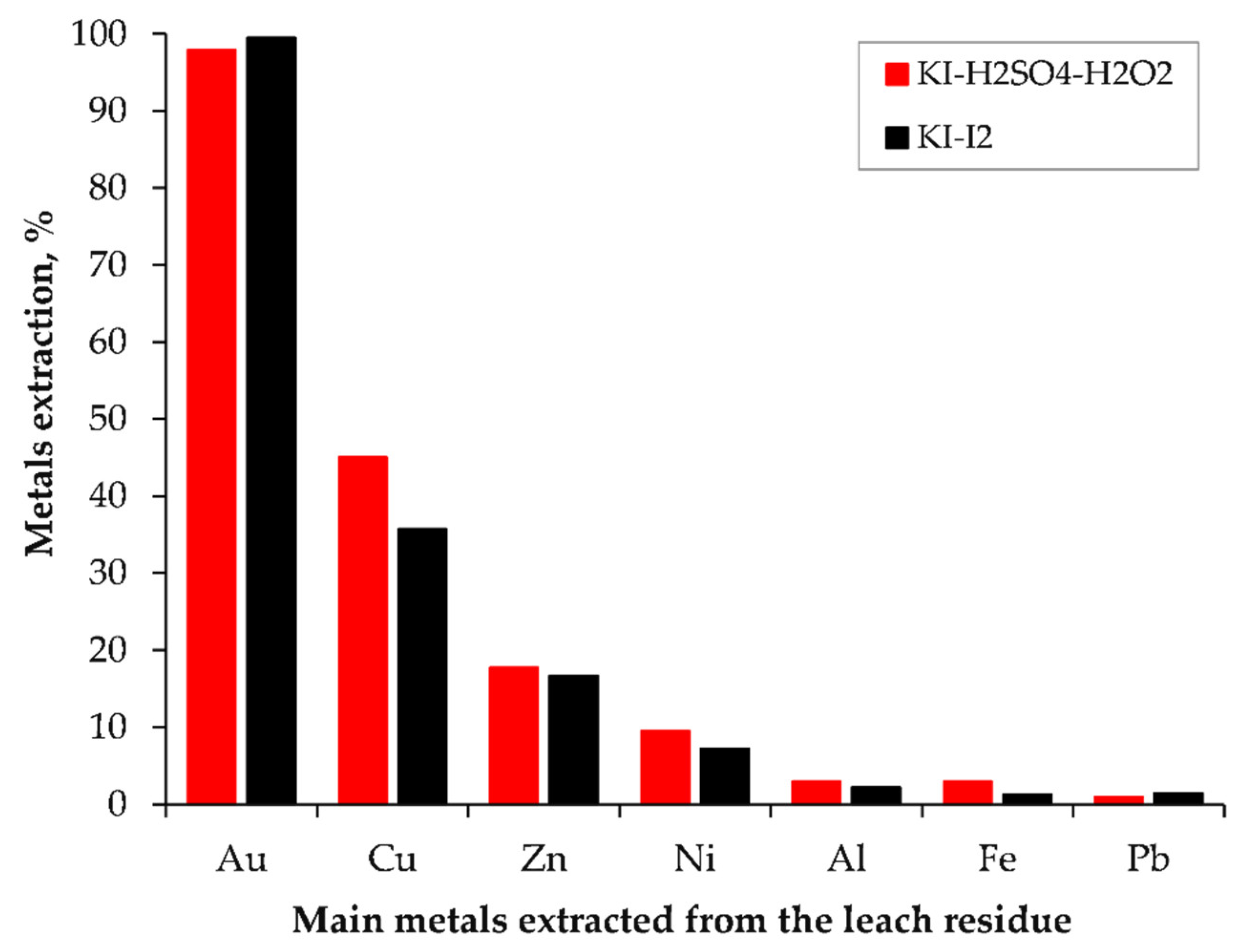
3.3. Extraction of Precious Metals from Leach Residue via Iodide Leaching
Effect of Pulp Density
3.4. Evaluation of Effectiveness of Iodide Leaching
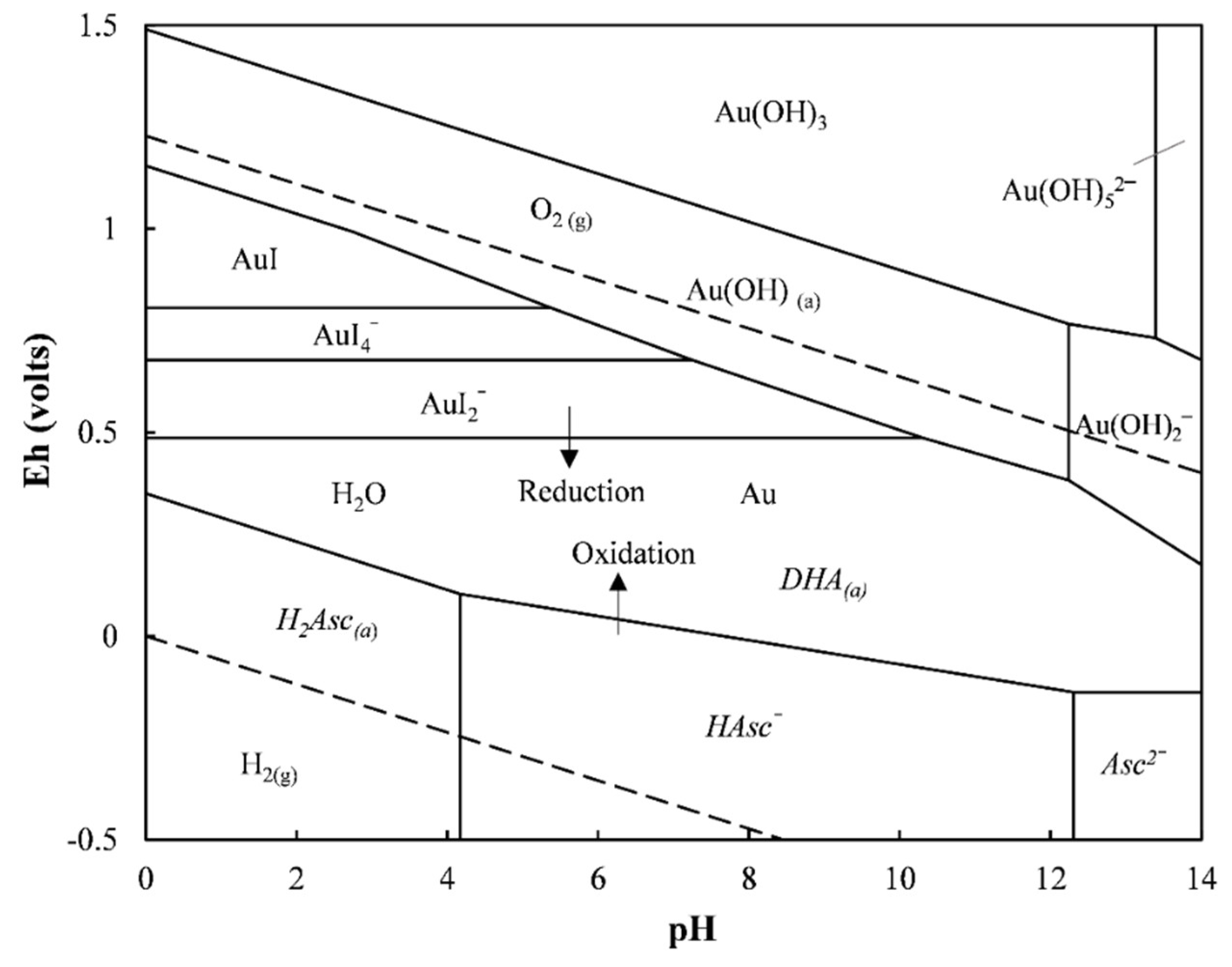
3.5. Recovery of Gold from the Pregnant Leach Solution
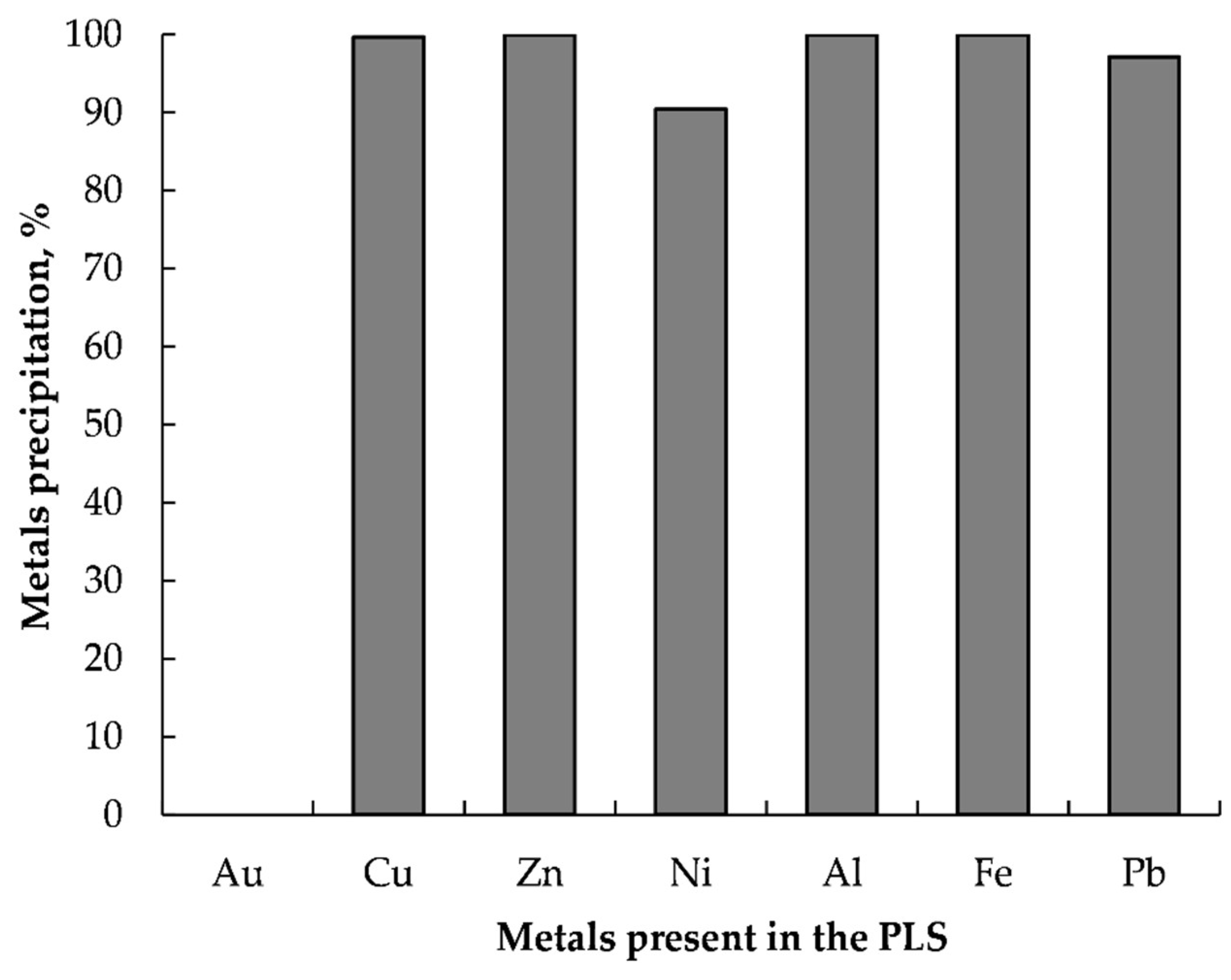
3.5.1. Removal of Metal Impurities from the PLS
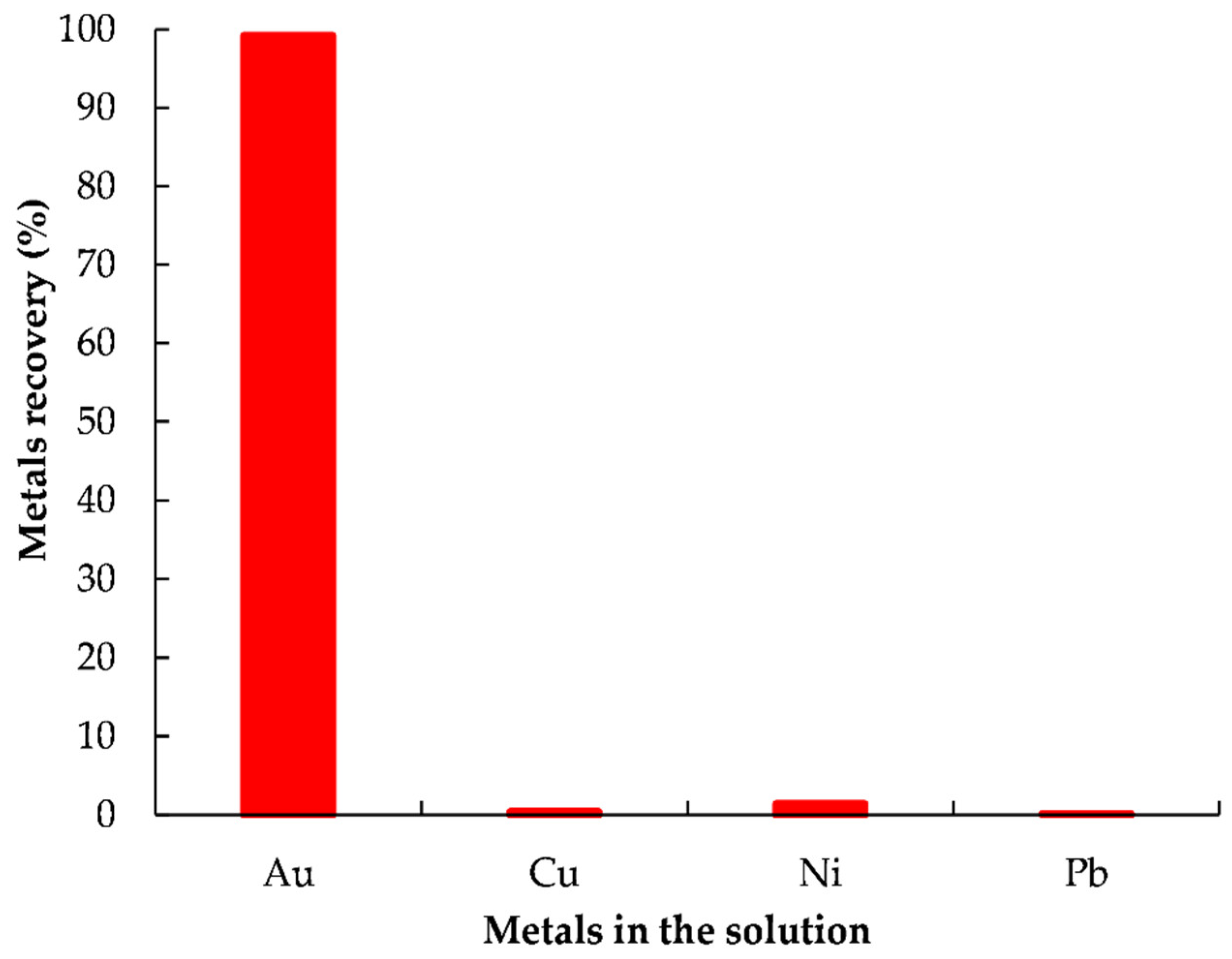
3.5.2. Recovery of Gold from the Solution by Reductive Precipitation Using L-AA
4. Conclusions
- -
- The maximum gold extraction of 1137 mg/L (99% extraction efficiency) was achieved from pure gold chips in KI–H2O2–H2SO4 media under the conditions determined through this study, while the molar ratio of KI–H2O2–H2SO4 reactants was 5:1:1.
- -
- The most effective extraction of Cu (99%), Zn (95.7%), Ni (91%), Al (87.3%), Co (82%), and Fe (70%) from the WPCBs ash were achieved in 1 M H2SO4 solution by HPOL pre-treatment at the defined conditions.
- -
- Over 95% of gold could be extracted from the leach residue from HPOL via iodide leaching under the conditions determined from the leaching of gold chips in the KI–H2O2–H2SO4 solution.
- -
- Results suggested that iodide leaching is an effective method for the extraction of gold from WPCBs.
- -
- Efficient gold recovery (>99.2%) could be achieved from the PLS by reductive precipitation using L-AA after selectively removed metal impurities via precipitation with NaOH at a pH of 9.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Masloboev, V.A.; Seleznev, S.G.; Svetlov, A.V.; Makarov, D.V. Hydrometallurgical Processing of Low-Grade Sulfide Ore and Mine Waste in the Arctic Regions: Perspectives and Challenges. Minerals 2018, 8, 436. [Google Scholar] [CrossRef]
- Choubey, P.K.; Lee, J.; Kim, M.; Kim, H. Conversion of chalcopyrite to copper oxide in hypochlorite solution for selective leaching of copper in dilute sulfuric acid solution. Hydrometallurgy 2018, 178, 224–230. [Google Scholar] [CrossRef]
- Battsengel, A.; Batnasan, A.; Narankhuu, A.; Haga, K.; Watanabe, Y.; Shibayama, A. Recovery of light and heavy rare earth elements from apatite ore using sulphuric acid leaching, solvent extraction and precipitation. Hydrometallurgy 2018, 179, 100–109. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Comprehensive characterization of printed circuit boards of various end-of-life electrical and electronic equipment for beneficiation investigation. Waste Manag. 2018, 75, 103–123. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Lenny Koh, S.C.; Rosa, P. Recycling of WEEEs: An economic assessment of present and future e-waste streams. Renew. Sust. Energ. Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef]
- Holgersson, S.; Steenari, B.-M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size Waste Electric and Electronic Equipment (WEEE) printed circuit boards—Part 1: Internet routers, mobile phones and smartphones. Resour. Conserv. Recycl. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Yamane, L.H.; Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Işıldar, A.; Rene, E.R.; Hullebusch, E.D.; Lens, P.N.L. Electronic waste as a secondary source of critical metals: Management and recovery technologies. Resour. Conserv. Recycl. 2018, 135, 296–312. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Lin, C.S.K.; Hui, C.W.; McKay, G. Waste printed circuit board recycling techniques and product utilization. J. Hazard. Mater. 2015, 283, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Two-step pre-processing enrichment of waste printed circuit boards: Mechanical milling and physical separation. J. Clean. Prod. 2018, 184, 1113–1124. [Google Scholar] [CrossRef]
- Işıldar, A.; Hullebusch, E.D.; Lenz, M.; Laing, G.D.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, B.; Pan, D.; Wu, Y.; Zuo, T. Recovery of waste printed circuit boards through pyrometallurgical processing: A review. Resour. Conserv. Recycl. 2017, 126, 209–218. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Jung, M.; Yoo, K.; Alorro, R.D. Dismantling of Electric and Electronic Components from Waste Printed Circuit Boards by Hydrochloric Acid Leaching with Stannic Ions. Mater. Trans. 2017, 58, 1076–1080. [Google Scholar] [CrossRef]
- Fleming, C.A. Hydrometallurgy of precious metals recovery. Hydrometallurgy 1992, 30, 127–162. [Google Scholar] [CrossRef]
- Anderson, C.G. Alkaline sulfide gold leaching kinetics. Miner. Eng. 2016, 92, 248–256. [Google Scholar] [CrossRef]
- Aylmore, M.G. Alternative lixiviants to cyanide for leaching gold ores. In Advances in Gold Ore Processing, 1st ed.; Adams, M.D., Wills, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 501–539. [Google Scholar]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Baghalha, M. The leaching kinetics of an oxide gold ore with iodide/iodine solutions. Hydrometallurgy 2012, 113–114, 42–50. [Google Scholar] [CrossRef]
- Sousa, R.; Futuro, A.; Fiúza, A.; Vila, M.C.; Dinis, M.L. Bromine leaching as an alternative method for gold dissolution. Miner. Eng. 2018, 118, 16–23. [Google Scholar] [CrossRef]
- Wang, H.; Sun, C.; Li, S.; Fu, P.; Song, Y.; Li, L.; Xie, W. Study on gold concentrate leaching by iodine-iodide. Int. J. Miner. Metall. Mater. 2013, 20, 323–328. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Akcil, A. Non-cyanide leaching processes in gold hydrometallurgy and iodine-iodide applications: A review. Miner. Process. Extr. Metall. Rev. 2015, 36, 198–212. [Google Scholar] [CrossRef]
- Altansukh, B.; Haga, K.; Huang, H.H.; Shibayama, A. Gold Recovery from Waste Printed Circuit Boards by Advanced Hydrometallurgical Processing. Mater. Trans. 2019, 60, 287–296. [Google Scholar] [CrossRef]
- Homick, R.P.; Slaon, H. Gold Reclamation Process. U.S. Patent US3,957,505, 18 May 1976. [Google Scholar]
- Qi, P.H.; Hiskey, J.B. Dissolution kinetics of gold in iodide solutions. Hydrometallurgy 1991, 27, 47–62. [Google Scholar] [CrossRef]
- Angelidis, T.N.; Kydros, K.A.; Matis, K.A. A fundamental rotating disk study of gold dissolution in iodine-iodide solutions. Hydrometallurgy 1993, 34, 49–64. [Google Scholar] [CrossRef]
- Altansukh, B.; Haga, K.; Ariunbolor, N.; Kawamura, S.; Shibayama, A. Leaching and Adsorption of Gold from Waste Printed Circuit Boards using Iodine-Iodide Solution and Activated Carbon. Eng. J. 2016, 20, 29–40. [Google Scholar] [CrossRef]
- Davis, A.; Tran, T.; Young, D.R. Solution chemistry of iodide leaching of gold. Hydrometallurgy 1993, 32, 143–159. [Google Scholar] [CrossRef]
- Meng, X.; Han, K.N. Dissolution kinetics of gold in moderate aqueous potassium iodide solutions with oxygen under pressure. Min. Metall. Explor. 1997, 14, 1–9. [Google Scholar] [CrossRef]
- Karrech, A.; Attar, M.; Oraby, E.A.; Eksteen, J.J.; Elchalakani, M.; Seibi, A.C. Modelling of multicomponent reactive transport in finite columns—Application to gold recovery using iodide ligands. Hydrometallurgy 2018, 178, 43–53. [Google Scholar] [CrossRef]
- Angelidis, T.N.; Kydros, K.A. Separation of Gold from Iodine-Iodide Solutions by Cementation on Zinc Particles. Sep. Sci. Technol. 1996, 31, 1105–1121. [Google Scholar] [CrossRef]
- Teirlinck, P.A.M.; Petersen, F.W. The nature of gold-iodide adsorption onto coconut-shell carbon. Miner. Eng. 1996, 9, 923–930. [Google Scholar] [CrossRef]
- Zhang, H.; Jeffery, C.A.; Jeffrey, M.I. Ion exchange recovery of gold from iodine-iodide solutions. Hydrometallurgy 2012, 125–126, 69–75. [Google Scholar] [CrossRef]
- Wong, G.T.F.; Zhang, L. The kinetics of the reactions between iodide and hydrogen peroxide in seawater. Mar. Chem. 2008, 111, 22–29. [Google Scholar] [CrossRef]
- Milenković, M.C.; Stanisavljev, D.R. Role of Free Radicals in Modeling the Iodide–Peroxide Reaction Mechanism. J. Phys. Chem. A 2012, 116, 5541–5548. [Google Scholar] [CrossRef]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Yoshida, T. Leaching of Zinc Oxide in Acidic Solution. Mater. Trans. 2003, 44, 2489–2493. [Google Scholar] [CrossRef]
- Altansukh, B.; Haga, K.; Shibayama, A. Recovery of Valuable Metals from Waste Printed Circuit Boards by Using Iodine-Iodide Leaching and Precipitation. In Rare Metal Technology 2018. TMS 2018. The Minerals, Metals & Materials Series, Proceedings of the TMS 2018, Phoenix, AZ, USA, 11–15 March 2018; Springer: Cham, Switzerland; pp. 131–142. [CrossRef]
- Schmid, G.M. Chapter 11: Copper, Silver, and Gold. In Standard Potentials in Aqueous Solution, 1st ed.; Bard, A.J., Parsons, R., Jordan, J., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 313–320. [Google Scholar]
- Mironov, I.V.; Belevantsev, V.I. Hydroxogold(I) complexes in aqueous Solution. Russ. J. Inorg. Chem. 2005, 50, 1210–1216. [Google Scholar]
- Matsui, T.; Kitagawa, Y.; Okumura, M.; Shigeta, Y. Accurate standard hydrogen electrode potential and applications to the redox potentials of vitamin C and NAD/NADH. J. Phys. Chem. A 2015, 119, 369–376. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Tu, Y.J.; Njus, D.; Schlegel, H.B. A theoretical study of ascorbic acid oxidation and HOO·/O2·− radical scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Serpe, A.; Rigoldi, A.; Marras, C.; Artizzu, F.; Mercuri, M.L.; Deplano, P. Chameleon behaviour of iodine in recovering noble-metals from WEEE: Towards sustainability and “zero” waste. Green Chem. 2015, 17, 2208–2216. [Google Scholar] [CrossRef]
| Main Metals and Non-Metal in WPCBs Ash, wt. % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Au | Ag | Pd | Cu | Zn | Ni | Al | Co | Fe | Pb | Sn | Si |
| 0.03 | 0.04 | 0.02 | 20.7 | 1.6 | 0.7 | 4.5 | 0.02 | 2.5 | 1.7 | 3.55 | 17.5 |
| Sample | Au | Ag | Pd | Cu | Zn | Ni | Al | Co | Fe | Pb | Sn | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leachate, (g/L) | 0 | 0 | 0 | 22.0 | 1.6 | 0.06 | 3.4 | 0.02 | 1.7 | 0.01 | 0 | 0 |
| Leach residue, (wt. %) | 0.06 | 0.21 | 0.02 | 0.23 | 0.07 | 0.04 | 0.48 | 0.004 | 0.7 | 1.67 | 7.7 | 24.4 |
| Pulp Density, g/L | Au | Cu | Zn | Ni | Al | Fe | Pb |
|---|---|---|---|---|---|---|---|
| 50 | 32.3 | 17.2 | 11.5 | 8.2 | 29.2 | 19.0 | 14.0 |
| 100 | 58.2 | 34.2 | 24.7 | 18.0 | 64.4 | 41.2 | 13.2 |
| 200 | 108 | 40.7 | 43.4 | 26.6 | 102.8 | 55.7 | 9.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batnasan, A.; Haga, K.; Huang, H.-H.; Shibayama, A. High-Pressure Oxidative Leaching and Iodide Leaching Followed by Selective Precipitation for Recovery of Base and Precious Metals from Waste Printed Circuit Boards Ash. Metals 2019, 9, 363. https://doi.org/10.3390/met9030363
Batnasan A, Haga K, Huang H-H, Shibayama A. High-Pressure Oxidative Leaching and Iodide Leaching Followed by Selective Precipitation for Recovery of Base and Precious Metals from Waste Printed Circuit Boards Ash. Metals. 2019; 9(3):363. https://doi.org/10.3390/met9030363
Chicago/Turabian StyleBatnasan, Altansukh, Kazutoshi Haga, Hsin-Hsiung Huang, and Atsushi Shibayama. 2019. "High-Pressure Oxidative Leaching and Iodide Leaching Followed by Selective Precipitation for Recovery of Base and Precious Metals from Waste Printed Circuit Boards Ash" Metals 9, no. 3: 363. https://doi.org/10.3390/met9030363
APA StyleBatnasan, A., Haga, K., Huang, H.-H., & Shibayama, A. (2019). High-Pressure Oxidative Leaching and Iodide Leaching Followed by Selective Precipitation for Recovery of Base and Precious Metals from Waste Printed Circuit Boards Ash. Metals, 9(3), 363. https://doi.org/10.3390/met9030363






