Dynamic Phase Transformation Behavior of a Nb-microalloyed Steel during Roughing Passes at Temperatures above the Ae3
Abstract
1. Introduction
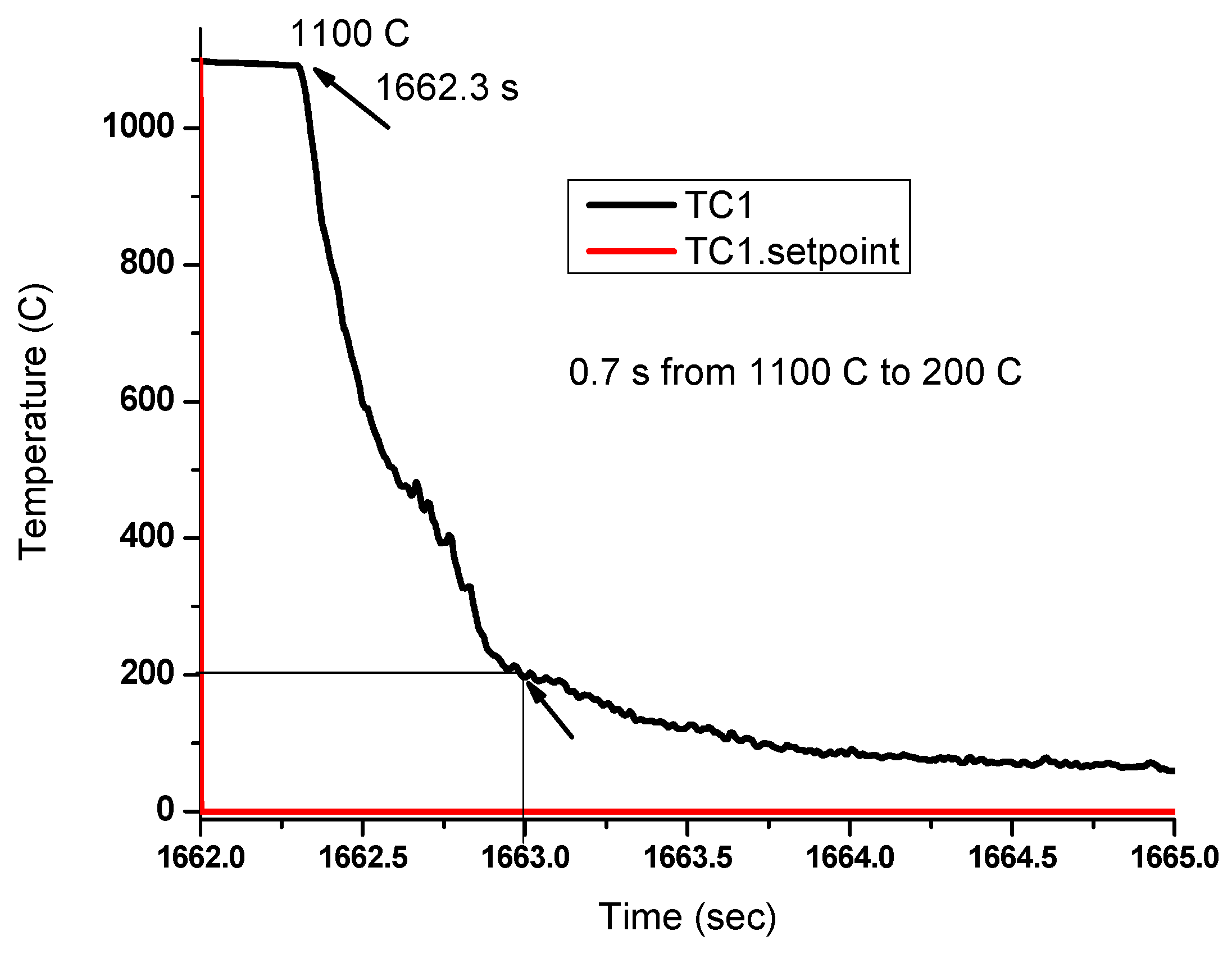
2. Materials and Methods
3. Results
3.1. Flow Curves and Mean Flow Stress
3.2. Microstrutural Results
3.3. Volume Fraction of Transformed Ferrite
4. Discussion
4.1. Cooling Rate and Continuous Cooling Transformation Curves
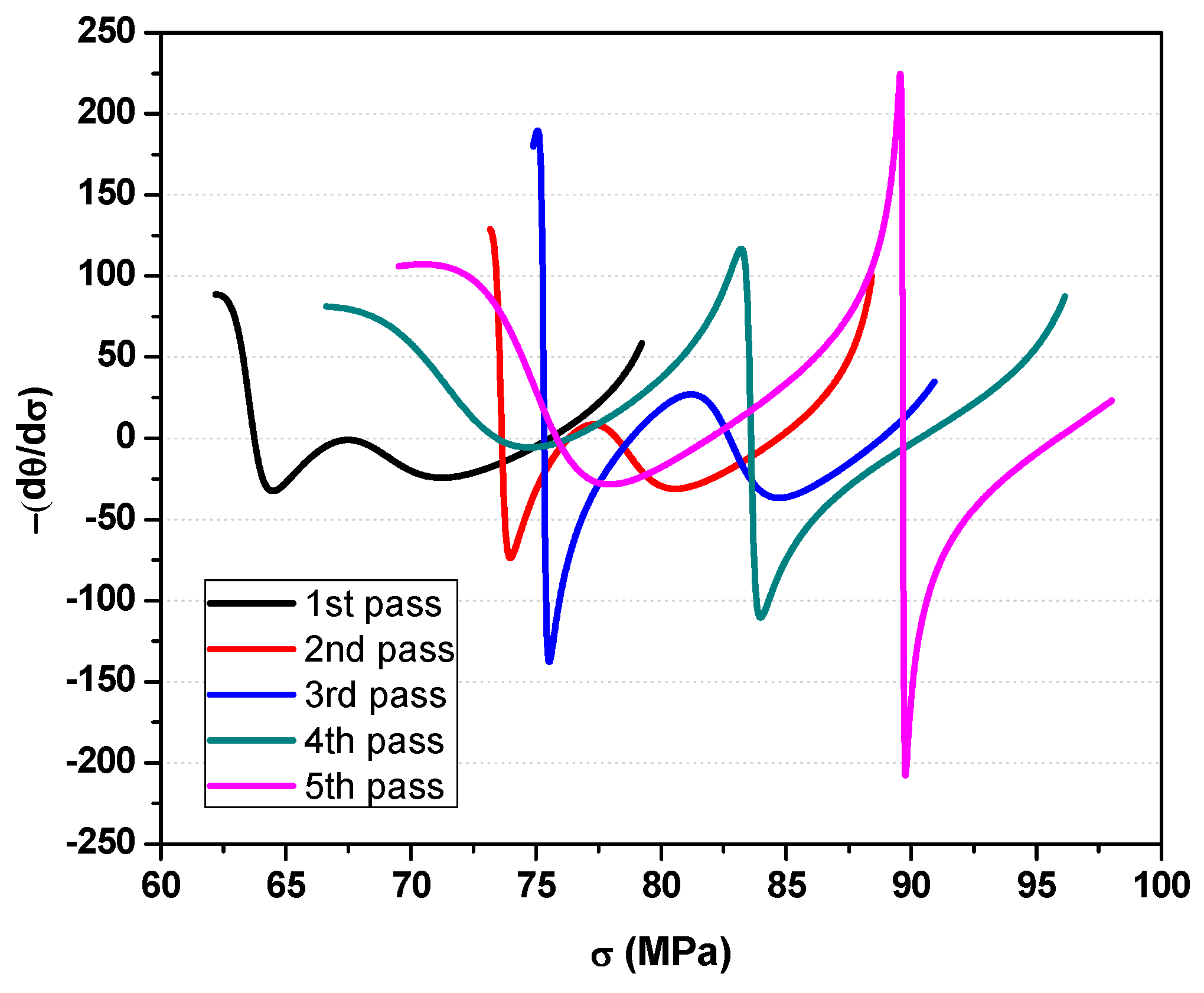
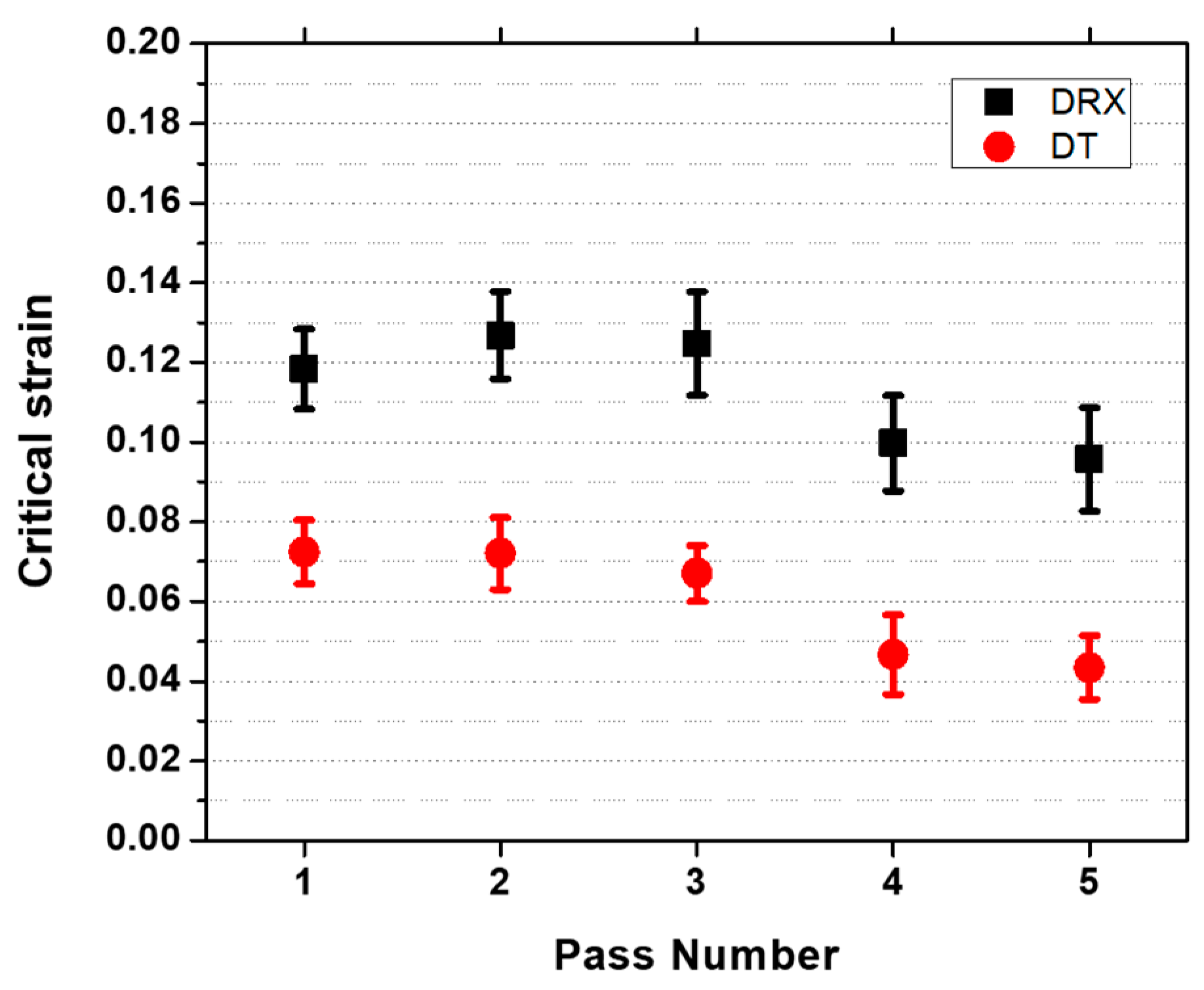
4.2. Critical Stresses and Strains
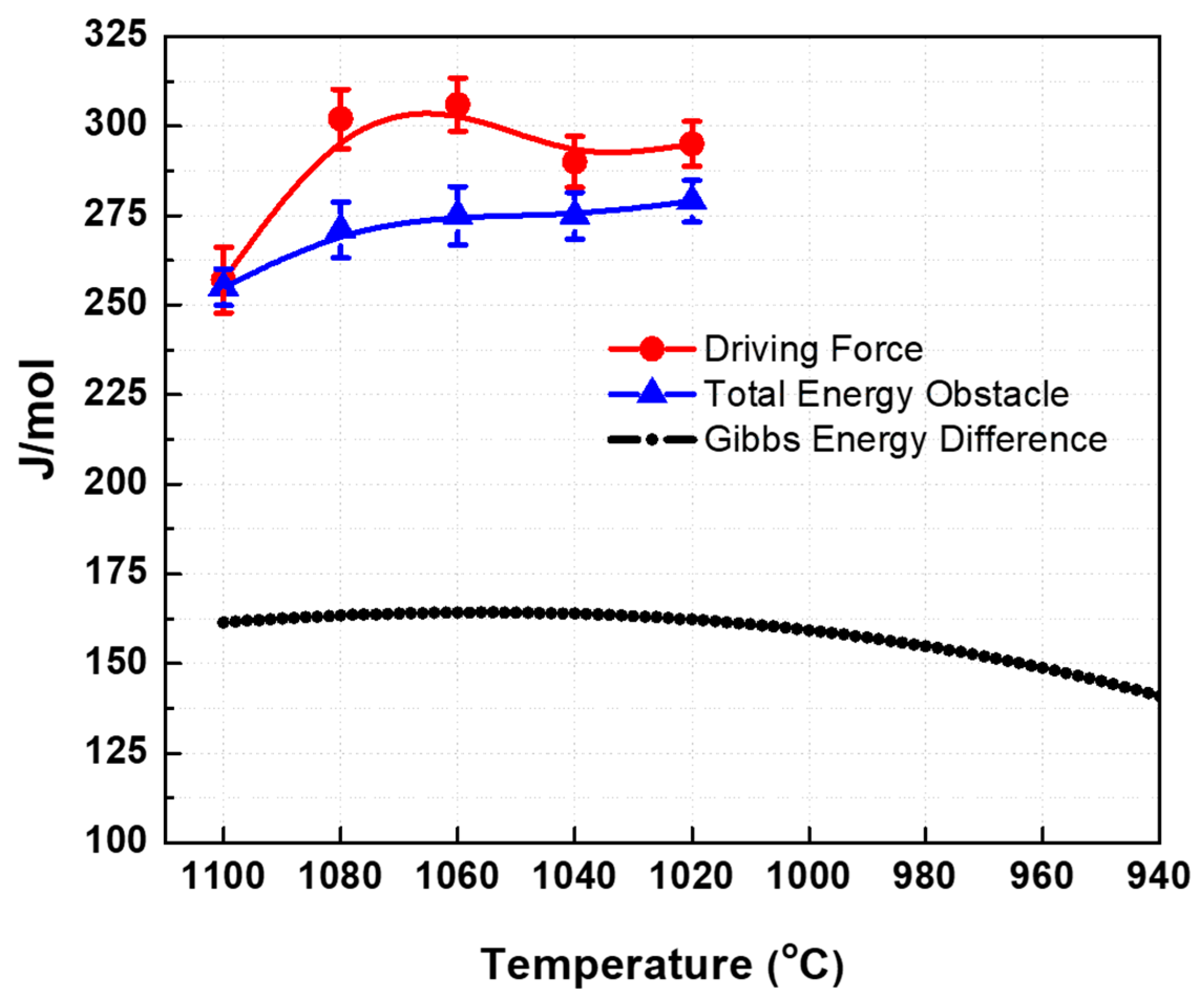
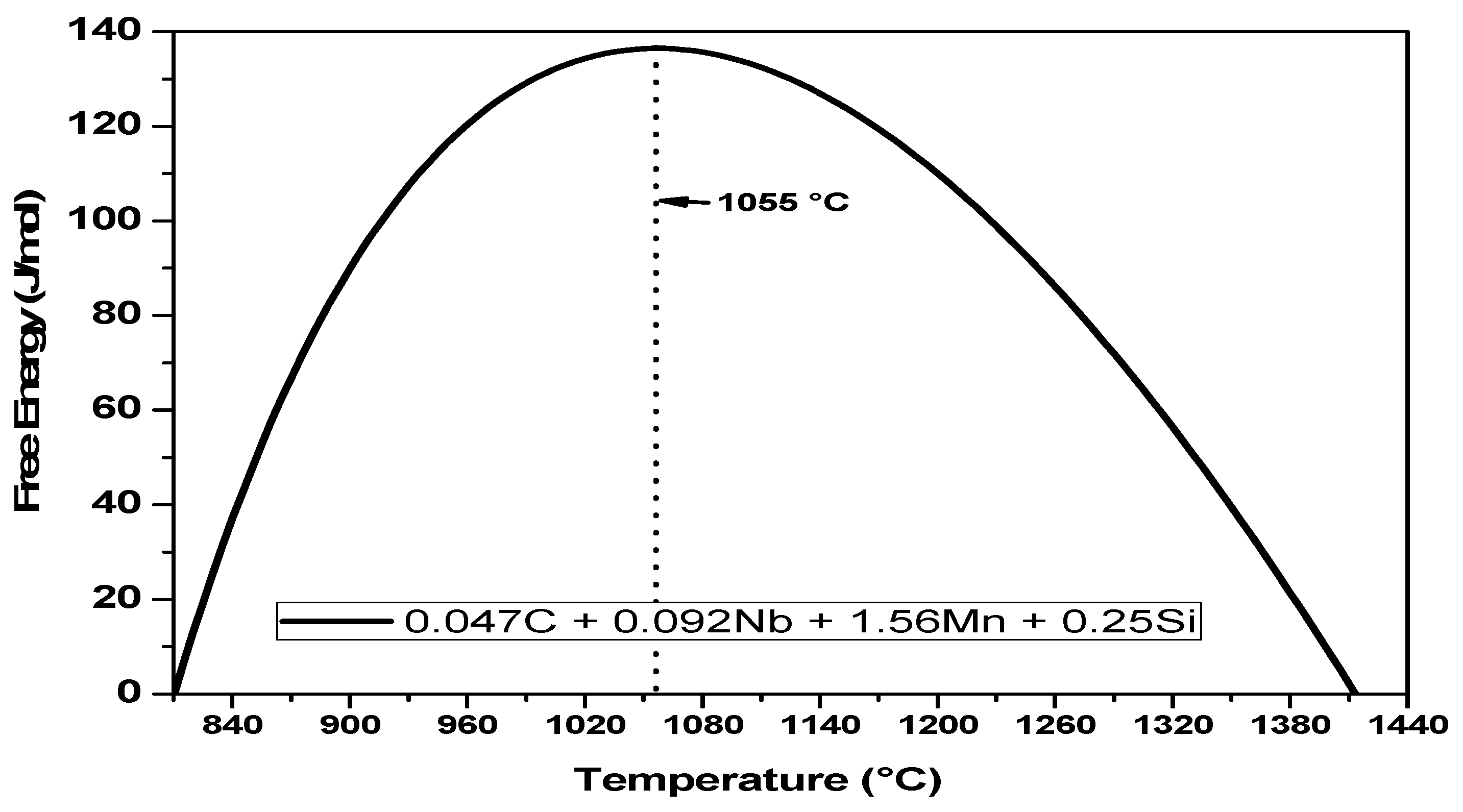
4.3. Total Energy Obstacles and Driving Force
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Grewal, R.; Aranas, C.; Chadha, K.; Shahriari, D.; Jahazi, M.; Jonas, J.J. Formation of Widmastatten ferrite at very high temperatures in the austenite phase field. Acta Mater. 2016, 109, 23–31. [Google Scholar] [CrossRef]
- Aranas, C.; Jung, I.H.; Yue, S.; Rodrigues, S.F.; Jonas, J.J. A metastable phase diagram for the dynamic transformation of austenite at temperatures above the Ae3. Int. J. Mater. Res. 2016, 107, 881–886. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yada, H. Evolution deformation of ultrafine- grained ferrite in hot successive deformation. Trans. ISIJ. 1987, 27, 492–498. [Google Scholar] [CrossRef]
- Yada, H.; Matsumura, Y.; Senuma, T. International conference on physical metallurgy of thermomechanical processing of steels and other metals. Thermec-88. Vol. 2. In Proceedings of the 1st Conference Physical Metallurgy of Thermomechanical Processing of Steels and other Metals, Tokyo, Japan, 6–10 June 1988; Tamura, I., Ed.; 1988; pp. 200–207. [Google Scholar]
- Yada, H.; Li, C.M.; Yamagata, H. Dynamic γ—A transformation during hot deformation in Iron-Nickel-Carbon alloys. ISIJ Int. 2000, 40, 200–206. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q. Dilatometric investigation on isothermal transformation after hot deformation. J. Iron Steel Res. Int. 2003, 10, 46–48. [Google Scholar]
- Liu, Z.; Li, D.; Lu, S.; Qiao, G. Thermal stability of high temperature deformation induced ferrite in a low carbon steel. ISIJ Int. 2007, 47, 289–293. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Dong, H.; Liu, Q.; Weng, Y. Microstructural evolution and kinetics for post-dynamic transformation in a plain low carbon steel. ISIJ Int. 2008, 48, 994–1000. [Google Scholar] [CrossRef]
- Basabe, V.V.; Jonas, J.J. The ferrite transformation in hot deformed 0.036% Nb austenite at temperature above the Ae3. ISIJ Int. 2010, 50, 1185–1192. [Google Scholar] [CrossRef]
- Ghosh, C.; Basabe, V.V.; Jonas, J.J.; Kim, Y.; Jung, I.; Yue, S. The dynamic transformation of deformed austenite at temperatures above the Ae3. Acta Mater. 2013, 61, 2348–2362. [Google Scholar] [CrossRef]
- Aranas, C.; Jonas, J.J. Effect of Mn and Si on dynamic transformation of austenite above the Ae3 temperature. Acta Mater. 2015, 82, 1–10. [Google Scholar] [CrossRef]
- Aranas, C.; Nguyen-Minh, T.; Grewal, R.; Jonas, J.J. Flow softening-based formation of Widmanstatten ferrite in a 0.06%C steel deformed above the Ae3. ISIJ Int. 2015, 55, 300–307. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Aranas, C.; Sun, B.; Siciliano, F.; Yue, S.; Jonas, J.J. Effect of grain size and residual strain on the dynamic transformation of austenite under plate rolling conditions. Steel Res. Int. 2018, 89, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Aranas, C.; Wang, T.; Jonas, J.J. Dynamic transformation of an X70 steel under plate rolling conditions. ISIJ Int. 2017, 57, 162–169. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Aranas, C.; Jonas, J.J. Retransformation behavior of dynamically transformed ferrite during the simulated plate rolling of a low C and an X70 Nb steel. ISIJ Int. 2017, 57, 929–936. [Google Scholar] [CrossRef]
- Aranas, C.; Wang, T.; Jonas, J.J. Effect of interpass time on the dynamic transformation of a plain C-Mn and Nb-microalloyed steel. ISIJ Int. 2015, 55, 647–654. [Google Scholar] [CrossRef]
- Bale, C.W.; Belisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.H.; Kang, Y.Y.; Melancon, J.; Pelton, A.D.; et al. FactSage thermomechanical software and database-recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Fields, D.S.; Backofen, W.A. Determination of strain hardening characteristics by torsion testing. Proc. Am. Soc. Test. Mater. 1957, 57, 1259–1272. [Google Scholar]
- Poliak, E.I.; Jonas, J.J. A one-parameter approach to determining the critical conditions for the initiation of dynamic recrystallization. Acta Mater. 1996, 44, 127–136. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ghosh, C.; Aranas, C.; Jonas, J.J. Dynamic transformation of deformed austenite at temperatures above the Ae. Prog. Mater. Sci. 2016, 82, 151–233. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Aranas, C.; Jonas, J.J. Dynamic Transformation during the Simulated Plate Rolling of a 0.09% Nb Steel. ISIJ Int. 2017, 57, 1102–1111. [Google Scholar] [CrossRef]
- Poliak, E.I.; Jonas, J.J. Critical strain for dynamic recrystallization in variable strain rate hot deformation. ISIJ Int. 2003, 43, 692–700. [Google Scholar] [CrossRef]
- Aranas, C.; Rodrigues, S.F.; Fall, A.; Jahazi, M.; Jonas, J.J. Determination of the critical stress associated with dynamic phase transformation in steels by means of free energy method. Metals 2018, 8, 360. [Google Scholar] [CrossRef]
- Jonas, J.J.; Ghosh, C. Role of mechanical activation in the dynamic transformation of austenite. Acta Mater. 2013, 61, 6125–6131. [Google Scholar] [CrossRef]
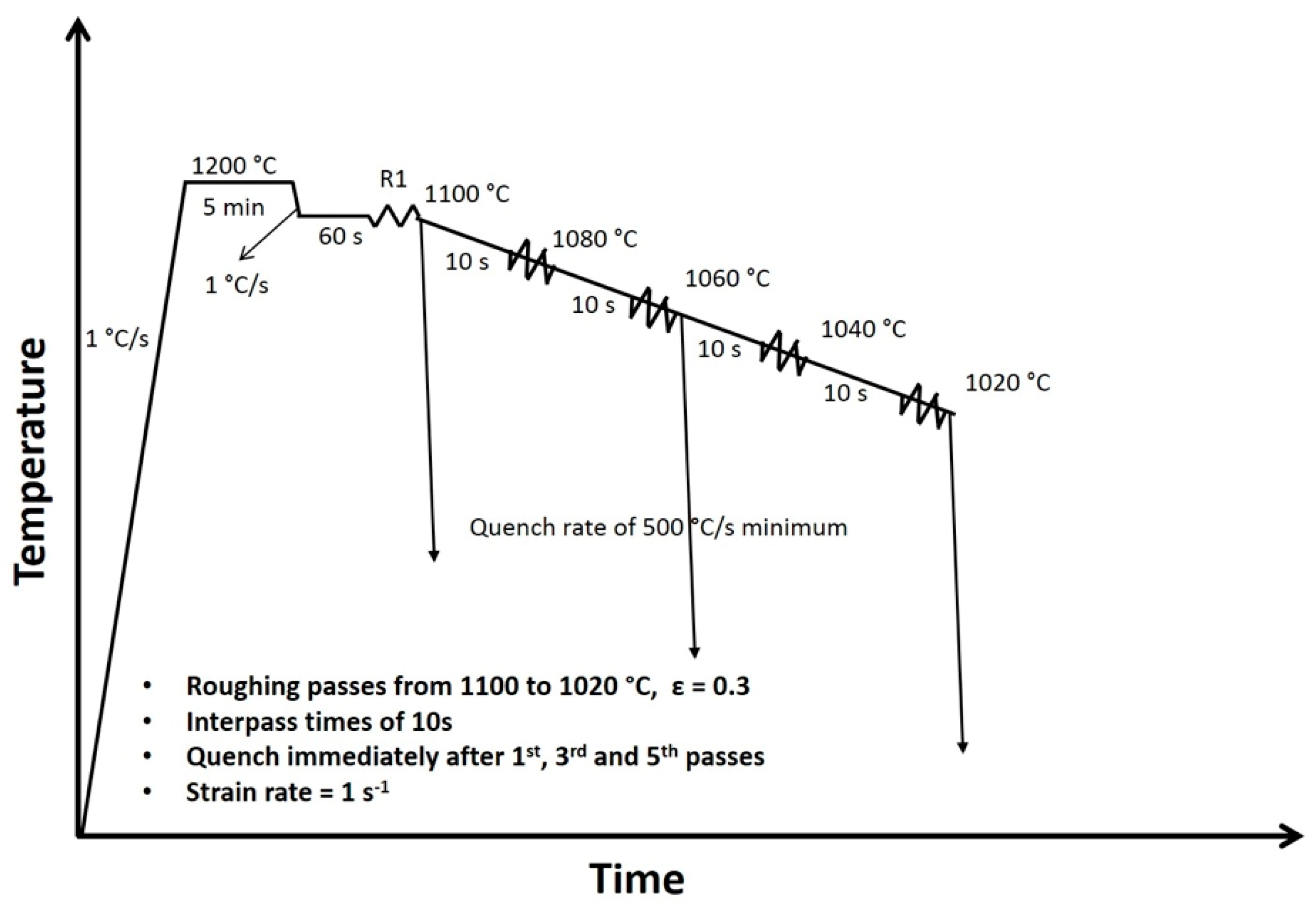
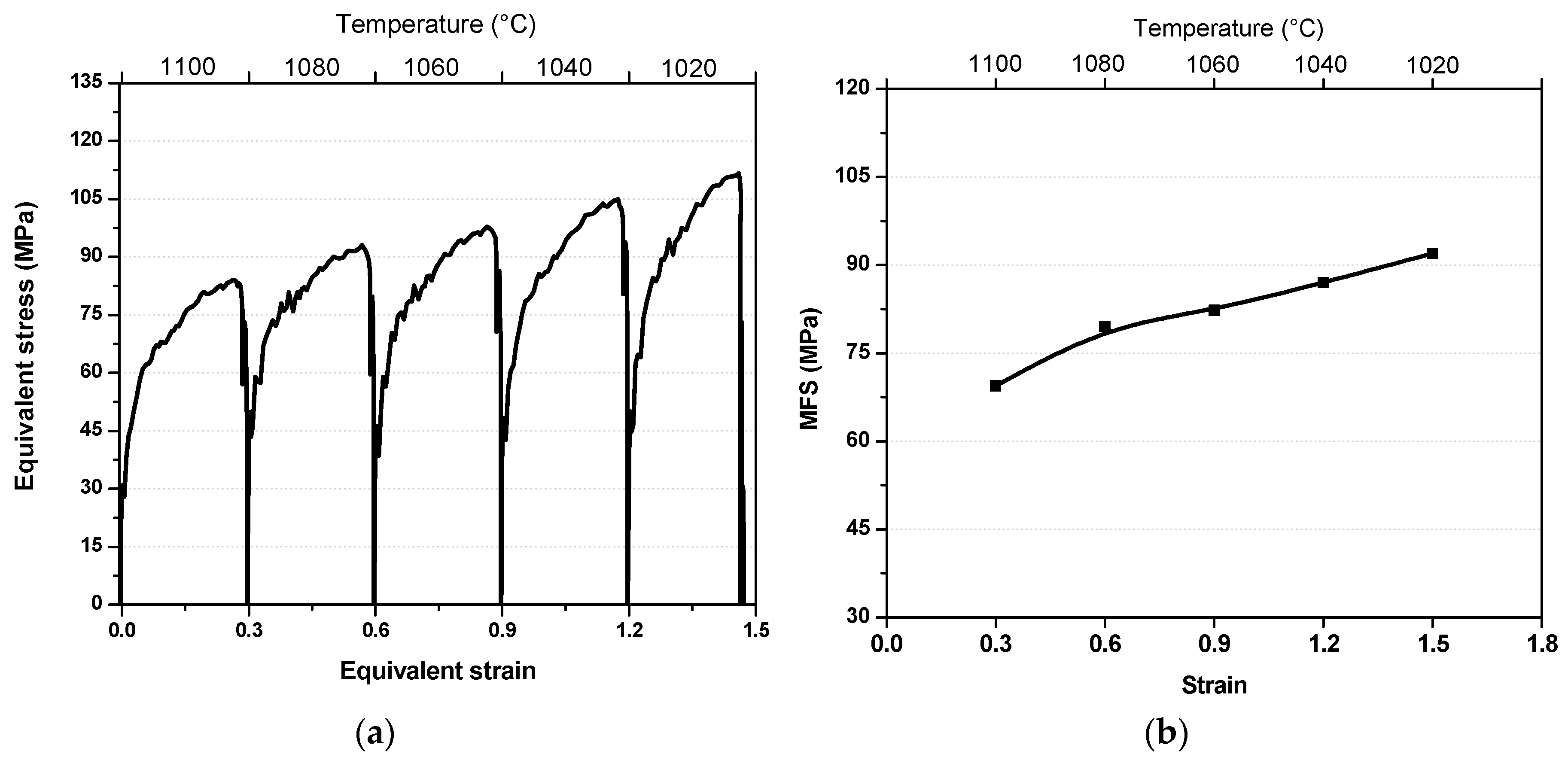
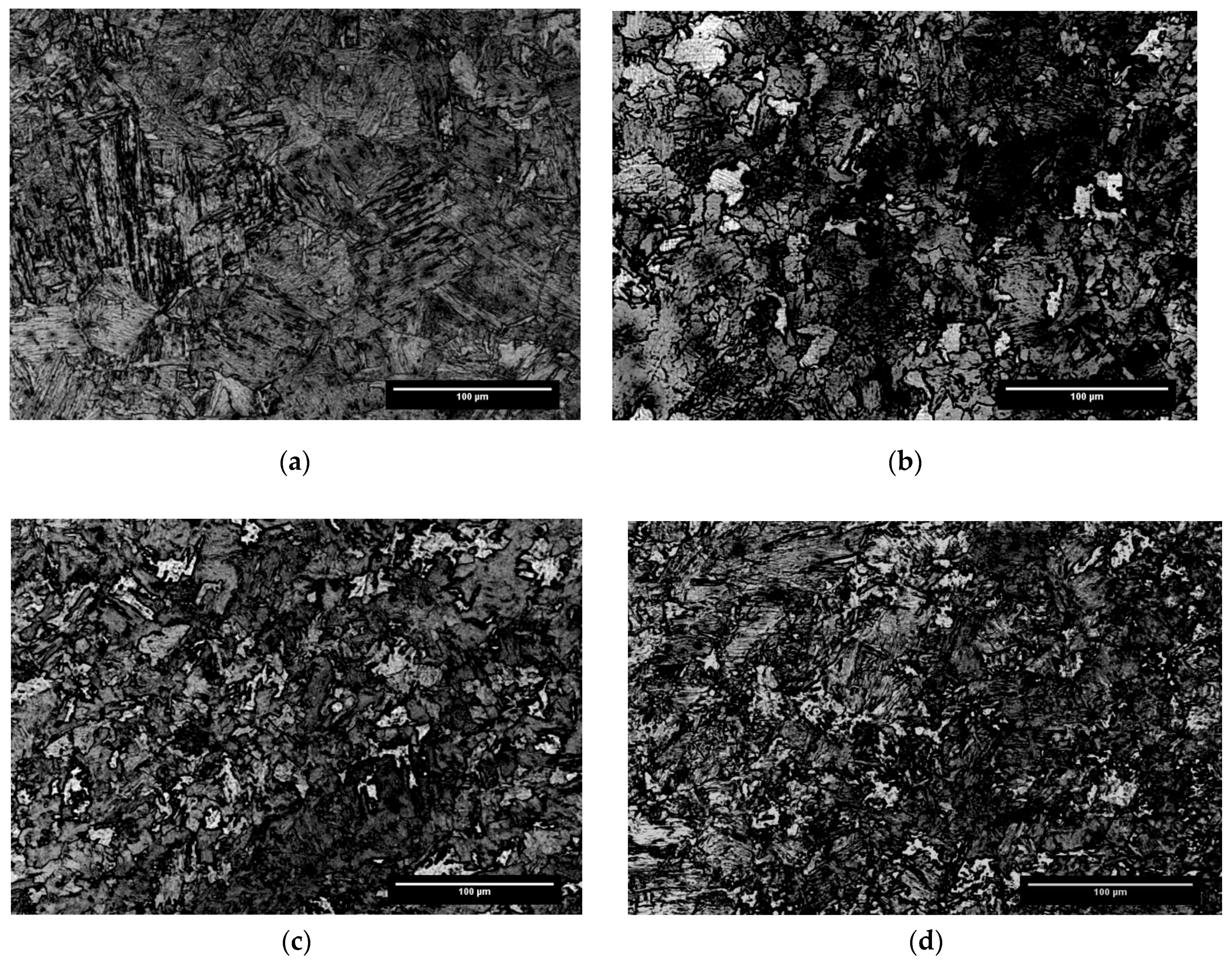
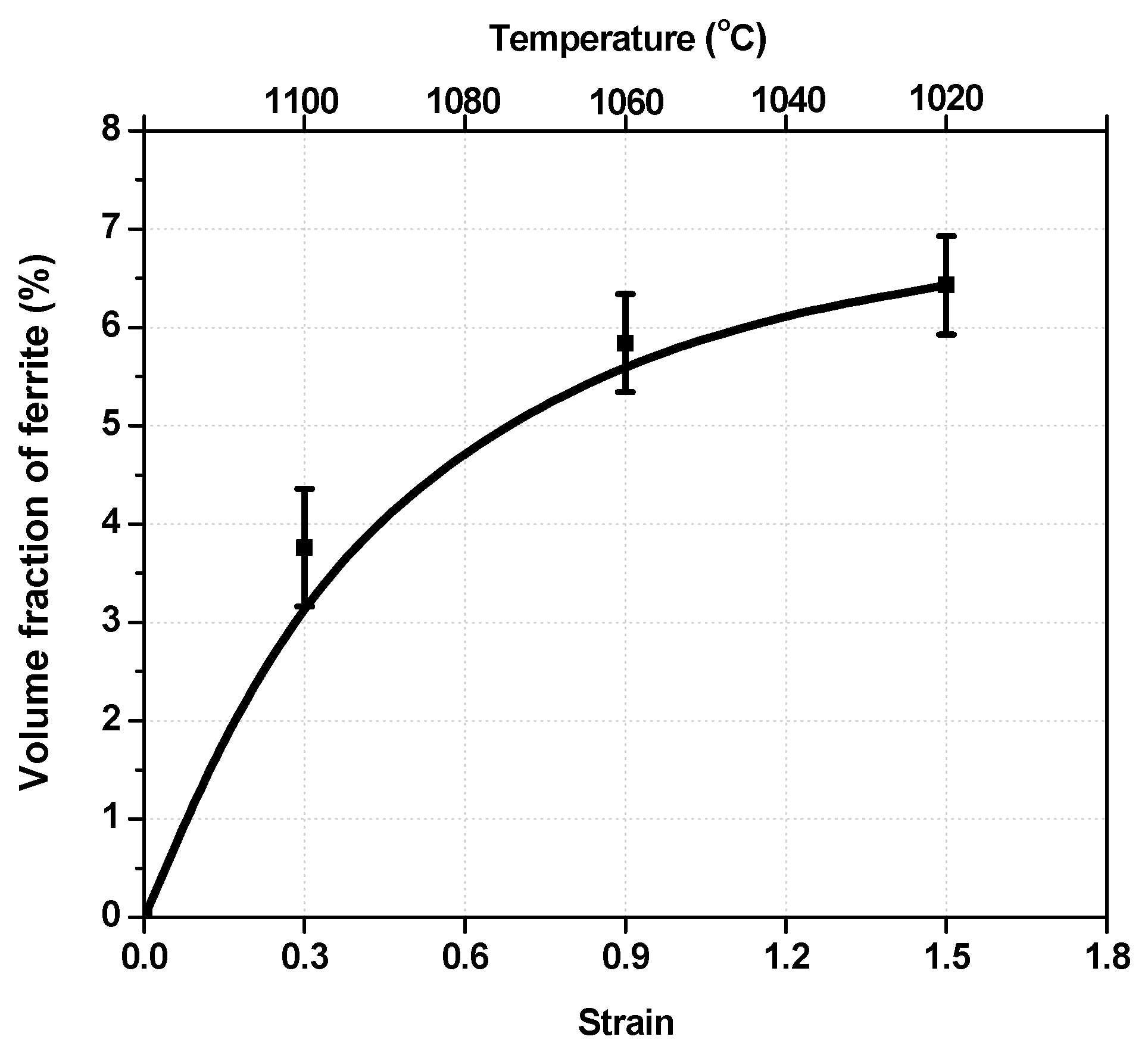

| C | Mn | Si | Cr | Nb | N | Orthoequilibrium Ae3 | Paraequilibrium Ae3 |
|---|---|---|---|---|---|---|---|
| 0.047 | 1.56 | 0.25 | 0.21 | 0.092 | 0.008 | 845 °C | 810 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, S.F.; Siciliano, F.; Aranas, C., Jr.; Silva, E.S.; Reis, G.S.; Jahazi, M.; Jonas, J.J. Dynamic Phase Transformation Behavior of a Nb-microalloyed Steel during Roughing Passes at Temperatures above the Ae3. Metals 2019, 9, 334. https://doi.org/10.3390/met9030334
Rodrigues SF, Siciliano F, Aranas C Jr., Silva ES, Reis GS, Jahazi M, Jonas JJ. Dynamic Phase Transformation Behavior of a Nb-microalloyed Steel during Roughing Passes at Temperatures above the Ae3. Metals. 2019; 9(3):334. https://doi.org/10.3390/met9030334
Chicago/Turabian StyleRodrigues, Samuel F., Fulvio Siciliano, Clodualdo Aranas, Jr., Eden S. Silva, Gedeon S. Reis, Mohammad Jahazi, and John J. Jonas. 2019. "Dynamic Phase Transformation Behavior of a Nb-microalloyed Steel during Roughing Passes at Temperatures above the Ae3" Metals 9, no. 3: 334. https://doi.org/10.3390/met9030334
APA StyleRodrigues, S. F., Siciliano, F., Aranas, C., Jr., Silva, E. S., Reis, G. S., Jahazi, M., & Jonas, J. J. (2019). Dynamic Phase Transformation Behavior of a Nb-microalloyed Steel during Roughing Passes at Temperatures above the Ae3. Metals, 9(3), 334. https://doi.org/10.3390/met9030334









