1. Introduction
Press hardening of boron alloyed steels has been used since the 1980s [
1] to produce beams, pillars, and safety-related components for cars [
2]. A six-fold increase in the adoption of the technique for component production was anticipated between 2006 and 2015 [
3] and the production reached 360 million components in 2015 [
4]. Strength levels achievable in boron steels in as quenched conditions are considered excellent (
Rm ≈ 1500 MPa) but the ductility is often limited (
A50 mm ≈ 6% or lower) as a result of the essentially martensitic microstructure of the steels [
5]. Tailor-welded blanks and differentiation of heat treatment are the methods that can be used to tailor and optimize the properties in different parts of a component [
6]. In addition, both ductility and toughness may be enhanced in these steels with the formation of carbide free bainitic (CFB) microstructures through austempering process and/or subjecting these steels to a novel concept of quenching and partitioning (QP) thermal treatment as described below. Formation of CFB microstructures can be facilitated in specially tailored steel compositions containing high levels of Si and/or Al (about 1.5–3 wt %), through austempering because both Si and or Al are strong graphitisers and hence, hinder or delay carbide formation in the steel structure. The microstructures of CFB steels comprise mainly of fine laths of bainitic ferrite and carbon enriched austenite divided finely between bainitic sheave [
7,
8] and martensite in some cases [
9]. Likewise, the QP treatment first described by Speer et al. [
10] also promotes formation of essentially martensitic microstructures with small fractions of finely divided, carbon-enriched interlath austenite [
11], besides a small fraction of bainitic ferrite and in some cases also carbides [
12]. Tensile properties typical of selected steels processed through QP technique are shown in
Table 1.
The twin benefits of the existing direct press hardening process applied to boron steels are (i) the combination of rapid forming through optimized processing and (ii) quenching of the component in the pressing tool. During austempering, austenite is isothermally transformed into lower bainite at a temperature slightly above the martensite start temperature (
Ms) for a duration adequate enough for complete austenite decomposition. However, slow kinetics of the austenite to bainite transformation at temperatures close to
Ms can have limitations in respect of the austempering process in combination with press hardening for commercial production of automotive components. In the QP process the steel is quenched to a temperature between the start (
Ms) and the finish (
Mf) of martensite reaction and subsequently either held at the quenching temperature or heated to just above or below
Ms temperature to facilitate partitioning of carbon from transformed supersaturated martensite into austenite or from the bainite that may form during subsequent partitioning step. The transformation rate has been shown to increase when the austempering temperature is lowered just below the
Ms temperature [
16]. A quench stop below
Ms allows a small amount of martensite to form prior to bainite transformation, thereby increasing the number of possible nucleation sites for bainite and thus its rate of formation [
16]. It has also been shown that the transformation from austenite to bainite can be accelerated if a small fraction of martensite can be formed from the austenite [
17] even though the rate of bainite formation following martensite formation remains unchanged and same amount of bainite would form following austenite decomposition [
18]. Utilization of the QP heat treatment thus provides the possibility to shorten the production cycle time. Press hardening with a QP treatment of boron steel has been shown to improve the ductility of the steel but with a marginal loss in yield strength [
19,
20], as compared with the properties obtained through conventional press hardening of 22MnB5 steel. This process has been repeated for low-carbon Si-Mn steels and the maximum volume fraction of retained austenite reached 17.2% with corresponding total elongation of 14.5% when hot stamping is done at 750 °C [
21]. Seo et al. [
22], designed two types of modified press hardening steels (PHS) by adding Si, and Si + Cr to 22MnB5 steel, followed by optimized QP processing to achieve improved properties. In the best QP conditions the ductility improved to 17% total elongation with 1032 and 1098 MPa yield and tensile strengths respectively, for the Si + Cr added (PHS) grade.
The aim of this work was to produce components with properties equal to or better than conventional press hardened boron steels, within a reasonable processing time for improved productivity. Various thermal treatments following the forming stage were investigated to achieve a fine multiphase microstructure. The quench stop temperature in the die was identified as a variable of interest along with the furnace temperature and the holding time of the heat treatment. Two variants of quench stop temperature were investigated, above and below the Ms. Both isothermal heat treatment above and below Ms and QP were investigated using thermal simulations for two cold rolled Fe-(0.15 and 0.26)C-1.5Si-2Mn-0.6Cr alloys. This paper reports an account of the mechanical properties obtained after press hardening experiments to produce hat-shaped profiles using QP heat treatments for high-silicon steels, in comparison with those of commercial 22MnB5 profiles. The effect of using QP treatments on austenite decomposition kinetics in comparison with austempering treatment is also studied.
4. Results
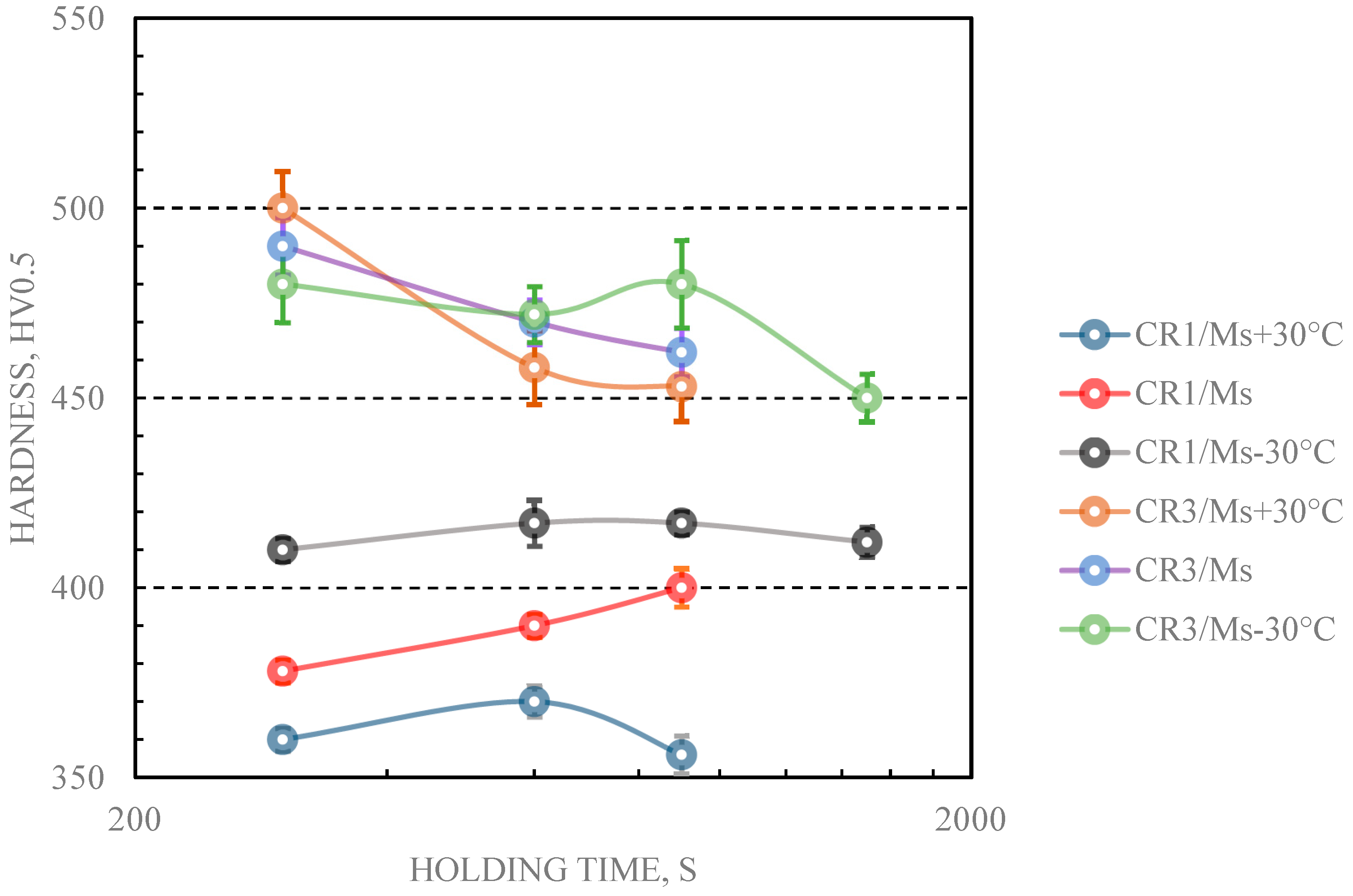
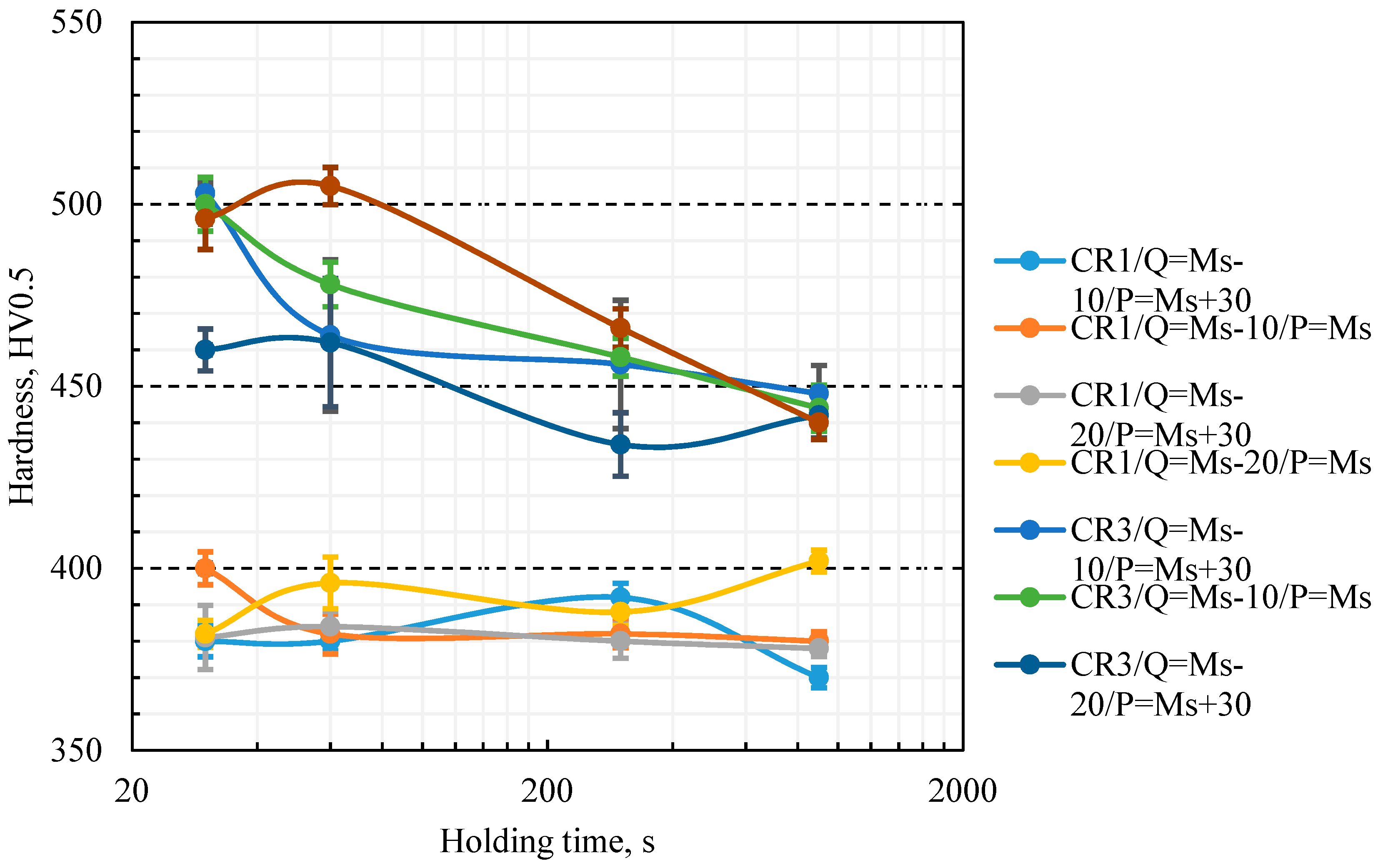
Microhardness results together with standard deviation values of Gleeble simulated samples are plotted in
Figure 3 and
Figure 4.
Figure 3 presents data for samples that experienced a quench stop above
Ms (i.e., austempering treatment) and
Figure 4 shows data for samples with a quench stop below
Ms (i.e., the QP treatment). The times for bainite formation at
Ms for steel CR1 and at
Ms + 30 °C for steel CR3 are given in
Table 2. These values were determined by dilatometric measurements. The hardness values for fully bainitic structures were in the ranges 370–410 HV for steel CR1 and 450–470 HV for steel CR3 as measured from the dilatometric test samples. The hardness values of the Gleeble-treated samples in
Figure 3 lay in the typical ranges of fully bainitic structures for the stated carbon levels in the steels. The effect of holding time on hardness remains difficult to analyze for 0.15C steel CR1 after isothermal transformation, but in general the lower the holding temperature is, the higher the hardness achieved. All hardness values of steel CR1 lay close to the expected values for a bainitic structure,
Figure 3. For the 0.26C steel CR3, longer holding times (10–25 min) gave results expected for a fully bainitic structure, whereas the shortest (5 min) holding time resulted in hardness values that exceeded the expected level for just bainite and, most likely is a result of untempered martensite formation during final cooling,
Figure 3.
Quenching with a cooling stop below
Ms followed by isothermal heat treatment resulted in the expected hardness typical of lower bainite for all 0.15C CR1 specimens,
Figure 4. For 0.26C CR3 steel the shortest holding time (0.5 min) appears to be insufficient for complete bainite transformation (
Figure 4), whereas a longer holding time led to the expected hardness typical of lower bainite. Isothermal heat treatment at
Ms appears to require a slightly longer time than the transformation at
Ms + 30 °C, obviously due to slower kinetics at lower temperature. No appreciable difference can be seen following quenching to
Ms − 10 °C or
Ms − 20 °C in respect of final hardness. For the quenching and post heat treatment process the initial formation of martensite appears to accelerate bainite transformation noticeably. There are also indications that bainite transformation is more rapid at a holding temperature of
Ms + 30 °C than at
Ms, though the results can become complex with the occurrence of other microstructural mechanisms, such as carbon partitioning and stabilization of a small fraction of austenite. The process variant selected for further investigation was die quenching to
Ms − 10 °C and subsequent post heat treatment at
Ms + 30 °C for 5 min before cooling to room temperature. The microstructures of steels CR1 and CR3 after Gleeble simulation by quenching to
Ms − 10 °C followed by holding at
Ms + 30 °C for 5 min are shown in
Figure 5. Both microstructures essentially consist of bainite and a small fraction of tempered martensite.
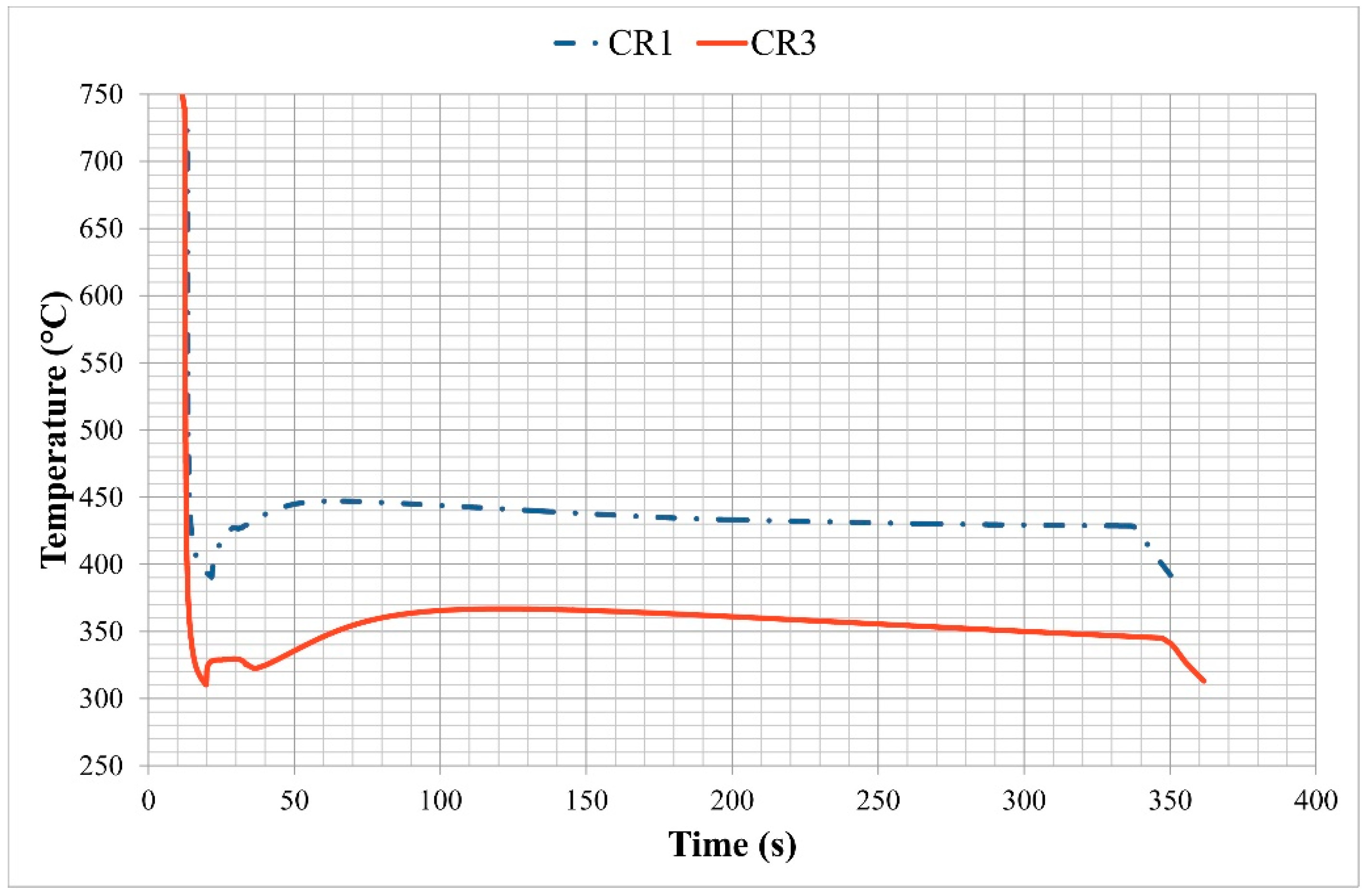
Temperature measurements from the pressed hat profiles showed that the latent heat of transformation is released during the formation of martensite. Some latent heat release was also observed during the bainite transformation, see
Figure 1. However, the targeted quench stop temperatures were achieved within about 10 °C. For most of the trials, the partitioning temperature was set lower than the targeted temperature (
Ms + 30 °C) to account for the latent heat generation and the corresponding increase in temperature. The highest temperatures caused by latent heat generation were
Ms + 60 °C. The targeted temperatures were achieved after approximately 5 min of isothermal holding in the case for the CR1 steel profiles. However, the partitioning temperature for CR3 steel profiles were estimated to be about 5–15 °C lower than the target value.
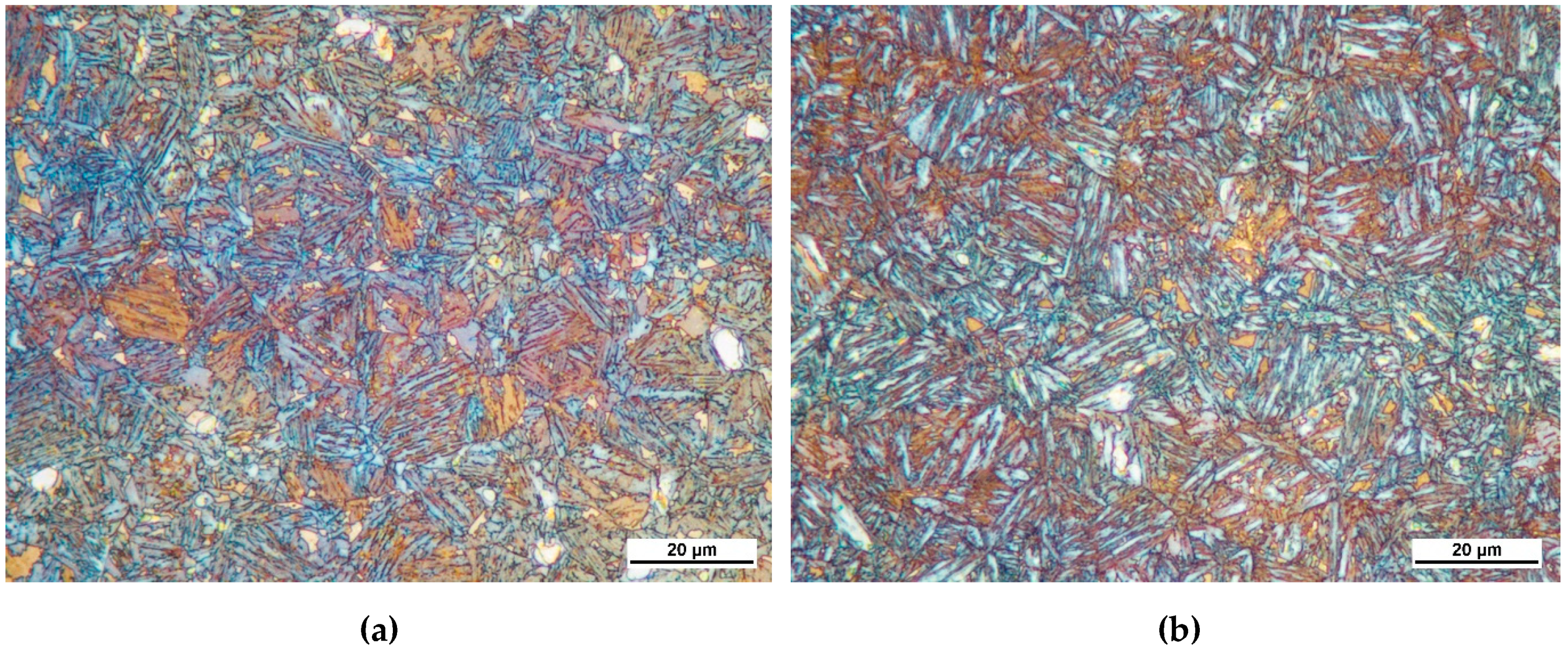
The microstructure obtained at the top of the profiles in CR1 and CR3 steels are shown in
Figure 6. Since it was not possible to distinguish between bainite, tempered martensite and retained austenite from the Nital etched samples, LePera etchant was used to reveal the microstructures. Following such etching, the white spots seen in the microstructure were identified as austenite or untempered martensite [
25]. Tempered martensite appeared as slightly brown-colored constituent and bainite often appeared as the blue-colored constituent.
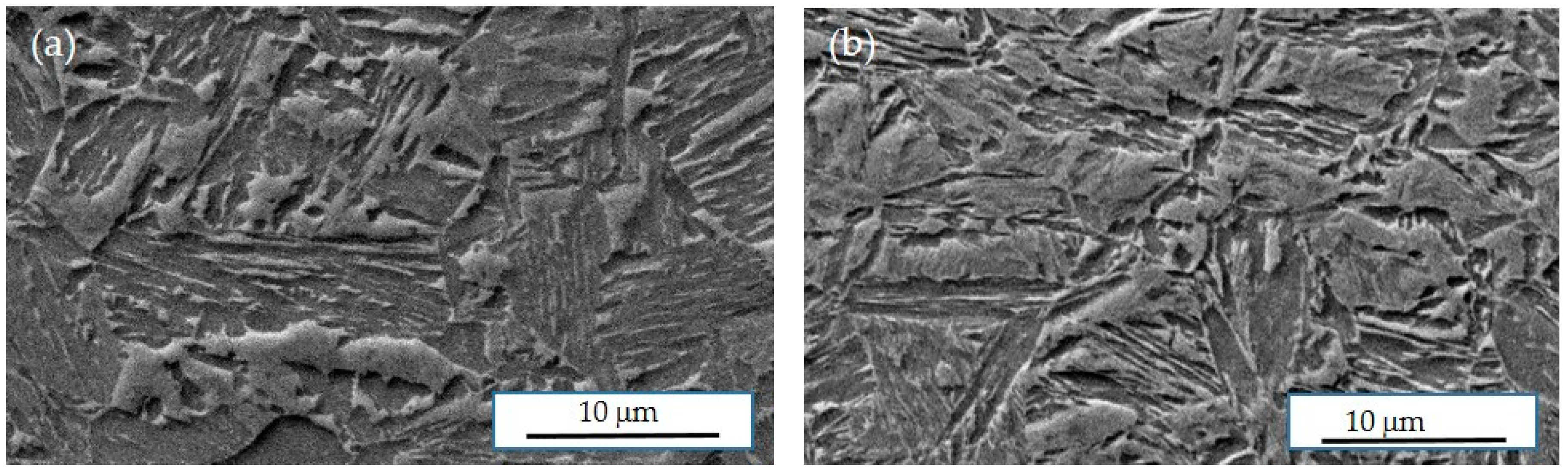
Typical SEM images from hat profiles are displayed in
Figure 7. It is seen that the microstructures comprise multiple phases such as a fine mixture of lath-like ferrite, retained austenite and some tempered martensite, and possibly also some untempered martensite formed during the final cooling.
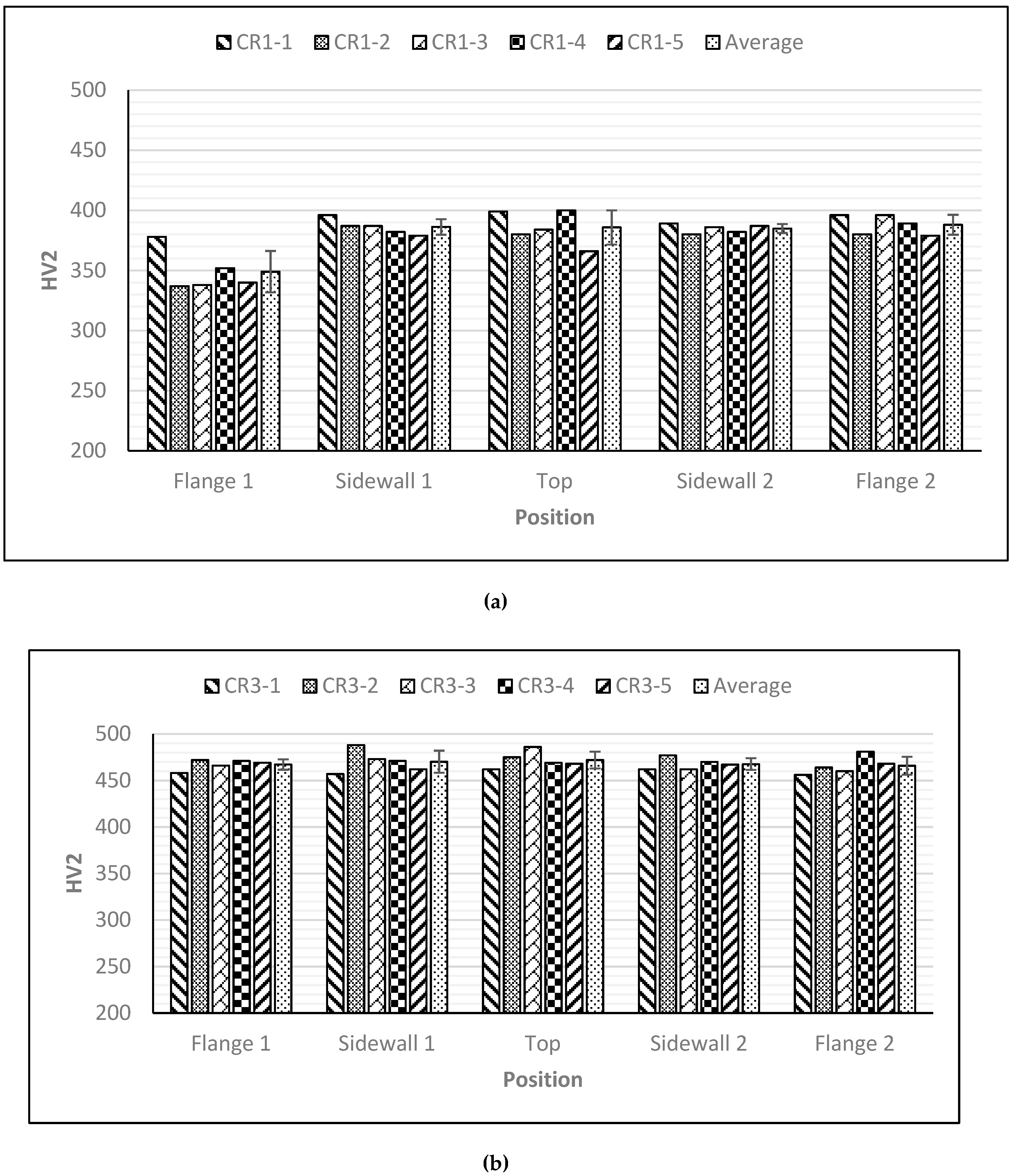
The volume fraction of retained austenite was determined by analysis the of X-ray diffraction data using two samples of each material. Accordingly, steel CR1 contained about 12% retained austenite and steel CR3 about 17%. Hardness values at different positions on the five hat-shaped profiles of each steel sort (according to
Figure 2) are shown in
Figure 8. Average values containing standard deviations are included for each position of the hat profiles in
Figure 8.
The average values of three measurements at each position are presented. The expected hardness values for bainite were achieved at all locations in the hat profile specimens of steel CR1, except for flange 1. This was presumably caused by slower cooling in that part of the die, which resulted in some ferrite formation. For steel CR3 the hardness values lie between 455 and 475 HV
2, which are close to the expected hardness of bainite (~450–470 HV) for this steel, as measured on isothermally treated samples. A few specimens showed higher hardness, up to 488 HV
2. It can be seen that the part with the lowest quenching stop temperature, CR3-5, has the most uniform hardness,
Figure 8.
Tensile properties were determined for the top sections of all the manufactured hat profiles.
Figure 9 shows a comparison between the tensile tests properties of CR1 and CR3 together with uncoated commercial boron 22MnB5 steel currently in use for press hardening. The presented values are the average of 10 measurements for steel CR1 and 5 measurements for steel CR3.
The targeted yield (1000–1300 MPa) and tensile (1400–1700 MPa) strength values were not achieved in the 0.15C CR1 steel. Though the targeted yield strength (1036–1093 MPa) was achieved in 0.26C CR3 steel, but the tensile strength was still below the target (1323–1404 MPa). On the other hand, the elongation to fracture for both steels (average values of 8.5% and 7.4% for CR1 and CR3 steels, respectively) was somewhat higher, and better than the targeted value of
A50mm > 5%. The measured total elongation was 4.9% for the tested 22MnB5 reference steel, which agrees well with the values found in the literature, see
Table 1. Furthermore, tensile tests were performed on sheets subjected to isothermal treatments, CR1 at 400 °C for 15 min and CR3 at 350 °C for 30 min to obtain reference values for bainitic structures of the steels. The elongation to fracture was higher for these samples in comparison to those of the hat profiles (CR1: 15.8% and CR3: 12.2%). The strength values of CR1 steel were approximately the same as for the hat profile, but for the CR3 steel the yield and tensile strength values were slightly higher for the isothermally treated samples. The shorter gauge length, 25 mm instead of 50 mm, for the austempered tensile test samples was identified as one reason for the difference in elongation to fracture.
5. Discussion
The hardness values and microstructures of the Gleeble simulated samples have been found to be similar to those of the final press hardened hat profiles. This indicates that physical simulation is a reliable means of determining press hardening parameters to achieve a desired microstructure. One complication encountered in press hardening of hat profiles was the increase in temperature caused mainly due to the exothermic heat generated by the decomposition of austenite to martensite and/or bainite. The isothermal transformations at different temperatures presented in
Figure 3 show that the time for completion of bainitic transformation is temperature dependent, and that longer holding time leads to higher fraction of bainite and it can also lead to more carbon enriched austenite and a decrease in the dislocation density by recovery mechanisms. Other microstructural mechanisms such as isothermal martensite formation below
Ms, carbide precipitation and carbon partitioning from supersaturated martensite or bainite to austenite can also take place, as reported in literature [
26]. The hardness values shown in
Figure 4 for the Gleeble samples quenched to a temperature below
Ms followed by a heating at a temperature above
Ms, show that the initial martensite formation shortens the time necessary for the bainite transformation. Two mechanisms that also influence the hardness value are the tempering of the initial martensite formed and the martensite that can be formed after final cooling to room temperature.
It is reasonable to assume that microstructural changes occurring during isothermal holding are essentially diffusion controlled, thus following a relationship similar in form to that of the Larson–Miller parameter LMP [
27]. Here the diffusion equation
D =
D0exp(−
Q/(
RT)) is used to calculate an effective ‘time-diffusivity’
tD (the sum of the individual diffusivities over the thermal cycle following quenching to desired temperature).
D is diffusivity (m
2/s),
D0 is a constant (m
2/s),
Q is the activation energy of diffusion (J/mol), and
R is the gas constant (8.31 J/molK). The time interval used in the calculation of
tD is from the point the samples reached their lowest temperature on quenching (
QT) to the point after isothermal treatment when the specimens had cooled to 200 °C, see Equation (1).
A pre-exponential diffusivity constant
D0 of 2.3 × 10
−5 m
2/s and an activation energy
Q of 148 kJ/mol for carbon diffusion in austenite were used in these calculations [
28]. The calculations may be used for an approximate comparison with the experimental data, but they do not take into account accurately the effect of the high silicon content on diffusivity in the steel.
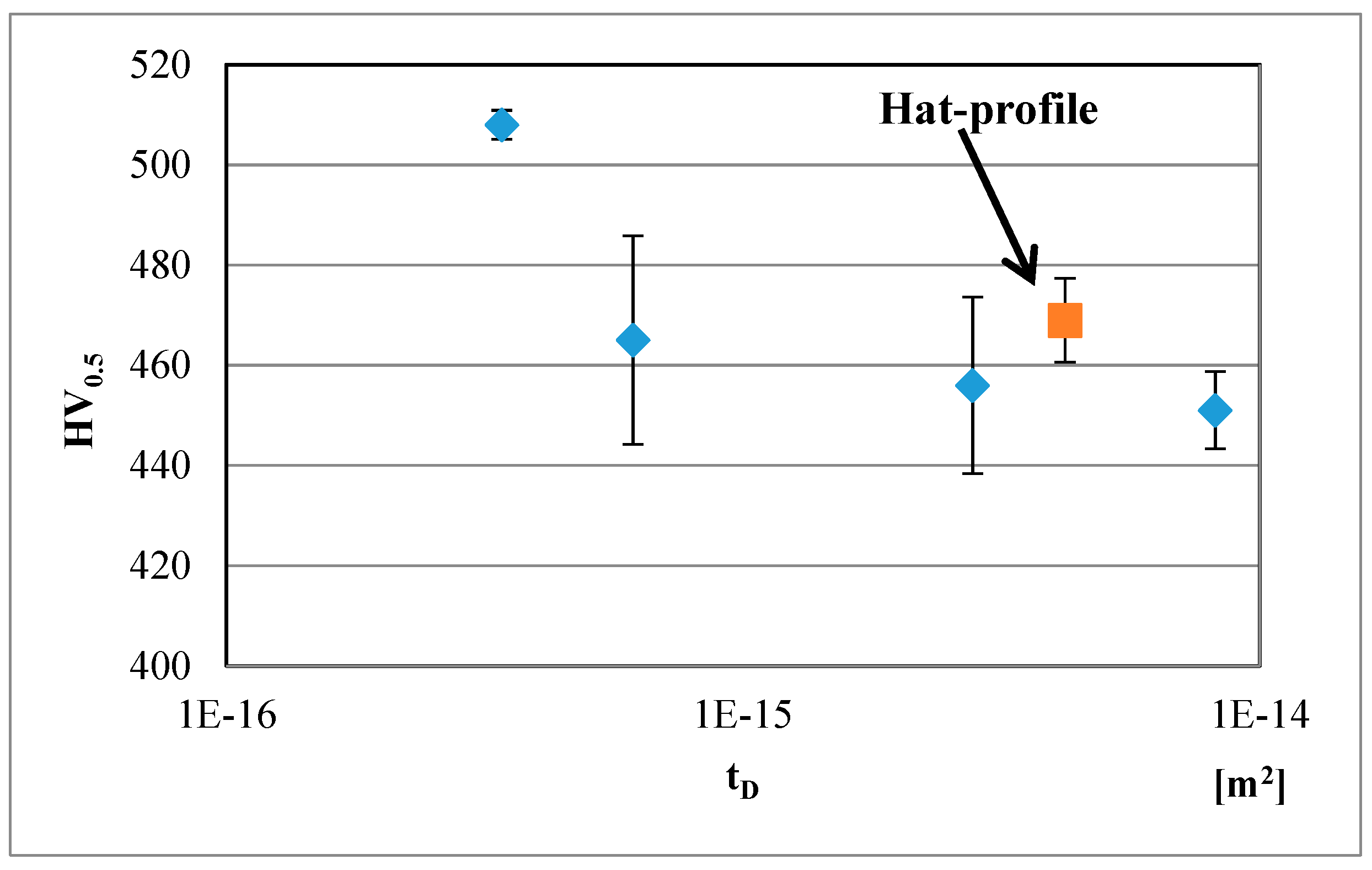
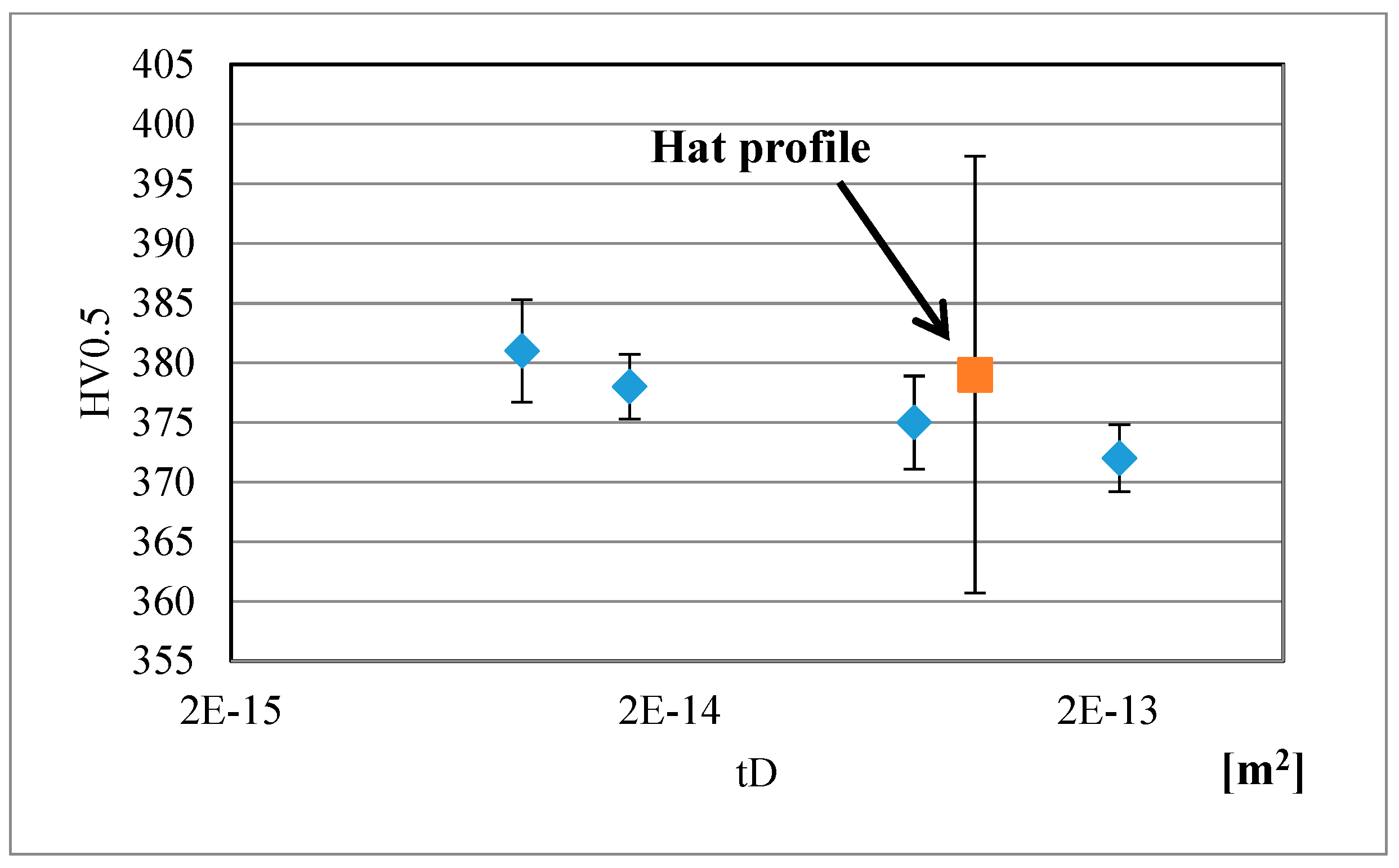
Figure 10 and
Figure 11 present variation of hardness with
tD for the Gleeble simulated QP type heat-treatments with a quench stop temperature of M
s − 10 °C and subsequent isothermal holding at M
s + 30 °C, carried out on CR3 and CR1 steels, respectively. The corresponding hardness values of the hat profiles are also included in the figures. As expected, a reduction in hardness with an increase in
tD is seen. It is clear that the hardness data for the press hardened hat profiles lie close to those obtained on the CR3 and CR1 steels subjected to equivalent Gleeble simulations, see
Figure 10 and
Figure 11. The calculations confirm the usefulness of the method for obtaining a rough estimate of the achievable hardness, though the results can easily be affected by the deformation applied in press hardening. The hardness after press hardening trials are 5–10 HV points higher in comparison with the Gleeble simulated samples. As stated above, one possible explanation could be the influence of the forming operation on the
Ms temperature during sheet deformation [
29]. The large standard deviation for CR1 sample probably is, caused by slow cooling of flange 1, resulting in ferrite formation, see
Figure 8a.
A comparison of the hardness data presented in
Figure 3 for bainitic microstructures following austempering, with those presented in
Figure 4 for samples subjected to prior QP type treatment and
Figure 8 for different locations on hat profiles indicates that it is possible to shorten the isothermal holding time without the risk of obtaining excessive hardness in the steel. If the partitioning period is too short, during which only a small fraction of the austenite has transformed to bainitic ferrite and/or stabilized as high carbon austenite, untempered high carbon martensite may form on final cooling resulting in high hardness. The results for steel CR1 indicate that even the minimum holding time gives the targeted hardness value. By comparing the ‘time-diffusivity’ calculations for different samples, it can be seen that a holding time of 5 min for steel CR3 at 350 °C is equivalent to about 0.5 min holding time for steel CR1 at 430 °C. Similarly, a holding time of 15 min for steel CR3 is equivalent to a holding time of 1 min for steel CR1 at the same test temperatures (350 and 430 °C) for the two steels.
The quench stop temperatures and subsequent temperature–time holding combinations investigated in this study resulted in mechanical properties that are promising for industrial application. The strength of 0.15C steel CR1 hat profile is lower than that for a conventional press hardened profile of 22MnB5 boron steel. The yield strength of 0.26C steel CR3, however, is comparable with that of the boron steel, though the tensile strength is lower. The elongation to fracture is, however, superior in the CR3 steel compared with that of the press hardened boron steel. Liu et al. [
19] applied hot stamping with QP type treatment and obtained tensile strength and ductility of the order of 1500–1600 MPa and 6.6–14.8%, respectively, though the yield strength was limited to 655–850 MPa. Hence in the present work, a higher yield strength was obtained. The improvement in ductility shown in the novel press hardening process provides the possibility to produce safety related components for cars with a possibility of reduction in weight. The hardness values after Gleeble simulated austempering cycles,
Figure 3, show that the hardness values reach the same levels as the fully austempered samples measured by dilatometry after 10–15 min. The Gleeble cycles simulating different QP type cycles reach same hardness levels after 1–5 min. The possibility of time reduction for processing CR1 hat profile is from ca. 8 min to between 1 and 5 min, and for sample CR3 from 23 to 1–5 min. The special QP type process simulated in Gleeble experiments and the subsequent production of the hat profiles created opportunities to shorten the processing time considerably in comparison to typical times of the conventional austempering process. The tests have also shown that the inevitable variations in the processing cycle with regard to reaching the desired quench stop temperature and subsequent holding to complete bainitic transformation in actual industrial component forming operation do not influence the properties of the final product significantly. On the other hand, a quick comparison of the strength and ductility properties reported in the literature for CFB and QP steels, as shown in
Table 1, reveals that the same levels of high strength and good ductility cannot be attained in press hardening, using the QP type treatment for realizing CFB microstructures in steels.
The novel press hardening technique for steels with increased Si content presented here together with the use of a QP type treatment to obtain a final CFB microstructure is shown to result in a combination of mechanical properties comparable with those of existing boron steels. It can be emphasized, however, that with further investigations of process variables, on the industrially produced sheets, and careful optimization of chemical composition to realize CFB microstructures for this kind of application are likely to provide property combinations far superior to those of boron bearing steels and closer to those obtained in CFB steels and QP-processed steel sheets themselves.