Effects of CeO2 on Viscosity, Structure, and Crystallization of Mold Fluxes for Casting Rare Earths Alloyed Steels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
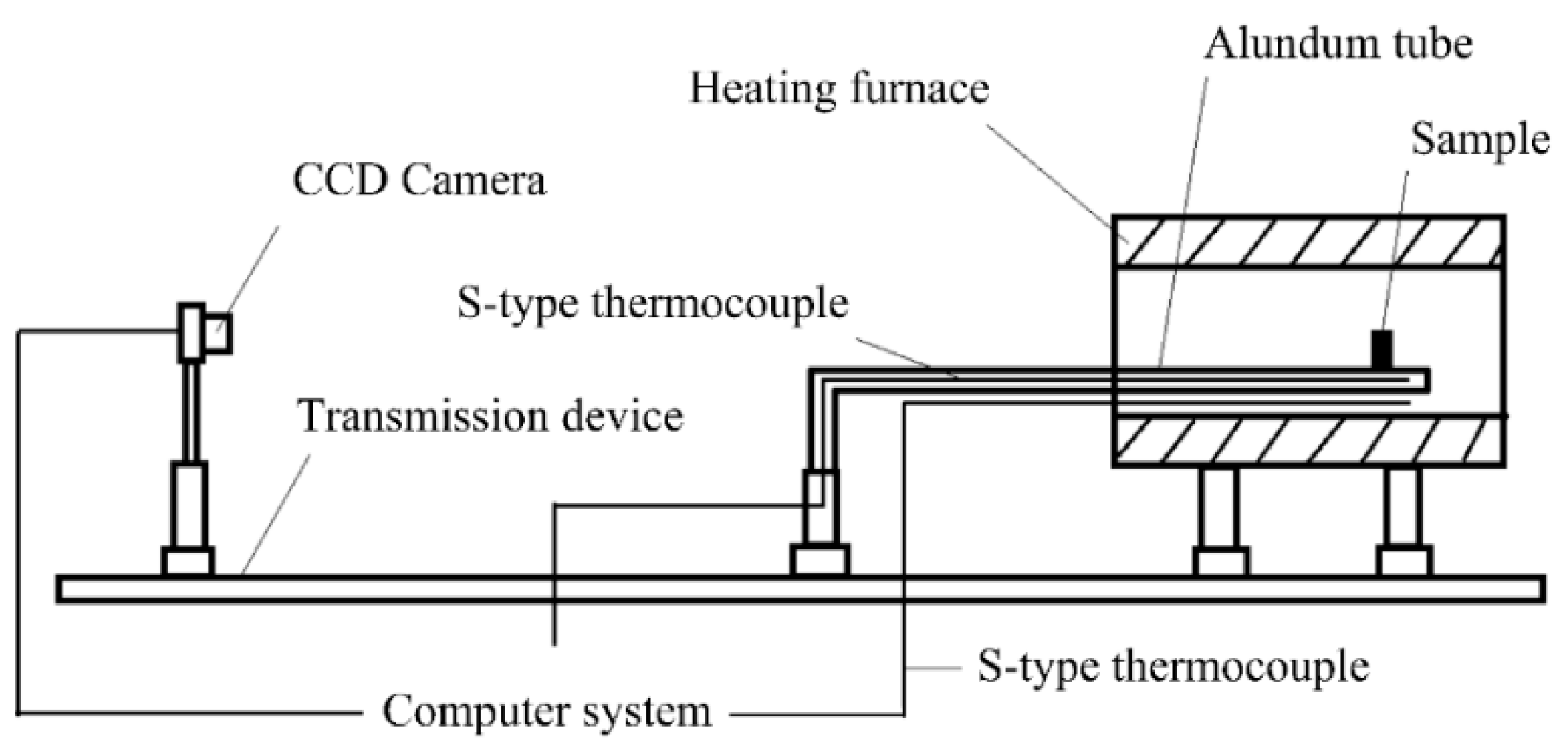
2.2. The Melting Temperature Test
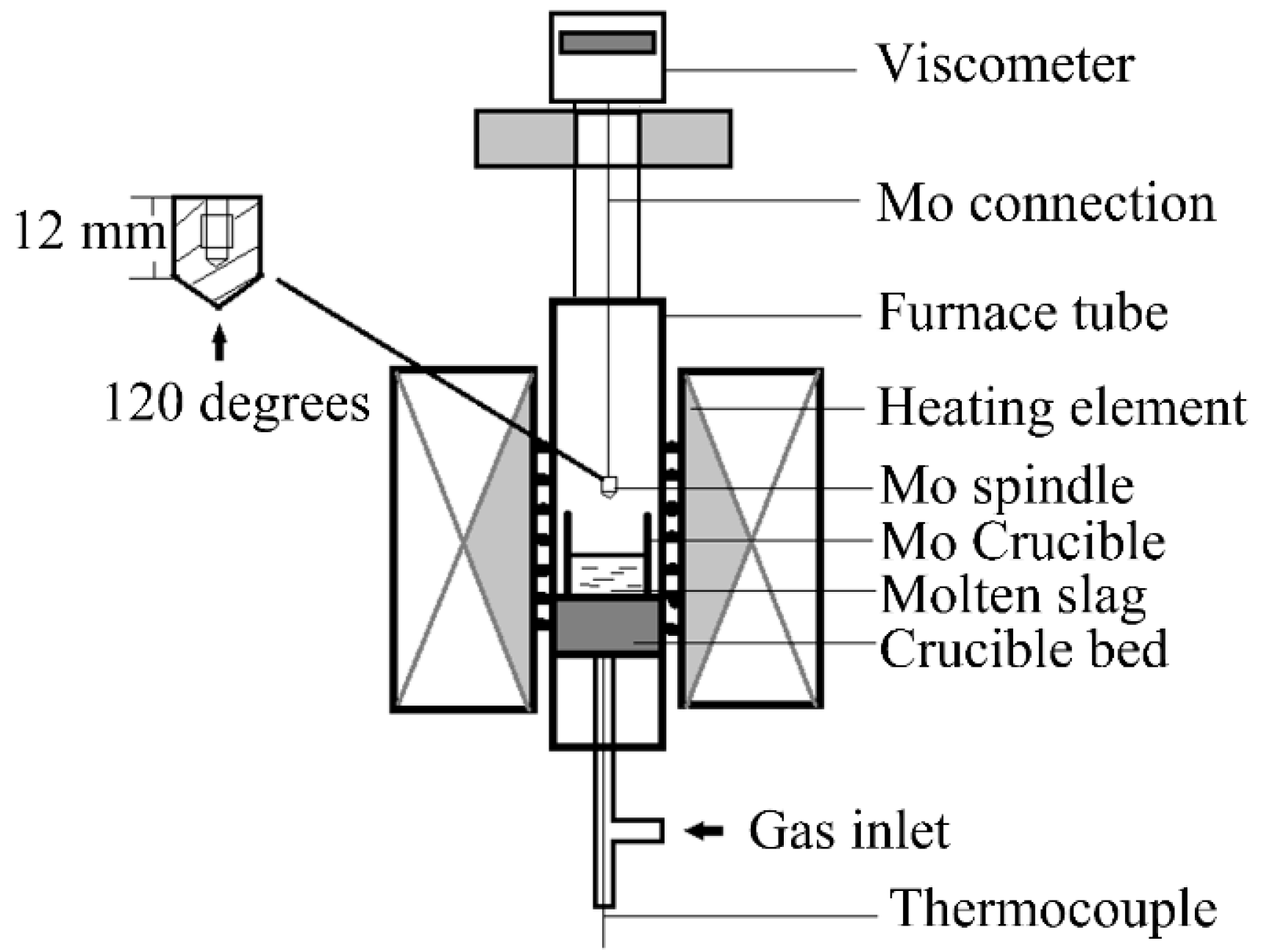
2.3. The Viscosity Test
2.4. The Structure Test
2.5. The Crystallization Test
3. Results and Discussions
3.1. Effects of CeO2 Content on Melting Temperature
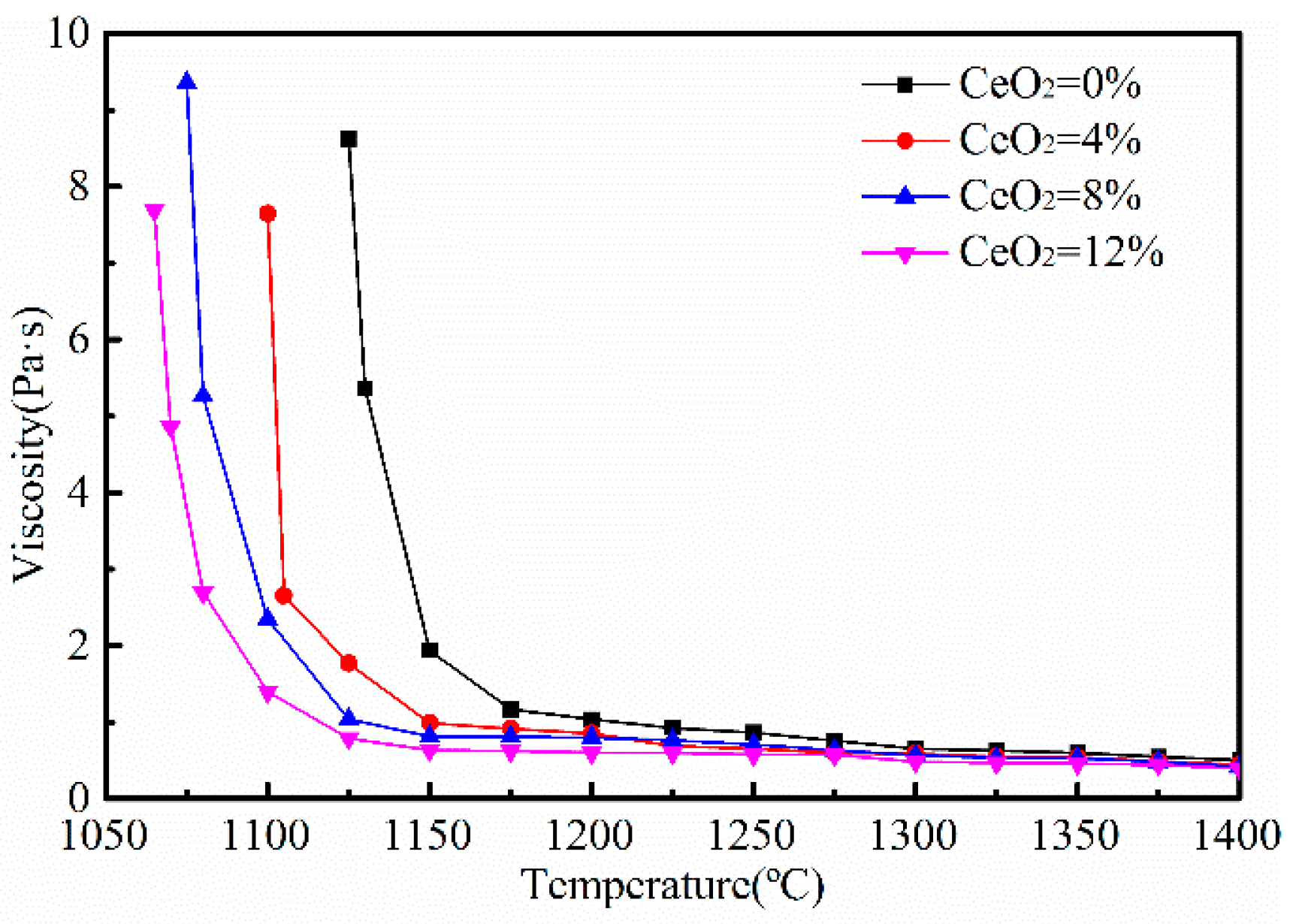
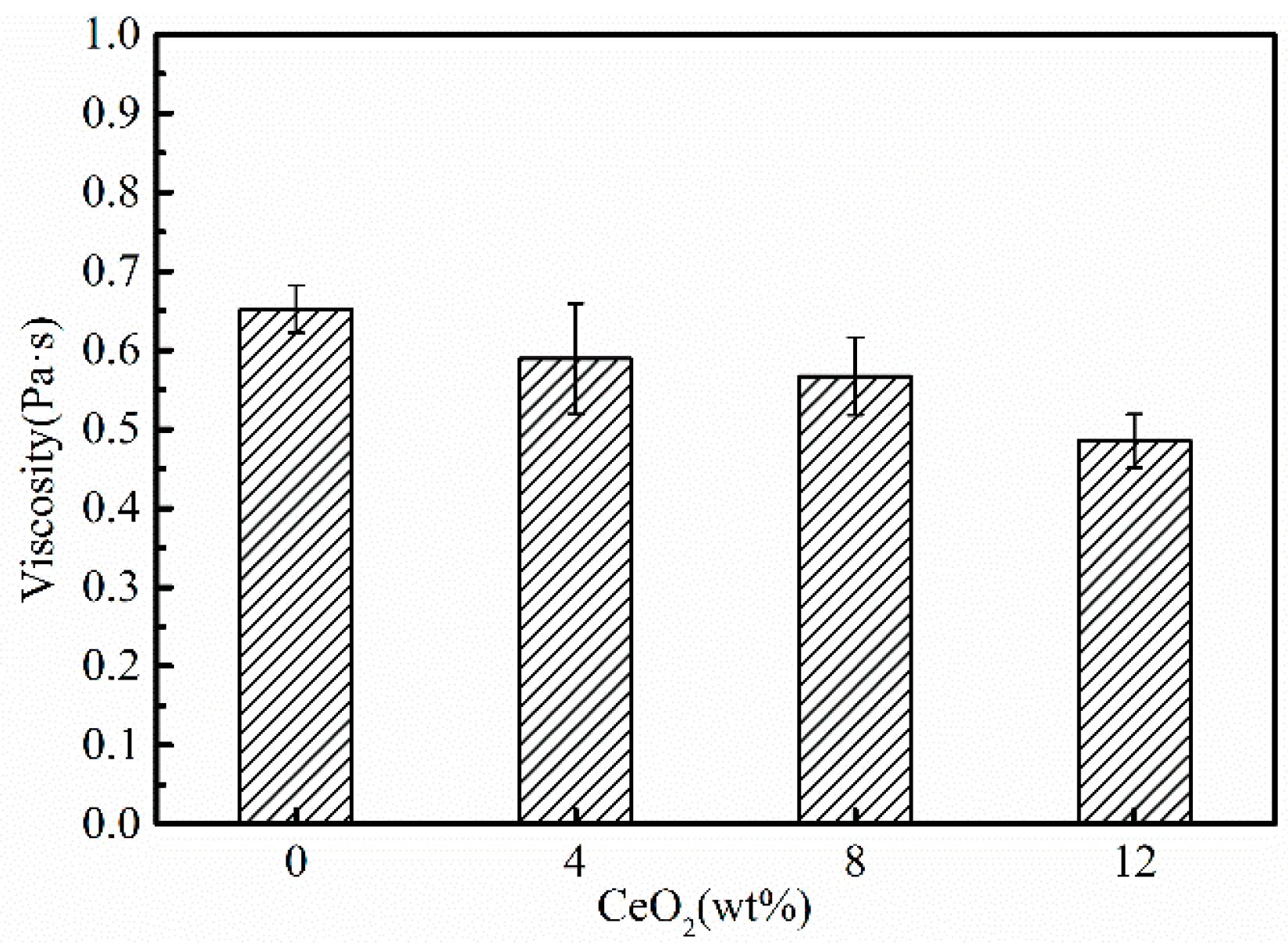
3.2. Effects of CeO2 Content on Viscosity
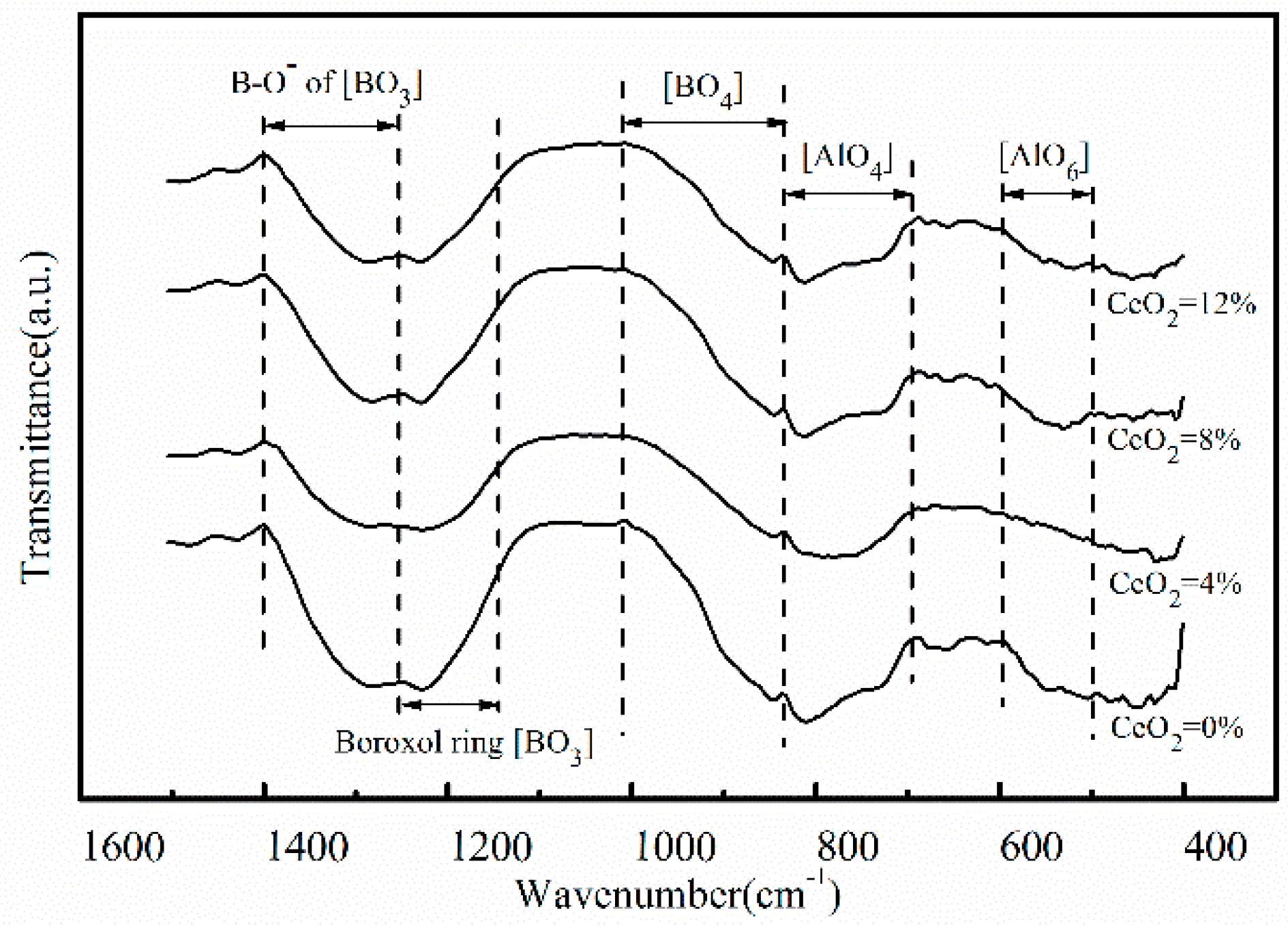
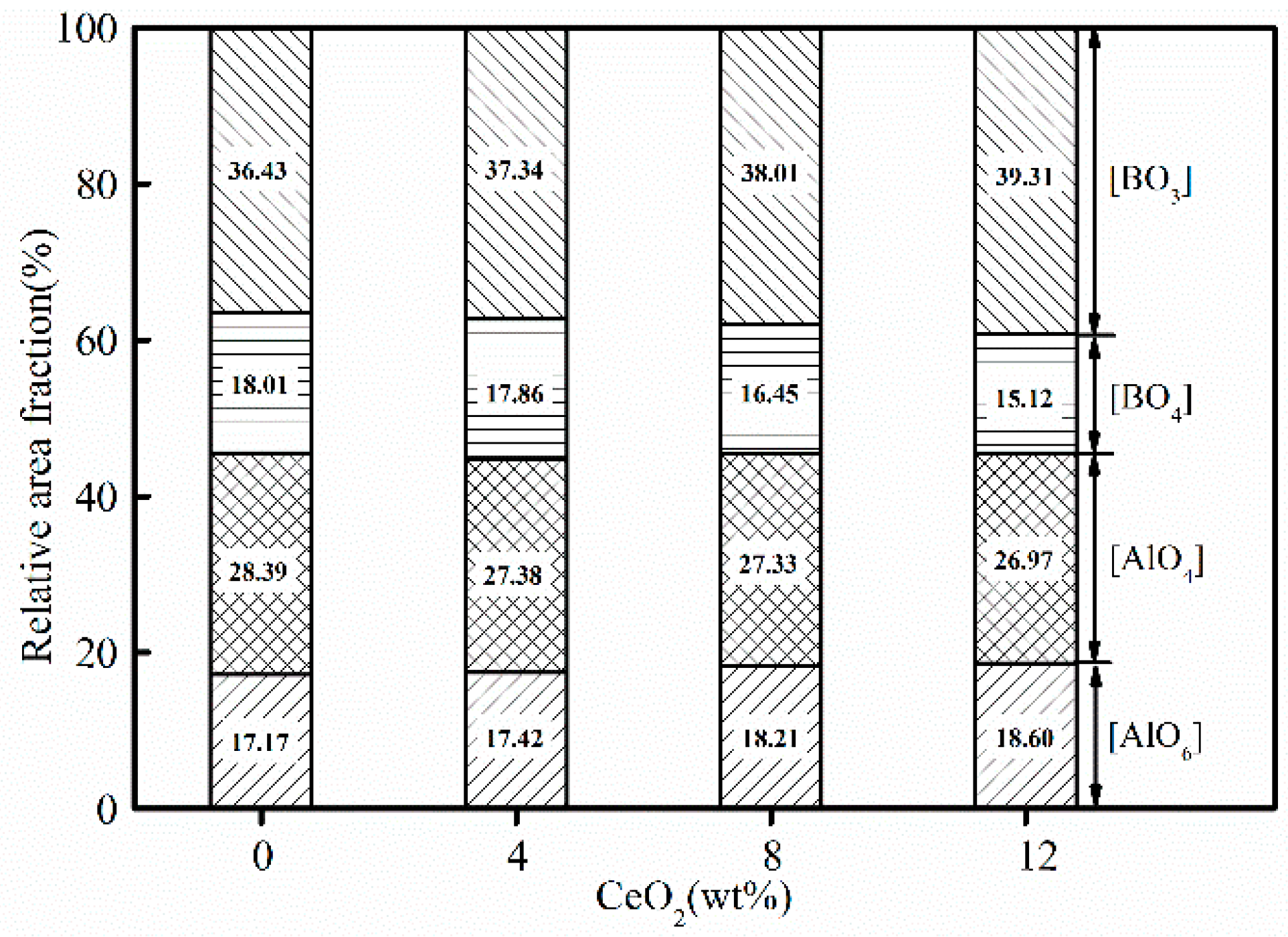
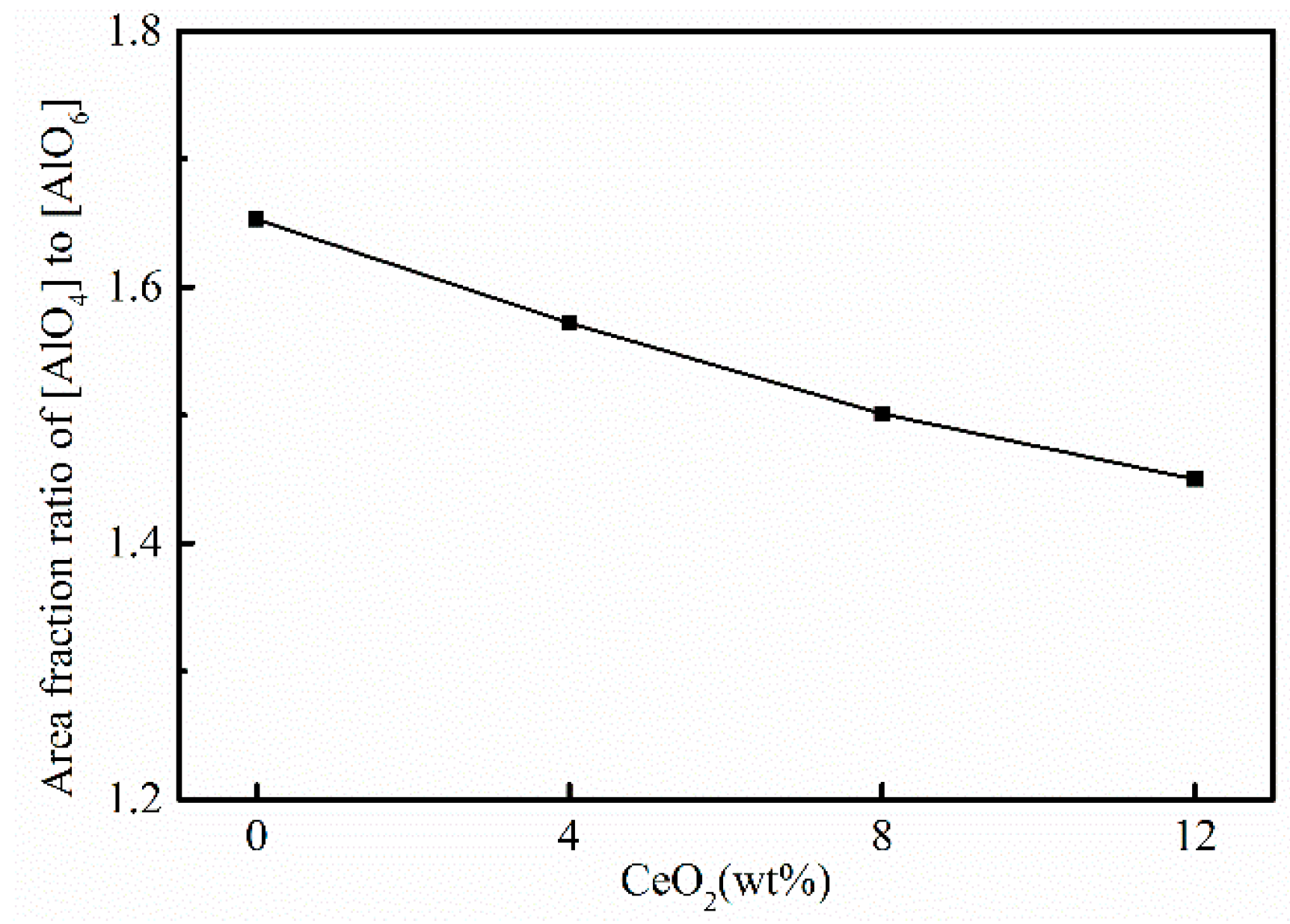
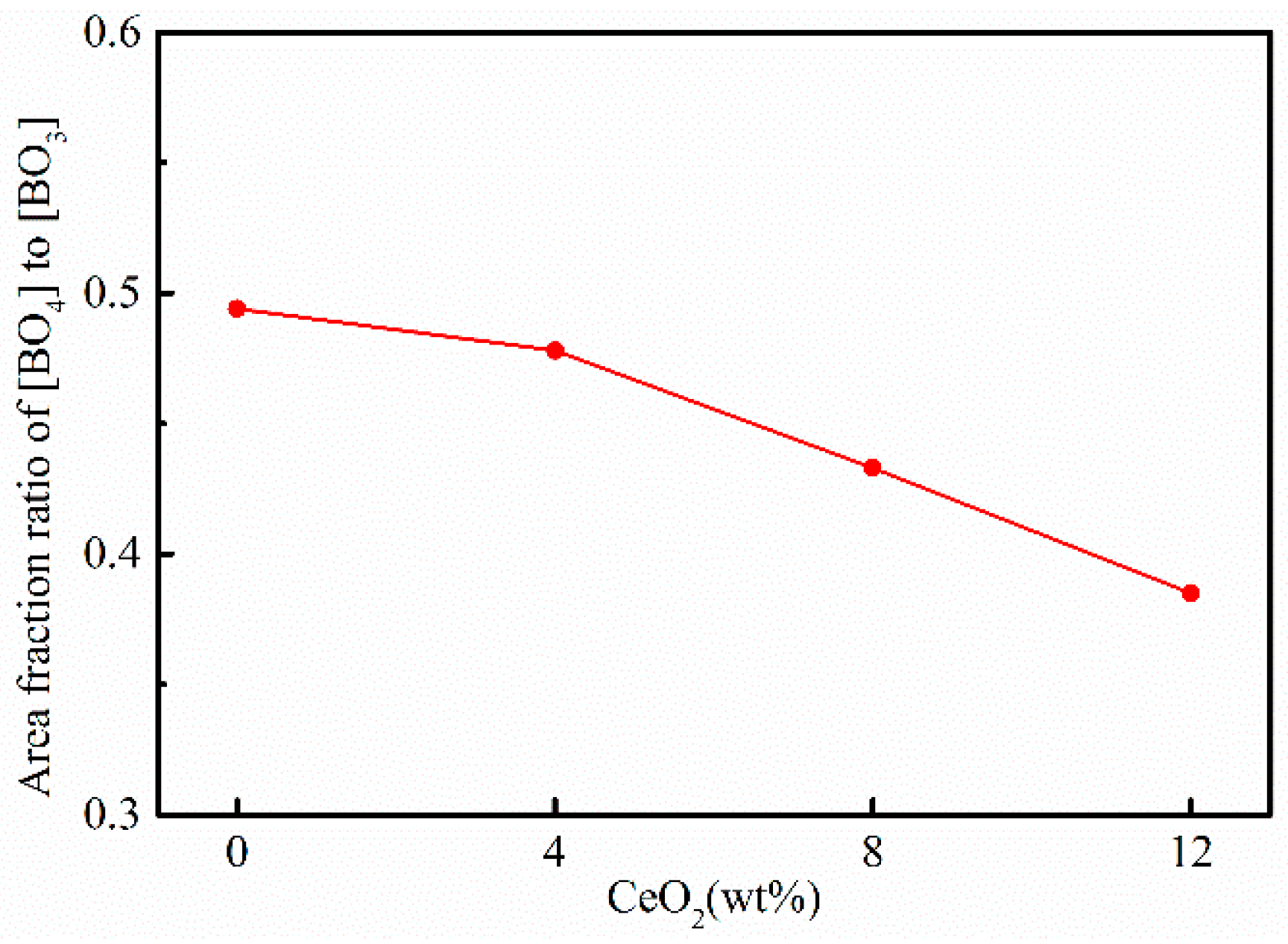
3.3. Effects of CeO2 Content on Microstructure
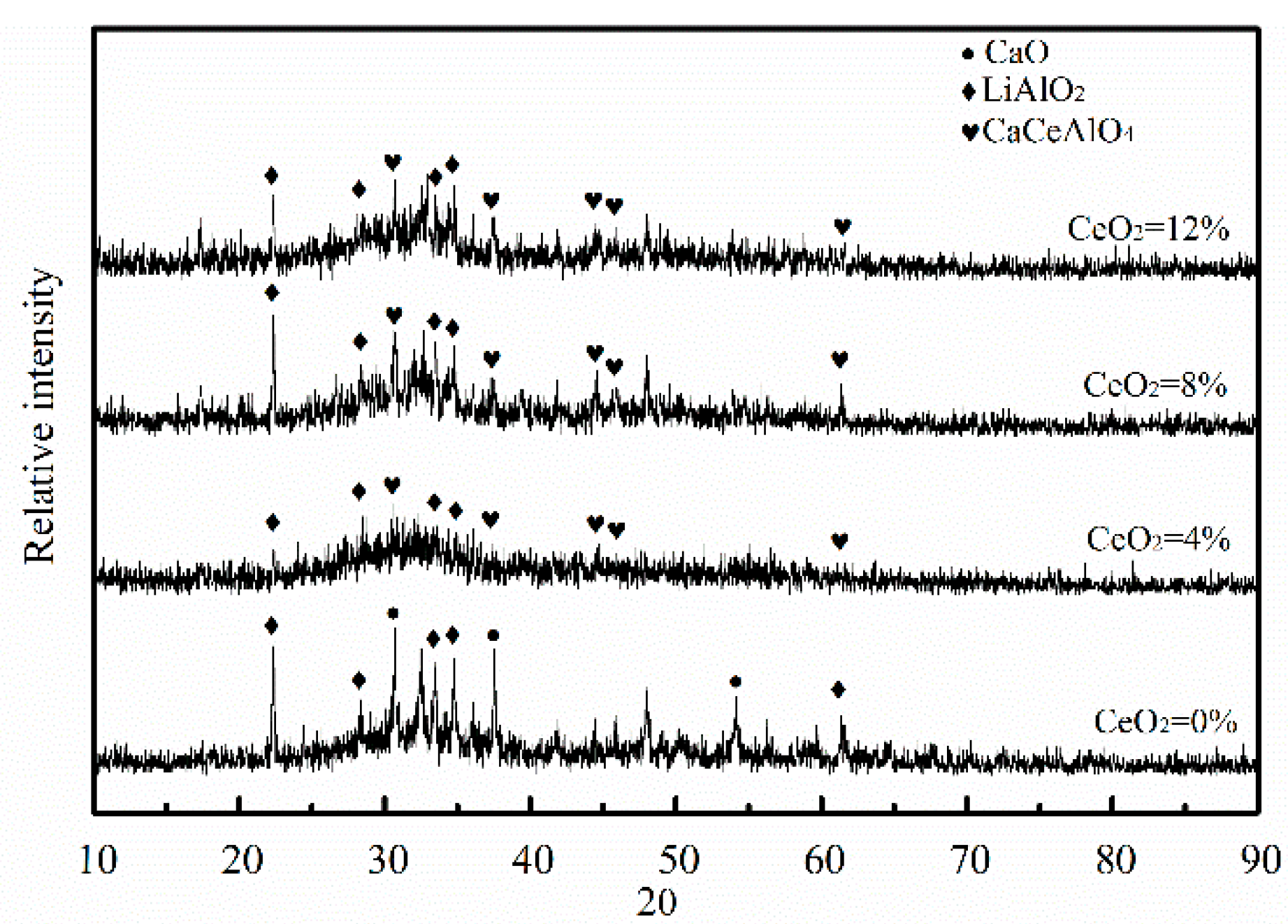
3.4. Effects of CeO2 Content on Crystallization
4. Conclusions
- The addition of CeO2 could increase the melting temperature, but decreased the viscosity and the break temperature of the mold fluxes for casting rare earth alloy heavy rail steels.
- CeO2 could increase the depolymerization of the melts, leading to a decrease in the viscosity of the mold fluxes above the break temperature.
- CeO2 could restrain the precipitation of CaO, but promoted the precipitation of CaCeAlO4, which decreases the break temperature.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.M.; Lin, Q.; Ji, J.W.; Lan, D.N. New study concerning development of application of rare earth metals in steels. J. Alloy. Compd. 2006, 48, 384–386. [Google Scholar] [CrossRef]
- Zhou, X.F.; Yin, X.Y.; Fang, F.; Jiang, J.Q.; Zhu, W.L. Influence of rare earths on eutectic carbides in AISI M2 high speed steel. J. Rare Earth. 2012, 30, 1075–1078. [Google Scholar] [CrossRef]
- Xing, X.G.; Han, Z.J.; Wang, H.F.; Lu, P.N. Electrochemical corrosion resistance of CeO2-Cr/Ti coatings on 304 stainless steel via pack cementation. J. Rare Earth. 2015, 33, 1122–1128. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, L.J.; Chou, K.C. Effect of cerium on the cleanliness of spring steel used in fastener of high-speed railway. J. Rare Earth. 2014, 32, 759–766. [Google Scholar] [CrossRef]
- Wang, D.Y.; Jiang, M.F.; Liu, C.J.; Shi, P.Y.; Yao, Y.; Wang, H.H. Effects of rare earth oxide on viscosity of mold fluxes for continuous casting. J. Rare Earth. 2005, 23, 68–72. [Google Scholar]
- Qi, J.; Liu, C.J.; Li, C.L.; Jiang, M.F. Viscous properties of new mold flux based on aluminate system with CeO2 for continuous casting of RE alloyed heat resistant steel. J. Rare Earth. 2016, 34, 328–335. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, C.; Wang, Y.C.; Dong, F.; Wu, M.Q. Influence of La2O3 on crystallization behavior of free-fluoride mold flux and heat transfer of slag films. J. Rare Earth. 2011, 29, 173–177. [Google Scholar] [CrossRef]
- Nichols, M.W.; Lingras, A.P.; Apelian, D. Viscosity characteristics of commercial fluxes for bottom poured ingots. In Proceedings of the Second International Symposium on Metallurgical Slags and Fluxes, Lake Tahoe, NV, USA, 11–14 November 1984; pp. 235–251. [Google Scholar]
- Seo, M.D.; Shi, C.B.; Baek, J.Y.; Cho, J.W.; Kim, S.H. Kinetics of isothermal melt crystallization in CaO-SiO2-CaF2-based mold fluxes. Metall. Mater. Trans. B 2015, 46, 2374–2383. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Leites, A.V.; Lrtvina, A.D.; Mogutnov, B.M. Investigation of the mould powder volatiles during continuous casting. Steel Res. Int. 1994, 65, 368–374. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.J.; Jiang, M.F. Properties investigation of CaO–Al2O3–SiO2–Li2O–B2O3–Ce2O3 mould flux with different w(CaO)/w(Al2O3) for heat-resistant steel continuous casting. Can. Metall. Quart. 2017, 56, 212–220. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.J.; Jiang, M.F. Role of Li2O on the structure and viscosity in CaO-Al2O3-Li2O-Ce2O3 melts. J. Non-Cryst. Solids 2017, 475, 101–107. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.J.; Jiang, M.F. Viscosity-structure-crystallization of the Ce2O3-bearing calcium-aluminate-based melts with different contents of B2O3. ISIJ Int. 2018, 58, 186–193. [Google Scholar] [CrossRef]
- Han, Z.H.; Xu, B.S.; Wang, H.J.; Zhou, S.K. A comparison of thermal shock behavior between currently plasma spray and supersonic plasma spray CeO2–Y2O3–ZrO2 graded thermal barrier coatings. Surf. Coat. Technol. 2007, 201, 5253–5256. [Google Scholar] [CrossRef]
- Sridhar, S.; Mills, K.C.; Afrange, O.D.C.; Lorz, H.P.; Carli, R. Break temperatures of mould fluxes and their relevance to continuous casting. Ironmak. Steelmak. 2000, 27, 238–242. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Q.; He, S.P.; Xu, J.F.; Long, X.; Liu, Y.J. Study on properties of alumina-based mould fluxes for high-al steel slab casting. Steel Res. Int. 2012, 83, 1194–1202. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. Structural investigation of CaO–Al2O3 and CaO–Al2O3–CaF2 slags via fourier transform infrared spectra. ISIJ Int. 2002, 42, 38–43. [Google Scholar] [CrossRef]
- Pernice, P.; Esposito, S.; Aronne, A.; Sigaev, V.N. Structure and crystallization behavior of glasses in the BaO–B2O3–Al2O3 system. J. Non-Cryst. Solids 1999, 258, 1–10. [Google Scholar] [CrossRef]
- McMillan, P.; Piriou, B. Raman spectroscopy of calcium aluminate glasses and crystals. J. Non-Cryst. Solids 1983, 55, 221–242. [Google Scholar] [CrossRef]
- McMillan, P.F.; Petuskey, W.T.; Coté, B.; Massiot, D.; Landron, C.; Coutures, J.P. A structural investigation of CaO-Al2O3 glasses via 27Al MAS-NMR. J. Non-Cryst. Solids 1996, 195, 261–271. [Google Scholar] [CrossRef]
- Kim, G.H.; Sohn, I. Role of B2O3 on the viscosity and structure in the CaO-Al2O3-Na2O-based system. Metall. Mater. Trans. B 2014, 45, 86–95. [Google Scholar] [CrossRef]
- Akagi, R.; Ohtori, N.; Umesaki, N. Raman spectra of K2O-B2O3 glasses and melts. J. Non-Cryst. Solids 2001, 293, 471–476. [Google Scholar] [CrossRef]
- Chryssikos, G.D.; Kamitsos, E.I.; Patsis, A.P.; Bitsis, M.S.; Karakassides, M.A. The devitrification of lithium metaborate: Polymorphism and glass formation. J. Non-Cryst. Solids 1990, 126, 42–51. [Google Scholar] [CrossRef]
- Frantza, J.D.; Mysen, B.O. Raman spectra and structure of BaO-SiO2, SrO-SiO2 and CaO-SiO2 melts to 1600 °C. Chem. Geol. 1995, 121, 155–176. [Google Scholar] [CrossRef]
- Mysen, B.O.; Frantz, J.D. Structure of silicate melts at high temperature: In-situ measurements in the system BaO-SiO2 to 1669 degrees C. Am. Mineral. 1993, 78, 699–709. [Google Scholar]


| Sample Number | Composition (wt. %) | |||||
|---|---|---|---|---|---|---|
| CaO | Al2O3 | Li2O | B2O3 | CeO2 | ||
| 1 | Calculated | 48.06 | 32.04 | 9.43 | 10.07 | 0 |
| Analyzed | 47.94 | 32.38 | 9.10 | 10.58 | 0 | |
| 2 | Calculated | 46.14 | 30.76 | 9.05 | 9.63 | 4.00 |
| Analyzed | 46.89 | 30.99 | 9.58 | 9.07 | 3.47 | |
| 3 | Calculated | 44.21 | 29.48 | 8.68 | 9.63 | 8.00 |
| Analyzed | 44.69 | 29.91 | 8.59 | 9.24 | 7.57 | |
| 4 | Calculated | 42.29 | 28.20 | 8.30 | 9.21 | 12.00 |
| Analyzed | 42.18 | 28.38 | 8.45 | 9.08 | 11.91 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Song, B.; Li, L.; Liu, Z.; Cui, X. Effects of CeO2 on Viscosity, Structure, and Crystallization of Mold Fluxes for Casting Rare Earths Alloyed Steels. Metals 2019, 9, 333. https://doi.org/10.3390/met9030333
Cai Z, Song B, Li L, Liu Z, Cui X. Effects of CeO2 on Viscosity, Structure, and Crystallization of Mold Fluxes for Casting Rare Earths Alloyed Steels. Metals. 2019; 9(3):333. https://doi.org/10.3390/met9030333
Chicago/Turabian StyleCai, Zeyun, Bo Song, Longfei Li, Zhen Liu, and Xiaokang Cui. 2019. "Effects of CeO2 on Viscosity, Structure, and Crystallization of Mold Fluxes for Casting Rare Earths Alloyed Steels" Metals 9, no. 3: 333. https://doi.org/10.3390/met9030333
APA StyleCai, Z., Song, B., Li, L., Liu, Z., & Cui, X. (2019). Effects of CeO2 on Viscosity, Structure, and Crystallization of Mold Fluxes for Casting Rare Earths Alloyed Steels. Metals, 9(3), 333. https://doi.org/10.3390/met9030333






