Inclusions Control and Refining Slag Optimization for Fork Flat Steel
Abstract
:1. Introduction
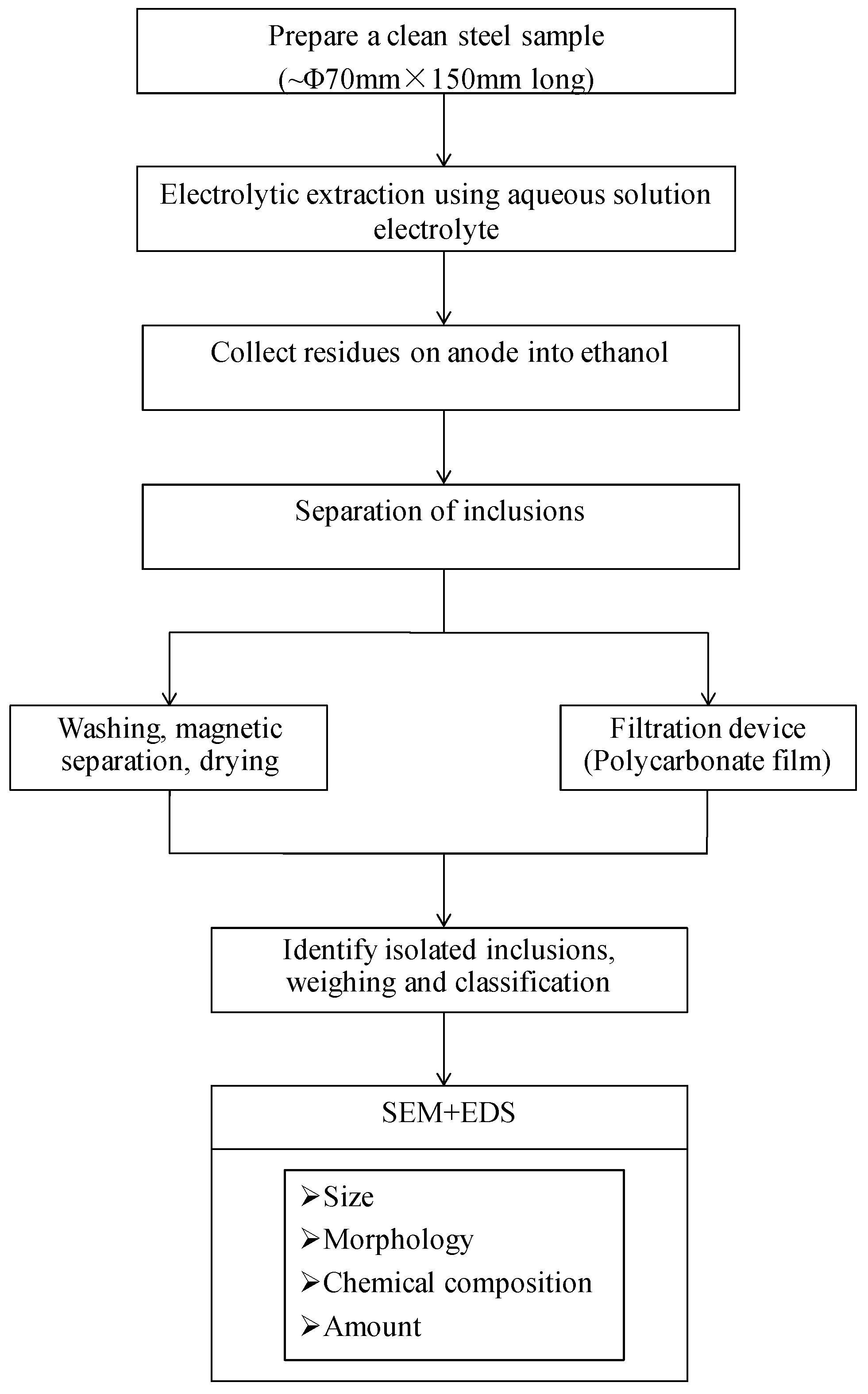
2. Materials and Experimental Methods
2.1. Fork Flat Steel Production Process
2.2. Experimental Methods
3. Analysis and Discussion
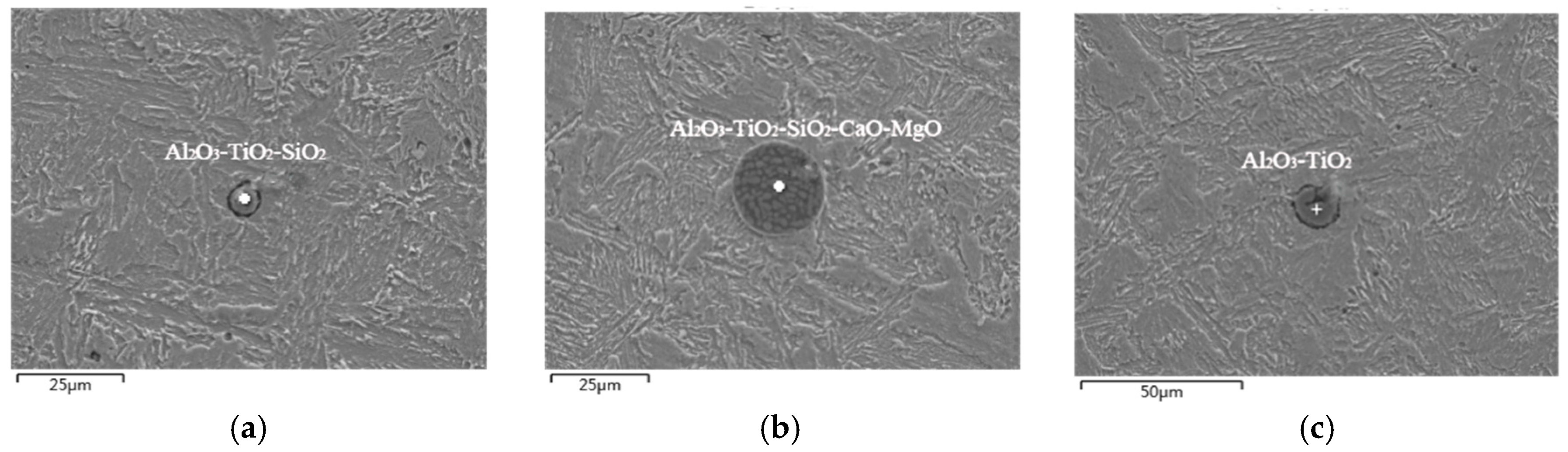
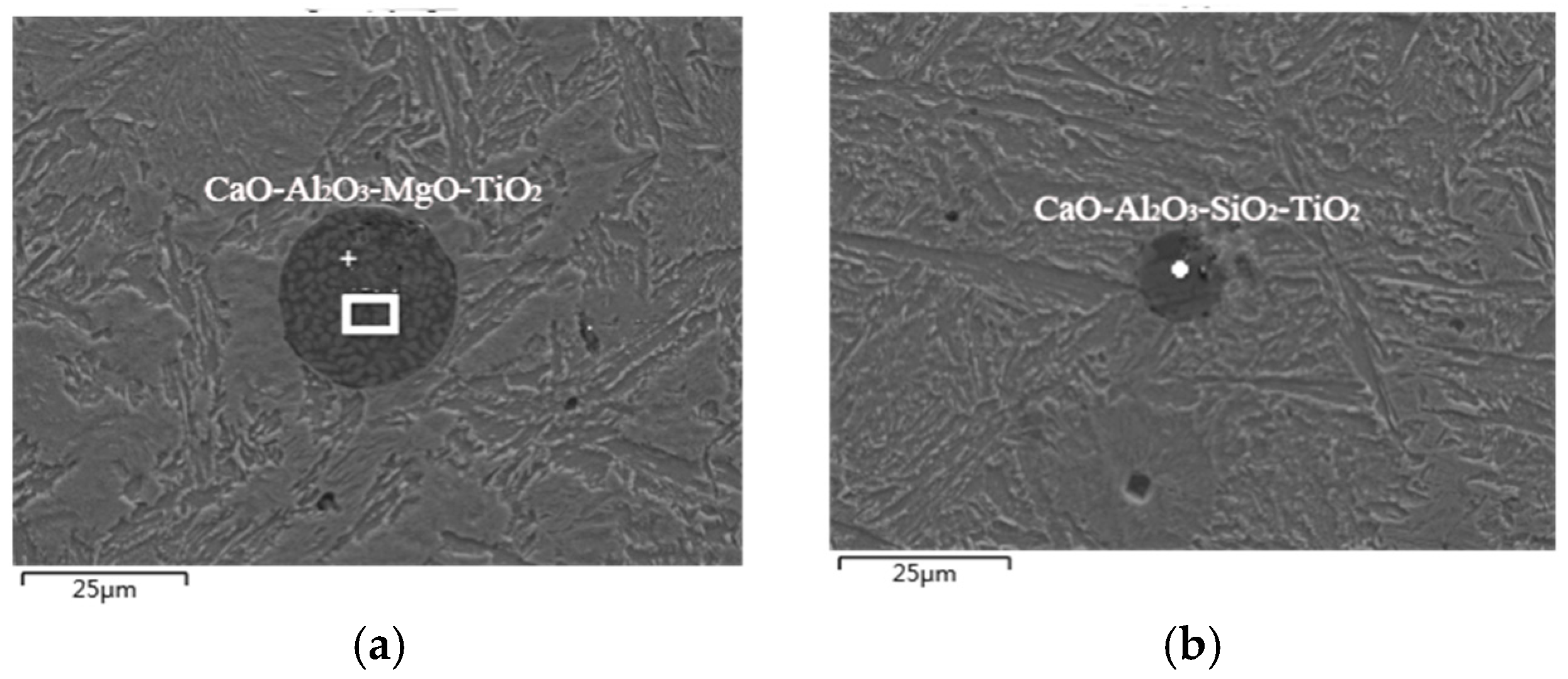
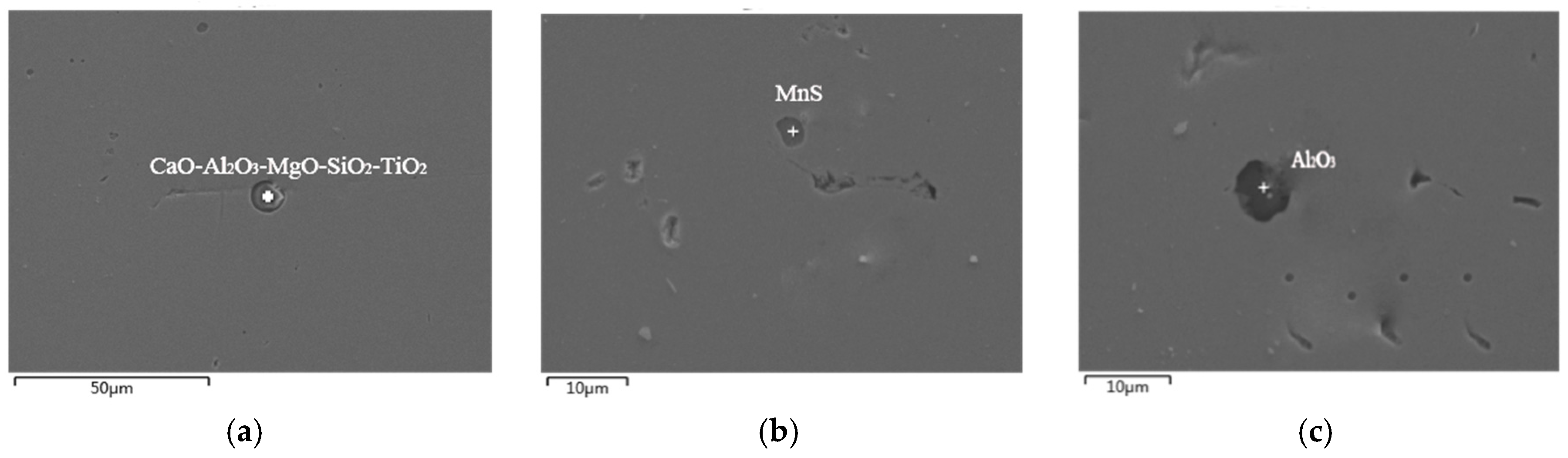
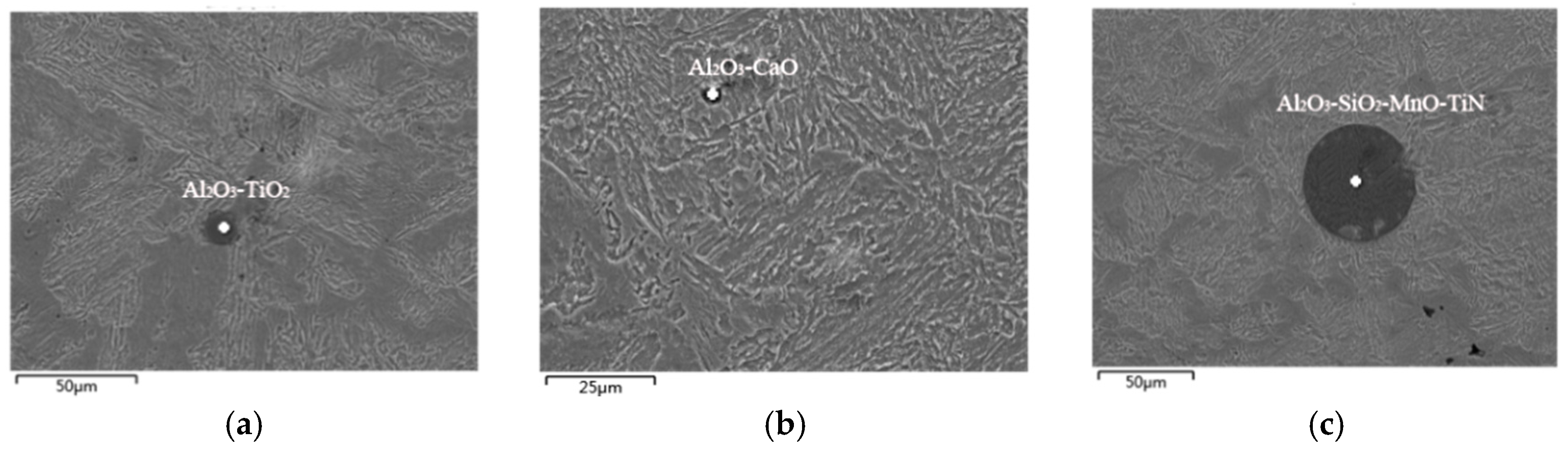
3.1. Exogenous Inclusions in Steel
3.2. Endogenous Inclusions in Steel
3.2.1. Pre-Refining of LF Furnace
3.2.2. Wire Feeding Process
3.2.3. Tundish Casting Process
3.2.4. Casting Billet
4. Control of Refining Slag and Precipitation Inclusions
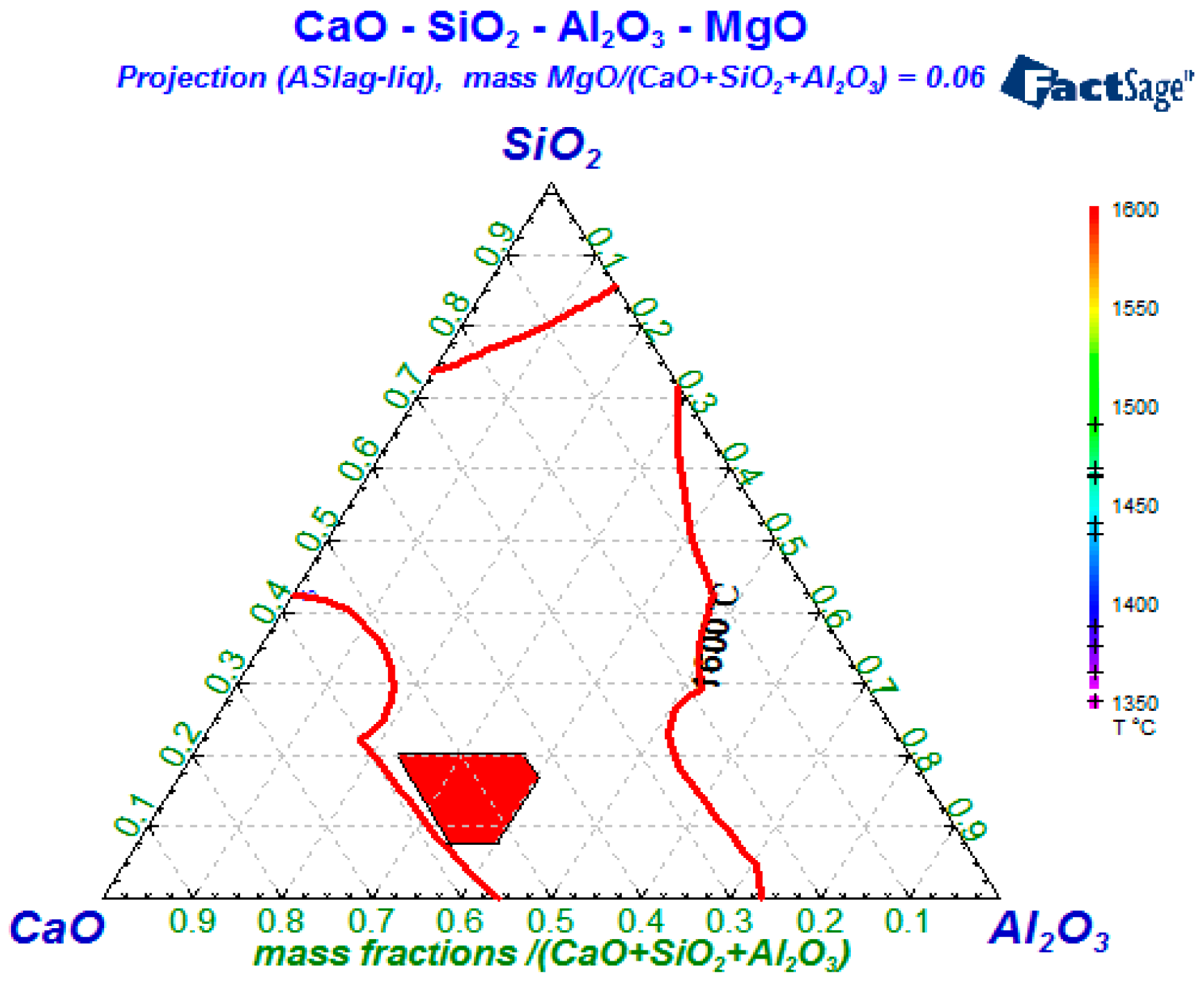
4.1. Compositions Control of Refining Slag
- (1)
- The melting-point of the final slag should be appropriate, i.e., lower than the temperature of molten steel at the tundish nozzle (about casting temperature −10 °C);
- (2)
- The refining slag should be fully reacted with the Al2O3 deoxidation product so that it can be assimilated and absorbed as much as possible. That is, the viscosity of refining slag and the initial activity of Al2O3 in the slag cannot be too large;
- (3)
- The slag should avoid chemical reactions with the steel, and the sulfur capacity should be as high as possible;
- (4)
- To ensure that the lining is not eroded by slag, the MgO content should be reduced as much as possible to reduce the melting point and viscosity of the slag. Wang et al. pointed out that the continuous increase of MgO content will reduce the saturation solubility of CaO, which will not only increase the melting point of the slag, but also reduce its desulfurization capacity when w(MgO) < 8% in the slag, its melting point and desulfurization ability are better [15]. Therefore, the MgO content is generally controlled at about 6% to 8%.
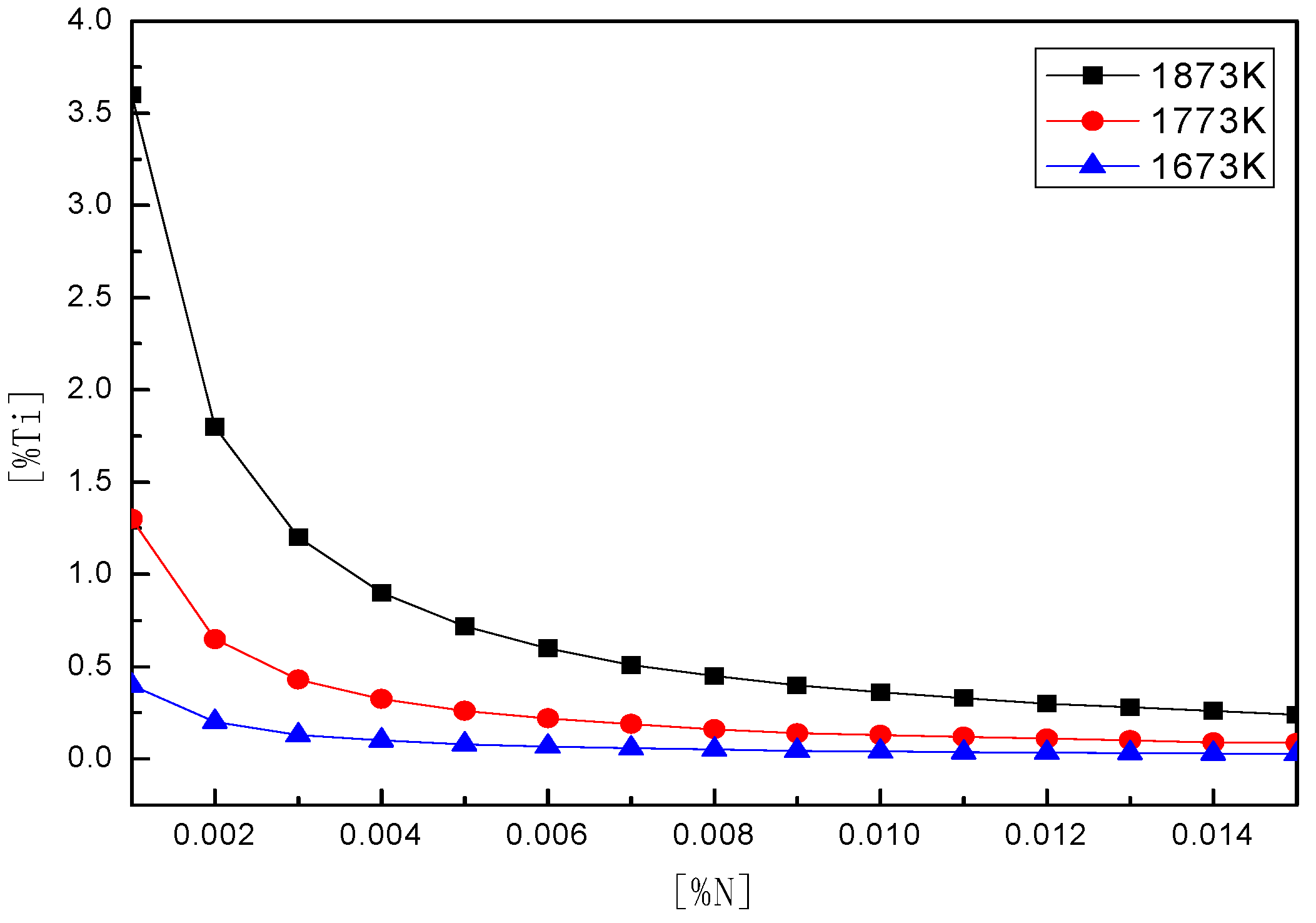
4.2. Thermodynamic Calculation of TiN Precipitations
5. Conclusions
- (1)
- There are lots of exogenous large inclusions in the billet, including: Al2O3, CaO-Al2O3, CaO-Al2O3-SiO2-TiO2, and CaO-MgO-Al2O3-SiO2-TiO2. Such large-scale inclusions mainly come from refractory materials, mold flux, and covering slag. The large-scale inclusions can generally be removed by optimizing the flow-field of mold and tundish, and adjusting the components of metallurgy auxiliary materials.
- (2)
- The typical endogenous oxides in molten steel are the Al2O3 and CaO-MgO-Al2O3-SiO2 systems. The size of endogenous inclusions are smaller than that of exogenous inclusions. By optimizing the composition of top slags in the refining process, the amount of Al2O3 and CaO-MgO-Al2O3-SiO2 inclusions can be effectively reduced. According to the thermodynamic calculations, the control range of refining slag should be: CaO/Al2O3 between 1.85 and 1.92, 7.5% < w(SiO2) < 20%, and 6% < w(MgO) < 8%.
- (3)
- The precipitation amount of TiN could be reduced during the cooling process of continuous casting by controlling the [Ti], [N] content in steel and rapid solidification.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Figueiredo, M.V.; Oliveira, F.M.F.; Goncalves, J.P.M.; de Castro, P.M.S.T.; Fernandes, A.A. Fracture analysis of a heavy duty lift truck. Eng. Fail. Anal. 2001, 8, 411–421. [Google Scholar] [CrossRef]
- Massone, J.M.; Boeri, R.E. Failure of forklift forks. Eng. Fail. Anal. 2010, 17, 1062–1068. [Google Scholar] [CrossRef]
- Wang, S.L. Strength structure and manufacture of forks for forklifts. Sci. Technol. Enterp. 2016, 9, 215–217. (In Chinese) [Google Scholar]
- Yin, X.G.; Wang, G.; Zhang, X.H. Development of low-alloy high-strength fork flat steel. Mod. Metall. 2009, 37, 13–15. (In Chinese) [Google Scholar]
- Wu, X.B.; Pang, B.H.; Wang, B.H.; Zhang, L. Analysis on the development of the material for forks. Heat Treat. Tech. Equip. 2006, 27, 20–22. (In Chinese) [Google Scholar]
- Yoshida, Y.; Funahashi, Y. On the extraction of large inclusions in steel by slime method and classification according to the size. J. Iron Steel Inst. Jpn. 1975, 61, 2489–2500. (In Japan) [Google Scholar] [CrossRef]
- Tunde, I.O.; Taiwo, E.A.; Augusta, I.E. Investigation of mechanical properties and parametric optimization of the dissimilar GTAW of AISI 304 stainless steel and low carbon steel. World Eng. 2018, 15, 584–591. [Google Scholar]
- Boya, O.; Danjuma, Y.; Ibraheem, S.; Dagwa, I.; David, O. Effects of electrode type on the mechanical properties of weldments of some steel samples produced in Nigeria. World Eng. 2014, 11, 95–106. [Google Scholar]
- Li, D.Z. Non-metal Inclusions in Steel, 1st ed.; Science China Press: Beijing, China, 1983; pp. 57–76. [Google Scholar]
- Zhao, S.; Ge, Y.Y.; Ma, L.; Yan, T.; Lyu, J.C.; Li, Z.S. Formation analysis of edge cracks of 33MnCrTiB fork steel. Metals 2018, 8, 587. [Google Scholar] [CrossRef]
- Huang, X.H. Metallurgical Principle of Iron and steel, 3rd ed.; Metallurgical Industry Press: Beijing, China, 2002; pp. 23–52. (In Chinese) [Google Scholar]
- Wang, Q.; He, S.P. Optimization of LF refining process and slag for low carbon aluminum containing steel. J. Univ. Sci. Technol. Beijing 2007, 29, 15–17. (In Chinese) [Google Scholar]
- Zhao, S.; He, S.P.; Chen, G.J.; Peng, M.M.; Wang, Q. Castability of molten steel and cleanliness of slab for high strength low alloy steel without calcium treatment. Ironmaking Steelmaking 2014, 41, 153–160. [Google Scholar] [CrossRef]
- Cui, L.Z. Control non-metal inclusion in steel by LF refining furnace. Hebei Metall. 2010, 179, 30–31. (In Chinese) [Google Scholar]
- Hao, N.; Wang, X.H.; Liu, J.G.; Wang, W.J. Effect of MgO content on desulphurization of CaO-Al2O3-SiO2-MgO slag. Steelmaking 2009, 25, 16–19. (In Chinese) [Google Scholar]
- Yang, J.; Wang, X.H.; Gong, Z.X.; Jiang, M.; Wang, G.C.; Huang, F.X. Precipitation thermodynamics analysis and control of titanium nitride inclusions in extra- low oxygen wheel steel. J. Univ. Sci. Technol. Beijing 2010, 32, 1139–1143. (In Chinese) [Google Scholar]
- Kang, Y.B.; Lee, H.G. Thermodynamic analysis of Mn-depleted zone near Ti oxide inclusions for intragranular nucleation of ferrite in steel. ISIJ Int. 2010, 50, 501–508. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S | Cr | Al | Ti | B |
|---|---|---|---|---|---|---|---|---|
| 0.33 | 0.26 | 1.36 | 0.011 | 0.005 | 0.40 | 0.009 | 0.034 | 0.0013 |
| 0.30–0.35 | 0.17–0.37 | 1.25–1.50 | ≤0.035 | ≤0.035 | 0.30–0.60 | - | ≥0.015 | 0.0005–0.0030 |
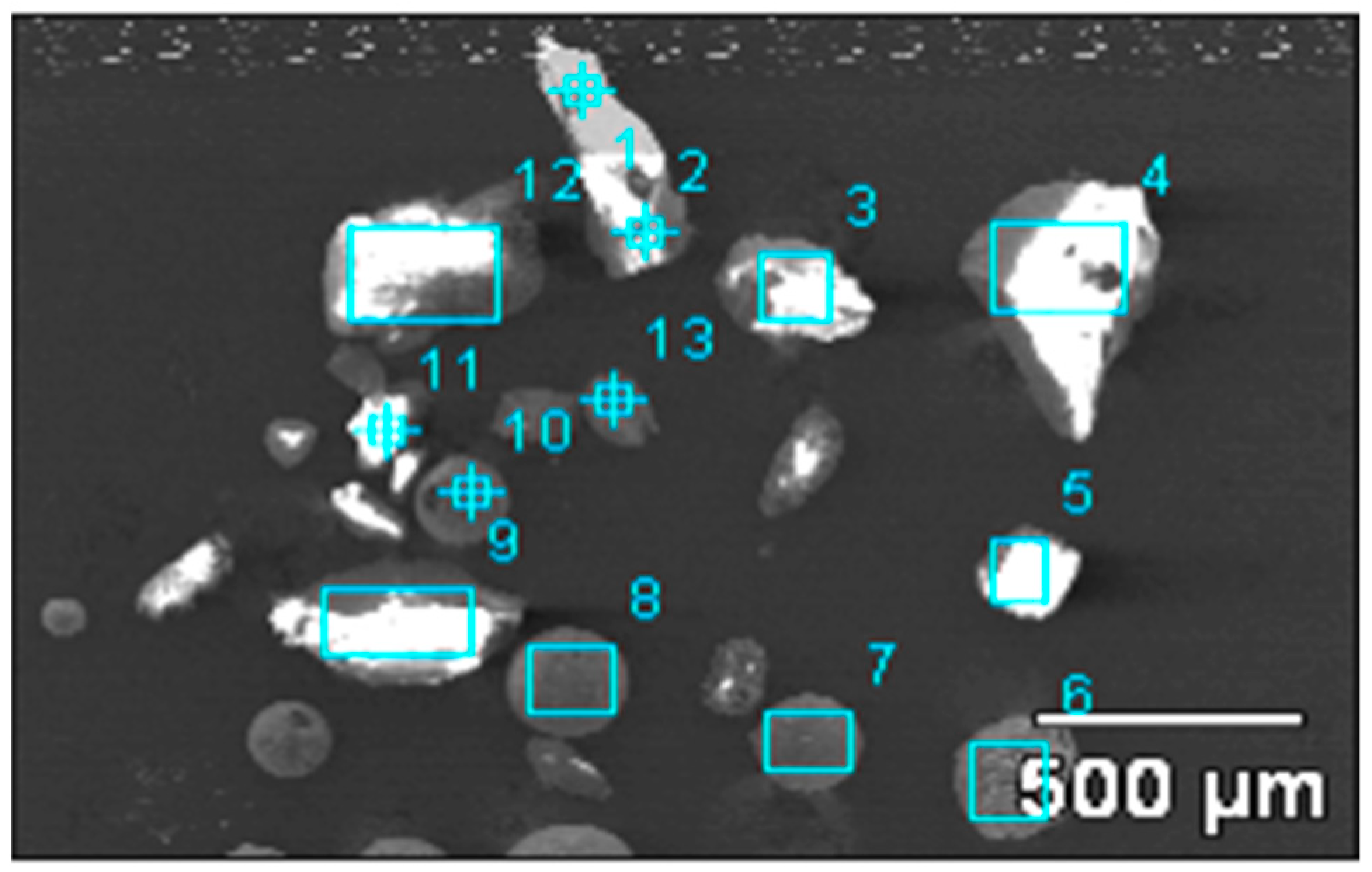
| No. | O | Mg | Al | Si | S | K | Ca | Ti | Cr | Mn | Fe | Cu | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50.85 | 1.33 | 12.76 | 23.22 | - | 3.22 | 3.05 | 1.85 | 0.52 | - | 3.2 | - | - |
| 2 | 60.39 | - | 22.58 | 3.21 | - | - | 5.03 | 1.71 | - | - | 7.09 | - | - |
| 3 | 50.12 | 0.85 | 23.33 | 12.08 | - | 1.62 | 3.87 | 3.06 | - | - | 5.09 | - | - |
| 4 | 45.98 | - | 34.43 | 7.55 | - | 0.98 | 0.95 | 2.31 | 0.51 | - | 7.28 | - | - |
| 5 | 60.89 | - | 21.2 | 2.61 | - | - | 9.83 | - | - | - | 5.46 | - | - |
| 6 | 20.85 | 2.03 | 7.18 | 1.03 | 1.11 | - | 6.03 | 6.56 | 3.46 | 5.01 | 46.74 | - | - |
| 7 | 29.42 | 8.6 | 24.96 | 0.78 | 2.15 | - | 11.16 | 12.71 | - | - | 7.78 | 2.45 | - |
| 8 | 24.45 | 3.47 | 25.24 | 0.86 | - | - | 18.2 | 18.15 | - | - | 2.19 | - | 7.44 |
| 9 | 48.79 | - | 51.21 | - | - | - | - | - | - | - | - | - | - |
| 10 | 14.14 | 1.09 | 23.22 | 3.56 | 5.47 | - | 32.27 | 14.55 | - | - | 5.7 | - | - |
| 11 | 44.09 | - | 55.91 | - | - | - | - | - | - | - | - | - | - |
| 12 | 60.67 | - | 20.78 | - | - | - | 10.03 | - | - | - | 8.52 | - | - |
| 13 | 42.75 | - | 32.08 | - | - | - | 25.17 | - | - | - | - | - | - |
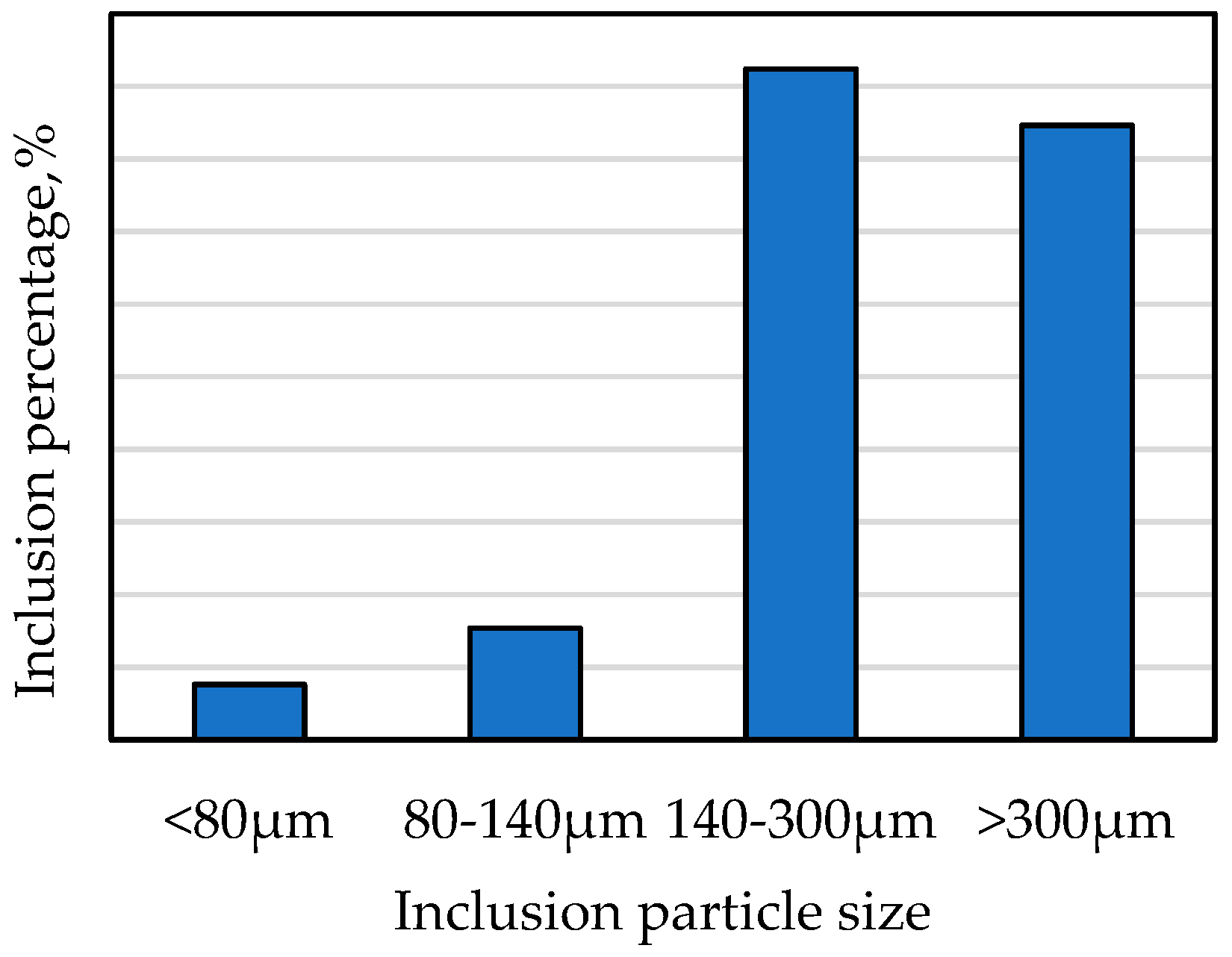
| Sample | Original Weight | Remaining Weight | Electrolytic Weight | Total Inclusions | Inclusion Particle Size Classification, µm | ||||
|---|---|---|---|---|---|---|---|---|---|
| <80 | 80~140 | 140~300 | >300 | ||||||
| kg | kg | kg | mg | mg/10 kg | mg | mg | mg | mg | |
| Y | 4.438 | 0.251 | 4.178 | 2.60 | 6.21 | 0.10 | 0.20 | 1.20 | 1.10 |
| Al | B | C | Cu | Ni | N | Mn | H | P | S | Si | Ti | Cr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 | - | 0.09 | 0.01 | - | −0.1 | - | 0.24 | - | 0.03 | 0.01 | - | - | |
| 0.04 | 0.13 | 0.11 | 0 | 0 | 0.01 | −0.026 | 0.12 | 0.03 | 0 | 0.06 | −0.1 | −0.011 | |
| 0.06 | 0.2 | 0.18 | 0.01 | 0.01 | 0.09 | 0.002 | 0.64 | 0.11 | 0.06 | 0.11 | - | −0.0003 | |
| - | - | −0.165 | - | - | −1.8 | 0.0043 | - | −0.0064 | −0.11 | 0.05 | 0.013 | 0.055 | |
| −0.3 | 0.094 | 0.13 | 0.009 | 0.01 | 0 | −0.021 | - | 0.045 | 0.007 | 0.047 | −0.53 | −0.047 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Zhao, S.; Ma, L.; Yan, T.; Li, Z.; Yang, B. Inclusions Control and Refining Slag Optimization for Fork Flat Steel. Metals 2019, 9, 253. https://doi.org/10.3390/met9020253
Ge Y, Zhao S, Ma L, Yan T, Li Z, Yang B. Inclusions Control and Refining Slag Optimization for Fork Flat Steel. Metals. 2019; 9(2):253. https://doi.org/10.3390/met9020253
Chicago/Turabian StyleGe, Yangyang, Shuo Zhao, Liang Ma, Tao Yan, Zushu Li, and Bin Yang. 2019. "Inclusions Control and Refining Slag Optimization for Fork Flat Steel" Metals 9, no. 2: 253. https://doi.org/10.3390/met9020253
APA StyleGe, Y., Zhao, S., Ma, L., Yan, T., Li, Z., & Yang, B. (2019). Inclusions Control and Refining Slag Optimization for Fork Flat Steel. Metals, 9(2), 253. https://doi.org/10.3390/met9020253




