A Phenomenological Mechanical Material Model for Precipitation Hardening Aluminium Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Examined Aluminium Alloy
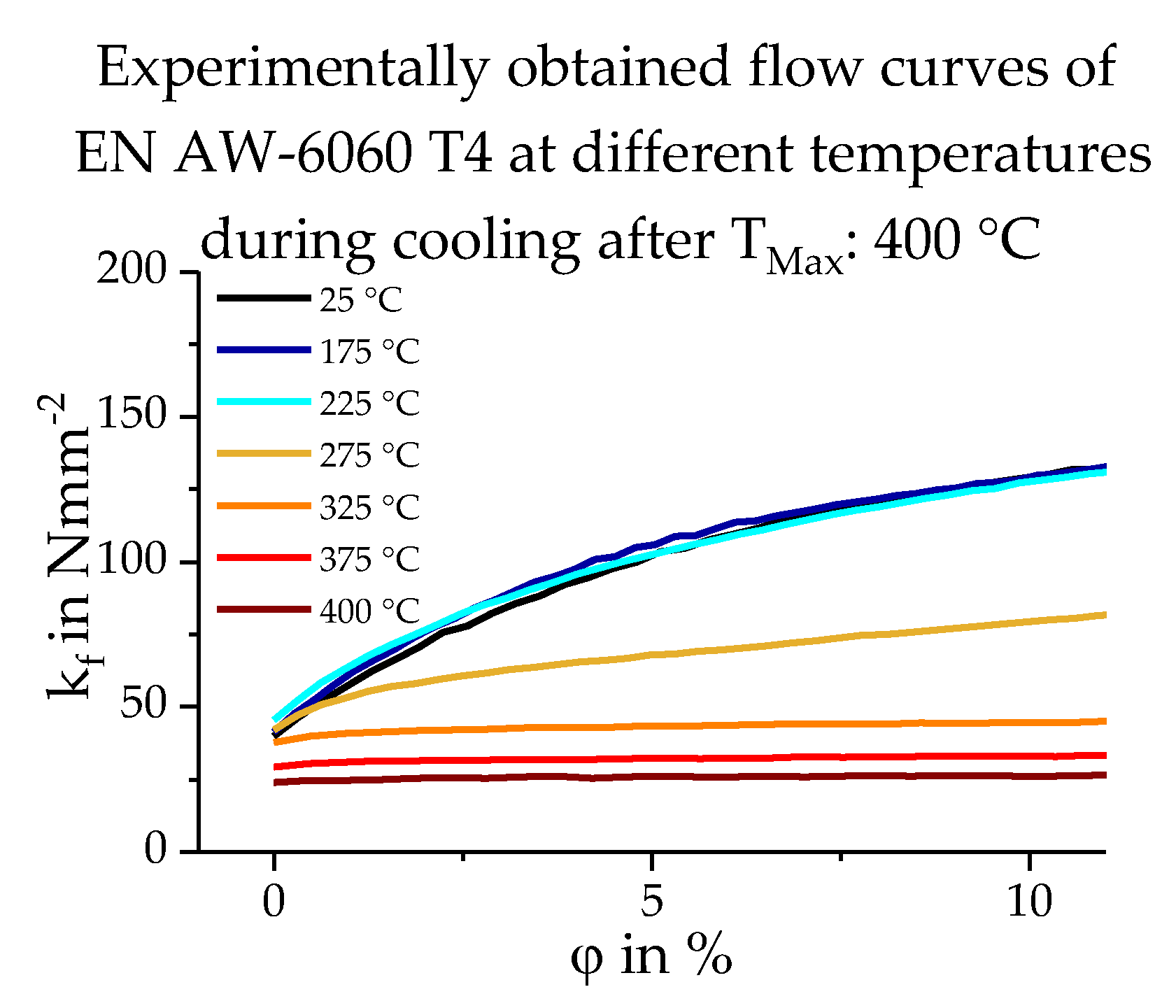
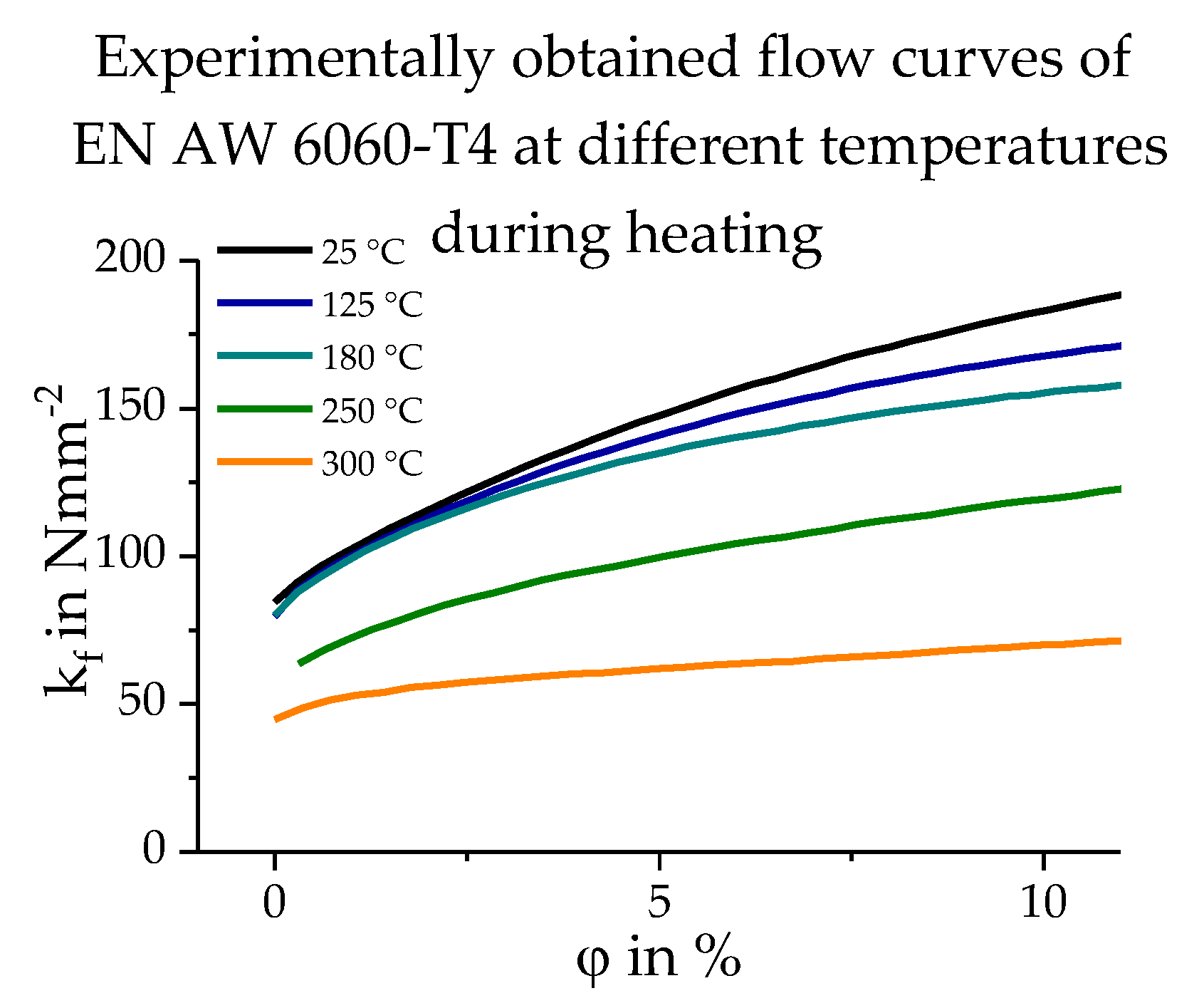
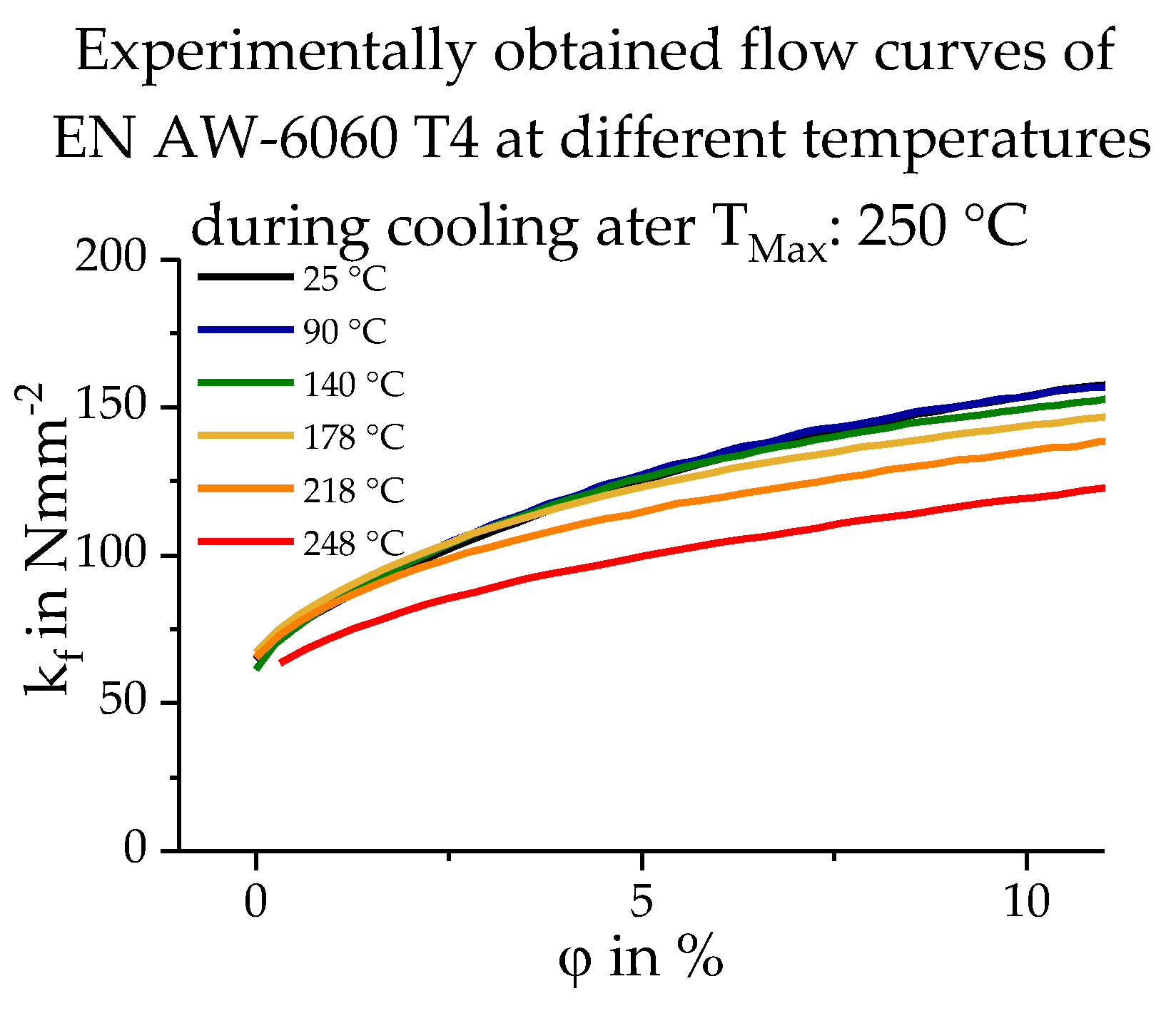
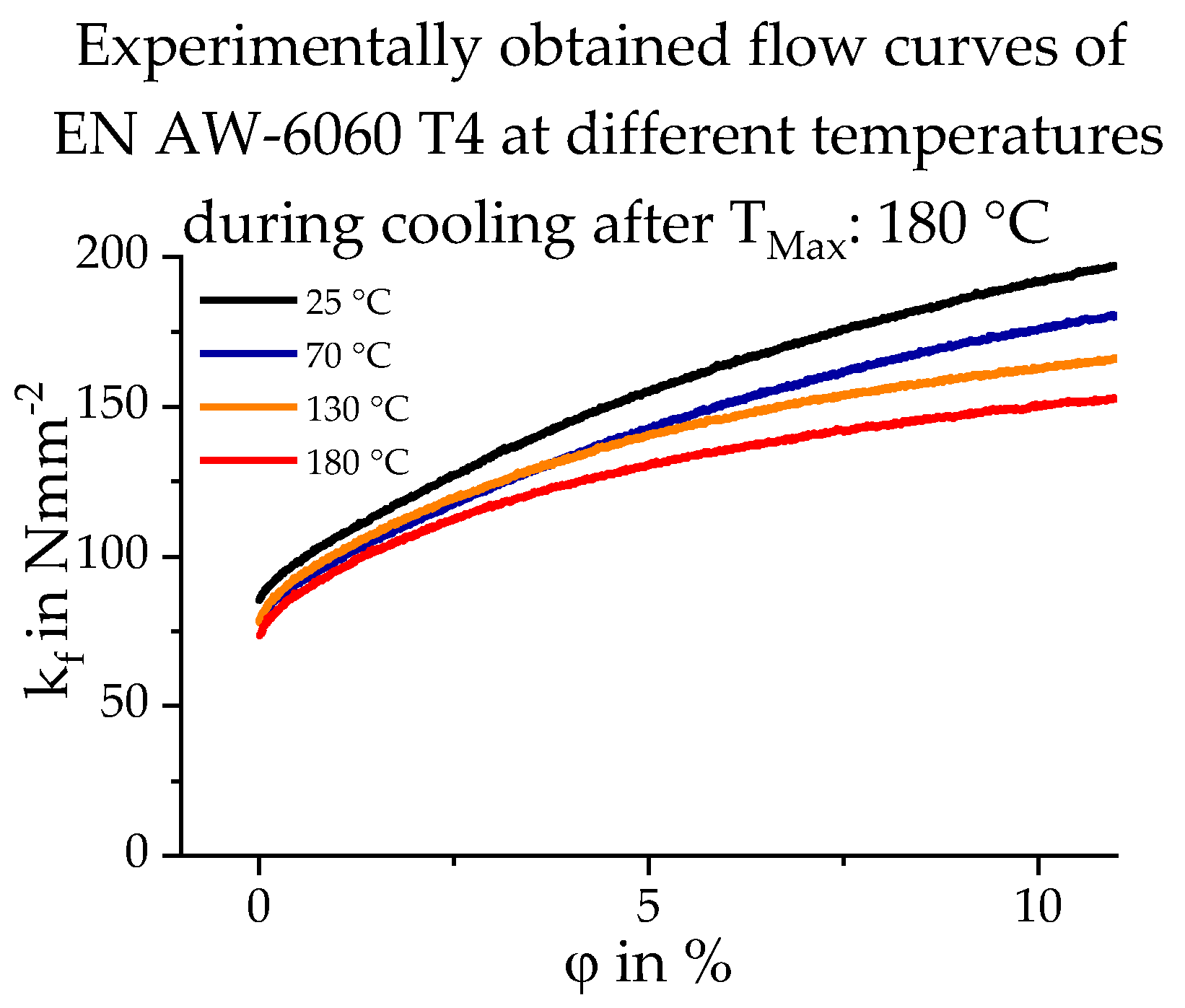
2.2. Database Used
3. Material Model Development
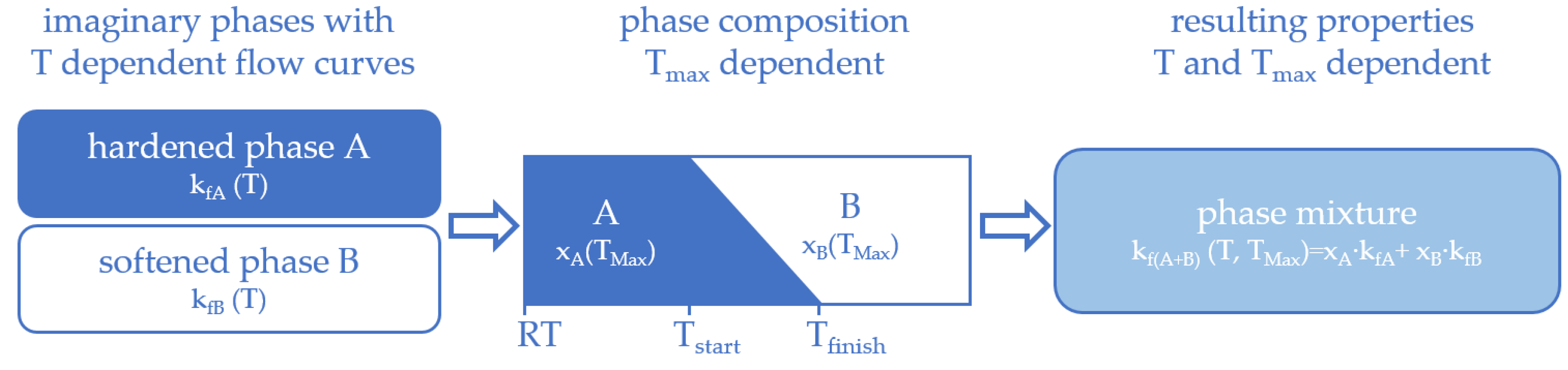
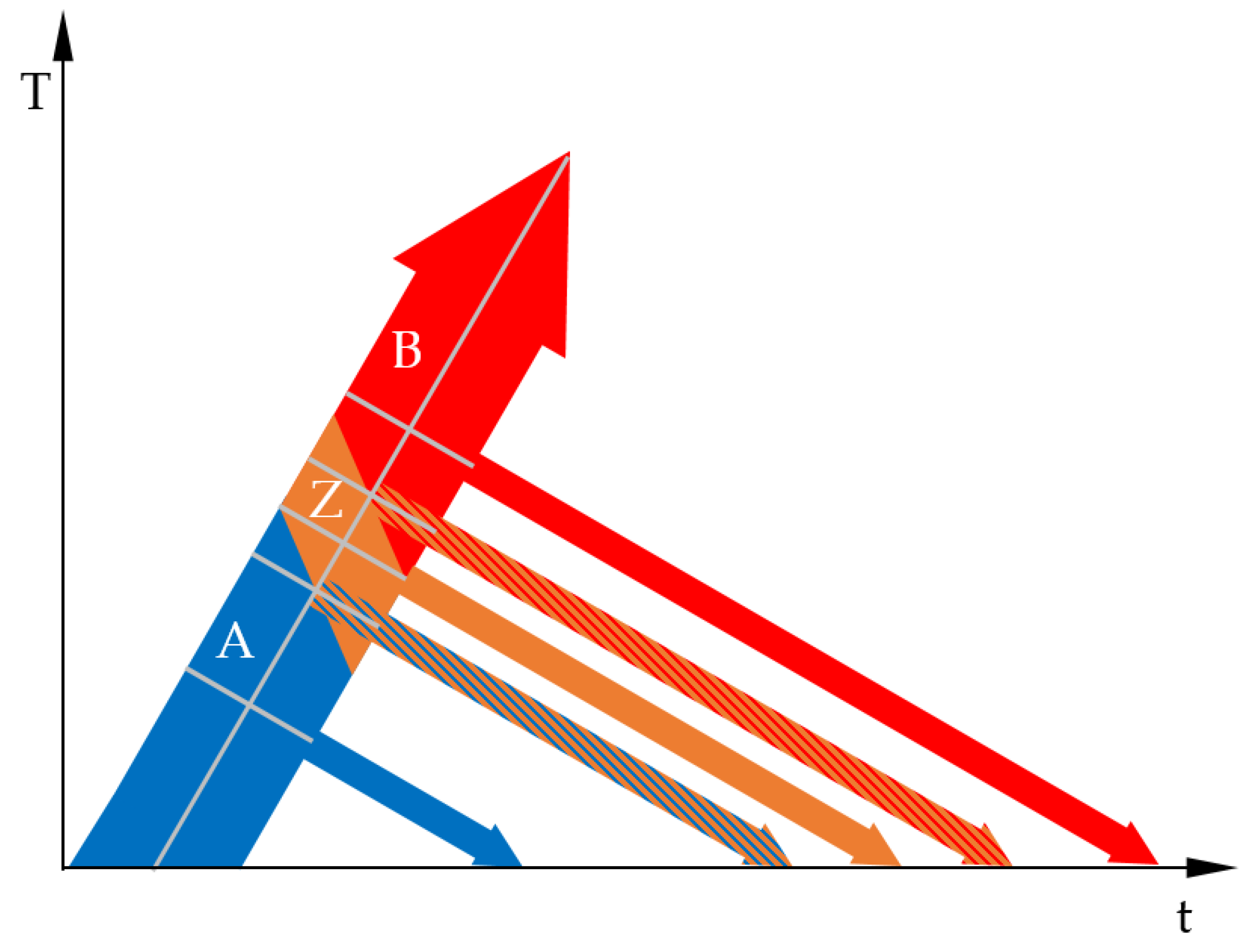
3.1. The Basic Idea
- The influence of the heating and cooling rate was not considered. Typical heating and cooling rates for short-term laser heat treatment were chosen.
- During cooling, no precipitation reaction took place. This assumption is realistic, as the critical cooling rate of alloy 6060 is far below the cooling rates of short-term laser heat treatment.
- Quenching followed directly after reaching the maximum temperature. There was no isothermal soaking.
- The influence of the strain rate on the mechanical properties was not considered.
- The maximum plastic strains were 10%.
- The influence of ageing after short-time heat treatment was not considered. The model was valid for the as-quenched state.
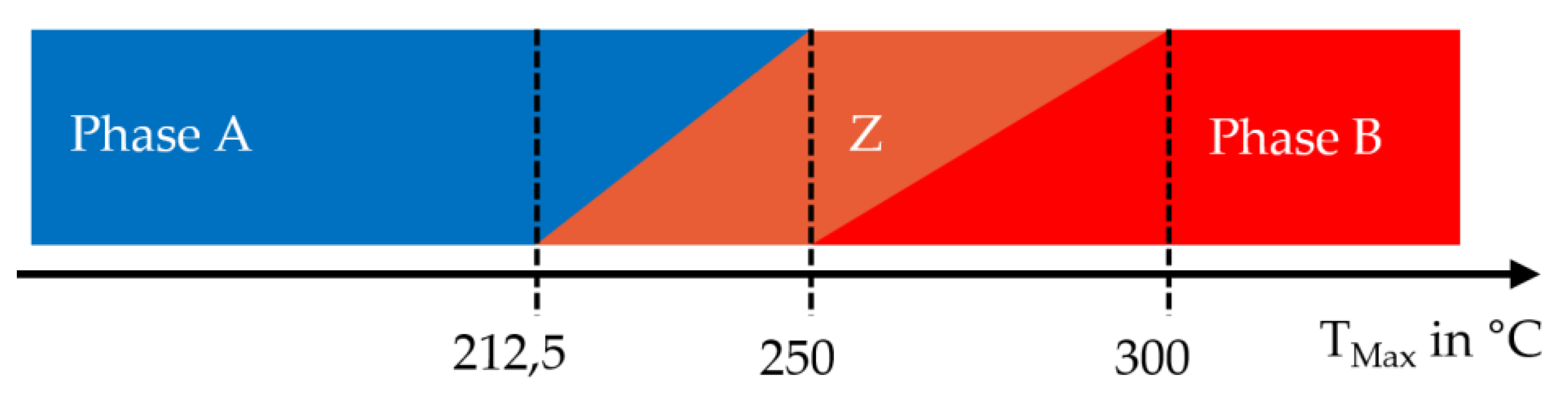
3.2. Determination of the Phase Transformation Temperatures
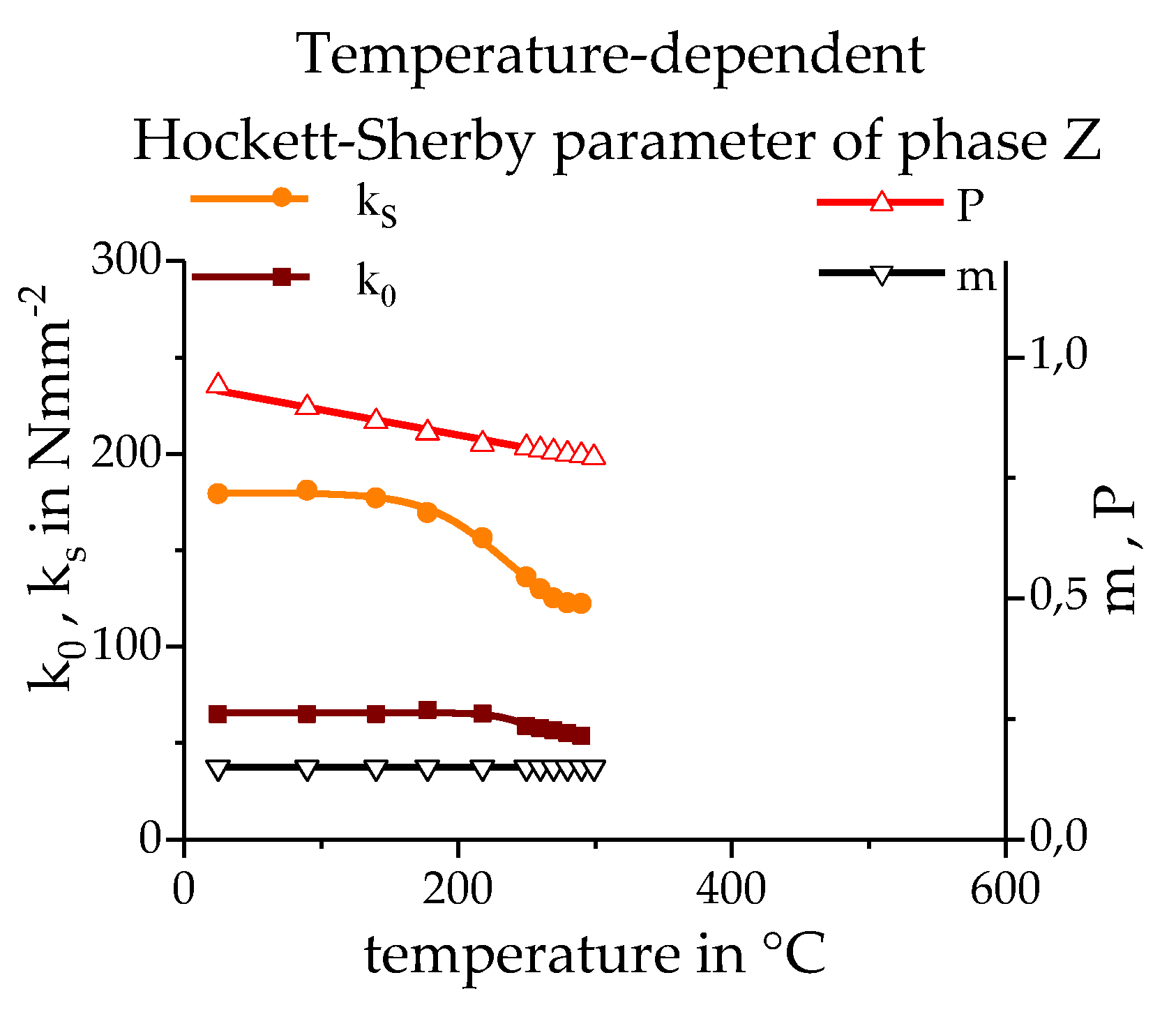
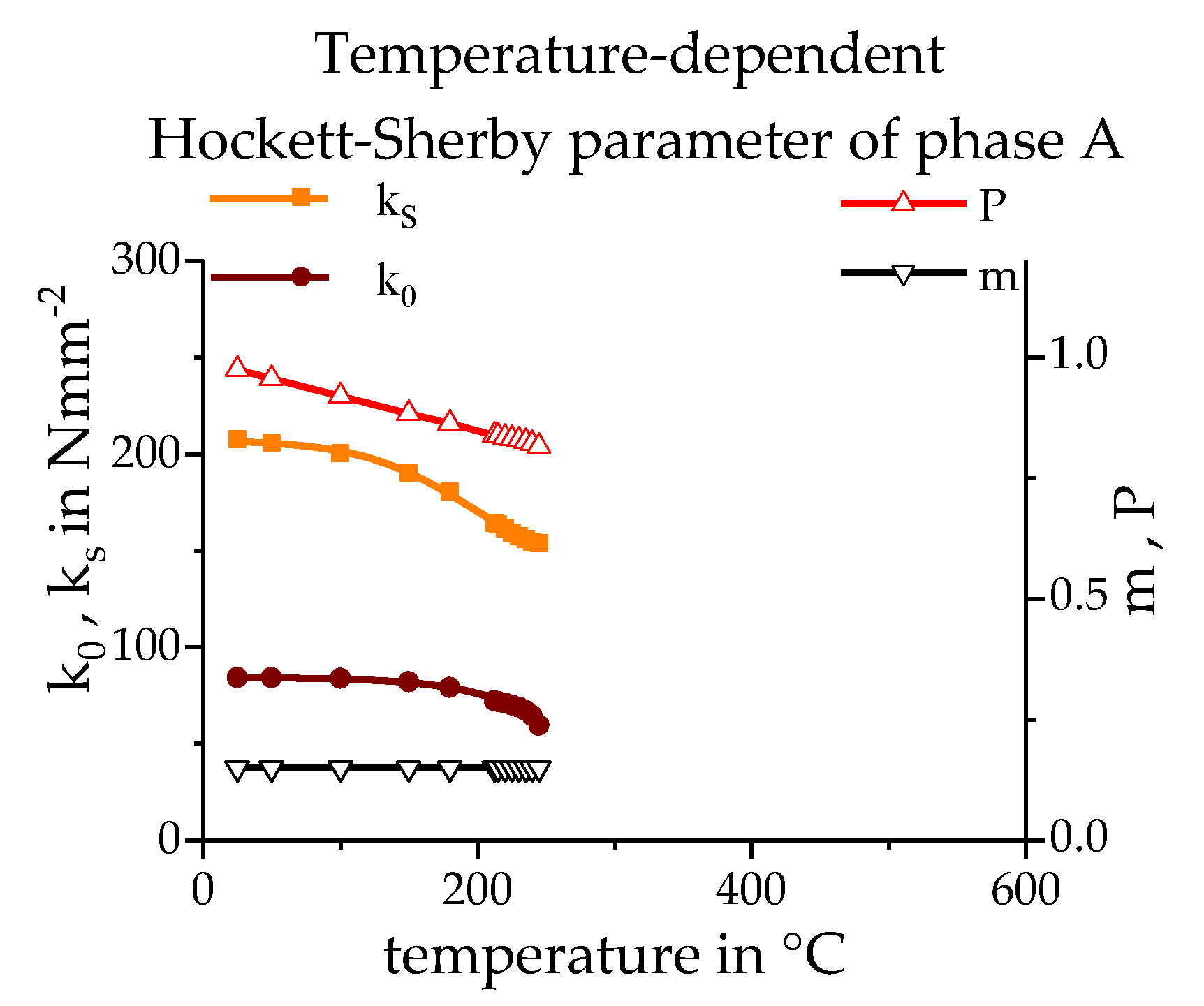
3.3. Calculating the Resulting Flow Curves
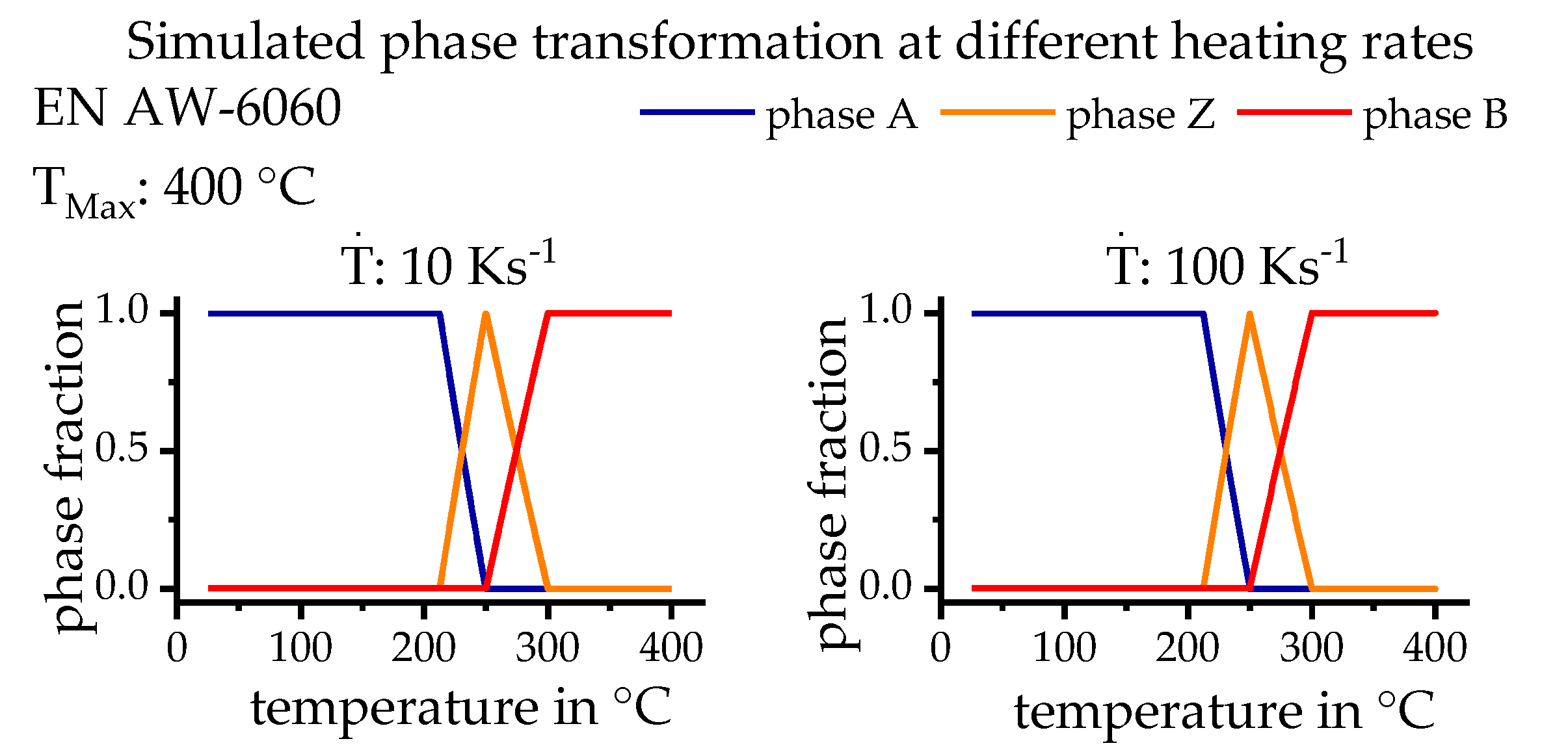
3.4. Phase Transformation
3.5. Simulation Model
4. Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mackerle, J. Finite element analysis and simulation of quenching and other heat treatment processes. Comput. Mater. Sci. 2003, 27, 313–332. [Google Scholar] [CrossRef]
- Oliveira, W.P.; de Savi, M.A.; Pacheco, P.M.C.L.; de Souza, L.F.G. Thermomechanical analysis of steel cylinders quenching using a constitutive model with diffusional and non-diffusional phase transformations. Mech. Mat. 2010, 42, 31–43. [Google Scholar] [CrossRef]
- Deng, X.; Ju, D. Modeling and simulation of quenching and tempering process in steels. Phys. Procedia 2013, 50, 368–374. [Google Scholar] [CrossRef]
- Carlone, P.; Palazzo, G.S.; Pasquino, R. Finite element analysis of the steel quenching process: Temperature field and solid-solid phase change. Comput. Math. Appl. 2010, 59, 585–594. [Google Scholar] [CrossRef]
- Reich, M.; Kessler, O. Numerical and experimental analysis of residual stresses and distortion in different quenching processes of aluminum alloy profiles. In ASM International; Quenching Control and Distortion; ASM International: Chicago, OH, USA, 2012; pp. 563–574. [Google Scholar]
- Reich, M.; Schöne, S.; Kessler, O.; Nowak, M.; Grydin, O.; Nürnberger, F.; Schaper, M. Simulation of gas and spray quenching during extrusion of aluminium alloys. Key Eng. Mater. 2010, 424, 57–64. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Nong, Z.; Lai, Z.; He, D. FEM simulation of quenching process in A357 aluminum alloy cylindrical bars and reduction of quench residual stress through cold stretching process. Comput. Mater. Sci. 2013, 69, 396–413. [Google Scholar] [CrossRef]
- Wang, M.J.; Gang, Y.A.N.G.; Huang, C.Q.; Bin, C.H.E.N. Simulation of temperature and stress in 6061 aluminum alloy during online quenching process. Trans. Nonferrous Met. Soc. China 2014, 24, 2168–2173. [Google Scholar] [CrossRef]
- Felde, I.; Simsir, C. Simulation trends in quenching technology for automotive components. Int. Heat Treat. Surf. Eng. 2014, 8, 42–48. [Google Scholar] [CrossRef]
- Deng, D.; Kiyoshima, S. FEM prediction of welding residual stresses in a SUS304 girth-welded pipe with emphasis on stress distribution near weld start/end location. Comput. Mat. Sci. 2010, 50, 612–621. [Google Scholar] [CrossRef]
- Donati, L.; Troiani, E.; Proli, P.; Tomesani, L. FEM analysis and experimental validation of friction welding process of 6xxx alloys for the prediction of welding quality. Mater. Today Proc. 2015, 2, 5045–5054. [Google Scholar] [CrossRef]
- Hömberg, D.; Liu, Q.; Montalvo-Urquizo, J.; Nadolski, D.; Petzold, T.; Schmidt, A.; Schulz, A. Simulation of multi-frequency-induction-hardening including phase transitions and mechanical effects. Finite Elem. Anal. Des. 2016, 121, 86–100. [Google Scholar] [CrossRef]
- Geiger, M.; Merklein, M.; Vogt, U. Aluminum tailored heat treated blanks. Prod. Eng. 2009, 3, 401–410. [Google Scholar] [CrossRef]
- Kahrimanidis, A.; Lechner, M.; Degner, J.; Wortberg, D.; Merklein, M. Process design of aluminum tailor heat treated blanks. Materials 2015, 8, 8524–8538. [Google Scholar] [CrossRef] [PubMed]
- Fröck, H.; Graser, M.; Reich, M.; Lechner, M.; Merklein, M.; Kessler, O. Linked Heat Treatment and Bending Simulation of Aluminium Tailored Heat Treated Profiles. In Proceedings of the 4th World Congress on Integrated Computational Materials Engineering; Mason, P., Fisher, C.R., Glamm, R., Manuel, M.V., Schmitz, G.J., Singh, A.K., Strachan, A., Eds.; Springer: Cham, Germany, 2017; pp. 237–248. [Google Scholar]
- Chen, X.; Xiao, N.; Li, D.; Li, G.; Sun, G. The finite element analysis of austenite decomposition during continuous cooling in 22MnB5 steel. Modell. Simul. Mater. Sci. Eng. 2014, 22, 65005. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J.; Poole, W.J. Modeling of precipitation hardening for the naturally aged Al-Mg-Si-Cu alloy AA6111. Acta Mater. 2003, 51, 3467–3481. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J.; Poole, W.J. A yield strength model for the Al-Mg-Si-Cu alloy AA6111. Acta Mater. 2003, 51, 2243–2257. [Google Scholar] [CrossRef]
- Fröck, H.; Graser, M.; Reich, M.; Lechner, M.; Merklein, M.; Kessler, O. Influence of short-term heat treatment on the microstructure and mechanical properties of EN AW-6060 T4 extrusion profiles: Part, A. Prod. Eng. 2016, 10, 383–389. [Google Scholar] [CrossRef]
- Fröck, H.; Reich, M.; Milkereit, B.; Kessler, O. Scanning rate extension of conventional DSCs through indirect measurements. Materials 2019, 12, 1085. [Google Scholar] [CrossRef]
- Fröck, H.; Graser, M.; Milkereit, B.; Reich, M.; Lechner, M.; Merklein, M.; Kessler, O. Precipitation behaviour and mechanical properties during short-term heat treatment for tailor heat treated profiles (THTP) of aluminium alloy 6060 T4. Mat. Sci. Forum 2016, 877, 400–406. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Tsao, C.S.; Chen, C.Y.; Jeng, U.S.; Kuo, T.Y. Precipitation kinetics and transformation of metastable phases in Al-Mg-Si alloys. Acta Mater. 2006, 54, 4621–4631. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 1998, 46, 893–3904. [Google Scholar] [CrossRef]
- Fröck, H.; Graser, M.; Reich, M.; Lechner, M.; Merklein, M.; Kessler, O. Influence of short-term heat treatment on the microstructure and mechanical properties of EN AW-6060 T4 extrusion profiles—Part, B. Prod. Eng. 2016, 10, 391–398. [Google Scholar] [CrossRef]
- Hockett, J.E.; Sherby, O.D. Large strain deformation of polycrystalline metals at low homologous temperatures. J. Mech. Phys. Solids 1975, 23, 87–98. [Google Scholar] [CrossRef]
- Loose, T.; Klöppel, T. An LS-DYNA material model for the consistent simulation of welding forming and heat treatment. In Proceedings of the 11th Int Seminar Numerical Analysis of Weldability, Seggau, Austria, 28 September 2015. [Google Scholar]
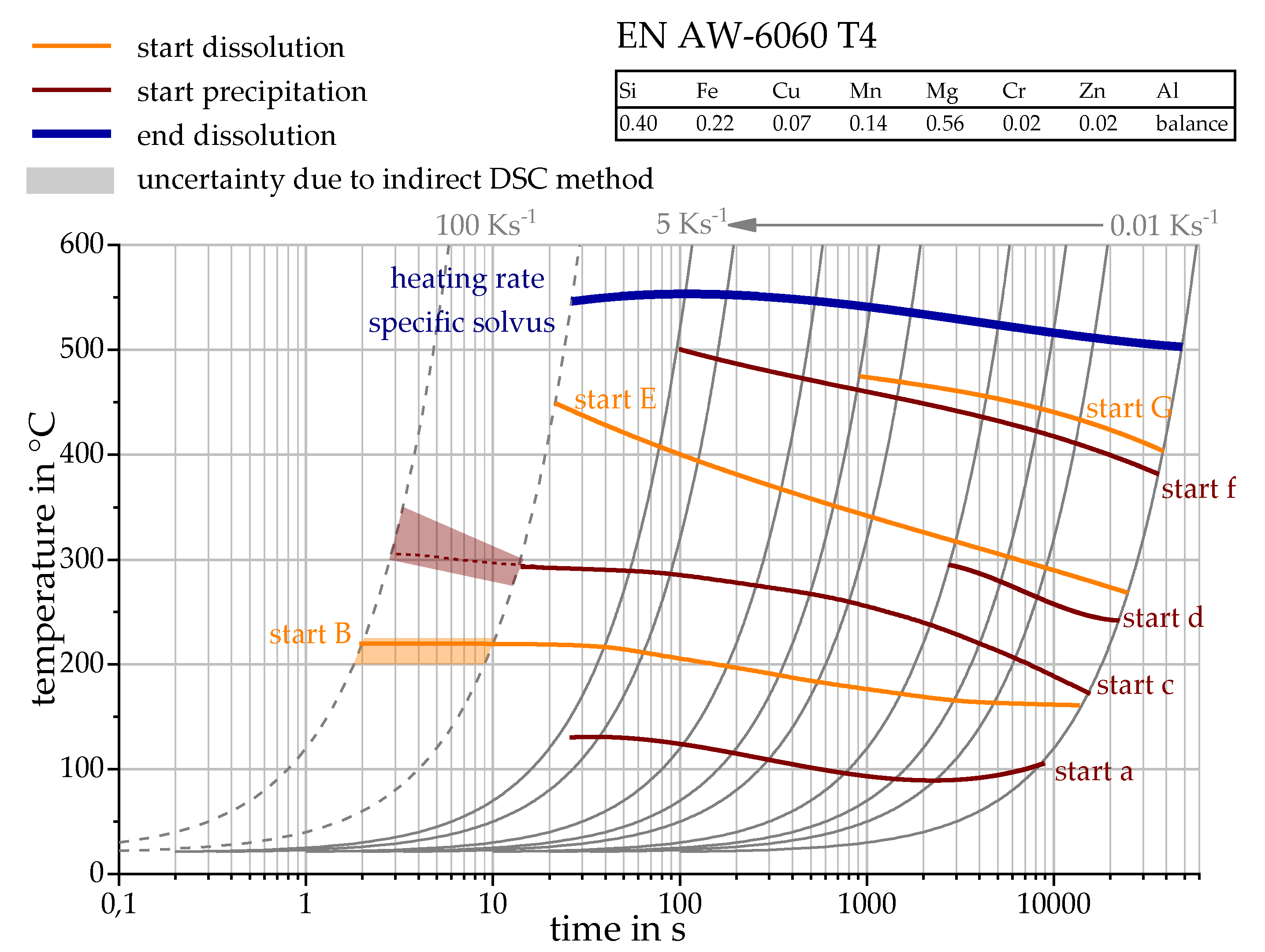
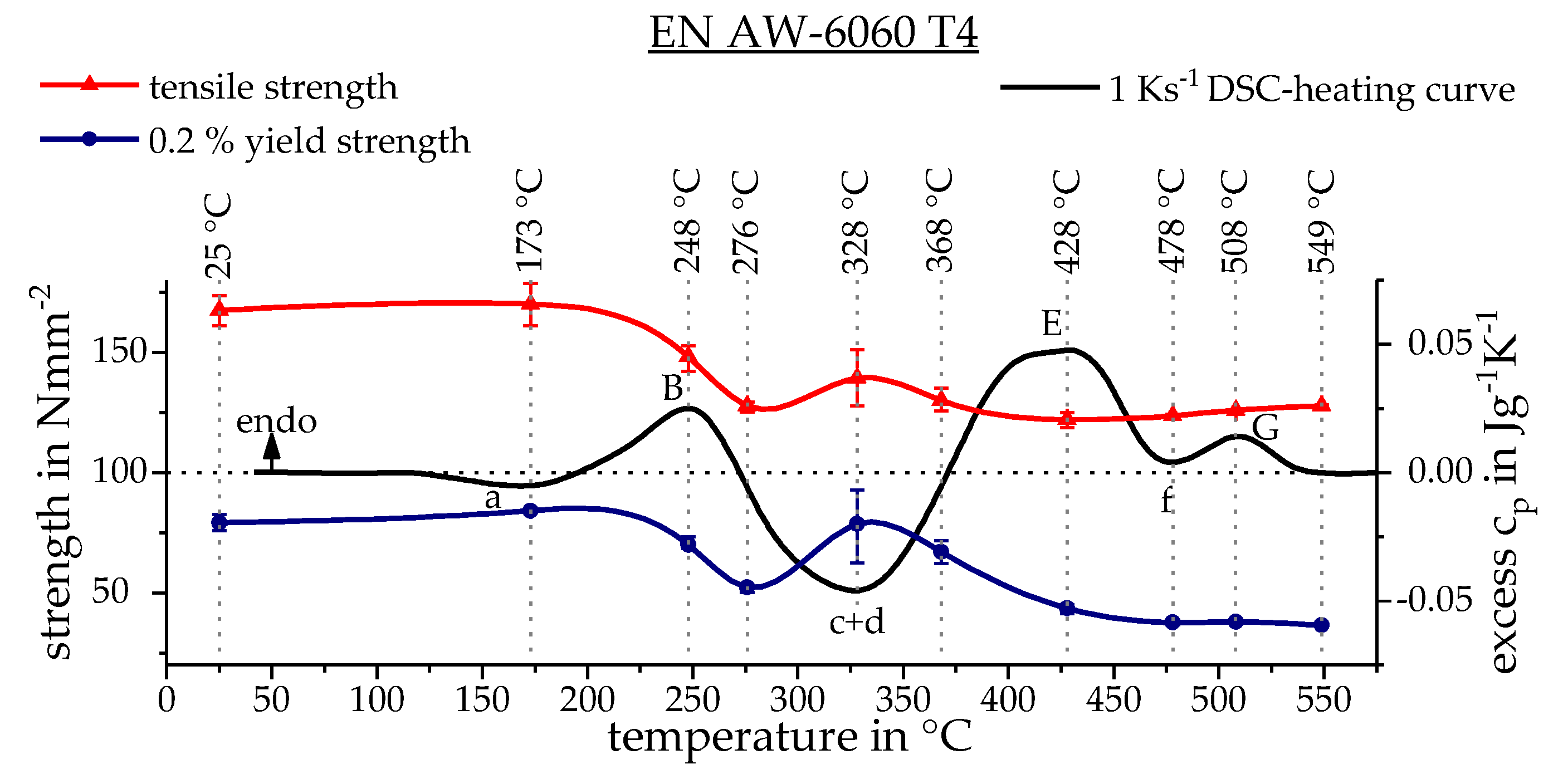

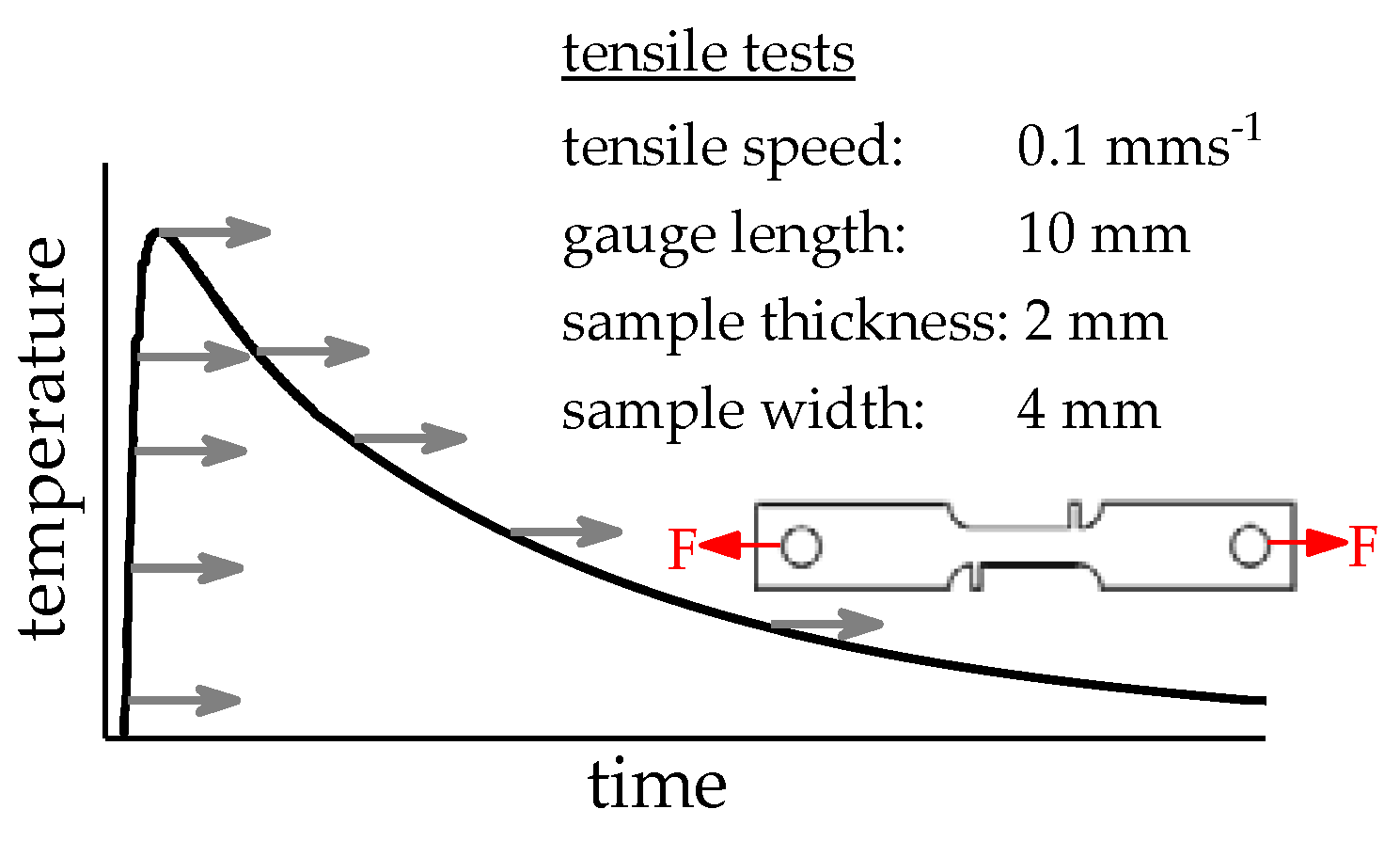
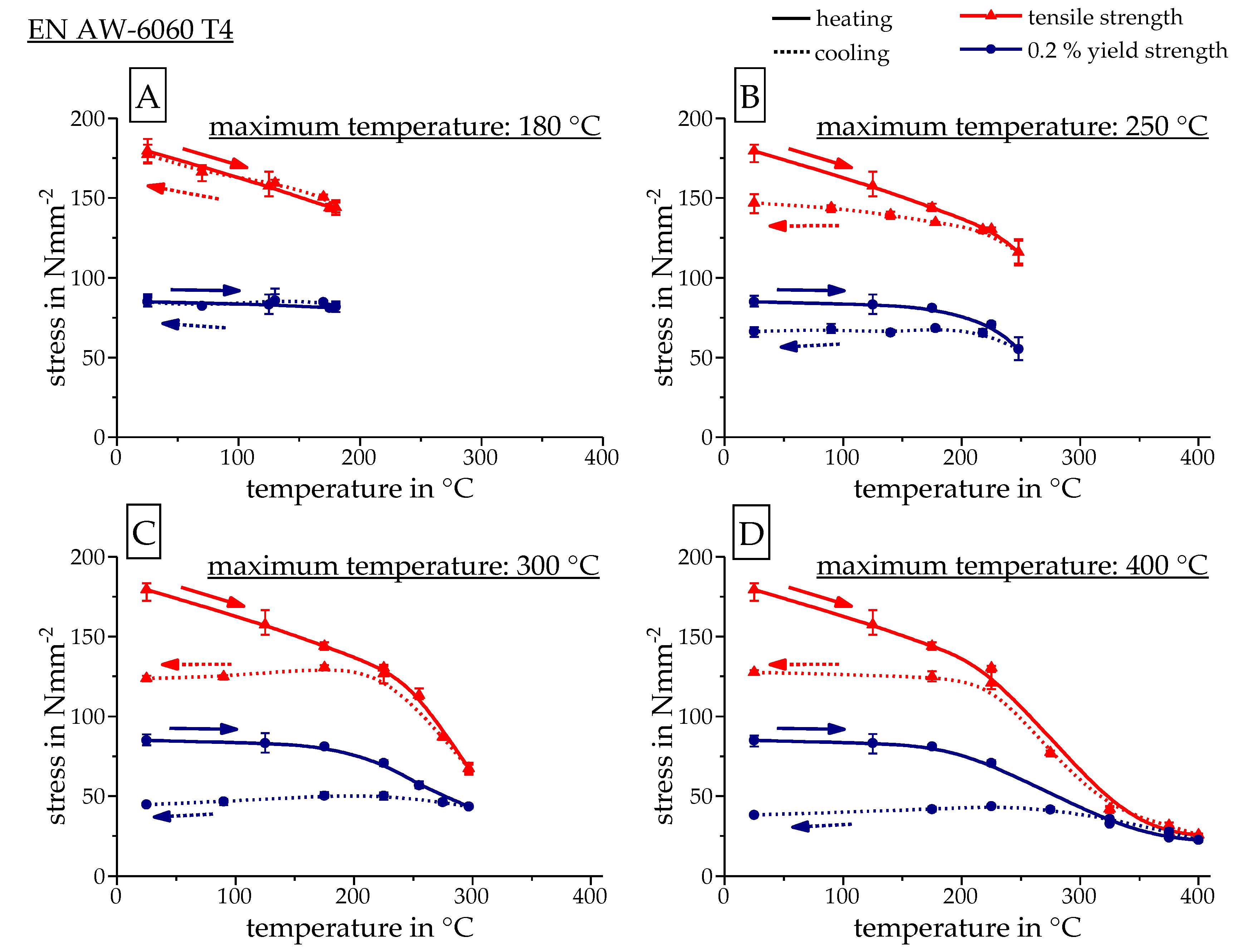



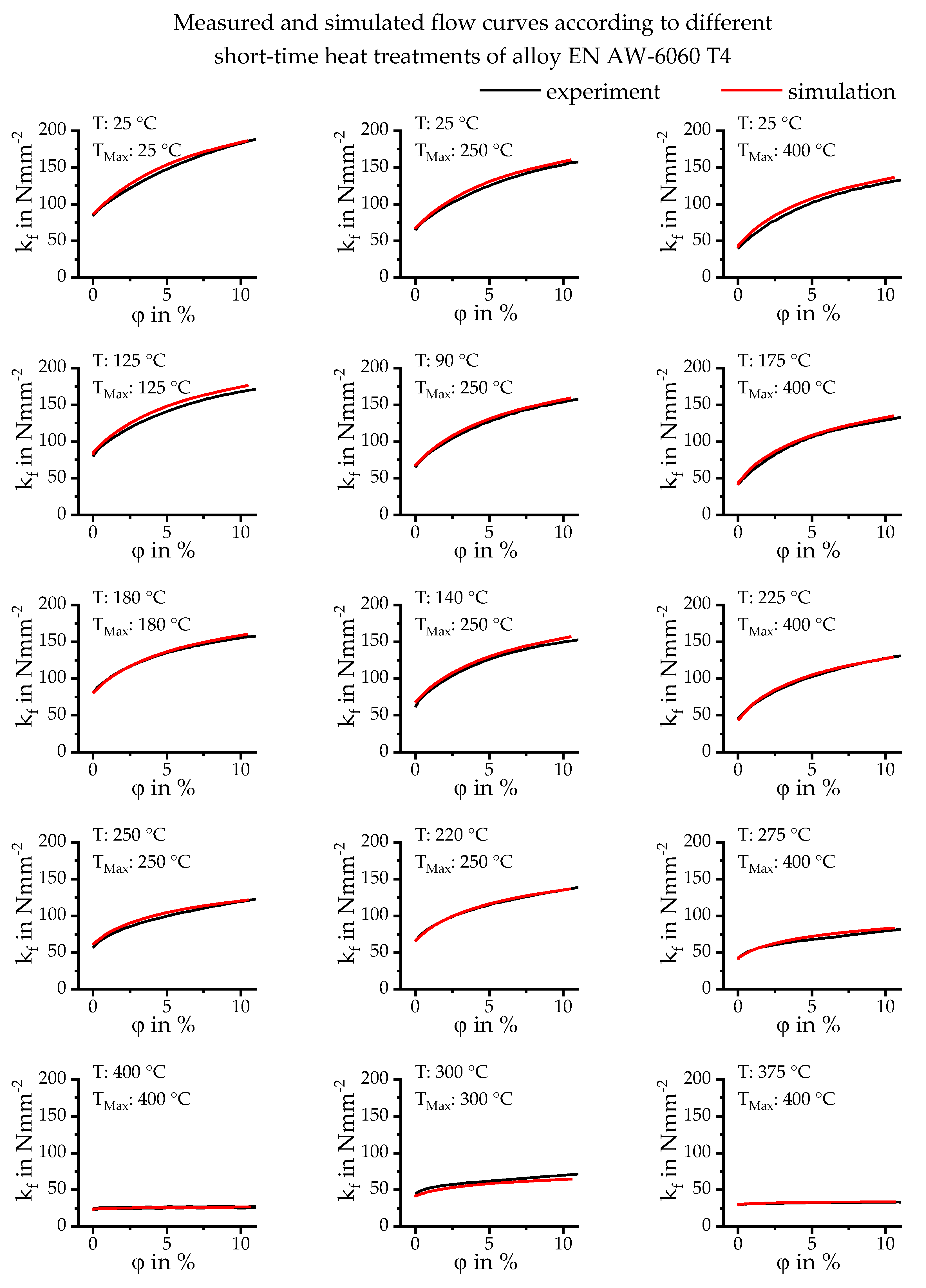

| Alloy | Mass Fraction in % | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Al | |
| OES EN AW-6060 T4 | 0.40 | 0.22 | 0.07 | 0.14 | 0.56 | 0.02 | 0.02 | balance |
| DIN EN 573-3 (6060) | 0.3–0.6 | 0.1–0.3 | ≤0.1 | ≤0.1 | 0.35–0.6 | ≤0.05 | ≤0.15 | balance |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröck, H.; Kappis, L.V.; Reich, M.; Kessler, O. A Phenomenological Mechanical Material Model for Precipitation Hardening Aluminium Alloys. Metals 2019, 9, 1165. https://doi.org/10.3390/met9111165
Fröck H, Kappis LV, Reich M, Kessler O. A Phenomenological Mechanical Material Model for Precipitation Hardening Aluminium Alloys. Metals. 2019; 9(11):1165. https://doi.org/10.3390/met9111165
Chicago/Turabian StyleFröck, Hannes, Lukas Vincent Kappis, Michael Reich, and Olaf Kessler. 2019. "A Phenomenological Mechanical Material Model for Precipitation Hardening Aluminium Alloys" Metals 9, no. 11: 1165. https://doi.org/10.3390/met9111165
APA StyleFröck, H., Kappis, L. V., Reich, M., & Kessler, O. (2019). A Phenomenological Mechanical Material Model for Precipitation Hardening Aluminium Alloys. Metals, 9(11), 1165. https://doi.org/10.3390/met9111165





