Analysis of Melt-Pool Behaviors during Selective Laser Melting of AISI 304 Stainless-Steel Composites
Abstract
1. Introduction
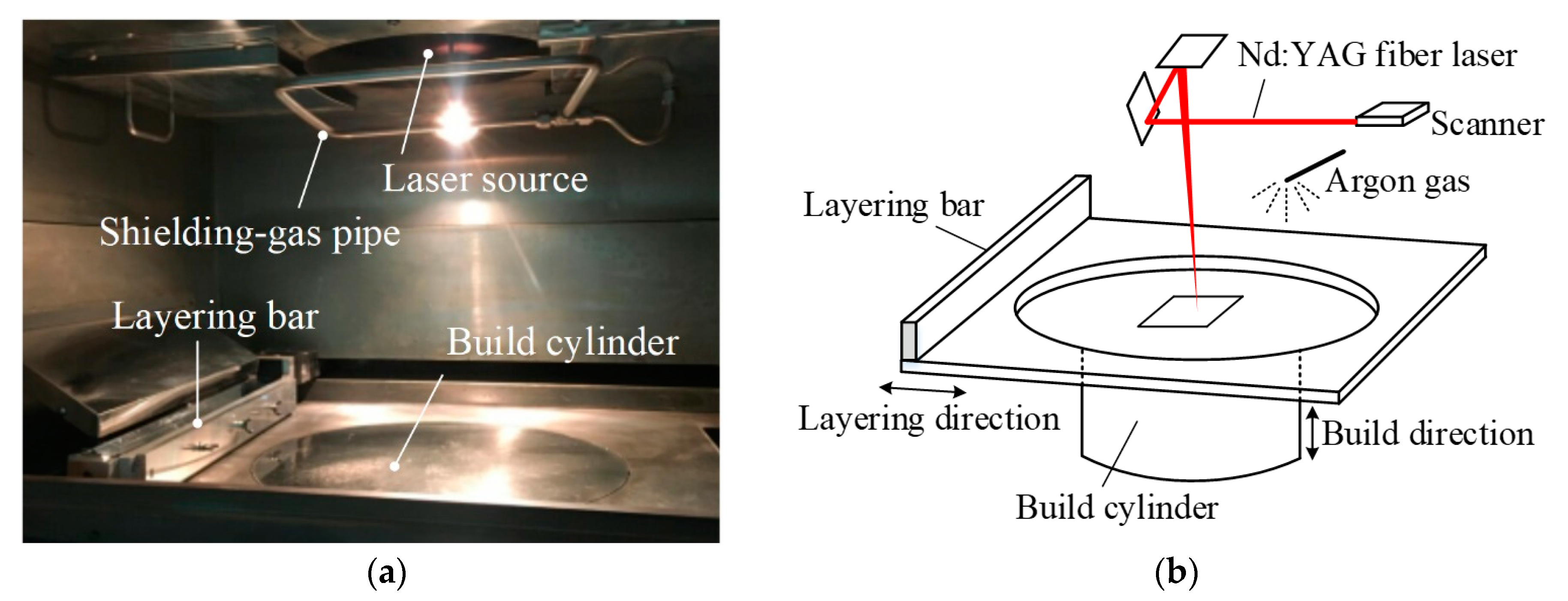
2. Materials and SLM System
3. FE Modeling
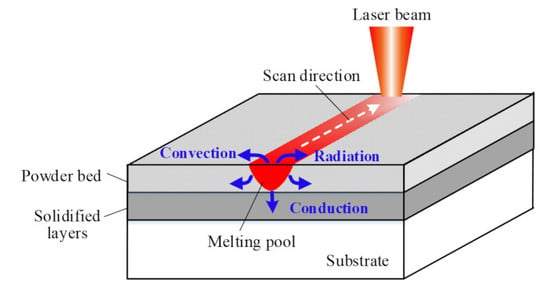
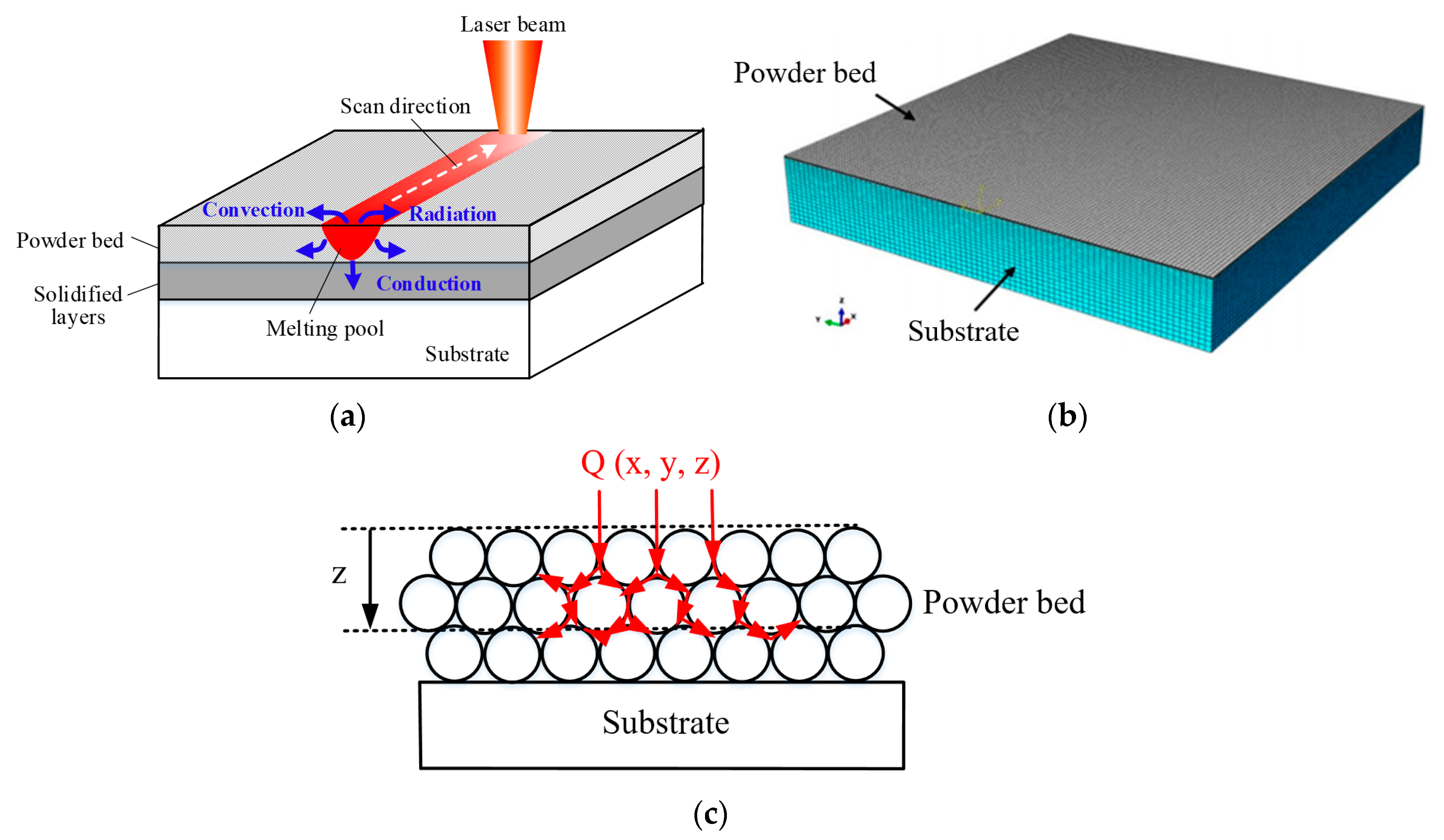
3.1. Physical Description of Model
3.2. Initial Governing Equation
3.3. Heat-Source Model
3.4. Physical Properties of Materials
4. Results and Discussion
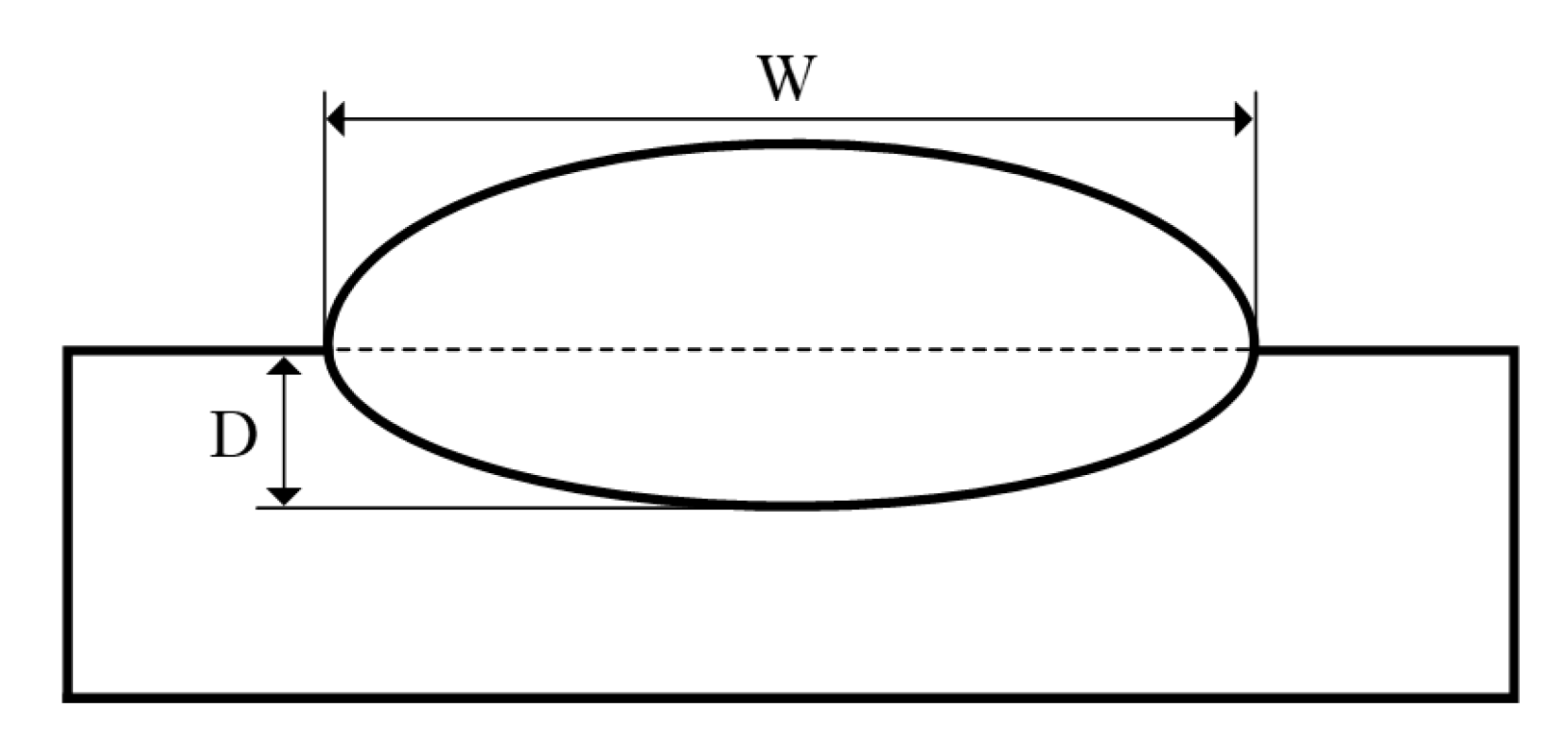
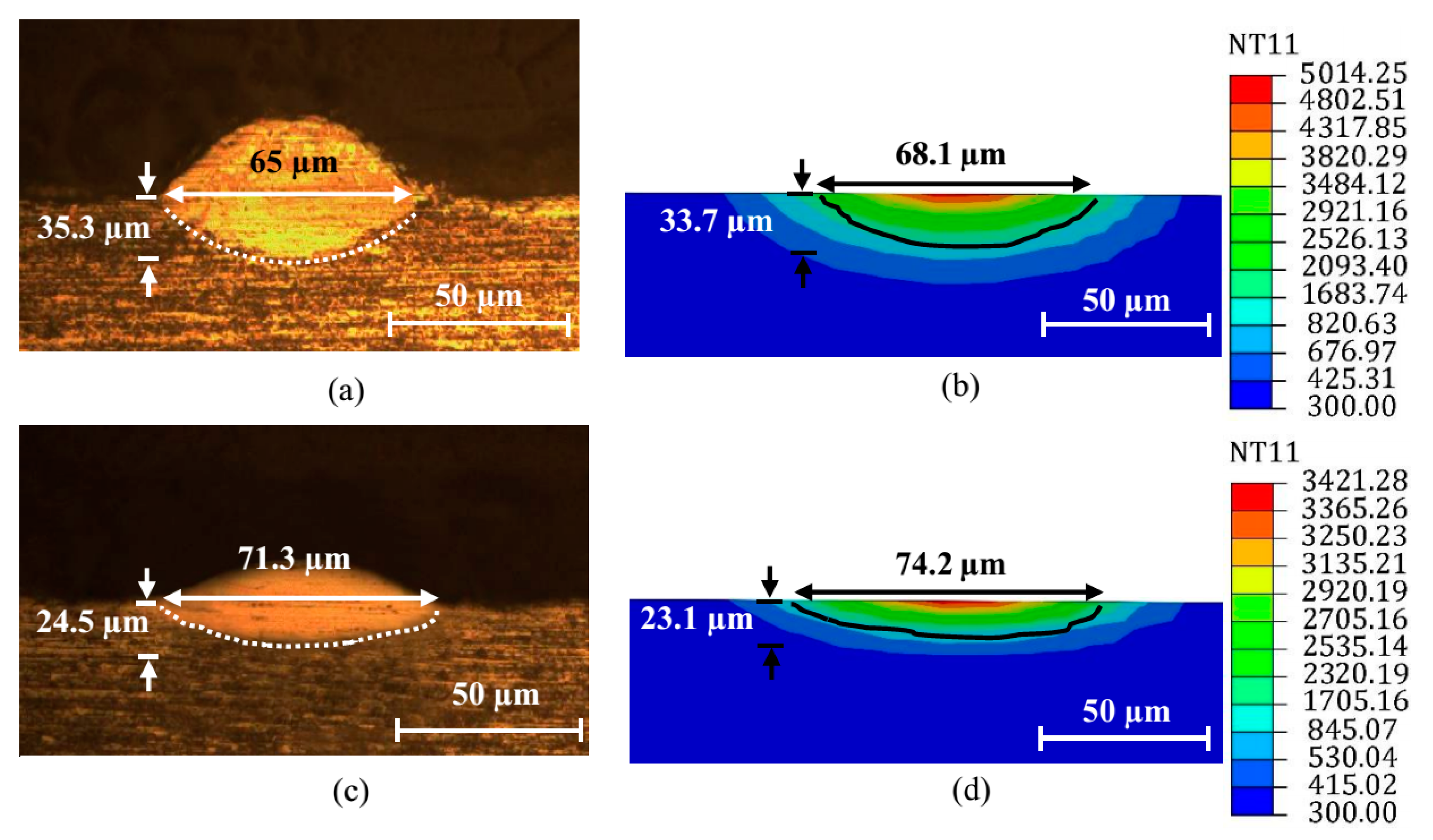
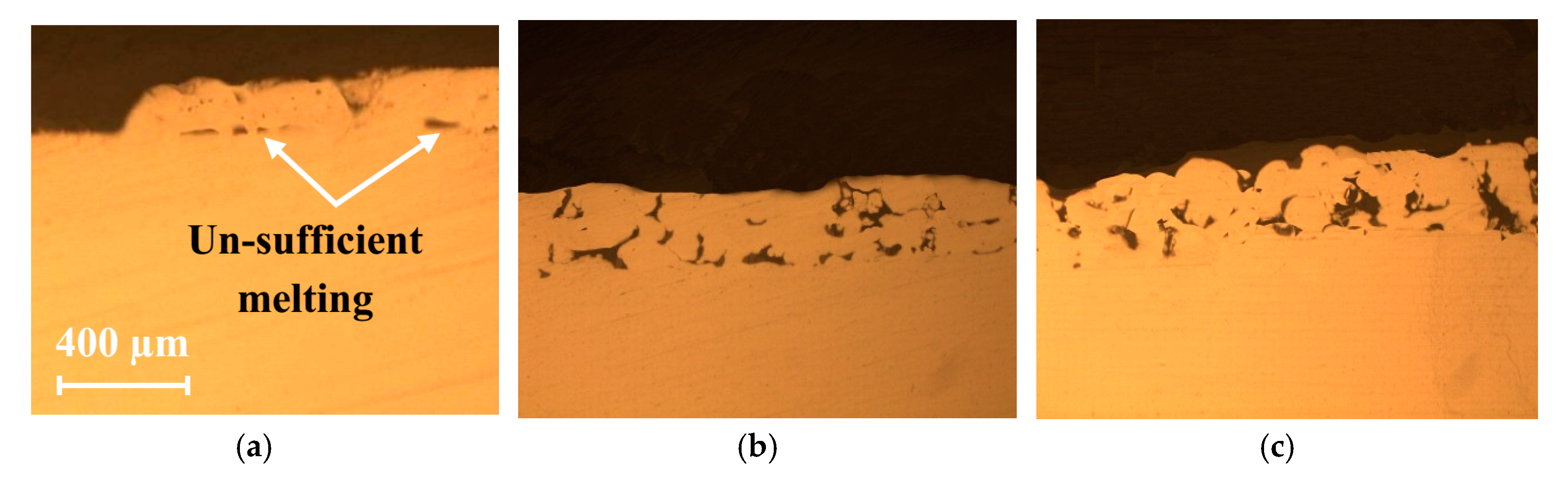
4.1. Numerical Validation and Melt-Pool Characterization
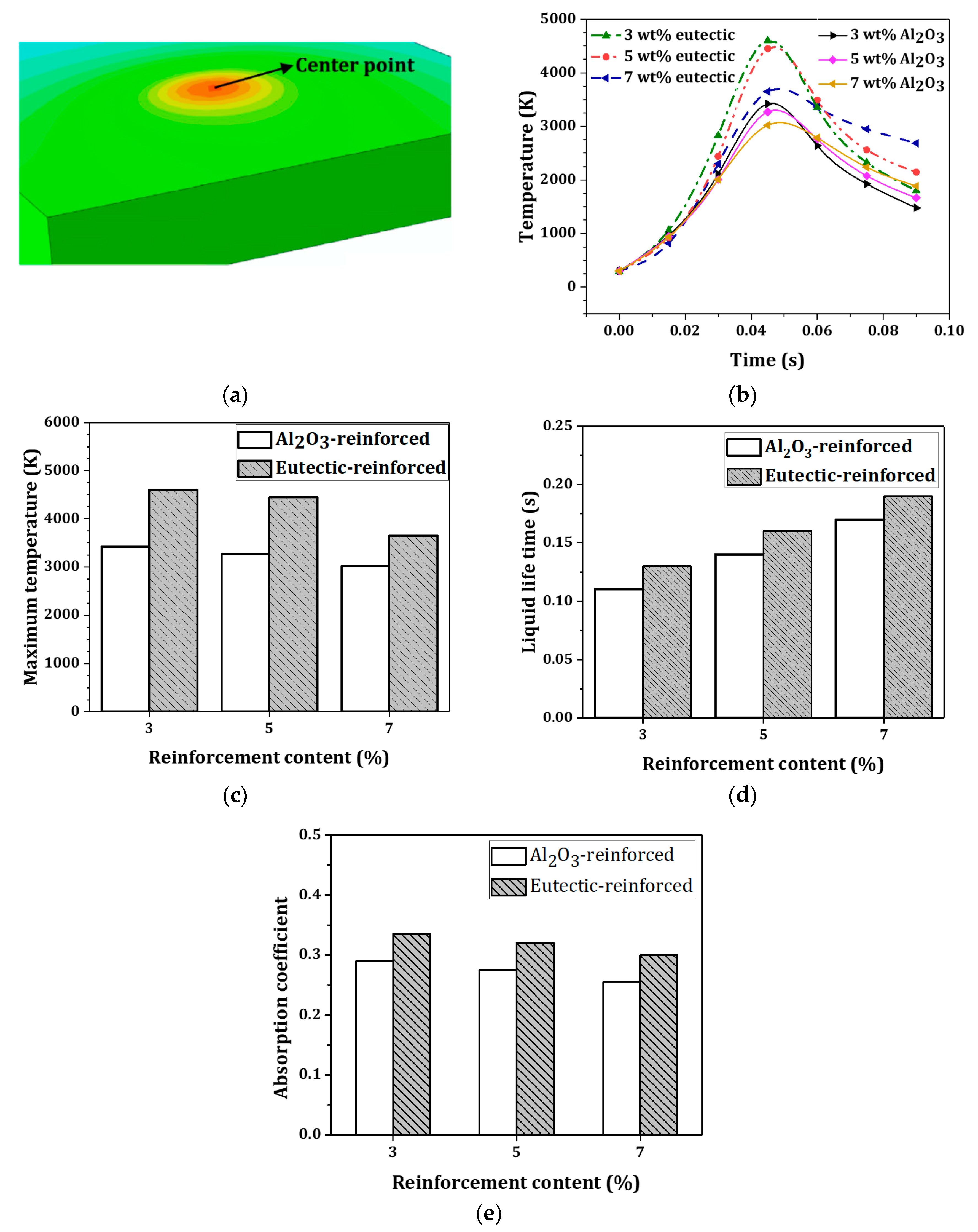
4.2. Temperature Distribution and Liquid Lifetime
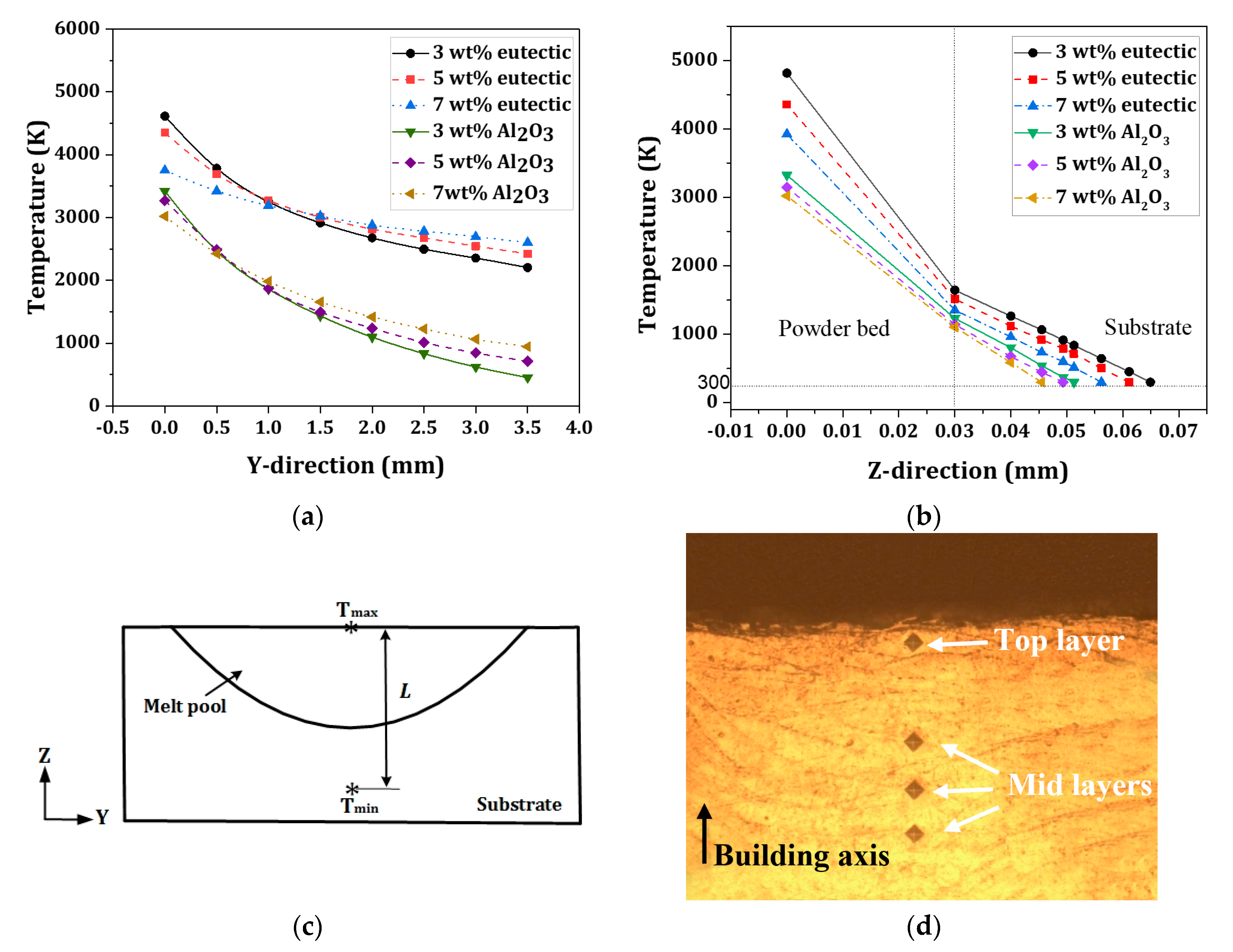
4.3. Temperature Gradient
5. Conclusions
- (1)
- A 3D FE model for the SLM of Al2O3-reinforced and eutectic Al2O3-ZrO2-reinforced AISI 304 steel composite powders was developed and successfully employed to compare the effects of the reinforcing materials on the melt-pool behaviors.
- (2)
- The width and depth of the melt pool were larger for the eutectic-reinforced sample, which is mainly attributed to the reduction in the melting point and thermal conductivity in this sample. With the use of the eutectic Al2O3-ZrO2 instead of the Al2O3 reinforcing particle, the maximum temperature is increased due to the reduction in the thermal conductivity and latent heat of the mixture.
- (3)
- As the reinforcement content increases, the cooling rate decreases. The liquid lifetime of the melt pool has the effect on the melting behavior, rather than the cooling rate, and the liquid lifetime increases with the use of a eutectic ratio of Al2O3-ZrO2 reinforcement. An average and moderate condition for the liquid lifetime was identified to be 5 wt% for the eutectic mixture.
- (4)
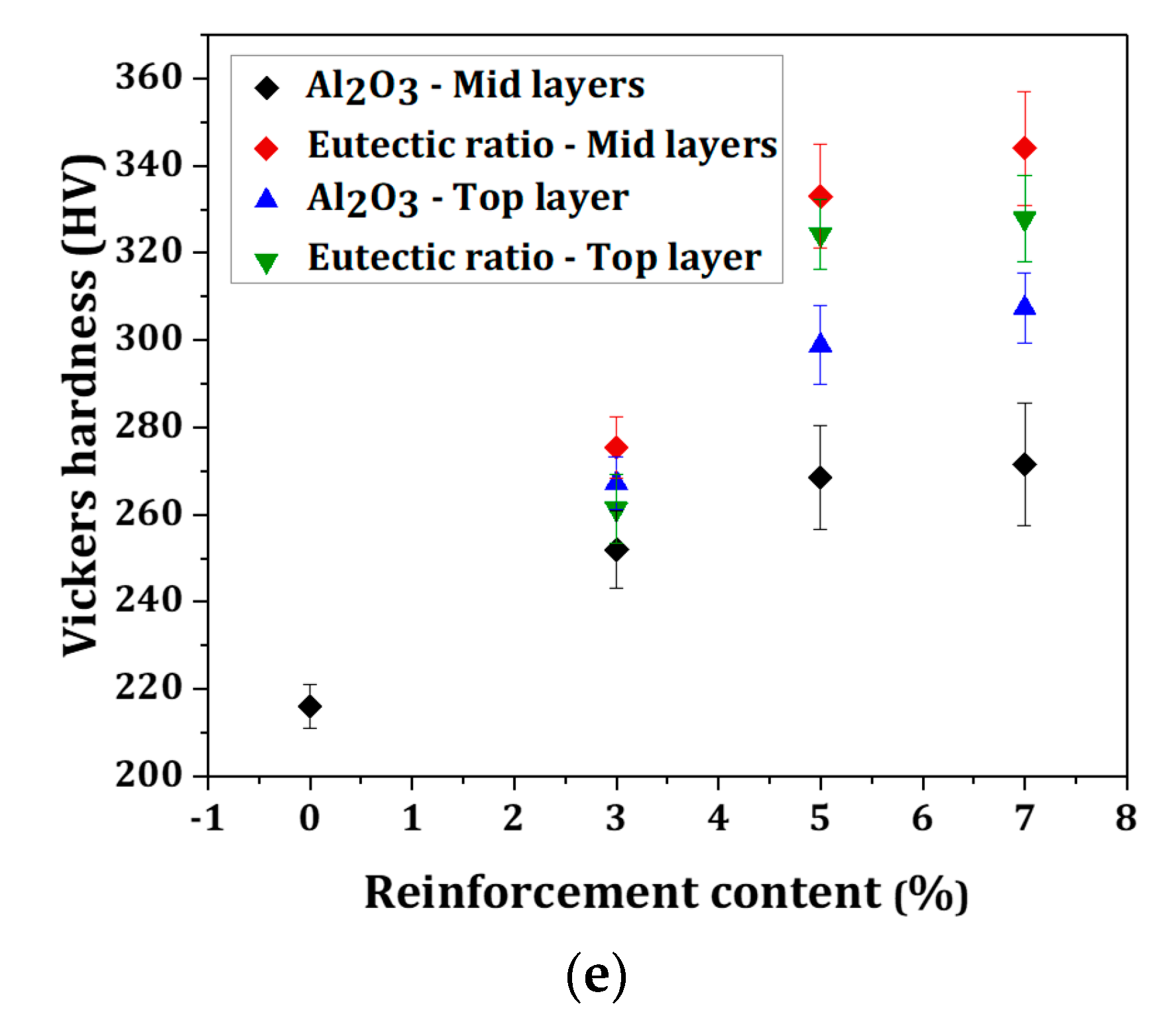
- With the use of a eutectic Al2O3-ZrO2 reinforcing particle, the temperature gradient at the top surface reduces compared with the Al2O3-reinforced sample, due to a wider melt pool and a larger temperature distribution. This led to a narrower discrepancy between the micro-hardness of Al2O3-reinforced samples and the eutectic ratio of Al2O3-ZrO2-reinforced samples at the top layer, indicating an improvement in reinforcement particles’ distribution towards the surface in Al2O3-reinforced samples. The molten-pool behaviors and the thermal evolution of AISI 304 stainless-steel composites during the selective laser melting process will provide a deep understanding of the effect of reinforcing particles on the shape accuracies and properties of fabricated products.
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Willy, H.J.; Chang, S.; Lu, W.; Herng, T.S.; Ding, J. Selective laser melting of stainless steel and alumina composite: Experimental and simulation studies on processing parameters, microstructure and mechanical properties. Mater. Des. 2018, 145, 1–10. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Yang, J.M. Selective laser melting of TiC reinforced 316L stainless steel matrix nanocomposites: Influence of starting TiC particle size and volume content. Mater. Des. 2016, 104, 141–151. [Google Scholar] [CrossRef]
- Yves-Christian, H.; Jan, W.; Wilhelm, M.; Konrad, W.; Reinhart, P. Net shaped high performance oxide ceramic parts by selective laser melting. Physics Procedia 2010, 5, 587–594. [Google Scholar] [CrossRef]
- Wilkes, J.; Hagedorn, Y.C.; Meiners, W.; Wissenbach, K. Additive manufacturing of ZrO2-Al2O3 ceramic components by selective laser melting. Rapid Prototyp. J. 2013, 19, 51–57. [Google Scholar] [CrossRef]
- Jang, J.H.; Joo, B.D.; Van Tyne, C.J.; Moon, Y.H. Characterization of deposited layer fabricated by direct laser melting process. Met. Mater. Int. 2013, 19, 497–506. [Google Scholar] [CrossRef]
- Hwang, T.W.; Woo, Y.Y.; Han, S.W.; Moon, Y.H. Fabrication of mesh patterns using a selective laser-melting process. Appl. Sci. 2019, 9, 1922. [Google Scholar] [CrossRef]
- Joo, B.D.; Jang, J.H.; Lee, J.H.; Son, Y.M.; Moon, Y.H. Selective laser melting of Fe-Ni-Cr layer on AISI H13 tool steel. Trans. Nonferrous Met. Soc. China 2009, 19, 921–924. [Google Scholar] [CrossRef]
- Altan, T.; Lilly, B.; Yen, Y.C.; Altan, T. Manufacturing of dies and molds. CIRP Ann. 2001, 50, 404–422. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joo, B.D.; Shin, S.; Van Tyne, C.J.; Moon, Y.H. Discrete layer hydroforming of three-layered tubes. Int. J. Mach. Tools Manuf. 2013, 68, 56–62. [Google Scholar] [CrossRef]
- Karnati, S.; Zhang, Y.; Liou, F.F.; Newkirk, J.W. On the feasibility of tailoring copper–nickel functionally graded materials fabricated through laser metal deposition. Metals 2019, 9, 287. [Google Scholar] [CrossRef]
- Han, S.W.; Woo, Y.Y.; Hwang, T.W.; Oh, I.Y.; Moon, Y.H. Tailor layered tube hydroforming for fabricating tubular parts with dissimilar thickness. Int. J. Mach. Tools Manuf. 2019, 138, 51–65. [Google Scholar] [CrossRef]
- Abazari, H.D.; Seyedkashi, S.M.H.; Gollo, M.H.; Moon, Y.H. Evolution of microstructure and mechanical properties of SUS430/C11000/SUS430 composites during the laser-forming process. Met. Mater. Int. 2017, 23, 865–876. [Google Scholar] [CrossRef]
- Zhao, J.R.; Hung, F.Y.; Lui, T.S.; Wu, Y.L. The relationship of fracture mechanism between high temperature tensile mechanical properties and particle erosion resistance of selective laser melting Ti-6Al-4V alloy. Metals 2019, 9, 501. [Google Scholar] [CrossRef]
- Hwang, T.W.; Woo, Y.Y.; Han, S.W.; Moon, Y.H. Functionally graded properties in directed-energy-deposition titanium parts. Opt. Laser Technol. 2018, 105, 80–88. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, J.H.; Joo, B.D.; Moon, Y.H. Flow characteristics of aluminum coated boron steel in hot press forming. T. Nonferr. Metal. Soc. 2009, 19, 913–916. [Google Scholar] [CrossRef]
- Seo, D.M.; Hwang, T.W.; Moon, Y.H. Carbonitriding of Ti-6Al-4V alloy via laser irradiation of pure graphite powder in nitrogen environment. Surf. Coat. Technol. 2019, 363, 244–254. [Google Scholar] [CrossRef]
- King, W.; Anderson, A.T.; Ferencz, R.M.; Hodge, N.E.; Kamath, C.; Khairallah, S.A. Overview of modelling and simulation of metal powder bed fusion process at Lawrence Livermore National Laboratory. Mater. Sci. Technol. 2015, 31, 957–968. [Google Scholar] [CrossRef]
- Schleifenbaum, H.; Meiners, W.; Wissenbach, K.; Hinke, C. Individualized production by means of high power selective laser melting. CIRP J. Manuf. Sci. Technol. 2010, 2, 161–169. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Cai, Q.Z.; Liu, J.H.; Zhang, L.; Li, R.D. Select laser melting of W–Ni–Fe powders: Simulation and experimental study. Int. J. Adv. Manuf. Technol. 2010, 51, 649–658. [Google Scholar] [CrossRef]
- Hussein, A.; Hao, L.; Yan, C.; Everson, R. Finite element simulation of the temperature and stress fields in single layers built without-support in selective laser melting. Mater. Des. 2013, 52, 638–647. [Google Scholar] [CrossRef]
- Dai, K.; Shaw, L. Thermal and mechanical finite element modeling of laser forming from metal and ceramic powders. Acta Mater. 2004, 52, 69–80. [Google Scholar] [CrossRef]
- Kundakcioglu, E.; Lazoglu, I.; Rawal, S. Transient thermal modeling of laser-based additive manufacturing for 3D freeform structures. Int. J. Adv. Manuf. Technol. 2016, 85, 493–501. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Cheng, J.; Ertas, Y. Thermal behavior of the molten pool, microstructural evolution, and tribological performance during selective laser melting of TiC/316L stainless steel nanocomposites: Experimental and simulation methods. J. Mater. Process. Technol. 2018, 257, 288–301. [Google Scholar] [CrossRef]
- Shi, Q.; Gu, D.; Xia, M.; Cao, S.; Rong, T. Effects of laser processing parameters on thermal behavior and melting/solidification mechanism during selective laser melting of TiC/Inconel 718 composites. Opt. Laser Technol. 2016, 84, 9–22. [Google Scholar] [CrossRef]
- Wang, L.; Jue, J.; Xia, M.; Guo, L.; Yan, B.; Gu, D. Effect of the Thermodynamic behavior of selective laser melting on the formation of in situ oxide dispersion-strengthened aluminum-based composites. Metals 2016, 6, 286. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, M.; Huang, H. Selective laser melting of alumina: A single track study. Ceram. Int. 2018, 44, 9484–9493. [Google Scholar] [CrossRef]
- Vastola, G.; Zhang, G.; Pei, X.Q.; Zhang, Y.W. Modeling the microstructure evolution during additive manufacturing of Ti6Al4V: A comparison between electron beam melting and selective laser melting. JOM 2016, 68, 1370–1375. [Google Scholar] [CrossRef]
- Gusarov, A.V.; Kruth, J.P. Modelling of radiation transfer in metallic powders at laser treatment. Int. J. Heat Mass Transfer 2005, 48, 3423–3434. [Google Scholar] [CrossRef]
- Sih, S.S.; Barlow, J.W. The prediction of the emissivity and thermal conductivity of powder beds. Part. Sci. Technol. 2004, 22, 427–440. [Google Scholar] [CrossRef]
- Boley, C.D.; Mitchell, S.C.; Rubenchik, A.M.; Wu, S.S.Q. Metal powder absorptivity: Modeling and experiment. Appl. Optics. 2016, 55, 6496. [Google Scholar] [CrossRef]
- Angle, J.P.; Wang, Z.; Dames, C.; Mecartney, M.L. Comparison of two-phase thermal conductivity models with experiments on dilute ceramic composites. J. Am. Ceram. Soc. 2013, 96, 2935–2942. [Google Scholar] [CrossRef]
- Spierings, A.B.; Dawson, K.; Heeling, T.; Uggowitzer, P.J.; Schäublin, R.; Palm, F.; Wegener, K. Microstructural features of Sc-and Zr-modified Al-Mg alloys processed by selective laser melting. Mater. Des. 2017, 115, 52–63. [Google Scholar] [CrossRef]
- Loh, L.E.; Chua, C.K.; Yeong, W.Y.; Song, J.; Mapar, M.; Sing, S.L.; Liu, Z.H.; Zhang, D.Q. Numerical investigation and an effective modelling on the Selective Laser Melting (SLM) process with aluminium alloy 6061. Int. J. Heat Mass Transfer 2015, 80, 288–300. [Google Scholar] [CrossRef]
- Mishra, A.K.; Aggarwal, A.; Kumar, A.; Sinha, N. Identification of a suitable volumetric heat source for modelling of selective laser melting of Ti6Al4V powder using numerical and experimental validation approach. Int. J. Adv. Manuf. Technol. 2018, 99, 2257–2270. [Google Scholar] [CrossRef]
- Jeon, T.J.; Hwang, T.W.; Yun, H.J.; Van Tyne, C.J.; Moon, Y.H. Control of porosity in parts produced by a direct laser melting process. Appl. Sci. 2018, 8, 2573. [Google Scholar] [CrossRef]
- Jeon, C.H.; Han, S.W.; Joo, B.D.; Van Tyne, C.J.; Moon, Y.H. Deformation analysis for cold rolling of Al-Cu double layered sheet by physical modeling and finite element method. Met. Mater. Int. 2013, 19, 1069–1076. [Google Scholar] [CrossRef]
- Gordon, W.A.; Van Tyne, C.J.; Moon, Y.H. Axisymmetric extrusion through adaptable dies—Part 1: Flexible velocity fields and power terms. Int. J. Mech. Sci. 2007, 49, 86–95. [Google Scholar] [CrossRef]
- Gordon, W.A.; Van Tyne, C.J.; Moon, Y.H. Axisymmetric extrusion through adaptable dies—Part 3: Minimum pressure streamlined die shapes. Int. J. Mech. Sci. 2007, 49, 104–115. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Borkar, T.; Yang, J.M. Densification behavior, microstructural evolution, and mechanical properties of TiC/316L stainless steel nanocomposites fabricated by selective laser melting. Mater. Des. 2018, 138, 119–128. [Google Scholar] [CrossRef]



| Ex. No. | wt% of Al2O3 | wt% of Al2O3-ZrO2 | wt% of 304SS |
|---|---|---|---|
| 1 | 0 | 0 | 100% |
| 2 | 3% | - | 97% |
| 3 | 5% | - | 95% |
| 4 | 7% | - | 93% |
| 5 | - | 3% | 97% |
| 6 | - | 5% | 95% |
| 7 | - | 7% | 93% |
| Powder | R(i) | D(i) (m) | ϕ(i) | ρbulk (kg/m3) | ρpowder (kg/m3) | Apowder |
|---|---|---|---|---|---|---|
| AISI 304 | 0.46 | 20 × 10−6 | 0.25 | 7900 | 5861.8 | 0.604 |
| Al2O3 | 0.79 | 3 × 10−6 | 0.65 | 3970 | 1580 | 0.173 |
| ZrO2 | 0.82 | 1 × 10−6 | 0.21 | 6000 | 4740 | 0.266 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abolhasani, D.; Seyedkashi, S.M.H.; Kang, N.; Kim, Y.J.; Woo, Y.Y.; Moon, Y.H. Analysis of Melt-Pool Behaviors during Selective Laser Melting of AISI 304 Stainless-Steel Composites. Metals 2019, 9, 876. https://doi.org/10.3390/met9080876
Abolhasani D, Seyedkashi SMH, Kang N, Kim YJ, Woo YY, Moon YH. Analysis of Melt-Pool Behaviors during Selective Laser Melting of AISI 304 Stainless-Steel Composites. Metals. 2019; 9(8):876. https://doi.org/10.3390/met9080876
Chicago/Turabian StyleAbolhasani, Daniyal, S. M. Hossein Seyedkashi, Namhyun Kang, Yang Jin Kim, Young Yun Woo, and Young Hoon Moon. 2019. "Analysis of Melt-Pool Behaviors during Selective Laser Melting of AISI 304 Stainless-Steel Composites" Metals 9, no. 8: 876. https://doi.org/10.3390/met9080876
APA StyleAbolhasani, D., Seyedkashi, S. M. H., Kang, N., Kim, Y. J., Woo, Y. Y., & Moon, Y. H. (2019). Analysis of Melt-Pool Behaviors during Selective Laser Melting of AISI 304 Stainless-Steel Composites. Metals, 9(8), 876. https://doi.org/10.3390/met9080876