An In Vivo Evaluation of Biocompatibility and Implant Accuracy of the Electron Beam Melting and Commercial Reconstruction Plates
Abstract
1. Introduction
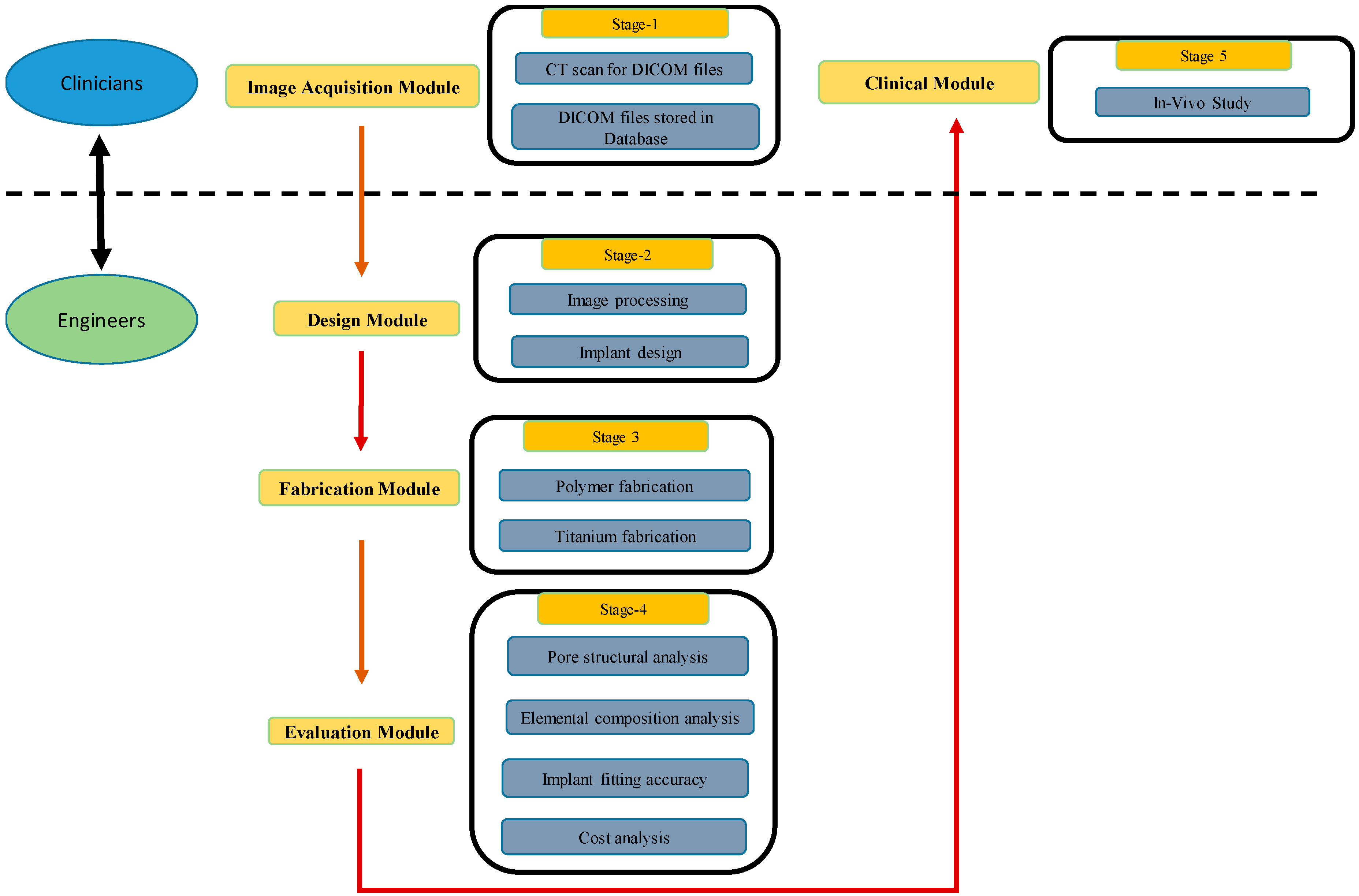
2. Methodology
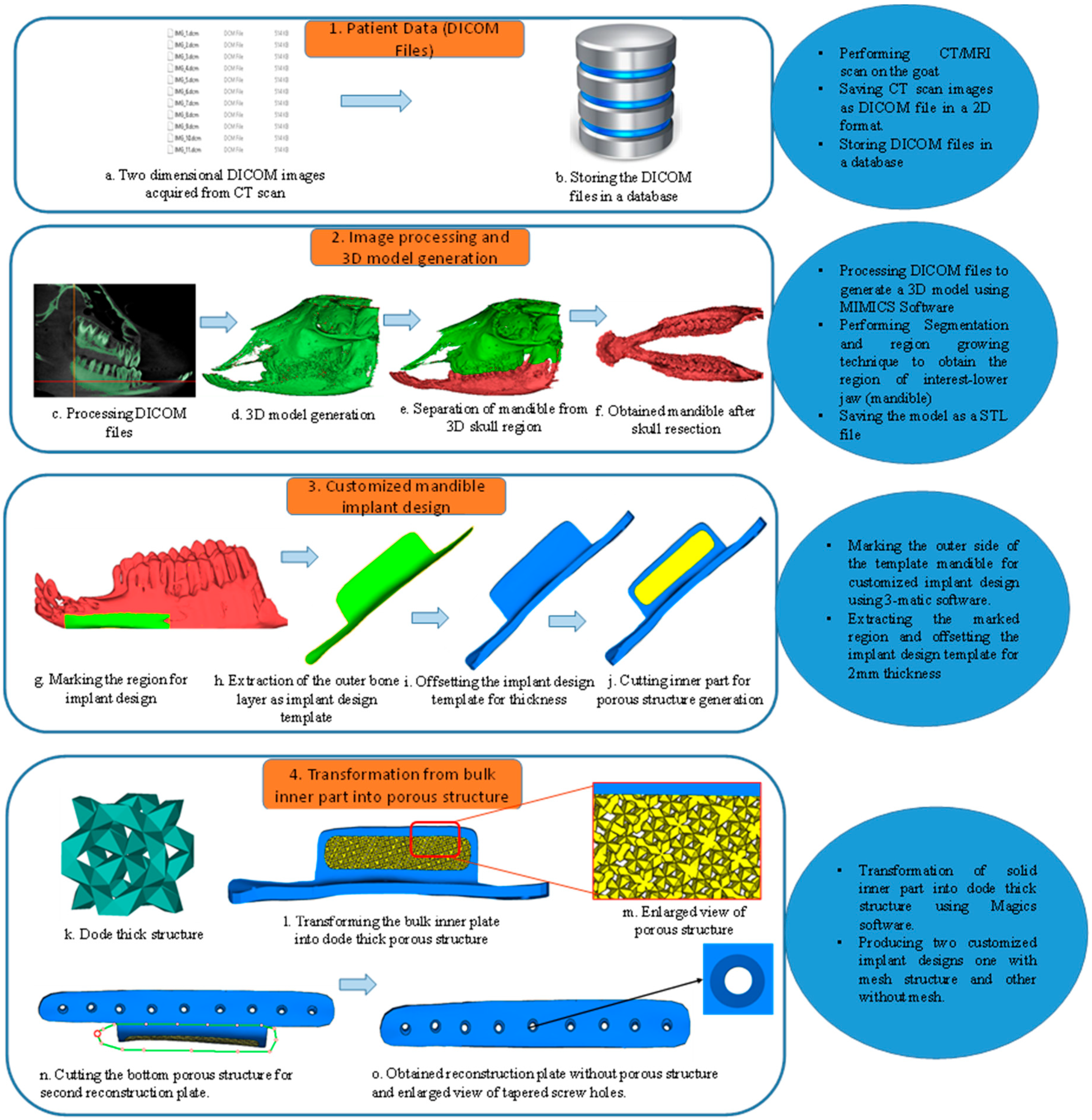
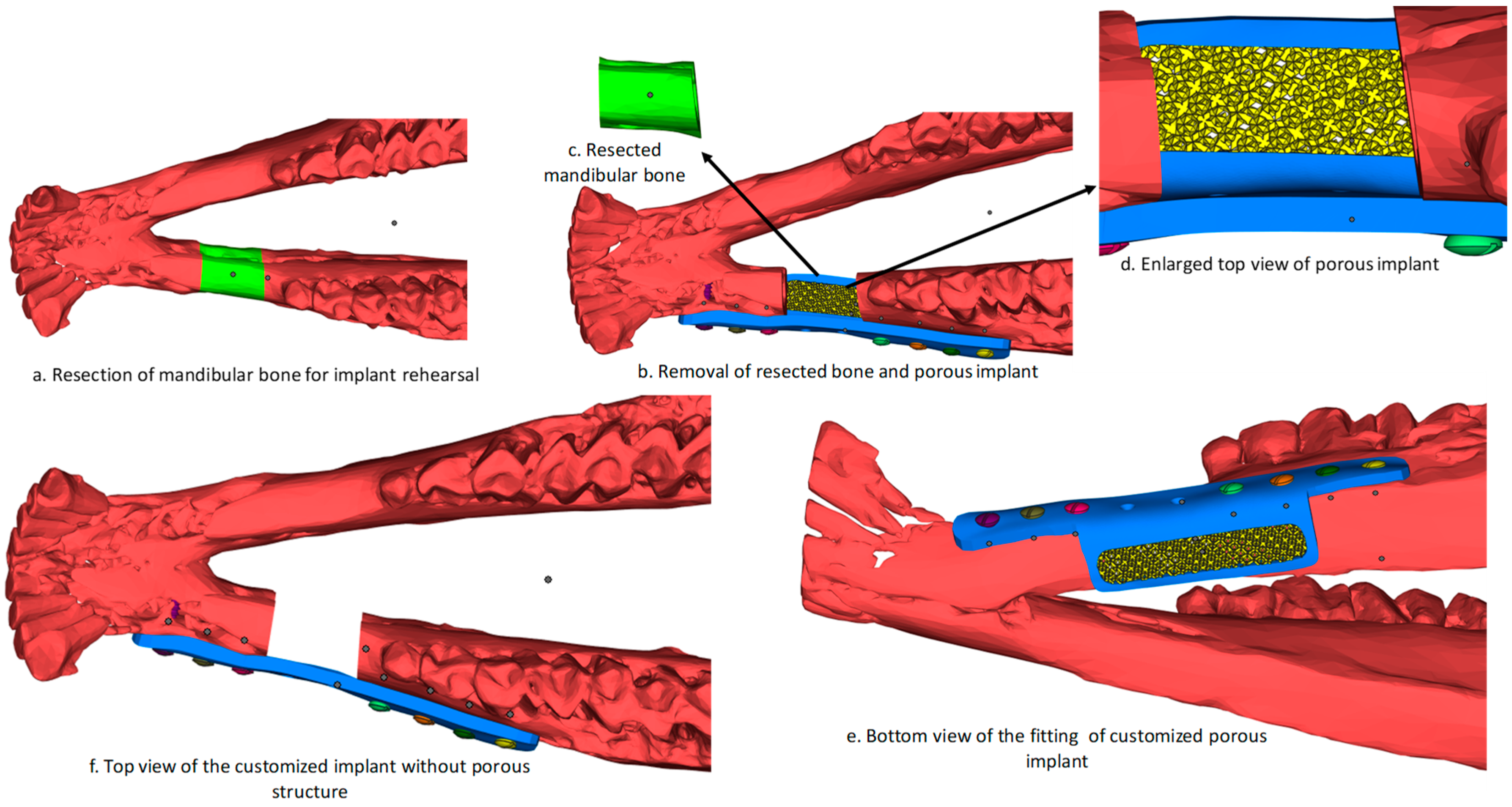
2.1. Image Processing and Implant Design
2.2. 3D Printing Reconstruction Plates
2.2.1. Fabrication of Polymer Model
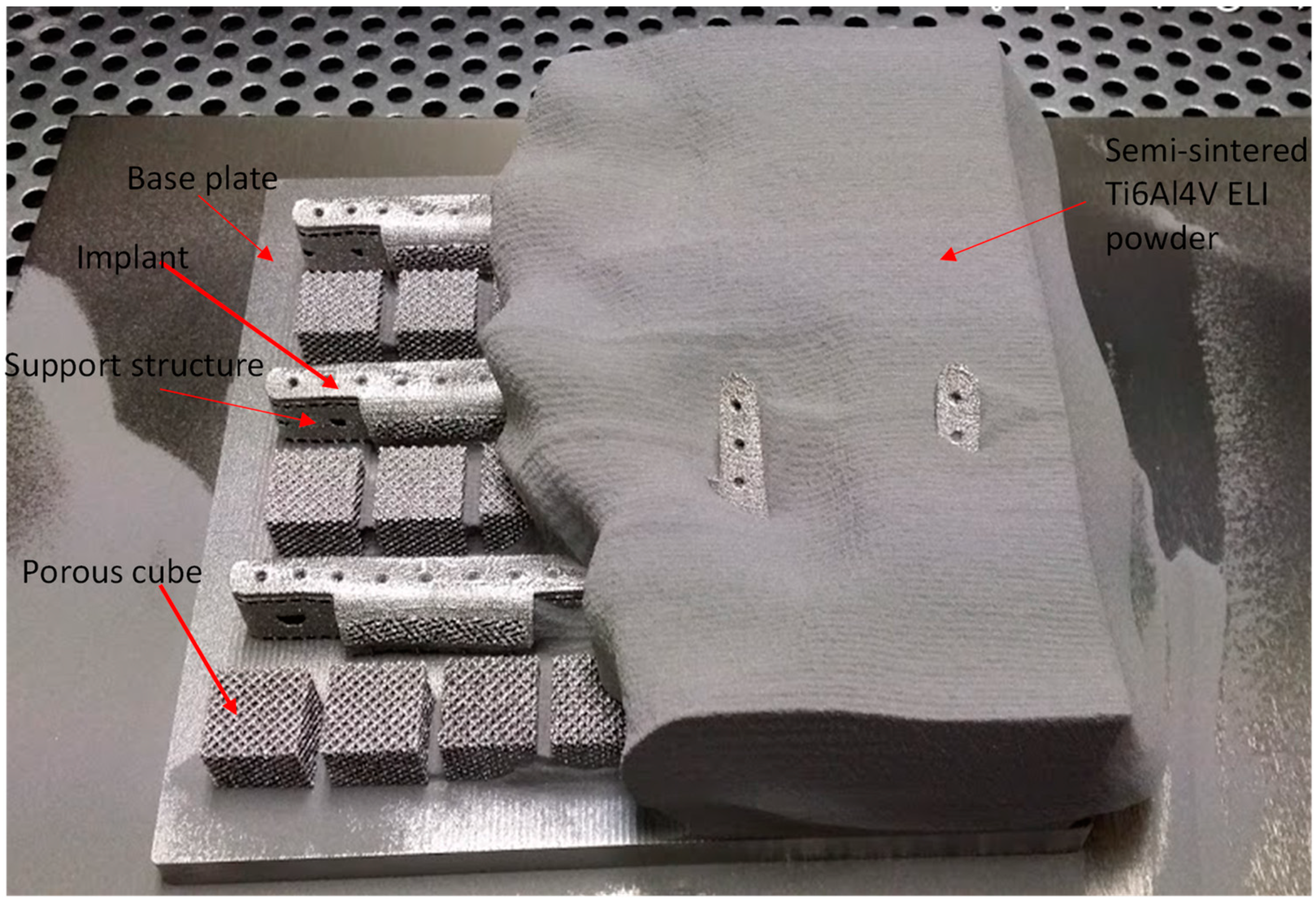
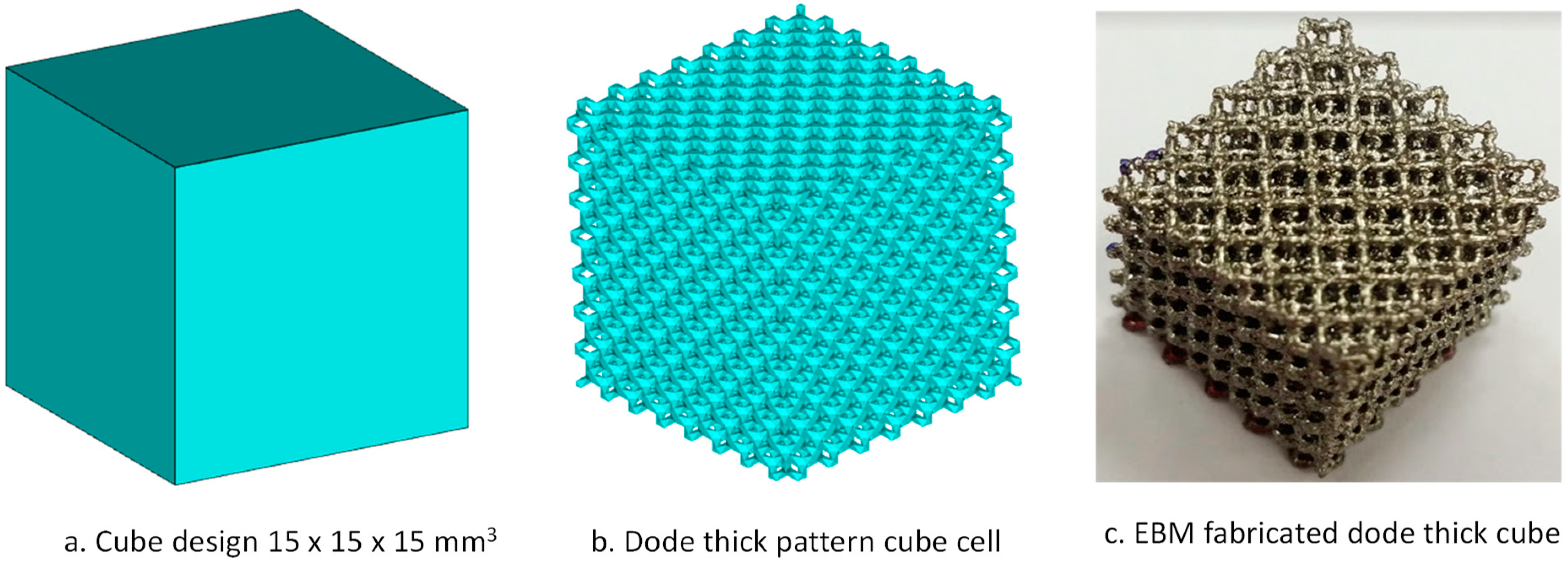
2.2.2. Fabrication of Titanium Implants
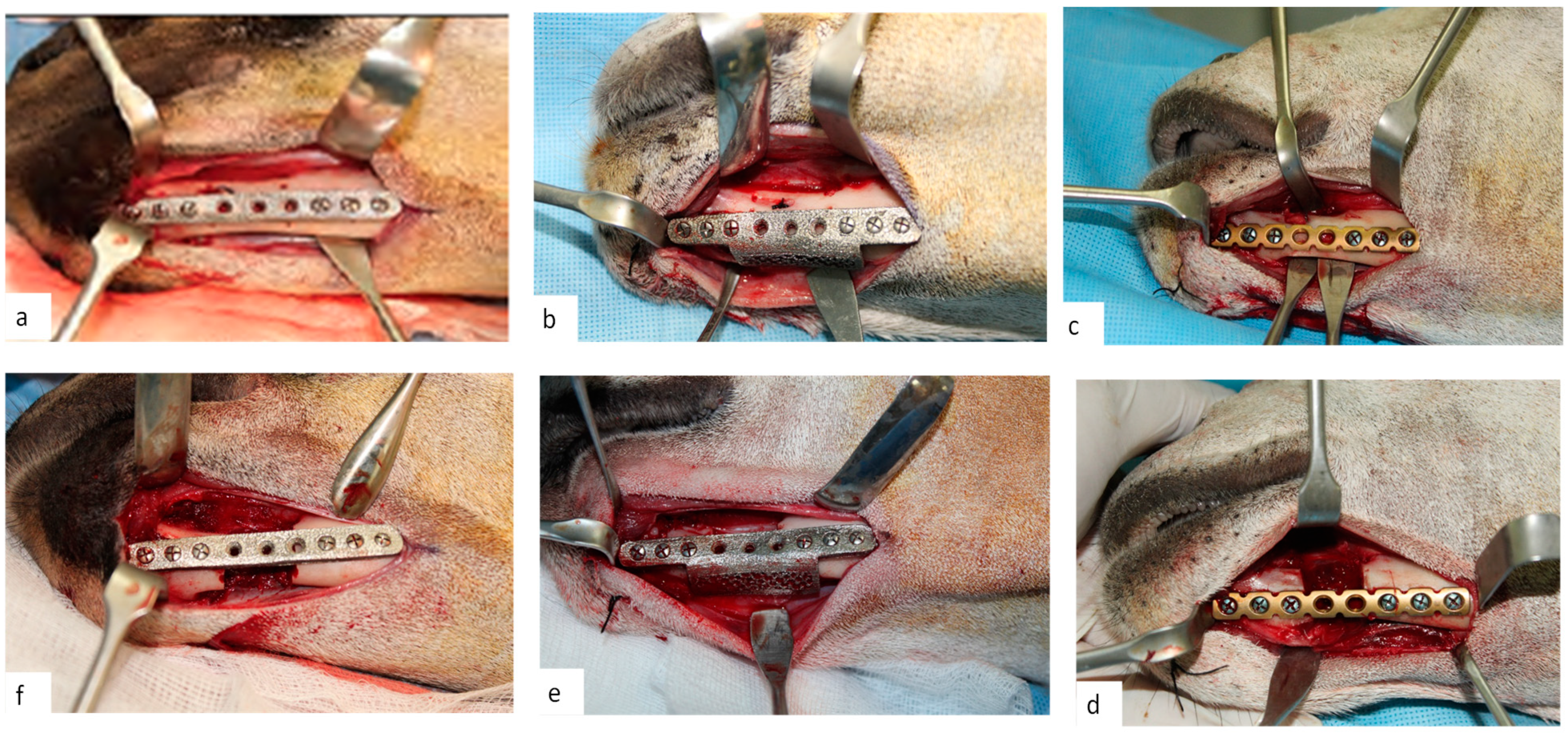
2.3. Implant Evaluation Study
2.3.1. Powder Metallurgical Study
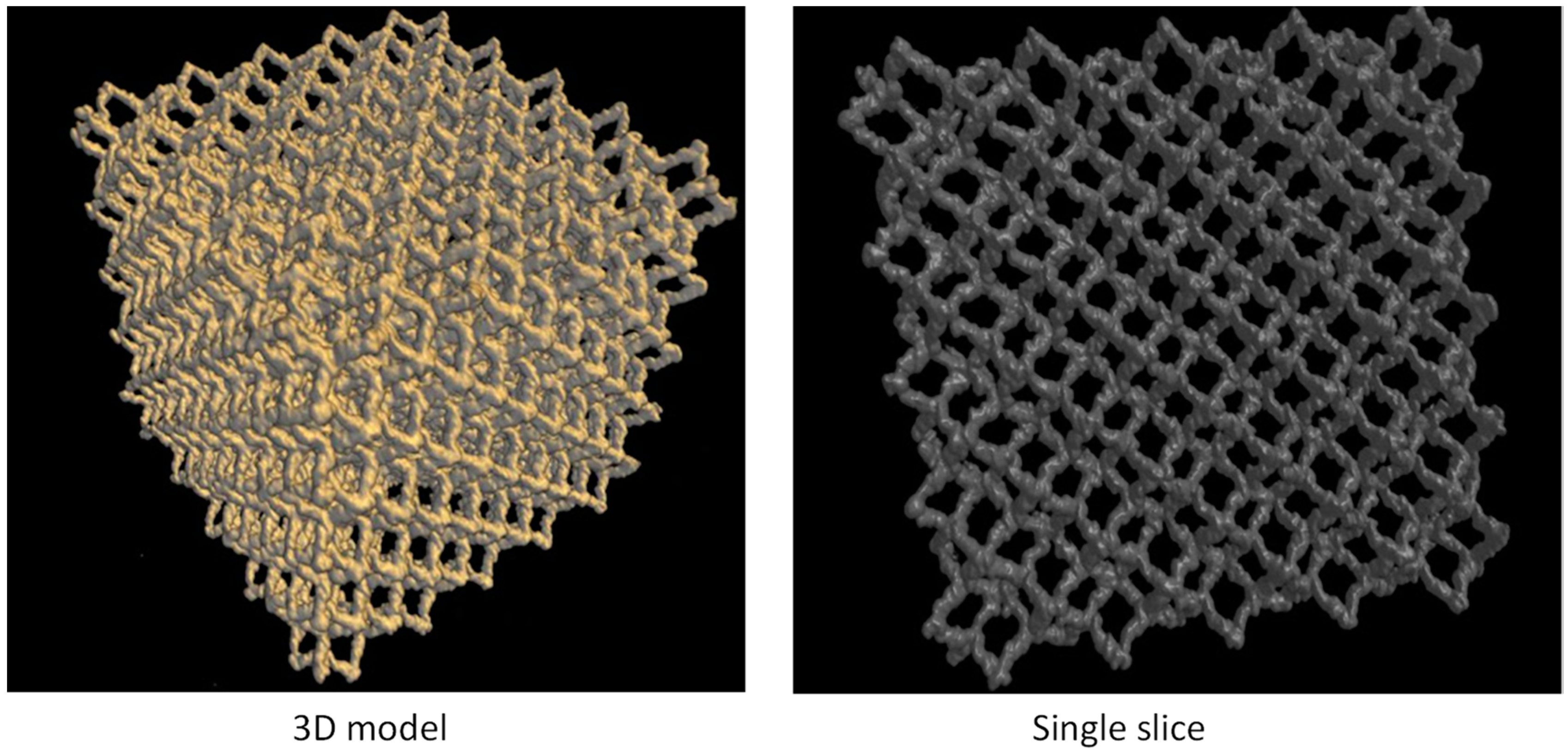
2.3.2. Pore Structural Analysis
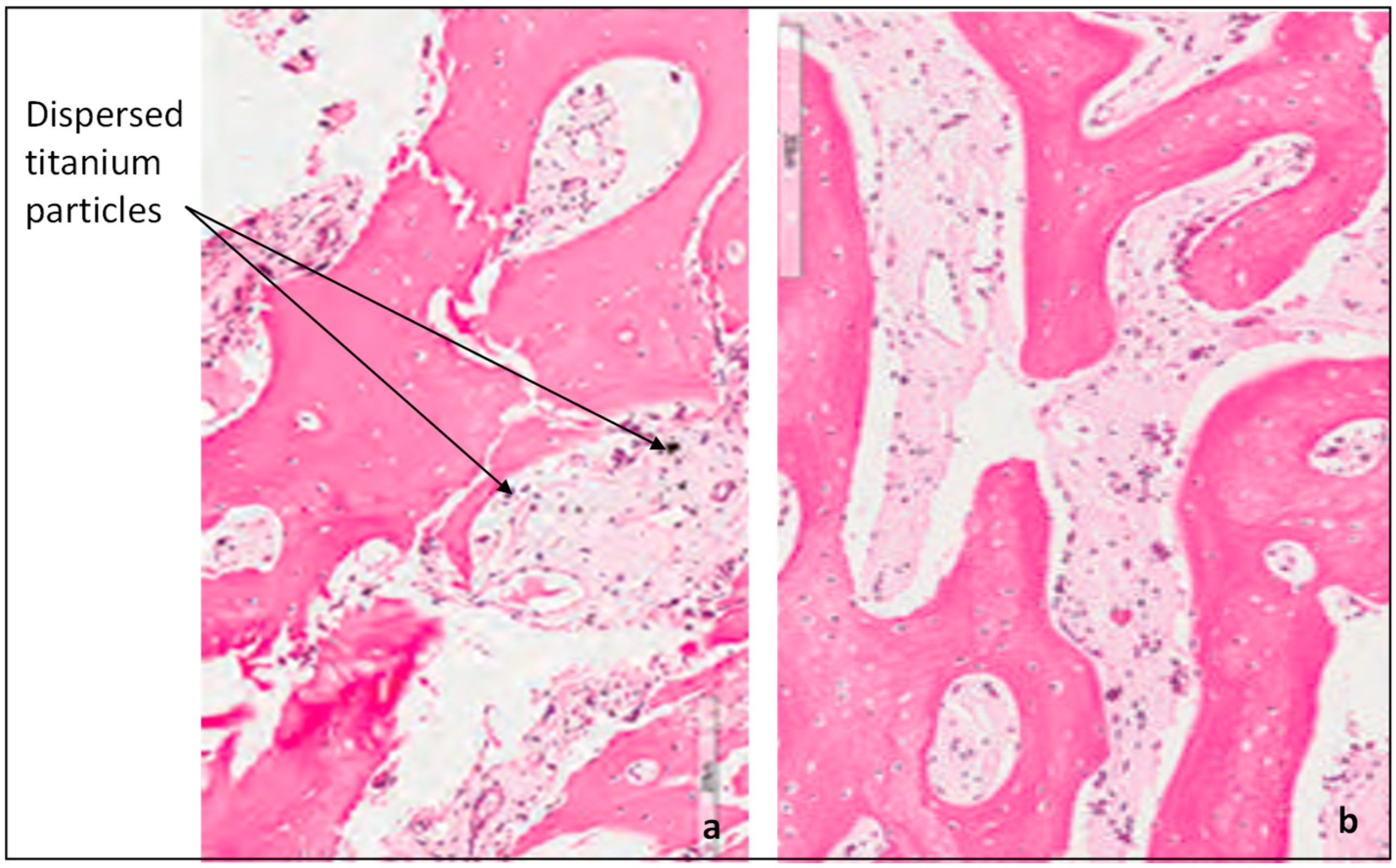
2.3.3. Clinical Study
2.3.4. Accuracy Analysis
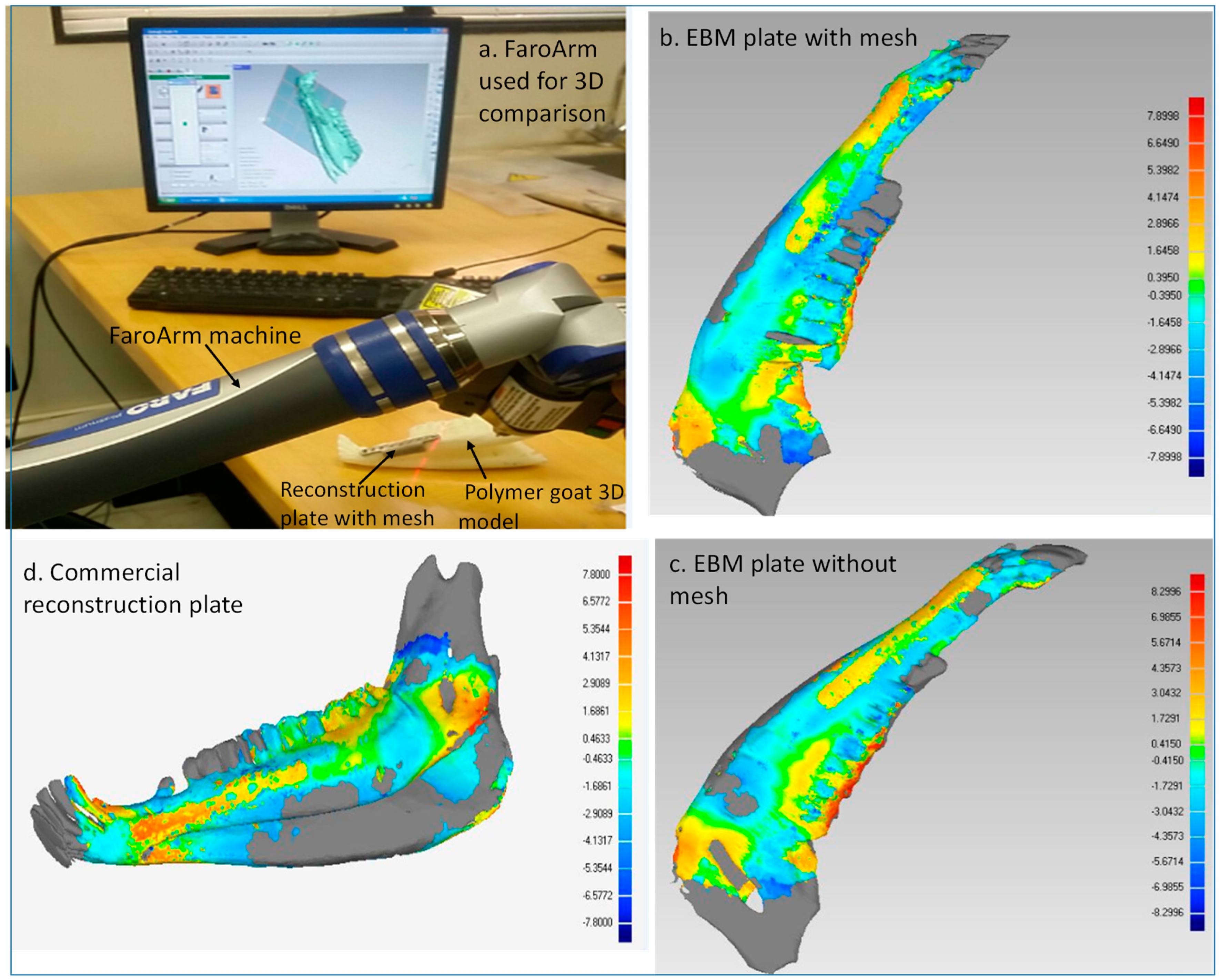
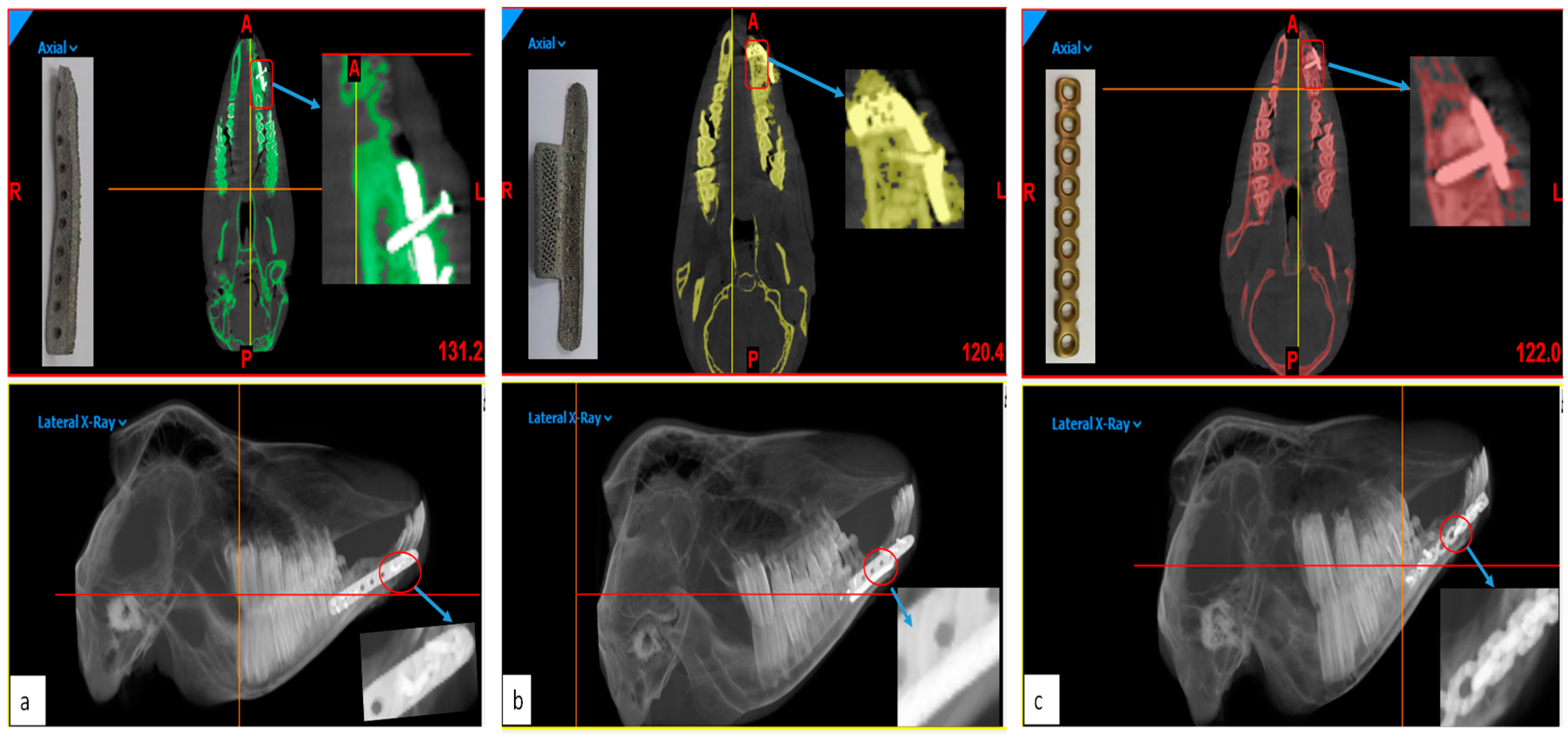
First Stage: Faro Arm Scans
- Acquisition of data: The data for the reconstructed implant mandible models was acquired in the form of the point cloud using a Faro Arm mounted scanner as shown in Figure 9a.
- Distinction of objects as test and reference: The point data for reconstructed implant mandible models from Faro Arm scanner were imported into the software as test models and the original mandible obtained from initial CT scan before surgery was considered to be the reference model.
- Alignment: The imported test mandibular models were aligned and superimposed with the reference mandible models.
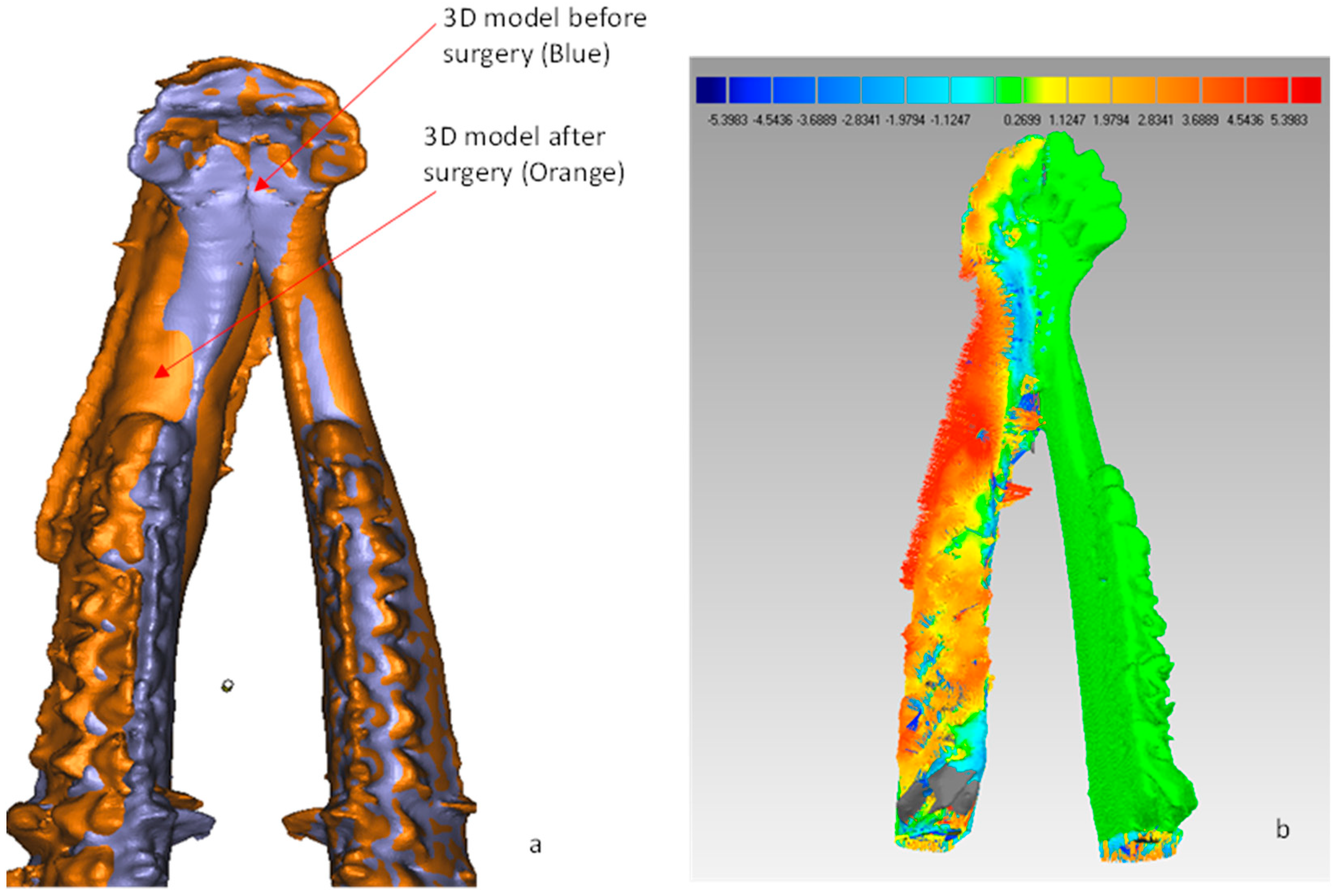
- 3D compare: Finally, the surface deviation between the test and the reference mandibular models (Figure 9b–d) were computed. The analysis data of the surface deviation were recorded.
Second Stage: CBCT Scans
2.3.5. Cost Analysis
- CIndirect—Indirect cost comprising EBM ownership cost and maintenance cost, measured in $/h,
- TBuild—EBM total build time,
- MPart—Mass of EBM-built parts including support structures,
- CRaw—Cost of the raw material (Ti6Al4V ELI) measured in $/g,
- EBuild—Energy consumption for EBM-built part,
- CEnergy—Energy consumption cost (Electricity cost for EBM process), measured in $/KWh.
- MHours—Manual hours spend in preparing the EBM build parts,
- CLabor—Cost of the labor/HR
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Maurer, P.; Eckert, A.W.; Kriwalsky, M.S.; Schubert, J. Scope and limitations of methods of mandibular reconstruction: a long-term follow-up. Br. J. Oral Maxillofac. Surg. 2010, 48, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in Biomedical Applications-Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Moiduddin, K.; Darwish, S.; Al-Ahmari, A.; ElWatidy, S.; Mohammad, A.; Ameen, W. Structural and mechanical characterization of custom design cranial implant created using additive manufacturing. Electron. J. Biotechnol. 2017, 29, 22–31. [Google Scholar] [CrossRef]
- Parthasarathy, J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Maxillofac. Surg. 2014, 4, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.; Pandit, A.; Apatsidis, D. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Ângelo, D.F.; Morouço, P.; Alves, N.; Viana, T.; Santos, F.; González, R.; Monje, F.; Macias, D.; Carrapiço, B.; Sousa, R.; et al. Choosing sheep (Ovis aries) as animal model for temporomandibular joint research: Morphological, histological and biomechanical characterization of the joint disc. Morphologie 2016, 100, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.K.; Chakraborty, A.; Banerjee, S. A micro-anatomical comparison of goat jaw cancellous bone with human mandible: Histomorphometric study for implant dentistry. J. Int. Clin. Dent. Res. Organ. 2014, 6, 20–23. [Google Scholar]
- Martola, M.; Lindqvist, C.; Hänninen, H.; Al-Sukhun, J. Fracture of titanium plates used for mandibular reconstruction following ablative tumor surgery. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 345–352. [Google Scholar] [CrossRef]
- Yang, W.F.; Choi, W.S.; Leung, Y.Y.; Curtin, J.P.; Du, R.; Zhang, C.Y.; Chen, X.S.; Su, Y.X. Three-dimensional printing of patient-specific surgical plates in head and neck reconstruction: A prospective pilot study. Oral Oncol. 2018, 78, 31–36. [Google Scholar] [CrossRef]
- Xilloc/Patient Specific Implants, Mandible Reconstruction Plate: Patient-Specific and 3D Printed. 27 August 2014. Available online: https://www.xilloc.com/products_services/mandible_recon/ (accessed on 21 May 2019).
- Ayoub, N.; Ghassemi, A.; Rana, M.; Gerressen, M.; Riediger, D.; Hölzle, F.; Modabber, A. Evaluation of computer-assisted mandibular reconstruction with vascularized iliac crest bone graft compared to conventional surgery: A randomized prospective clinical trial. Trials 2014, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, N.; da Assuncao, R.; Hancock, N.J.; Lau, A.; Walsh, W.R. Influence of Electron Beam Melting Manufactured Implants on Ingrowth and Shear Strength in an Ovine Model. J. Arthroplast. 2012, 27, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Cronskär, M.; Rännar, L.E.; Bäckström, M. Implementation of Digital Design and Solid Free-Form Fabrication for Customization of Implants in Trauma Orthopaedics. J. Med. Biol. Eng. 2012, 32, 91. [Google Scholar] [CrossRef]
- Lethaus, B.; ter Laak, M.P.; Laeven, P.; Beerens, M.; Koper, D.; Poukens, J.; Kessler, P. A treatment algorithm for patients with large skull bone defects and first results. J. Cranio-Maxillofac. Surg. 2011, 39, 435–440. [Google Scholar] [CrossRef]
- Suska, F.; Kjeller, G.; Tarnow, P.; Hryha, E.; Nyborg, L.; Snis, A.; Palmquist, A. Electron Beam Melting Manufacturing Technology for Individually Manufactured Jaw Prosthesis: A Case Report. J. Oral Maxillofac. Surg. 2016, 74, 1706.e1–1706.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jung, Y.-G. Additive Manufacturing: Materials, Processes, Quantifications and Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-812155-9. [Google Scholar]
- Jones, D.B.; Sung, R.; Weinberg, C.; Korelitz, T.; Andrews, R. Three-Dimensional Modeling May Improve Surgical Education and Clinical Practice. Surg. Innov. 2016, 23, 189–195. [Google Scholar]
- De Viteri, V.S.; Fuentes, E. Titanium and Titanium Alloys as Biomaterials. In Tribology-Fundamentals and Advancements; IntechOpen: Rijeka, Croatia, 2013; pp. 155–181. [Google Scholar]
- Hopkinson, N.; Dickens, P. Emerging Rapid Manufacturing Processes. In Rapid Manufacturing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 55–80. [Google Scholar]
- Murr, L.; Esquivel, E.; Quinones, S.; Gaytan, S.; López, M.; Martinez, E.; Medina, F.; Hernandez, D.; Martinez, E.; Martinez, J.; et al. Microstructures and mechanical properties of electron beam-rapid manufactured Ti–6Al–4V biomedical prototypes compared to wrought Ti–6Al–4V. Mater. Charact. 2009, 60, 96–105. [Google Scholar] [CrossRef]
- Heinl, P.; Korner, C.; Singer, R.F. Selective Electron Beam Melting of Cellular Titanium: Mechanical Properties. Adv. Eng. Mater. 2008, 10, 882–888. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Karp, J.M. Biology and Engineering of Stem Cell Niches; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Luthardt, R.G.; Koch, R.; Rudolph, H.; Walter, M.H. Qualitative computer aided evaluation of dental impressions in vivo. Dent. Mater. 2006, 22, 69–76. [Google Scholar] [CrossRef]
- Geraedts, J.; Doubrovski, E.; Verlinden, J. Three Views on Additive Manufacturing: Business, Research, and Education. In Proceedings of the Tools and Methods of Competitive Engineering, Karlsruhe, Germany, 7–11 May 2012; Horváth, I., Albers, A., Behrendt, M., Rusák, Z., Eds.; Delft University of Technology: Delft, The Netherlands, 2012. [Google Scholar]
- Facchini, L.; Magalini, E.; Robotti, P.; Molinari, A. Microstructure and mechanical properties of Ti-6Al-4V produced by electron beam melting of pre-alloyed powders. Rapid Prototyp. J. 2009, 15, 171–178. [Google Scholar] [CrossRef]
- Arcam EBM system, Ti6Al4V ELI Titanium Alloy. 14 January 2014. Available online: http://www.arcam.com/wp-content/uploads/Arcam-Ti6Al4V-ELI-Titanium-Alloy.pdf (accessed on 3 June 2019).
- Rustemeyer, J.; Melenberg, A.; Sari-Rieger, A. Costs incurred by applying computer-aided design/computer-aided manufacturing techniques for the reconstruction of maxillofacial defects. J. Craniomaxillofac. Surg. 2014, 42, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, A.; Snis, A.; Emanuelsson, L.; Browne, M.; Thomsen, P. Long-term biocompatibility and osseointegration of electron beam melted, free-form–fabricated solid and porous titanium alloy: Experimental studies in sheep. J. Biomater. Appl. 2013, 27, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.; Malmström, J.; Emanuelsson, L.; Rene, M.; Snis, A. Electron beam-melted, free-form-fabricated titanium alloy implants: Material surface characterization and early bone response in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Metzler, P.; Geiger, E.J.; Alcon, A.; Ma, X.; Steinbacher, D.M. Three-dimensional virtual surgery accuracy for free fibula mandibular reconstruction: planned versus actual results. J. Oral Maxillofac. Surg. 2014, 72, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.J.; Phillips, C.V.; Gateno, J.; Teichgraeber, J.F.; Christensen, A.M.; Gliddon, M.J.; Lemoine, J.J.; Liebschner, M.A. Cost-Effectiveness Analysis for Computer-Aided Surgical Simulation in Complex Cranio-Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2006, 64, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.M. Process planning for the rapid machining of custom bone implants. Master’s Thesis, Iowa State University, Ames, IA, USA, 2011. [Google Scholar]
- Fazel, R. Biomedical Engineering-from Theory to Applications; InTechOpen: Rijeka, Croatia, 2011. [Google Scholar]


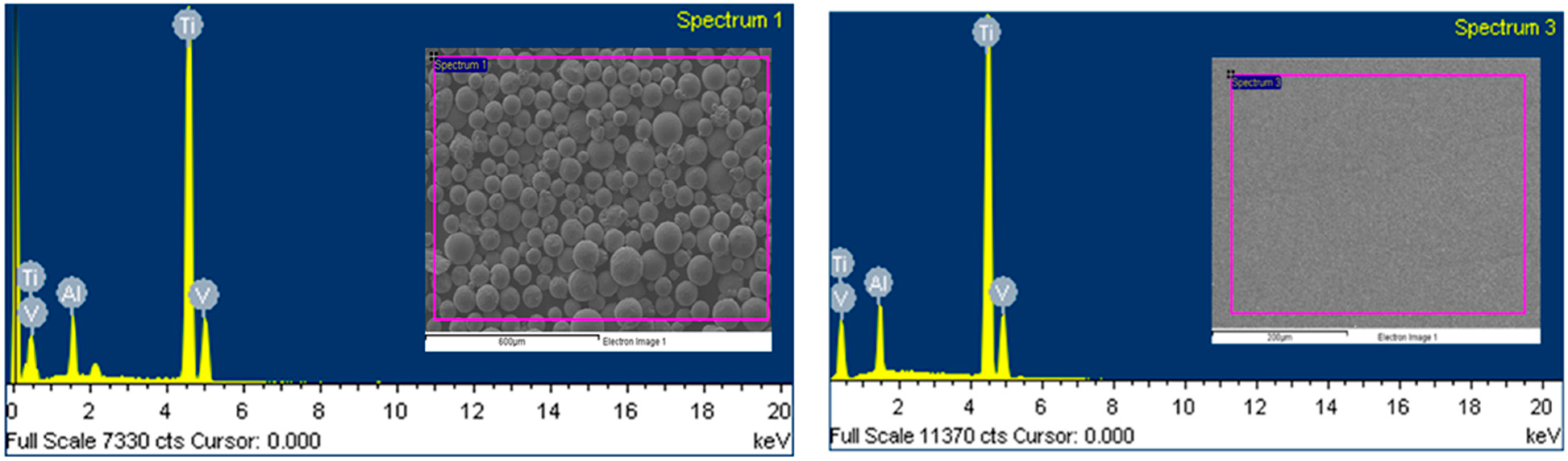
| Chemical Specification (wt. %) | Al (Aluminum) | V (Vanadium) | Ti (Titanium) |
|---|---|---|---|
| ASTM [F136] standards [27] Feedstock powder | 5.5–6.5 6.4 | 3.5–4.5 4.0 | 88–91 89.6 |
| EBM Fabrication Specimen | 6.24 | 3.89 | 89.87 |
| Study Group | RMS (mm) | MAE (mm) | ||
|---|---|---|---|---|
| First Stage | Second Stage | First Stage | Second Stage | |
| Group-1 (EBM titanium reconstruction plate with mesh) | 2.5270 | 2.0616 | 1.951 | 1.362 |
| Group-2 (EBM titanium reconstruction plate without mesh) | 2.6545 | 2.2696 | 2.118 | 1.461 |
| Group-3 (Commercial reconstruction plate) | 2.6692 | 2.2880 | 2.149 | 1.593 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiduddin, K.; Hammad Mian, S.; Alkindi, M.; Ramalingam, S.; Alkhalefah, H.; Alghamdi, O. An In Vivo Evaluation of Biocompatibility and Implant Accuracy of the Electron Beam Melting and Commercial Reconstruction Plates. Metals 2019, 9, 1065. https://doi.org/10.3390/met9101065
Moiduddin K, Hammad Mian S, Alkindi M, Ramalingam S, Alkhalefah H, Alghamdi O. An In Vivo Evaluation of Biocompatibility and Implant Accuracy of the Electron Beam Melting and Commercial Reconstruction Plates. Metals. 2019; 9(10):1065. https://doi.org/10.3390/met9101065
Chicago/Turabian StyleMoiduddin, Khaja, Syed Hammad Mian, Mohammed Alkindi, Sundar Ramalingam, Hisham Alkhalefah, and Osama Alghamdi. 2019. "An In Vivo Evaluation of Biocompatibility and Implant Accuracy of the Electron Beam Melting and Commercial Reconstruction Plates" Metals 9, no. 10: 1065. https://doi.org/10.3390/met9101065
APA StyleMoiduddin, K., Hammad Mian, S., Alkindi, M., Ramalingam, S., Alkhalefah, H., & Alghamdi, O. (2019). An In Vivo Evaluation of Biocompatibility and Implant Accuracy of the Electron Beam Melting and Commercial Reconstruction Plates. Metals, 9(10), 1065. https://doi.org/10.3390/met9101065





