Effect of T6 Heat Treatment on Microstructure and Hardness of Nanosized Al2O3 Reinforced 7075 Aluminum Matrix Composites
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
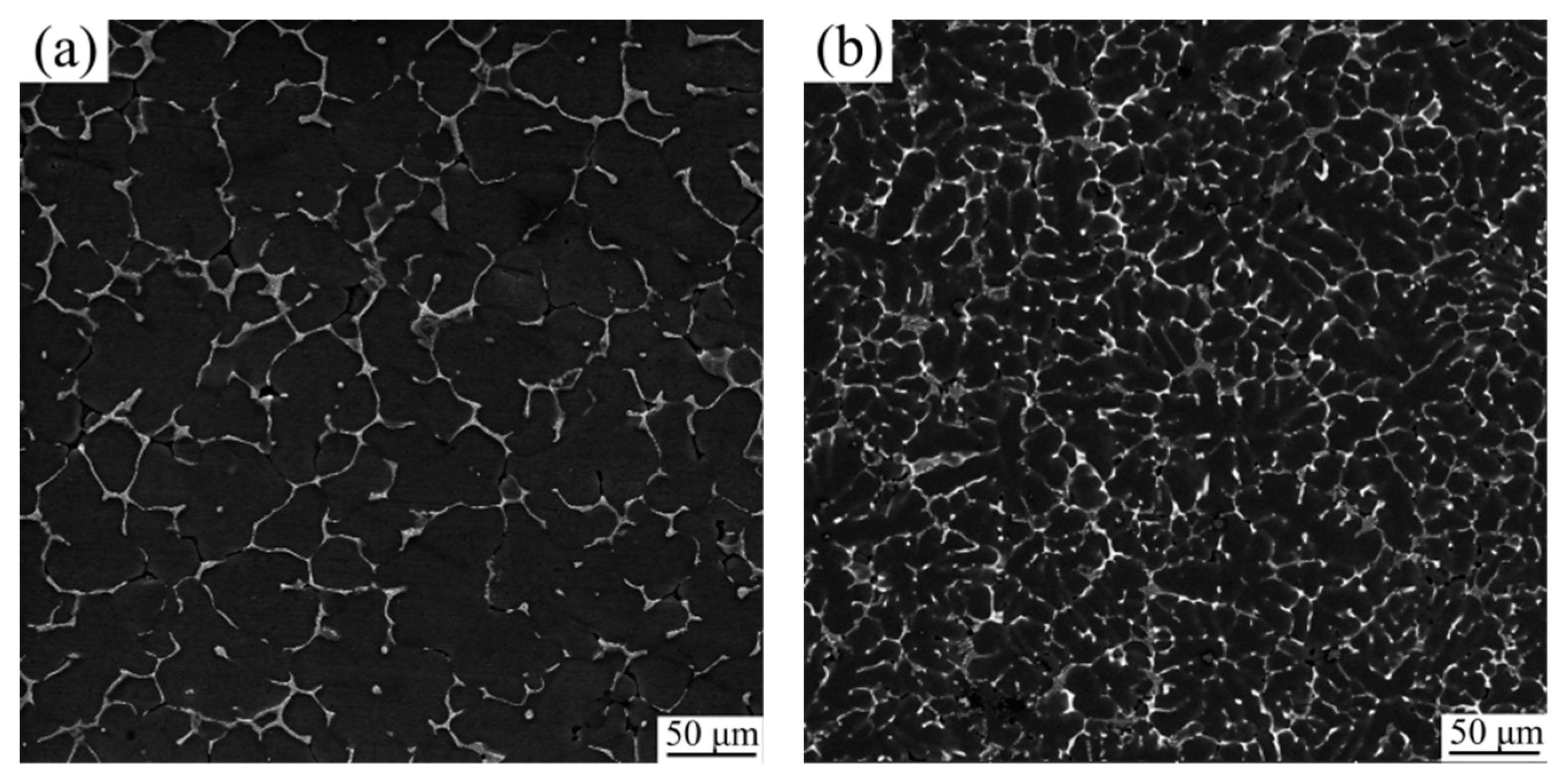
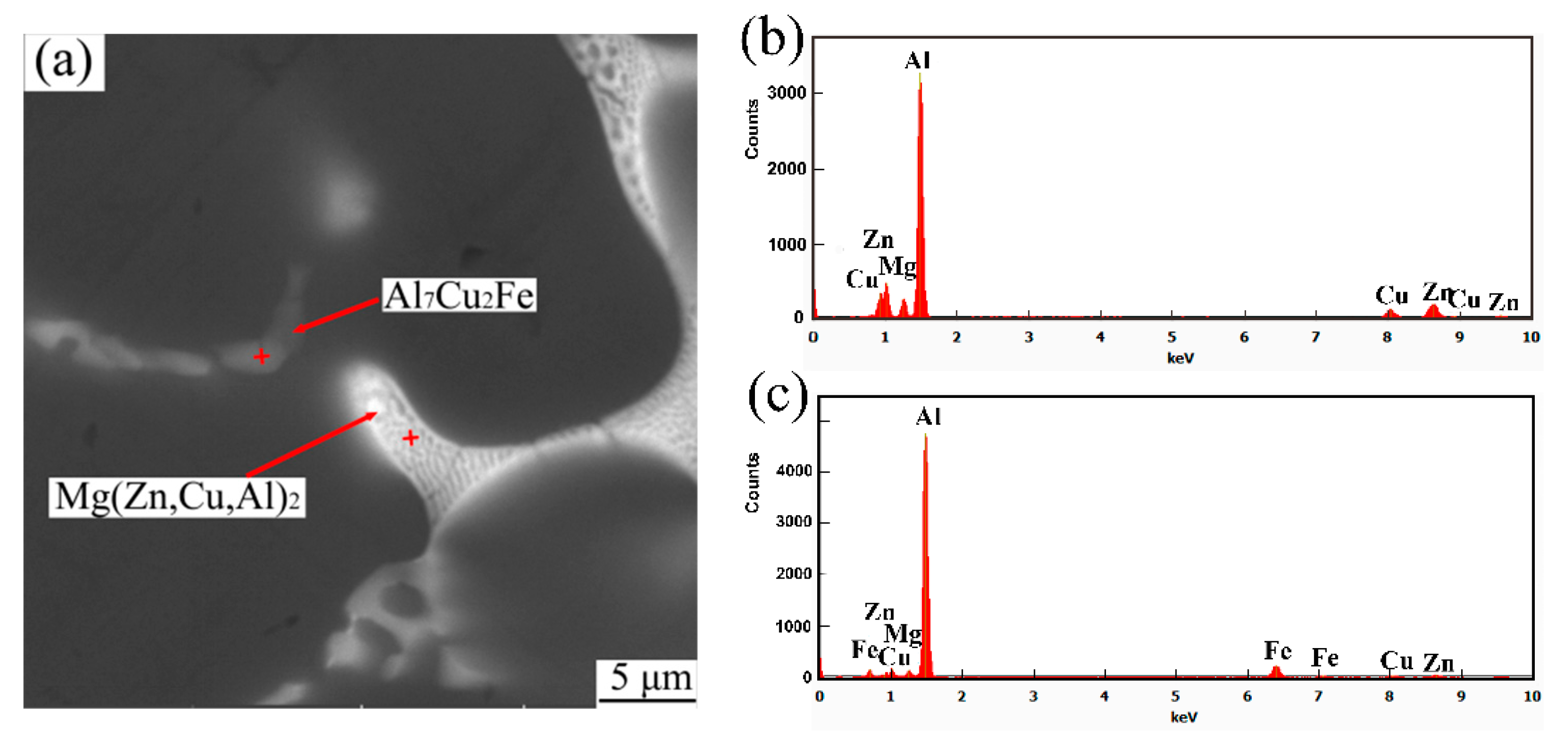
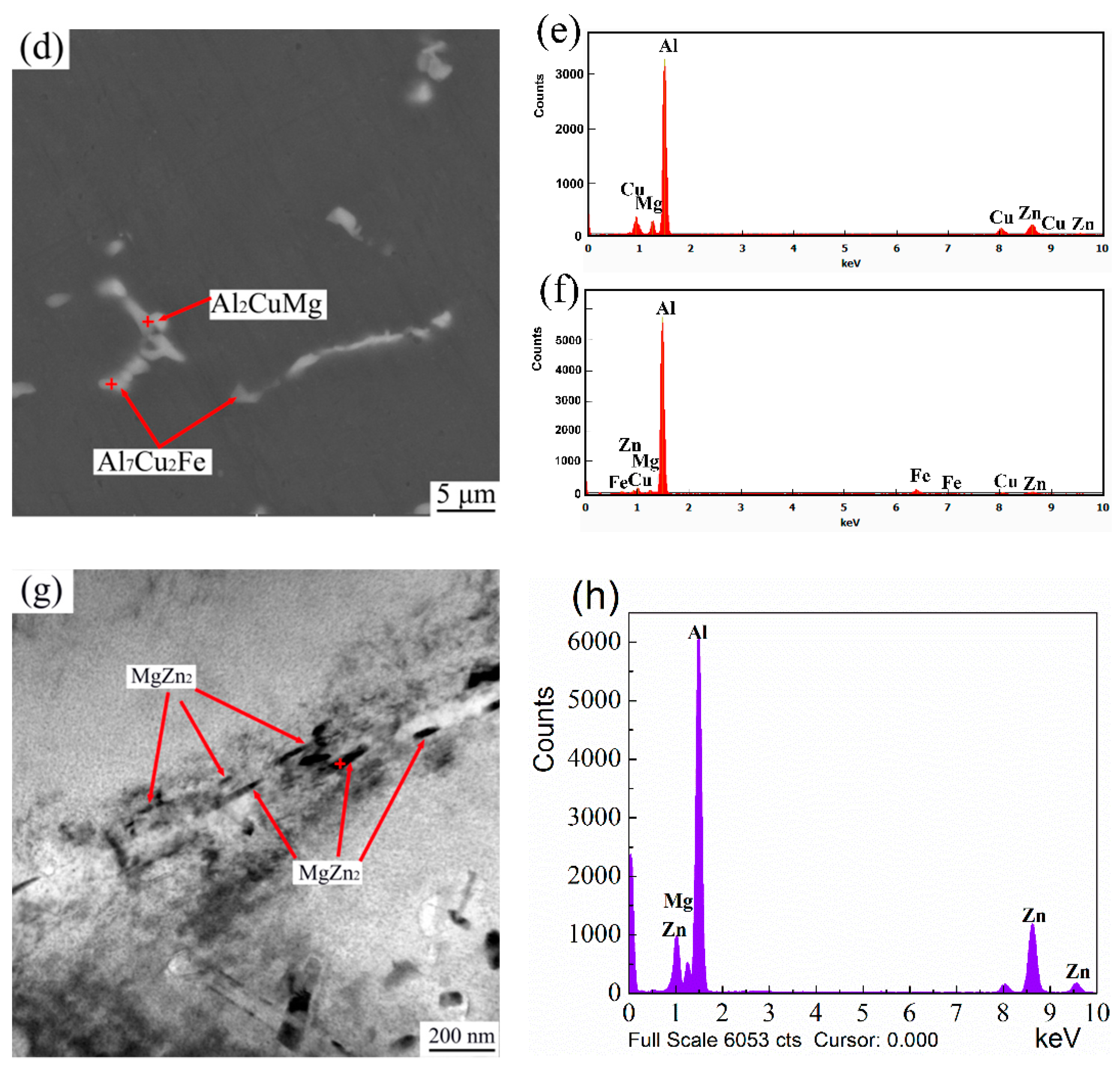
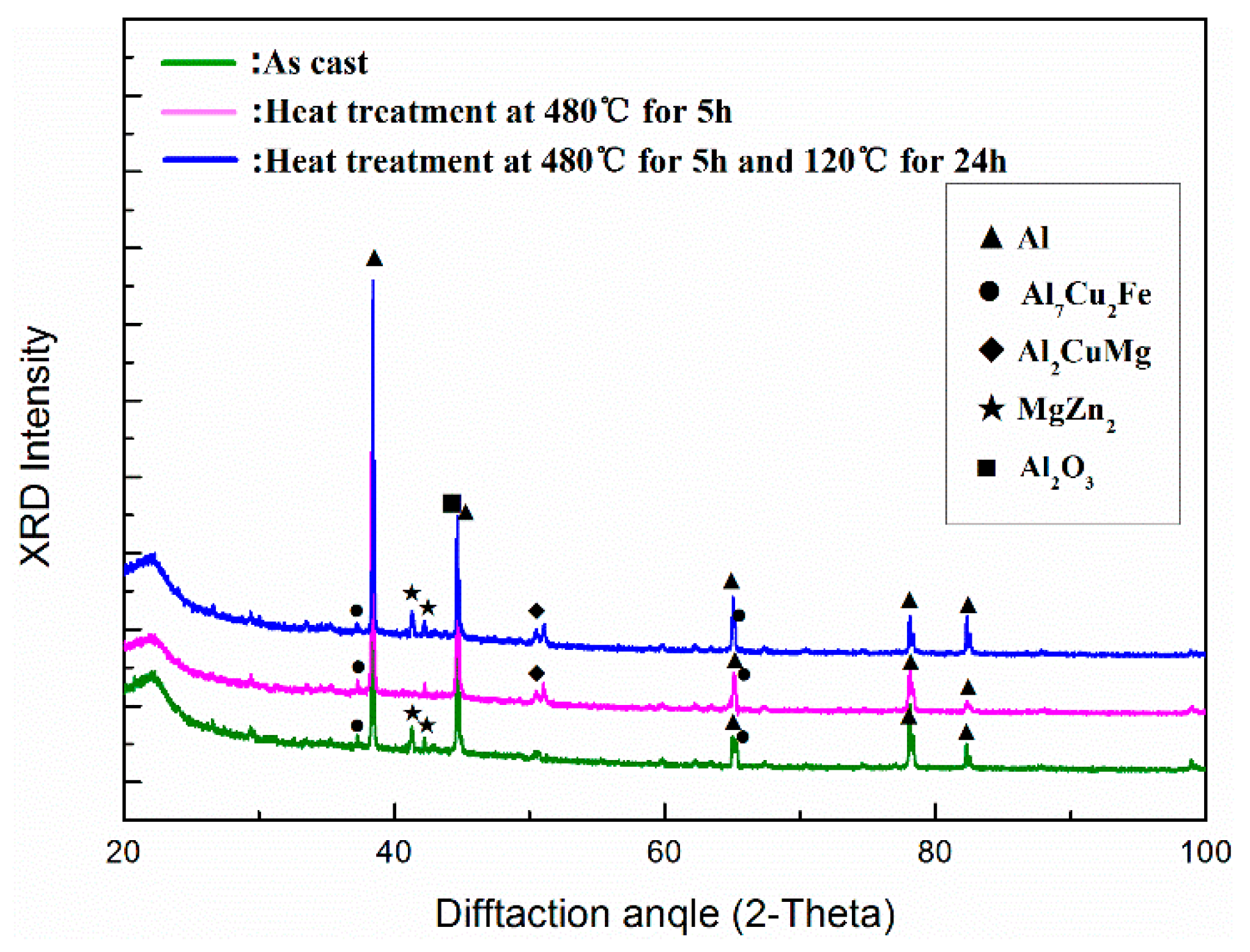
3.1. Microstructure Evolution
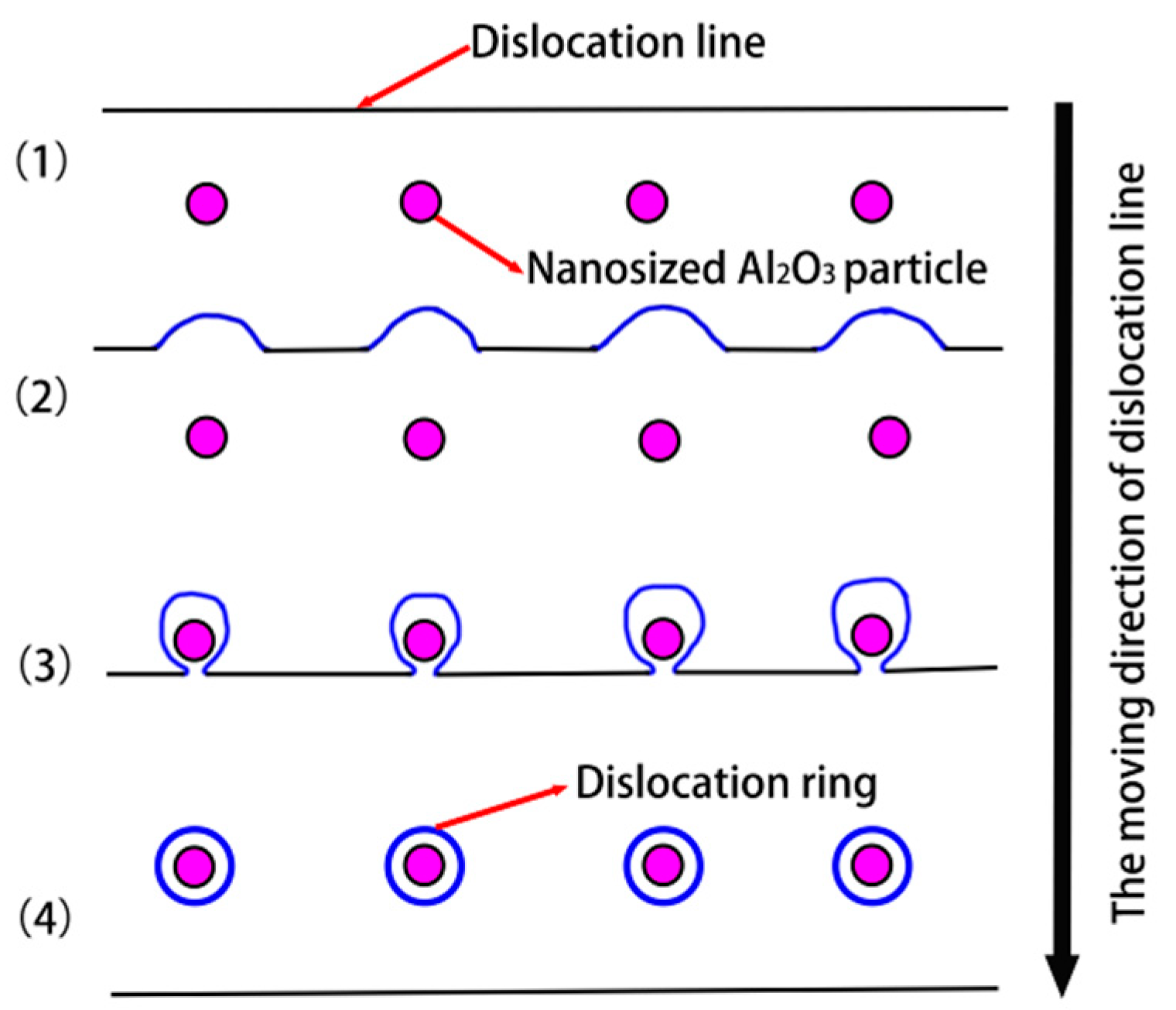
3.2. Strengthening Mechanism and Hardness
4. Conclusions
- (1)
- The as-cast microstructure of Al2O3np/7075 composites consists of α-Al, Al2O3np particles and eutectic phases Mg(Zn,Cu,Al)2 and Al7Cu2Fe.
- (2)
- In response to solution treatment at 480 °C for 5 h, Mg(Zn,Cu,Al)2 phases gradually dissolve into the α-Al matrix and a new eutectic phase Al2CuMg forms. The morphology and size of Al7Cu2Fe phases remain nearly unchanged. After aging treatment, small MgZn2 phases separate out.
- (3)
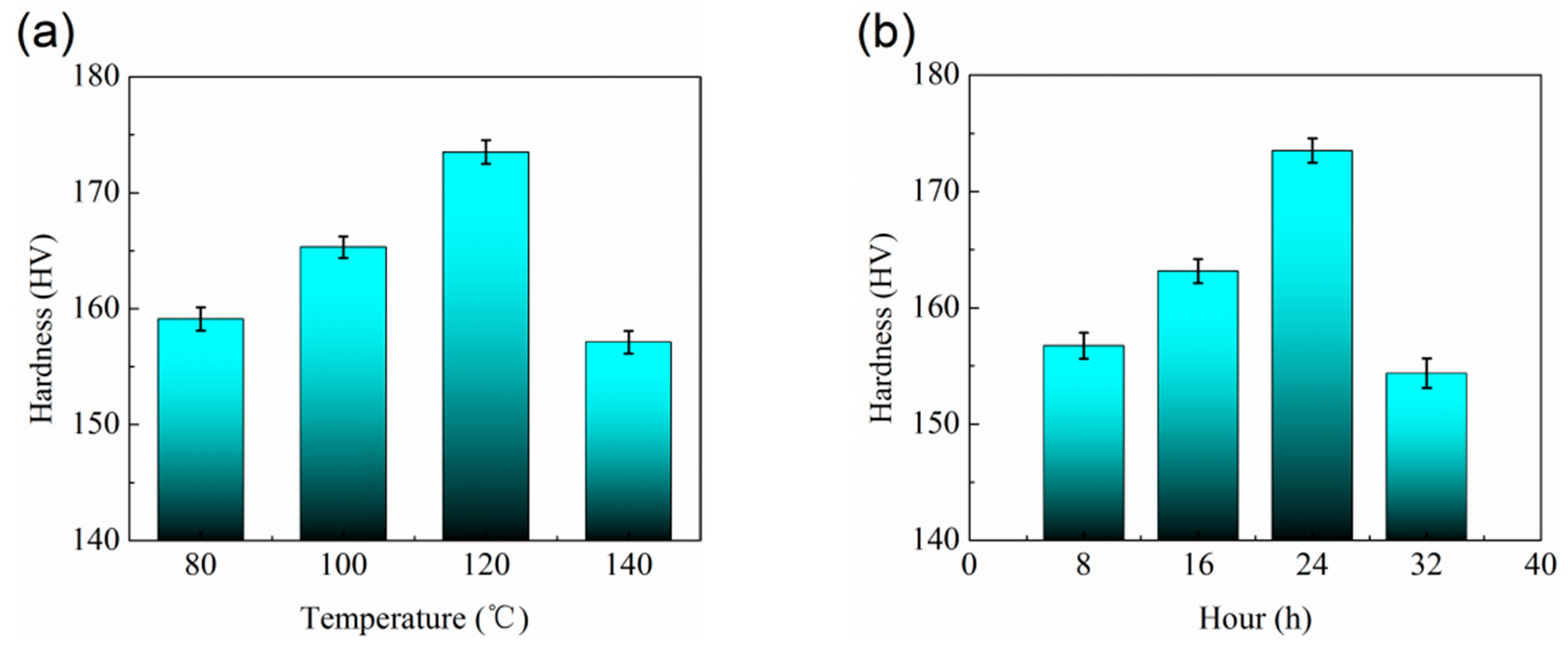
- The optimum T6 heat treatment situation for Al2O3np/7075 composites is the solution treatment at 480 °C for 5 h and aging treatment at 120 °C for 24 h.
- (4)
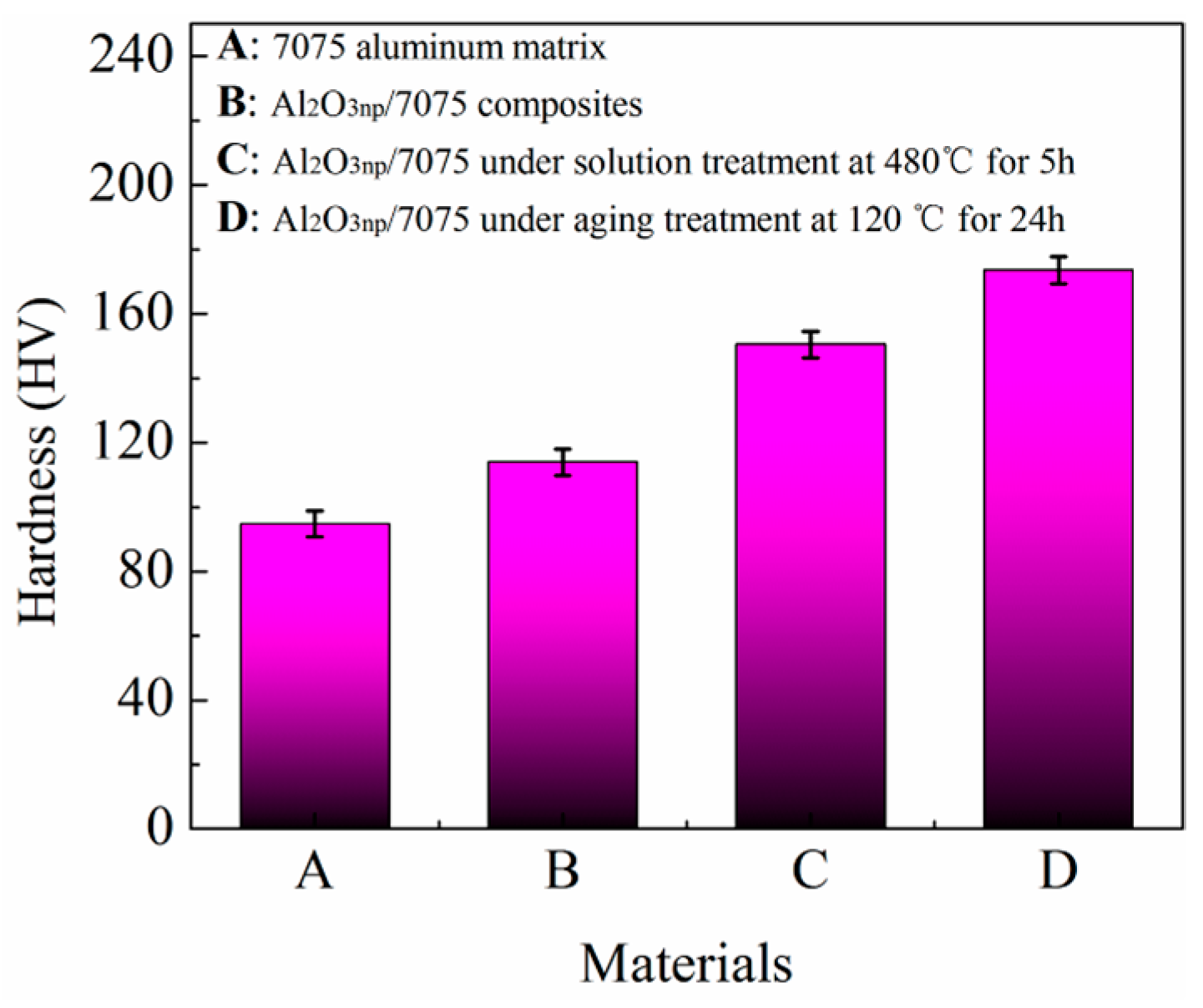
- Compared to the hardness of 7075 aluminum alloy matrix (94.7 HV), the hardness of Al2O3np/7075 composites (113.8 HV) increased ~20%. Compared to the hardness of Al2O3np/7075 composites (113.8 HV), the hardness of Al2O3np/7075 composites under solution treatment (150.4 HV) at 480 °C for 5 h increased ~32% and the hardness of Al2O3np/7075 composites under optimum T6 heat treatment (173.5 HV) increased ~52%.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.H.; Yan, H. Constitutive behavior of Al2O3np/Al7075 composites with a high solid fraction for thixoforming. J. Alloys Compd. 2017, 708, 751–762. [Google Scholar] [CrossRef]
- Alam, S.N.; Kumar, L. Mechanical properties of aluminium based metal matrix composites reinforced with graphite nanoplatelets. Mater. Sci. Eng. A 2016, 667, 16–32. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, G.; Che, C.; Wang, Y. Microstructure, mechanical properties and wear behavior of the rheoformed 2024 aluminum matrix composite component reinforced by Al2O3 nanoparticles. Metals 2018, 8, 460. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, G.Z.; Cui, W.; Ren, S.B.; Wang, Q.J.; Qu, X.H. Effect of Al2O3sf addition on the friction and wear properties of (SiCp + Al2O3sf)/Al2024 composites fabricated by pressure infiltration. Int. J. Miner. Metall. Mater. 2018, 25, 375–382. [Google Scholar] [CrossRef]
- Qiu, F.; Tong, H.T.; Gao, Y.Y.; Zou, Q.; Dong, B.X.; Li, Q.; Chu, J.G.; Chang, F.; Shu, S.L.; Jiang, Q.C. Microstructures and compressive properties of Al matrix composites reinforced with bimodal hybrid in-situ nano-/micro-sized TiC particles. Materials 2018, 11, 1284. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Gallardo, C.; Estrada-Guel, I.; López-Meléndez, C.; Ledezma-Sillas, E.; Castañeda-Balderas, R.; Pérez-Bustamante, R.; Herrera-Ramírez, J.M. B4C particles reinforced Al2024 composites via mechanical milling. Metals 2018, 8, 647. [Google Scholar] [CrossRef]
- Walczak, M.; Pieniak, D.; Zwierzchowski, M. The tribological characteristics of SiC particle reinforced aluminium composites. Arch. Civ. Mech. Eng. 2015, 15, 116–123. [Google Scholar] [CrossRef]
- Chen, X.H.; Yan, H.; Jie, J.P. Effects of Ti addition on microstructure and mechanical properties of 7075 alloy. Int. J. Cast Met. Res. 2015, 28, 151–157. [Google Scholar] [CrossRef]
- Vencl, A.; Bobic, I.; Arostegui, S.; Bobic, B.; Marinković, A.; Babić, M. Structural, mechanical and tribological properties of A356 aluminium alloy reinforced with Al2O3, SiC and SiC + graphite particles. J. Alloys Compd. 2010, 506, 631–639. [Google Scholar] [CrossRef]
- Issa, H.K.; Taherizadeh, A.; Maleki, A.; Ghaei, A. Development of an aluminum/amorphous nano-SiO2 composite using powder metallurgy and hot extrusion processes. Ceram. Int. 2017, 43, 14582–14592. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; Li, X. Study on bulk aluminum matrix nano-composite fabricated by ultrasonic dispersion of nano-sized SiC particles in molten aluminum alloy. Mater. Sci. Eng. 2004, 380, 378–383. [Google Scholar] [CrossRef]
- Liu, P.; Wang, A.Q.; Xie, J.P.; Hao, S.M. Effect of heat treatment on microstructure and mechanical properties of SiCp/2024 aluminum matrix composite. J. Wuhan Univ. Technol. 2015, 30, 1229–1233. [Google Scholar] [CrossRef]
- Li, N.; Yan, H.; Wang, Z.W. Effects of heat treatment on the tribological properties of SiCp/Al-5Si-1Cu-0.5Mg composite processed by electromagnetic stirring method. Appl. Sci. 2018, 8, 372. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Zangeneh, S.; Shahmir, H.; Saghafi, M.; Emamy, M. Heat treatment effect on the microstructure, tensile properties and dry sliding wear behavior of A356–10%B4C cast composites. Mater. Des. 2010, 39, 4414–4422. [Google Scholar] [CrossRef]
- Albiter, A.; León, C.A.; Drew, R.A.L.; Bedolla, E. Microstructure and heat-treatment response of Al-2024/TiC composites. Mater. Sci. Eng. A 2000, 289, 109–115. [Google Scholar] [CrossRef]
- Chen, X.H.; Yan, H. Effect of nanoparticle Al2O3 addition on microstructure and mechanical properties of 7075 alloy. Int. J. Cast Met. Res. 2016, 28, 337–344. [Google Scholar] [CrossRef]
- Chen, X.H.; Yan, H. Fabrication of nanosized Al2O3 reinforced aluminum matrix composites by subtype multifrequency ultrasonic vibration. J. Mater. Res. 2015, 30, 2197–2209. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Ma, L.; Yi, J. Effects of solution treatment on microstructure and high-cycle fatigue properties of 7075 aluminum alloy. Metals 2017, 7, 193. [Google Scholar] [CrossRef]
- Fan, X.G.; Jiang, D.M.; Meng, Q.C.; Zhong, L. The microstructural evolution of an Al-Zn-Mg-Cu alloy during homogenization. Mater. Lett. 2006, 60, 1475–1479. [Google Scholar] [CrossRef]
- Zou, X.L.; Yan, H.; Chen, X.H. Evolution of second phases and mechanical properties of 7075 Al alloy processed by solution heat treatment. Trans. Nonferr. Met. Soc. China 2017, 27, 2146–2155. [Google Scholar] [CrossRef]
- Deng, Y.L.; Wan, L.; Wu, L.H.; Zhang, Y.Y.; Zhang, X.M. Microstructural evolution of Al-Zn-Mg-Cu alloy during homogenization. J. Mater. Sci. 2011, 46, 875–881. [Google Scholar] [CrossRef]
- Schultz, B.F.; Ferguson, J.B.; Rohatgi, P.K. Microstructure and hardness of Al2O3 nanoparticle reinforced Al-Mg composites fabricated by reactive wetting and stir mixing. Mater. Sci. Eng. A 2011, 530, 87–97. [Google Scholar] [CrossRef]
- You, G.L.; Ho, N.J.; Kao, P.W. In-situ formation of Al2O3 nanoparticles during friction stir processing of Al/SiO2 composite. Mater. Charact. 2013, 80, 1–8. [Google Scholar] [CrossRef]
- Hu, Z.H.; Peng, X.; Wu, G.H.; Cheng, D.Q.; Liu, W.C.; Zhang, L.; Ding, W.J. Microstructure evolution and mechanical properties of rheo-processed ADC12 alloy. Trans. Nonferr. Met. Soc. China 2016, 26, 3070–3080. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Jayalakshmi, S.; Gupta, M. Effect of addition of mutually soluble and insoluble metallic elements on the microstructure, tensile and compressive properties of pure magnesium. Mater. Sci. Eng. A 2011, 530, 149–160. [Google Scholar] [CrossRef]
- Lei, Z.B.; Zhao, K.; Wang, Y.G.; An, L.N. Thermal expansion of Al matrix composites reinforced with hybrid micro-/nano-sized Al2O3 particles. J. Mater. Sci. Technol. 2013, 40, 1–4. [Google Scholar] [CrossRef]
- Fleischer, R.L. Substitutional solution hardening durcissement de solution par substitution verfestigung in substitutionsmischkkistallen. Acta Metall. Sin. 1963, 11, 203. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- He, Z.J. Mechanical Properties of Metals, 1st ed.; Metallurgical Industry Press: Beijing, China, 1989. [Google Scholar]
- Orowan, E. Internal Stresses in Metals and Alloys, 1st ed.; Institute of Metals: London, UK, 1948. [Google Scholar]
- Karabay, S.; Bayraklillar, M.S.; Balci, E. Influence of different heat treatments on the solid particle erosion behavior of aluminum alloy AA7075 in industrial applications. Acta Phys. Pol. A 2015, 127, 1052–1054. [Google Scholar] [CrossRef]
- Ku, M.H.; Hung, F.Y.; Lui, T.S.; Lai, J.J. Enhanced formability and accelerated precipitation behavior of 7075 Al alloy extruded rod by high temperature aging. Metals 2018, 8, 648. [Google Scholar] [CrossRef]


| Zn | Mg | Cu | Mn | Cr | Si | Fe | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| 5.23 | 2.10 | 1.45 | 0.30 | 0.23 | 0.22 | 0.22 | 0.16 | Balance |
| No. | Heat Treatment Processes Scheme |
|---|---|
| 1 | (480 °C, 5 h) + water quench (25 ± 5 °C) |
| 2 | (480 °C, 5 h) + water quench (25 ± 5 °C) + (120 °C, 8 h) |
| 3 | (480 °C, 5 h) + water quench (25 ± 5 °C) + (120 °C, 16 h) |
| 4 | (480 °C, 5 h) + water quench (25 ± 5 °C) + (120 °C, 24 h) |
| 5 | (480 °C, 5 h) + water quench (25 ± 5 °C) + (120 °C, 32 h) |
| 6 | (480 °C, 5 h) + water quench (25 ± 5 °C) + (80 °C, 24 h) |
| 7 | (480 °C, 5 h) + water quench (25 ± 5 °C) + (100 °C, 24 h) |
| 8 | (480 °C, 5 h) + water quench (25 ± 5 °C) + (140 °C, 24 h) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.-X.; Yan, H.; Liu, W.; Zou, X.-L.; Tang, B.-B. Effect of T6 Heat Treatment on Microstructure and Hardness of Nanosized Al2O3 Reinforced 7075 Aluminum Matrix Composites. Metals 2019, 9, 44. https://doi.org/10.3390/met9010044
Zhang P-X, Yan H, Liu W, Zou X-L, Tang B-B. Effect of T6 Heat Treatment on Microstructure and Hardness of Nanosized Al2O3 Reinforced 7075 Aluminum Matrix Composites. Metals. 2019; 9(1):44. https://doi.org/10.3390/met9010044
Chicago/Turabian StyleZhang, Peng-Xiang, Hong Yan, Wei Liu, Xiu-Liang Zou, and Bin-Bing Tang. 2019. "Effect of T6 Heat Treatment on Microstructure and Hardness of Nanosized Al2O3 Reinforced 7075 Aluminum Matrix Composites" Metals 9, no. 1: 44. https://doi.org/10.3390/met9010044
APA StyleZhang, P.-X., Yan, H., Liu, W., Zou, X.-L., & Tang, B.-B. (2019). Effect of T6 Heat Treatment on Microstructure and Hardness of Nanosized Al2O3 Reinforced 7075 Aluminum Matrix Composites. Metals, 9(1), 44. https://doi.org/10.3390/met9010044





